Abstract
Dihydroxyeicosatrienoic acids (DHETs), which are metabolites of arachidonic acid (AA) and epoxyeicosatrienoic acids (EETs), have been identified as highly potent endogenous vasodilators, but the mechanisms by which DHETs induce relaxation of vascular smooth muscle are unknown. Using inside-out patch clamp techniques, we examined the effects of DHETs on the large conductance Ca2+-activated K+ (BK) channels in smooth muscle cells from rat small coronary arteries (150–300 μm diameter).
11,12-DHET potently activated BK channels with an EC50 of 1.87 ± 0.57 nm (n = 5). Moreover, the three other regioisomers 5,6-, 8,9- and 14,15-DHET were equipotent with 11,12-DHET in activating BK channels. The efficacy of 11,12-DHET in opening BK channels was much greater than that of its immediate precursor 11,12-EET. In contrast, AA did not significantly affect BK channel activity.
The voltage dependence of BK channels was dramatically modulated by 11,12-DHET. With physiological concentrations of cytoplasmic Ca2+ (200 nm), the voltage at which the channel open probability was half-maximal (V1/2) was shifted from a baseline of 115.6 ± 6.5 mV to 95.0 ± 10.1 mV with 5 nm 11,12-DHET, and to 60.0 ± 8.4 mV with 50 nm 11,12-DHET.
11,12-DHET also enhanced the sensitivity of BK channels to Ca2+ but did not activate the channels in the absence of Ca2+. 11,12-DHET (50 nm) reduced the Ca2+ EC50 of BK channels from a baseline of 1.02 ± 0.07 μm to 0.42 ± 0.11 μm.
Single channel kinetic analysis indicated that 11,12-DHET did not alter BK channel conductance but did reduce the first latency of BK channel openings in response to a voltage step. 11,12-DHET dose-dependently increased the open dwell times, abbreviated the closed dwell times, and decreased the transition rates from open to closed states.
We conclude that DHETs hyperpolarize vascular smooth muscle cells through modulation of the BK channel gating behaviour, and by enhancing the channel sensitivities to Ca2+ and voltage. Hence, like EETs, DHETs may function as endothelium-derived hyperpolarizing factors.
Large conductance Ca2+-activated potassium (BK) channels provide the means to link the cellular metabolic state and Ca2+ homeostasis with cellular excitability. BK channels are K+ selective with a large unitary conductance, sense changes in both membrane voltage and intracellular Ca2+ concentration, and are particularly sensitive to blockade by iberiotoxin (Latorre et al. 1989; Brayden & Nelson, 1992; Nelson et al. 1995; Nelson & Quayle, 1995; Carl et al. 1996; Vergara et al. 1998; Wallner et al. 1999). Because of their large conductance and high density in vascular smooth muscle, the activity of BK channels is a key determinant in controlling the resting membrane potential and vascular tone. Moreover, in arterial smooth muscle cells, BK channels are activated by Ca2+‘sparks’ (Nelson et al. 1995) giving rise to spontaneous transient outward currents (STOCs), which lead to membrane hyperpolarization (Bolton & Imaizumi, 1996; Bychkov et al. 1997). Thus, blockade of BK channels in smooth muscle cells results in membrane depolarization and increased contractile tone (Anwer et al. 1993; Heppner et al. 1997).
Recently, the cytochrome P450 metabolites of arachidonic acid (AA) were found to be highly potent activators of vascular BK channels and are candidates for endothelium-derived hyperpolarizing factors (EDHFs) (Hecker et al. 1994; Campbell & Harder, 1999; Fisslthaler et al. 1999). Cytochrome P450 mono-oxygenases convert AA to four epoxyeicosatrienoic acid regioisomers (EETs): 5,6-, 8,9-, 11,12- and 14,15-EET, as well as several allylic oxidation products, including 19- and 20-hydroxyeicosatetraenoic acids (HETEs) (Fitzpatrick & Murphy, 1988; McGiff, 1991; Oliw et al. 1994). In contrast to these HETEs, EETs are almost exclusively vasodilators (Miura & Gutterman, 1998; Oltman et al. 1998), and Gebremedhin et al. (1992) showed that dilatation by EETs was mediated by the opening of K+ channels in vascular smooth muscle cells. This observation was confirmed by multiple investigators who further demonstrated that EETs specifically enhanced the activity of BK channels (Hu & Kim, 1993; Campbell et al. 1996; Zou et al. 1996; Li et al. 1997; Li & Campbell, 1997).
Like EETs, DHETs are vasoactive. For example, micromolar concentrations of DHETs and EETs dilate coronary conduit vessels (Campbell et al. 1996; Fang et al. 1996; Weintraub et al. 1997). Moreover, all four EET regioisomers dilate the downstream arterioles at EC50 values between 1 and 100 pm, which is about 1000- to 10 000-fold lower than the values for the upstream conduit vessels. In contrast, the EC50 for 11,12-DHET was 10−15m in the coronary arterioles, establishing it among the most potent of identified vasodilators (Oltman et al. 1998). However, the cellular ionic mechanisms through which DHETs induce vasorelaxation are unknown.
In the present study, 50 nm 11,12-DHET hyperpolarized the resting membrane potential of freshly isolated myocytes from rat coronary arteries. Moreover, using patch clamp techniques, we showed that nanomolar concentrations of DHETs increased the opening probability (Po) of the BK channels severalfold. The maximum BK channel Po (Po,max) attained by 11,12-DHET was much greater than that attained by its precursor 11,12-EET. 11,12-DHET modulated channel gating by enhancing the BK channel sensitivity to cytoplasmic Ca2+ and to depolarization. These observations raise the possibility that DHETs may also be potent EDHFs.
METHODS
Solutions
All concentrations are millimolar.
Cell dissociation solution contained: NaCl 110.0, KCl 5.0, CaCl2 0.16, MgCl2 2.0, Hepes 10.0, NaHCO3 10.0, NaH2PO4 0.5, KH2PO4 0.5, glucose 10.0, EGTA 0.49 and taurine 10.0, adjusted to pH 6.9 with NaOH.
KB solution contained: KOH 70.0, KCl 40.0, l-glutamic acid 50.0, taurine 20.0, MgCl2 0.5, K2HPO4 1.0, EGTA 0.5, Hepes 10.0, creatine 5.0, pyruvic acid 5.0 and Na2ATP 5.0, adjusted to pH 7.38 with KOH.
The pipette (extracellular) solution for BK channel recordings in inside-out patches contained: KCl 140.0, CaCl2 1.0, MgCl2 1.0, Hepes 10.0 and EGTA 1.0, adjusted to pH 7.4 with KOH.
The bath (cytoplasmic) solution for BK channel recordings in inside-out patches contained: KCl 140.0, EGTA 1.0 and Hepes 10.0, adjusted to pH 7.35 with KOH. Various amounts of Ca2+ were added in the form of CaCl2 to give the desired concentrations of free Ca2+ (from 10−9 to 10−4m) as calculated using Chelator software (Theo J. M. Schoenmakers, Department of Animal Physiology, University of Nijmagen, Toernooiveld, The Netherlands).
The intracellular solution for whole-cell current clamp recordings contained: KCl 140.0, EGTA 1.0, CaCl2 0.018 (200 nm free Ca2+), GTP 0.5, ATP 5.0, Hepes 10.0 and MgCl2 1.0, pH 7.35.
The extracellular solution for whole-cell current clamp recordings contained: NaCl 140.0, KCl 5.4, CaCl2 1.8, MgCl2 0.5, Na2HPO4 1.0, glucose 5.0 and Hepes 5.0, pH 7.4.
Isolation of arterial smooth muscle cells from rat coronary microvessels
The use of animals and the procedures involved with tissue isolation were approved by the Animal Care and Use Committee at The University of Iowa. Single coronary artery smooth muscle cells were prepared as described by Carrier et al. (1997). Briefly, male Sprague-Dawley rats (200-250 g body wt) were anaesthetized with methoxyfluorane. Hearts were exposed with a mid-sternotomy and the animals were killed by rapid excision of the hearts, which were placed in ice-cold cell dissociation solution. The secondary and tertiary branches of the septal coronary arteries (150-300 μm intraluminal diameter, i.d.) in the interventricular septum were isolated under a dissection microscope and freed of surrounding myocardium and connective tissue as described previously (Miller et al. 1999). The small arteries were then placed in 1 ml of cell dissociation solution containing 1.3 mg ml−1 papain (11.9 units mg−1, Sigma Chemical Co., St Louis, MO, USA), 0.64 mg ml−1 dithiothreitol (Boehringer Mannheim Co., Indianapolis, IN, USA), 0.4 mg ml−1 collagenase (CLS-2, 364 units mg−1, Worthington Biochemical Co., Lakewood, NJ, USA) and bovine serum albumin (0.2 % w/v). After 40 min of incubation at 37 °C, the vessels were transferred into 1 ml KB solution, and then gently triturated with a fire-polished glass pipette until the cells were completely dissociated. The resulting smooth muscle cell suspension was left at room temperature and used within 8 h. All solutions were vigorously oxygenated for 30 min before starting the experiment.
Whole-cell current clamp recordings
Resting membrane potentials were recorded at 37 °C in isolated single smooth muscle cells from small rat coronary arteries using the current clamp technique. After baseline resting membrane potentials were obtained, the cells were exposed to 11,12-DHET (50 nm) and the changes in membrane potential were recorded continuously. After the resting potential had reached steady state, 200 nm of iberiotoxin was added to the 11,12-DHET.
