Abstract
Extracellular application of ATP generates two whole-cell currents in toad gastric smooth muscle cells: an immediate inward non-selective cation current (due to the activation of a P2X or P2Z-like receptor) and a slowly developing outward K+ current. The inward non-selective cation current depends on the continuous presence of ATP while the outward K+ current can last for minutes after ATP application ceases.
In cell-attached patches, application of ATP to the extra-patch membrane can activate K+ channels in the patch indicating that a diffusible cellular messenger may be involved. The characteristics of these K+ channels are similar to those of a previously described fatty acid-activated K+ channel that is also a stretch-activated channel.
This whole-cell K+ current can be induced by ATP in the absence of extracellular Ca2+ (with EGTA present to chelate trace amounts). However, the current generated in the presence of extracellular Ca2+ is considerably larger.
The pharmacological profiles for the activation of the non-selective cation current and the K+ current are similar, suggesting that the same P2Z-like receptor could be mediating both responses. This type of plasma membrane receptor/channel-channel coupling by a process that does not appear to involve Ca2+ flow through the receptor/channel or a subsequent membrane potential change may be representative of a new class of signalling mechanisms.
ATP is now widely recognized as a neurotransmitter or co-transmitter released from the nerve endings that innervate many tissues including smooth muscles (Burnstock, 1995). Biological effects of extracellular ATP have been documented in virtually every tissue or major organ that has been studied and various types of ATP receptors have been reported (see Dubyak & El-Moatassim, 1993, and the references therein). The subclass of purinoceptors activated by extracellular ATP, designated as P2 receptors, can be further divided into two groups: ionotropic (P2X and P2Z) and metabotropic (P2Y) (for reviews see Dubyak & El-Moatassim, 1993; Barnard et al. 1994). Metabotropic receptor activation has been associated with the activation of several second messenger systems and ion channels. Ion channel activation associated with this type of receptor is thought to be G-protein mediated and, in some cases, secondary to an increase in intracellular Ca2+ (for review see Harden et al. 1995). The ionotropic receptors, on the other hand, are ligand-gated non-selective cation channels (Surprenant et al. 1995). There have also been reports that multiple types of P2 receptors may co-exist in the same cell (for examples see Firestein et al. 1996; Najbar et al. 1996; Ryan et al. 1999).
Decades ago it was suggested that ATP was the neurotransmitter for the vagal neurons that innervate gastric smooth muscle in the toad Bufo Marinus (Campbell, 1969; Burnstock et al. 1970). We have recently characterized a purinergic receptor in smooth muscle cells isolated from this tissue (Ugur et al. 1997) with some properties similar to the P2Z or P2X7 (suggested to be a cloned variant of the P2Z receptor; Surprenant et al. 1996) purinoceptors. Activation of this toad stomach purinoceptor opens a Ca2+-permeable non-selective cation channel with a conductance of about 22 pS and with a zero current (or reversal) potential near 0 mV. The inward current response does not desensitize with repeated or prolonged application of ATP. However, divalent cations, such as Ca2+ and Mg2+, decrease the whole-cell P2Z-like receptor-mediated current. Although this receptor has many features in common with the P2Z or P2X7 receptors, unlike those receptors, it does not form pores passing large molecular weight molecules (Ugur et al. 1997).
A fatty acid-activated K+ (FAAK) channel, which has been shown to be directly activated by fatty acids and other negatively charged single chain lipids like lysophosphatidic acid, as well as membrane stretch, also exists in these toad stomach smooth muscle cells (Ordway et al. 1989, 1995; Petrou et al. 1994). This FAAK channel resembles in its gating properties the two-pore-domain K+ channel TREK-1 (Fink et al. 1996; Patel et al. 1998; Maingret et al. 1999). Both channels are activated by negatively charged single chain lipids, membrane stretch, and an increase in intracellular H+ concentration (Petrou et al. 1994; Ordway et al. 1995). To date, although some of the molecules activating this FAAK channel have been identified and suggested to be second messengers, the physiological stimulus or initiator, other than possibly membrane stretch, is not known. The results presented here suggest that extracellular ATP, besides opening ligand-gated non-selective cation channels, may be one of the initiators of FAAK channel activation. Moreover, the opening of non-selective cation channels and of FAAK channels appears to be mediated by the same P2Z-like receptor. A brief preliminary report of some of the findings presented here has been published in abstract form (Zou et al. 1997).
METHODS
Single smooth muscle cell preparation
Smooth muscle cells were enzymatically dispersed from the stomach of the toad, Bufo marinus, as described previously (Fay et al. 1982; Lassignal et al. 1986) and used on the same day. The toads used in these studies were killed by decapitation according to the guidelines of the Animal Care Committee of the University of Massachusetts Medical School.
Patch-clamp recordings and data analysis
Single channel currents, whole-cell currents and membrane potentials were recorded using standard patch-clamp techniques with an EPC-7 patch-clamp amplifier (List-Medical). Data were initially low-pass filtered at 3 kHz and sampled at 44 kHz onto videotape using a Sony PCM digital audio processor. Membrane potential changes were controlled and recordings were processed using a custom computer software suite (kindly supplied by Dr Michel Vivaudou). For the records displayed in the figures, data were further low-pass filtered at 20 Hz and 1 kHz for whole-cell current recordings and single channel current recordings, respectively, and resampled at 100 Hz and 4 kHz. The capacitive transients in the whole-cell current recordings were removed for clarity.
When experiments were carried out in the whole-cell recording configuration, at least 5 min passed after rupturing the patch membrane before collecting data. An equilibration period of 10 min or longer was used when larger molecules (e.g. GTPγS, GDPβS or higher concentrations of BAPTA) were included in the patch pipette. The measured resistance for the patch pipettes was in the range 2-5 MΩ. Series resistance compensation was not used for the whole-cell current data presented here. Therefore, with larger inward currents the cell membrane potential might have become less negative, and with the larger outward currents the membrane potential might have become more negative. However, this would not affect the conclusions drawn from the results.
There was a large variation in the magnitude and time course of the outward currents making precise quantitative analysis difficult. However, the peak current and the time to peak (mean ±s.e.m.) are provided for general comparison purposes. There were over 70 cells that responded to ATP, but only those cells (39 cells) where at least one other agent was applied or that were used as a control for a specific experimental manipulation were selected for this analysis.
