Abstract
Homomeric cyclic nucleotide-gated (CNG) channels composed of α2 subunits from bovine cone photoreceptors were heterologously expressed in the human embryonic kidney (HEK) 293 cell line. Modulation of cGMP sensitivity by protein kinase C (PKC)-mediated phosphorylation and by binding of calmodulin (CaM) was investigated in inside-out patches.
A peptide encompassing the putative CaM-binding site within the N-terminus of the channel protein binds Ca2+-CaM with high affinity, yet the ligand sensitivity of α2 channels is not modulated by CaM.
PKC-mediated phosphorylation increased the activation constant (K1/2) for cGMP from 19 to 56 μm and decreased the Hill coefficient (from 2.5 to 1.5). The change in ligand sensitivity involves phosphorylation of the serine residues S577 and S579 in the cGMP-binding domain. The increase in K1/2 was completely abolished in mutant channels in which the two serine residues were replaced by alanine.
An antibody specific for the δ isoform of PKC strongly labels the cone outer segments.
Modulation of cGMP affinity of bovine α2 CNG channels by phosphorylation could play a role in the regulation of photoreceptor sensitivity.
In retinal rod and cone photoreceptors and olfactory sensory neurons (OSNs), cyclic nucleotide-gated (CNG) channels mediate the electrical response to stimulation by light and odorants, respectively. CNG channels are composed of different subunits, generically termed α and β (for review see Finn et al. 1996). The native channel in rod photoreceptors is composed of the principal subunit, designated here α1 (Kaupp et al. 1989; Dhallan et al. 1992; Pittler et al. 1992; Bönigk et al. 1993) and the β1a subunit, one of several splice forms derived from the β1 gene (Chen et al. 1993; Körschen et al. 1995). Cone photoreceptors express a different α subunit, designated α2 (Bönigk et al. 1993; Weyand et al. 1994; Yu et al. 1996; Wissinger et al. 1997), and perhaps a different β subunit (Gerstner et al. 2000), here designated β2. However, the subunit composition of CNG channels in cone photoreceptors has not been studied as extensively as in rods. In rat OSNs, three different subunits form the native CNG channel: the principal subunit α3, which can form functional homomeric channels on its own (Dhallan et al. 1990; Ludwig et al. 1990) and the modulatory subunits α4 (Bradley et al. 1994; Liman & Buck, 1994) and β1b, a short splice form derived from the β1 gene (Sautter et al. 1998; Bönigk et al. 1999).
The ligand sensitivity of CNG channels is modulated by phosphorylation and by Ca2+ binding proteins. The modulatory action of CaM is believed to serve as a feedback mechanism that helps to terminate the responses to light or odorants and that controls adaptation. The physiological significance of channel phosphorylation is not clear. In the rod CNG channel, the ligand sensitivity is affected by both serine/threonine and tyrosine phosphorylation (Gordon et al. 1992; Molokanova et al. 1997, 1999) and by binding of Ca2+-calmodulin (CaM) (Hsu & Molday, 1993; Gordon et al. 1995; Bauer, 1996). The effects are relatively small; both tyrosine phosphorylation and Ca2+-CaM increase the constant of half-maximal activation (K1/2) maximally 2-fold. The CaM action involves an unconventional CaM-binding site (Grunwald et al. 1998; Weitz et al. 1998) in the N-terminal region of the β1a subunit (Chen et al. 1994; Körschen et al. 1995). The tyrosine residue (Y498) that is involved in the phosphorylation-induced change in ligand sensitivity resides within the nucleoside 3′,5′-cyclic monophosphate (cNMP)-binding site of the α1 subunit.
Modulation of CNG channels in OSNs occurs by way of different subunits and different mechanisms. The olfactory α3 subunit harbours a classical CaM-binding site in the N-terminus (Liu et al. 1994). In both native olfactory CNG channels and in heterologously expressed α3 homooligomers, binding of CaM decreases the apparent ligand affinity up to 50-fold, i.e. the modulatory range is much larger than in rod CNG channels (Chen & Yau, 1994; Liu et al. 1994; Balasubramanian et al. 1996). The Ca2+-induced decrease in ligand sensitivity has been identified as the principal mechanism of odorant adaptation in OSNs (Kurahashi & Menini, 1997). Moreover, the ligand sensitivity of the α3 subunit is enhanced severalfold by protein kinase C (PKC)-mediated phosphorylation of a serine residue in the α3 N-terminus (Müller et al. 1998), whereas tyrosine phosphorylation seems to be absent (Molokanova et al. 1999).
We know much less about the regulation of ligand sensitivity of the CNG channels from cones. In the N-terminus of α2 orthologues, a CaM-binding motif was identified resembling that in the α3 subunit (Bönigk et al. 1996; Grunwald et al. 1999). However, some cone CNG channels are insensitive to CaM (native catfish: Haynes & Stotz, 1997; human α2: Yu et al. 1996; Grunwald et al. 1999), whereas others are modulated by CaM though not as vigorously as CNG channels in OSNs (heterologously expressed chicken α2: Bönigk et al. 1996; native cone CNG channels of striped bass: Hackos & Korenbrot, 1997). Nothing is known about the regulation by phosphorylation. Here we investigate the action of CaM and phosphorylation on the ligand sensitivity of homomeric bovine α2 channels. We show that α2 and α3 homomeric channels, despite their strikingly similar structural features, are modulated in completely different ways.
METHODS
Surface plasmon resonance spectroscopy
Sensorgrams were recorded with a BIAcore 1000 system using the BIAcore 1.2 control software (Biacore, Sweden). Synthesis of peptides, immobilization of CaM on the chip surface, and surface plasmon resonance (SPR) instrumentation have been described elsewhere (Weitz et al. 1998; Koch, 2000). Calmodulin (CaM) from spinach was immobilized on the surface of a CM-5 sensorchip (Pharmacia) by thiol coupling via a single cysteine residue (C26) (Weitz et al. 1998). The density of immobilized CaM on the surface of the sensor chip was 0.65-0.9 ng mm−2. The running buffer contained 150 mm NaCl, 10 mm Hepes-KOH at pH 7.4, 10 mm MgCl2, 2 mm CaCl2 and 0.005 % Tween-20. A synthetic peptide, Pα2, encompassing the hypothetical CaM-binding site of the bovine α2 subunit (F65-H89) was applied in running buffer at 8, 17, 25, 33, 67, 100 and 167 nm concentrations. The flow rate of the mobile phase was 30 μl min−1. Complex formation between the immobilized CaM and the peptide was recorded as a sensorgram (expressed in response units (RU) as a function of time). Dissociation of the CaM-peptide complex was initiated by injecting running buffer without peptide. After each cycle, the sensor chip was regenerated by injection of 30 μl running buffer without CaCl2, but in the presence 2 mm EDTA.
