Abstract
It has been demonstrated that both salicylic acid and fungal elicitors activate a 48-kDa mitogen-activated protein kinase termed salicylic acid-induced protein kinase (SIPK) in tobacco suspension cells. Here, we show that infiltration of these agents into tobacco leaves also activates SIPK. Of particular interest, infiltration of water alone activated a kinase of the same size, possibly because of wounding and/or osmotic stresses. The kinetics of kinase activation, however, differ for these different treatments. Various mechanical stresses, including cutting and wounding by abrasion, also activated a 48-kDa kinase. By using an immune-complex kinase assay with antibodies specific for SIPK or wounding-induced protein kinase, we demonstrate that this wounding-activated 48-kDa kinase is SIPK, rather than wounding-induced protein kinase, as reported [Seo, S., Okamoto, M., Seto, H., Ishizuka, K., Sano, H. & Ohashi, Y. (1995) Science 270, 1988–1992]. Activation of SIPK after wounding was associated with tyrosine phosphorylation but not with increases in SIPK mRNA or protein levels. Thus, the same mitogen-activated protein kinase, SIPK, appears to facilitate signaling for two distinct pathways that lead to disease resistance responses and wounding responses.
Plants have developed an impressive array of defense responses that help minimize or prevent damage caused by a variety of stresses, such as mechanical wounding, UV light exposure, or pathogen attack. Some of these defense responses, including ion fluxes and the generation of reactive oxygen species, occur within minutes and may involve events that occur primarily at the post-translational level (1–4). Within a few hours, these immediate early responses are followed by the activation of enzymes involved in the biosynthesis of phytoalexins and cell wall components such as hydroxyproline-rich glycoproteins; these responses require transcriptional activation (5). Meanwhile, secondary signaling molecules such as ethylene, jasmonates, and/or salicylic acid (SA) are produced. These signals lead to the induction of various late responses, such as the activation of genes encoding protease inhibitors, chitinases, glucanases, and/or other pathogenesis-related proteins (6, 7).
In yeast and animal cells, mitogen-activated protein (MAP) kinases have been shown to play important roles in regulating stress responses (8–10). They comprise the bottom tier of a cascade that is composed of at least three functionally interlinked kinases and that participates in the transmission of extracellular signals through the cytoplasm to the nucleus. Activation of MAP kinase requires the dual phosphorylation of threonine and tyrosine residues in a TXY motif by an upstream MAP kinase kinase. Similarly, the activity of MAP kinase kinase is regulated by an upstream MAP kinase kinase kinase through phosphorylation (11).
In plants, several kinase activities believed to be MAP kinases [based on the fact that they preferentially phosphorylate myelin basic protein (MBP) and are themselves phosphorylated on tyrosine residues on activation] have been shown (12–16) to be activated by stress stimuli. These kinases include the tobacco wounding (cutting)-activated 46-kDa kinase (12, 13), the fungal elicitor-activated 47-kDa kinase from tobacco (14), the harpin-activated 49-kDa kinase from tobacco (15), and the wounding, systemin, and oligosaccharide-activated 48-kDa kinase from tomato (16). In addition, a gene encoding a tobacco MAP kinase homolog, designated WIPK, has been isolated and shown to be rapidly induced at the mRNA level by wounding. Based on this response, WIPK has been hypothesized to encode the 46-kDa kinase that is activated rapidly by wounding (12). Recently, evidence using an antibody against the C-terminal peptide of the alfalfa MMK4 has linked the alfalfa MMK4 to cold, drought, and mechanical stresses (17, 18). The same antibody also was used to demonstrate that parsley ERMK may encode the 45-kDa kinase activated by Pep25 elicitor derived from the Phytophthora sojae glycoprotein elicitor (19).
We previously have identified a gene that encodes an SA-activated MAP kinase by purifying the protein and cloning the corresponding gene based on peptide sequence (20). This gene was termed SIPK (for SA-induced protein kinase). With the use of an antibody raised against a peptide corresponding to the unique N terminus of SIPK, it was shown that a fungal cell wall-derived carbohydrate elicitor and two elicitins from Phytophthora spp. activate SIPK in tobacco suspension cells (21). In this report, we demonstrate that both SA and a fungal cell wall-derived elicitor are able to activate SIPK in tobacco plants, although they do so with distinct kinetics. Of more importance, it was found that the wounding-activated kinase previously thought to be encoded by WIPK (12) actually is encoded by SIPK. These results suggest that SIPK is involved in both disease resistance and response to wounding.
