Abstract
Disturbances of the in utero environment are associated with an increased risk of cardiovascular disease in adulthood. In this study we have determined whether abnormal vascular function in the adult offspring of rats fed a high saturated fat diet in pregnancy is associated with altered plasma lipids or vascular fatty acid content.
Female Sprague-Dawley rats were fed a breeding diet (4 % fat) or a diet high in saturated fat (20 % fat) for 10 days prior to and throughout pregnancy, and during weaning. Female offspring were then fed a maintenance diet (3 % fat) until 160 days of age.
Endothelium-dependent relaxation induced by acetylcholine was blunted in isolated branches of the femoral artery from 160-day-old female offspring of dams fed the saturated fat diet when compared with female offspring of dams fed the breeding diet. These offspring exhibited elevated plasma triglyceride and reduced plasma high density lipoprotein cholesterol concentrations.
The fatty acid composition of the aortas was abnormal, with a marked reduction in the content of arachidonic and docosahexaenoic acids.
This study demonstrates that a high fat diet in pregnant rats produces abnormal vascular function, plasma lipid disturbances and altered vascular fatty acid content in their female offspring during adulthood.
Increasing evidence suggests that disturbances of the in utero environment may ‘programme’ the fetus for development of cardiovascular disease in later life. Retrospective population studies have suggested that low birth weight is associated with an increased risk of coronary heart disease, stroke and non-insulin-dependent diabetes in adulthood (Barker, 1997). Experimentally, this association has been investigated by inducing fetal growth retardation in animals through poor nutrition. Prospective investigations of the ‘fetal programming’ hypothesis have reported hypertension in adulthood (Langley & Jackson, 1994) and disorders of glucose (Ozanne et al. 1996) and lipid metabolism (Lucas et al. 1996) in offspring of pregnant rats subjected to protein deprivation in utero. Our group has recently reported that a 30 % reduction of dietary intake in pregnant rats is associated with vascular dysfunction and hypertension in adult offspring, which were more pronounced in male animals (Ozaki et al. 2001). Fewer studies have investigated possible adverse influences on the fetus of diets more obviously associated with cardiovascular disease, but which are unrelated to growth retardation. In an earlier investigation we showed that 15- and 60-day-old offspring of rats fed saturated fat during pregnancy demonstrate vascular dysfunction and plasma lipid abnormalities (Koukkou et al. 1998), and we also reported that 1- and 15-day-old offspring exhibited profound irregularities in the fatty acid composition of the liver (Ghebremeskel et al. 1999).
In the present study we have investigated vascular function in 160-day-old offspring of rats with simultaneous evaluation of parameters of lipid status. We have determined the plasma lipid profile, and because altered fatty acid composition could undermine many aspects of vascular function, have examined the composition of membrane fatty acids in the vasculature.
Vascular function was assessed by measuring responses to constrictor agonists and to the endothelium-dependent vasodilator acetylcholine (ACh) in femoral arteries of offspring using a small-vessel wire myograph. Fatty acid composition was measured in aortas of offspring using thin-layer chromatography to separate the phosphoglycerides, and subsequent gas-liquid chromatography to determine individual fatty acid species.
METHODS
The animal procedures in the following protocol were carried out under Home Office (UK) licence No. PPL 90 765.
Dietary protocol
Female Sprague-Dawley rats (12–14 weeks old) were fed either a standard breeding diet (fat 4 %, protein 22 %, carbohydrate 51 %, fibre 5 %; principal fat constituents: palmitic acid 0.35 %, stearic acid 0.10 %, palmitoleic acid 0.18 %, oleic acid 1.04 %, linoleic acid 1.25 %, α-linolenic acid 0.19 %, arachidonic acid 0.24 %) or a diet substituted with animal lard (final composition fat 20 %, protein 18 %, carbohydrate 41 %, fibre 3 %; principal fat constituents: palmitic acid 4.50 %, stearic acid 1.99 %, palmitoleic acid 0.12 %, oleic acid 6.86 %, linoleic acid 2.58 %, α-linolenic acid 0.21 %, arachidonic acid 0.16 %) for 10 days prior to mating, throughout pregnancy and for 21 days postpartum. After weaning, the offspring were fed a standard chow diet (fat 3 %, protein 15 %, carbohydrate 62 %, fibre 5 %; principal fat constituents: palmitic acid 0.32 %, stearic acid 0.04 %, palmitoleic acid 0.10 %, oleic acid 0.76 %, linoleic acid 0.71 %, α-linolenic acid 0.06 %, arachidonic acid 0.13 %) until 160 days old, when offspring were killed by CO2 inhalation and cervical dislocation, and vascular function was assessed in isolated arteries from the femoral circulation. The aortas were also removed, dissected free of adventitia, snap frozen and stored at −70 °C for subsequent determination of fatty acid composition.