Immunohistochemistry
Adult rats were deeply anaesthetized with fluothane and rapidly decapitated in agreement with national ethics committee guidelines. Eyes were removed, the anterior pole with the vitreous body was discarded and the eyecup with the retina was fixed by immersion in 4 % paraformaldehyde-0.1 m phosphate buffer (pH 7.4) (PB) for 15 min. Subsequently, retinae were washed in buffer, dissected free and cryoprotected in 30 % sucrose-PB for 3 h. Retinae were frozen in OCT compound (Miles Scientific, Elkhard, IN, USA). Vertical sections of 16 μm thickness were cut on a Cryostat and collected on gelatinized slides. Sections were preincubated for 1 h in 10 % normal goat serum (NGS), 0.5 % Triton X-100 in PB. Sections were incubated overnight in primary antibodies raised against PKC isoforms (Transduction Laboratories). Dilution of antibody stocks in 5 % NGS, 0.5 % Triton X-100 in PB were: α, 1:250; β, 1:500; γ, 1:1000; δ, 1:150; ε, 1:50; λ, 1:100; μ, 1:150; ι, 1:50; ζ, 1:50. After several rinses in PB, sections were incubated with the secondary antibody (anti-mouse-Alexa488, Molecular Probes, 1:500) for 1 h. After rinsing in PB, sections were coverslipped in Mowiol solution (Hoechst) and examined by epifluorescence. Some sections were double-labelled using a mixture of PKC δ antibodies and either one of the antisera JH455 (directed against the short-wavelength cone opsin) or JH492 (against the middle- to long-wavelength cone opsin) (both 1:5000, kindly provided by J. Nathans), followed by a mixture of the secondary antibodies (anti-mouse-Alexa488 and anti-rabbit-Alexa568, respectively, Molecular Probes, 1:500). Images were taken with a confocal laser scanning microscope (Leica TCS) and processed and printed using Corel Draw 8.
Electrophysiological measurements of apparent ligand sensitivity
Cells of the human embryonic kidney (HEK) 293 cell line were transfected with DNA encoding wild-type or mutant bovine α2 subunits (Weyand et al. 1994) and the β1 subunit (Körschen et al. 1995) using the expression vector pcDNA1 (Invitrogen) as described previously (Baumann et al. 1994). During the experiments, cells were kept at room temperature in a bath solution containing (mm): 135 NaCl, 5 KCl, 10 EGTA-NaOH, 10 Hepes-NaOH at pH 7.4, which was continuously exchanged at a rate of 2 ml min−1. The CaM sensitivity was studied in inside-out patches. Patches were excised and moved to a perfusion system several millimetres away from the cells. Patches were first superfused for 1-2 min with a solution containing (mm): 100 KCl, 10 Hepes-KOH at pH 7.4, and 10 EGTA-KOH to remove all endogenous CaM that might have adhered to the channel in the HEK 293 cell. The same solution was used as the pipette solution. Subsequently, the patch was exposed to solutions containing (mm): 100 KCl, 10 Hepes-KOH at pH 7.4, 2 nitrilotriacetic acid-0.8 CaCl2 (to give 50 μm free Ca2+), and the indicated concentrations of CaM (Calbiochem) and cGMP (Sigma). Using voltage ramps from -80 to +80 mV, current-voltage relations were obtained. Leak currents recorded in the absence of cGMP were subtracted.
During treatment with phorbol esters, cells were kept at 37 °C in a solution containing (mm): 135 NaCl, 5 KCl, 10 MgCl2, 10 Hepes-NaOH at pH 7.4. Phorbol 12-myristate 13-acetate (4β-PMA, Sigma), 4α-PMA (Alexis, Germany), and staurosporin (Alexis) were dissolved in DMSO at 1 mg ml−1 and used at a final concentration of 0.5-1 μm. Cells were incubated with phorbol esters for 30-60 min, followed by thorough washing. Patch-clamp experiments were carried out for up to 2 h at room temperature in the absence of phorbol esters. The cGMP sensitivity of the channels was tested in inside-out patches taken from PMA-treated and from control cells. Both external (pipette) and internal (bath) solutions contained (mm): 100 KCl, 10 EGTA-KOH, 10 Hepes-KOH at pH 7.4 plus the indicated concentrations of cGMP. Dose-response relations were analysed for each patch by fitting to the data a Hill-type function:
where Imax is the current at saturating concentrations of cGMP, C the cGMP concentration, nH the Hill coefficient, and K1/2 the concentration for half-maximal channel activation (least-squares fit). Results at a membrane potential (Vm) of -60 mV are given as means ±s.d. (n, numbers of experiments).
Construction of mutant channels
The channel mutants were constructed by PCR using plasmid pCGTEF (Weyand et al. 1994) as the template. The point mutations at position S577 and S579 of the α2 subunit were introduced according to Herlitze & Koenen (1990) using synthetic oligonucleotides that contained the desired nucleotide substitutions. For the truncated mutant Δ105, a 5′-adapter primer (containing an EcoRV restriction site, a consensus sequence for eukaryotic ribosomal-binding site (Kozak, 1984) and 18 nucleotides following the start codon) and a gene-specific 3′-primer were used. PCR fragments and pCGTEF were digested with suitable restriction endonucleases, and the wild-type fragments were replaced by the corresponding mutant fragments. All mutations were verified by sequencing of the inserts with the dideoxynucleotide chain-termination method. Recombinant cDNAs were subcloned into pcDNAI vector (Invitrogen) for expression in HEK 293 cells.
RESULTS
The putative CaM-binding site of the bovine α2 CNG channel subunit binds CaM with high affinity
The N-terminal regions of α2 subunits from various species contain a sequence motif with high similarity to the CaM-binding site of the olfactory α3 subunit (Fig. 1A). We studied the binding of CaM to this putative CaM-binding site using surface plasmon resonance (SPR) spectroscopy.
Figure 1. Analysis of the CaM-binding site.

A, sequence of the peptides Pα2 and Pα3 encompassing the putative CaM-binding sites. B, binding of peptide Pα2 to immobilized CaM (0.9 ng mm−2). Sensorgrams display an association and dissociation phase at increasing concentrations (8, 17, 25, 33, 67, 100 and 167 nm) of Pα2 in the running buffer (filled bar). Application of running buffer without Pα2 is indicated by open bars, and without Ca2+ but with 2 mm EDTA by the hatched bar. The lower inset shows a sensorgram in the presence of 100 nm Pα2 but without Ca2+ and with 2 mm EDTA.
