Abstract
A hyperpolarization-activated non-specific cation current, Ih, was examined in bushy cell bodies and their giant presynaptic terminals (calyx of Held). Whole-cell patch clamp recordings were made using an in vitro brain slice preparation of the cochlear nucleus and the superior olivary complex. The aim was to characterise Ih in identified cell bodies and synaptic terminals, to examine modulation by presynaptic cAMP and to test for modulatory effects of Ih activation on synaptic transmission.
Presynaptic Ih was activated by hyperpolarizing voltage-steps, with half-activation (V1/2) at –94 mV. Activation time constants were voltage dependent, showing an e-fold acceleration for hyperpolarizations of –32 mV (time constant of 78 ms at –130 mV). The reversal potential of Ih was –29 mV. It was blocked by external perfusion of 1 mm CsCl but was unaffected by BaCl2.
Application of internal cAMP shifted the activation curve to more positive potentials, giving a V1/2 of –74 mV; hence around half of the current was activated at resting membrane potentials. This shift in half-activation was mimicked by external perfusion of a membrane-permeant analogue, 8-bromo-cAMP.
The bushy cell body Ih showed similar properties to those of the synaptic terminal; V1/2 was –94 mV and the reversal potential was –33 mV. Somatic Ih was blocked by CsCl (1 mm) and was partially sensitive to BaCl2. Somatic Ih current density increased with postnatal age from 5 to 16 days old, suggesting that Ih is functionally relevant during maturation of the auditory pathway.
The function of Ih in regulating presynaptic excitability is subtle. Ih had little influence on EPSC amplitude at the calyx of Held, but may be associated with propagation of the action potential at branch points. Presynaptic Ih shares properties with both HCN1 and HCN2 recombinant channel subunits, in that it gates relatively rapidly and is modulated by internal cAMP.
Hyperpolarization-activated non-specific cation currents (Ih) are mixed Na+/K+ conductances which are thought to play a variety of roles in regulating excitability, oscillatory behaviour and integration. They are expressed in muscle (Ludwig et al. 1999; Okabe et al. 1999), photoreceptors (Bader et al. 1982) and neurones (Pape, 1996; Santoro et al. 2000). Ih is involved in pacemaker activity in the heart (Brown & DiFrancesco, 1980; DiFrancesco & Tromba, 1988; DiFrancesco, 1993), and influences oscillatory behaviour in the brain, for instance in thalamic neurones (McCormick & Pape, 1990a,b) and inferior olivary neurones (Bal & McCormick, 1997). Ih influences resting membrane potentials (Maccaferri et al. 1993), triggers rebound action potentials (Crepel & Penit-Soria, 1986), modulates activity following inhibition (Luo & Perkel, 1999) and contributes to resting conductance, oscillatory firing (Pape, 1996; Luthi & McCormick, 1998) and synaptic integration in dendrites (Magee, 1998, 1999).
A family of four hyperpolarization-activated cyclic nucleotide-sensitive cation channels (HCN1-4) have been cloned which give rise to Ih (Santoro et al. 1997; Gauss et al. 1998; Ludwig et al. 1998; Seifert et al. 1999; Ishi et al. 1999). This channel family share significant sequence homology with ether-à-go-go (EAG) potassium channels and possess a cyclic nucleotide binding site (Ludwig et al. 1998; Gauss et al. 1998) which modulates channel activation. Ih channels open relatively slowly on hyperpolarization and do not inactivate (Clapham, 1998).
Immunohistochemical labelling of mHCN1 (Santoro et al. 1997) and patch-clamp recording from the axon terminals of cerebellar basket cells (Southan et al. 2000) demonstrate that Ih is present at this inhibitory synaptic terminal. A hyperpolarization-activated conductance has also been observed in the giant cholinergic terminal of the avian ciliary ganglion (Fletcher & Chiapenelli, 1992) and presynaptic Ih is present in the crayfish neuromuscular junction, where its activation is linked to an enhancement of synaptic transmission (Beaumont & Zucker, 2000). Our aim here is to characterise an Ih from a mammalian excitatory synaptic terminal, to compare it with that recorded from bushy cell bodies, from which the terminals arise, and to examine its possible function. We have developed methods for patch-clamp recording from a giant excitatory synapse in the superior olivary complex (Forsythe, 1994). This synapse was first documented by Held (1893) and is known as the calyx of Held. The calyx arises from axons of globular bushy cells in the anterior ventral cochlear nucleus (aVCN, Tolbert et al. 1982; Friauf & Ostwald, 1988) and forms around the soma of principal neurones in the medial nucleus of the trapezoid body (MNTB; Forsythe 1994). This relay forms part of the binaural auditory pathways concerned with sound source localisation (Oertel, 1999) and transmission is mediated by glutamate receptors (Forsythe & Barnes-Davies, 1993a,b; Barnes-Davies & Forsythe 1995). We have used this preparation both as a general model for transmission at central fast excitatory synapses and to study synaptic transmission in the brainstem auditory pathway.