Ca2+ measurements
Intracellular Ca2+ concentration was measured using a computerized high temporal resolution microfluorimeter as described previously (Yagi et al. 1988; Becker et al. 1989). Fura-2 (50 μm), used as the fluorescent indicator for Ca2+, was introduced into the cytosol through the patch pipette. A filter wheel, spinning at 135 r.p.s., provided excitation wavelengths at 340 and 380 nm (≈10 nm bandwidth). The ratio of fluorescence emission intensities at 510 nm (≈10 nm bandwidth) was measured and converted to a Ca2+ concentration using the method of Grynkiewicz et al. (1985). In these experiments, an Axoclamp-2A amplifier (Axon Instruments) was used to record the whole-cell membrane potential or current. Data were digitally collected at a sampling frequency of 300 Hz after being low-pass filtered at 100 Hz (Ugur et al. 1997).
Solutions and chemicals
Since divalent cations were found to inhibit the ATP-induced activation of P2Z-like channels in these cells (Ugur et al. 1997), most of the whole-cell experiments were carried out in a low divalent cation bathing solution − solution A (see Table 1 for contents of all the solutions). TEA was present to suppress any possible Ca2+-activated K+ current. Some experiments were carried out using a normal divalent cation solution (solution B). The patch pipette solution usually contained solution C. Sometimes 5 or 10 mm EGTA was used in place of 0.5 mm BAPTA. Na2ATP (1 mm) was also, at times, included in the pipette solution. For the experiments with 20 mm BAPTA in the pipette, the KCl concentration was 80 mm. For the experiments monitoring the intracellular Ca2+ concentration, solution D was used as the patch pipette solution. Most of the single channel current recordings from cell-attached patches were carried out with the pipette containing solution A and cells bathed in solution E to set the cell membrane potential to 0 mV. These solutions were modified when experiments were carried out for specific purposes as indicated in Results.
Table 1.
Compositions of the solutions (mm) used in the experiments
| A | B | C | D | E | |
|---|---|---|---|---|---|
| NaCl | 120 | 120 | — | — | — |
| KCl | 3 | 3 | 128 | 130 | 130 |
| CaCl2 | 0.1 | 1.8 or 2 | — | — | 0.1 |
| MgCl2 | — | 1 | 1 | 1 | — |
| Hepes | 5 | 5 | 20 | 20 | 5 |
| TEACl | 10 | 10 | — | — | 10 |
| Glucose | 11 | 11 | — | — | 11 |
| K4BAPTA | — | — | 0.5 | — | — |
| Na2ATP | — | — | — | 3 | — |
| Na3GTP | — | — | — | 1 | — |
| Fura-2 | — | — | — | 0.05 | — |
| pH | 7.6 | 7.6 | 7.2 | 7.2 | 7.6 |
Except when otherwise specified, agents were dissolved in the corresponding bathing solutions and applied by pressure ejection from a glass micropipette using a picospritzer (General Valve Corp.) as described by Lassignal et al. (1986). The pH values of the solutions were re-adjusted when necessary after dissolving these agents. As a control, the bathing solution was applied to some of the cells during whole-cell recordings in the same way. While there was usually no obvious effect when the bathing solution was applied before ATP application, transient effects on the amplitude of the ATP-induced whole-cell FAAK current, either enhancement or inhibition, were sometimes seen. In some experiments in the cell-attached patch configuration, the tip of the patch pipette was first filled with solution A and then the rest of the pipette was filled with solution A plus 1 mm Na2ATP in order to delay the effect of ATP on the channels in the patch membrane (‘back-filled pipette’). Except for the experiments measuring intracellular Ca2+ concentrations, the cell chamber was constantly perfused with the bathing solution. All experiments were carried out at room temperature.
Concentrations of free divalent cations and free ATP in bathing solutions A, B and E were estimated using a computer program (MaxChelator) based on the method of Bers et al. (1994), and the values are presented in Table 2. Free Ca2+ concentrations for some of the experimental solutions were measured with 10 μm mag-fura-2, using an approach similar to that for measuring the intracellular Ca2+ concentration. Since some of the Ca2+ in the solutions would bind to mag-fura-2, the measured free Ca2+ concentration is a minimum estimate of that in the absence of mag-fura-2.
Table 2.
Estimates of free ATP and divalent cation concentrations (mm) when ATP was added to the bathing solutions (total/free)
| [ATP] | [Ca2+] | [Mg2+] | |
|---|---|---|---|
| Solution A | 1.00/0.45 | 0.10/0.03 | 0.00/0.00 |
| Solution B | 2.50/0.31 | 1.80/0.64 | 1.00/0.29 |
| Solution E | 1.00/0.49 | 0.10/0.03 | 0.00/0.00 |
Arachidonic acid was dissolved in DMSO as a stock solution (20 mm). It was kept in a −20 °C freezer and diluted into the bathing solutions immediately before the experiments.
Sources for the reagents used in the experiments were as follows: 2-methylthio ATP tetra-sodium salt (2-MeSATP) from RBI (Natick, MA, USA); arachidonic acid from Nu Check Prep (Elysian, MN, USA); fura-2, mag-fura-2 and BAPTA from Molecular Probes (Eugene, OR, USA); ultrapure ATP (sodium salt), α,β-methyleneadenosine 5′-triphosphate lithium salt (AMP-CPP), p1,p5-di(adenosine-5′)pentaphosphate sodium salt (AP5A), adenosine 5′-O-(3-thiotriphosphate) tetra-lithium salt (ATPγS), 2′- and 3′-O-(4-benzoylbenzoyl) ATP triethyl-ammonium salt (BzATP) and fatty-acid free bovine serum albumin from Sigma (St Louis, MO,USA). All of the other chemicals were either from Sigma or Fluka Chemical Co. (Buchs, Switzerland).
RESULTS
Extracellular ATP generates two whole-cell currents with different time courses
When extracellular ATP was applied to the toad gastric smooth muscle cells, two currents were activated (Zou et al. 1997). The first current is inward at negative holding potentials (Fig. 1A), and the second current can be seen in isolation as an outward current when the cell membrane potential is held at 0 mV (near the reversal potential for the first current, Fig. 1B). The inward current is due to the opening of non-selective cation channels from activation of a P2Z-like purinoceptor (Ugur et al. 1997). The outward current is due to the opening of K+ channels (see below). The appearance of the outward current is often delayed a few seconds after the activation of the P2Z-like receptor, reaching a maximum of 987 ± 73 pA (39 cells) after the ATP application was terminated. However, there was considerable variation in the magnitude (from 400 to 2150 pA) and time course (time to peak ranging from 18 s to over 1 min and not necessarily correlating with the magnitude of the current) preventing effective quantitative analysis.
Figure 1. Extracellular ATP (1 mm) generates two whole-cell currents with different time courses: an inward current (due to the activation of a non-selective cation channel) and an outward current (due to the activation of a K+-selective channel).