Calmodulin was immobilized on a sensor chip and the synthetic peptide Pα2 (see Fig. 1A), encompassing the putative CaM-binding site, was applied in the mobile phase. The running buffer contained 2 mm CaCl2 to ensure maximal interaction between CaM and the peptide. Sensorgrams consisted of three parts, a pre-running phase without peptide Pα2 (Fig. 1B, open bar), an association phase when Pα2 was present in the running buffer (filled bar), and a dissociation phase (open bar) after removal of Pα2 from the running buffer. The time-dependent increase of resonance units (RU) indicates the formation of a CaM-peptide complex, and the decrease of signal amplitude indicates the dissociation of the complex. Figure 1B shows a set of sensorgrams for Pα2 concentrations ranging from 8 to 167 nm. The Pα2 bound reversibly to CaM in a concentration-dependent and saturable fashion. Both the association rate and the amplitude of the binding signal increased with the CaM concentration. The formation of the CaM-Pα2 complex required Ca2+: in the absence of Ca2+ and in the presence of 2 mm EDTA, no complex formation was observed (Fig. 1B, lower inset). The maximal amplitude of ≈100 RU corresponds to a binding stoichiometry CaM:Pα2 of roughly 1:0.6 (at a CaM density of 0.9 ng mm−2). After removal of Pα2 from the running buffer, a slow dissociation signal commenced. Dissociation was much more rapid and virtually complete in the presence of the Ca2+ chelator EDTA (Fig. 1B, hatched bar).
To quantify the binding of Pα2 to CaM, the signal amplitudes at equilibrium (Req) were determined from sensorgrams using seven different CaM concentrations. The dependence of Req on the Pα2 concentration was analysed in a Scatchard plot (Req/C vs. Req). The analysis of four independent experiments all resulted in non-linear Scatchard plots (data not shown), which do not permit determination of the true dissociation constants KD (Klotz, 1989). Curvilinear Scatchard plots may have resulted from: (1) inhomogeneity in the accessibility or binding affinity of the immobilized CaM; such a mechanism is suggested by a sub-optimal CaM:Pα2 ratio of 1:0.6; (2) concentration-dependent interaction of peptides with themselves which would lower the effective concentration of free peptide; or (3) a combination of both effects. Whatever the mechanism(s) might be, the binding signal saturates at 100 nm Pα2 and, therefore, the true KD value must be < 100 nm. A further independent manifestation of the high stability of the CaM-Pα2 complex is the slow dissociation of Pα2 from the immobilized CaM. A peptide Pα3, encompassing the CaM-binding site of the α3 CNG channel subunit, displayed a similarly slow dissociation signal and yielded a KD value of 12.4 nm (see Fig. 4 of Weitz et al. 1998).
Figure 4. cGMP sensitivity is modulated by PKC activity.

A, I-Vm relations obtained from a cell after treatment with 0.5 μm of the inactive isomer 4α-PMA. Ligand sensitivity of channels was similar to that of untreated control. B, I-Vm relations obtained from a cell after treatment with 0.5 μm PMA in the presence of 1 μm staurosporin. No modulation of channel activity was observed. C, comparison of channel sensitivity of control cells (dotted line, taken from Fig. 3C), cells treated with 4α-PMA (○, continuous line), cells treated with PMA in the presence of staurosporin (•, dashed line), and cells treated with PMA (dash-dotted line, taken from Fig. 3C).
Binding of CaM to Pα2 was tested by a second independent approach using a preparation of photoreceptor outer/inner segments that contain a nitric oxide synthase (NOS) and a soluble guanylyl cyclase (sGC) (Koch et al. 1994; Weitz et al. 1998). This assay probes the CaM-peptide interaction in solution and not on a dextran-water interface as with SPR spectroscopy. The Ca2+-CaM-dependent activation of NOS was monitored by the cGMP synthesis of NO-activated sGC (Zoche et al. 1996; Weitz et al. 1998). The activity of neuronal NOS is strictly dependent on Ca2+-CaM; peptides that sequester CaM inhibit NOS activity and thereby also cGMP synthesis. In fact, Pα2 inhibited cGMP production by complexing CaM in a concentration-dependent fashion. Half-maximal inhibition (IC50) was observed at roughly 1 μm (data not shown). Although this semi-quantitative assay does not allow the determination of true KD constants, it is still suitable to establish a rank order of relative binding affinities among different CaM targets. The same analysis, using peptides Pα3 and P2β1, which represent the CaM-binding sites of the α3 subunit from OSNs and of the β1 subunit from rods, yielded IC50 values of 0.37 μm and 11.5 μm, respectively (Weitz et al. 1998). In conclusion, Pα2 is more akin to Pα3 than to P2β1 with respect to both sequence similarity and CaM-binding affinity.
The ligand sensitivity of bovine α2 homomeric channels is not modulated by Ca2+-CaM
Considering the high CaM-binding affinity of Pα2 and the sequence similarity of Pα2 and Pα3, we examined whether CaM modulates the ligand affinity of bovine α2 channels in a similar way as it modulates the α3 channel (Chen & Yau, 1994; Liu et al. 1994). As a positive control, we measured the CaM action on the chicken α2 channel. Figure 2A shows macroscopic current-voltage (I-Vm) relations of chicken α2 channels recorded in inside-out membrane patches in the presence of 50 μm free Ca2+. At saturating cGMP concentration (500 μm), current recordings with CaM (1.2 μm) and without CaM in the bath are identical (compare traces 1 and 2), whereas at 20 μm cGMP (i.e. a concentration close to K1/2), CaM lowered the current amplitude to about 50 % (compare traces 3 and 4). In a more detailed analysis, we found that this corresponds to a shift in K1/2 value from 20 μm to about 31 μm at +80 mV (Bönigk et al. 1996). The CaM action developed gradually and was complete within approximately 1 min. The decrease of current was only observed in the presence of CaM, and it was fully reversible in a solution containing 10 mm EGTA and no Ca2+. In contrast, bovine α2 homomeric channels were not affected by CaM in the presence of 10 μm cGMP (traces 3 and 4; Fig. 2B). Normalized currents (I10 μm/Imax) in the presence of CaM were 96 ± 17 % of normalized currents in the absence of CaM (n= 9; +80 mV). In a few patches, we observed a current decrease during the perfusion with CaM (up to 5 μm), which we suspect represents an unspecific run-down, because it resisted wash-out by EGTA and it was observed in a few control patches in the absence of Ca2+-CaM.
Figure 2. Modulation of cGMP sensitivity of CNG channels by CaM.