Single BK channel recordings
Unitary membrane currents in single smooth muscle cells from small rat coronary arteries were recorded using standard patch clamp techniques in the inside-out configuration (Hamill et al. 1981). In brief, isolated vascular smooth muscle cells were placed in a 0.5 ml chamber on the stage of an inverted microscope (Olympus CK2, Olympus America Inc., Mellville, NY, USA), and were superfused at 1-2 ml min−1 using a direct current-powered pump (model 700, Instech Laboratories Inc., Plymouth Meeting, PA, USA). Thus, all bath solution exchanges were complete within 30-60 s. Borosilicate glass capillaries (Corning 7056, Warner Instrument Corp., Hamden, CT, USA) were used to fabricate patch pipettes. When filled with the pipette solution, each electrode had a tip resistance between 2 and 5 MΩ, and the typical seal resistance was greater than 10 GΩ. Single BK channel currents were recorded with an Axopatch 200B integrating amplifier (Axon Instruments, Foster City, CA, USA), and the output of the amplifier was filtered through an 8-pole low-pass Bessel filter unit (902 LPF, Frequency Devices Inc., Haverhill, MA, USA) at 5 kHz and digitized at 20 kHz (12-bit resolution, Digidata 1200, Axon Instruments). pCLAMP software (version 6.05, Axon Instruments) was used to generate voltage-clamp protocols and the resulting current recordings were stored on a Pentium-based personal computer (Dimension XPS T450, Dell Computer Corp., Round Rock, TX, USA) for further analysis. All experiments were performed at 21-23 °C. BK channels were identified by the unitary conductance, voltage and Ca2+ sensitivity, and by inhibition with 50-100 nm iberiotoxin. After patch excision in all experiments, we routinely examined the channel sensitivity to Ca2+ by removing and replenishing Ca2+ in the bath solution, and in some experiments, exposure of Ca2+ to the channels was performed in a graded manner (200 nm and 1 μm). Only patches containing BK channels that demonstrated sensitivity to Ca2+, with effects that were rapid in onset and reversible upon Ca2+ washout and replenishment, were used in our experiments.
Unitary BK current amplitude (i) was determined from amplitude histograms fitted with a Gaussian function. Single channel conductance in symmetrical 140 mm K+ solutions was determined by performing voltage clamp recordings from a membrane potential of -160 mV to +140 mV in 20 mV increments. The unitary conductance (γ) was determined from the slope of a least-square linear fit of the unitary current amplitude-voltage (i-V) relationship. BK channel activity in inside-out patches was determined by measuring the channel Po at a membrane potential of +60 mV, which was slightly above the voltage threshold for BK channel activation in the presence of 200 nm Ca2+. Po was determined by:
where Po is the single channel open probability, T is the duration of recording, tj is the time spent with j = 1, 2, …N channel openings and N is the maximal number of simultaneous channel openings observed when Po was high. An N value less than 5 was used for all the Po studies.
The effects of membrane voltage on BK channel Po were characterized by the Boltzmann equation:
where Po,max represents the maximal Po, V½ is the voltage at which Po is half of Po,max, Vm is the membrane potential and k is the slope factor. k is equal to RT/zF, where z is the equivalent charge movement associated with the Boltzmann distribution, F is the Faraday constant, R is the universal gas constant and T is the absolute temperature.
The electron charge movement associated with each rate constant was estimated by fitting the voltage dependence of each transition rate to the exponential function:
where τo represents each component of the BK channel open dwell time distribution, k0 represents the value of the voltage-dependent rate constant at 0 mV and δ is the electrical distance through the membrane.
Dose-response curves were fitted using a Hill equation of the following form:
where S represents the concentration of the chemical, EC50 is the concentration at half-maximal effect and nH is the Hill coefficient.
Single BK channel kinetics analysis
All kinetics analyses were performed in membrane patches containing only one BK channel. The opening and closing transitions of BK channels were detected by the half-amplitude threshold method using TAC software (version x4.0.9, Bruxton Inc., Seattle, WA, USA). Data were digitally filtered at 1.0 kHz bandwidth to achieve the appropriate signal-to-noise ratio. The open and closed dwell times were fitted with the sums of exponential probability density functions using the maximum likelihood with simplex optimization. Each fit was corrected for the dead time of the recording system as implemented in TAC. The dead time was estimated as 0.253/f, where f represents the filter cut-off frequency in hertz (-3 dB). In these studies, the open and closed dwell times were presented using log-binning transformation. Only dwell times longer than the rising time were fitted. The number of exponential components was established using the likelihood ratio test and any additional exponential component was added only when the probability exceeded 0.95.
The first latency was assessed by the probability density function of the first BK channel opening after a depolarizing voltage step from a holding potential of -60 mV to a test potential of +60 mV. First latency measurements were corrected for the filter dead time, and curve fitting of the first latency histograms was performed using the pSTAT Marquardt-Levenberg algorithm. The data were fitted using a multiple exponential equation. The number of exponential components was determined as described above.
Curve fittings were performed using Igor Pro 3.16 software (WaveMetrics Inc., Lake Oswego, OR, USA).
Materials
11,12-EET, and 5,6-, 8,9-, 11,12- and 14,15-DHET were obtained from Cayman Chemical Co. (Ann Arbor, MI, USA). AA was purchased from Nu-Chek-Prep Inc. (Elysian, MN, USA). Iberiotoxin and other chemicals were obtained from Sigma Chemical Co. AA, 11,12-EET and the DHETs were prepared in absolute ethanol as a 5 mm stock solution and stored under nitrogen at -20 °C. The chemicals were administered with a final concentration of ethanol of less than 0.0001 %.
Statistical methods
Data are presented as means ±s.e.m. Student's paired t test was used to compare data obtained before and after each intervention. A one-way ANOVA followed by contrast testing was used to compare data from multiple groups. Statistically significant differences were defined as P < 0.05.
RESULTS
Effects of 11,12-DHET on BK channel activities
The effects of 11,12-DHET on the resting potential of isolated rat coronary arterial myocytes were determined using whole-cell current clamp techniques. Figure 1A shows a representative experiment. The smooth muscle cell had a resting potential of -53 mV and exposure to 50 nm 11,12-DHET was accompanied by hyperpolarization of the membrane potential to -64 mV. The effects of 11,12-DHET reached a steady state in about 4 min, and addition of 200 nm iberiotoxin, a selective BK channel blocker, reversed these effects, bringing the resting potential to -56 mV. Figure 1B shows a bar graph of the collective data. Under control conditions, the resting potential of isolated rat small coronary arterial smooth muscle myocytes was -54.2 ± 1.3 mV (n = 7) and exposure to 50 nm 11,12-DHET hyperpolarized the resting potential to -69.1 ± 1.9 mV (P < 0.05vs. control). Addition of 200 nm iberiotoxin significantly inhibited the effects of 11,12-DHET, reversing the resting potential to -60.2 ± 1.6 mV (P < 0.05vs. 11,12-DHET alone and P = n.s. vs. control).
Figure 1. Effects of 11,12-DHET on the resting membrane potential and on the BK channel activity in smooth muscle cells from rat small coronary arteries.
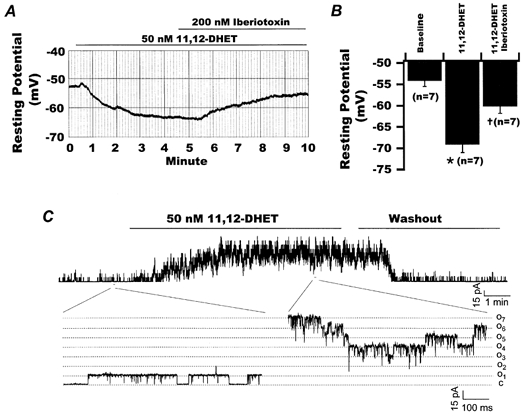
A, recordings of resting membrane potential of a smooth muscle myocyte isolated from small rat coronary artery. At baseline, the resting potential was -53 mV. Superfusion with 50 nm 11,12-DHET resulted in hyperpolarization of the membrane potential to -64 mV. This effect of 11,12-DHET was reversed upon the addition of 200 nm iberiotoxin, bringing the resting potential to -56 mV. B, results from seven experiments measuring the resting membrane potentials of smooth muscle cells isolated from rat small coronary arteries at baseline (-54.2 ± 1.3 mV), in the presence of 50 nm 11,12-DHET (-69.1 ± 1.9 mV) and with 200 nm iberiotoxin added to the 11,12-DHET (-60.2 ± 1.6 mV). *P < 0.05vs. baseline control; †P < 0.05vs. 11,12-DHET alone. C, continuous BK channel recordings in an inside-out patch at +60 mV and in the presence of 1 μm cytoplasmic Ca2+. Application of 50 nm 11,12-DHET to the bath stimulated BK channel activities, which were rapidly reversed upon washout of the diol with bath solution containing fatty acid-free albumin (0.2 % w/v). Selected sections of current tracings before and after application of 50 nm 11,12-DHET are expanded to show BK channel behaviour in greater detail. C represents the baseline when no channels were opened, and O1 and O7 represent the current amplitudes at which one and seven channels were opened, respectively.
To examine the mechanism of membrane hyperpolarization by 11,12-DHET, we tested the effects of the diol on BK channels, which are major determinants of vascular membrane potential and tone. Membrane patches from freshly dissociated rat coronary arterial smooth muscle cells were richly endowed with large conductance K+ channels that were activated by Ca2+. At a membrane potential of +60 mV, no channel activity was detectable in the absence of Ca2+. However, in the presence of 1 μm cytoplasmic Ca2+, the channel activities were robust and could be inhibited by 100 nm iberiotoxin (data not shown). Application of 50 nm 11,12-DHET dramatically enhanced BK channel activity (Fig. 1C), which rapidly resumed basal levels upon 11,12-DHET washout.
11,12-DHET activated the BK channels in a dose-dependent manner. Raw tracings of BK channels activated by four concentrations of 11,12-DHET, in the presence of 200 nm and 1 μm Ca2+ are shown in Fig. 2A. The relationship between BK channel Po and 11,12-DHET concentration is shown in Fig. 2B. In the presence of 200 nm Ca2+, the EC50 for 11,12-DHET was 1.87 ± 0.57 nm(n = 5, nH = 1.3), and 100 nm 11,12-DHET increased the Po,max 12-fold to 0.50 ± 0.02 (n = 5, P < 0.05vs. baseline). In the presence of 1 μm free Ca2+, the EC50 for 11,12-DHET was reduced to 0.93 ± 0.18 nm(n = 5, P < 0.05vs. 200 nm Ca2+, nH = 1.4), and 100 nm 11,12-DHET increased the Po,max to 0.70 ± 0.04 (n = 5, P < 0.05vs. baseline). Thus, in the presence of physiological concentrations of cytoplasmic Ca2+, 11,12-DHET is a potent activator of the coronary smooth muscle BK channels, and it is an even more potent agonist under conditions of increased cytoplasmic concentrations of Ca2+.