At delivery, litter size was standardised to five to six pups. Animals were weighed every 2 days until 20-21 days gestation, then weekly until the time of culling.
Evaluation of vascular function
To accord with our previous study (Koukkou et al. 1998) only female offspring were studied (one from each litter). Animals were killed by CO2 inhalation and cervical dislocation and third order branches of the femoral artery from the offspring were mounted as ring preparations on a small-vessel myograph (Mulvany & Halpern, 1977). Arteries failing to produce tension equivalent to a pressure of 100 mmHg in response to 5 μm noradrenaline (NA) in KPSS (125 mm KCl-substituted physiological salt solution, PSS) were rejected. Concentration-response curves were constructed to NA (10−7–10−5m) and to the thromboxane mimetic U46619 (10−10−3 × 10−6m). Arteries were then pre-constricted with NA to achieve approximately 80 % maximum response and relaxation induced by ACh (10−9–10−6m) was then assessed. A similar protocol was followed to evaluate relaxation induced by the nitric oxide (NO) donor spermine NONOate (10−9–10−5m).
Determination of plasma lipids
Following an overnight fast, blood samples were obtained by cardiac puncture and plasma was stored at −70 °C prior to analysis. Total plasma cholesterol and triglyceride concentrations were determined using assays based on the colorimetric evaluation of enzyme activity using commercially available kits ‘Unimate CHOL’ (cholesterol oxidase) and ‘Unimate TRIG’ (glycerol phosphate dehydrogenase) respectively (Roche Diagnostic Systems, Welwyn Garden City, UK) High density lipoprotein (HDL) was measured using a similar assay. Non-esterified fatty acids (NEFA) were quantified using a Wako NEFA C test kit (Eastleigh, Hants, UK) based on an acyl-coA synthetase-acyl-coA oxidase enzymatic colorimetric method.
Analysis of fatty acid in the aorta
Total lipid was extracted from each aorta by homogenising the samples in chloroform and methanol (2:1 v/v) containing 0.01 % butylated hydroxytoluene (BHT) as an antioxidant under N2, as previously described (Folch et al. 1957). Lipid classes were separated by thin-layer chromatography on 20 cm × 20 cm, 20 μm thick, silica gel plates (K6 Silica Gel 60A plates, Merck Ltd, Lutterworth, UK). Chloroform, methanol and water (60:30:4 v/v/v), containing 0.01 % BHT, were used as developing solvents for the separation of choline phosphoglyceride (CPG) and ethanolamine phosphoglyceride (EPG) reflecting the outer and inner components of the cell membrane, respectively. The phospholipid bands were detected by spraying with a methanolic solution of 2,7-dichloroflurescein (0.01 % w/v) and identified by the use of standards.
We have previously reported the method used to analyse fatty acids in CPG and EPG (Ghebremeskel et al. 1995). Fatty acid methyl esters (FAMEs) were prepared by heating the phosphoglycerides (CPG and EPG) with 5 ml of 15 % acetyl chloride (Sigma Aldrich) in methanol in a sealed vial at 70 °C for 3 h under N2. FAMEs were separated by a gas chromatograph (HRGC MEGA 2 Series, Fisons Instruments, Italy) fitted with a BP20 capillary column (25 mm × 0.32 mm i.d., 0.25 μm film; SGE Ltd, Milton Keynes, UK). Hydrogen was used as a carrier gas, and the injector, oven and detector temperatures were 230, 210 and 260 °C respectively. FAMEs were identified by comparison of retention times with authentic standards and interpretation of equivalent chain length values. Peak areas were quantified by a computer chromatography data system (EZChrom Chromatography Data System, Scientific Software, Inc., CA, USA).
Chemicals and diets
Chemicals used to assess vascular function were noradrenaline (Sanofi Winthrop Ltd, Guildford, UK), spermine NONOate (Alexis Corporation, Nottingham, UK), and acetylcholine and U46619 (Sigma Aldrich). All chemicals for PSS, which contained (mm): NaCl 119, NaHCO3 25, d-glucose 5.5, KH2PO4 1.18, MgSO4.7H2O 1.17, KCl 4.7, CaCl2 2.5, ethylenediaminetetraacetic acid disodium salt (EDTA) 0.026, were obtained from BDH, UK.
For fatty acid analyses all the solvents and chemicals were obtained from Sigma Aldrich. The high fat diet and normal breeding diet (no. 3 breeding diet) were purchased from Special Diet Services, Witham, UK.
