Abstract
The Ca2+ indicator dye fluo-5F was excited by an argon ion laser to measure changes in free Ca2+ concentration ([Ca2+]i) in the outer segments of isolated salamander rods rapidly exposed to a 0 Ca2+, 0 Na+ solution designed to minimise surface membrane Ca2+ fluxes. Over 30-60 s of laser illumination, the fluorescence first increased rapidly and then declined at a rate that was much slower than in Ringer solution and consistent with previous physiological evidence that 0 Ca2+, 0 Na+ solution greatly retards light-induced changes in [Ca2+]i.
The initial increase in fluorescence was investigated with a sequence of 100 ms laser flashes presented at 5 s intervals. The fluorescence evoked by the second laser flash was on average 30 % larger than the first, and subsequent responses exhibited a slow decline like that measured with continuous laser exposures. The initial increase in fluorescence did not depend upon the timing of exposure to 0 Ca2+, 0 Na+ solution but appeared to be evoked by exposure to the laser light.
Both the increase and subsequent decline in fluorescence measured with brief laser flashes could be reduced by incorporation of the Ca2+ chelator BAPTA. This and other results indicate that the fluorescence increase was unlikely to have been caused by a change in the affinity of fluo-5F for Ca2+ or an increase in the quantity of incorporated dye available to bind Ca2+ but reflects an actual release of intracellular Ca2+ within the outer segment.
The pool of Ca2+ available to be released could be decreased if, before the first laser flash, the rod was exposed to light bright enough to bleach a substantial fraction of the photopigment. The releasable pool could also be depleted by exposure to saturating light of much lower intensity if delivered in Ringer solution but not if delivered in 0 Ca2+, 0 Na+ solution. We conclude that Ca2+ can be released within the outer segment both by the bleaching of rhodopsin and by the reduction in [Ca2+]i which normally accompanies illumination in Ringer solution.
The activation of rhodopsin appears somehow to induce the release of Ca2+ from a binding site or store within the outer segment. Substantial release, however, required stimulating light of an intensity sufficient to bleach a considerable fraction of the visual pigment. It therefore seems unlikely that such release contributes to the normal Ca2+-mediated modulation of transduction during light adaptation. The mechanism and physiological function of light-induced Ca2+ release are unknown.
The cytoplasmic free Ca2+ concentration ([Ca2+]i) in vertebrate photoreceptors is believed to be governed by the balance of Ca2+ fluxes across the outer segment membrane. Ca2+ enters as a component of the dark current through the cyclic nucleotide-gated channels (Yau & Nakatani, 1984a; Hodgkin et al. 1985) and is extruded via Na+-Ca2+,K+ exchange (Yau & Nakatani, 1984b; Hodgkin, et al. 1987; Cervetto, et al. 1989). When the dark current is suppressed during the response to light, this balance is upset and [Ca2+]i falls (Yau & Nakatani, 1985). The reduction in [Ca2+]i plays a crucial role in the modulation of sensitivity in vertebrate photoreceptors during light and dark adaptation (for recent reviews see Pugh et al. 1999; Fain et al. 2001).
A light-induced decrease in [Ca2+]i was first measured directly with the Ca2+-sensitive photoprotein aequorin (McNaughton et al. 1986) and then with the Ca2+-sensitive fluorescent dyes fura-2 (Ratto et al. 1988; McCarthy et al. 1994, 1996), indo-1 (Gray-Keller & Detwiler, 1994) and fluo-3 (Sampath et al. 1998). All of these measurements appear to show that Ca2+ declines monotonically when the cyclic nucleotide-gated channels close after light stimulation, with a time course variously described by the sum of one to three exponential functions, thought to reflect the time course of Ca2+ extrusion by Na+-Ca2+,K+ exchange and buffering by Ca2+ binding proteins in the outer segment. These experiments, together with more recent studies which suggest little or no communication of Ca2+ between the inner and outer segments (Krizaj & Copenhagen, 1998), lend support to the notion that the Ca2+ economy of the outer segment is dominated by the dynamic balance between Ca2+ influx and Ca2+ efflux across the surface membrane.
It seemed possible, however, that additional mechanisms of Ca2+ homeostasis might be revealed if these normally-dominant fluxes were prevented. We therefore measured [Ca2+]i in rods whose outer segments were exposed to a 0 Ca2+, 0 Na+ solution designed to minimise surface membrane fluxes of Ca2+ (Matthews et al. 1988; Nakatani & Yau, 1988; Fain et al. 1989). The removal of external Na+ suppresses Ca2+ efflux via Na+-Ca2+,K+ exchange, while Ca2+ influx through the cyclic nucleotide-gated channels is minimised in darkness by the absence of external Ca2+, and additionally during measurement of [Ca2+]i by the suppression of the dark current by the intense light from the laser used to excite the fluorescent Ca2+ indicator. Physiological evidence suggests that exposure of the outer segment to such a solution substantially retards the normal light-induced fall in [Ca2+]i, produced by flashes presented either in darkness or during steady light (Fain et al. 1989).
Having suppressed Ca2+ fluxes across the outer segment membrane, we discovered a light-dependent increase in Ca2+ indicator fluorescence which could be suppressed by BAPTA incorporation or exposure to prior illumination. This fluorescence increase is likely to reflect a light-induced release of Ca2+ within the outer segment (Schröder & Fain, 1984; Fain & Schröder, 1990). Preliminary results of this study have been reported to the Physiological Society (Matthews & Fain, 1999a, 2000a), the Association for Research in Vision and Ophthalmology (Matthews & Fain, 1999b), and the American Society of Biophysics (Fain & Matthews, 2000).
METHODS
Preparation
Aquatic tiger salamander (Ambystoma tigrinum) were dark-adapted overnight, killed by decapitation and pithing, and enucleated. Photoreceptors were dissociated mechanically from the isolated retina under infrared illumination. Dissociated cells were transferred to the recording chamber and incubated for 30 min with 10 μm fluo-5F acetoxymethyl ester (fluo-5F AM; Molecular Probes, Inc., Eugene, OR, USA) in amphibian Ringer solution (Sampath et al. 1998), then excess unhydrolysed dye was removed by bath perfusion.
An isolated rod photoreceptor was drawn inner segment first into a suction pipette so that the outer segment was exposed to the bathing solution. Rapid solution changes were effected by laterally translating the boundary between two streams of solution across the exposed outer segment (Matthews, 1996). The 0 Ca2+, 0 Na+ solution was identical in composition to the ‘0 Ca2+, 0 Mg2+, 0 Na+ solution’ used previously (Matthews, 1995, 1996) in which choline was substituted for Na+. In a few experiments guanidinium was used instead of choline as a Na+ substitute (solution G1 of Fain et al. 1989). All experiments were performed at 22 °C.
Ca2+ measurement
Ca2+ was measured as described previously using a laser spot technique (Sampath et al. 1998, 1999; Matthews & Fain, 2000b) derived from a method originally developed for measuring changes in free Ca2+ in skeletal muscle (Escobar et al. 1994). Briefly, an argon ion laser tuned to 514 nm (Model 60, American Laser Corporation, Salt Lake City, UT, USA) was used to illuminate a pinhole which was imaged by the high-numerical-aperture objective lens of the microscope to evoke fluorescence from a spot ≈10 μm in diameter on the outer segment of a rod loaded with the Ca2+ indicator fluo-5F.
In comparison with our previous work, the experimental procedure incorporated a number of important modifications (Matthews & Fain, 2000b, c). First, we used a Nikon Eclipse 300 microscope with a × 40 oil-immersion infinity-corrected objective (1.3NA, CFI 60, Nikon UK), that has a working distance of more than 200 μm above the cover slip that serves as the floor of the chamber. The rod could therefore be brought into focus sufficiently above the chamber floor to allow ample flow around the outer segment, permitting rapid solution exchange. Second, fluorescence was detected with a photomultiplier tube (Model 9130/100A, Electron Tubes Ltd, UK) instead of a photodiode (as was the case in Sampath et al. 1998), so as to allow the intensity of laser illumination to be decreased with reflective neutral density filters (New Focus Inc, Santa Clara, CA, USA and Newport Corporation, Irvine, CA, USA) to reduce bleaching of the calcium indicator dye. In experiments which employed continuous laser exposures (as in Fig. 2A), the intensity of the laser was attenuated to around 3 × 109 photons μm−2 s−1, a factor of 50 times lower than used previously (Sampath et al. 1998). At this intensity, even a 60 s laser exposure produced no more than a 1 % decrease in the magnitude of the fluorescence from a dye film (see below). For laser flash protocols as in Fig. 3, which delivered 100 ms laser exposures at 5 s intervals, the laser intensity was typically raised to around 3 × 1010 photons μm−2 s−1 to increase the signal-noise ratio. The precise laser intensities used in each experiment are given in the figure legends. Laser exposures were controlled using a fast electromagnetic shutter (Model LS6, Vincent Associates, Rochester, NY, USA).
Figure 2. Fluo 5F fluorescence recorded from a salamander rod in 0 Ca2+, 0 Na+ solution.
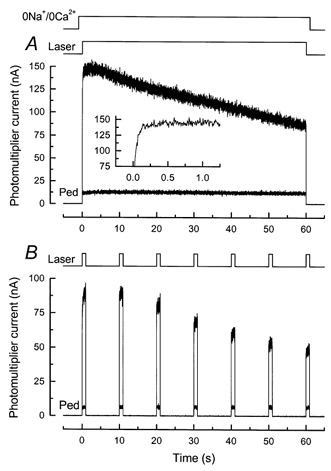
Fluorescence magnitude is represented by the current recorded from the photomultiplier. A, dark-adapted rod was stepped to 0 Ca2+, 0 Na+ solution (see Methods) 1 s before beginning of laser exposure. Laser shutter was opened for 60 s (laser intensity, 2.5 × 109 photons μm−2 s−1), giving the larger of the two traces. One second after laser exposure, the rod was rapidly returned to Ringer solution; approximately 45 s later, the outer segment was stepped a second time to 0 Ca2+, 0 Na+solution and the laser shutter opened for 60 s to give smaller trace (Ped). Inset, larger of two traces at higher temporal resolution, showing time-dependent increase in fluorescence (compare to inset of Fig. 1A). B, same protocol and laser intensity as in A for another rod, but instead of continuous laser illumination the rod was repeatedly exposed to the laser for 1 s at 10 s intervals, effectively reducing the total exposure of the rod to the laser by a factor of 10. Larger signals were recorded from dark-adapted rod; smaller signals (Ped) from a second presentation after return of the outer segment to Ringer solution.