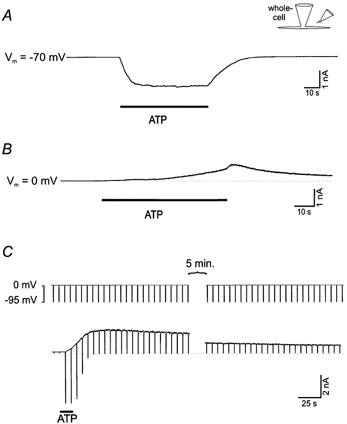
A, extracellular ATP activates a P2Z-like non-selective cation channel, which is best seen at negative holding potentials as an inward current. B, extracellular ATP also activates a delayed current, which is best seen as an outward current when the cell membrane potential is held at 0 mV (near the reversal potential for the P2Z-like channel current). C, the cell membrane potential was held at 0 mV to record the outward current and briefly (for 0.5 s every 6 s) changed to −95 mV (the calculated reversal or equilibrium potential for the K+ current) to record the non-selective cation current. The inward cation current depends on the continued presence of ATP while the outward current at 0 mV lasts for minutes after ATP application is terminated. Solutions A and C were used for these experiments. The reason for the brief additional increase of the outward current in B (sometimes) seen when the ATP application ceased is not clear. It may be partially due to some or all of the following: the series resistance, an increase in the extracellular Ca2+ concentration (see Fig. 4) as ATP (which chelates Ca2+, Table 2) is washed away, or an effect of cessation of pressure ejection. The stimulus protocol used for C was also used for Figs 3B, 4 and 5.
To demonstrate that the outward current was carried by K+, experiments were carried out using voltage ramps to measure whole-cell current reversal potentials after ATP application in different concentrations of extracellular K+ (K+ substituted for Na+). To do this, the current ramp obtained before ATP application was subtracted from the current ramp obtained after the ATP-induced non-selective cation current had deactivated and while there was still a substantial outward current. The measured reversal potentials (from 6 cells) were −78 ± 1, −27 ± 2 and −2 ± 2 mV in extracellular K+ concentrations of 3, 40 and 130 mm, respectively, close to the corresponding calculated K+ equilibrium potentials of −95, −30 and 0 mV. From linear regression analysis of the data, a slope of 47 mV for a 10-fold change in extracellular K+ concentration was obtained (r2 = 0.99). The ratio of the permabilities for K+ and Na+ (PK/PNa) for this channel is approximately 40 based on fitting the Goldman-Hodgkin-Katz equation to the measured reversal potential values (Hille, 1992). These results indicate that the delayed outward current is mainly due to K+ efflux.
The general experimental protocol used to monitor both of these currents (Fig. 1C) was to hold the cell membrane potential at 0 mV to observe the outward current and briefly (for 0.5 s every 6 s) change it to −95 mV (the calculated reversal potential for the K+ current) to observe the cation selective current. The inward cation current depends on the continued presence of ATP while the outward K+ current can last for many minutes after ATP application is terminated (Fig. 1C).
Extracellular ATP activates FAAK channels
To determine the properties of the channel underlying the K+ current and how it might be activated, single channel currents were recorded in cell-attached patches. Cells were superfused with a solution containing 130 mm K+ (solution E, 12 cells) to set the cell membrane potential to 0 mV. As shown in Fig. 2A, ATP application to the extra-patch membrane (see illustration in Fig. 2A) induced an increase in activity of the K+ channels within the patch. (Responses were also obtained in two other patches using modified solutions with 0 Ca2+ and 5 mm EGTA (and 1 mm Mg2+).) When 1 mm ATP was included in the ‘back-filled pipette’ (see Methods) in cell-attached patch experiments (3 cells), an increase in K+ channel activity was also observed. It occurred when the activation of P2Z-like receptors in the same patch became evident (Fig. 2B), which varied considerably from patch to patch. These results suggest that the effect of ATP on the activation of K+ channels not only occurs locally but can also be mediated by a diffusible cellular messenger, with the same messenger presumably acting in both cases.
Figure 2. Extracellular ATP activates K+ channels via a messenger molecule.
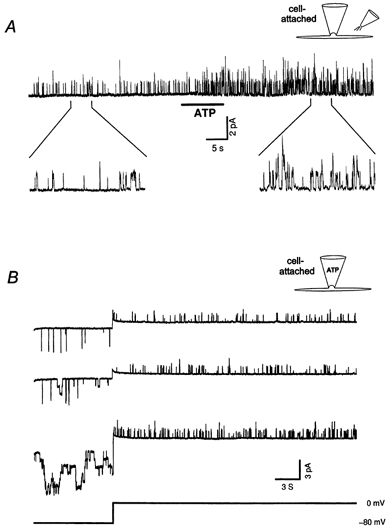
A, application of ATP (1 mm) to the extra-patch membrane of a cell-attached patch activates K+ channels in the patch (pipette potential was 0 mV). The duration of the two expanded traces is 5 s. B, ATP (1 mm, ‘back-filled pipette’, see Methods) in the cell-attached patch pipette can activate the K+ channels in the patch. The patch membrane potential was held at 0 mV to record the unitary outward currents and changed to −80 mV to record the unitary non-selective cation currents. The top trace was recorded about 5 min before the first appearance of the cation channel openings. The middle trace was recorded when the first cation channel openings appeared and no obvious change in the K+ channel activity could be seen. The bottom trace was recorded 2 min 40 s afterwards when both the cation channel and the K+ channel activity were greatly increased. The number of K+ channel openings in the first, second and third trace is 62, 73 and 144, respectively. The very brief inward currents were due to openings of another channel having a current amplitude and open time distinct from the P2Z-like receptor channel. Experiments were carried out using solutions A and E as pipette and bath solutions, respectively. Additional filtering was applied to the current traces by digital filtering with a 5 ms square wave.
The unitary current amplitude for the K+ channel activated by ATP was examined in cell-attached patches using voltage step-ramps with a bathing solution containing 130 mm K+ (solution E without TEA) and the pipette solution containing 3 mm K+ (solution A). The obtained unitary conductance (22.8 ± 0.4 pS, 5 cells) was close to that of the FAAK channel reported by Ordway (1990, 20 pS), who used essentially the same solutions. In addition, when ATP and arachidonic acid were applied to the extra-patch membrane of the same cell sequentially, the resulting respective unitary currents were identical (5 cells). Moreover, like the FAAK channel, this ATP-activated K+ channel is also relatively insensitive to TEA (in the presence of 10 mm external TEA, the unitary currents were suppressed by only 30 %).