Our results demonstrate that Ih is expressed at the bushy cell soma and in the giant synaptic terminals of the calyx of Held. Ih appears not to be closely linked with synaptic efficacy but may play a role in regulating the resting presynaptic conductance. Preliminary accounts of part of this work have been published in abstract form (Rusznák et al. 1996; Owens et al. 1999).
METHODS
Lister Hooded rats were killed by decapitation and brain slices of the ventral cochlear nucleus (VCN) or the superior olivary complex (SOC) were prepared as described previously (Barnes-Davies & Forsythe, 1995, Rusznák et al. 1997). Brief details are as follows.
VCN brain slices were prepared from 5- to 14-day-old rats. Sagittal sections of the brainstem were cut (200 μm thick) in a low sodium artificial cerebrospinal fluid (ACSF) and at a temperature of about 0 °C, the slices were immediately transferred to an incubation chamber containing normal ACSF bubbled with 95 % O2-5 % CO2 and maintained at 37 °C for 1 h. Transverse SOC slices (100-150 μm thick) containing the medial nucleus of the trapezoid body (MNTB) were prepared using the same methods from 9- to 16-day-old rats. Data on presynaptic Ih were obtained mainly from 9- to 12-day-old animals, with an additional data set from one 16-day old. Following incubation at 37 °C for 1 h, the chamber and slices were allowed to passively cool to room temperature (18-23 °C). The composition of the normal ACSF was (mm): NaCl, 125.0; KCl, 2.5; NaHCO3, 26.0; glucose, 10.0; NaH2PO4, 1.25; sodium pyruvate, 2.0; myo-inositol, 3.0; CaCl2, 2.0; MgCl2, 1.0; ascorbic acid, 0.5 (pH 7.4 when saturated with 95 % O2-5 % CO2). For the low-Na+ ACSF, 250 mm sucrose was substituted for NaCl, and CaCl2 and MgCl2 concentrations were changed to 1 and 2 mm, respectively.
Whole-cell voltage clamp recordings from presynaptic terminals and postsynaptic neurones in the MNTB were made using an Axopatch 200A (Axon Instruments, USA) amplifier; some of the aVCN recordings were made using an EPC7 amplifier (List). Neurones and terminals were visualised using Nomarski optics on an Axioskop FS microscope (Zeiss, Germany or M2A microscope, MicroInstruments) with water immersion objectives (Zeiss × 63, 0.7 NA or Nikon × 40, 0.55 NA). Whole-cell pipettes had input resistances of 4-8 MΩ when filled with (mm): potassium gluconate, 100; KCl, 33; MgCl2, 1; Hepes, 10; EGTA, 5 (pH to 7.2 with KOH) or KCl, 130; Hepes, 10; MgCl2, 1; EGTA, 5. Series resistances varied from 4 to 33 MΩ and were compensated by 60-90 %. Bushy cells in the aVCN were identified using the following criteria: soma diameter (15-20 μm); their location close to the entry point and between fibre bundles of the acoustic nerve; intracellular filling with Lucifer Yellow (Friauf & Ostwald, 1988); and in some cases by current clamp recording indicating a type II action potential firing response (Manis & Marx, 1991). It was not possible to distinguish between spherical and globular bushy cells, but since all the recordings showed Ih, this does not effect our conclusions. EPSCs were evoked by stimulation of the trapezoid body fibre with a bipolar stimulating electrode positioned at the mid-line. MNTB neurones were voltage clamped at a holding potential (HP) of -60 mV and trains of supra-threshold stimuli delivered at 50 Hz for 2 s. Whole-cell patch recordings from MNTB neurones were made with patch solutions containing (mm): potassium gluconate, 97.5; KCl, 32.5; EGTA, 5; Hepes, 10; MgCl2, 1. In additional synaptic experiments (n = 4) the postsynaptic patch solution had CsCl substituted for potassium gluconate.