Figure 3. Fluo 5F fluorescence recorded from a salamander rod in 0 Ca2+, 0 Na+ solution in response to brief laser flashes.
A, dark-adapted rod was stepped to 0 Ca2+, 0 Na+ solution 1 s before a series of 5 laser flashes 100 ms in duration, separated by 5 s intervals. Laser intensity, 2.4 × 1010 photons μm−2 s−1. Fluorescence amplitudes to same laser flash protocol during a second exposure to 0 Ca2+, 0 Na+ solution after return to Ringer solution are indicated by the bars (Ped). B, same stimulus protocol as in A except that first laser pulse was omitted, so that the time between exposure to 0 Ca2+, 0 Na+ solution and the first laser flash was 6 s instead of 1 s.
A final modification in experimental procedure was the use of the dye fluo-5F instead of fluo-3. Fluo 5F offers a number of advantages for these experiments. Since its Kd is higher than for fluo-3 (nominally 2.3 μmvs. 400 nm), changes in fluorescence are a more linear function of Ca2+i, both within the normal physiological range for a rod (about 30-700 nm, see Gray-Keller & Detwiler, 1994; Sampath et al. 1998) and also for the increases in [Ca2+]i which are the focus of this paper. This dye also exhibits a very high ratio of maximum to minimum fluorescence (Fmax/Fmin), even after incorporation into the rod outer segment. For fluo-3, previous absolute calibrations of the fluorescence signal in situ with solutions containing ionomycin and high or low concentrations of Ca2+ (Sampath et al. 1998) gave a value for Fmax/Fmin of 7.3 ± 0.6. In contrast, preliminary experiments of this kind with fluo-5F yielded a mean value for Fmax/Fmin of 55.8 ± 6.4 (n= 5 rods), a value nearly as high as measured previously for fluo-3 in the cuvette. Even this value may be underestimated because of the difficulty of increasing [Ca2+]i in the intact outer segment to a level sufficiently high to saturate fluo-5F. It would therefore appear that fluo-5F yields little Ca2+-insensitive fluorescence in the rod outer segment, suggesting that little fluo-5F is bound or compartmentalised. We shall describe the absolute calibration of fluorescence signals with fluo-5F in more detail in a later publication.
Recording and light stimulation
The fluorescence signal recorded by the photomultiplier was amplified with a low-noise current-voltage converter (Model PDA700, Terahertz Technology Inc., Oriskany, NY, USA), filtered at 50 Hz with an active, 8 pole Bessel filter (Model VBF8 Mk. 4, Kemo Ltd, UK), and digitally sampled at 200 Hz by a PC-compatible computer equipped with an intelligent interface card (Cambridge Research Systems, UK). Suction pipette currents were recorded with a patch-clamp amplifier (Model PC501-A, Warner Instruments Co., Hamden, CT, USA), filtered at 20 Hz and sampled at 200 Hz with the same apparatus used for recording the dye signals.
Light stimuli were delivered from a metal halide discharge lamp (Model HLS-24W, Welch-Allyn, Skaneateles Falls, NY, USA) via a dual-beam optical bench, coupled to the microscope via a bifurcated fibre optic bundle (CUDA Products Corp., Jacksonville, FL, USA) to illuminate an area of the chamber 3 mm in diameter. Stimulus duration was controlled by fast electromagnetic shutters (Model LS6, Vincent Associates, Rochester, NY, USA), and absolute intensity was calibrated at regular intervals with a silicon photodiode optometer (Model S370, Graseby Optronics, Orlando, FL, USA). The brightest intensity which could be delivered by the optical stimulator at 500 nm was of the order of 6-7 × 106 photons μm−2 s−1, which was typically attenuated with calibrated neutral density filters. In some experiments white light was used to stimulate the rods instead of 500 nm light. The effective intensity of the white light was estimated by comparing the relative sensitivity of the electrical response of rods to dim flashes of white and 500 nm light. Rhodopsin bleaching was estimated from the photosensitivity for a vitamin A2-based pigment in solution (Dartnall, 1972), corrected for the difference in dichroism in free solution and in disk membranes (Jones et al. 1993).
To estimate the amplitude of the fluorescence evoked by 100 ms laser flashes, the photomultiplier current was averaged over a time window from 20 ms after the beginning of the flash until the end of the flash. The time of 20 ms was chosen as the settling time for the 50 Hz Bessel filter used in recording the photomultiplier current (Horowitz & Hill, 1980). Similarly, for the few experiments in which we used 10 ms laser flashes, recordings were made with a 500 Hz Bessel filter and data were averaged between 2 and 10 ms.
Fluorescence measurements from dye films
As a control for the kinetics of rod responses and for dye bleaching, we examined the fluorescence evoked from a film of free fluo-5F under conditions for laser stimulation and signal recording identical to those used for measurements from outer segments. Ringer solution was heated to boiling to dissolve 0.5 % agar (BDH Chemicals, UK) and then allowed to cool. Before the solution gelled, free fluo5F (Molecular Probes) was added at a final concentration of 50 μm and the resulting mixture was placed between two glass cover slips, which were separated by a gap of approximately 130 μm created by a further pair of cover slips placed to either side of the gel.
Figure 1A shows the average of eight responses of the dye film to a 60 s continuous laser exposure at a laser intensity similar to that used for the rod outer segment (see e.g. Fig. 2A). The variation in the amplitude of the signal during the 60 s laser exposure was estimated by dividing the response into six 10 s segments and calculating the mean for each segment. The amplitudes of the means were within 1 % of each other for all segments. This indicates that dye bleaching was minimal over this duration at this laser intensity. A small but consistent ‘rounding’ of the fluorescence signal was observed over the first 3-4 s of laser illumination (Fig. 1A, inset). This effect was also visible in measurements of reflected light without a dye film, suggesting that it might represent a time-dependent non-linearity in the photomultiplier when used in current mode. It is, however, quantitatively rather small when compared to the time-dependent increase in fluorescence recorded from a dark-adapted outer segment at a similar temporal resolution (Fig. 2A, inset), representing only around 1.5 % of the total fluorescence signal.
Figure 1. Fluorescence measured from a dye film.
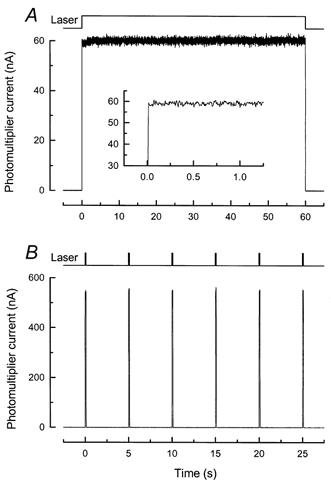
Fluorescence measured from a gel film approximately 130 μm thick containing 50 μm free fluo-5F in Ringer solution with 0.5 % Agar (see Methods). A, continuous 60 s exposure of the dye film to the laser spot at an intensity of 2.2 × 109 photons μm−2 s−1, similar to that used for measurements with continuous laser illumination from rod outer segments (see e.g. Fig. 2A). Trace is average of 8 measurements. Timing of laser illumination is indicated by upper trace. Inset, same data at higher temporal resolution. B, exposure of same dye film to 6 laser flashes 100 ms in duration repeated at 5 s intervals. Intensity of laser was 2.2 × 1010 photons μm−2 s−1, similar to that used for this laser flash protocol on rod outer segments (see e.g. Fig. 3A). Trace is average of 4 measurements. Amplitudes of fluorescence responses were within 1-2 % of one another and showed no evidence of a systematic decrease indicative of dye bleaching. Timing of laser illumination is indicated by upper trace.
Figure 1B shows the average response of the dye film to a series of four presentations of six 100 ms laser flashes at 5 s intervals, at a laser intensity of 2.2 × 1010 photons μm−2 s−1, which is similar to that used for an outer segment with this stimulation protocol (see e.g. Fig. 3A). The amplitudes of the responses of the dye film were averaged between 20 and 100 ms, as for similar responses from outer segments. The mean amplitudes were again within 1-2 % of one another and showed no evidence of systematic dye bleaching.
RESULTS
When a salamander rod outer segment is rapidly superfused with a 0 Ca2+, 0 Na+ solution in which Na+ is replaced by choline or guanidinium and the free Ca2+ concentration has been buffered to a low level, both the influx and efflux of Ca2+ across the outer segment plasma membrane are believed to be largely suppressed (Matthews et al. 1988; Nakatani & Yau, 1988; Fain et al. 1989). Superfusion with such a solution would be expected to have little effect on [Ca2+]i in darkness and, for the most part, to prevent the large and rapid fall in [Ca2+]i which normally accompanies exposure to steady light and the closure of the cyclic nucleotide-gated channels in the plasma membrane (Yau & Nakatani, 1985). If there were little or no movement of Ca2+ between the inner and outer segment (Krizaj & Copenhagen, 1998), the intensity of fluo-5F fluorescence might be expected to remain relatively constant under these conditions, provided the dye faithfully recorded [Ca2+]i.
The results of Fig. 2A show, however, that this expectation was not fulfilled. In this experiment, a dark-adapted rod outer segment was exposed to 0 Ca2+, 0 Na+ solution in which choline had been substituted for sodium. Then, 1 s after the solution change, the outer segment was illuminated by the argon ion laser, which excited both the visual pigment rhodopsin and the previously incorporated fluorescent indicator dye fluo-5F. The laser will have bleached the vast majority of the photopigment within the area of the illuminated spot, while scattered light will have bleached a lesser amount of rhodopsin throughout the rest of the outer segment, together resulting in the rapid and complete suppression of the circulating current (recorded but not shown; see e.g. Sampath et al. 1998). This was accompanied by an initial rise in fluo-5F fluorescence followed by a gradual decline. This decline was considerably slower than would normally be seen following illumination in Ringer solution (see for example Fig. 4A), consistent with physiological evidence showing that superfusion with low Ca2+, 0 Na+ solution greatly retards changes in [Ca2+]i (Fain et al. 1989).