Figure 2. Dose-dependent effects of 11,12-DHET on BK channels.
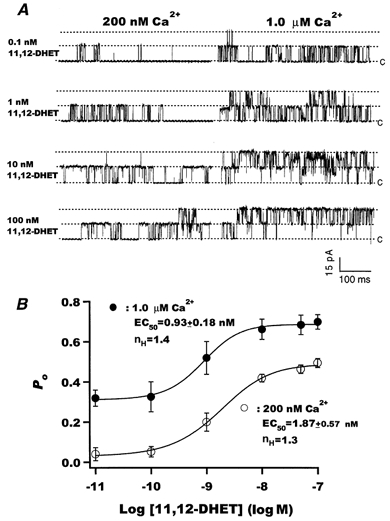
A, raw current tracings of BK channel activities recorded at a membrane potential of +60 mV in the presence of various concentrations of 11,12-DHET (10−10 to 10−7m) with either 200 nm (left) or 1.0 μm free Ca2+ (right) in the bath solution. The dotted line labelled c represents the baseline when no channels were open and the other two dotted lines represent the current amplitudes at which one and two channels were open. B, dose-response relationships for the effect of 11,12-DHET on BK channel opening probability in the presence of 200 nm (○) and 1 μm (•) free Ca2+. The data were fitted using a Hill equation. EC50 represents the diol concentration at half-maximal effect, and nH represents the Hill coefficient (n = 5).
We also examined the effects of 11,12-EET, the immediate precursor of 11,12-DHET, on BK channel activity. 11,12-EET is a potent vasodilator in dog coronary arterioles (Oltman et al. 1998) and activates BK channels in bovine coronary arteries (Campbell et al. 1996). At +60 mV and 200 nm Ca2+, BK channels were exposed to various 11,12-EET concentrations, with or without 0.5 mm GTP (Fig. 3A). The normalized dose-response curve for 11,12-DHET is included for ready comparison. We confirmed that 11,12-EET is a potent BK channel activator in rat coronary smooth muscle cells with an EC50 of 2.48 ± 0.81 nm(n = 6), similar to that of 11,12-DHET. Addition of GTP (0.5 mm) did not alter the effects of 11,12-EET or 11,12-DHET on BK channel Po (Fig. 3A and B). However, the Po attained with 11,12-DHET was at least 2- to 3-fold higher at all BK-activating concentrations; for example, the Po,max in the presence of 100 nm 11,12-EET was only 0.22 ± 0.06 (n = 6), whereas the Po,max at 100 nm 11,12-DHET was 0.46 ± 0.07 (n = 6, P < 0.05vs. 11,12-EET). Figure 3B shows results from a separate set of experiments in which the inside-out patches were first exposed to 50 nm 11,12-EET and then to 50 nm 11,12-DHET, both in the presence of 200 nm free Ca2+. 11,12-EET (50 nm) increased the BK channel Po 3.1-fold (n = 8, P < 0.05vs. baseline), and subsequent addition of 11,12-DHET increased the BK channel Po an additional 8.7-fold (n = 8, P < 0.05vs. 11,12-EET; Fig. 3B). Thus, not only is 11,12-DHET a more efficacious BK channel activator than its precursor, 11,12-EET, but 11,12-DHET is also capable of further activating BK channels after saturating effects of 11,12-EET have been reached.
Figure 3. Effects of AA, 11,12-EET, the four DHET regioisomers and GTP on BK channel activities.
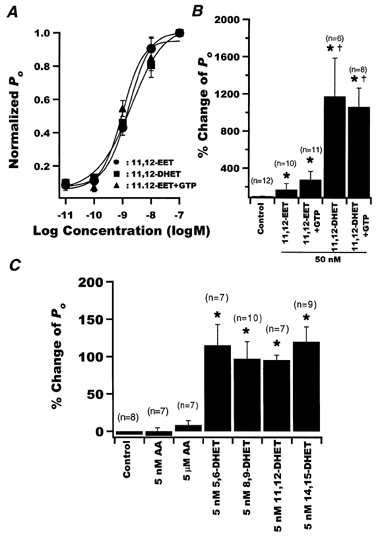
A, dose-response curves for the effect of 11,12-DHET (▪) and 11,12-EET in the presence (▴) or absence (•) of 0.5 mm GTP in the bath solution, on BK channel opening probability. The results were normalized to the Po,max of each intervention and fitted using a Hill equation. EC50 was 1.87 ± 0.57 nm for 11,12-DHET and 2.48 ± 0.81 nm for 11,12-EET (n = 6). Addition of 0.5 mm GTP to the bath solution did not change the responsiveness of BK channels to 11,12-EET (EC50 was 1.31 ± 0.91 nm). B, percentage change of BK channel Po with 50 nm 11,12-EET and 11,12-DHET in the presence and absence of 0.5 mm GTP. *P < 0.05vs. ethanol control. †P < 0.05vs. 11,12-EET. Data are presented as means ±s.e.m. C, percentage change in BK channel Po with application of ethanol (Control), AA and the four DHET regioisomers. Data are presented as means ±s.e.m. *P < 0.05vs. ethanol control. There was no significant difference among the DHET regioisomers.
Figure 3C summarizes the percentage changes of Po measured at a membrane potential of +60 mV and in the presence of 1 μm Ca2+ that were produced by the ethanol vehicle (control), AA at 5 nm and 5 μm, and the four DHET regioisomers at 5 nm. Neither the ethanol vehicle nor AA (n = 14) activated the BK channels. However, the four DHET regioisomers, at a concentration close to the EC50 of 11,12-DHET, were equipotent activators of BK channels, producing a 2-fold increase in Po(n = 7-10, P < 0.05vs. ethanol control). Further experiments were performed using only 11,12-DHET.
Effects of 11,12-DHET on the voltage dependence of BK channels
Figure 4A shows representative raw current tracings of BK channels in the same patch, with 200 nm Ca2+ and at different membrane potentials (+20 to +120 mV), before and after the application of 50 nm 11,12-DHET. Under control conditions, BK channels had a threshold for activation at around +40 mV, and this was shifted to around -20 mV in the presence of 50 nm 11,12-DHET. The relationship of normalized Povs. voltage is plotted in Fig. 4B and the data were fitted with a Boltzmann equation. The membrane potential at half-maximal Po(V1/2) shifted from 115.6 ± 6.5 mV at baseline to 95.0 ± 10.1 mV with 5 nm 11,12-DHET (n = 7, P < 0.05vs. control), and to 60.0 ± 8.4 mV with 50 nm 11,12-DHET (n = 7, P < 0.05vs. 5 nm 11,12-DHET; Fig. 4B). However, 11,12-DHET did not alter the slope factors of the curves (k = 13-15 mV per e-fold change); thus, the equivalent of 1.7-1.9 electron charges (zδ) moved through the membrane electrical field during the activation transition of each channel, which is consistent with previous reports (Cui et al. 1997; Horrigan et al. 1999b). In summary, 11,12-DHET changed the BK channel V½ towards physiological potentials, making the channel more sensitive to activation by depolarization.
Figure 4. Effects of membrane potential on BK channels in the presence and absence of 11,12-DHET.
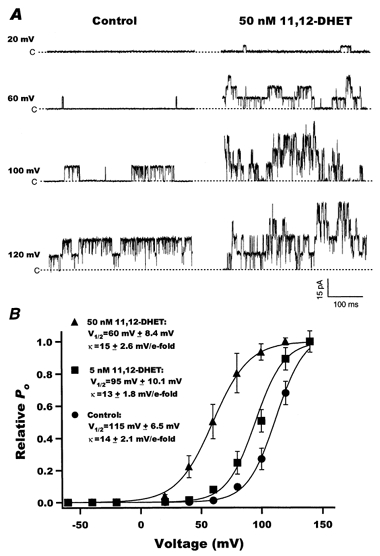
A, raw current traces of rat coronary BK channel activities recorded at different membrane potentials (+20 to +120 mV), with 200 nm Ca2+, before (left) and after (right) application of 50 nm 11,12-DHET. B, the relationships between BK channel opening probability and membrane potential at baseline (•, Control) and with 5 nm (▪) and 50 nm (▴) 11,12-DHET. The data were fitted using a Boltzmann equation. V½ represents the voltage at half-maximum opening probability, and k represents the slope factor (n = 7).
We examined the effects of 11,12-DHET on single BK channel conductance by measuring the unitary current amplitudes (i) at various membrane potentials (V). In these experiments (data not shown), high concentrations of Ca2+ (1 and 50 μm) were selected to facilitate BK channel openings at negative potentials. The relationship of unitary current amplitude and voltage (i-V) was linear with a weakly inward rectification occurring only at very high depolarized potentials (> +100 mV). The slope conductance was 245.4 ± 4.6 pS at baseline (n = 10), which was similar to values reported by other laboratories (Latorre et al. 1989; McManus, 1991; Nelson & Quayle, 1995; Carl et al. 1996). 11,12-DHET (50 nm) did not alter either the weak inward rectification or the single channel conductance of the BK channel (248.3 ± 2.5 pS, n = 10, P = n.s. vs. control). Thus, as reported for EETs, 11,12-DHET did not alter the K+ permeability properties of the BK channels.
