Abstract
In order to use electrical stimulation or biological repair to attempt to alleviate spinal cord dysfunction, a key problem will be how to target the intervention. Since the majority of inputs to spinal motoneurones originate from intrinsic spinal premotor interneurones, these are key targets for interventions that may help restore function. Information on the organisation of these neurones could thus be crucially important to determine how to proceed. Understanding the organisation of spinal interneurones is no easy task. In this article I review evidence of the connectivity of some of the groups of spinal premotor interneurones that have been studied, focusing particularly on whether they form subgroups and how these can be identified.
Two competing strategies for restoring function after CNS damage are the manipulation of biological systems (by growing neurones or repairing damaged neurones) and intervention with engineering solutions to generate the missing neuronal activation patterns (e.g. with electrical stimulation). In the case of restoration of function following spinal cord injury both of these approaches may succeed by default, for example if axons induced to grow produce either a connectivity that is functionally useful or a pattern of connectivity that facilitates plastic processes to restore function, or, in the case of electrical stimulation, if an array of stimulation sites activates a range of elementary movements that can be assessed empirically and combined into functionally useful patterns. However, interventions of this type may have a better chance of success if they are guided by information on the organisation of the intrinsic spinal circuitry that provides the large majority of the inputs to spinal interneurones. A great deal of effort has been directed at understanding the organisation of spinal interneurones, but this has been difficult to study because of the need to identify connectivity in circuits of small neurones in close proximity to their target motoneurones. Progress depends on an ability to gather information about functional connectivity in the spinal cord, which limits the use of in vitro methods which have been so informative in understanding synaptic mechanisms. Much of the information available to date gives the appearance of a complex organisation, making it difficult to make generalisations. Further complication arises from the fact that alternative pathways may operate in different situations (see McCrea, 1992, 2001). Progress in understanding spinal circuits is likely to require a better understanding of functional roles of different groups of neurones. Here I review some findings on spinal premotor interneurones in the mammalian spinal cord, focusing on the grouping of neurones on the basis of their inputs. The general organisation of this connectivity may have some implications for the organisation of neuronal circuits more generally.
Investigations of the organisation of spinal interneurones, particularly last order interneurones, has relied on the identification of characteristic connections, for example recurrent collaterals of motoneurones onto Renshaw cells, and from primary muscle spindle afferents onto Ia inhibitory interneurones. Giving ‘nicknames’ to groups of interneurones on the basis of a characteristic input gives a simple indicator to which group they belong, but it is important to remember that what the neurones actually do depends on all of the inputs, as well as on the outputs. An infrequently emphasised feature common to all groups of premotor interneurones studied to date is that they receive a complex multisensory input from afferents of many different types and from different origins (Lundberg, 1979; Baldissera et al. 1981). Analysis of input to specific groups of neurones has usually been summarised in the form of schematic stick diagrams. Figure 1 illustrates in schematic form the convergence onto two well-studied groups of spinal premotor interneurones: neurones responsible for ‘non-reciprocal’ group I inhibition of extensors (see Harrison & Jankowska, 1985a Harrison & Jankowska, 1985b Jankowska, 1992) and midlumbar interneurones (see Jankowska, 1992; Davies & Edgley, 1994; Aggelopoulos et al. 1995). Similar diagrams for Renshaw cells and neurones that mediate group Ia reciprocal inhibition can be found in Baldissera et al. (1981). In all cases the convergence of afferent inputs to these neurones is complex and multisensory. Furthermore, the picture we have is incomplete since not all sources of afferents have been examined for all groups of neurones, and certainly not all possible origins of afferents have been examined. For example inputs from hip muscles are difficult to assess, but provide a powerful input to midlumbar interneurones (Aggelopoulos et al. 1996). For most neurones the generalisation that they receive inputs from ‘cutaneous afferents’ does not specify which types of afferents or from which skin region. Furthermore, although Fig. 1 provides an overall picture of the range of inputs seen by a group of interneurones, it does not indicate the relative frequency of these inputs. Thus a group of interneurones with a common ‘nickname’ which reflects one characteristic of their many inputs might disguise the existence of different subgroups of neurones based on inputs from separate afferent systems. An example is provided by the midlumbar interneurones of Fig. 1. The characteristic feature of these neurones is the potent input from group II muscle afferents (Edgley & Jankowska, 1987). More than 80 % of midlumbar neurones receive monosynaptic input from descending motor pathways (Davies & Edgley, 1994), which often evoke large EPSPs in them. However, the descending connections to these neurones are organised such that two subpopulations of interneurones can be identified. Interneurones receive monosynaptic inputs from either ventral (reticulospinal or vestibulospinal) pathways or dorsolateral (rubrospinal and corticiospinal) pathways, but very rarely from both (Davies & Edgley, 1994). Thus midlumbar neurones include at least two different subpopulations, one with input from ventromedial descending pathways, the other with input from dorsolateral descending pathways.