Figure 4. Effect of BAPTA incorporation on the time course of fluo-5F fluorescence decrease in Ringer solution.
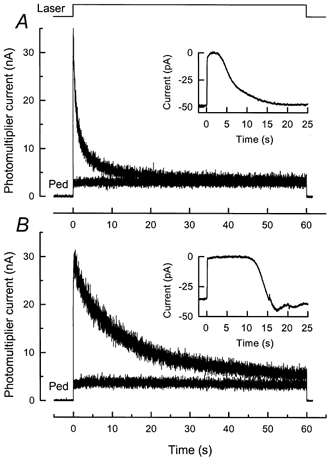
Laser intensity, 2.4 × 109 photons μm−2 s−1. A, fluorescence decrease for a dark-adapted rod in Ringer solution for 60 s laser exposure. Smaller-amplitude trace is for second laser exposure 45 s after first (Ped). The inset shows the suction pipette current response of the same rod to 20 ms flash of 500 nm light of intensity 310 photons μm−2. Response was measured before laser exposure from dark-adapted cell. B, identical protocol for a rod from another piece of retina from the same animal as in A except that the fluo-5F incubation solution also included 50 μm of the acetoxymethyl ester of the Ca2+ chelator BAPTA (BAPTA AM). Note slower decline of fluorescence during the first laser exposure, and the greatly prolonged duration and undershooting recovery of the suction current response (inset).
After the laser exposure, the outer segment was returned to Ringer solution, and then 45 s later the laser exposure was repeated, again in 0 Ca2+, 0 Na+ solution. In contrast to the first exposure of the dark-adapted rod to laser light, only a low and virtually constant level of fluorescence was recorded (Fig. 2A, Ped). This apparently was the result of a substantial reduction in [Ca2+]i by extrusion via Na+-Ca2+,K+ exchange, during the period of exposure of the bleached rod to Ringer solution between the two laser measurements (Sampath et al. 1998).
For the cell of Fig. 2A the fall in fluorescence could be adequately fitted by a single exponential with a time constant of 115 s. Qualitatively similar results were obtained from a further seven cells with 60 s laser exposures and 10 cells with 30 s exposures. The time course of the fluorescence decline was rather variable and in many cells showed more than one temporal component which could not be fitted satisfactorily by a simple sum of exponentials. Experiments similar to those in Fig. 2A with 60 s of laser illumination were also performed on eight cells exposed to a low Ca2+, 0 Na+ solution identical in composition to that used previously (Matthews et al. 1988; Fain et al. 1989), in which sodium was replaced with guanidinium. The results were indistinguishable from those with choline.
Every effort was made in these experiments to reduce the laser intensity to a value that would keep the bleaching of fluo-5F to a minimum (see Methods). Nevertheless, as a further control against a contribution by dye bleaching to the slow decrease in fluorescence, the experiment of Fig. 2B was performed. For this cell the intensity of the laser was the same as in Fig. 2A, but instead of continuous laser illumination, 1 s laser flashes were presented at 10 s intervals, reducing the total light exposure by an order of magnitude. The similar time course for the fluorescence change indicates that bleaching of fluo-5F is unlikely to have made a significant contribution to our measurements.
If the slow decline in fluorescence reflects a gradual and progressive decrease in [Ca2+]i, then it is of undoubted interest, since it suggests that mechanisms may be present for Ca2+ efflux, sequestration or diffusion from the outer segment other than the well-characterised and normally dominant fluxes across the outer segment surface membrane via the cyclic nucleotide-gated channels and Na+/ Ca2+-K+ exchanger. Nevertheless, we shall postpone a study of this phenomenon to a later publication and, in this paper, investigate only the origin of the initial rounding of the fluorescence signal, indicative of a time-dependent increase in [Ca2+]i.
Light-dependent increases in fluorescence
The initial increase in fluorescence in the records of Fig. 2 can be demonstrated more clearly with brief flashes of laser light. An example of such an experiment is shown in Fig. 3A, where a rod exposed to 0 Ca2+, 0 Na+ solution was illuminated with 100 ms laser flashes repeated at 5 s intervals. This stimulus protocol revealed a clear and consistent increase in fluorescence between the first and second flashes, followed by a slow decline with a time course similar to that for the cells in Fig. 2. In a sample of 57 cells loaded with fluo-5F and tested with this flash protocol, the ratio of the amplitude of fluorescence evoked by the second flash to that evoked by the first was 1.30 ± 0.02 (mean ±s.e.m.) suggesting a substantial increase in [Ca2+]i during the first 5 s after laser illumination.
In many of the experiments in the remainder of the paper, we have employed a laser flash protocol similar to that of Fig. 3A to explore the nature and properties of the fluorescence increase and have estimated the amplitude of the fluorescence responses by averaging over most of the laser flash duration (see Methods). This procedure probably underestimates the amplitude of the fluorescence increase, which seems to have quite a rapid onset (see Fig. 2A, inset). When the laser flash duration was reduced further to 10 ms (with flashes again separated by 5 s intervals), the ratio of the amplitude of the fluorescence evoked by the second laser flash to that evoked by the first increased to 1.64 ± 0.07 (7 cells). We nevertheless chose to use the longer flash duration in this study, since responses were more easily and accurately measured and provided a consistent and reproducible measurement of the fluorescence increase.
The bars at the bottom of each fluorescence response labelled Ped (pedestal) denote the amplitude of the fluorescence evoked by an identical solution change and sequence of laser flashes presented approximately 45 s after the cell was returned to Ringer solution. As for steady laser light, this low level of fluorescence reflects the substantially reduced value for [Ca2+]i which results after the outer segment has been exposed to intense illumination and then returned to Ringer solution. For the same sample of 57 cells, this pedestal had an average amplitude of 0.13 ± 0.01 of the fluorescence evoked by the first laser flash in 0 Ca2+, 0 Na+ solution. Results qualitatively similar to those of Fig. 3 were also obtained from 58 rods using fluo-3 (Matthews & Fain, 1999a,b). However, since the Kd for fluo-3 is below [Ca2+]i in the dark-adapted outer segment (see Methods), it seems likely that the magnitude of the laser-induced increase in fluorescence was underestimated with this dye.
The data in Fig. 3B were obtained from another cell in response to a laser flash protocol similar to that employed in Fig. 3A, with the sole difference that the first flash in the sequence was omitted. This had the effect of first illuminating the outer segment with laser light 6 s after the beginning of exposure to 0 Ca2+, 0 Na+ solution instead of 1 s after the solution change. Despite this alteration in timing, there was no apparent change in the relative amplitudes of the fluorescence signals. In other experiments to be described later in the paper (see Figs 9 and 10), the delay from the solution change to the first laser flash was increased to as long as 30 s, but fluorescence increases with relative amplitudes similar to those in Fig. 3A were still observed. The increase in fluo-5F fluorescence from the first to the second flash seems therefore to be triggered by laser illumination per se rather than by exposure to the 0 Ca2+, 0 Na+ solution.
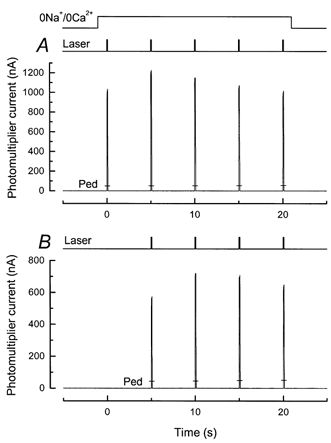
Figure 9. Calcium release after prior exposure to light in 0 Ca2+, 0 Na+ solution.
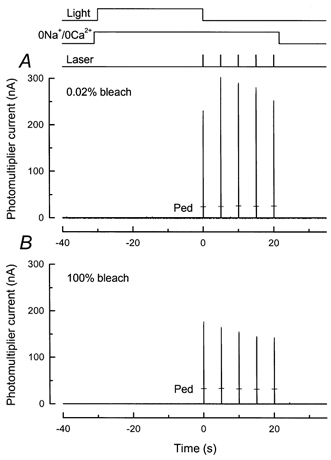
Protocol for experiment is illustrated by the light and solution monitors at top of the figure. The rod outer segment was stepped into 0 Ca2+, 0 Na+ solution and 1 s later was illuminated for 30 s with a light of variable intensity. Then, 100 ms after this light was extinguished, the first of 5 laser flashes was delivered, each 100 ms in duration and separated by 5 s intervals. The outer segment remained in 0 Ca2+, 0 Na+ solution for all of the laser flashes. Responses to a second exposure to 0 Ca2+, 0 Na+ solution and series of laser flashes after return to Ringer solution are indicated by the bars (Ped). Laser intensity, 2.6 × 1010 photons μm−2 s−1. A, intensity of pre-exposure, 1.29 × 103 photons μm−2 s−1. Total bleach after 30 s was 0.02 %. B, intensity of pre-exposure, 1.68 × 108 photons μm−2 s−1, given in equivalent photons at 500 nm for white light, calibrated as described in the Methods. The total bleach calculated after 30 s approached 100 %. Note the suppression of fluorescence increase.
Figure 10. Summary of effect of pre-exposure to light in 0 Ca2+, 0 Na+ solution on the magnitude of the fluo-5F fluorescence increase.