Effects of 11,12-DHET on the Ca2+ dependence of BK channels
To determine the effects of 50 nm 11,12-DHET on the Ca2+ dependence of BK channel activities, recordings were performed at +60 mV in the presence of 10−9 to 10−4m cytoplasmic free Ca2+. Without DHET, BK channels barely opened in the presence of 100 nm Ca2+ (Po = 0.03± 0.02, n = 6). When Ca2+ was increased to 100 μm, the channel Po increased to a maximum of 0.64 ± 0.06 (n = 6). At baseline, the EC50 of Ca2+ was 1.02 ± 0.07 μm with a Hill coefficient of 1.5 (n = 6). These values are similar to those reported for human coronary smooth muscle BK channels (Tanaka et al. 1997). Application of 50 nm 11,12-DHET increased the BK channel Po at all tested Ca2+ concentrations, to a maximum of 0.80 ± 0.03 (n = 6, P < 0.05vs. control) at 100 μm Ca2+. The Ca2+ dependence of the BK channel is shown in Fig. 5A. Thus, 50 nm 11,12-DHET shifted the Po-[Ca2+] curve leftward and upward and reduced the Ca2+ EC50 by 58.8 % to 0.42 ± 0.10 μm(n = 6, P < 0.05vs. control). 11,12-DHET did not alter the Hill coefficient (nH = 1.3). Surprisingly, in the absence of Ca2+, 50 nm 11,12-DHET failed to activate BK channels, indicating that the presence of Ca2+ is obligatory for DHET modulation of BK channels. In summary, these results raised the possibility that 11,12-DHET modulates BK channel activity by increasing the channel sensitivity to Ca2+.
Figure 5. Effects of free cytoplasmic [Ca2+] on BK channels in the presence and absence of 11,12-DHET.
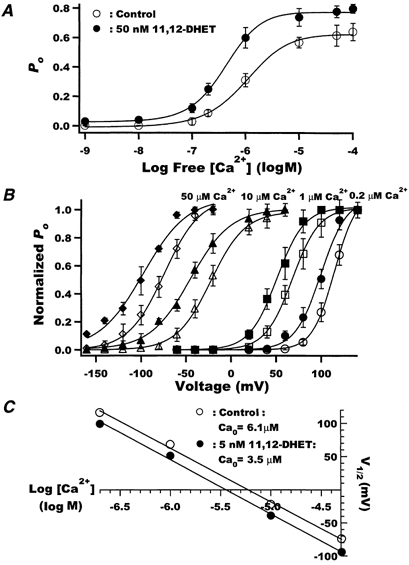
A, dose-response relationships between free [Ca2+] and BK channel Po at baseline (○) and with 50 nm 11,12-DHET (•). The data were fitted using a Hill equation. EC50, the Ca2+ concentration at half-maximal stimulation, was 1.02 ± 0.07 μm at baseline, but was reduced to 0.42 ± 0.10 μm with 11,12-DHET (P < 0.05vs. control, n = 6). The Hill coefficient and was 1.5 for control and 1.3 for 11,12-DHET (P = n.s.). B, the effects of 5 nm 11,12-DHET (filled symbols) on the relationship between normalized open probability and membrane voltage in the presence of 0.2 μm (circles), 1 μm (squares), 10 μm (triangles) and 50 μm (diamonds) free Ca2+. Open symbols show control data in the absence of 11,12-DHET. The data were fitted using a Boltzmann equation (n = 5). C, the membrane potential at half-maximum open probability (V½) plotted against the logarithm of free Ca2+ concentration was fitted using a linear equation (○, control; •, 5 nm 11,12-DHET).
To clarify whether 11,12-DHET directly alters BK channel sensitivity to Ca2+ or indirectly alters Ca2+ sensitivity by enhancing BK channel voltage sensitivity, the Ca2+ set point (Ca0) was determined. Ca0 is the concentration of Ca2+ required to produce half-maximal Po at a membrane potential of 0 mV (Carl et al. 1996). Figure 5B shows the effects of 5 nm 11,12-DHET on the BK channel Po-V relationship in the presence of 0.2, 1, 10 and 50 μm of cytoplasmic free Ca2+. 11,12-DHET shifted the BK channel V½ by 18-24 mV in the hyperpolarizing direction, while the slope factor, k, was not significantly altered. Thus, 11,12-DHET shifted the Po-V curves to the left at all Ca2+ concentrations examined. The increased Ca2+ sensitivity of BK channels was also apparent by a reduced Ca0. In the absence of DHET, the V½-log[Ca2+] curve was linear (slope = 83.0 mV (log m)−1) with an intercept of 6.1 μm (Fig. 5C). In the presence of 5 nm 11,12-DHET, the curve was also linear (slope = 82.2 mV (log m)−1) but had an intercept of 3.5 μm. Thus, 11,12-DHET produced a parallel leftward shift in the curve by 17-20 mV and lowered the Ca0 set point by 43 %. These results clearly established that 11,12-DHET directly enhanced the BK channel sensitivity to Ca2+.
Effect of 11,12-DHET on the first latency of BK channel opening
In these studies, the time from the start of a depolarizing pulse to the first channel opening (first latency analysis) was measured. A voltage step from -60 mV to +60 mV was applied for 650 ms at 0.03 Hz. At baseline, the first opening of the BK channels in response to the voltage step was slow and some sweeps showed first channel opening late into the pulse. However, on exposure to 50 nm 11,12-DHET, there was a dramatic reduction in the first latency, with the channels opening promptly upon voltage activation (Fig. 6A). The first latency distribution histograms are presented in Fig. 6B. The distribution of the first latency for control was fitted using a three-exponential equation, whereas that for 11,12-DHET required only a two-exponential fit. The control fast (τ1), intermediate (τ2) and slow (τ3) time constant components and their relative weights (in parentheses) were: 10 ms (0.73), 20 ms (0.06) and 156.3 ms (0.21), respectively. In the presence of 50 nm 11,12-DHET, the first latency was much shorter (note the difference in time scales in Fig. 6B); τ1 and τ2 and their relative weights were: 2.4 ms (0.93) and 20.7 ms (0.07). Hence, 11,12-DHET not only accelerates the fast transition rates but also eliminates the slow rate component. These results indicate that 11,12-DHET facilitates the response of channel opening to voltage activation. This finding corroborates the observation that 11,12-DHET enhances the voltage sensitivity of the BK channels.
Figure 6. Effect of 11,12-DHET on the first latency of BK channel activation.
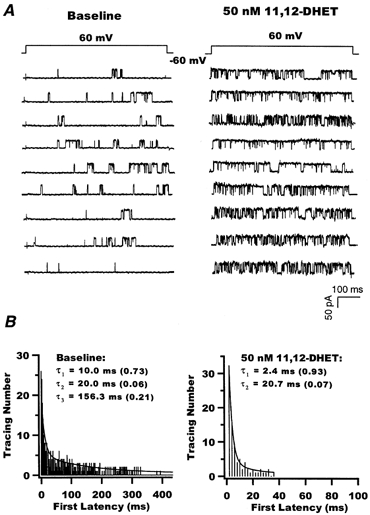
A, raw current tracings of single BK channels elicited at a membrane potential of +60 mV from a holding potential of -60 mV, in the presence of 200 nm Ca2+, before (left) and after application of 50 nm 11,12-DHET (right). B, histograms of BK channel first latency in opening before (left) and after (right) application of 50 nm 11,12-DHET. The data were best fitted using a three-exponential equation for baseline and a two-exponential equation for 11,12-DHET; τ1, τ2 and τ3 represent the time constants derived from curve fitting. The relative weight of each time constant is given in parentheses.
Kinetic analysis of the dose-dependent effects of 11,12-DHET on BK channels
To assess the effects of 11,12-DHET on the kinetic properties of BK channels, membrane patches with only one channel were selected. Figure 7A shows raw current tracings of BK channel activities at +60 mV in the presence of 200 nm Ca2+ and 0, 10 and 100 nm 11,12-DHET. In the absence of DHET, the BK channels showed low levels of opening, as well as a low frequency of fast opening and closing transitions, known as ‘flickerings’ (first and second tracing). In the presence of 11,12-DHET, BK channels opened more frequently and showed more channel flickerings (third to sixth tracings, Fig. 7A). Figure 7B shows the histograms of open and closed dwell time distributions derived from the same experiment as in Fig. 7A. Curve fitting results demonstrated that the distribution of open dwell times consisted of three time constants, designated the fast (τo,1), intermediate (τo,2) and slow (τo,3) components. Likewise, the distribution of closed dwell times consisted of four time constants, designated the fast (τc,1), intermediate (τc,2), slow (τc,3) and very slow (τc,4) components. Group data showing the dose-dependent effects of 11,12-DHET on the open time and closed time constants, as well as their relative weights, from four experiments are summarized in Table 1. From these results, we made the following observations. (1) 11,12-DHET prolonged all three BK channel open time constants, τo,1, τo,2 and τo,3, in a dose-dependent manner, accompanied by an increase in the relative weights of the fast and the intermediate time constants (Ao,1 and Ao,2), but a decrease in that of the slow time constant (Ao,3). (2) 11,12-DHET shortened the fast, intermediate and slow (τc,1, τc,2 and τc,3) components of channel closed times, and increased the relative weight of the fast component (Ac,1), but did not significantly alter those of the intermediate (Ac,2) or the slow components (Ac,3) of the closed time constants. (3) 11,12-DHET dramatically shortened the very slow closed time constant (τc,4) and reduced its relative weight (Ac,4). The net result of the 11,12-DHET effects was very frequent flickerings within bursts of channel openings and the disappearance of the long quiescent periods.
Figure 7. Effects of 11,12-DHET on BK channel kinetics.
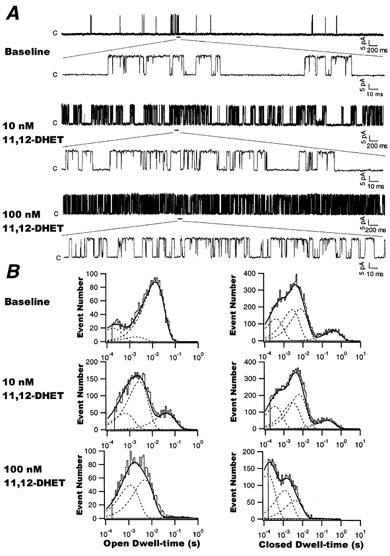
A, recordings showing the effects of various concentrations of 11,12-DHET (0, 10 and 100 nm) on single BK channel activity, at a membrane potential of +60 mV and in the presence of 200 nm cytoplasmic Ca2+. Selected segments of recordings are expanded to illustrate BK channel behaviour in greater detail. B, histograms of BK channel open dwell times (left) and closed dwell times (right) at baseline and in the presence of 10 and 100 nm 11,12-DHET. The dashed lines represent the distributions of the exponential components determined by the likelihood ratio test. The data were fitted as described in Methods.