A, current-voltage (I-Vm) relations recorded from an inside-out patch from HEK 293 cells expressing chicken α2 channels (chα2). No significant difference was observed between trace 1 (500 μm cGMP) and trace 2 (500 μm cGMP plus 1.2 μm CaM). The current at low cGMP concentration was reduced by CaM (trace 3: 20 μm cGMP; trace 4: 20 μm cGMP plus 1.2 μm CaM). B, I-Vm relations from an inside-out patch from HEK 293 cells expressing bovine α2 homomeric channels (bα2). CaM did not reduce channel activity (trace 1: 1 mm cGMP; trace 2: 1 mm cGMP plus 1 μm CaM; trace 3: 10 μm cGMP; trace 4: 10 μm cGMP plus 1 μm CaM). C, I-Vm relations from an inside-out patch from HEK 293 cells expressing bovine α2β1 heteromeric channels (bα2β1). CaM modulation of channel activity is similar to that in A (traces 1-4, conditions as in B).
In rod CNG channels, the CaM sensitivity requires the β1 subunit (Hsu & Molday, 1993; Chen et al. 1994; Körschen et al. 1995). Moreover, in mammalian sperm, the α2 subunit is co-expressed with short splice forms of the β1 subunit, suggesting that these two subunits may co-assemble to form functional channels (Wiesner et al. 1998). Although there is no evidence for the involvement of the rod β1 subunit or one of its splice variants in forming the native CNG channel of cones, we considered the possibility that a related subunit endows this channel with CaM sensitivity. Therefore, we examined whether the β1 subunit, when co-expressed with the bovine α2 subunit, confers CaM sensitivity upon the heteromeric channels. Co-expression of α1 and β1 subunits occasionally results in mixtures of homomeric α1 and heteromeric α1β1 channels (Körschen et al. 1995). The α1 and α1β1 channel species are distinguished by their different sensitivities to blockage by l-cis-diltiazem. This drug blocks α1β1 heteromeric channels at micromolar concentrations, whereas the α1 homomeric channel requires 50- to 100-fold higher concentrations (Kaupp et al. 1989; Chen et al. 1993). Like α1 channels, homomeric α2 channels were only weakly blocked by 10 μml-cis-diltiazem, whereas co-expression of α2 and β1 subunits produced channels that were inhibited by 50 % at +80 mV (data not shown). From this result we conclude that α2 and β1 subunits coassemble and that the β1 subunit significantly enhances sensitivity to l-cis-diltiazem. The CaM action on α2β1 channels (Fig. 2C) was similar to that shown in Fig. 2A for chicken α2 and similar to that described for α1β1 channels (Körschen et al. 1995; Grunwald et al. 1998; Weitz et al. 1998). In the presence of CaM, the normalized current decreased to 59 ± 10 % (n= 2; +80 mV). In conclusion, bovine α2 homomeric channels are insensitive to modulation by CaM, but CaM sensitivity can be conferred to heteromeric channels by the β1 subunit of rod CNG channels.
Phorbol ester treatment decreases the apparent ligand affinity of α2 homomeric channels
To study the effects of PKC-mediated phosphorylation, HEK 293 cells expressing bovine α2 subunits were incubated for 30-60 min with 0.5 μm phorbol 12-myristate 13-acetate (PMA), a phorbol ester that specifically activates most PKC isoforms (Nishizuka, 1986). The ligand sensitivity was tested in PMA-free solution in inside-out patches exposed to various concentrations of cGMP. Figure 3A shows I-Vm relations in the presence of several cGMP concentrations in a patch from an untreated control cell. The average value for K1/2 was 19.3 ± 1.8 μm cGMP, and the Hill coefficient, nH, was 2.5 ± 0.2 at Vm= -60 mV (8 experiments). The data are in good agreement with an earlier report on the cGMP sensitivity of the α2 subunit (Weyand et al. 1994). Figure 3B shows I-Vm relations recorded from a cell after PMA treatment. The cGMP sensitivity was markedly diminished, i.e. higher concentrations of cGMP were necessary to activate the channel. The shift of the K1/2 value persisted for 2 h after removal of PMA. In addition, the Hill coefficient was significantly lower. We determined average values for K1/2 of 55.8 ± 13 μm cGMP, and for nH of 1.5 ± 0.2 (14 experiments). The dose-response relations in Fig. 3C illustrate both the shift of channel activation to higher cGMP concentrations and the smaller Hill coefficient upon PMA treatment. K1/2 values measured in inside-out patches from PMA-treated cells ranged from 40 to 80 μm cGMP. This range of K1/2 values is larger than expected from normal experimental variations. We interpret the excess variability to indicate that the patches contained mixed channel populations with different ratios of phosphorylated to unphosphorylated channels.
Figure 3. Modulation of cGMP sensitivity of bovine α2 homomeric CNG channels by treatment with phorbol ester.

A, I-Vm relations of untreated α2 homomeric channels. B, I-Vm relations obtained from a cell after incubation with 0.5 μm PMA, demonstrating a decrease of cGMP sensitivity. Here and in the other figures, the cGMP concentrations (μm) are indicated to the right of each trace. C, dose-response relations at Vm= -60 mV in control (○) and PMA-treated (•) cells. Continuous lines represent fits of a Hill-type function to normalized currents (see Methods). Fitting parameters were K1/2= 19.3 ± 1.8 μm; nH= 2.5 ± 0.2 (8 patches) for control and K1/2= 55.8 ± 13 μm; nH= 1.5 ± 0.2 (14 patches) for PMA-treated cells.
The following experiments were performed to control for unspecific effects of the phorbol ester. First, treatment of the cells with 4α-PMA, a stereo-isomer that does not activate PKC (Van Duuren et al. 1979), did not change the cGMP sensitivity of the channels (Fig. 4A, K1/2= 18.6 ± 1.4 μm, nH= 2.6 ± 0.5, 11 experiments). Second, the kinase inhibitor staurosporin (1 μm) completely prevented the shift of ligand sensitivity by the active isomer 4β-PMA (Fig. 4B, K1/2= 20.3 ± 1.7 μm, nH= 2.8 ± 0.1, 3 experiments). We conclude that the PMA action was specifically mediated by activation of PKC.