MATERIALS AND METHODS
Treatment of Tobacco.
Tobacco plants (Nicotiana tabacum cv. “Xanthi nc”) were grown at 22°C in a growth room programmed for a 14-hr light cycle. Seven- to eight-week-old plants were used for experiments. For water, SA (1 mM), or fungal elicitor (100 μg glucose equivalents per milliliter) treatment, one leaf from each plant was injected with solution by using a syringe until the entire leaf was infiltrated. Wounding experiments were performed according to Usami et al. (13) and Seo et al. (12) for either cutting or rubbing with carborundum. The fungal cell wall elicitor was prepared from a heat-released cell wall fraction of the fungal pathogen Phytophthora parasitica and was quantitated as described (22).
Preparation of Protein Extracts.
Leaf discs (four discs, each ≈1 cm in diameter) were first ground to a fine powder in 1.5-ml microcentrifuge tubes by using small plastic pestles. After adding 0.25 ml of extraction buffer (100 mM Hepes, pH 7.5/5 mM EDTA/5 mM EGTA/10 mM DTT/10 mM Na3VO4/10 mM NaF/50 mM β-glycerophosphate/1 mM phenylmethylsulfonyl fluoride/5 mg/ml antipain/5 mg/ml aprotinin/5 mg/ml leupeptin/10% glycerol) (20), the mixture was sonicated for 15 sec with a W-375 Sonicator (Heat System/Ultrasonics) fitted with a microprobe at setting 4 and 80% duty cycle. After centrifugation at 13,000 rpm for 30 min in a microfuge, the supernatant was frozen in liquid nitrogen and stored at −80°C.
In-Gel Kinase Activity Assay.
The in-gel kinase activity assay was performed as described (20).
Antibody Production.
The peptide p44N (MADANMGAGGGQFPDFPS), which corresponds to the N terminus of the wounding-induced protein kinase (WIPK), was synthesized and conjugated to keyhole limpet hemacyanin carrier. Polyclonal antisera were raised in rabbits and were purified by affinity column chromatography (Zymed).
Immunoprecipitation, Immunoblot Analysis, and Immune-Complex Kinase Assay.
These procedures were performed as described (21).
RNA Blot Analysis.
RNA isolation and analysis were performed as described (21).
RESULTS
Infiltration of Water, SA, or a Fungal Cell Wall Elicitor Activates a 48-kDa Kinase in Tobacco Leaves, Each with Distinct Kinetics.
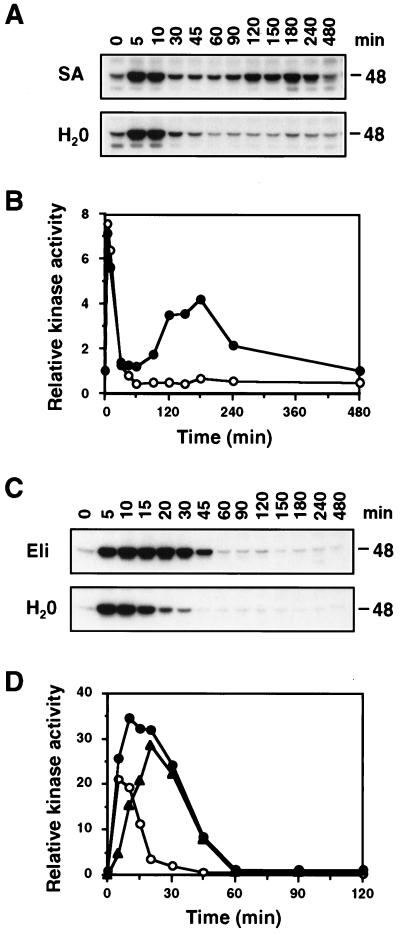
It was demonstrated (20, 21) in tobacco suspension cells that both SA and fungal elicitors activate the 48-kDa SIPK. To test whether these agents can activate SIPK in tobacco plants, leaves were infiltrated with either SA or a fungal cell wall elicitor, and samples were taken at the indicated times. After extracting total protein, the kinase activity in these samples was examined by an in-gel kinase activity assay with MBP as the substrate. Infiltration of SA was observed to activate a 48-kDa kinase with biphasic kinetics (Fig. 1 A and B). Of interest, the first peak, occurring ≈5 min after infiltration, also was present in samples from the water-infiltrated control, suggesting that it might be caused by either the wounding and/or osmotic stresses associated with infiltration (Fig. 1 A and B). The second peak, which was detected ≈180 min after treatment, was specific to SA treatment (Fig. 1 A and B). Infiltration with the fungal cell wall elicitor also activated a 48-kDa kinase. This activation was of a higher magnitude and occurred over a longer time period than that seen with the water control (Fig. 1 C and D). The difference between the relative kinase activities detected in fungal elicitor-infiltrated and water-infiltrated leaves may represent the level of kinase activity specifically caused by fungal elicitor and is depicted as the triangle-marked curve (Fig. 1D).