Table 1.
Effects of 11,12-DHET on the open and closed time constants of BK channels in rat coronary vascular smooth muscle cells
| Control | 1 nM DHET | 10 nM DHET | 100 nM DHET | |
|---|---|---|---|---|
| τo,1(ms) | 0.36 ± 0.02 | 0.50 ± 0.02* | 0.56 ± 0.02* | 0.93 ± 0.02* |
| (Ao,1) | (0.16 ± 0.04) | (0.21 ± 0.05) | (0.33 ± 0.06)* | (0.45 ± 0.15)* |
| τo,2(ms) | 2.06 ± 0.55 | 2.74 ± 0.42 | 3.64 ± 0.40 | 7.30 ± 0.32* |
| (Ao,2) | (0.24 ± 0.10) | (0.50 ± 0.14) | (0.57 ± 0.13) | (0.49 ± 0.14) |
| τo,3(ms) | 10.9 ± 2.5 | 12.6 ± 5.8 | 43.1 ± 9.2* | 126.3 ± 32.4* |
| (Ao,3) | (0.60 ± 0.12) | (0.32 ± 0.16) | (0.16 ± 0.15)* | (0.08 ± 0.06)* |
| τc,1(ms) | 0.48 ± 0.05 | 0.45 ± 0.05 | 0.34 ± 0.07 | 0.20 ± 0.07* |
| (Ac,1) | (0.15 ± 0.03) | (0.16 ± 0.05) | (0.25 ± 0.04)* | (0.32 ± 0.02)* |
| τc,2(ms) | 2.79 ± 0.35 | 2.33 ± 0.51 | 1.70 ± 0.30 | 1.24 ± 0.45 |
| (Ac,2) | (0.31 ± 0.06) | (0.35 ± 0.07) | (0.35 ± 0.05) | (0.38 ± 0.01) |
| τc,3(ms) | 7.69 ± 1.53 | 6.41 ± 1.57 | 6.33 ± 1.52 | 4.56 ± 1.10 |
| (Ac,3) | (0.32 ± 0.04) | (0.28 ± 0.08) | (0.31 ± 0.07) | (0.30 ± 0.08) |
| τc,4(ms) | 495.2 ± 55.1 | 245.2 ± 79.0 | 195.2 ± 73.3* | 76.9 ± 6.7* |
| (Ac,4) | (0.21 ± 0.08) | (0.14 ± 0.07) | (0.10 ± 0.04) | (0.01 ± 0.01) |
Data represent the mean ± S.E.M., n = 4
P < 0.05 vs. control. The time constants (τ) and the relative areas or weights (A, in parentheses) are presented. τo,1, τo,2 and τo,3 represent the fast, intermediate and slow components of the BK channel open times and Ao,1, Ao,2 and Ao,3 represent the relative weights of τo,1, τo,2 and τo,3, respectively, where Ao,1 + Ao,2 + Ao,3 = 1. τc,1, τc,2, τc,3and τc,4 represent the fast, intermediate, slow and very slow components of the BK channel closed times and Ao,1, Ao,2, Ao,3 and Ao,4 represent the relative weights of τc,1, τc,2, τc,3 and τc,4, respectively, where Ac,1 + Ac,2 + Ac,3 + Ac,4 = 1.
Kinetic analysis of the voltage-dependent effects of 11,12-DHET on BK channels
Figure 8A shows recordings of single BK channel currents elicited from membrane potentials of +60, +80 and +100 mV in the presence of 200 nm cytoplasmic Ca2+. These results showed that membrane potential depolarization increased BK channel openings as well as channel flickerings. The baseline kinetic characteristics of BK channels from a typical experiment are shown in Fig. 8B. Again, the open dwell time histograms consisted of three time constant components, and the closed dwell time distributions consisted of four time constant components as designated above. Depolarized membrane potentials were associated with a prolongation of all three open time constants, τo,1, τo,2 and τo,3, and an abbreviation of the closed time constants τc,2, τc,3 and τc,4, but not τc,1. Group data showing these time constants and their corresponding weights in relation to membrane potentials are summarized in Table 2. The following observations were made on the effects of membrane potential depolarization on BK channel kinetics. (1) All three BK channel open time constants, τo,1, τo,2 and τo,3, were prolonged, with a decrease in Ao,1 and a reciprocal increase in Ao,2, while Ao,3 was only slightly reduced. (2) There was little effect on τc,1 or Ac,1. (3) τc,2, τc,3 and τc,4 were all reduced, with a small increase in Ac,3 and a reciprocal decrease in Ac,4, while Ac,2 was not affected by membrane potential over the range studied. These changes indicate that membrane depolarization prolonged BK channel opening durations and shortened the channel closed intervals.
Figure 8. Effects of 11,12-DHET and membrane voltage on the BK channel kinetics.
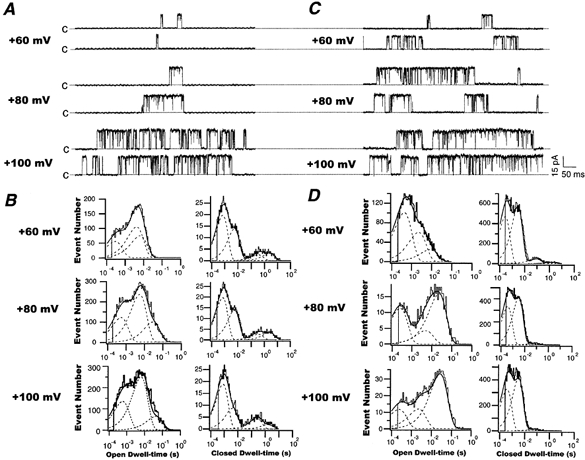
Representative current tracings of single BK channels recorded at +60, +80 and +100 mV with 200 nm cytoplasmic Ca2+ and in the absence (A) or presence (C) of 5 nm 11,12-DHET. B and D, histograms of open dwell times (left) and closed dwell times (right) of BK channels at the membrane potentials shown in A and C, respectively. The dashed lines represent the distributions of the exponential components determined by the likelihood ratio test.
Table 2.
Effects of membrane potential on the open and closed time constants of rat coronary microvessel BK channels in the presence and absence of 5 nM 11,12–DHET
| 40 mV | 80 mV | 120 mV | ||||
|---|---|---|---|---|---|---|
| Time constants(relative weights) | Control | DHET | Control | DHET | Control | DHET |
| τo,1(ms) | 0.22 ± 0.02 | 0.36 ± 0.09 | 0.33 ± 0.03 | 0.87 ± 0.03 * | 0.39 ± 0.04 | 1.25 ± 0.01 * |
| (Ao,1) | (0.54 ± 0.01) | (0.59 ± 0.07) | (0.29 ± 0.06) | (0.30 ± 0.05) | (0.22 ± 0.03) | (0.10 ± 0.05) * |
| τo,2(ms) | 1.87 ± 0.23 | 4.08 ± 0.63 * | 3.10 ± 0.75 | 9.09 ± 1.28 * | 4.50 ± 1.00 | 16.7 ± 2.43 * |
| (Ao,2) | (0.25 ± 0.01) | (0.37 ± 0.10) | (0.45 ± 0.05) | (0.28 ± 0.05) | (0.52 ± 0.07) | (0.15 ± 0.03) * |
| τo,3(ms) | 5.00 ± 1.32 | 22.2 ± 2.4 * | 13.8 ± 6.81 | 33.3 ± 4.7 * | 32.7 ± 10.7 | 50.0 ± 8.8 |
| (Ao,3) | (0.32 ± 0.07) | (0.09 ± 0.06) * | (0.27 ± 0.09) | (0.47 ± 0.07) | (0.19 ± 0.09) | (0.67 ± 0.13) * |
| τc,1(ms) | 0.31 ± 0.00 | 0.50 ± 0.07 | 0.32 ± 0.02 | 0.48 ± 0.02 * | 0.32 ± 0.01 | 0.48 ± 0.01 * |
| (Ac,1) | (0.62 ± 0.11) | (0.40 ± 0.04) | (0.62 ± 0.04) | (0.45 ± 0.08) | (0.63 ± 0.04) | (0.50 ± 0.07) |
| τc,2(ms) | 9.09 ± 1.14 | 5.96 ± 1.60 | 4.35 ± 0.54 | 3.13 ± 0.86 | 3.55 ± 0.79 | 2.33 ± 0.32 |
| (Ac,2) | (0.40 ± 0.01) | (0.56 ± 0.07) | (0.42 ± 0.04) | (0.54 ± 0.07) | (0.38 ± 0.02) | (0.48 ± 0.08) |
| τc,3(ms) | 588 ± 167 | 109 ± 68 | 238 ± 34 | 50.0 ± 25.6 * | 125 ± 4 | 31.3 ± 4.93 * |
| (Ac,3) | (0.06 ± 0.03) | (0.006 ± 0) | (0.09 ± 0.03) | (0.002 ± 0) * | (0.15 ± 0.02) | (0.001 ± 0) * |
| τc,4(ms) | 3500 ± 296 | 2100 ± 550 * | 1520 ± 108 | 1100 ± 342 | 435 ± 50 | 120 ± 24 |
| (Ac,4) | (0.10 ± 0.02) | (0.008 ± 0) * | (0.08 ± 0.02) | (0.003 ± 0) | (0.03 ± 0.00) | (0.0003 ± 0) |
Data are means ± s.e.m., n = 3
P < 0.05 vs. control. The symbols for time constants and relative weights are as described in Table 1.