Statistical analysis
All values are given as the mean ±s.e.m. To account for small differences in vessel diameter and resultant variability in tension development, noradrenaline-induced tension was expressed as percentage of the maximum contractile response to KPSS. Relaxation induced by acetylcholine and spermine NONOate was expressed as percentage of the noradrenaline-induced pre-contraction. The negative log of the concentration (mm) of a drug required to produce 50 % of the maximum response (pEC50) was calculated after fitting data to a sigmoidal curve (GraphPad Software Inc., San Diego, CA, USA). Two arteries from each animal were studied and the mean pEC50 used in analysis. One way analysis of variance and Student's independent t test were used for single comparisons between groups of arteries, and comparisons of total cholesterol and triglyceride levels (‘Instat’; GraphPad, San Diego, CA, USA). Student's t test was also used for comparison of individual fatty acids in the aortas of experimental and control groups. Significance was assumed if P < 0.05.
RESULTS
Weight and plasma analyses
Maternal weights on the day before delivery tended to be higher in the dams fed the high fat diet than those fed the normal breeding diet during pregnancy but the results were not significantly different (312.2 ± 6.0 g, n = 10 vs. controls 293.9 ± 4.1 g, n = 10). The birthweights of the female offspring studied were not significantly different between groups (680 ± 50 mg, n = 10 vs. controls 730 ± 40 mg, n = 10) and the weights of the 160-day-old female offspring were also similar between groups (303.5 ± 9.0 g, n = 10 vs. controls 291.2 ± 6.8 g, n = 10). The concentration of plasma triglycerides was significantly higher in the offspring of the fat-fed dams than in controls (P < 0.05) and the concentration of plasma high density lipoprotein cholesterol was lower (P < 0.05). The concentrations of plasma total cholesterol and non-esterified fatty acids were not significantly different between the two groups (Table 1).
Table 1. Plasma lipids in 160-day-old offspring.
| Control dams | High fat-fed dams | |
|---|---|---|
| Total cholesterol (mM) | 1.49 ± 0.05 | 1.44 ± 0.09 |
| High density lipoprotein cholesterol (mM) | 1.38 ± 0.08 | 1.01 ± 0.06* |
| Non-esterified fatty acids (mM) | 0.29 ± 0.04 | 0.33 ± 0.03 |
| Total triglycerides (mM) | 1.85 ± 0.85 | 2.46 ± 0.24* |
Values are given as means ± S.E.M.; n = 10 for both groups.
P < 0.05 vs. control.
Vascular function
Constrictor responses
There was no significant difference in the size of the arteries from the two groups of offspring; normalised diameter was 198 ± 18 μm (n = 10) for offspring of high fat-fed dams compared with 183 ± 17 μm (n = 10) for controls. Constriction induced by a depolarising solution of K+ (125 mm) in the branches of the femoral artery was not significantly different between arteries from offspring of dams fed the high fat diet and those from controls fed the breeding diet (1.88 ± 0.37 mN mm−1, n = 10 vs. controls 2.66 ± 0.41 mN mm−1, n = 10). There were no significant differences between the arteries from the offspring of high fat-fed dams and controls in constrictor responses to the addition of NA (pEC50, 5.96 ± 0.11 vs. controls 6.06 ± 0.10; maximal response, 108.22 ± 7.81 % of constrictor response to 125 mm KCl vs. controls 94.39 ± 5.76 %; n = 10 for all groups) (Fig. 1) or U46619 (pEC50, 6.57 ± 0.15 vs. controls 6.20 ± 0.05; maximal response, 105.57 ± 8.33 %vs. controls 102.74 ± 7.51 %; n = 10 for all groups).
Figure 1. Noradrenaline-induced tension development in branches of the femoral arteries of the 160-day-old offspring.
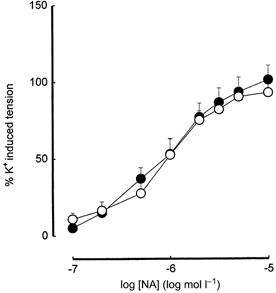
○, offspring of control dams on breeding diet; •, offspring of high fat-fed dams. When absent, error bars (s.e.m.) lie within symbols.
Endothelium-dependent relaxation
NA-induced preconstriction was similar between the arteries from offspring of high fat-fed dams and controls. Arteries from the 160-day-old offspring of 20 % fat-fed dams demonstrated decreased maximal relaxation induced by ACh (60.31 ± 2.56 % of NA-induced preconstriction, n = 10 vs. controls 70.53 ± 3.09 %, n = 10; P < 0.05). Sensitivity to ACh was not significantly different between the two groups (pEC50 6.81 ± 0.12, n = 10 vs. controls 7.15 ± 0.22, n = 10; Fig. 2).
Figure 2. Acetylcholine-induced relaxation in femoral arteries of 160-day-old offspring.