Identification of PKC phosphorylation sites
We set out to identify the sites of phosphorylation by mutagenesis of consensus sites for PKC phosphorylation. In twelve α2 mutants the serine or threonine residues of putative PKC phosphorylation sites were replaced by alanine residues, either one by one (for example in mutant S79A, the serine residue at position 79 was exchanged for alanine), or in various combinations. In addition, a truncated mutant, Δ105, was constructed which lacked the N-terminus up to residue 105. The mutant channels were studied in HEK 293 cells for PMA-induced shifts of the K1/2 constant and Hill coefficient. The results are presented in Table 1. Under control conditions, mutant channels S79A, Δ105, S132A and S438A showed K1/2 values similar to that of the wild-type channel. Mutants S97A, S84A, S79A-S84A, and S111A showed a slightly higher cGMP sensitivity; only mutant T495A showed a significantly lower cGMP sensitivity. Residue T495 is located next to D494, a residue that has been identified among several other residues in the linker region between transmembrane segment 6 and the cGMP-binding domain, which control the gating and thereby the cGMP sensitivity in α2 homomeric channels (Zong et al. 1998). All nine mutants responded with a shift of K1/2 to PMA treatment, indicating that phosphorylation of these residues does not control ligand sensitivity.
Table 1.
The action of PMA on wild-type and mutant channels
| K1/2 (μM) | nH | |||
|---|---|---|---|---|
| Control | PMA | Control | PMA | |
| Wild-type | 19.3 ± 1.8 (8) | 55.8 ± 13 (14) | 2.5 ± 0.2 (8) | 1.5 ± 0.2 (14) |
| S97A | 16 ± 3.5 (3) | 45 (1) | 2.2 ± 0.2 (3) | 1.2 (1) |
| S79A | 20.8 ± 3.6 (4) | 62 (1) | 2.4 ± 0.1 (4) | 1.4 (1) |
| S84A | 16.5 ± 2.8 (10) | 56.3 ± 14.8 (8) | 2.0 ± 0.2 (10) | 1.4 ± 0.2 (8) |
| S79A–S84A | 14 ± 2.6 (10) | 49.4 ± 0.14 (5) | 2.2 ± 0.4 (10) | 1.1 ± 0.2 (5) |
| Δ105 | 22.3 ± 3 (3) | 103 ± 20.8 (3) | 1.9 ± 0.1 (3) | 1.2 ± 0.2 (3) |
| S111A | 13.8 ± 1.5 (6) | 41.0 ± 9.4 (4) | 1.9 ± 0.2 (6) | 1.2 ± 0.2 (4) |
| S132A | 17.3 ± 1.15 (3) | 78.3 ± 19.9 (3) | 2.2 ± 0.3 (3) | 1.4 ± 0.1 (3) |
| S438A | 18.3 ± 0.6 (3) | 54.6 ± 23.9 (3) | 2.0 ± 0.3 (3) | 1.3 ± 0.1 (3) |
| T495A | 96.0 ± 18.0 (16) | 186.0 ± 25.0 (13) | 1.8 ± 0.2 (16) | 1.4 ± 0.3 (13) |
| S577A | 18.4 ± 6.1 (8) | 19.3 ± 5.7 (8) | 2.5 ± 0.4 (8) | 1.9 ± 0.2 (8) |
| S579A | 21.1 ± 3.3 (7) | 26.3 ± 4.45 (10) | 2.3 ± 0.3 (7) | 1.8 ± 0.2 (10) |
| S577A–S579A | 81.4 ± 12 (5) | 75.4 ± 7.1 (6) | 2.6 ± 0.2 (5) | 2.3 ± 0.3 (6) |
Values are given as means ± S.D. (number of experiments).
Two serine residues, S577 and S579, were identified within the cGMP-binding domain as being crucial for the PMA action. In mutant S577A, the K1/2 value of 18.4 ± 6.1 μm and nH of 2.5 ± 0.4 were not significantly different from the respective values of the wild-type channel under control conditions. PMA treatment did not alter the K1/2 value (K1/2= 19.3 ± 5.7 μm), and nH was only slightly lower (1.9 ± 0.2) (Fig. 5). This result shows that S577 is essential for the PMA action on ligand sensitivity. The adjacent residue, S579, was of similar importance. In control patches, the K1/2 value of mutant S579A was 21.1 ± 3.3 μm, and nH was 2.3 ± 0.3. In PMA-treated cells, the K1/2 value increased insignificantly to 26.3 ± 4.5 μm, and nH was 1.8 ± 0.2. In conclusion, both mutations abolish the PMA action on the cGMP sensitivity, but a small decrease in the Hill coefficient was still noticeable.
Figure 5. PMA-induced decrease of cGMP sensitivity is abolished in mutant S577A.
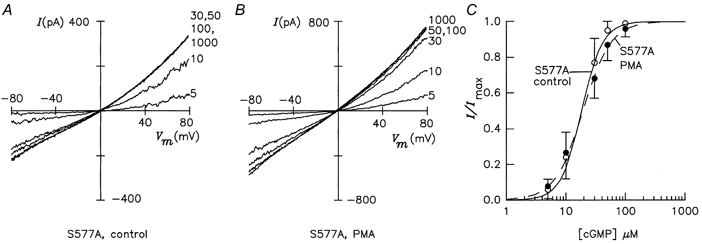
A, I-Vm relations of untreated mutant channels. B, I-Vm relations of mutant channels after PMA treatment. C, dose-response relations of channel activity in control (○, continuous line; K1/2= 18.4 ± 6.1 μm, nH= 2.5 ± 0.4) and PMA-treated (•, dashed line; K1/2= 19.3 ± 5.7 μm, nH= 1.9 ± 0.2) cells.
In the double mutant lacking both serines (S577A-S579A), K1/2 was 81.4 ± 11.9 μm, and nH was 2.6 ± 0.2 under control conditions. Upon PMA treatment, the values of K1/2 and nH were not changed significantly (75.4 ± 7.1 μm, and 2.3 ± 0.3) (Fig. 6). This lack of a PMA effect in the double mutant further supports the idea that PKC-mediated phosphorylation of S577 and S579 is responsible for the PMA-induced decrease of ligand sensitivity in the wild-type channel.
Figure 6. PMA-induced decrease of cGMP sensitivity is abolished in double mutant S577A-S579A.

A, I-Vm relations of untreated mutant channels. B, I-Vm relations of mutant channels after PMA treatment. C, comparison of dose-response relations in the untreated control (○, continuous line; K1/2= 81.4 ± 12 μm, nH= 2.6 ± 0.2) and PMA-treated (•, dashed line; K1/2= 75.4 ± 7.1 μm, nH= 2.3 ± 0.3) cells.