Figure 1. Schematic diagram of some of the inputs to non-reciprocal inhibitory interneurones and midlumbar interneurones.
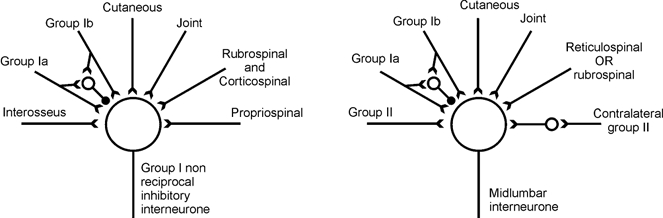
These diagrams summarise some of the inputs established for group I non-reciprocal inhibitory interneurones (based on Harrison & Jankowska, 1985a), Harrison & Jankowska, 1985b and midlumbar interneurones with group II input (based on Edgley & Jankowska (1987), Davies & Edgley (1994) and other studies (see Jankowska, 1992)). Excitation is represented by lines with a forked end, inhibition by the line ending with a filled circle. Although these diagrams are complex enough, they do not show all connections and do not represent the specific origins of the afferents (specific muscles for proprioceptors, specific skin areas for cutaneous afferents).
Observations such as these raise the question: how homogeneous are the groups of interneurones which have been described? Neurones considered to form a group under one criterion may look very different if viewed from the perspective of another, since any individual interneurone from the population will have only a proportion of the inputs present in the whole population. This question has been addressed in a study of fractionation of the input to interneurones that mediate group I ‘non-reciprocal’ inhibition (Harrison & Jankowska, 1985a).Harrison & Jankowska, 1985b These interneurones mediate inhibition in motoneurones under anaesthesia or during static posture, but this inhibition is not seen during locomotion (see McCrea, 2001). These neurones are identified by their location in laminae V-VI of the L6 and L7 segments of the spinal cord and by the characteristic ascending projection to Clarke's column (see Harrison & Jankowska, 1985a).Harrison & Jankowska, 1985b Interneurones of this group are monosynaptically excited and disynaptically inhibited by group I afferents of many different extensor muscles, but particularly the ankle extensors, as well as receiving a range of other inputs including those from joint and cutaneous afferents as well as descending systems (see Fig. 1 and Harrison & Jankowska, 1985a).Harrison & Jankowska, 1985b Both muscle spindle primary (Ia) afferent and tendon organ (Ib) afferent inputs converge onto individual interneurones (Jankowska & McCrea, 1983). Most individual interneurones receive input from only a fraction of these possible sources, raising the possibility that the group contains subpopulations, each with specific patterns of input. The possibility that subgroups exist was tested by comparing the distribution of input patterns observed in individual neurones with the expected frequency of those patterns given different model distributions of the inputs within the population. For example if two specific inputs, A and B, are each found in 50 % of the neurones in a population, these inputs could be associated with each other, in which case the distribution would have 50 % of neurones with both input A and B, the other 50 % having neither. Conversely, they could be segregated, in which case 50 % of neurones would have input A (but not B) and the other 50 % would have input B (but not A). Both of these possibilities involve subpopulations of neurones, the former with specific inputs associated, the latter with the inputs segregated. If the interneurones sampled were drawn from a single population without subgroups, then the distribution of inputs should be independent, i.e. the probability of an individual interneurone having any one input would be independent of its having another. In the example with inputs A and B, under this condition 25 % of the interneurones would have both input A and input B, 25 % would have input A alone, 25 % would have input B alone and 25 % would have neither input.