Figure 1.
Infiltration of SA, fungal cell wall elicitor, and water activate a 48-kDa kinase in tobacco leaves. (A) Tobacco leaves were infiltrated with either SA (1 mM) or water, and samples were taken at the indicated times. Kinase activity was determined with an in-gel kinase activity assay by using MBP as the substrate. The size of the activated kinase is given in kilodaltons (kDa). (B) The 48-kDa kinase activities detected in SA-infiltrated (•) and water-infiltrated (○) leaves were quantitated by using a PhosphorImager (Molecular Dynamics), and the relative activities were plotted against time. Kinase activities were normalized to the level present at the zero time point, which was given a value of 1. (C) Tobacco leaves were infiltrated with either a cell wall elicitor (Eli) from P. parasitica (100 μg of glucose equivalent per milliliter) or water, and samples were taken at the indicated times and analyzed as in A. (D) The 48-kDa kinase activities in fungal cell wall elicitor-infiltrated (•) and water-infiltrated (○) leaves were quantitated by using a PhosphorImager, and the relative activities were plotted against time as in B. The difference between elicitor- and water-infiltrated leaves, which presumably represents the elicitor effect, is depicted as a line with triangular symbol (▴).
The 48-kDa Kinase Activated in Tobacco Leaves by SA and the Fungal Elicitor Is SIPK.
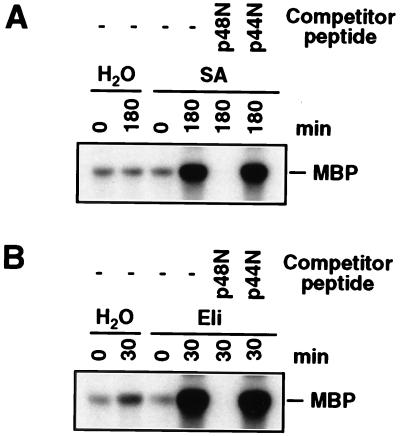
To test whether the SA and fungal elicitor-activated 48-kDa kinase in tobacco leaves are encoded by SIPK, as in suspension cells, protein extracts from leaves treated with SA or the fungal elicitor for 180 min or 30 min, respectively, were subjected to an immune-complex kinase assay (21). At these times, the activity of the 48-kDa kinase was high whereas its activity in the water control had returned to basal level (Fig. 1). Protein extracts first were reacted with the SIPK-specific antibody Ab-p48N. The resultant antigen–antibody complexes were precipitated with protein A-agarose beads and washed before addition to a kinase assay mixture with [γ-32P]-ATP and MBP as substrates. The reaction mixture, including the phosphorylated MBP, then was fractionated by SDS/PAGE and was subjected to autoradiography. The 48-kDa kinase activated by SA and the fungal elicitor both were immunoprecipitated by the SIPK-specific antibody (Fig. 2), indicating that they are, indeed, SIPK. Further evidence that these 48-kDa kinases are SIPK came from the demonstration that the competitor peptide p48N, which corresponds to the unique N terminus of SIPK and was used to generate this antibody, was able to compete with this kinase for binding whereas the competitor peptide p44N, which corresponds to the unique N terminus of the WIPK, could not.
Figure 2.
The 48-kDa kinase activated by SA and fungal cell wall elicitor corresponds to SIPK. (A) Protein extracts (50 μg) from water-infiltrated (0 and 180 min) and SA-infiltrated (0 and 180 min) leaves were immunoprecipitated with the SIPK-specific antibody Ab-p48N in the absence or presence of a competitor peptide (either p48N or p44N). Kinase activity of the immune-complex subsequently was determined with an in-solution kinase assay by using MBP as the substrate, and the phosphorylated MBP was visualized by autoradiography after SDS/PAGE. (B) Protein extracts (50 μg) from water-infiltrated (0 and 30 min) and fungal cell wall elicitor (Eli)-infiltrated (0 and 30 min) leaves were immunoprecipitated with antibody Ab-p48N in the absence or presence of a competitor peptide. Kinase activity of the immune-complex subsequently was determined as described in A.