Experiments were performed in normal ACSF at 26 °C, which was regulated by feedback control of a Peltier device. Voltage commands, acquisition and analysis were performed using a Digidata 1200 interface, the pCLAMP 6.03 software suite (Axon Instruments) and a data acquisition and analysis program developed by Dr N. W. Davies using AxoBasic software (Axon Instruments) running on a PC. Activation curves were constructed from tail current amplitudes and fitted with a Boltzmann function:
| (1) |
where I is the current, Imax is the maximal current, V is the potential, V1/2 is the half-activation voltage, and s is the slope factor. Data were sampled at 2-13 kHz and filtered at the appropriate Nyquist limit (1-5 kHz). Data are presented as means ±s.e.m. Antagonists were applied by perfusion in the ACSF. Most chemicals and drugs were supplied by Sigma UK. 2-Amino-5-phosphovalerate (d,l-AP5), MK801 and ZD7288 were supplied by Tocris.
RESULTS
Presynaptic Ih
Whole-cell voltage-clamp recordings were made from 41 identified presynaptic terminals in the MNTB. All terminals possessed a slowly activating current upon hyperpolarization steps from a holding potential of -60 or -70 mV (Fig. 1A and B). The maximum conductance ranged from 2.1 to 10.0 nS.
Figure 1. A hyperpolarization-activated inward current is present at presynaptic nerve terminals.
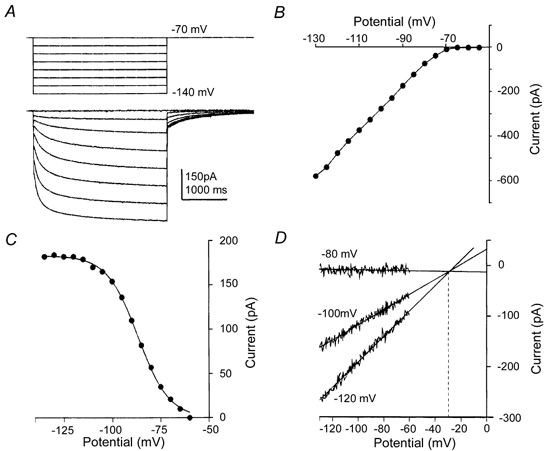
A, hyperpolarizing voltage steps (above) from a holding potential (HP) of -70 mV revealed a slowly activating and non-inactivating inward current (lower traces) which deactivated slowly on return to the HP. B, a plot of the leak-subtracted current-voltage relationship from a terminal shows activation on hyperpolarizations negative to -65 mV. C, the activation curve is plotted for another terminal. The tail current magnitude (measured on return to a HP of -60 mV) is plotted against the prior command voltage (applied for 3 s). The continuous line is the fit to a Boltzmann distribution with half-activation of -93 mV and slope of 8.0 mV. D, the reversal potential was estimated by extrapolation of 10 ms voltage ramps applied over a range of holding potentials straddling the activation voltage (-80, -100 and -120 mV). The reversal potential is indicated by linear regression of the three leak subtracted voltage ramps (continuous lines) and was found to be -30 mV (dotted line). The characteristics of this current are consistent with the conclusion that it is the hyperpolarization-activated cation current, Ih.
The voltage dependence of activation was measured as the tail current magnitude (recorded at -70 mV) following voltage step commands over a voltage range of -140 to -50 mV (Fig. 1C). In seven terminals the activation curve was fitted by a Boltzmann distribution which gave a mean half-activation voltage of -94.4 ± 1.7 mV and a slope factor of 8.4 mV. Similar observations from the postsynaptic neurones in the medial nucleus of the trapezoid body (MNTB) also displayed a hyperpolarization-activated current (n = 4; half-activation, 91.8 ± 1.1 mV; slope factor, 8.4 mV; data not shown), similar to that reported previously (Banks et al. 1993).
The reversal potential for the presynaptic current was estimated using fast (10 ms) ramps generated from a range of holding potentials that extended across the activation range (-80 to -140 mV). Leak subtraction, followed by linear regression of multiple I-V relationships recorded from three different holding potentials intersected at the reversal potential of the current. Direct measurement of the reversal potential was not possible since voltage-gated potassium currents activated at potentials positive to -60 mV (Fig. 1D). The mean reversal potential was -30 ± 4 mV (n = 7). The current proved to be insensitive to 1 mm barium chloride (100 ± 4 % of control, n = 4) but was blocked by extracellular perfusion of 1 mm caesium chloride (5 ± 2 % of control, n = 4, Fig. 2).
Figure 2. The presynaptic Ih is caesium sensitive and barium insensitive.