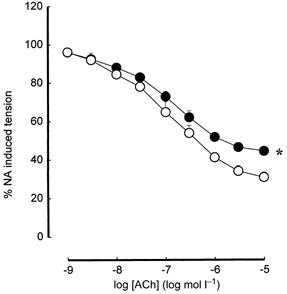
○, offspring of control dams on breeding diet; •, offspring of high fat-fed dams. * P < 0.05 maximum relaxation in offspring of high fat-fed dams vs. offspring of control dams on breeding diet. When absent, error bars (s.e.m.) lie within symbols.
Endothelium-independent relaxation
Relaxation induced by spermine NONOate was not significantly different between 160-day-old offspring of 20 % fat-fed dams and controls (pEC50, 5.77 ± 0.08, n = 10 vs. controls 5.76 ± 0.09, n = 10; maximum relaxation, 43.20 ± 3.09 % of NA-induced constriction, n = 10 vs. controls 37.07 ± 2.95 %, n = 10; Fig. 3).
Figure 3. Relaxation induced by the nitric oxide donor, spermine NONOate in femoral arteries of the 16-day-old offspring.
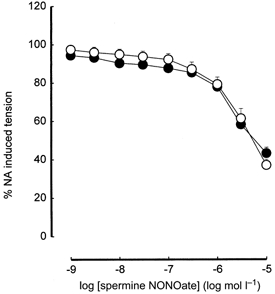
○, offspring of control dams on breeding diet; •, offspring of high fat-fed dams. When absent, error bars (s.e.m.) lie within symbols.
Aorta fatty acid composition
Saturated and monounsaturated fatty acids
The palmitic acid (16:0), palmitoleic (16:1) and oleic acid (18:0) contents of the CPG and EPG fractions, expressed as a percentage of the total fatty acids in each fraction, were higher in the aortas of offspring from high fat-fed dams compared with controls (P < 0.05, P < 0.001 and P < 0.001, respectively). In contrast, stearic acid (18:0) was lower (P < 0.001) in CPG and EPG fractions in the aortas of offspring from high fat-fed dams (Fig. 4).
Figure 4. Palmitic acid (16:0), stearic acid (18:0), palmitoleic acid (16:1) and oleic acid (18:1) in choline phosphoglycerides (CPG) and ethanolamine phosphoglycerides (EPG) in aortas of 160-day-old offspring.
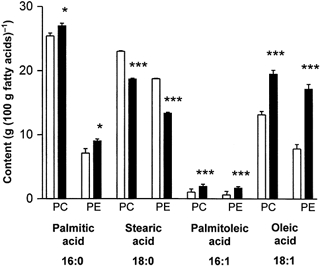
Values are expressed as a percentage of the total fatty acids in each fraction. □, controls: offspring of rats fed normal breeding diet in pregnancy; ▪, offspring of rats fed high fat diet during pregnancy. * P < 0.05 vs. controls; *** P < 0.001vs. controls.
n−6 polyunsaturated fatty acids
The content of linoleic acid (18:2n−6), the parent n−6 fatty acid, was elevated in both CPG and EPG fractions (P < 0.001) in the aortas of the offspring from high fat-fed rats compared with controls. In contrast, the content of the metabolites, arachidonic acid (20:4n−6) and adrenic acid (22:4n−6) were reduced in CPG (P < 0.001 and P < 0.01, respectively) and EPG (P < 0.001 for both) fractions in the offspring from the high fat-fed dams (Fig. 5).
Figure 5. Linoleic acid (18:2n−6), arachidonic acid (20:4n−6) and adrenic acid (22:4n−6) in choline phosphoglycerides (CPG) and ethanolamine phosphoglycerides (EPG) in aortas of 160-day-old offspring.
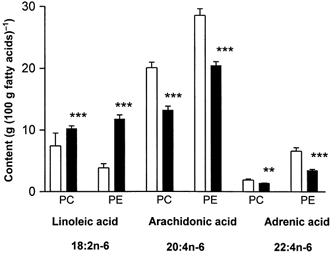
Values are expressed as a percentage of the total fatty acids in each fraction. □, controls: offspring of rats fed normal breeding diet in pregnancy; ▪, offspring of rats fed high fat diet during pregnancy. ** P < 0.01 vs. controls; *** P < 0.001 vs. controls.
n−3 polyunsaturated fatty acids
.The content of the parent n−3 fatty acid, α-linolenic acid (18:3n−3) was raised in both CPG and EPG lipid fractions in the aortas of the offspring from high fat-fed rats compared with controls (P < 0.001). However, the proportion of docosahexaenoic acid (22:6n−3), the major n−3 metabolite, was lower in both CPG (P < 0.01) and EPG (P < 0.001) fractions from the offspring of the high fat-fed dams (Fig. 6).