Since albumin has been shown to decrease the FAAK channel activity induced by cell membrane stretch (Ordway et al. 1995), its effect was also tested on the ATP-induced K+ current. Both the cell-attached patch single channel activity and the whole-cell outward current were suppressed by extracellular application of albumin (10 or 20 μm, Fig. 3). The inhibition of K+ current by albumin was more effective in the whole-cell configuration (9 of 9 cells showed inhibition) than in cell-attached patches (4 of 10 patches showed inhibition). This could be due to the fact that, for the cell-attached patches, the effect of albumin is indirect in that albumin applied to the extra-patch membrane has to affect events in the cell-attached patch. In addition, the position of the patch membrane in the pipette may also affect the response. After application of albumin, K+ currents could be activated again by application of 10 or 20 μm arachidonic acid (Fig. 3A and B, 3 of 3 cells in the whole-cell recording configuration and 3 of 3 cell-attached patches). Soybean trypsin inhibitor (15 μm) was used as a control for any possible non-specific effects of proteins (Ordway et al. 1995) and found to have only a slight inhibitory effect on the ATP-induced whole-cell K+ current (94 ± 3 % of the current remained compared to a mere 7 ± 3 % after albumin application, 3 cells).
Figure 3. Cell-attached patch single channel (A) and whole-cell (B) current recordings demonstrate that albumin (20 μm) suppresses the FAAK channel activity induced by ATP (1 mm) and arachidonic acid (10 μm) restores it.
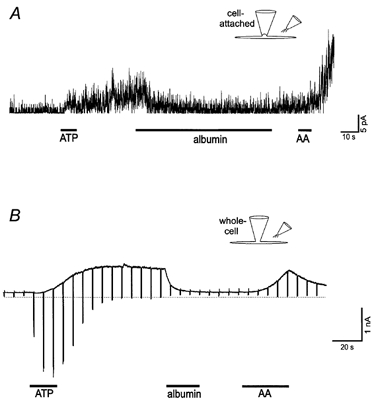
Solutions A and E (as pipette and bath solutions, respectively) were used for the cell-attached patch recording, while solutions A and C (as bath and pipette solutions, respectively) were used for the whole-cell voltage-clamp recording. For A the pipette potential was 0 mV.
In summary, the outward current induced by extracellular ATP is due to openings of FAAK channels and a cellular messenger molecule most probably mediates this effect.
Ca2+ affects the FAAK current induced by extracellular ATP
As illustrated in Fig. 4, activation of the FAAK current by ATP was affected by the presence of extracellular Ca2+. After the outward FAAK current was induced by ATP, chelating extracellular Ca2+ by applying an EGTA containing solution (5 mm EGTA added to solution A) immediately suppressed the outward current (6 cells, Fig. 4A). This suppression is unlikely to be due to a direct effect of EGTA on FAAK channels because these channels can be activated by arachidonic acid or membrane stretch in the presence of EGTA (Ordway et al. 1989, 1995). In addition, a similar inhibition of the FAAK current was also observed using 5 mm BAPTA in place of EGTA (3 cells).
Figure 4. Extracellular Ca2+ helps maintain or enhances the ATP-induced whole-cell K+ current but Ca2+ does not have to pass through P2Z-like channels.

A, brief application of a 5 mm EGTA-containing solution eliminates the outward K+ current induced by extracellular ATP (1 mm) application. Solutions A and C were used for this experiment. B, in an EGTA-containing Ca2+-free bath solution, ATP (2 mm in the same bath solution) application induced a substantially diminished outward current. This current was then increased when a 100 μm Ca2+-containing solution (solution A) was applied to the cell membrane after the inward current declined. Application of the Ca2+-containing solution prior to ATP application did not activate the outward K+ current. The Ca2+-free bath solution contained (mm): 127 NaCl, 3 KCl, 1 MgCl2, 10 Hepes, 5 EGTA, 11 glucose, pH 7.6.
The outward current induced by extracellular ATP (1 or 2 mm) was also recorded in a bathing solution containing 5 or 10 mm EGTA (essentially solution B with EGTA and 0 Ca2+) to chelate trace amounts of Ca2+. It was either smaller (ranging from 30 to 250 pA with an average current being 87 ± 18 pA, 14 cells) or substantially diminished (less than 30 pA with an average current being 12 ± 2 pA, 15 cells). When a Ca2+ (100 μm) containing solution (solution A) was applied to the same cells (11 of the above-mentioned 29 cells) after the inward current declined, the average outward current increased either 6-fold from the first group (4 of 4 cells) or 60-fold from the second group (5 of 7 cells, Fig. 4B). Among those nine cells which responded to Ca2+ after ATP, the Ca2+ containing solution was also applied to eight of them before ATP application and none showed an increase in outward current (Fig. 4B).
These observations suggest that, although important for the enhancement or maintenance of the ATP-induced FAAK current, the presence of extracellular Ca2+ alone is not sufficient to induce the FAAK current. On the other hand, the ATP-induced FAAK current, although smaller, could still be obtained in EGTA containing solutions (28 of 29 cells) suggesting that extracellular Ca2+ is not required for FAAK current induction. Moreover, Ca2+ passing through the P2Z-like receptor/channel is not essential for the enhancement effect since it can occur after the inward current has declined.
When 5 mm (5 cells) or 20 mm (2 cells) BAPTA was included in the whole-cell patch pipette to further chelate intracellular Ca2+, the ATP-induced outward current (603 ± 51 pA, 400-850 pA), although at the low end, was still in the range of the currents obtained using the usual pipette solution. Thus, the enhancement effect of Ca2+ is not likely to be due to a substantial rise in global intracellular Ca2+ concentration.
G-proteins are apparently not involved in the activation of FAAK channels
To determine whether G-proteins were involved in the signalling pathway by which ATP activates the FAAK channel, experiments were carried out with either GTPγS (a non-hydrolysable GTP analogue which activates G-proteins) or GDPβS (a GDP analogue which inhibits G-proteins) included in the whole-cell patch pipette. One millimolar GTPγS (4 cells) or 1 mm GDPβS (5 cells) alone did not activate the outward current, and outward currents could still be induced by application of ATP (data not shown).
The pharmacological profile for the activation of the FAAK channel is similar to that for the activation of the P2Z-like non-selective cation channel
Various ATP analogues (all at 1 mm) were tested for their effects on whole-cell current (Fig. 5). ATPγS (2 cells), BzATP (8 cells) and 2-MeSATP (4 cells) activated both the inward P2Z-like receptor cation current and the outward FAAK current. On the other hand, adenosine (2 cells), ADP (3 cells), AMP-CPP (5 cells), AP5A (2 cells), GTP (6 cells) and UTP (5 cells), causing little or no P2Z-like receptor non-selective cation current (see also Ugur et al. 1997), did not activate the FAAK current (Fig. 5). The cells that did not respond to this latter group of ATP analogues all responded to applications of ATP. Thus, the pharmacological profile for activation of the FAAK current was similar to that for activation of the P2Z-like receptor non-selective cation current (Ugur et al. 1997). The activation of the outward K+ current by 2-MeSATP, BzATP and especially ATPγS application occurred with a longer delay when compared to ATP, the time to peak being 122 ± 29, 184 ± 11, 236 and 56 ± 4 s, respectively. In addition, the peak current induced by 2-MeSATP (178 ± 41 pA) was smaller than that induced by ATP (987 ± 73 pA), BzATP (816 ± 168 pA) or ATPγS (825 pA).