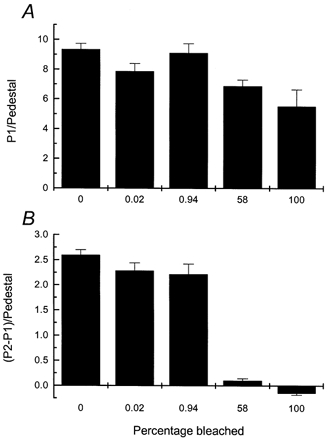
Protocol as for cells in Fig. 9. Each histogram column gives mean ±s.e.m. for a different pre-exposure intensity, given below each column as the percentage of photopigment bleached by the 30 s exposure, calculated as described in the Methods. Columns labelled ‘0’ give paired dark controls for which there was no light exposure before the first laser flash. Cell numbers were as follows: bleach of 0 %, n= 23; bleach of 0.02 %, n= 11; bleach of 0.94 %, n= 10; bleach of 58 %, n= 8; and bleach of 100 %, n= 6. A, magnitude of the fluo-5F fluorescence response to the first laser pulse (P1) divided by the mean value of the pedestal. B, difference between the fluorescence responses to first and second laser pulses (P2 - P1) divided by the mean value of the pedestal.
BAPTA incorporation
If the fluorescence increase in 0 Ca2+, 0 Na+ solution reflects an increase in [Ca2+]i, it would be considerably reduced if the Ca2+ chelator BAPTA were introduced into the rods at the same time as fluo-5F. BAPTA has been previously shown to produce a characteristic delay in the recovery phase of the light response (Matthews et al. 1985; Korenbrot & Miller, 1986; Matthews, 1991), which reflects a slowing in the Ca2+-mediated modulation of transduction responsible for adaptation (Torre et al. 1986). Rods were therefore incubated in Ringer solution containing 50 μm BAPTA as well as 10 μm fluo-5F, both as their AM esters (see Methods).
The records in Fig. 4, from rods from different pieces of retina from the same animal, compare photocurrent responses to saturating light flashes (insets) and fluorescence signals in Ringer solution after incorporation of either fluo-5F alone (Fig. 4A) or both fluo-5F and BAPTA (Fig. 4B). As in previous experiments (Matthews et al. 1985; Torre et al. 1986; Matthews, 1991), BAPTA retarded the recovery phase of the light response and evoked a characteristic undershoot upon return of the current toward the original dark level. In these same rods, BAPTA also produced a pronounced slowing of the light-induced decline in fluo-5F fluorescence in Ringer solution.
For five cells from this animal without BAPTA incorporation, the mean values for the time constants from a two-exponential fit to the fluorescence decrease were 0.94 ± 0.06 and 7.73 ± 0.55 s. In contrast, for nine cells from this animal after BAPTA incorporation, the mean time constants for the decline in fluorescence were 4.15 ± 0.52 and 21.3 ± 1.73 s. Comparison of these values reveals that both the short and long time constants were prolonged by a similar factor of 3-4 following BAPTA incorporation. Furthermore, the ratio of the amplitude of the component which decays with the short to that which decays with the long time constant decreased from 2.21 ± 0.36 without BAPTA to 0.57 ± 0.09 after BAPTA incorporation. These data are most simply interpreted as indicating that BAPTA at this concentration produces a pronounced slowing in the decline of [Ca2+]i in the rod outer segment following closure of the cyclic nucleotide-gated channels by light, which is reflected in the time course of the decrease in the fluo-5F fluorescence signal.
We then examined the effect of BAPTA on the fluorescence response in 0 Ca2+, 0 Na+ solution. Figure 5A and B shows typical results for two cells from another animal, either after incorporation of fluo-5F alone (Fig. 5A) or fluo-5F with BAPTA (Fig. 5B), at the same concentrations of dye and chelator as in Fig. 4. As a control for the consistency of BAPTA incorporation in the experiments of Figs 4 and 5, the photocurrent responses to a saturating flash were first measured from each rod used subsequently for the fluorescence measurements, yielding responses very similar to that in the inset of Fig. 4B. Thus changes in [Ca2+]i are likely to have been retarded similarly in both groups of cells.
Figure 5. Effect of BAPTA incorporation on fluo-5F responses to brief laser flashes in rods exposed to 0 Ca2+, 0 Na+ solution.
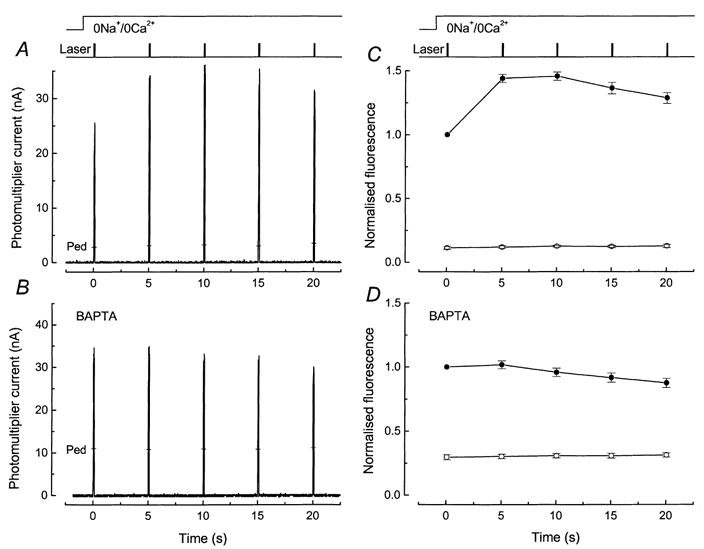
Laser intensity, 2.4 × 1010 photons μm−2 s−1. A and B, show sample fluorescence responses from dark-adapted rods in 0 Ca2+, 0 Na+ solution in the absence and presence of BAPTA. C and D show mean ±s.e.m. under equivalent conditions from rods dissociated from the same animal. A, protocol identical to Fig. 3A: 5 laser flashes of 100 ms duration were delivered at 5 s intervals commencing 1 s after stepping to 0 Ca2+, 0 Na+ solution. Pedestal values measured 45 s after return to Ringer solution are indicated by the bars (Ped). B, protocol as for A but fluo-5F incubation solution included 50 μm BAPTA AM. Pedestal values are indicated by the bars (Ped). C, mean ±s.e.m. for 6 rods as in A (without BAPTA). For each cell the fluorescence signal has been normalised to the fluorescence elicited by the first laser flash. First exposure of dark-adapted rod to laser flashes in 0 Ca2+, 0 Na+ solution (•); pedestal values (○), for which the standard errors were smaller than the symbol width. D, mean ±s.e.m. for 8 rods as in B (after BAPTA incorporation). Note much smaller fluorescence increase and somewhat slower decline. Larger relative magnitude of pedestal values suggests either that BAPTA buffering has altered rod [Ca2+]i in darkness or that light-induced Ca2+ release is underestimated with this pulse protocol, or both.
Comparison of the responses in Fig. 5A and B reveals that the incorporation of BAPTA greatly reduced the increase in fluorescence evoked by the second laser flash in comparison to the first, and it also prolonged the time course for the decline of fluorescence over subsequent laser flashes. This impression is confirmed in Fig. 5C and D, which shows mean values obtained from six rods loaded only with fluo-5F (Fig. 5C) and eight rods loaded with both fluo-5F and BAPTA (Fig. 5D). The ratio of the fluorescence evoked by the second laser flash to that evoked by the first decreased from 1.44 ± 0.03 in Fig. 5C to 1.02 ± 0.04 in Fig. 5D. Furthermore, following BAPTA incorporation the rise time of the fluorescence increase evoked by the first laser flash in 0 Ca2+, 0 Na+ solution was virtually indistinguishable from that evoked by subsequent laser flashes, in contrast to the measurements in cells not loaded with the chelator (see e.g. Fig. 2). The simplest explanation for these results is that intense illumination by the laser triggers a release of Ca2+ within the outer segment which is then gradually removed or sequestered. In the presence of BAPTA, the magnitude of the rise in [Ca2+]i is reduced and the kinetics of the subsequent decline are retarded.
Pre-exposure to bright light
These results are consistent with the notion that the increase in fluo-5F fluorescence represents the release of Ca2+ from some buffer or sequestered pool following laser illumination. They do not, however, exclude the possibility that part of the fluorescence increase might be produced either by a light-dependent release of previously bound and Ca2+-insensitive fluo-5F or by an increase in the affinity of the dye for Ca2+ (see Baylor & Hollingworth, 2000). Either of these changes in the properties of the dye would increase the total fluorescence signal without a rise in [Ca2+]i. To exclude these possibilities, we examined the effect of decreasing [Ca2+]i by prior exposure of the entire outer segment to saturating light before the onset of laser illumination.
The results of such an experiment are illustrated in Fig. 6, which compares the increase in fluorescence in a dark-adapted rod (Fig. 6A) with that in another rod from the same animal which had been pre-exposed to saturating light before the change to 0 Ca2+, 0 Na+ solution (Fig. 6B). Pre-exposure to light reduced the fluorescence evoked by the first laser flash, reflecting a fall in the initially recorded [Ca2+]i as a result of the closure of the cyclic nucleotide-gated channels and Ca2+ extrusion from the outer segment in Ringer solution before the solution change. A more quantitative comparison of the fluorescence evoked by the first laser flash is given for a number of such experiments in Fig. 6C, where the value of the response to the first laser flash (P1) has been divided by the mean pedestal value to correct for differences in dye loading between different photoreceptors. A clear decline in fluorescence is produced by the saturating light exposure in Ringer solution.
Figure 6. Effect of prior illumination in Ringer solution on the magnitude of fluo-5F fluorescence increase in 0 Ca2+, 0 Na+ solution.