Figure 6. α-Linolenic acid (18:3n−3), eicosapentaenoic acid (20:5n−3), and docosahexaenoic acid (22:6n−3) in choline phosphoglycerides (CPG) and ethanolamine phosphoglycerides (EPG) in aortas of 160-day-old offspring.
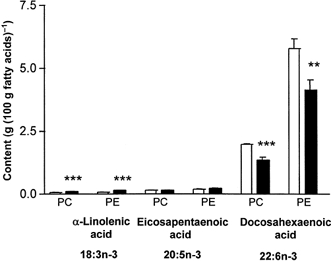
Values are expressed as a percentage of the total fatty acids in each fraction. □, controls: offspring of rats fed normal breeding diet in pregnancy; ▪, offspring of rats fed high fat diet during pregnancy. ** P < 0.01 vs. controls; *** P < 0.001 vs. controls.
DISCUSSION
This study demonstrates that a diet high in saturated fat fed to rats during pregnancy and the weaning period results in vascular dysfunction and plasma lipid and aorta fatty acid abnormalities in the adult offspring. These defects occurred despite the offspring being reared on a normal diet.
There are, to our knowledge, no studies other than our previous report (Koukkou et al. 1998) and the present investigation, which have directly assessed vascular function in adult offspring of pregnant rats fed a diet rich in supplemented fat. However, young adult offspring of dams fed a diet rich in coconut oil (predominantly saturated fatty acids) have been shown to be hypertensive (Langley-Evans, 1996). Additionally, fetal insulin resistance has been reported in the offspring of saturated fat-fed dams (Guo & Jen, 1995), while the offspring of rats fed a diet rich in corn oil exhibit abnormal cholesterol metabolism (Brown et al. 1990). Taken together, these studies strongly suggest that raised fat intake in pregnant rats may induce abnormalities in the offspring associated with hypertension and atherogenesis.
The branches of the femoral arteries from the 160-day-old female offspring of the pregnant rats fed a diet enriched with lard exhibited impaired maximal relaxation induced by acetylcholine. This observation is consistent with findings we have made previously in younger (15- and 60-day-old) offspring (Koukkou et al. 1998). Poor endothelium-dependent relaxation in response to acetylcholine is associated with a range of cardiovascular disorders (Drexler & Hornig, 1999), and has been proposed to be a primary event in the aetiology of atherosclerosis. In hypertensive disorders, it may be secondary to the development of hypertension. The impaired response observed could be attributable to a reduction in nitric oxide (NO), prostacyclin and/or endothelium-derived hyperpolarising factor production as all are implicated in vasodilatation induced by acetylcholine in the rat (Edwards et al. 1998; Fleming & Busse, 1999). The defect is unlikely to be due to NO insensitivity, because relaxation induced by the NO donor spermine NONOate was unaffected. The origin of the defect is unknown but the abnormal plasma lipid profile and highly disordered vascular fatty acids are likely, and possibly related, contributors. Plasma triglycerides, implicated in endothelial cell dysfunction (Lewis et al. 1999), were significantly raised in the 160-day-old offspring of high fat-fed dams and we and others have also reported hypertriglyceridaemia in younger offspring of rats fed a similar diet (Guo & Jen, 1995; Koukkou et al. 1998). Plasma HDL cholesterol was also reduced in the offspring of the fat-fed dams, which together with the hypertriglyceridaemia presents an atherogenic lipid profile (Cooke & Creager, 2000). The suggestion that maternal dietary lipids and fat metabolism may be involved in programming of endothelial dysfunction is supported by the recent investigation by Napoli et al. (2000) in which hypercholesterolaemic pregnant rabbits produced offspring with atherosclerotic-like lesions. In the newborn offspring, these lesions had a raised content of oxidised fatty acids which were associated with higher plasma concentrations of peroxidative end products. Lipid peroxidation is considered to be pivotal in the endothelial dysfunction associated with atherosclerosis in man (Dart & Chin-Dusting, 1999). Interestingly, the administration of vitamin E to the pregnant rabbits reversed the size of the lesions and the oxidation of the lipids. We have recently reported that pregnant rats fed the same diet rich in saturated fat as used in this study demonstrate oxidative stress as assessed by the measurement of the isoprostane 8-epi PGF2α (Gerber et al. 1999). Another recent study has shown in rats that glucocorticoid exposure in utero, reported to ‘programme’ adulthood hypertension in the offspring (Lindsay et al. 1996), increases the sensitivity of cultured cerebellar cells from the brains of 1-week-old offspring to oxidative stress and cell death (Ahlborn et al. 2000). We suggest that oxidative stress may be a common mechanistic pathway in these rat and rabbit models of the fetal programming of vascular disorders. Future work is planned to investigate whether antioxidant supplementation in pregnancy may reverse the endothelial defects observed in our rat model.