Figure 5. The pharmacological profiles for the activation of the P2Z-like receptor and for the activation of the FAAK channel are similar.
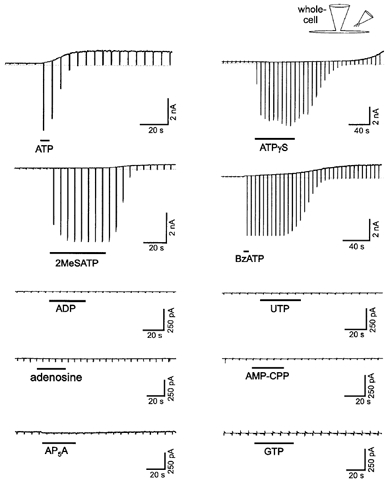
Agents that activate the K+ channel (ATP, ATPγS, BzATP and 2-MeSATP) are also P2Z-like receptor agonists; while agents that show little or no effect on the P2Z-like receptor (adenosine, ADP, AMP-CPP, AP5A, GTP and UTP) do not activate the K+ channel. The concentration for all of the ATP analogues used here was 1 mm dissolved in the bath solutions. Solutions A and C were used in the experiments.
Thus, it appears that ATP activates both currents by occupying a single type of P2Z-like receptor.
Effects of extracellular ATP on the smooth muscle cell membrane potential and intracellular Ca2+ concentration
To mimic what might occur when ATP is released at the neuromuscular junction in vivo (see Discussion), experiments were also carried out in a bathing solution containing normal concentrations of divalent cations (solution B) while ATP (1 mm) was applied in the low divalent cation solution (solution A; containing 100 μm Ca2+ and no added Mg2+) normally used for most of the other experiments. When ATP was applied to cells that were not ‘patched’, 12 of the 34 cells contracted and then gradually relaxed upon the removal of ATP. The ATP-induced contraction was repeatable with those cells that contracted.
In the current-clamp configuration (with no current injection), application of ATP using the same solutions as above caused the cell membrane potential to approach 0 mV from the resting level consistent with the opening of P2Z-like non-selective cation channels. When ATP was removed, the membrane potential shifted towards the equilibrium potential for K+ (3 cells) consistent with the activation of FAAK channels. When the intracellular Ca2+ concentration was measured using fura-2 in four additional cells, it increased while the cell membrane was depolarized by the application of ATP and decreased towards baseline values upon the cessation of ATP application (Fig. 6A). For two of these cells 2.5 mm ATP was applied in solution B. The same experiment was also carried out in the low divalent cation bathing solution (solution A), instead of the normal divalent containing solution (solution B), without monitoring the intracellular Ca2+ concentration; and similar membrane potential changes were observed (7 cells). When albumin (10 or 20 μm) and arachidonic acid (10 or 20 μm) were applied sequentially to these seven cells after ATP application ceased, the membrane potential shifted towards less negative or more negative values, respectively (Fig. 6B). These results are consistent with those obtained under voltage clamp (see Fig. 3) and illustrate what may occur in vivo.
Figure 6. Extracellular ATP causes membrane depolarization and an intracellular Ca2+ concentration increase which is followed by hyperpolarization and restoration of the intracellular Ca2+ concentration when ATP is removed.
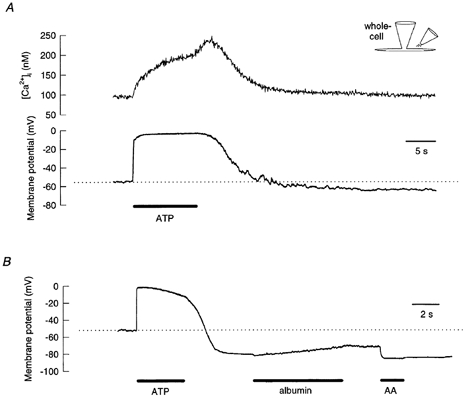
A and B, in the current-clamp configuration, the cell membrane potential approaches 0 mV from the resting potential in the presence of extracellular ATP, and it goes towards the equilibrium potential for K+ when ATP is removed. A, before returning to baseline, the intracellular Ca2+ concentration further increased when the application of ATP ceased. This increase is probably from accelerated Ca2+ entry due to the extracellular Ca2+ concentration being restored upon the removal of ATP (which chelates Ca2+, Table 2). B, when albumin (20 μm) and arachidonic acid (20 μm) were applied to the cell sequentially after ATP application, the membrane potential shifted towards less negative and more negative values, respectively. Solutions B and D were used in the experiment shown in A and solutions A and C were used in the experiment shown in B. ATP (1 mm) was dissolved in solution A for both experiments.
DISCUSSION
Results from this study demonstrate that, in freshly isolated toad stomach smooth muscle cells, extracellular ATP activates at least two channels: a non-selective cation channel and the fatty acid-activated K+ (FAAK) channel. The immediate activation of the first will cause membrane depolarization while the delayed activation of the second, repolarization (Fig. 6). While the activation of the second channel seems to occur after the activation of the first, it does not require Ca2+ influx through the first or a membrane potential change due to the inward current.
A diffusible cellular messenger may mediate the effect of ATP on FAAK channels
Results presented here suggest that, in toad gastric smooth muscle cells, stimulation of the P2Z-like receptor activates a K+ channel by means of a second messenger molecule. That the K+ channels in cell-attached patches can be activated by ATP application to the extra-patch membrane suggests that a diffusible messenger molecule is involved. With their ability to freely diffuse within and across cell membranes, fatty acids and other negatively charged single chain lipids are suitable candidates for such a messenger molecule. Evidence for the involvement of these molecules includes the facts that: (1) this K+ channel is the same channel as the FAAK channel previously shown to be directly activated by fatty acids and other negatively charged single chain lipids (Ordway et al. 1989; Petrou et al. 1994) and (2) albumin, which can bind fatty acids and other such lipids and remove them from cell membranes (Ordway et al. 1995), suppressed the ATP-induced FAAK currents. The inhibitory effect of albumin is not likely to be non-specific because soybean trypsin inhibitor, used as a control for any non-specific effects, had only a minimal effect on the ATP-induced outward current. Although these results are consistent with fatty acids or other lipids being the second messengers other possibilities cannot be ruled out.