Wounding Activates a 48-kDa Kinase That Is Recognized by the SIPK-Specific Antibody Ab-p48N.
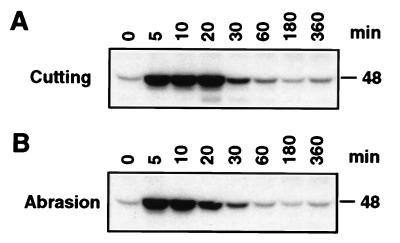
Our previous experiments indicated that water infiltration activates a 48-kDa kinase, possibly via a wounding pathway. Thus, we tested whether two other methods of wounding that have been shown to activate a kinase of similar size (12, 13) would activate this kinase. Both cutting and wounding by rubbing with carborundum were observed to activate rapidly and transiently a 48-kDa kinase (Fig. 3). Activation occurred within 5 min, and activity returned to basal level in ≈1 hr.
Figure 3.
Wounding by cutting or abrasion also activates a 48-kDa kinase. (A) Leaf discs were punched out of fully expanded tobacco leaves and floated on Hepes buffer as described by Usami et al. (13). At the indicated times, leaf discs were harvested, and protein extracts were prepared. Kinase activity was determined by an in-gel kinase activity assay. (B) Fully expanded tobacco leaves were wounded by rubbing with carborundum as described by Seo et al. (12) and analyzed as in A.
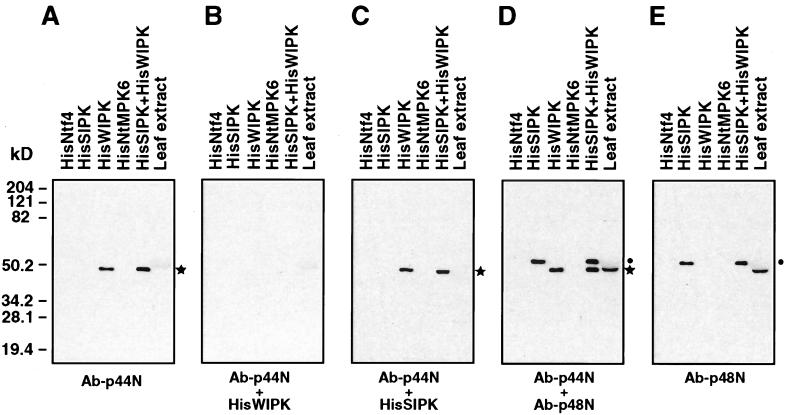
It was proposed that wounding-activated kinase activity is encoded by WIPK, a MAP kinase gene whose transcript is induced by wounding (12), but a rigorous demonstration of this has been lacking. To determine which gene encodes the wounding-activated kinase, we used antibodies directed against peptides corresponding to the unique N termini of SIPK and WIPK. Antibody against p44N was prepared as described for SIPK-specific antibody Ab-p48N (21). The specificity of the p44N antibody (Ab-p44N) was assessed by immunoblot analyses against different His-tagged recombinant tobacco MAP kinases. Ab-p44N only reacted with HisWIPK, not with HisSIPK, HisNtf4, or HisNtMPK6 (Fig. 4A). Furthermore, Ab-p44N preadsorbed with HisWIPK failed to detect the HisWIPK whereas preadsorption with HisSIPK had no effect (Fig. 4 B and C). At the same dilution (0.5 μg/ml), both Ab-p44N and Ab-p48N recognized their corresponding recombinant protein equally well (Fig. 4D), suggesting they had similar affinities. The failure of Ab-p44N to detect a band in the total protein extract [whereas Ab-p48N could under the same condition (Fig. 4 A, D, and E)] indicates that WIPK is much less abundant than SIPK in tobacco leaves.
Figure 4.
Specificity of antibody Ab-p44N raised against a peptide (p44N) corresponding to the N terminus of WIPK. One nanogram each of HisSIPK, HisNtf4, HisWIPK, and HisNtMPK6, equal mixture of HisSIPK and HisWIPK, or 20 μg of protein extracts from fully expanded tobacco leaf were separated in 10% SDS/polyacrylamide gel. Duplicated blots were subjected to immunoblot analysis by using the antibodies listed below: (A) Ab-p44N (0.5 μg/ml); (B) Ab-p44N preadsorbed with HisWIPK; (C) Ab-p44N preadsorbed with HisSIPK; (D) Ab-p44N (0.5 μg/ml) plus Ab-p48N (0.5 μg/ml); (E) Ab-p48N (0.5 μg/ml). The bands corresponding to HisWIPK and HisSIPK are indicated with asterisks (∗) and dots (⋅), respectively. Recombinant proteins migrate ≈3.6-kDa slower than their endogenous counterparts because of the His-tag; thus, HisWIPK and SIPK migrate similarly as seen in D.