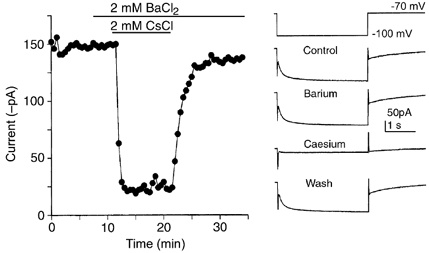
A hyperpolarizing voltage step from -70 to -120 mV lasting 3 s was applied to a presynaptic terminal every 30 s. The amplitude of the inward current measured at the end of the voltage step is plotted against time. Example traces are shown on the right. Addition of BaCl2 (2 mm) to the ACSF had no effect on the current amplitude or kinetics, whereas CsCl (2 mm) reversibly blocked the hyperpolarization-activated current.
Activation of the presynaptic Ih was relatively fast, exhibiting an activation time constant of 78.4 ± 10.1 ms on stepping to -130 mV (n = 6, holding potential of -60 mV). The rate of activation was voltage dependent, accelerating with negative voltage steps, with an e-fold change in the activation time constant for a potential change of 32 mV (see Fig. 3). A second, slower time constant exceeding 2000 ms accounted for less than 10 % of the peak magnitude, but because of the sampling duration a more accurate estimate was not made. Deactivation of the presynaptic current was also voltage dependent and relatively slow at membrane potentials around rest (τ= 346 ± 11.5 ms, n = 5, measured at a holding potential of -70 mV).
Figure 3. Voltage dependence of Ih activation.
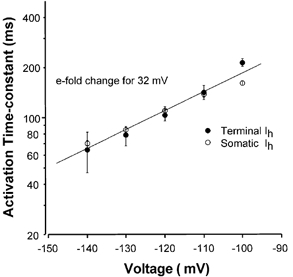
Mean activation time constants are plotted on a logarithmic axis against voltage. Data for both the presynaptic terminal (•) and the bushy cell soma (○) are similar, showing an e-fold change for 32 mV (continuous line). Data are means ±s.e.m. with n = 13-20 for the bushy cell soma and n = 3-6 for the presynaptic calyx of Held terminal.
The combination of barium insensitivity, caesium block, negative activation range, reversal potential positive to EK and slow activation kinetics identify the current as the non-specific cation conductance, Ih. The resting membrane potential of the calyx of Held (-73 mV, Forsythe, 1994) is more negative than that of the postsynaptic MNTB cell (-62 mV, Forsythe & Barnes-Davies, 1993a,b). Hence, the presynaptic Ih will make a larger contribution to the resting conductance in the terminal than in the postsynaptic neurone (for a given level of modulatory drive, see below).
Previous reports indicate that modulation by cAMP can dramatically shift the activation voltage of Ih in many cells, including MNTB neurones (DiFrancesco & Tortora, 1991; Banks et al. 1993). To determine whether a similar phenomenon occurs at presynaptic sites, we examined Ih following addition of cAMP to the intracellular pipette solution. Addition of 1 mm cAMP shifted the half-activation voltage of Ih from -94.4 ± 1.7 mV in control (n = 7) to -74.1 ± 2.8 mV (n = 8). This 20 mV positive shift means that around half of the current is activated at resting membrane potentials (Fig. 4). In some experiments the holding potential was changed to -50 mV and tetrodotoxin (1 μm) and 4-aminopyridine (1 mm) added to block presynaptic voltage-gated sodium channels and potassium channels, respectively (Forsythe, 1994). Under these conditions the activation curve from -50 to -70 mV was measured with little contamination from low voltage-activated currents. The half-activation voltage for these responses was similar to those measured at holding potentials of -70 mV and so the data are pooled in the average curves.
Figure 4. Intracellular cAMP induces a positive shift in the voltage dependence of Ih activation.
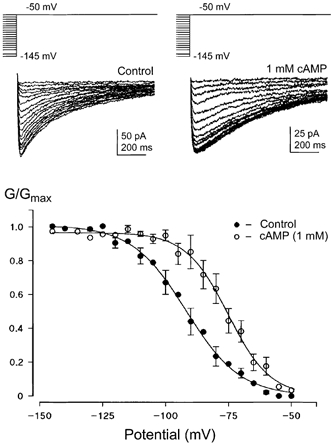
Activation curves were measured from Ih tail currents (as in Fig. 1) in different terminals with or without cAMP (1 mm) in the internal patch solution. Sample traces in different terminals are shown with voltage protocols above. Activation curves were measured from a HP of either -70 mV or in 4 cases -50 mV following the addition of TTX (1 μm) and TEA (10 mm) to the ACSF. No significant difference was found between these two protocols and so the data were pooled. Control half-activation was -94.4 ± 1.7 mV with a slope of 10.8 mV (n = 3-7; only data points with n > 3 have error bars). In terminals dialysed with cAMP, Ih half-activation was -74.1 ± 2.8 mV with a slope of 8.4 mV (n = 8). This shift in activation results in about half of the current being activated at resting membrane potentials in the calyx.