Harrison & Jankowska (1985b) examined the distribution of inputs to a population of 18 non-reciprocal group I inhibitory interneurones, in each of which a wide range of the potential sources of input were tested. In the population of interneurones they sampled from, the observed input patterns were a very close match to the patterns expected if the inputs were distributed randomly among neurones rather than in specific combinations (see Harrison & Jankowska, 1985b, Fig. 2). The absence of subgroups of interneurones with specific input patterns suggests that the interneurones were sampled from a common functional population.
Figure 2. Distributions of inputs to midlumbar interneurones.
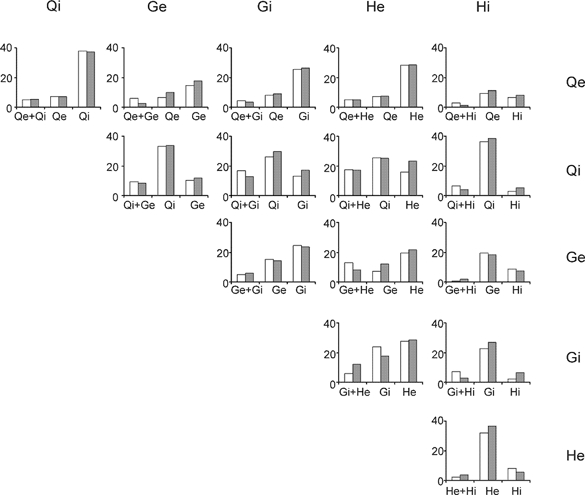
The figure compares the observed patterns of input from group I afferents of different nerves to midlumbar interneurones (open bars) with the patterns expected based on the frequency of occurrence of each individual input if the inputs were distributed randomly among the interneurones (grey bars). Each bar chart compares 2 sources of input and the 3 sets of columns shown represent the proportion of the population (percentage of neurones) with both inputs, the first alone and the second alone. The combinations of inputs compared in each bar chart are shown by the labels at the top and side of the figure. The figure takes the same format as Fig. 2 of Harrison & Jankowska (1985b) and reveals the same pattern: for all possible combinations of inputs the frequency of occurrence of each possible convergence pattern matches closely the pattern expected if the inputs were distributed independently. Abbreviations: Qe, quadriceps group I EPSPs; Qi, quadriceps group I IPSPs; Ge, gastrocnemius-soleus group I EPSPs; Gi, gastrocnemius-soleus group I IPSPs; He, hamstring group I EPSPs; Hi, hamstring group I IPSPs.
This analysis can also be applied to the inputs to midlumbar interneurones with group II inputs, in which group I afferents also evoke monosynaptic EPSPs and disynaptic IPSPs, and other sources of afferents also provide inputs (Edgley & Jankowska, 1987). The group I inputs to these neurones are found with very different frequencies than in the non-reciprocal group I inhibitory interneurones examined by Harrison & Jankowska (1985b). In the latter, inputs from ankle and digit extensors are much more frequent than from knee and hip extensors, whereas in midlumbar interneurones inputs from hip and knee extensors are the most common (Edgley & Jankowska, 1987). An analysis of the pattern of inputs from a large population of midlumbar interneurones sampled from several different studies (Bajwa et al. 1992; Davies & Edgley, 1994; Aggelopuoulos et al. 1996) is presented in Fig. 2. The figure follows the format of Fig. 2 of Harrison & Jankowska (1985a). In each histogram the open columns show the proportion of neurones with each input pattern found in a population of 183 sampled interneurones. The shaded columns show the proportions expected to have each pattern of inputs if they were distributed independently. The key point is that, as was found for non-reciprocal inhibitory interneurones, the observed convergence patterns are a close match to those expected if the inputs were distributed independently. Thus these data are consistent with the midlumbar neurones sampled forming a single population, rather than different subgroups. Similar analyses of the distribution of inputs from descending systems to the same group of interneurones does reveal a subgrouping from ventral (reticulospinal or vestibulospinal) and dorsolateral (rubrospinal and corticiospinal) pathways (see Davies & Edgley, 1994).