PKC δ is specifically expressed in cone outer segments
We investigated the expression of PKC in the rat retina using a set of nine monoclonal antibodies raised against different isoforms. While antibodies against several PKC isoforms stained bipolar and amacrine cells in the inner retina (data not shown), only an antibody against PKC δ stained cone photoreceptors. Figure 7 shows confocal images of a vertical section through the retina viewed with Nomarski optics (A) or epifluorescence (B and C). The outer nuclear layer (ONL) harbours the somata of rods and cones. The inner segments (IS) of rods and cones are located above the ONL. The uppermost layer represents the rod outer segments (ROS), and cone outer segments are located closer to the inner segment layer. Figure 7B shows the immunofluorescence detection of PKC δ (visualized by green fluorescence). Two kinds of structures are labelled. Rods are immunopositive at the transition from inner to outer segments, whereas the distal parts of the rod outer segments are immunonegative. In addition, four putative cone outer segments are strongly PKC δ positive (arrows). Figure 7C shows the immunofluorescence detection of the middle- to long-wavelength-sensitive cone opsin (detected by antiserum JH492, red fluorescence). The same four structures are clearly marked, unequivocally identifying them as cone outer segments (arrows). Cone outer segments labelled with the antiserum JH455 against the short-wavelength-sensitive opsin also were PKC δ immunoreactive (data not shown). In conclusion, all cone types were found to show PKC δ immunoreactivity.
Figure 7. Localization of PKC δ-like immunoreactivity in vertical sections of the rat retina.

A, microphotograph of the photoreceptor layer taken with differential interference contrast optics. ROS, rod outer segments; IS, inner segments; ONL, outer nuclear layer. B, confocal image showing immunofluorescence detection of PKC δ. Immunoreactivity is seen at the transition from rod inner to outer segments. Four putative cone outer segments (arrows) are strongly labelled. C, confocal image of cone opsin immunoreactivity. The four structures from B (arrows) are unequivocally identified as cone outer segments. Scale bar, 20 μm.
DISCUSSION
Modulation of cone CNG channels by CaM
In the present study we show that the putative CaM-binding site in the N-terminus of the bovine α2 subunit binds CaM with high affinity, yet α2 homomeric channels are not modulated by CaM. This result disagrees with another study, which reported modulation of the same bovine α2 subunit by CaM (Biel et al. 1996). The reason for this discrepancy is not evident. Our experimental protocol reveals robust and reversible CaM modulation of rat and bovine α3 homomeric channels (Müller et al. 1998), chicken α2 homomeric channels (Fig. 2A), and bovine α1β1 and α2β1 heteromeric channels (Körschen et al. 1995, and Fig. 2C), but not of bovine α2 homomeric channels. Our results agree, however, with two reports showing that the human α2 orthologue is insensitive to CaM (Yu et al. 1996; Grunwald et al. 1999). The action of CaM on native cone CNG channels has been studied in two species. In striped bass CaM has a weak effect (Hackos & Korenbrot, 1997), whereas CNG channels of catfish cones are insensitive to CaM (Haynes & Stotz, 1997).
It seems odd that CaM-binding motifs are functional in the CNG channels of cones from some but not all species and that a similar motif in the olfactory α3 subunit mediates an order of magnitude larger effect. In the olfactory α3 channel, an intramolecular interaction between the N-terminal domain and the C-terminal cNMP-binding domain promotes channel opening after ligand binding (Goulding et al. 1994; Gordon & Zagotta, 1995; Varnum & Zagotta, 1997). Binding of Ca2+-CaM perturbs the interdomain interaction and thereby diminishes the apparent ligand affinity. Deletion of the CaM-binding site (Liu et al. 1994) or the entire N-terminal domain (Müller et al. 1998) increases the K1/2 constant to the same extent as does binding of CaM. The CaM-binding domain may not exclusively provide the interaction between N- and C-termini. Additional residues downstream of the CaM-binding site are important for the CaM-sensitive component of channel gating (Liu et al. 1994; Müller et al. 1998; Grunwald et al. 1999). As an example, phosphorylation by PKC of a serine residue (S93) downstream of the CaM-binding site enhances cGMP sensitivity roughly 10-fold (Müller et al. 1998). In α2 CNG channels, the interaction between N- and C-termini seems to be less pronounced. Removal of the N-terminus did not change the cGMP sensitivity (see deletion mutant Δ105). Mutation of serine 97, the homologue of residue 93 in α3, neither substantially altered the apparent ligand affinity nor abolished the PMA effect. Thus, the different sensitivities to CaM may depend on differences in the amino acid sequence outside the CaM-binding site. Interestingly, when several residues in this downstream region of human α2 are replaced by the respective residues of α3, the channel chimera becomes CaM sensitive (Grunwald et al. 1999).
A quite different line of evidence suggests that an unknown endogenous factor modulates the cone CNG channel in situ and that CaM acts as a partial agonist that is unable to shift the ligand sensitivity as much as the authentic ligand (Rebrik & Korenbrot, 1998). An endogenous factor other than CaM has also been invoked in the control of CNG channels from rod photoreceptors (Gordon et al. 1995).
Modulation of CNG channels by PKC-mediated phosphorylation
Activation of PKC decreases the ligand sensitivity of bovine α2 subunits brought about by phosphorylation of two amino acid residues within the cGMP-binding domain. It is plausible that changes in the cGMP-binding domain might either perturb ligand binding or the gating mechanism, both resulting in higher activation constants. We show here that PKC δ is expressed in rat cone outer segments. This enzyme belongs to a class of ‘novel’ Ca2+-independent PKC isoforms whose regulatory mechanisms are not known. Our findings raise the possibility that the CNG channel is a target for this PKC isoform and that phosphorylation of the CNG channel could serve as an adaptive mechanism that controls light sensitivity. PKC activation was reported to have no effect on the light response of rod photoreceptors (for a comprehensive discussion see Xiong et al. 1997). A similarly incisive study is lacking in cones. Future work needs to define the functional significance of this PKC isoform in intact cone photoreceptors and its mechanism of activation.
CNG channel phosphorylation has also been studied in rod photoreceptors. Gordon et al. (1992) reported that the cGMP sensitivity of CNG channels in membrane patches excised from rod outer segments is enhanced by Ser/Thr phosphatase activity, indicating that the channels are phosphorylated in situ. Molokanova et al. (1997, 1999) found that tyrosine phosphorylation lowers cGMP sensitivity of α1 subunits expressed in oocytes about 2-fold. The action of tyrosine phosphorylation is much less pronounced in native CNG channels from rods (maximally 15 % change in K1/2; Molokanova et al. 1997). In conclusion, the physiological significance of phosphorylation, whether in rods or cones or whether by PKC or tyrosine kinases, is still enigmatic and requires further study in intact photoreceptor cells.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft to K.-W.K. and U.B.K. We thank M. Bruns for technical assistance in cell culture and A. Eckert for preparing the manuscript. We thank J. Nathans, Baltimore, for kindly providing the antisera JH455 and JH492.