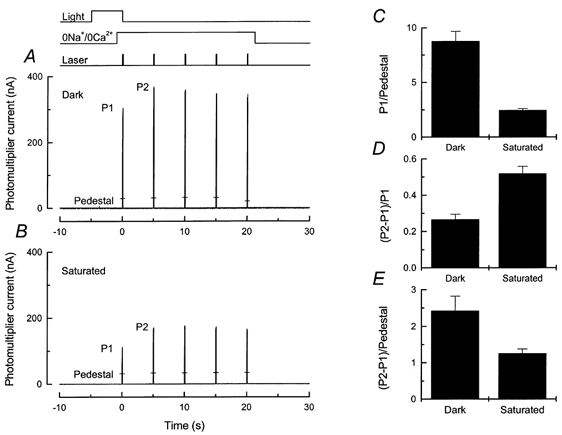
Fluo 5F fluorescence responses from two different rods evoked by 100 ms laser flashes repeated at 5 s intervals are shown in A and B; protocol and laser intensity as for Fig. 3A. The responses to the first and second laser flashes have been labelled P1 and P2. Responses to a second series of laser flashes in 0 Ca2+, 0 Na+ solution 45 s after a return to Ringer solution are indicated for each trace by the bars (Ped). A, without pre-exposure to illumination. B, with 5 s prior illumination of sufficient intensity to saturate the photocurrent during the light exposure. Timing of illumination given by light monitor at the top of the figure. Note that the rod was stepped into 0 Ca2+, 0 Na+solution 4 s after the onset of illumination. Intensity of the 500 nm saturating light was 1.55 × 103 photons μm−2 s−1. C-E, mean ±s.e.m. for 20 cells treated as in A with no pre-illumination (Dark) and 28 cells treated as in B with steady exposure to saturating light of intensity 1.4-1.6 × 103 photons μm−2 s−1 (Saturated). Histograms are as follows: C, the amplitude of the fluorescence response to the first laser pulse (P1) has been divided by the mean value of the pedestal; D, the difference between the responses to the first and second laser pulses (P2 - P1) has been divided by P1; and E, (P2 - P1) has been divided by the mean value of the pedestal.
When the responses to the first two laser flashes are compared, it can be seen that fluo-5F fluorescence increased by a larger proportion after light exposure than if the rod was kept in darkness. This can be seen more clearly in Fig. 6D, which shows that in the 28 rods from which recordings were made following steady illumination in Ringer solution, the fluorescence increased between the first and second laser flashes by 0.52 ± 0.04 ((P2 - P1)/P1; mean ±s.e.m.), as compared with 0.27 ± 0.03 in 20 dark-adapted rods. This difference is significant at greater than the 1 % level (Student's t test, t= 4.93).
These results are consistent with the notion that the increase in fluorescence between the first and second laser flashes is primarily the result of an increase in [Ca2+]i within the outer segment. In contrast, if the increase in fluorescence were entirely the result of a release of previously bound fluo-5F or a change in its affinity, then (P2 - P1)/P1 would not have changed after prior illumination, since the response of fluo-5F is nearly linear in the region of the previously reported dark [Ca2+]i (650-700 nm; see Gray-Keller & Detwiler, 1994; Sampath et al. 1998). These results, together with the effect on the fluorescence signal of BAPTA incorporation (Fig. 5), show that the increase in fluorescence is primarily the result of a release of Ca2+ within the outer segment.
It is, however, of some interest that the magnitude of the rise in fluorescence, when normalised to the pedestal ((P2 - P1)/Pedestal; Fig. 6E), was clearly reduced by prior saturating illumination. This result suggests that the amount of Ca2+ available to be released must also have been reduced, either directly by light or indirectly by the reduction in [Ca2+]i which light produces in Ringer solution. We therefore extended the experimental protocol of Fig. 6 to investigate how the magnitude of the pool of Ca2+ available to be released depends on the intensity and duration of previous light exposure.
Dependence on intensity of prior illumination
We first investigated the dependence of the increase in fluorescence on the intensity of prior illumination using a laser flash protocol similar to that of Fig. 6. To minimise any time-dependent change in the releasable Ca2+ pool, the duration of prior illumination was fixed for all the cells in this protocol to 2 s in Ringer solution before the change to 0 Ca2+, 0 Na+ solution, giving 3 s total light exposure. Collected results from such experiments are shown in Fig. 7, in which the same parameters used in Fig. 6C-E are plotted as functions of the percentage of photopigment bleached by the light delivered before the first laser flash (see Fig. 7 legend and Methods).
Figure 7. Magnitude of Ca2+ release as a function of the intensity of prior light exposure.
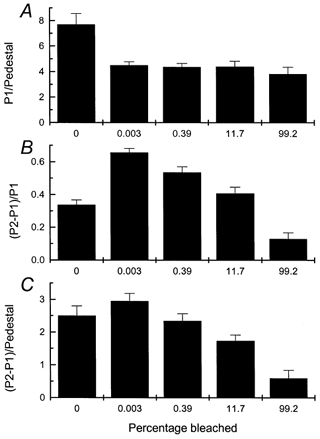
Same protocol as for Fig. 6 except that total light exposure was 3 s long (2 s in Ringer solution) and the light intensity was varied. Intensities are given as percentage of visual pigment bleached, calculated over the entire duration of the light exposure as described in the Methods; even the dimmest intensity was sufficient to saturate the rod (i.e. close all of the cyclic nucleotide-gated channels). A bleach of zero indicates paired controls for which no light exposure was given before the first laser flash. Each histogram column gives mean ±s.e.m.n = 8. A, amplitude of fluorescence response to the first laser pulse (P1) divided by mean value of pedestal. B, difference between the responses to first and second laser pulses (P2 - P1) divided by P1. C, (P2 - P1) divided by the mean value of the pedestal.
Figure 7A shows that prior light exposure in Ringer solution reduced the normalised fluorescence evoked by the first laser flash (P1/Pedestal). The reason for this is that even the dimmest light used in the experiments of Fig. 7 was sufficiently bright to close all of the cyclic nucleotide-gated channels, causing a reduction in [Ca2+]i via the Na+-Ca2+,K+ exchanger. Although P1 was reduced, Fig. 7B shows that P2 - P1 became a larger fraction of the fluorescence evoked by the first laser flash provided the bleach was not very large (cf. Fig. 6D).
If the intensity of the prior illumination and therefore the percentage of photopigment bleached was increased, P1/Pedestal showed little further change, indicating that the decrease in [Ca2+]i was similar for all of these pre-exposures, which were all of the same duration and sufficiently bright to have saturated the photocurrent. However, P2 - P1 became progressively smaller, whether normalised to P1 (Fig. 7B) or to the mean pedestal value (Fig. 7C). This observation is consistent with the notion that the releasable pool of Ca2+ can be discharged in a graded manner either by prior illumination from the optical bench or by a brief flash of intense laser light; if a part of this pool has already been released by prior illumination, then less is available for release by the laser. Furthermore, it is apparent that light of sufficient intensity to bleach the majority of the photopigment can discharge a considerable fraction of this pool of Ca2+.
Dependence on duration of prior illumination
The dependence of the increase in fluorescence on the duration of prior illumination in Ringer solution is examined in Fig. 8. In this experiment individual rods were exposed to the same modest but saturating steady intensity for a total duration of 5-30 s, and the outer segment was stepped into 0 Ca2+, 0 Na+ solution 1 s before the end of this light exposure. As the duration of illumination in Ringer solution was increased, both the normalised fluorescence evoked by the first laser flash (P1/Pedestal; Fig. 8A) and the normalised magnitude of the increase in fluorescence ((P2 - P1)/Pedestal; Fig. 8B) progressively decreased.
Figure 8. Magnitude of Ca2+ release as a function of the duration of prior light exposure in Ringer solution.
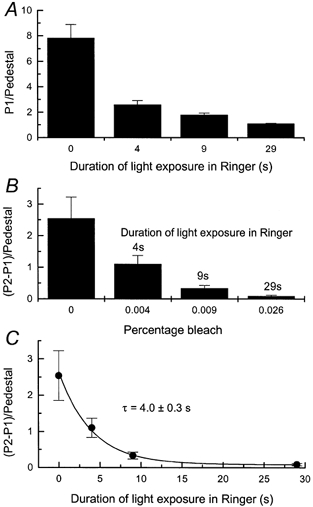
Protocol as for Fig. 6A and B but duration of light exposure was varied. A, ratio of fluo-5F fluorescence evoked by first laser pulse (P1) divided by mean pedestal response. Each histogram column gives mean ±s.e.m.; n= 11. First histogram column is for paired controls for which no light exposure was given (as in Fig. 6A), and following three columns are for 4, 9 and 29 s exposure in Ringer solution to a saturating light of intensity of 1.37 × 103 photons μm−2 s−1. Since the outer segment was stepped into 0 Ca2+, 0 Na+ solution 1 s before the end of illumination, the total durations of illumination were 5, 10 and 30 s respectively. Even the 30 s exposure produced a bleach of less than 0.03 % of the visual pigment. B, mean ±s.e.m. for the difference in the fluo-5F fluorescence evoked by the first and second laser pulses (P2 - P1) divided by the mean value of the pedestal. Data from the same experiments as in An = 11. Bleaches were calculated as described in the Methods for the total duration of each light exposure (i.e. for 5, 10 and 30 s exposures). C, data from B replotted as a function of the duration of the light exposure in Ringer solution. Data have been fitted with a single exponential curve of time constant of 4.0 ± 0.3 s using a weighted least-squares algorithm.
When replotted in Fig. 8C as a function of illumination duration in Ringer solution, these data can be fitted by a single exponential decay with a time constant of 4.0 ± 0.3 s, corresponding to the virtually complete depletion of the releasable pool after 29 s of saturating illumination in Ringer solution. It is important to note that the light intensity used in this experiment was sufficiently low that even the longest exposure resulted in a bleach of less than 0.1 % of the visual pigment. In contrast, in the experiments of Fig. 7, high levels of photopigment bleaching produced a substantial depletion of the releasable pool of Ca2+ even for rather short light exposures.
The effect of prior illumination in 0 Ca2+, 0 Na+ solution
The depletion of the releasable pool of Ca2+ in Fig. 8 by long exposures to light of modest but saturating intensity could most readily be explained if the release of Ca2+ were caused, not by the small amount of photopigment bleaching, but instead by the progressive fall in [Ca2+]i which accompanied the complete suppression of the circulating current. This possibility was addressed by changing the solution bathing the outer segment from Ringer solution to 0 Ca2+, 0 Na+ solution before exposure to saturating illumination, thereby greatly retarding the light-induced fall in [Ca2+]i which would have taken place if the rod had been illuminated in Ringer solution (compare Figs 2A and 4A).