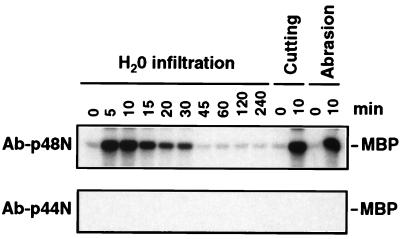
After the specificity and sensitivity of Ab-p44N were established, the immune-complex kinase assay was used to identify the wounding-activated kinase. Protein extracts from water-infiltrated, cut, or abraded leaves were immunoprecipitated with either Ab-p48N or Ab-p44N, and the kinase activity of the immune-complex was assayed in solution by using MBP as the substrate. For all three treatments, the 48-kDa kinase was immunoprecipitated by Ab-p48N, but not Ab-p44N (Fig. 5). Based on these results, the water infiltration- and wounding-activated kinase is encoded by SIPK rather than WIPK.
Figure 5.
The 48-kDa kinase activated by water infiltration and wounding is encoded by SIPK rather than WIPK. Protein extracts (50 μg) from water-infiltrated, cutting- or abrasion-wounded leaves were immunoprecipitated with either the SIPK-specific antibody Ab-p48N (Upper) or the WIPK-specific antibody Ab-p44N (Lower). Kinase activity of the resultant immune-complexes subsequently was determined as described in the text.
Water Infiltration and Wounding Activate SIPK Post-Translationally via a Phosphorylation Event.
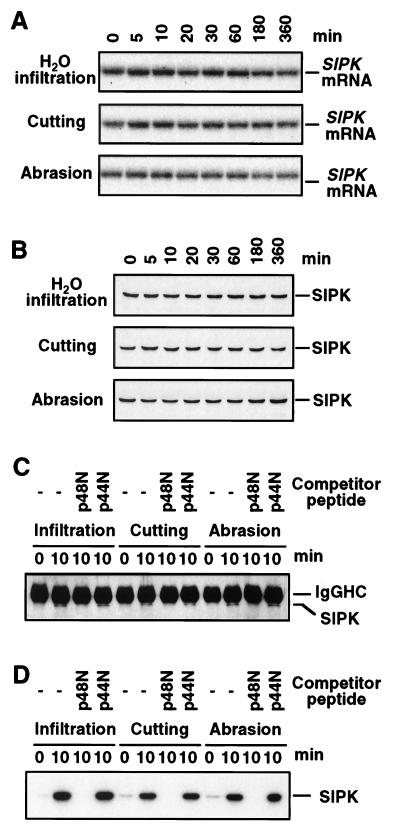
Activation of SIPK by water infiltration or either type of wounding was not associated with increases in mRNA or protein levels (Fig. 6 A and B), suggesting that SIPK is activated post-translationally. To assess phosphorylation state and activity level, total proteins were immunoprecipitated with the SIPK-specific antibody Ab-p48N, and the resultant immune-complexes were either subjected to immunoblot analysis with the phosphotyrosine-specific antibody 4G10 or monitored for kinase activity. Coordinated increases in tyrosine phosphorylation and kinase activity were detected after water infiltration and wounding treatments (Fig. 6 C and D). Therefore, SIPK activation by these stresses appears to be regulated post-translationally by phosphorylation. The ability of peptide p48N but not peptide p44N to block immunoprecipitation by SIPK-specific antibody Ab-p48N further argues that wounding-activated MBP kinase is encoded by SIPK instead of WIPK (Fig. 6 C and D).
Figure 6.
Activation of SIPK by water infiltration or wounding is regulated at the post-translational level by phosphorylation. (A) Total RNA was extracted from water-infiltrated or wounded leaves and was subjected to RNA blot analysis. Blots were hybridized sequentially with the 5′ untranslated region and then the full-length SIPK cDNA. Because both probes yielded the same result, only the autoradiogram produced with the full length cDNA probe is shown. (B) Protein extracts (20 μg) were subjected to immunoblot analysis by using the SIPK-specific antibody Ab-p48N. (C) Protein extracts (50 μg) from water-infiltrated or wounded leaves (0 and 10 min post-treatment) were immunoprecipitated with Ab-p48N in the absence or the presence of the competitor peptides p48N or p44N. Phosphotyrosine residues in the immune-complex were detected by reaction with the phosphotyrosine-specific monoclonal antibody 4G10. The upper band corresponds to the heavy chain of rabbit IgG (IgGHC). (D) The kinase activity in the immune-complex from (C) was determined by an in-gel kinase assay by using MBP as the substrate.