References
- Balasubramanian S, Lynch JW, Barry PH. Calcium-dependent modulation of the agonist affinity of the mammalian olfactory cyclic nucleotide-gated channel by calmodulin and a novel endogenous factor. Journal of Membrane Biology. 1996;152:13–23. doi: 10.1007/s002329900081. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Cyclic GMP-gated channels of bovine rod photoreceptors: affinity, density and stoichiometry of Ca2+-calmodulin binding sites. Journal of Physiology. 1996;494:675–685. doi: 10.1113/jphysiol.1996.sp021523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann A, Frings S, Godde M, Seifert R, Kaupp UB. Primary structure and functional expression of a Drosophila cyclic nucleotide-gated channel present in eyes and antennae. EMBO Journal. 1994;13:5040–5050. doi: 10.1002/j.1460-2075.1994.tb06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M, Zong X, Ludwig A, Sautter A, Hofmann F. Molecular cloning and expression of a modulatory subunit of the cyclic nucleotide-gated cation channel. Journal of Biological Chemisty. 1996;271:6349–6355. doi: 10.1074/jbc.271.11.6349. [DOI] [PubMed] [Google Scholar]
- Bönigk W, Altenhofen W, Müller F, Dose A, Illing M, Molday RS, Kaupp UB. Rod and cone photoreceptor cells express distinct genes for cGMP-gated channels. Neuron. 1993;10:865–877. doi: 10.1016/0896-6273(93)90202-3. [DOI] [PubMed] [Google Scholar]
- Bönigk W, Bradley J, Müller F, Sesti F, Boekhoff I, Ronnett GV, Kaupp UB, Frings S. The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits. Journal of Neuroscience. 1999;19:5332–5347. doi: 10.1523/JNEUROSCI.19-13-05332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bönigk W, Müller F, Middendorff R, Weyand I, Kaupp UB. Two alternatively spliced forms of the cGMP-gated channel α-subunit from cone photoreceptor are expressed in the chick pineal organ. Journal of Neuroscience. 1996;16:7458–7468. doi: 10.1523/JNEUROSCI.16-23-07458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J, Li J, Davidson N, Lester HA, Zinn K. Heteromeric olfactory cyclic nucleotide-gated channels: A new subunit that confers increased sensitivity to cAMP. Proceedings of the National Academy of Sciences of the USA. 1994;91:8890–8894. doi: 10.1073/pnas.91.19.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-Y, Illing M, Molday LL, Hsu Y-T, Yau K-W, Molday RS. Subunit 2 (or β) of retinal rod cGMP-gated cation channel is a component of the 240-kDa channel-associated protein and mediates Ca2+-calmodulin modulation. Proceedings of the National Academy of Sciences of the USA. 1994;91:11757–11761. doi: 10.1073/pnas.91.24.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-Y, Peng Y-W, Dhallan RS, Ahamed B, Reed RR, Yau K-W. A novel subunit of the cyclic nucleotide-gated cation channel in retinal rods. Biophysical Journal. 1993;64:A133. doi: 10.1038/362764a0. (Abstract) [DOI] [PubMed] [Google Scholar]
- Chen T-Y, Yau K-W. Direct modulation by Ca2+-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994;368:545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- Dhallan RS, Macke JP, Eddy RL, Shows TB, Reed RR, Yau K-W, Nathans J. Human rod photoreceptor cGMP-gated channel: Amino acid sequence, gene structure, and functional expression. Journal of Neuroscience. 1992;12:3248–3256. doi: 10.1523/JNEUROSCI.12-08-03248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhallan RS, Yau K-W, Schrader KA, Reed RR. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- Finn JT, Grunwald ME, Yau K-W. Cyclic nucleotide-gated ion channels: An extended family with diverse functions. Annual Review of Physiology. 1996;58:395–426. doi: 10.1146/annurev.ph.58.030196.002143. [DOI] [PubMed] [Google Scholar]
- Gerstner A, Zong X, Hofmann F, Biel M. Molecular cloning and functional characterization of a new modulatory cyclic nucleotide-gated channel subunit from mouse retina. Journal of Neuroscience. 2000;20:1324–1332. doi: 10.1523/JNEUROSCI.20-04-01324.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SE, Brautigan DL, Zimmerman AL. Protein phosphatases modulate the apparent agonist affinity of the light-regulated ion channel in retinal rods. Neuron. 1992;9:739–748. doi: 10.1016/0896-6273(92)90036-d. [DOI] [PubMed] [Google Scholar]
- Gordon SE, Downing-Park J, Zimmerman AL. Modulation of the cGMP-gated ion channel in frog rods by calmodulin and an endogenous inhibitory factor. Journal of Physiology. 1995;486:533–546. doi: 10.1113/jphysiol.1995.sp020832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SE, Zagotta WN. A histidine residue associated with the gate of the cyclic nucleotide-activated channels in rod photoreceptors. Neuron. 1995;14:177–183. doi: 10.1016/0896-6273(95)90252-x. [DOI] [PubMed] [Google Scholar]
- Goulding EH, Tibbs GR, Siegelbaum SA. Molecular mechanism of cyclic-nucleotide-gated channel activation. Nature. 1994;372:369–374. doi: 10.1038/372369a0. [DOI] [PubMed] [Google Scholar]
- Grunwald ME, Yu W-P, Yu H-H, Yau K-W. Identification of a domain on the β-subunit of the rod cGMP-gated cation channel that mediates inhibition by calcium-calmodulin. Journal of Biological Chemistry. 1998;273:9148–9157. doi: 10.1074/jbc.273.15.9148. [DOI] [PubMed] [Google Scholar]
- Grunwald ME, Zhong H, Lai J, Yau K-W. Molecular determinants of the modulation of cyclic nucleotide-activated channels by calmodulin. Proceedings of the National Academy of Sciences of the USA. 1999;96:13444–13449. doi: 10.1073/pnas.96.23.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackos DH, Korenbrot JI. Calcium modulation of ligand affinity in the cyclic GMP-gated ion channels of cone photoreceptors. Journal of General Physiology. 1997;110:515–528. doi: 10.1085/jgp.110.5.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LW, Stotz SC. Modulation of rod, but not cone, cGMP-gated photoreceptor channels by calcium-calmodulin. Visual Neuroscience. 1997;14:233–239. doi: 10.1017/s0952523800011378. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Koenen M. A general and rapid mutagenesis method using polymerase chain reaction. Gene. 1990;91:143–147. doi: 10.1016/0378-1119(90)90177-s. [DOI] [PubMed] [Google Scholar]