Extracellular Ca2+ affects the FAAK current induced by ATP
We have provided evidence that the presence of extracellular Ca2+ is important for the enhancement or maintenance of the FAAK current induced by ATP. However, Ca2+ does not seem to be necessary for the response to occur. An ATP-induced FAAK current, albeit often smaller, could still develop in the EGTA containing bathing solution.
We found no strong evidence for the involvement of Ca2+ influx in the modulation of FAAK channel activity. When ATP was applied in an EGTA-containing bathing solution, the effect of extracellular Ca2+ on FAAK current development or enhancement could still occur long after the P2Z-like cation current had deactivated (Fig. 4B). Therefore, the effect of Ca2+ on the FAAK channel does not require Ca2+ influx through the P2Z-like channel. Moreover, membrane depolarization alone in a Ca2+-containing bath solution does not induce the current as can be seen in the figures before ATP application. Consistent with Ca2+ acting on an extracellular site is the observation that high concentrations of intracellular BAPTA failed to abolish the response suggesting that changes in global levels of intracellular Ca2+ are not required for FAAK current development. However, we cannot completely rule out the possibility that Ca2+ is acting intracellularly in a restricted space near the plasma membrane where it cannot be effectively chelated by intracellular Ca2+ chelators.
This effect of Ca2+ on the outward current and the fact that ATP is a Ca2+ chelator (Table 2) may partially explain why there was a slow development of the current during the application of ATP and why the peak current occurred after ATP application was terminated (Fig. 1 and Fig. 3–5).
It is unclear whether Ca2+ is involved in the signal transduction pathway between P2Z-like receptor activation and FAAK current development or whether it directly augments the FAAK current or both. The increase in the average number of endogenous FAAK channels open in cell-attached patches with 1.8 mm Ca2+ in the pipette instead of 0 Ca2+, 5 mm EGTA (Ordway, 1990) suggests that a direct effect of Ca2+ on the current is possible.
As an aside, it is unlikely that the inhibitory effect of albumin was due to a marked lowering of the extracellular Ca2+ concentration due to its ability to bind Ca2+. The reason is that the affinity of albumin for Ca2+ is quite low (Kd > 1 mm; See Moore, 1969) predicting that it would have a relatively small effect on the low concentration of Ca2+ (100 μm) used for these experiments. Consistent with this is that the free Ca2+ concentration (measured using mag-fura-2; see Methods) was approximately 68 μm in solution A containing 20 μm albumin (compared with the measured value of approximately 98 μm in the absence of albumin). In addition, soybean trypsin inhibitor, which might also bind Ca2+ in a similar way, had only a slight effect on the outward current (see Results).
The receptor activating the FAAK channel appears to be the P2Z-like receptor
Co-existence of multiple types of purinoceptors has been demonstrated in many cell types (Najbar et al. 1996; Firestein et al. 1996; Boarder & Hourani, 1998). Therefore, it is possible that FAAK channel activation is caused by activation of a metabotropic P2 receptor (i.e. a G-protein-coupled P2Y receptor) as shown in other cell types (see Ryan et al. 1999 and references therein). However, the following experimental results do not support this possibility. (1) Every nucleotide analogue tested either activated both the P2Z-like and the FAAK currents or showed little or no effect on either of them. (2) BzATP activated the FAAK current but adenosine, UTP, ADP or AP5A had no or very little effect. These observations indicate a role for a P2Z-like receptor and argue against the involvement of P1-type receptors or some of the P2Y receptor subclasses (otherwise known as P2U, P2T or P2D receptors; for definitions see Fredholm et al. 1994; Alexander & Peters, 2000). Moreover, where the data are available, all of the known P2Y subtypes are activated by UTP or ADP either more potently or at least as potently as ATP (Alexander & Peters, 2000). (3) Neither GTPγS nor GDPβS had an obvious effect on ATP induction of the FAAK current suggesting, along with the lack of effect of P2Y agonists, that G-proteins or metabotropic receptors are not involved in the activation process. However, the possible involvement of G-proteins cannot be ruled out since we do not have an independent assay to determine whether GTPγS or GDPβS affected G-protein activity. In summary, although we cannot definitively rule out a second type of purinoceptor for activating FAAK channels, our results suggest that the receptor which causes the activation of these channels is very similar to or is the same as the P2Z-like receptor itself.
Having another current develop from the activation of an ionotropic receptor, yet independent of ion flux through the receptor, is what distinguishes this study from previous studies where current development requires a metabotropic receptor (for example see Matsuura et al. 1996), or where more than one purinoceptor was involved in the development of multiple currents (for example see Fitz & Sostman, 1994), or where the current passing through one receptor would activate other currents either by depolarization of the cell membrane (for example see Benham, 1989) or by increasing intracellular Ca2+ concentration (for example see Friedrich et al. 1989).
Coupling of P2Z receptor activation to metabolic responses
There have been previous reports suggesting that the P2Z or P2X7 receptor/channel is also coupled to a second response. The second response was first reported as the activation of phospholipase D (PLD) in a macrophage cell line by El-Moatassim & Dubyak (1992, 1993). Other second responses include the activation of PLD in human lymphocytes (Gargett et al. 1996), phospholipase C (PLC) in rat parotid acinar cells (Jorgensen et al. 1995), and phospholipase A2 (PLA2) in rat submandibular gland ductal cells (Alzola et al. 1998). Although in most of these studies an increase in intracellular Ca2+ concentration was observed, activation of phospholipases after P2Z stimulation was not simply due to Ca2+ influx through P2Z channels because, in some cases, activation was found to be Ca2+ independent (Jorgensen et al. 1995; Alzola et al. 1998). Furthermore, El-Moatassim & Dubyak (1993) reported that the P2Z receptor's pore-forming effect can be separated from its activation of PLD; and Gargett et al. (1996) suggested that PLD activation via P2Z receptors depends on Ca2+ influx but not on global intracellular Ca2+ levels. For the latter, the relationship between PLD activation and the timing of Ca2+ influx is not clear.
The reports linking P2Z or P2X7 receptors to enzyme activation bear a strong resemblance to the studies we report here on activation of the FAAK channels. Taking together these published reports and our results, some purinoceptors with the P2Z-like phenotype, besides passing non-selective cation currents, can be coupled to effectors (e.g. some lipase) by some unknown mechanism. In our case, since a second messenger is most probably involved in the activation of FAAK channels by ATP, the enzyme or transduction mechanism responsible for its production might reside in the P2Z-like receptor itself, in a yet-to-be-determined subunit, or in another molecule coupled to the purinoceptor. In line with these possibilities, the β-subunit of a voltage-gated K+ channel bears structural similarity to aldo-keto reductases suggesting a direct link between ion channel activation and enzyme activity (Gulbis et al. 1999).