Wounding and Water Infiltration Lead to a Transient Increase in WIPK mRNA Levels but Little or No Accumulation of WIPK Protein.
Seo and coworkers (12) reported that WIPK transcripts accumulate after wounding and thus argued that WIPK encodes the wounding-activated kinase. However, studies detailed in this report indicate that the tobacco wounding-activated kinase is encoded by SIPK rather than WIPK. What, then, is the function of WIPK in the wounding response? To address this question, we first tested whether increases in WIPK mRNA levels lead to an accumulation of WIPK protein. Water infiltration and both methods of wounding led to a transient elevation of WIPK mRNA levels, which was first detected ≈20–30 min after wounding (Fig. 7A). This increase occurred significantly later than the wounding activation of the 48-kDa kinase, i.e., SIPK. Immunoblot analysis with the WIPK-specific antibody Ab-p44N demonstrated that there was little or no increase in the level of WIPK protein (Fig. 7B). Of more importance, we were unable to detect the WIPK activity in protein extracts from water-infiltrated or wounded leaves (Fig. 5). Therefore, we suspect that WIPK is not involved in the wounding response although its mRNA is induced transiently by wounding.
Figure 7.
Water infiltration and wounding induce transient increases in WIPK mRNA levels but little or no increases in WIPK protein level. (A) Total RNA was extracted at the indicated times from water-infiltrated or wounded leaves and was subjected to RNA blot analysis. Blots were hybridized sequentially with the 3′ untranslated region and then the full length WIPK cDNA. Both probes yielded the same result; thus, only the autoradiogram produced with the full-length cDNA probe is shown. (B) Protein extracts (20 μg) were subjected to immunoblot analysis with the WIPK-specific antibody Ab-p44N.
DISCUSSION
SIPK was first identified as an SA-activated MAP kinase. However, we have observed since that it is activated in tobacco suspension cells and/or plants after treatment with various agents that induce disease resistance responses, including SA, a fungal cell wall elicitor, elicitins, and TMV (20, 21, 23). Here, we demonstrated that SIPK also is activated by wounding. Thus, the same MAP kinase appears to be involved in two distinct pathways, one leading to disease resistance responses and the other leading to activation of wounding responses. In mammals, there is considerable precedence for induction of different cellular responses through the same MAP kinase. For instance, activation of the stress-activated protein kinase/Jun N-terminal kinase, a mammalian MAP kinase, by a variety of stress stimuli is rapid and transient and leads to responses that allow cells to adapt to their environment (9). In contrast, prolonged activation of this kinase initiates apoptosis (10, 24).
SIPK was activated rapidly in tobacco suspension cells treated with SA. Why, then, is the SA-mediated activation of SIPK delayed in tobacco leaves (Fig. 1)? One possible explanation is that infiltrating an SA solution into leaves actually sends two signals, one caused by wounding/osmotic stress and one caused by SA. If these two stimuli initially activate signaling pathways that share common components found in limited quantities, the more rapidly activated wounding response pathway might be able to sequester this component and thereby initially suppress the SA-mediated activation of SIPK. Alternatively, the rate of SA uptake by suspension cells and leaf cells might be different. In contrast, activation of SIPK by the fungal cell wall elicitor was concurrent with its activation by wounding (Fig. 1). A 49-kDa kinase also has been shown to exhibit similar activation kinetics when tobacco leaves are infiltrated with harpin, a bacterial elicitor derived from Erwinia amylovora (15). The identity of this 49-kDa kinase is unknown; however, we suspect that it is encoded by SIPK as well.
Multiple MAP kinase genes have been cloned from several plant species, indicating that MAP kinase are encoded by a multigene family in plants, as in yeast and animals (25, 26). Analysis of a phylogenetic tree based on sequence homology among all of the cloned plant MAP kinases indicates that there are several distinct groups (20). The distribution of different MAP kinase family members from a given species among the different groups suggests that the groups have different functions and the kinase within a group have a similar function(s). Currently, three orthologs of tobacco WIPK have been identified in other plant species that form a distinct group. These orthologs include Arabidopsis AtMPK3, alfalfa MMK4, and parsley ERMK (18, 19, 27). Of interest, the mRNA levels of these four genes are induced transiently by various stresses although increases of protein levels have yet to be demonstrated (12, 17–19, 27).