One way to envisage this organisation of inputs is from the perspective of a developing axon. During development growing axons from group I afferents (and from inhibitory interneurones activated by group I afferents) make connections with a given proportion of the population of neurones, but do so regardless of their inputs from group I afferents of other muscles. Thus the growing afferents treat the neurones as a functional population and make connections with a given proportion of them. On the other hand, growing rubrospinal and corticospinal axons also seek to make connections with a given proportion of midlumbar interneurones, but are very specific as to which interneurones they contact, only making connections with interneurones that do not receive inputs from ventral pathways. The peripheral input to the interneurones with rubrospinal and corticospinal inputs is very similar to the peripheral input to interneurones with reticulospinal and vestibulospinal inputs, suggesting that the subgroups of midlumbar interneurones with different descending inputs are both activated (by peripheral afferents) in the same functional context. This may have a simple origin, in that the population of midlumbar interneurones includes both inhibitory and excitatory premotor neurones (Cavallari et al. 1987); rubrospinal and corticospinal axons may connect with one group, reticulo and vestibulospinal fibres with the other.
The analysis of the distribution of inputs to both non-reciprocal inhibitory interneurones and midlumbar interneurones lends weight to the argument that these represent functional groups of neurones, on the basis of their input connections. Key data which are currently missing for these and other neurones relate to the circumstances in which these interneurones are active during movement. Assumptions about the information carried by spinal neurones based on patterns of inputs assessed electrophysiologically may be misleading. For example, on the basis of their inputs the neurones in Clarke's column were considered to carry information relating to the state of a limited number of muscles, whereas analysis of their activity during passive limb movement implies a more global role, namely to signal whole-limb position and movement (see Osborn & Poppele, 1993; Bosco et al. 2000).
From the point of view of function, the most important information needed is on the output connectivity of the neurones. This is difficult information to obtain directly, although indirectly the outputs can be inferred from the reflex actions of the afferents (for midlumbar interneurones, see Cavallari et al. 1987). This indirect evidence indicates that most interneurones target multiple motoneurone pools, a conclusion that is well supported by analysis of the collateral branching patterns of individual labelled interneurones or their axons, which show terminals in different motor nuclei (see e.g. Bras et al. 1989 for midlumbar interneurones). This suggests that the output of the interneurones should generate synergistic multijoint movement within the limb, rather than single muscle-related actions.
A different approach to identifying the output of spinal circuits has been to activate spinal systems electrically and examine the resultant multijoint movement. In the frog spinal cord, this approach has revealed that intraspinal microstimulation activates many muscles leading to multijoint movement (see e.g. Bizzi et al. 1995). The pattern of forces evoked is consistent for given regions of the spinal cord, leading to the view that the spinal circuitry is modular, the output of each module driving the limb into a particular posture. This approach has been used to argue for the concept that movement is guided using an equilibrium point method (see Bizzi et al. 1992), but it remains controversial (see Gomi & Kawato, 1996; Aoyagi et al. 2000). Regardless of the significance of these observations for equilibrium point control, these studies have highlighted the problem of the paucity of information on the output of spinal interneurone populations and yet have provided data suggestive of a modular organisation of spinal circuits. A similar modular organisation originally shown in frogs has recently been shown to exist in the rat spinal cord (Tresch & Bizzi, 1999). However, the use of electrical microstimulation of the spinal cord grey matter seems crude. Firstly, electrical stimuli will activate many structures, including axons and terminals, which have lower thresholds than neuronal cell bodies (see e.g. Gustafsson & Jankowska, 1976). Axon reflex activation in local fibres (as well as descending or peripheral afferent fibres) can contribute to the activation of muscles. Secondly, there are mutual interconnections between spinal interneurone populations which have been studied (see Jankowska, 1992), so stimuli that activate the terminals of any one group of interneurones may have an influence on other groups of interneurones that is unlikely to be physiological. The extent of these mutual interconnections is hard to assess, but they can be seen for example as monosynaptic EPSPs and IPSPs in premotor interneurones evoked in response to stimuli delivered in the motor nuclei (see Edgley & Jankowska, 1987). Nevertheless a similar pattern of muscle activation was produced with microinjection of NMDA in the frog spinal cord (Saltiel et al. 1998), which would have been evoked through the activation of local neurones, rather than axons and terminals. Key questions that need to be addressed are thus, whether the existence of these output modules can be verified and whether they represent specific groups of premotor interneurones.