Therefore, the coupling of the P2Z-like receptor to FAAK channels in toad stomach smooth muscle cells may be representative of a new class of signalling mechanisms between plasma membrane ion channels, where a ligand-gated channel affects the behaviour of a second channel type by a process that does not involve Ca2+ flow through the channel or a membrane potential change. The mechanism of this possible channel-channel interaction (or receptor/channel-channel coupling), which would include clearly identifying the putative second messenger, remains to be determined.
Physiological significance of extracellular ATP
Campbell (1969) reported that stimulation of the vagal roots caused an inhibitory response of toad stomach as indicated by a decrease in intragastric pressure. The inhibitory innervation is neither cholinergic nor adrenergic. Burnstock et al. (1970) demonstrated that ATP or a related nucleotide is the transmitter substance released by these inhibitory nerves. They also showed that, in response to ATP perfusion, toad stomach smooth muscle tissue could either contract or relax, and the variation in the response was not dependent on ATP concentration. More interestingly, some preparations could contract transiently (as indicated by an increase in intragastric pressure) and then relax below the initial pressure; and the relaxation took minutes to recover after ATP was removed. Although this has been known for more than 30 years, the mechanism underlying the inhibitory effect of ATP is still unknown.
Recently, we characterized the purinoceptor in these smooth muscle cells as a P2Z-like receptor, a ligand-gated ion channel that is permeable to Ca2+ and other cations (Ugur et al. 1997). Since the current passing through this P2Z-like receptor reversed around 0 mV, activation of this receptor will depolarize the cell membrane and cause the cell to contract. The latter most probably occurs due to increases in the concentration of intracellular Ca2+ from Ca2+ passing through the channel as well as the subsequent depolarization activating voltage-gated Ca2+ channels. This, however, does not explain the inhibitory effect of ATP perfusion or vagal nerve stimulation on toad stomach (Campbell, 1969; Burnstock et al. 1970). Findings from the present study, that activation of the P2Z-like receptor also activates the FAAK channel causing hyperpolarization of the cell membrane which persists for a long time after the removal of ATP, may provide the cellular mechanism for this second physiological response to neuronal stimulation. As shown in Fig. 6, binding of ATP to P2Z-like receptors opens non-selective cation channels causing depolarization of the cell membrane, which in turn increases intracellular Ca2+ concentration leading to smooth muscle contraction. The opening of FAAK channels, which is secondary to the stimulation of P2Z-like receptors, will hyperpolarize the cell membrane and cause relaxation. The fact that the FAAK current starts with a delay, develops slowly and persists for minutes after ATP application stops (see Fig. 1) also agrees with the time course for muscle relaxation after stimulation of the vagal root (Campbell, 1969). Such effects of extracellular ATP on smooth muscle contraction and relaxation provide another example of the balance between the Yin and Yang of physiological function.
Normal release of ATP from nerve endings in the confined synaptic or extracellular space would lead to chelation of extracellular Mg2+ and Ca2+, possibly leaving sufficient free ATP to act on P2Z-like receptors even as the ATP level declines. This decline would restore the free extracellular Ca2+, which would enhance the development of the FAAK current. This sequence of events, which may occur during neuronal transmitter release, supplied the rationale for the design of the experiments shown in Fig. 6. Moreover, it may explain how low divalent cation and high free ATP concentrations, which are required for activation of the toad stomach P2Z-like receptor (Ugur et al. 1997), can be attained in the extracellular space under physiological conditions.
In conclusion, this study suggests that extracellular ATP, besides opening P2Z-like receptor cation channels, activates the FAAK channel. The activation of the FAAK channel appears to occur via a diffusible cellular messenger molecule. This molecule may be a fatty acid, another lipid, or a non-lipid substance. ATP could be a ‘first messenger’ that causes the opening of FAAK channels, and this effect appears to be mediated by the P2Z-like receptor. In other words, the occupancy of the P2Z-like receptor by ATP causes two processes to occur in toad gastric smooth muscle cells. One is the opening of a cation channel inherent to the P2Z-like receptor itself, which would cause membrane depolarization. The other is the production of a second messenger molecule activating the FAAK channel which would lead to subsequent membrane hyperpolarization. Further studies are needed to explore the exact mechanism underlying the activation of the FAAK channel by extracellular ATP.
Acknowledgments
We thank Jeff Carmichael, Rebecca McKinney, Brian Packard and Paul Tilander for excellent technical assistance, Thomas W. Honeyman for helpful discussions, and Ann R. Rittenhouse for providing comments on an earlier version of this manuscript. This work was supported by the National Institutes of Health, grant DK31620.