11,12-DHET (5 nm) enhanced the single BK channel activities at all membrane potentials positive to BK channel activation threshold (Fig. 8C). The histograms of BK channel open and closed dwell times at +60, +80 and +100 mV in the presence of 5 nm 11,12-DHET are shown in Fig. 8D. Group data on the voltage-dependent effects of 11,12-DHET on BK channel kinetic properties from three experiments are summarized in Table 2. Similar to the baseline findings, 11,12-DHET increased all BK channel open time constants and reduced all closed time constants at all membrane potentials studied, except τc,1 at 5 nm 11,12-DHET, which remained unchanged. There are a number of prominent features that should be highlighted. First, in the presence of 5 nm 11,12-DHET, Ao,1, instead of being enhanced by membrane depolarization, was significantly reduced, whereas Ao,3 instead of being reduced by membrane depolarization, was dramatically increased. Second, 5 nm 11,12-DHET significantly reduced τc,2, τc,3 and τc,4 at all membrane voltages compared to baseline. Third, in the presence of 5 nm 11,12-DHET, Ac,3 and Ac,4 contributed to less than 1 % of the total (compared with 3-15 % at baseline, Table 2) and these parameters were further reduced with increases in membrane depolarization; thus, 11,12-DHET virtually eliminated the long closed states. The combined effects of 11,12-DHET and membrane depolarization, however, do not result in increased flickerings but rather in a prolongation of channel openings. These results suggest that the effects of 11,12-DHET on the BK channel voltage dependence kinetics are complex, but the net effect is a prolonged duration of channel openings and shortened periods of channel closings in association with membrane depolarization (Fig. 8C). These results also suggest that 11,12-DHET is an even more effective hyperpolarizing agent at depolarized membrane potentials.
Figure 9 shows the effect of 11,12-DHET on the reciprocals of open dwell time constants (1/τo) plotted against membrane potentials. These relationships could be fitted with a single-exponential equation: 1/τo = k0exp(zδFV/RT), representing the rate of channel transition from the open to the closed state. The voltage-dependent effect on the fast rate constant at the membrane potential of 0 mV, k0,-1, was not altered by 11,12-DHET (Fig. 9A). In contrast, that for the intermediate rate constant, k0,-2, was decreased from 1.12 ms−1 with control to 0.45 ms−1 with 5 nm 11,12-DHET (Fig. 9B). But the most dramatic effect was observed in the slow rate constant, k0,-3, which was decreased from 0.54 to 0.08 ms−1 with 5 nm 11,12-DHET (Fig. 9C). Moreover, the results in Fig. 9 indicate that BK channel activation involves gating charges (zδ) of 0.5-0.63 in the presence of 200 nm Ca2+, and this is similar to that measured in mSlo channels (Cui et al. 1997; Horrigan et al. 1999a,b). However, application of 5 nm 11,12-DHET reduced the gating charge to 0.2 for the long open state transition (Fig. 9C), but did not alter those involving the other opening transitions (Fig. 9A and B). These results suggest that the major effect of 11,12-DHET on the voltage-dependent kinetics of BK channel gating was on the long open state. Through mechanisms of markedly reducing the voltage-dependent transition rate from the long open state to the closed state (small k0) as well as limiting the gating charge movements of such a transition (small zδ), 11,12-DHET prolonged the duration of the BK channel open states.
Figure 9. Effects of 50 nm 11,12-DHET on the open to closed state transition rates of BK channels.
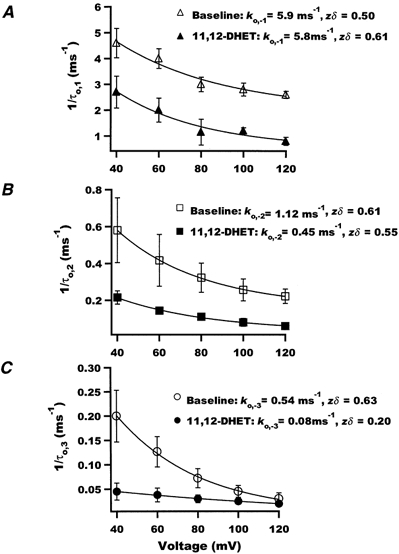
Reciprocals of the fast (A), the intermediate (B) and the slow (C) components of the open time constants were plotted against membrane voltage. Baseline and 5 nm 11,12-DHET conditions are represented by open and filled symbols, respectively. The relationships were fitted using a single-exponential equation to assess the corresponding rate constants (k0,-1, k0,-2 and k0,-3) when the membrane potential was 0 mV. zδ represents the associated gating charges (n = 3).
DISCUSSION
In this study, we have demonstrated that 11,12-DHET potently activated BK channels and hyperpolarized the resting membrane potential in rat coronary arteriole myocytes. In the presence of physiological concentrations of cytoplasmic free Ca2+, 11,12-DHET activated the BK channels with an EC50 in the 10−9m range. Moreover, as a BK channel activator, 11,12-DHET was several times more efficacious than its precursor 11,12-EET, a putative EDHF. 11,12-DHET modulated the BK channel gating behaviour through changing the voltage and Ca2+ dependence of the BK channels, and permitted them to open at more physiological membrane potentials and at reduced cytoplasmic concentrations of Ca2+.
BK channels are present in high densities in vascular smooth muscles and their regulation is critical for maintaining adequate tissue perfusion. BK channels are known to be modulated by various endogenous vasoactive agents (Nelson & Quayle, 1995; Wallner et al. 1999). β-Adrenergic agonists activate BK channels directly through G proteins and indirectly via cAMP-dependent protein kinase stimulation (Kume et al. 1989, 1992; Scornik et al. 1993). Similarly, nitric oxide can activate BK channels directly or through cGMP-dependent phosphorylation (Robertson et al. 1993; Bolotina et al. 1994). The BK channel activators would produce membrane hyperpolarization, inactivating the voltage-sensitive L-type Ca2+ channels, and promoting relaxation of vascular smooth muscle cells (Nelson & Quayle, 1995). In addition, vasorelaxation can also be enhanced by a reduced sensitivity of contractile elements to Ca2+ and attenuation of the mobilization of Ca2+ from intracellular stores (Drummond & Cocks, 1996; Mombouli & Vanhoutte, 1997). Whether DHETs have these properties is not known.
DHETs are metabolites of EETs, which arise from AA through the cytochrome P450 epoxygenase pathway. EETs are well established coronary vasodilators, but their potency depends upon the vessel size, with an EC50 of around 1.0 μm for large conduit vessels (Campbell et al. 1996; Weintraub et al. 1997), and an EC50 in the low picomolar range for resistance microvessels (Oltman et al. 1998). EETs hyperpolarize most of the coronary smooth muscle cells studied to date and thus may be EDHFs (Quilley & McGiff, 2000). When injected intravascularly, the EETs are rapidly converted in vivo to DHETs (Catella et al. 1990). In in vitro studies, EETs are also converted rapidly by vascular endothelial and smooth muscle cell epoxide hydrolases to DHETs (VanRollins et al. 1993; Fang et al. 1995). Studies in cultured vascular endothelial and smooth muscle cells also indicate that EETs and DHETs are both incorporated into cellular phospholipids (VanRollins et al. 1993, 1996; Fang et al. 1996; Weintraub et al. 1997). Once incorporated, the EETs and DHETs can be rapidly released from cultured endothelial cells in response to the Ca2+ ionophore A23187 (Weintraub et al. 1999) and bradykinin (M. VanRollins, E. J. Yoder, F. M. Faraci & S. A. Moore, unpublished data). Like EETs, DHETs are potent coronary vasodilators, and their EC50 decreases from micromolar to picomolar values with decreasing vessel size (Weintraub et al. 1997; Oltman et al. 1998). In conduit coronary vessels, DHETs were at best equipotent with their EET precursors. But in coronary microvessels, at least one DHET regioisomer (11,12-DHET) was significantly more potent than its precursor EET (Oltman et al. 1998).
In the present study, we delineated an ionic mechanism for DHET-induced vasodilatation. We demonstrated that 11,12-DHET activates BK channels with an EC50 of 1.87 ± 0.57 nm in rat coronary smooth muscle cells (150-300 μm i.d.) in the presence of 200 nm Ca2+. With 1 μm Ca2+, the 11,12-DHET EC50 is reduced to 0.93 ± 0.18 nm. Thus, DHETs may become even more potent vasodilators under conditions of intracellular Ca2+ overload, such as during ischaemia, acidosis and anoxia. Moreover, 11,12-DHET is more efficacious than the precursor 11,12-EET as a BK channel activator, consistent with the effects of these metabolites on canine coronary arteriole vasorelaxation (Oltman et al. 1998). In addition, 5,6-, 8,9- and 14,15-DHET are as effective as 11,12-DHET in activating BK channels. The inability of AA to activate the BK channels indicates that the effects of the DHETs are not due to a non-specific fatty acid effect.
Since 11,12-DHET could further activate BK channels after saturating effects of 11,12-EET have been reached, these two important endogenous vasoactive metabolites probably have different sites of action. Although the concentration of DHETs in blood or in the vessel wall is unknown, it has been reported that activation of phospholipase A2 could lead to the release of micromolar concentrations of EETs from human platelets (Zhu et al. 1995). EETs are converted rapidly by vascular endothelial and smooth muscle cells to DHETs (VanRollins et al. 1993; Fang et al. 1995), which are chemically more stable than EETs. Also, DHETs are still present in the plasma 60 min after EETs are injected intravenously (Catella et al. 1990). Hence, a nanomolar EC50 for DHETs to activate BK channels should be a highly relevant physiological finding.
We showed that DHETs activate BK channels in inside-out patches, but we have not examined the effects of DHETs on BK channels through activation of cellular second messenger systems and other signal transduction pathways. Several laboratories reported that activation of vascular BK channels by EET only occurred in cell-attached but not in inside-out patch preparations, so additional cytosolic factors may be necessary for mediating the EET effects (Hu & Kim, 1993; Li & Campbell, 1997; Hayabuchi et al. 1998). Li & Campbell (1997) suggested that EET activation of BK channels in vascular smooth muscle myocytes can be mediated through Gsα. Xiao et al. (1998) also reported that EETs enhanced the L-type Ca2+ channel activity in ventricular myocytes through enhancement of cytosolic cAMP levels. However, EETs have been reported to open BK channels in inside-out patches from cultured vascular endothelial cells (Baron et al. 1997), pituitary GH3 cells (Wu et al. 2000) and reconstituted bovine airway smooth muscle (Dumoulin et al. 1998). These findings demonstrate that EETs could directly interact with BK channels. In the present study, the activation of BK channels by 11,12-EET and by the DHETs indicated a similar direct action in inside-out patches from rat coronary smooth muscle. It is likely that the mechanism through which DHETs directly activate the vascular BK channels is quite different from that of EETs, as indicated by the ability of DHETs to enhance BK channel opening probability above and beyond that attained by EETs.