We shall also determine whether the offspring are hypertensive and investigate an alternative explanation, that the defects in endothelium-dependent relaxation observed could be secondary to an increase in the blood pressure.
The fatty acids, analysed in the polar phosphoglyceride fractions of cell membranes isolated from the offsprings’ aortas, demonstrated reduced arachidonic acid (AA), adrenic acid and docosahexaenoic acid (DHA) content in both the ethanolamine and choline phosphoglyceride fractions. AA and DHA are key constituents of plasma membranes in vascular smooth muscle and endothelium. Due to the predominance of vascular smooth muscle, the majority of the fatty acids must originate from this cell type, but there would also be a contribution from the endothelium. AA is the precursor of a host of vasoactive substances and may also promote vasodilatation by inhibition of the delayed rectifier K+ current (Smirnov & Aaronson, 1996) and through opening of Ca2+-activated K+ channels (Ahn et al. 1994). DHA is considered to be cardioprotective (Christine et al. 1998) and antiarrythmic (Xiao et al. 1997) and reduces endothelial cell susceptibility to oxidative stimuli (Chen et al. 1994; Crosby et al. 1996). DHA also profoundly affects the membrane fluidity of vascular endothelial cells (Hashimoto et al. 1999) and DHA deficiency may lead to substantial alteration of endothelial cell function. Given the magnitude of the fatty acid abnormalities observed it would be unlikely if these pathways, including those contributing to acetylcholine-induced relaxation, were unaffected. However, in view of the well recognised heterogeneity of the endothelium between vascular beds and arteries of different size, it will be necessary to establish whether the endothelial defect observed in the small femoral arteries is also present in the aorta of these animals before the potential association between endothelial cell dysfunction and fatty acid disorders can be confirmed.
The mechanism by which the fat content of the maternal diet ‘programmes’ the vascular and lipid disorders in adulthood warrants detailed further study. The potential role of oxidative stress has been discussed (see above) but additional, possibly consequent mechanisms may contribute to the fatty acid disturbances observed. First, placental transfer of DHA to the fetus may be compromised because of competition with the saturated fatty acids in maternal blood. The developing fetus is highly dependent on maternal AA and DHA (Ruyle et al. 1990), and we have reported reduced DHA in livers of the newborn of high fat-fed dams (Ghebremeskel et al. 1999). However, the adult synthesises DHA and therefore this acquired deficiency would be unlikely to persist beyond neonatal life. Second, there is increasing evidence of protein-lipid specificity in the cell membrane whereby protein-lipid matching plays an important role in membrane organisation and function (Dumas et al. 1999). A deficiency of fetal DHA could permanently alter a membrane protein, preventing normal docking with DHA-containing molecular species. Third, a maternal high fat diet may ‘programme’ the activity of enzymes involved in fatty acid metabolism and/or transport. AA and DHA are derived from linoleic and α-linolenic acid respectively by a rate-limited process of desaturation and chain elongation. The pattern of the fatty acid abnormalities in the offspring of the fat-fed dams was highly suggestive of a desaturase enzyme defect as linoleic and α-linolenic acid content in the aortas were raised in the offspring of high fat-fed dams whereas levels of longer chain members of the n−3 and n−6 series were reduced. The desaturases may be particularly susceptible to fetal ‘programming’ since offspring of rats fed a protein restricted diet demonstrate reduced Δ5 desaturase activity associated with deficiencies in DHA (Ozanne et al. 1998). Another transporter enzyme, fatty acid translocase, encoded by the Cd36 (fat) gene, has recently been implicated in the genesis of disorders of fatty acid and glucose metabolism in hypertensive rats (Aitman et al. 1999) and investigation of this enzyme in the vasculature of the adult offspring is warranted. The raised palmitic acid (16:1) and oleic acid (18:1) contents of the arteries could also play a role as high concentrations of both these fatty acids inhibit endothelial NO synthesis (Davda et al. 1995; Moers & Schrezenmeir, 1997).
In conclusion, this study provides strong evidence that a maternal diet high in saturated fat can evoke vascular endothelial dysfunction, abnormalities in the plasma lipid profile and a highly disturbed vascular fatty acid profile in adult female offspring reared on a normal diet. Further studies are underway to determine whether these offspring demonstrate other characteristics of the ‘metabolic syndrome’, i.e. whether they are hypertensive or insulin-resistant, and also to establish whether males are similarly affected.