Of the WIPK orthologs, alfalfa MMK4 has been suggested to encode a wounding-activated MAP kinase (17). Support for this conclusion came from the demonstration that this activated kinase was recognized by the M7 antibody, which was raised against a 10-aa peptide corresponding to the C terminus of alfalfa MMK4 (17, 18). The specificity of this M7 antibody was established by testing against recombinant MMK2, MMK3, and MMK4 by using immunoblot analysis (17, 18). However, it is unclear whether this antibody also recognizes alfalfa MMK1. MMK1 is a member of another distinct group of plant MAP kinase represented by SIPK from tobacco (20, 28, 29). MMK1 shares 8 of 10 C-terminal amino acids with MMK4. Another WIPK ortholog, the parsley ERMK gene, has been proposed to encode a 45-kDa MAP kinase that is activated rapidly by Pep25 (19). This conclusion was based on the ability of the M7 antibody to immunodetect both the parsley Pep25 elicitor-activated kinase and recombinant ERMK protein (19). However, because ERMK shares 7 of 10 C-terminal amino acids with alfalfa MMK4, and MMK1 shares 8 of 10 C-terminal amino acids, it seems likely that this antibody reacts with members of both the WIPK and SIPK groups. Thus, further analysis is needed to establish firmly the relationship between these genes and the activated kinases.
In this report, we have used specific antibodies raised against the unique N termini of SIPK and WIPK to demonstrate that the wounding-activated MAP kinase in tobacco is encoded by SIPK instead of WIPK. If other members of the SIPK group similarly are activated by wounding, it seems likely that the alfalfa wounding-activated MAP kinase is encoded by MMK1 instead of MMK4. Possibly, the confusion as to which gene encodes this wounding-activated kinase stems from an ability of the M7 antibody to crossreact with both MMK1 and MMK4. Based on a serial dilution of recombinant His-tagged WIPK, the basal level of WIPK in tobacco plants was estimated to be only ≈0.0005% of the total protein, which is substantially lower than that of SIPK (≈0.004%; ref. 20 and data not shown). If the relative abundance of MMK1 and MMK4 in alfalfa cells is similar to that of SIPK and WIPK in tobacco, then most of the protein precipitated by the M7 antibody likely would be MMK1 instead of MMK4. We also have demonstrated that SIPK is activated in tobacco by treatment with a cell wall elicitor or either of two elicitins from Phytophthora spp. (21). The possibility that other SIPK orthologs are regulated analogously further underscores the question as to whether the parsley Pep25-activated kinase is encoded by an ortholog of WIPK or SIPK.
The function of SIPK in wounded tobacco or in plants treated with pathogens or pathogen-derived elicitors is currently unknown. In yeast and animal systems, activated MAP kinases have been shown to be translocated into the nucleus, where they phosphorylate transcription factors, thereby activating gene expression (11, 30). Therefore, it is possible that wounding and infection activate defense genes through an analogous mechanism. Furthermore, mammalian MAP kinases also are known to phosphorylate and thus activate cytosolic proteins, such as cytoplasmic phospholipase A2 (cPLA2), during injury and pathogen infection (31). As part of the inflammatory response, cPLA2 cleaves phospholipids in the membrane to release arachidonic acid, which is the precursor for prostaglandin and leucotriene biosynthesis. In plants, wounding and fungal elicitors activate an analogous pathway leading to the release of linolenic acid from membranes and the subsequent conversion of linolenic acid to jasmonic acid via the octadecanoid pathway. Both jasmonic acid and its methyl ester are important secondary signals involved in the induction of certain plant defense responses (6, 32). A cytosolic phospholipase A activity that is induced by elicitors has been identified in soybeans (33). The possibility that SIPK regulates the activation of such a phospholipase is intriguing. However, further analyses, including identification of SIPK substrates, will be required to elucidate the function of this MAP kinase in response to wounding and infection.
Acknowledgments
We thank Joe Chappell for providing the cell wall preparation from P. parasitica and D’Maris Dempsey for assistance preparing this manuscript. This work was supported by Grants MCB-9310371 and MCB-9723952 from the National Science Foundation.