Despite an EC50 in the 10−9m range, there is a 1000-fold difference in 11,12-DHET potency between its effects on BK channels in this study and those on dog coronary arteriole dilatation (Oltman et al. 1998). This discrepancy raises the possibility that DHET activation of BK channels may involve other cellular factors, but it could also be due to differences in the species studied, size of vessels used and the temperatures of the experiments. In our preparations, 11,12-EET activated BK channels in inside-out patches, and we could not identify a GTP requirement for such channel activation. We suspect the lack of effect of EET in inside-out patches reported by Hu & Kim (1993) was due to the low Ca2+ concentrations (10−8m) used in their experiments, as we did not observe EET effects at that Ca2+ concentration in our preparations. The reason for the discrepancy between our results and those reported by Li & Campbell (1997) and Hayabuchi et al. (1998) is not immediately clear. It could be due to variability in the expression of different BK channel isoforms and accessory unit coupling, since there seem to be substantial differences in the voltage dependence of the BK channels depending upon the species and tissue source. While definitive delineation of the disparity will require further experiments, it will not change the major conclusions of our findings. In summary, our results indicate that DHETs hyperpolarize vascular smooth muscle cells by direct and potent activation of BK channels through modulation of channel gating behaviour.
At least two mechanisms underlie BK channel activation by the DHETs. In the first mechanism, 11,12-DHET enhances BK channel sensitivity to cytoplasmic Ca2+, based on the following observations. First, 11,12-DHET required at least some Ca2+ for BK channel activation. Second, 50 nm 11,12-DHET shifted the Ca2+ dose-response curve of BK channel activation leftward and upward. This shift resulted in a reduction in the Ca2+ EC50 by more than 50 %, and increased the maximum opening probability of BK channels at saturating Ca2+ effects. Moreover, 11,12-DHET increased the channel opening probability at all Ca2+ concentrations tested. In particular, in the presence of a physiological level of 200 nm Ca2+, 50 nm 11,12-DHET increased the BK channel Po 6-fold. Third, 5 nm 11,12-DHET reduced the Ca0 by 43 %, allowing the BK channels to be more sensitive to Ca2+ activation. 11,12-DHET only altered the intercept but not the slope of the V½-log[Ca2+] relationship (Fig. 5C), suggesting that the DHET affected only the Ca0, and not the rate of channel activation by Ca2+.
Reciprocal modulation also occurs, such that Ca2+ enhances the effects of 11,12-DHET on BK channels. The EC50 of 11,12-DHET in the presence of 1 μm Ca2+ was 50 % lower than that in the presence of 200 nm Ca2+. A synergistic relationship between 11,12-DHET and Ca2+ in the activation of BK channels is likely: the Po,max in the presence of either Ca2+ or 11,12-DHET alone could be further increased when the other was added (Fig. 2 and Fig. 5). While the binding site for DHETs (or EETs) on the BK channel is unknown, the C-terminus of the BK channel contains a domain for Ca2+ sensing, the so-called ‘calcium bowl’, with many negatively charged residues that are highly conserved (Wei et al. 1994; Schreiber & Salkoff, 1997). It is conceivable that the carboxy moiety of the DHET increases the negativity of the bowl or that the Ca2+ binding site(s) could be allosterically modulated by 11,12-DHET. Our observation that 11,12-DHET could increase the maximum opening probability of BK channels at saturating Ca2+ effects suggests that the DHETs probably do not bind directly to the Ca2+ binding site. It is more likely that DHETs facilitate the recruitment of dormant channels or augment the effects of Ca2+ binding on BK channel gating, resulting in enhanced opening of activated channels.
A second mechanism explaining BK channel activation is that 11,12-DHET modulates the voltage-dependent activation of the channel. First, the absolute channel opening probability was enhanced by 11,12-DHET at all channel-activating potentials. Second, the voltage-dependent modulation effects were dependent on DHET concentration, and were sensitive to nanomolar concentrations of 11,12-DHET. For example, 5 and 50 nm 11,12-DHET shifted the V½ of the BK channel by -20 and -55 mV, respectively. Third, 11,12-DHET lowered the voltage threshold for BK channel activation, allowing the channels to open towards physiological resting potentials (Fig. 4). Fourth, 11,12-DHET reduced the first latency of channel opening not only by increasing the transition rates, but also by reducing the number of channel transitions from closed to open states (Fig. 6). In summary, 11,12-DHET makes the BK channels much more sensitive to voltage activation, and allows them to open expediently and at much less depolarized membrane potentials.
In addition, single channel kinetic analysis demonstrated that 11,12-DHET modulates BK channel activity by altering gating behaviour in both dose- and voltage-dependent manners. Increases in 11,12-DHET concentration and membrane depolarization promote both the prolongation of BK channel open times and the abbreviation of the channel closed times, resulting in an enhancement of channel activity. 11,12-DHET dose-dependently increases the weights of the fast components of both channel open and closed time constants, leading to increased channel flickerings (Fig. 7 and Table 1). Also, 11,12-DHET markedly reduces the very slow closed time constant and its weight, leading to a reduction and virtual elimination of the long closed states. However, the effects of 11,12-DHET on the weight of each time constant were very complicated in the dose- and voltage-dependent experiments. In independent experiments, increasing the 11,12-DHET concentrations or membrane depolarizations alone would lead to a progressive increase in Ao,2 and/or Ao,1 with a reciprocal reduction in Ao,3. However, in the presence of 5 nm 11,12-DHET, an increase in membrane depolarization instead would increase Ao,3 and decrease Ao,1 and Ao,2, suggesting that the voltage-dependent and DHET-dependent activation of the BK channels impart different effects on channel gating mechanisms. The net result of the 11,12-DHET effects is a prolongation of BK channel openings with elimination of long periods of channel quiescence. Our findings also suggest that 11,12-DHET would markedly and voltage-dependently slow the exit of the BK channel from the long open state, and less markedly from the intermediate open state as well (Fig. 9).
The molecular mechanism mediating the DHET effects is not known. DHETs may directly interact with the voltage sensor and the Ca2+-sensitive regions (Stefani et al. 1997), or with the β subunit of BK channels (McManus et al. 1995; Tanaka et al. 1997). As part of the ionic filter, the residues of TXXTXGYGD in the K+ channel pore region are conserved among all K+ channels (Heginbotham et al. 1994). Mutations in the pore region, such as the Shaker T442S mutant, exhibit greatly prolonged open dwell times and channel activation is shifted towards negative voltages (Yool & Schwarz, 1991). These features are similar to those observed with the DHET effects on the BK channel. The β subunit is also important in mediating the BK channel sensitivity to cytosolic Ca2+. It is possible that DHET modulates the BK channel through its effects on one or more of these sites. Site-directed mutagenesis and channel binding experiments are needed to clarify these issues.
In conclusion, we have provided compelling evidence that DHETs hyperpolarize vascular smooth muscle cells by potently modulating the pharmacological, electrophysiological and kinetic properties of vascular BK channels. While the chemical identity of EDHF remains in question and multiple EDHFs are probably present, DHETs should be considered prime candidates for EDHF in coronary arterioles.
Acknowledgments
We thank Beena Padanilam and Papri Chatterjee for technical assistance. This work was supported by a Merit Review Award from the Department of Veterans Affairs, a RO1 Grant (HL63754) from the National Institute of Health, a Grant-in-Aid award from the American Heart Association Heartland Affiliates (0051311Z), and a Program Project Grant (HL-49264) from NIH. M.V. is supported by the American Heart Association (Grant-in-Aid, 96012380) and by the NIH (RO1 HL-56670). N.L.W. is a Clinician-Scientist awardee of the American Heart Association.