An example of such an experiment is shown in Fig. 9, for which the rod was stepped into 0 Ca2+, 0 Na+ solution 1 s before the onset of a 30 s exposure to saturating light. If the steady intensity was sufficiently low to cause little photopigment bleaching (Fig. 9A), then a sizeable fluorescence increase was observed between the first and second laser flashes, indicating little prior depletion of the releasable Ca2+ pool. If, however, the intensity of the steady light was increased so as to bleach virtually all of the photopigment (Fig. 9B), then the laser-induced release of Ca2+ was abolished.
Collected data from a number of such experiments are shown in Fig. 10. The normalised fluorescence after prior illumination in 0 Ca2+, 0 Na+ solution changed only slightly over a wide intensity range (Fig. 10A; P1/pedestal), in contrast to the substantial decline in this parameter for a similar period of just-saturating illumination in Ringer solution (Fig. 8A). This observation is consistent with our previous experiments (Fig. 2A and Fain et al. 1989) showing that the fall in [Ca2+]i which normally accompanies the response to steady illumination is greatly retarded in 0 Ca2+, 0 Na+ solution.
A quite different result was obtained for the normalised increase in fluorescence between the first and the second laser flashes (Fig. 10B; (P2 - P1)/pedestal). This parameter was little affected in 0 Ca2+, 0 Na+ solution by light exposures which bleached only a small fraction of the photopigment but was dramatically reduced following more substantial bleaches. These results demonstrate that when [Ca2+]i is prevented from falling, the releasable pool of [Ca2+]i within the rod outer segment can still be depleted, but only by light stimuli sufficiently intense to bleach a substantial fraction of the photopigment. Taken together with the results of Figs 7 and 8, they indicate that the releasable Ca2+ pool within the rod outer segment can be discharged both by prior bleaching and by the reduction in [Ca2+]i which accompanies illumination in Ringer solution.
Ca2+ release can alter the time course of fluorescence decrease in Ringer solution
When a rod is exposed to saturating light in Ringer solution, [Ca2+]i declines monotonically with no apparent indication of an initial increase. A typical result for a dark-adapted rod in Ringer solution after incorporation of fluo-5F is shown in Fig. 11A (see also Fig. 4A). As in previous experiments (Gray-Keller & Detwiler, 1994; Sampath et al. 1998), the decline in fluorescence evoked by the continuous laser exposure could be adequately fitted by the sum of two exponentials, with time constants for the cells from this animal of 0.84 ± 0.04 and 7.07 ± 0.38 s (n= 7, mean ±s.e.m.). The amplitude of the faster exponential component was 1.51 ± 0.13 times that of the slower component. These time constants, although longer than those published previously for isolated rods (Gray-Keller & Detwiler, 1994; Sampath et al. 1998), seem likely to be more accurate, since fluo-5F has a Kd higher than the dyes used previously, with a value several times greater than the [Ca2+]i reported previously in darkness (Gray-Keller & Detwiler, 1994; Sampath et al. 1998). Furthermore, we used longer laser exposures of lower intensity, to permit a more accurate exponential fit with a minimum of dye bleaching.
Figure 11. Effect of prior laser exposure in 0 Ca2+, 0 Na+ solution on the time course of fluo-5F fluorescence decrease in Ringer solution.
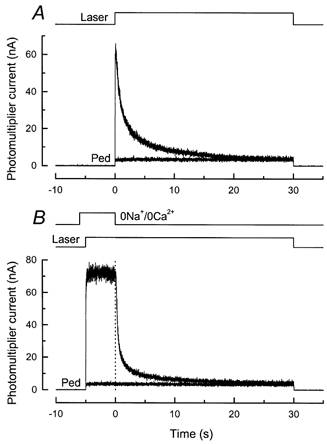
A, fluorescence decrease in Ringer solution. Larger-amplitude current trace is for first of two 30 s laser exposures for a dark-adapted rod in Ringer solution. Smaller-amplitude trace (Ped) was recorded 45 s after end of first trace. Laser intensity, 2.5 × 109 photons μm−2 s−1. B, another dark-adapted rod from the same animal was exposed to 0 Ca2+, 0 Na+ solution for 6 s, and continuous laser illumination commenced 1 s after beginning of solution change. Initial 5 s of laser illumination show the increase and slow decrease in fluorescence characteristic of a rod in 0 Ca2+, 0 Na+ solution (see Fig. 2A). The rod was then stepped back to Ringer solution, and fluorescence declined rapidly as Ca2+ was extruded from the outer segment by Na+-Ca2+,K+ exchange. Note faster decline of fluorescence than in A. Smaller-amplitude trace (Ped) is the fluorescence during repetition of this stimulus protocol for same rod 45 s after end of first laser exposure.
The monotonic exponential fall of Ca2+ may initially seem surprising, since exposure to intense laser light will have bleached the overwhelming majority of the photopigment within the area of the outer segment illuminated by the laser spot. The results presented above indicate that such a substantial bleach would be expected also to evoke a release of Ca2+ within the outer segment. A possible explanation is that the release of Ca2+ might be masked in Ringer solution by the rapid decline in [Ca2+]i produced by Ca2+ extrusion via the Na+-Ca2+,K+ exchanger. The release of Ca2+ evoked by the laser should nevertheless overlap with and alter the time course of the decline in [Ca2+]i. We therefore measured the light-induced decline of [Ca2+]i in Ringer solution for rods first exposed to laser illumination in 0 Ca2+, 0 Na+ solution for a period of time apparently sufficient to discharge much of the light-releasable Ca2+ pool.
An example of such an experiment is shown in Fig. 11B. The rod was stepped to 0 Ca2+, 0 Na+ solution for 6 s and then exposed to the laser for 35 s, beginning 1 s after the solution change. The fluorescence signal in 0 Ca2+, 0 Na+ solution was quite similar to the initial part of the record in Fig. 2A, which was obtained under similar circumstances. The solution bathing the outer segment was then rapidly restored to Ringer solution, thereby re-exposing the cell to the normal Na+ concentration and thus re-activating Na+-Ca2+,K+ exchange. The fluorescence then declined with a time course visibly more rapid than that for the cell in Fig. 11A. For the seven cells treated in this manner, the time course of fluorescence decline after the return to Ringer solution could again be fitted with two exponentials but with time constants of 0.33 ± 0.03 and 5.79 ± 0.45 s, with a ratio of the amplitudes of the faster to the slower components of 3.33 ± 0.31. Thus pre-exposure of the outer segment to the laser resulted in an acceleration of the fluorescence decline when the cell was re-exposed to Na+. This result is consistent with the notion that, for a dark-adapted rod in Ringer solution, the light-induced decline in [Ca2+]i is slowed by the concomitant release of Ca2+ if the light used to measure [Ca2+]i is sufficiently intense to induce Ca2+ release.
In order to estimate the time course of release, we made measurements similar to those in Fig. 11B using the dye fluo-3 instead of fluo-5F and varying the duration of laser exposure in 0 Ca2+, 0 Na+ solution before the return to Ringer solution. If the rod outer segment was exposed to the laser in 0 Ca2+, 0 Na+ solution for 5 or 2 s before the return to Ringer solution, the time course of the subsequent decline in [Ca2+]i was faster than for cells in Ringer solution without pre-exposure to the laser. If, however, the cell was exposed to the laser for only 100 ms in 0 Ca2+, 0 Na+ solution before the return to Ringer solution, the time constants of the fitted exponentials were little altered from those obtained in Ringer solution. These results are broadly consistent with those of Figs 2 and 3, suggesting that Ca2+ release occurs over a time course of a few seconds following laser illumination. However, this approach is not sufficiently precise to determine the time course of release accurately. We are therefore currently improving the temporal resolution of our fluorescence measurements, and shall describe these results in a subsequent publication.
DISCUSSION
These experiments demonstrate that the outer segment of a salamander rod loaded with a fluorescent Ca2+ indicator dye and illuminated with a visible laser shows a time-dependent increase in fluorescence if superfused with a 0 Ca2+, 0 Na+ solution designed to minimise surface membrane Ca2+ fluxes. This increase in fluorescence is evoked directly by illumination of the photoreceptor and not, for example, by exposure to the 0 Ca2+, 0 Na+ solution, since a similar increase in fluorescence is seen regardless of the duration of pre-exposure to 0 Ca2+, 0 Na+ (Figs 3B and 9A). Furthermore, a short period of bleaching illumination can reduce or prevent the fluorescence increase evoked by subsequent laser light (Figs 7C, 9B and 10B).
Properties of the light-induced increase in fluorescence
Our evidence supports the interpretation that the light-induced increase in fluorescence is produced primarily by an increase in [Ca2+]i. Experiments in which BAPTA was incorporated into the cytoplasm (Figs 5 and 6) support this notion, since the magnitude of the fluorescence increase was greatly reduced in the presence of this chelator. Furthermore, the increase in fluorescence is unlikely to be solely the result of a change in the Kd of fluo-5F or an increase in the quantity of dye available to bind Ca2+. In that case the increase (P2 - P1) would have been expected to remain a constant fraction of the initial fluorescence (P1) regardless of the initial [Ca2+]i, since [Ca2+]i is likely to have been sufficiently below the Kd of fluo-5F (nominally 2.3 μm) for the dye to have behaved in an approximately linear fashion. When, however, the initial [Ca2+]i was altered by prior saturating illumination in Ringer solution, the ratio (P2 - P1)/P1 was not fixed but changed as a function of the intensity of the pre-illumination (Figs 6 and 7B). This increase in fluorescence must therefore principally represent the release of a pool of Ca2+ which is bound or sequestered within the outer segment.
The pool of Ca2+ available to be released by laser illumination is strongly dependent upon previous illumination. Exposure to light sufficiently intense to bleach a substantial fraction of the photopigment reduced the size of the releasable pool, irrespective of whether this prior illumination was delivered in Ringer solution (Figs 6 and 7) or in 0 Ca2+, 0 Na+ solution (Figs 9 and 10). Even in the absence of substantial rhodopsin bleaching, however, the size of the pool could be reduced by exposing the rod to saturating illumination in Ringer solution, probably as an indirect consequence of the fall in [Ca2+]i which accompanies the suppression of the outer segment conductance in Na+-containing solution. If this interpretation is correct, the value of the time constant for this depletion (4 s, see Fig. 8C) may reflect both the time course of the [Ca2+]i decrease in the outer segment and the kinetics of the release of Ca2+ from the pool.