References
- Alexander S P H, Peters JA. Receptor and ion channel nomenclature supplement. Trends in Pharmacological Sciences. 2000;11:73–75. [Google Scholar]
- Alzola E, Pérez-Etxebarria A, Kabré E, Fogarty DJ, Métioui M, Chaib N, Macarulla JM, Matute C, Dehaye JP, Marino A. Activation by P2X7 agonists of two phospholipases A2 (PLA2) in ductal cells of rat submandibular gland. Journal of Biological Chemistry. 1998;273:30208–30217. doi: 10.1074/jbc.273.46.30208. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Burnstock G, Webb TE. G protein-coupled receptors for ATP and other nucleotides: a new receptor family. Trends in Pharmacological Sciences. 1994;15:67–70. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Becker PL, Singer JJ, Walsh JV, Jr, Fay FS. Regulation of calcium concentration in voltage-clamped smooth muscle cells. Science. 1989;244:211–214. doi: 10.1126/science.2704996. [DOI] [PubMed] [Google Scholar]
- Benham CD. ATP-activated channels gate calcium entry in single smooth muscle cells dissociated from rabbit ear artery. Journal of Physiology. 1989;419:689–701. doi: 10.1113/jphysiol.1989.sp017893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D, Patton C, Nuccitelli R. A practical guide to the preparation of Ca buffers. In: Nuccitelli R, editor. Methods in Cell Biology. Vol. 40. San Diego, CA, USA: Academic Press; 1994. pp. 3–29. [DOI] [PubMed] [Google Scholar]
- Boarder MR, Hourani S M O. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends in Pharmacological Sciences. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Noradrenaline and ATP: cotransmitters and neuromodulators. Journal of Physiology and Pharmacology. 1995;46:365–384. [PubMed] [Google Scholar]
- Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. British Journal of Pharmacology. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. The autonomic innervation of the stomach of a toad (Bufo marinus) Comparative Biochemistry and Physiology. 1969;31:693–706. doi: 10.1016/0010-406x(69)92069-6. [DOI] [PubMed] [Google Scholar]
- Dubyak GR, El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. American Journal of Physiology. 1993;265:C577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- El-Moatassim C, Dubyak GR. A novel pathway for the activation of phospholipase D by P2Z purinergic receptors in BAC1. 2F5 macrophages. Journal of Biological Chemistry. 1992;267:23664–23673. [PubMed] [Google Scholar]
- El-Moatassim C, Dubyak GR. Dissociation of the pore-forming and phospholipase D activities stimulated via P2Z purinergic receptors in BAC1. 2F5 macrophages. Journal of Biological Chemistry. 1993;268:15571–15578. [PubMed] [Google Scholar]
- Fay FS, Hoffman R, Leclair S, Merriam P. Preparation of individual smooth muscle cells from the stomach of Bufo marinus. Methods in Enzymology. 1982;85:284–292. doi: 10.1016/0076-6879(82)85027-1. [DOI] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO Journal. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Firestein BL, Xing M, Hughes RJ, Corvera CU, Insel PA. Heterogeneity of P2U- and P2Y-purinergic receptor regulation of phospholipases in MDCK cells. American Journal of Physiology. 1996;271:F610–618. doi: 10.1152/ajprenal.1996.271.3.F610. [DOI] [PubMed] [Google Scholar]
- Fitz JG, Sostman AH. Nucleotide receptors activate cation, potassium, and chloride currents in a liver cell line. American Journal of Physiology. 1994;266:G544–553. doi: 10.1152/ajpgi.1994.266.4.G544. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. Normenclature and classification of purinoceptors. Pharmacological Reviews. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- Friedrich F, Weiss H, Paulmichl M, Lang F. Activation of potassium channels in renal epithelioid cells (MDCK) by extracellular ATP. American Journal of Physiology. 1989;256:C1016–1021. doi: 10.1152/ajpcell.1989.256.5.C1016. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Cornish EJ, Wiley JS. Phospholipase D activation by P2Z-purioceptor agonists in human lymphocytes is dependent on bivalent cation influx. Biochemical Journal. 1996;313:529–535. doi: 10.1042/bj3130529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gulbis JM, Mann S, MacKinnon R. Structure of a voltage-dependent K+ channel β subunit. Cell. 1999;97:943–952. doi: 10.1016/s0092-8674(00)80805-3. [DOI] [PubMed] [Google Scholar]
- Harden TK, Boyer JL, Nicholas RA. P2-purinergic receptors: subtype-associated signaling responses and structure. Annual Review of Pharmacology and Toxicology. 1995;35:541–579. doi: 10.1146/annurev.pa.35.040195.002545. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2. Sunderland, MA, USA: Sinauer Associates Inc.; 1992. [Google Scholar]
- Jorgensen TD, Gromada J, Tritsaris K, Nauntofte B, Dissing S. Activation of P2Z purinoceptors diminishes the muscarinic cholinergic-induced release of inositol 1,4,5-trisphosphate and stored calcium in rat parotid acini. Biochemical Journal. 1995;312:457–464. doi: 10.1042/bj3120457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassignal NL, Singer JJ, Walsh JV., Jr Multiple neuropeptides exert a direct effect on the same isolated single smooth muscle cell. American Journal of Physiology. 1986;250:C792–798. doi: 10.1152/ajpcell.1986.250.5.C792. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. Journal of Biological Chemistry. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- Matsuura H, Tsuruhara Y, Sakaguchi M, Ehara T. Enhancement of delayed rectifier K+ current by P2-purinoceptor stimulation in guinea-pig atrial cells. Journal of Physiology. 1996;490:647–658. doi: 10.1113/jphysiol.1996.sp021174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EW. Studies with ion-exchange calcium electrodes in biological fluids: some applications in biomedical research and clinical medicine. In: Durst RA, editor. Ion-Selective Electrodes. Washington, DC, USA: National Bureau of Standards; 1969. pp. 215–285. [Google Scholar]
- Najbar AT, Li CG, Rand MJ. Evidence for two distinct P2-purinoceptors subserving contraction of the rat anococcygeus smooth muscle. British Journal of Pharmacology. 1996;118:537–542. doi: 10.1111/j.1476-5381.1996.tb15435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway RW. PhD Thesis. Worcester, MA, USA: University of Massachusetts Medical School; 1990. Fatty acids directly activate K+ channels in isolated gastric and vascular smooth muscle cells. [Google Scholar]
- Ordway RW, Petrou S, Kirber MT, Walsh JV, Jr, Singer JJ. Stretch activation of a toad smooth muscle K+ channel may be mediated by fatty acids. Journal of Physiology. 1995;484:331–337. doi: 10.1113/jphysiol.1995.sp020668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway RW, Walsh JV, Jr, Singer JJ. Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science. 1989;244:1176–1179. doi: 10.1126/science.2471269. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honoré E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO Journal. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou S, Ordway RW, Hamilton JA, Walsh JV, Jr, Singer JJ. Structural requirement for charged lipid molecules to directly increase or suppress K+ channel activity in smooth muscle. Effects of fatty acids, lysophosphatidate, acyl coenzyme A and sphingosine. Journal of General Physiology. 1994;103:471–486. doi: 10.1085/jgp.103.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JS, Baldridge WH, Kelly M E M. Purinergic regulation of cation conductances and intracellular Ca2+ in cultured rat retinal pigment epithelial cells. Journal of Physiology. 1999;520:745–759. doi: 10.1111/j.1469-7793.1999.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Buell G, North RA. P2X receptors bring new structure to ligand-gated ion channels. Trends in Neurosciences. 1995;18:224–229. doi: 10.1016/0166-2236(95)93907-f. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Ugur M, Drummond RM, Zou H, Sheng P, Singer JJ, Walsh JV., Jr An ATP-gated cation channel with some P2Z-like characteristics in gastric smooth muscle cells of toad. Journal of Physiology. 1997;498:427–442. doi: 10.1113/jphysiol.1997.sp021869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi S, Becker PL, Fay FS. Relationship between force and Ca2+ concentration in smooth muscle as revealed by measurements on single cells. Proceedings of the National Academy of Sciences of the USA. 1988;85:4109–4113. doi: 10.1073/pnas.85.11.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Ugur M, Walsh JV, Jr, Singer JJ. Extracellular ATP increases the activity of a fatty acid- and stretch activated K+ channel: possible role for fatty acids as second messengers. Biophysical Journal. 1997;72:A264. [Google Scholar]