The data presented here strongly support the hypothesis that disturbance of the in utero environment through maternal nutrition may predispose offspring to cardiovascular disease in adulthood.
Acknowledgments
The authors would like to thank Tommy's Campaign and the British Heart Foundation for supporting this work.
References
- Ahlborn E, Gogvadze V, Chen M, Celsi G, Ceccatelli S. Prenatal exposure to high levels of glucocorticoids increases the susceptibility of cerebellar granule cells to oxidative stress-induced cell death. Proceedings of the National Academy of Sciences of the USA. 2000;97:14726–14730. doi: 10.1073/pnas.260501697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn DS, Kim YB, Lee YH, Kang BS, Kang DH. Fatty acids directly increase the activity of Ca2+-activated K+ channels in rabbit coronary smooth muscle cells. Yonsei Medical Journal. 1994;35:10–24. doi: 10.3349/ymj.1994.35.1.10. [DOI] [PubMed] [Google Scholar]
- Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, Al-Majali KM, Trembling PM, Mann CJ, Shoulders CC, Graf D, St Lezin E, Kurtz TW, Kren V, Pravanec M, Ibrahimi A, Abumrad NA, Stanton LW, Scott J. Indentification of CD36 (fat) as an insulin-resistance gene causing defective fatty acids and glucose metabolism in hypertensive rats. Nature Genetics. 1999;21:76–83. doi: 10.1038/5013. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal nutrition and cardiovascular disease in later life. British Medical Bulletin. 1997;53:96–108. doi: 10.1093/oxfordjournals.bmb.a011609. [DOI] [PubMed] [Google Scholar]
- Brown SA, Rogers LK, Dunn JK, Gotto AM, Patsch W. Development of cholesterol homeostatic memory in the rat is influenced by maternal diets. Metabolism Clinical and Experimental. 1990;39:468–473. doi: 10.1016/0026-0495(90)90004-v. [DOI] [PubMed] [Google Scholar]
- Chen LY, Lawson DL, Mehta JL. Reduction in human neutrophil superoxide anion generation by n-3 polyunsaturated fatty acids: role of cyclooxygenase products and endothelium-derived relaxing factor. Thrombosis Research. 1994;76:317–322. doi: 10.1016/0049-3848(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Christine M, Albert CM, Hennekens CH, O'donnell CJ, Ajani UJ, Carey VJ, Willett WC, Ruskin JN, Manson JE. Fish consumption and risk of sudden cardiac death. Journal of the American Medical Association. 1998;279:23–28. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Creager MA. Hypercholesterolaemia, atherosclerosis and the NO synthase pathway. In: Vallance PJ T, Webb DJ, editors. Vascular Endothelium in Human Physiology and Pathophysiology. Harwood Academic Publishers; 2000. pp. 147–172. [Google Scholar]
- Crosby AJ, Wahle KW, Duthie GG. Modulation of glutathione peroxidase activity in human vascular endothelial cells by fatty acids and the cytokine interleukin-1 beta. Biochimica et Biophysica Acta. 1996;1303:187–192. doi: 10.1016/0005-2760(96)00093-8. [DOI] [PubMed] [Google Scholar]
- Dart AM, Chin-Dusting JP. Lipids and the endothelium. Cardiovascular Research. 1999;43:308–322. doi: 10.1016/s0008-6363(99)00150-9. [DOI] [PubMed] [Google Scholar]
- Davda RK, Stepniakowski KT, Lu G, Ullian ME, Goodfriend TL, Egan BM. Oleic acid inhibits nitric oxide synthase by a protein kinase C-independent mechanism. Hypertension. 1995;26:764–770. doi: 10.1161/01.hyp.26.5.764. [DOI] [PubMed] [Google Scholar]
- Drexler H, Hornig B. Endothelial dysfunction in human disease. Journal of Molecular and Cellular Cardiology. 1999;31:51–60. doi: 10.1006/jmcc.1998.0843. [DOI] [PubMed] [Google Scholar]
- Dumas F, Lebrun MC, Tocanne JF. Is the protein lipid hydrophobic matching principle relevant to membrane organisation and functions. FEBS Letters. 1999;458:271–277. doi: 10.1016/s0014-5793(99)01148-5. [DOI] [PubMed] [Google Scholar]
- Fleming I, Busse R. NO: the primary EDRF. Journal of Molecular and Cellular Cardiology. 1999;31:5–14. doi: 10.1006/jmcc.1998.0839. [DOI] [PubMed] [Google Scholar]
- Folch JL, Lees M, Sloane-Stanley GH S. A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- Gerber RT, Holemans K, O'brien-Coker I, Mallet A, van Bree R, van Assche FA, Poston L. Cholesterol independent endothelial dysfunction in virgin and pregnant rats fed a high in saturated fat diet. Journal of Physiology. 1999;517:607–616. doi: 10.1111/j.1469-7793.1999.0607t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebremeskel K, Bitsanis D, Koukkou E, Lowy C, Poston L, Crawford MA. Saturated fat maternal diet in the pregnant rat reduces docosahexaenoic acid in liver lipids of the neonate and suckling pups. British Journal of Nutrition. 1999;81:395–404. [PubMed] [Google Scholar]