ABBREVIATIONS
- MAP
mitogen-activated protein
- MBP
myelin basic protein
- SA
salicylic acid
- SIPK
salicylic acid-induced protein kinase, WIPK, wounding-induced protein kinase
References
- 1.Mehdy M. Plant Physiol. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yahraus T, Chandra S, Legendre L, Low P S. Plant Physiol. 1995;109:1259–1266. doi: 10.1104/pp.109.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hahlbrock K, Scheel D, Logemann E, Nürnberger T, Parniske M, Reinold S, Sacks W R, Schmelzer E. Proc Natl Acad Sci USA. 1995;92:4150–4157. doi: 10.1073/pnas.92.10.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamb C, Dixon R A. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 5.Dixon R A, Lamb C J. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:339–367. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 6.Bergey D R, Howe G, Ryan C A. Proc Natl Acad Sci USA. 1996;93:12053–12058. doi: 10.1073/pnas.93.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, Shah J, Klessig D F. Genes Dev. 1997;11:1621–1639. doi: 10.1101/gad.11.13.1621. [DOI] [PubMed] [Google Scholar]
- 8.Herskowitz I. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 9.Kyriakis J M, Avruch J. BioEssays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 10.Kyriakis J M, Avruch J. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 11.Seger R, Krebs E G. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 12.Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. Science. 1995;270:1988–1992. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- 13.Usami S, Banno H, Ito Y, Nishihama R, Machida Y. Proc Natl Acad Sci USA. 1995;92:8660–8664. doi: 10.1073/pnas.92.19.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki K, Shinshi H. Plant Cell. 1995;7:639–647. doi: 10.1105/tpc.7.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adám A L, Pike S, Hoyos M E, Stone J M, Walker J C, Novacky A. Plant Physiol. 1997;115:853–861. doi: 10.1104/pp.115.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stratmann J W, Ryan C A. Proc Natl Acad Sci USA. 1997;94:11085–11089. doi: 10.1073/pnas.94.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bögre L, Ligterink W, Meskiene I, Barker P J, Heberle-Bors E, Huskisson N S, Hirt H. Plant Cell. 1997;9:75–83. doi: 10.1105/tpc.9.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonak C, Kiegeri S, Ligterink W, Barker P J, Huskisson N S, Hirt H. Proc Natl Acad Sci USA. 1996;93:11274–11279. doi: 10.1073/pnas.93.20.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ligterink W, Kroj T, zur Nieden U, Hert H, Scheel D. Science. 1997;276:2054–2057. doi: 10.1126/science.276.5321.2054. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Klessig D F. Plant Cell. 1997;9:809–824. doi: 10.1105/tpc.9.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Du H, Klessig D F. Plant Cell. 1998;10:435–449. doi: 10.1105/tpc.10.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, Sheng J, Liu Y, Mehdy M C. Plant Cell. 1993;5:1089–1099. doi: 10.1105/tpc.5.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, S. & Klessig, D. F. (1998) Proc. Natl. Acad. Sci. USA, in press.
- 24.Chen Y-R, Meyer C F, Tan T-H. J Biol Chem. 1996;271:631–634. doi: 10.1074/jbc.271.2.631. [DOI] [PubMed] [Google Scholar]
- 25.Hirt H. Trends Plant Sci. 1997;2:11–15. [Google Scholar]
- 26.Mizoguchi T, Ichimura K, Shinozaki K. Trends Biotechnol. 1997;15:15–19. doi: 10.1016/S0167-7799(96)10074-3. [DOI] [PubMed] [Google Scholar]
- 27.Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumota K, Shinozaki K. Proc Natl Acad Sci USA. 1996;93:765–769. doi: 10.1073/pnas.93.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duerr B, Gawienowski M, Ropp T, Jacobs T. Plant Cell. 1993;5:87–96. doi: 10.1105/tpc.5.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonak C, Páy A, Bögre L, Hirt H, Heberle-Bors E. Plant J. 1993;3:611–617. doi: 10.1046/j.1365-313x.1993.03040611.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang X Z, Ron D. Science. 1996;272:1347–1349. doi: 10.1126/science.272.5266.1347. [DOI] [PubMed] [Google Scholar]
- 31.Lin L-L, Wartmann M, Lin A Y, Knopf J L, Seth A, Davis R J. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 32.Doares S H, Syrovets T, Weiler E W, Ryan C A. Proc Natl Acad Sci USA. 1995;92:4095–4098. doi: 10.1073/pnas.92.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandra S, Heinstein P F, Low P S. Plant Physiol. 1996;110:979–986. doi: 10.1104/pp.110.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]