When a dark-adapted rod was exposed to laser light in 0 Ca2+, 0 Na+ solution, fluo-5F fluorescence increased by about 30 % between the first and second laser flashes. We have not attempted to translate this value into a precise magnitude for the releasable pool of Ca2+ for two reasons. First, it seems likely that the 100 ms laser flashes which we used in these experiments may not have adequately resolved the kinetics of the rise in fluorescence. Second, since the laser bleached only a 10 μm spot within the outer segment, the majority of the bleach-evoked release may have occurred in a similarly restricted area, with the Ca2+ then diffusing along the outer segment thereafter (Gray-Keller et al. 1999). Both considerations would suggest that our measurements underestimate the magnitude of the [Ca2+]i increase.
Decline of [Ca2+]i in 0 Ca2+, 0 Na+ solution
The rapid increase in [Ca2+]i produced by light-dependent release is followed in 0 Ca2+, 0 Na+ solution by a much more gradual decline of fluorescence with a time constant of the order of 100 s (Fig. 2), considerably slower than in Ringer solution (Figs 4A and 11A). This fluorescence decline is slowed even further by BAPTA incorporation (see Fig. 5) and is likely to reflect a slow decrease in [Ca2+]i.
Indirect evidence for a slow decrease in [Ca2+]i in 0 Ca2+, 0 Na+ solution has been previously provided by the gradual increase in circulating current during just-saturating illumination in low Ca2+, 0 Na+ solution (see Fig. 3 of Fain et al. 1989). At present we do not know whether this gradual decline in [Ca2+]i represents residual activity of Na+-Ca2+,K+ exchange, re-uptake of Ca2+ into a light-releasable pool, or slow diffusion from the outer segment into the inner segment. Regardless of its mechanism, its slow time course is consistent with previous indications that rod outer segment [Ca2+]i remains reasonably stable in 0 Ca2+, 0 Na+ solution for a period of 10-15 s, at least in the presence of saturating illumination.
Comparison to previous measurements
Light-dependent increases in [Ca2+]i in the outer segment have not previously been observed in our earlier measurements with the Ca2+ indicator fluo-3 (Sampath et al. 1998, 1999). There are two possible reasons for this. First, when a rod is illuminated with intense light in Ringer solution, the time course of the increase produced by Ca2+ release overlaps that of the decrease produced by extrusion via Na+-Ca2+,K+ exchange (see Fig. 11). Unless Na+-Ca2+,K+ exchange is suppressed by superfusion with 0 Ca2+, 0 Na+ solution, released Ca2+ would be rapidly extruded across the outer segment membrane. Consequently, a rise in [Ca2+]i would not be evident, at least at the temporal resolution used in these experiments. Second, our measurements (Figs 7C and 10B) indicate that rather intense lights are required to produce a substantial Ca2+ release, although it should be noted that such intensities are typical of those required to make Ca2+ measurements from single cells with fluorescent indicator dyes (Gray-Keller & Detwiler, 1994; Sampath et al. 1998, 1999; Gray-Keller et al. 1999). Even when the intact retina was continuously superfused with 0 Ca2+, 0 Na+ solution Younger et al. (1996) found no sign of Ca2+ release on exposure to just-saturating light. In contrast Gray-Keller & Detwiler (1994) observed a light-induced increase in the fluorescence of the Ca2+-bound form of free indo-1 when the outer segment was stimulated in Ringer solution. Gray-Keller & Detwiler (1994) also showed, however, that if instead of the free form of indo-1 the rods were dialysed with a dextran-bound form of this indicator, the measured light-induced decline in fluorescence was very similar to that found in Ringer solution with fluo-3 (Sampath et al. 1998) or fluo-5F (Figs 4A and 11A). The simplest interpretation of this observation is that the rise in fluorescence seen with free indo does not represent a rise in [Ca2+]i (Gray-Keller & Detwiler, 1994) but is rather the result of some change in the properties of the indicator.
Other approaches have, however, provided indirect evidence for light-dependent Ca2+ release. Early measurements with extracellular Ca2+-sensitive microelectrodes from intact retina showed an increase in extracellular Ca2+ concentration, which was initially interpreted to indicate an increase in Ca2+ efflux from the rod (Gold & Korenbrot, 1980;Yoshikami et al. 1980) but subsequently shown mostly to result from a light-dependent decrease in Ca2+ influx caused by the closing of the cGMP-gated channels (Yau & Nakatani, 1985). More recent experiments (Knopp & Rüppel, 1996), however, appear to indicate that bright light produces a maintained increase in extracellular Ca2+ concentration that persists even 5 min after the onset of illumination. This extracellular increase decays when the light is extinguished, indicating the re-entry of Ca2+ into the cell; but less Ca2+ re-enters than was extruded, resulting in a decrease in total rod Ca2+ content suggestive of a light-dependent release.
Light-dependent Ca2+ release has also been reported from rod outer segments disrupted by centrifugation or sonication; these results were interpreted to indicate Ca2+ release from outer segment disks (Kaupp et al. 1980; George & Hagins, 1983). Finally, measurements of the total calcium content of the rod outer segment with laser micro-mass analysis showed that rod outer segments contain a pool of calcium which in darkness exchanges only very slowly with extracellular (Schröder & Fain, 1984) and intracellular (Fain & Schröder, 1987) Ca2+. The quantity of calcium in this pool can be reduced by light exposure, and the rate of its depletion increases with increasing light intensity, both consistent with a light-induced release of Ca2+ from within the outer segment (Schröder & Fain, 1984; Fain & Schröder, 1990).
Mechanism of Ca2+ release
Our experiments indicate that the release of Ca2+ is likely to be initiated in some way by the bleaching of rhodopsin. While it is possible that Ca2+ release is triggered by transducin or some component of the G protein-coupled transduction cascade, this seems to us unlikely. Light bright enough to saturate transduction but not to bleach a significant fraction of the photopigment has little effect on the light-releasable pool of Ca2+ in 0 Ca2+, 0 Na+ solution. This suggests that rhodopsin might initiate the release of Ca2+ by some mechanism requiring activation by light but independent of the normal transduction cascade.
At present, it is not possible to say with certainty whether the increase in Ca2+ that we measure is produced by release from a binding site or from some intracellular compartment within the outer segment. Rod outer segments are known to contain intrinsic buffers for Ca2+ (see for example Lagnado et al. 1992) and appear also to be able to accumulate Ca2+ within some intracellular compartment, perhaps the disks (Schnetkamp, 1979, 1995; Fain & Schröder, 1987). The rod outer segment has been shown to express an isoform of phospholipase C (PLCβ) (Peng et al. 1997), the enzyme that hydrolyses phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) and, in other cells, plays a crucial role in Ca2+ release from internal stores. However, knocking out the isoform PLCβ4 (Jiang et al. 1996), or treatment with phorbol esters (Xiong et al. 1997) seems to have little effect on the photoreceptor light response. Furthermore, there are few if any IP3 receptors in the outer segment (Day et al. 1993). Measurements of Ca2+ release in mouse photoreceptors may make it possible to use genetically modified animals to elucidate the mechanism of Ca2+ release.
Functional consequences of Ca2+ release
There is, as yet, no indication of a physiological role for light-induced Ca2+ release. Previous experiments have shown that the changes in sensitivity and response waveform that constitute light adaptation are prevented by exposure to 0 Ca2+, 0 Na+ solution. Exposure to this solution opposes Ca2+ fluxes across the rod outer segment plasma membrane (Matthews et al. 1988; Nakatani & Yau, 1988; Fain et al. 1989) and could only serve to increase any change in [Ca2+]i produced by Ca2+ release. Furthermore, a measurable release of Ca2+ appears only to be evoked by light sufficiently intense to bleach a substantial fraction of the photopigment. These considerations would seem to leave little role for Ca2+ release in the modulation of sensitivity. Nevertheless, it remains possible that Ca2+ release may play a role in some other, as yet unidentified Ca2+-dependent process in these cells.
Despite the uncertainty as to its physiological role, the release of Ca2+ induced by intense light has potentially significant consequences for our understanding of changes in [Ca2+]i during the responses to more moderate light intensities. Light bright enough to be used to make measurements of Ca2+ from single cells with fluorescent indicator dyes will evoke Ca2+ release with a time course which overlaps that of Ca2+ extrusion via Na+-Ca2+,K+ exchange. This seems likely to lead to a poorly resolved initial rise in [Ca2+]i at early times, followed by a slowing of its subsequent decline (see Fig. 11). In contrast, dimmer, just-saturating light would evoke much less Ca2+ release, but the subsequent fall in [Ca2+]i would gradually deplete the releasable Ca2+ pool. Therefore, it seems probable that both the kinetics and magnitude of the apparent decline in [Ca2+]i measured with presently available techniques will be influenced by the release of Ca2+ induced by the intense light required to make the measurement.
Acknowledgments
This work was supported by a grant from The Wellcome Trust (to H.R.M.) and by a grant from the National Eye Institute of the National Institutes of Health (EY-01844), a fellowship from the John Simon Guggenheim Memorial foundation, and an Overseas Visiting Scholarship from St John's College, Cambridge (to G.L.F.).