- Ghebremeskel K, Leighfield M, Leaf A, Costeloe K, Crawford MA. Fatty acid composition of plasma and red cell phospholipids of preterm babies fed on breast milk and formulae. European Journal of Pediatrics. 1995;154:46–52. [PubMed] [Google Scholar]
- Guo F, Jen KC L. High fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiology and Behaviour. 1995;57:681–686. doi: 10.1016/0031-9384(94)00342-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hossain S, Yamasaki H, Masumura S. Effects of eicosapentaenoic acid and docosahexaenoic acid on plasma membrane fluidity of aortic endothelial cells. Lipids. 1999;34:1297–1304. doi: 10.1007/s11745-999-0481-6. [DOI] [PubMed] [Google Scholar]
- Koukkou E, Ghosh P, Lowy C, Poston L. Offspring of normal and diabetic rats fed saturated fat in pregnancy demonstrate vascular dysfunction. Circulation. 1998;98:2899–2904. doi: 10.1161/01.cir.98.25.2899. [DOI] [PubMed] [Google Scholar]
- Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diet. Clinical Sciences. 1994;86:217–222. doi: 10.1042/cs0860217. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Intrauterine programming of hypertension in the rat: nutrient interactions. Comparative Biochemistry and Physiology. 1996;114:327–333. doi: 10.1016/0300-9629(96)00018-7. [DOI] [PubMed] [Google Scholar]
- Lewis TV, Dart AM, Chin-Dusting JP. Endothelium-dependent relaxation by acetylcholine is impaired in hypertriglyceridemic humans with normal levels of plasma LDL cholesterol. Journal of the American College of Cardiology. 1999;33:805–812. doi: 10.1016/s0735-1097(98)00667-6. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Lindsay RM, Edwards CR W, Seckl JR. Inhibition of 11β-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;27:1200–1204. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- Lucas A, Baker BA, Desai M, Hales CN. Nutrition in pregnant or lactating rats programs lipid metabolism in the offspring. British Journal of Nutrition. 1996;76:605–612. doi: 10.1079/bjn19960066. [DOI] [PubMed] [Google Scholar]
- Moers A, Schrezenmeir J. Palmitic acid but not stearic acid inhibits NO-production in endothelial cells. Experimental and Clinical Endocrinology and Diabetes. 1997;105:78–80. doi: 10.1055/s-0029-1211804. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance arteries in spontaneously hypertensive and normotensive rats. Circulation Research. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Napoli C, Witzum JL, Calara F, De Nigris F, Palinski W. Maternal hypercholesterolaemia enhances atherogenesis in normocholesterolaemic rabbits, which is inhibited by antioxidant or lipid-lowering intervention during pregnancy. Circulation Research. 2000;87:946–952. doi: 10.1161/01.res.87.10.946. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. Journal of Physiology. 2001;530:141–153. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne SE, Martensz ND, Petry CJ, Liozou CL, Hales CN. Maternal low protein diet in rats programmes fatty acid desaturase activities in the offspring. Diabetologia. 1998;41:1337–1342. doi: 10.1007/s001250051074. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Smith GD, Tikerpae J, Hales CN. Altered regulation of hepatic glucose output in the male offspring of protein-malnourished dams. American Journal of Physiology. 1996;270:E559–564. doi: 10.1152/ajpendo.1996.270.4.E559. [DOI] [PubMed] [Google Scholar]
- Ruyle M, Connor WE, Anderson GJ, Lowensohn RL. Placental transfer of essential fatty acids in humans: venous-arterial difference for docosahexaenoic acid in fetal umbilical erythrocytes. Proceedings of the National Academy of Sciences of the USA. 1990;87:7902–7905. doi: 10.1073/pnas.87.20.7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov SV, Aaronson PI. Modulatory effects of arachidonic acid on the delayed rectifier K+ current in rat pulmonary arterial myocytes. Structural aspects and involvement of protein kinase C. Circulation Research. 1996;79:20–31. doi: 10.1161/01.res.79.1.20. [DOI] [PubMed] [Google Scholar]
- Xiao YF, Gomez AM, Morgan JP, Lederer WJ, Leaf A. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proceedings of the National Academy of Sciences of the USA. 1997;94:4182–4187. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]