References
- Anwer K, Oberti C, Perez GJ, Perez-Reyes N, McDougall JK, Monga M, Sanborn BM, Stefani E, Toro L. Calcium-activated K+ channels as modulators of human myometrial contractile activity. American Journal of Physiology. 1993;265:C976–985. doi: 10.1152/ajpcell.1993.265.4.C976. [DOI] [PubMed] [Google Scholar]
- Baron A, Frieden M, Beny JL. Epoxyeicosatrienoic acids activate a high-conductance, Ca2+-dependent K+ channel on pig coronary artery endothelial cells. Journal of Physiology. 1997;504:537–543. doi: 10.1111/j.1469-7793.1997.537bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Bolton TB, Imaizumi Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium. 1996;20:141–152. doi: 10.1016/s0143-4160(96)90103-7. [DOI] [PubMed] [Google Scholar]
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Bychkov R, Gollasch M, Reid C, Luft FC, Haller H. Regulation of spontaneous transient outward potassium currents in human coronary arteries. Circulation. 1997;95:503–510. doi: 10.1161/01.cir.95.2.503. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circulation Research. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Harder DR. Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circulation Research. 1999;84:484–488. doi: 10.1161/01.res.84.4.484. [DOI] [PubMed] [Google Scholar]
- Carl A, Lee HK, Sanders KM. Regulation of ion channels in smooth muscles by calcium. American Journal of Physiology. 1996;271:C9–34. doi: 10.1152/ajpcell.1996.271.1.C9. [DOI] [PubMed] [Google Scholar]
- Carrier GO, Fuchs LC, Winecoff AP, Giulumian AD, White RE. Nitrovasodilators relax mesenteric microvessels by cGMP-induced stimulation of Ca-activated K channels. American Journal of Physiology. 1997;273:H76–84. doi: 10.1152/ajpheart.1997.273.1.H76. [DOI] [PubMed] [Google Scholar]
- Catella F, Lawson JA, Fitzgerald DJ, Fitzgerald GA. Endogenous biosynthesis of arachidonic acid epoxides in humans: Increased in pregnancy-induced hypertension. Proceedings of the National Academy of Sciences of the USA. 1990;87:5893–5897. doi: 10.1073/pnas.87.15.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Cox DH, Aldrich RW. Intrinsic voltage dependence and Ca2+ regulation of mSlo large conductance Ca2+-activated K+ channels. Journal of General Physiology. 1997;109:647–673. doi: 10.1085/jgp.109.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin M, Salvail D, Gaudreault SB, Cadieux A, Rousseau E. Epoxyeicosatrienoic acids relax airway smooth muscles and directly activate reconstituted KCa channels. American Journal of Physiology. 1998;275:L423–431. doi: 10.1152/ajplung.1998.275.3.L423. [DOI] [PubMed] [Google Scholar]
- Drummond GR, Cocks TM. Evidence for mediation by endothelium-derived hyperpolarizing factor of relaxation to bradykinin in the bovine isolated coronary artery independently of voltage-operated Ca2+ channels. British Journal of Pharmacology. 1996;117:1035–1040. doi: 10.1111/j.1476-5381.1996.tb16693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Kaduce TL, Weintraub NL, VanRollins M, Spector AA. Functional implications of a newly-characterized pathway of 11,12-epoxyeicosatrienoic acid metabolism in arterial smooth muscle. Circulation Research. 1996;79:784–793. doi: 10.1161/01.res.79.4.784. [DOI] [PubMed] [Google Scholar]
- Fang X, VanRollins M, Kaduce TL, Spector AA. Epoxyeicosatrienoic acid metabolism in arterial smooth muscle cells. Journal of Lipid Research. 1995;36:1236–1246. [PubMed] [Google Scholar]
- Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–496. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick FA, Murphy RC. Cytochrome P-450 metabolism of arachidonic acid: formation and biological actions of “epoxygenase”-derived eicosanoids. Pharmacological Reviews. 1988;40:229–241. [PubMed] [Google Scholar]
- Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. American Journal of Physiology. 1992;263:H519–525. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- Hayabuchi Y, Nakaya Y, Matsuoka S, Kuroda Y. Endothelium-derived hyperpolarizing factor activates Ca2+-activated K+ channels in porcine coronary artery smooth muscle cells. Journal of Cardiovascular Pharmacology. 1998;32:642–649. doi: 10.1097/00005344-199810000-00018. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakman B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hecker M, Bara AT, Bauersachs J, Busse R. Characterization of endothelium-derived hyperpolarizing factor as a cytochrome P450-derived arachidonic acid metabolite in mammals. Journal of Physiology. 1994;481:407–414. doi: 10.1113/jphysiol.1994.sp020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+ channel signature sequence. Biophysical Journal. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. American Journal of Physiology. 1997;273:C110–117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- Horrigan FT, Aldrich RW. Allosteric voltage gating of potassium channels II: mSlo channel gating charge movement in the absence of Ca2+ Journal of General Physiology. 1999a;114:305–336. doi: 10.1085/jgp.114.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan FT, Cui J, Aldrich RW. Allosteric voltage gating of potassium channels I: mSlo ionic currents in the absence of Ca2+ Journal of General Physiology. 1999b;114:277–304. doi: 10.1085/jgp.114.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Kim HS. Activation of K+ channel in vascular smooth muscles by cytochrome P450 metabolites of arachidonic acid. European Journal of Pharmacology. 1993;230:215–221. doi: 10.1016/0014-2999(93)90805-r. [DOI] [PubMed] [Google Scholar]
- Kume H, Graziano MP, Kotlikoff MI. Stimulatory and inhibitory regulation of calcium-activated potassium channels by guanine nucleotide-binding proteins. Proceedings of the National Academy of Sciences of the USA. 1992;89:11051–11055. doi: 10.1073/pnas.89.22.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H, Takai A, Tokuno H, Tomita T. Regulation of Ca2+-dependent K+ channel activity in tracheal myocytes by phosphorylation. Nature. 1989;341:152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Latorre R, Oberhauser A, Labarca P, Alvarez O. Varieties of calcium-activated potassium channels. Annual Review of Physiology. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Li P, Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circulation Research. 1997;80:877–884. doi: 10.1161/01.res.80.6.877. [DOI] [PubMed] [Google Scholar]
- Li P, Zou A, Campbell WB. Regulation of potassium channels in coronary arterial smooth muscle by endothelium-derived vasodilators. Hypertension. 1997;29:262–267. doi: 10.1161/01.hyp.29.1.262. [DOI] [PubMed] [Google Scholar]
- McGiff JC. Cytochrome P-450 metabolism of arachidonic acid. Annual Review of Pharmacology and Toxicology. 1991;31:339–369. doi: 10.1146/annurev.pa.31.040191.002011. [DOI] [PubMed] [Google Scholar]
- McManus OB. Calcium-activated potassium channels: regulation by calcium. Journal of Bioenergetics and Biomembranes. 1991;23:537–560. doi: 10.1007/BF00785810. [DOI] [PubMed] [Google Scholar]
- McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the β subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- Miller AW, Katakam PVG, Ujhelyi MR. Impaired endothelium-mediated relaxation in coronary arteries from insulin-resistant rats. Journal of Vascular Research. 1999;36:385–392. doi: 10.1159/000025678. [DOI] [PubMed] [Google Scholar]
- Miura H, Gutterman DD. Human coronary arteriolar dilation to arachidonic acid depends on cytochrome P-450 monooxygenase and Ca2+-activated K+ channels. Circulation Research. 1998;83:501–507. doi: 10.1161/01.res.83.5.501. [DOI] [PubMed] [Google Scholar]
- Mombouli JV, Vanhoutte PM. Endothelium-derived hyperpolarizing factor(s): updating the unknown. Trends in Pharmacological Sciences. 1997;18:252–256. [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. American Journal of Physiology. 1995;268:C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Oliw EH. Oxygenation of polyunsaturated fatty acids by cytochrome P450 monooxygenases. Progress in Lipid Research. 1994;33:329–354. doi: 10.1016/0163-7827(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circulation Research. 1998;83:932–939. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- Quilley J, McGiff JC. Is EDHF an epoxyeicosatrienoic acid? Trends in Pharmacological Sciences. 2000;21:121–124. doi: 10.1016/s0165-6147(00)01445-0. [DOI] [PubMed] [Google Scholar]
- Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca2+ -activated K+ channels in cerebral artery smooth muscle cells. American Journal of Physiology. 1993;265:C299–303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophysical Journal. 1997;73:1355–1363. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scornik FS, Codina J, Birnbaumer L, Toro L. Modulation of coronary smooth muscle KCa channels by Gsα independent of phosphorylation by protein kinase A. American Journal of Physiology. 1993;265:C708–713. doi: 10.1152/ajpheart.1993.265.4.H1460. [DOI] [PubMed] [Google Scholar]
- Stefani E, Ottolia M, Noceti F, Olcese R, Wallner M, Latorre R, Toro L. Voltage-controlled gating in a large conductance Ca2+-sensitive K+ channel (hslo) Proceedings of the National Academy of Sciences of the USA. 1997;94:5427–5431. doi: 10.1073/pnas.94.10.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Meera P, Song M, Knaus H, Toro L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant α+β subunit complexes. Journal of Physiology. 1997;502:545–557. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRollins M, Kaduce TL, Fang X, Knapp HR, Spector AA. Arachidonic acid diols produced by cytochrome P-450 monooxygenases are incorporated into phospholipids of vascular endothelial cells. Journal of Biological Chemistry. 1996;271:14001–14009. doi: 10.1074/jbc.271.24.14001. [DOI] [PubMed] [Google Scholar]
- VanRollins M, Kaduce TL, Knapp HR, Spector AA. 14,15-epoxyeicosatrienoic acid metabolism in endothelial cells. Journal of Lipid Research. 1993;34:1931–1942. [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Current Opinion in Neurobiology. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L. Calcium-activated potassium channels in muscle and brain. Current Topics in Membranes. 1999;46:117–140. [Google Scholar]
- Wei A, Solaro C, Lingle C, Salkoff L. Calcium sensitivity of BK-type KCa channels determined by a separable domain. Neuron. 1994;13:671–681. doi: 10.1016/0896-6273(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Weintraub NL, Fang X, Kaduce TL, VanRollins M, Chatterjee P, Spector AA. Potentiation of endothelium-dependent relaxation by epoxyeicosatrienoic acids. Circulation Research. 1997;81:258–267. doi: 10.1161/01.res.81.2.258. [DOI] [PubMed] [Google Scholar]
- Weintraub NL, Fang X, Kaduce TL, VanRollins M, Chatterjee P, Spector AA. Epoxide hydrolases regulate epoxyeicosatrienoic acid incorporation into coronary endothelial phospholipids. American Journal Physiology. 1999;277:H2098–2108. doi: 10.1152/ajpheart.1999.277.5.H2098. [DOI] [PubMed] [Google Scholar]
- Wu S-N, Li H-F, Chiang H-T. Actions of epoxyeicosatrienoic acid on large-conductance Ca2+-activated K+ channels in pituitary GH3 cells. Biochemical Pharmacology. 2000;60:251–262. doi: 10.1016/s0006-2952(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Xiao Y-F, Huang L, Morgan JP. Cytochrome P450: a novel system modulating Ca2+ channels and contraction in mammalian heart cells. Journal of Physiology. 1998;508:777–792. doi: 10.1111/j.1469-7793.1998.777bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yool AJ, Schwarz TL. Alteration of ionic selectivity of a K+ channel by mutation of the H5 region. Nature. 1991;349:700–704. doi: 10.1038/349700a0. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Schieber B, Mcgiff JC, Balazy M. Identification of arachidonate P-450 metabolites in human platelet phospholipids. Hypertension. 1995;25:854–859. doi: 10.1161/01.hyp.25.4.854. [DOI] [PubMed] [Google Scholar]
- Zou A, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K+-channel activity. American Journal of Physiology. 1996;270:F822–832. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]