References
- Baylor SM, Hollingworth S. Measurement and interpretation of cytoplasmic [Ca2+] signals from calcium-indicator dyes. News in Physiological Sciences. 2000;15:19–26. [PubMed] [Google Scholar]
- Cervetto L, Lagnado L, Perry RJ, Robinson DW, McNaughton PA. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989;337:740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Dartnall HJA. Photosensitivity. In: Dartnall HJA, editor. Handbook of Sensory Physiology. Berlin: Springer; 1972. pp. 122–145. [Google Scholar]
- Day NS, Koutz CA, Anderson RE. Inositol-1,4,5-trisphosphate receptors in the vertebrate retina. Current Eye Research. 1993;12:981–992. doi: 10.3109/02713689309029224. [DOI] [PubMed] [Google Scholar]
- Escobar AL, Monck JR, Fernandez JM, Vergara JL. Localization of the site of Ca2+ release at the level of a single sarcomere in skeletal-muscle fibers. Nature. 1994;367:739–741. doi: 10.1038/367739a0. [DOI] [PubMed] [Google Scholar]
- Fain GL, Lamb TD, Matthews HR, Murphy RLW. Cytoplasmic calcium concentration as the messenger for light adaptation in salamader rods. Journal of Physiology. 1989;416:215–243. doi: 10.1113/jphysiol.1989.sp017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Matthews HR. Light-evoked calcium release in vertebrate photoreceptors. Biophysical Journal. 2000;78:143A. [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiological Reviews. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- Fain GL, Schröder WH. Calcium in dark-adapted toad rods: evidence for pooling and cyclic guanosine-3′,5′-monophosphate-dependent release. Journal of Physiology. 1987;389:361–384. doi: 10.1113/jphysiol.1987.sp016661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Schröder WH. Light-induced calcium release and re-uptake in toad rods. Journal of Neuroscience. 1990;10:2238–2249. doi: 10.1523/JNEUROSCI.10-07-02238.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JS, Hagins WA. Control of Ca2+ in rod outer segment disks by light and cyclic GMP. Nature. 1983;303:344–348. doi: 10.1038/303344a0. [DOI] [PubMed] [Google Scholar]
- Gold GH, Korenbrot JI. Light-induced Ca release by intact retinal rods. Proceedings of the National Academy of Sciences of the USA. 1980;77:5557–5561. doi: 10.1073/pnas.77.9.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Keller M, Denk W, Shraiman B, Detwiler PB. Longitudinal spread of second messenger signals in isolated rod outer segments of lizards. Journal of Physiology. 1999;519:679–692. doi: 10.1111/j.1469-7793.1999.0679n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Keller MP, Detwiler PB. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994;13:849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, McNaughton PA, Nunn BJ. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. Journal of Physiology. 1985;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, McNaughton PA, Nunn BJ. Measurement of sodium-calcium exchange in salamander rods. Journal of Physiology. 1987;391:347–370. doi: 10.1113/jphysiol.1987.sp016742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz P, Hill W. The Art of Electronics. Cambridge: Cambridge University Press; 1980. [Google Scholar]
- Jiang HP, Lyubarsky A, Vardi N, Pugh EN, Chen J, Xu J, Simon MI. Phospholipase beta-4-knockout mouse exhibits retinal phenotype. Investigative Ophthalmology and Visual Science. 1996;37:3719. [Google Scholar]
- Jones GJ, Fein A, MacNichol EFJ, Cornwall MC. Visual pigment bleaching in isolated salamander retinal cones. Microspectrophotometry and light adaptation. Journal of General Physiology. 1993;102:483–502. doi: 10.1085/jgp.102.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp UB, Schnetkamp PP, Junge W. Metarhodopsin I/metarhodopsin II transition triggers light-induced change in calcium binding at rod disk membranes. Nature. 1980;286:638–640. doi: 10.1038/286638a0. [DOI] [PubMed] [Google Scholar]
- Knopp A, Rüppel H. Ca2+ fluxes and channel regulation in rods of the albino-rat. Journal of General Physiology. 1996;107:577–595. doi: 10.1085/jgp.107.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot JI, Miller DL. Calcium ions act as a modulator of intracellular information flow in retinal rod phototransduction. Neuroscience Research. 1986;4:11–34S. doi: 10.1016/0168-0102(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Krizaj D, Copenhagen DR. Compartmentalization of calcium extrusion mechanisms in the outer and inner segments of photoreceptors. Neuron. 1998;21:249–256. doi: 10.1016/s0896-6273(00)80531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L, Cervetto L, McNaughton PA. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. Journal of Physiology. 1992;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy ST, Younger JP, Owen WG. Free calcium concentrations in bullfrog rods determined in the presence of multiple forms of fura-2. Biophysical Journal. 1994;67:2076–2089. doi: 10.1016/S0006-3495(94)80691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy ST, Younger JP, Owen WG. Dynamic, spatially nonuniform calcium regulation in frog rods exposed to light. Journal of Neurophysiology. 1996;76:1991–2004. doi: 10.1152/jn.1996.76.3.1991. [DOI] [PubMed] [Google Scholar]
- McNaughton PA, Cervetto L, Nunn BJ. Measurement of the intracellular free calcium concentration in salamander rods. Nature. 1986;322:261–263. doi: 10.1038/322261a0. [DOI] [PubMed] [Google Scholar]
- Matthews HR. Incorporation of calcium chelator into guinea-pig rods shows that calcium mediates mammalian photoreceptor light adaptation. Journal of Physiology. 1991;436:93–105. doi: 10.1113/jphysiol.1991.sp018541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR. Effects of lowered cytoplasmic calcium concentration and light on the responses of salamander rod photoreceptors. Journal of Physiology. 1995;484:267–286. doi: 10.1113/jphysiol.1995.sp020664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR. Static and dynamic actions of cytoplasmic Ca2+ in the adaptation of responses to saturating flashes in salamander rods. Journal of Physiology. 1996;490:1–15. doi: 10.1113/jphysiol.1996.sp021123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR, Fain GL. Light-induced changes in Ca when outer segment Ca2+ fluxes are prevented in isolated salamander rods. Journal of Physiology. 1999a;520.P:45P. [Google Scholar]
- Matthews HR, Fain GL. Light-induced changes in intracellular Ca2+ after blocking Na+/Ca2+-K+ exchange. Investigative Ophthalmology and Visual Science. 1999b;40(suppl.):238S. [Google Scholar]
- Matthews HR, Fain GL. Intense light induces a rise in [Ca2+]i when outer segment Ca2+ fluxes are prevented in isolated salamander rods. Journal of Physiology. 2000a;525.P:58P. [Google Scholar]
- Matthews HR, Fain GL. Laser spot confocal technique to measure cytoplasmic calcium concentration in photoreceptors. In: Palczewski K, editor. Methods in Enzymology. San Diego: Academic Press; 2000b. pp. 146–163. [DOI] [PubMed] [Google Scholar]
- Matthews HR, Fain GL. Rapid solution changes during measurement of [Ca2+]i by a laser spot technique from rod photoreceptors isolated from the tiger salamander. Journal of Physiology. 2000c;527.P:2P. [Google Scholar]
- Matthews HR, Murphy RLW, Fain GL, Lamb TD. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988;334:67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- Matthews HR, Torre V, Lamb TD. Effects on the photoresponse of calcium buffers and cyclic GMP incorporated into the cytoplasm of retinal rods. Nature. 1985;313:582–585. doi: 10.1038/313582a0. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Yau K-W. Calcium and light adaptation in retinal rods and cones. Nature. 1988;334:69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Peng YW, Rhee SG, Yu WP, Ho YK, Schoen T, Chader GJ, Yau KW. Identification of components of a phosphoinositide signaling pathway in retinal rod outer segments. Proceedings of the National Academy of Sciences of the USA. 1997;94:1995–2000. doi: 10.1073/pnas.94.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh EN, Jr, Nikonov S, Lamb TD. Molecular mechanisms of vertebrate photoreceptor light adaptation. Current Opinion in Neurobiology. 1999;9:410–418. doi: 10.1016/S0959-4388(99)80062-2. [DOI] [PubMed] [Google Scholar]
- Ratto GM, Payne R, Owen WG, Tsien RY. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with fura-2. Journal of Neuroscience. 1988;8:3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath AP, Matthews HR, Cornwall MC, Bandarchi J, Fain GL. Light-dependent changes in outer segment free Ca2+ concentration in salamander cone photoreceptors. Journal of General Physiology. 1999;113:267–277. doi: 10.1085/jgp.113.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath AP, Matthews HR, Cornwall MC, Fain GL. Bleached pigment produces a maintained decrease in outer segment Ca2+ in salamander rods. Journal of General Physiology. 1998;111:53–64. doi: 10.1085/jgp.111.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetkamp PPM. Calcium translocation and storage of isolated intact cattle rod outer segments in darkness. Biochimica et Biophysica Acta. 1979;554:441–459. doi: 10.1016/0005-2736(79)90383-3. [DOI] [PubMed] [Google Scholar]
- Schnetkamp PPM. How does the retinal rod Na-Ca+K exchanger regulate cytosolic free Ca2+? Journal of Biological Chemistry. 1995;270:13231–13239. doi: 10.1074/jbc.270.22.13231. [DOI] [PubMed] [Google Scholar]
- Schröder WH, Fain GL. Light-dependent calcium release from photoreceptors measured by laser micro-mass analysis. Nature. 1984;309:268–270. doi: 10.1038/309268a0. [DOI] [PubMed] [Google Scholar]
- Torre V, Matthews HR, Lamb TD. Role of calcium in regulating the cyclic GMP cascade of phototransduction in retinal rods. Proceedings of the National Academy of Sciences of the USA. 1986;83:7109–7113. doi: 10.1073/pnas.83.18.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong WH, Nakatani K, Ye B, Yau K-W. Protein kinase C activity and light sensitivity of single amphibian rods. Journal of General Physiology. 1997;110:441–452. doi: 10.1085/jgp.110.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K-W, Nakatani K. Cation selectivity of light-sensitive conductance in retinal rods. Nature. 1984a;309:352–354. doi: 10.1038/309352a0. [DOI] [PubMed] [Google Scholar]
- Yau K-W, Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984b;311:661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yau K-W, Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985;313:579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- Yoshikami S, George JS, Hagins WA. Light-induced calcium fluxes from outer segment layer of vertebrate retinas. Nature. 1980;286:395–398. doi: 10.1038/286395a0. [DOI] [PubMed] [Google Scholar]
- Younger JP, McCarthy ST, Owen WG. Light-dependent control of calcium in intact rods of the bullfrog Rana catesbeiana. Journal of Neurophysiology. 1996;75:354–366. doi: 10.1152/jn.1996.75.1.354. [DOI] [PubMed] [Google Scholar]