- Hsu Y-T, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993;361:76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Niidome T, Tanabe T, Terada S, Bönigk W, Stühmer W, Cook NJ, Kangawa K, Matsuo H, Hirose T, Miyata T, Numa S. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989;342:762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Klotz IM. Ligand-protein binding affinities. In: Creighton TE, editor. Protein Function - A Practical Approach. Oxford: IRL Press; 1989. pp. 25–54. [Google Scholar]
- Koch K-W. Identification and characterization of calmodulin binding sites in cGMP-gated channel using surface plasmon resonance spectroscopy. Methods in Enzymology. 2000;315:785–797. doi: 10.1016/s0076-6879(00)15882-3. [DOI] [PubMed] [Google Scholar]
- Koch K-W, Lambrecht H-G, Haberecht M, Redburn D, Schmidt HHHW. Functional coupling of a Ca2+/calmodulin-dependent nitric oxide synthase and a soluble guanylyl cyclase in vertebrate photoreceptor cells. EMBO Journal. 1994;13:3312–3320. doi: 10.1002/j.1460-2075.1994.tb06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körschen HG, Illing M, Seifert R, Sesti F, Williams A, Gotzes S, Colville C, Müller F, Dosé A, Godde M, Molday L, Kaupp UB, Molday RS. A 240 kDa protein represents the complete β subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron. 1995;15:627–636. doi: 10.1016/0896-6273(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Research. 1984;12:857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- Liman ER, Buck LB. A second subunit of the olfactory cyclic nucleotide-gated channel confers high sensitivity to cAMP. Neuron. 1994;13:611–621. doi: 10.1016/0896-6273(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Liu M, Chen T-Y, Ahamed B, Li J, Yau K-W. Calcium-calmodulin modulation of the olfactory cyclic nucleotide-gated cation channel. Science. 1994;266:1348–1354. doi: 10.1126/science.266.5189.1348. [DOI] [PubMed] [Google Scholar]
- Ludwig J, Margalit T, Eismann E, Lancet D, Kaupp UB. Primary structure of cAMP-gated channel from bovine olfactory epithelium. FEBS Letters. 1990;270:24–29. doi: 10.1016/0014-5793(90)81226-e. [DOI] [PubMed] [Google Scholar]
- Molokanova E, Savchenko A, Kramer RH. Noncatalytic inhibition of cyclic nucleotide-gated channels by tyrosine kinase induced by genistein. Journal of General Physiology. 1999;113:45–56. doi: 10.1085/jgp.113.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E, Trivedi B, Savchenko A, Kramer RH. Modulation of rod photoreceptor cyclic nucleotide-gated channels by tyrosine phosphorylation. Journal of Neuroscience. 1997;17:9068–9076. doi: 10.1523/JNEUROSCI.17-23-09068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Bönigk W, Sesti F, Frings S. Phosphorylation of mammalian olfactory cyclic nucleotide-gated channels increases ligand sensitivity. Journal of Neuroscience. 1998;18:164–173. doi: 10.1523/JNEUROSCI.18-01-00164.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:306–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Pittler SJ, Lee AK, Altherr MR, Howard TA, Seldin MF, Hurwitz RL, Wasmuth JJ, Baehr W. Primary structure and chromosomal localization of human and mouse rod photoreceptor cGMP-gated cation channel. Journal of Biological Chemistry. 1992;267:6257–6262. [PubMed] [Google Scholar]
- Rebrik TI, Korenbrot JI. In intact cone photoreceptors, a Ca2+-dependent, diffusible factor modulates the cGMP-gated ion channels differently than in rods. Journal of General Physiology. 1998;112:537–548. doi: 10.1085/jgp.112.5.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautter A, Zong X, Hofmann F, Biel M. An isoform of the rod photoreceptor cyclic nucleotide-gated channel β subunit expressed in olfactory neurons. Proceedings of the National Academy of Sciences of the USA. 1998;95:4696–4701. doi: 10.1073/pnas.95.8.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duuren BL, Tseng SS, Segal A, Smith AC, Melchionne S, Seidman I. Effects of structural changes on the tumor-promoting activity of phorbol myristate acetate on mouse skin. Cancer Research. 1979;39:2644–2646. [PubMed] [Google Scholar]
- Varnum MD, Zagotta WN. Interdomain interactions underlying activation of cyclic nucleotide-gated channels. Science. 1997;278:110–113. doi: 10.1126/science.278.5335.110. [DOI] [PubMed] [Google Scholar]
- Weitz D, Zoche M, Müller F, Beyermann M, Körschen HG, Kaupp UB, Koch K-W. Calmodulin controls the rod photoreceptor CNG channel through an unconventional binding site in the N-terminus of the β-subunit. EMBO Journal. 1998;17:2273–2284. doi: 10.1093/emboj/17.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand I, Godde M, Frings S, Weiner J, Müller F, Altenhofen W, Hatt H, Kaupp UB. Cloning and functional expression of a cyclic-nucleotide-gated channel from mammalian sperm. Nature. 1994;368:859–863. doi: 10.1038/368859a0. [DOI] [PubMed] [Google Scholar]
- Wiesner B, Weiner J, Middendorf R, Hagen V, Kaupp UB, Weyand I. Cyclic nucleotide-gated channels on the flagellum control Ca2+ entry into sperm. Journal of Cell Biology. 1998;142:473–484. doi: 10.1083/jcb.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissinger B, Müller F, Weyand I, Schuffenhauer S, Thanos S, Kaupp UB, Zrenner E. Cloning, chromosomal localization and functional expression of the gene encoding the α-subunit of the cGMP-gated channel in human cone photoreceptors. European Journal of Neuroscience. 1997;9:2512–2521. doi: 10.1111/j.1460-9568.1997.tb01680.x. [DOI] [PubMed] [Google Scholar]
- Xiong W-H, Nakatani K, Ye B, Yau K-W. Protein kinase C activity and light sensitivity of single amphibian rods. Journal of General Physiology. 1997;110:441–452. doi: 10.1085/jgp.110.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W-P, Grunwald ME, Yau K-W. Molecular cloning, functional expression and chromosomal localization of a human homolog of the cyclic nucleotide-gated ion channel of retinal cone photoreceptor. FEBS Letters. 1996;393:211–215. doi: 10.1016/0014-5793(96)00889-7. [DOI] [PubMed] [Google Scholar]
- Zoche M, Bienert M, Beyermann M, Koch K-W. Distinct molecular recognition of calmodulin-binding sites in the neuronal and macrophage nitric oxide synthases: A surface plasmon resonance study. Biochemistry. 1996;35:8742–8747. doi: 10.1021/bi960445t. [DOI] [PubMed] [Google Scholar]
- Zong X, Zucker H, Hofmann F, Biel M. Three amino acids in the C-linker are major determinants of gating in cyclic nucleotide-gated channels. EMBO Journal. 1998;17:353–362. doi: 10.1093/emboj/17.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]