From the perspectives of both the input to interneurones and the output from interneurones to motoneurones there are indications of a modular organisation of the spinal cord. For the potential interventions aimed at restoring spinal cord function, a modular organisation has distinct advantages: a consistent distribution of functional circuits between different individuals should be identifiable and would provide targets for interventions to restore function. Understanding the organisation of these circuits, especially their output, needs to be a priority for spinal research.
References
- Aggelopoulos NC, Bawa P, Edgley SA. Inputs to midlumbar interneurones from anterior hindlimb muscles in the cat. Journal of Physiology. 1996;497:795–802. doi: 10.1113/jphysiol.1996.sp021810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi Y, Mushahwar VK, Stein RB, Prochazka A. Are movement primitives determined by spinal cord circuitry or biomechanics. Society for Neuroscience Abstracts. 2000;26:260. [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal poathways. In: Brooks VB, editor. Handbook of Physiology,The Nervous System, Motor Systems. II. Washington, DC: American Physiological Society; 1981. pp. 509–595. section 1. [Google Scholar]
- Bajwa S, Edgley SA, Harrison PJ. Crossed actions on group II activated midlumbar propriospinal neurones in the cat. Journal of Physiology. 1992;455:205–217. doi: 10.1113/jphysiol.1992.sp019297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi E, Hogan N, Mussa-Ivaldi FA, Giszter S. Does the nervous system use equilibrium point control to guide single and multiple joint movement. Behavioural and Brain Sciences. 1992;15:603–613. doi: 10.1017/S0140525X00072538. [DOI] [PubMed] [Google Scholar]
- Bosco G, Poppele RE, Eian J. Reference frames for spinal proprioception: limb endpoint based or joint-level based. Journal of Neurophysiology. 2000;83:2931–2945. doi: 10.1152/jn.2000.83.5.2931. [DOI] [PubMed] [Google Scholar]
- Cavalliari P, Edgley SA, Jankowska E. Postsynaptic actions of mid-lumbar interneurones of hindlimb muscles in the cat. Journal of Physiology. 1987;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Projections to midlumbar neurones from descending motor pathways in the cat. Journal of Physiology. 1994;479:463–474. doi: 10.1113/jphysiol.1994.sp020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the mid-lumbar segments of the cat spinal cord. Journal of Physiology. 1987;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi H, Kawato M. Equilibrium-point control hypothesis examined by measured arm stiffness during multijoint movement. Science. 1996;272:117–120. doi: 10.1126/science.272.5258.117. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. Journal of Physiology. 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Organization of input to the interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. Journal of Physiology. 1985a;361:403–418. doi: 10.1113/jphysiol.1985.sp015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. Journal of Physiology. 1985b;361:379–401. doi: 10.1113/jphysiol.1985.sp015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiology. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. Interactions between pathways controlling posture and gait at the level of the spinal cord. Progress in Brain Research. 1993;97:161–170. doi: 10.1016/s0079-6123(08)62274-8. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Mccrea DA. Shared reflex pathways from Ib tendon organ afferents and Ia muscle spindle afferents in the cat. Journal of Physiology. 1983;338:99–111. doi: 10.1113/jphysiol.1983.sp014663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A. Multisensory control of spinal reflex pathways. Progress in Brain Research. 1979;50:11–28. doi: 10.1016/S0079-6123(08)60803-1. [DOI] [PubMed] [Google Scholar]
- McCrea DM. Can sense be made of spinal interneuron circuits. Behavioural and Brain Sciences. 1992;15:633–643. [Google Scholar]
- Osborn CE, Poppele RE. Sensory integration by the dorsal spinocerebellar tract circuitry. Neuroscience. 1993;54:945–956. doi: 10.1016/0306-4522(93)90586-5. [DOI] [PubMed] [Google Scholar]
- Saltiel P, Tresch MC, Bizzi E. Spinal cord modular organization and rhythm generation: an NMDA iontophoretic study in the frog. Journal of Neurophysiology. 1998;80:2323–2339. doi: 10.1152/jn.1998.80.5.2323. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Bizzi E. Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Experimental Brain Research. 1999;129:401–416. doi: 10.1007/s002210050908. [DOI] [PubMed] [Google Scholar]