Modulation of Ih was also observed in whole terminal recordings following the extracellular perfusion of 500 μm 8-bromo-cAMP (8-Br-cAMP), a membrane-permeant cAMP analogue. 8-Br-cAMP induced a positive shift in the activation curve in all terminals examined, with an average shift of 7.8 ± 2.0 mV (control V1/2= -92.6 ± 1.1 mV; plus 8-Br-cAMP, V1/2= 84.6± 1.3 mV, n = 4); see Fig. 5.
Figure 5. Extracellular perfusion of 8-Br-cAMP mimics the action of internal cAMP.
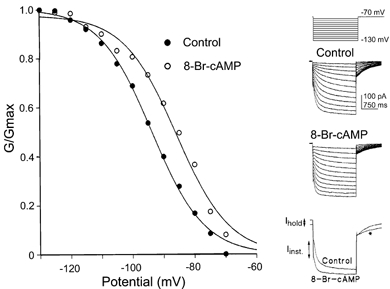
Perfusion of the membrane permeable analogue of cAMP, 8-Br-cAMP (500 μm), produced a positive shift in the activation curve similar to that observed by including cAMP in the patch pipette (Fig. 4). In this example a positive shift of 9.3 mV is induced by 8-Br-cAMP; similar results were seen in 4 terminals. Overlaid data traces are shown on the right, with voltage protocols shown above. The activation curves measured from tail currents in this terminal are plotted on the right. Other changes, consistent with a shift in half-activation, were an increase in the holding current (Ihold), an increase in the instantaneous current (Iinst), and a slowing in tail current deactivation kinetics at -70 mV (*) as shown in the overlaid traces on the lower right.
Ih activation and synaptic transmission
The cAMP-induced positive shift in Ih activation was used to induce activation of the current at resting membrane potentials to study the role of Ih in the presynaptic terminal. Whole-cell patch clamp recordings were made from postsynaptic MNTB neurones at a holding potential of -60 mV using a KCl-based patch solution (see Methods). Following stimulation of the trapezoid body, EPSCs from the calyx of Held synapse were evoked in MNTB neurones. NMDA receptors were blocked by perfusion of 50 μm AP5 and 10 μm MK801. In early experiments, single EPSCs were evoked at a frequency of 0.3 Hz in the presence of 10 μm forskolin and then 1 mm CsCl was used to block Ih. No changes were observed in EPSC amplitude in three cases (data not shown). In further experiments using trains of stimuli, Ih was activated by external perfusion of 8-Br-cAMP (500 μm) and then antagonised by combined application of 8-Br-cAMP and ZD7288 (10 μm), which is a specific antagonist of Ih channels (BoSmith et al. 1993; Harris & Constanti, 1995). An increase in the holding current of the postsynaptic MNTB neurone (at -60 mV) was measured on application of 8-Br-cAMP which was blocked by ZD7288, confirming that Ih was indeed activated by this protocol (Fig. 6D). Trains of EPSCs were evoked at a frequency of 50 Hz for 2 s as shown in Fig. 6, and repeated at 1 min intervals. Within each train, EPSCs depressed rapidly to around 20 % of the peak amplitude of the first EPSC (Fig. 6A and B) and this was unaffected by activation of Ih. Some run-down in EPSC amplitude was observed over the course of the experiment (over 20 min); this experiment entailed stimulation of the presynaptic axon by between 2000 and 4000 stimuli. In three experiments the first EPSC declined to 77 ± 2 % control amplitude following 8-Br-cAMP and remained at 75 ± 2 % of control after ZD7288. The 50th EPSC declined to 88 ± 2 % of control on perfusion with 8-Br-cAMP and 78 ± 3 % following ZD7288. These data show that in contrast to the crustacean neuromuscular junction, activation of Ih by cAMP anologues caused no potentiation of transmitter release at the calyx of Held.
Figure 6. Ih has no effect on EPSC amplitude or synaptic depression.
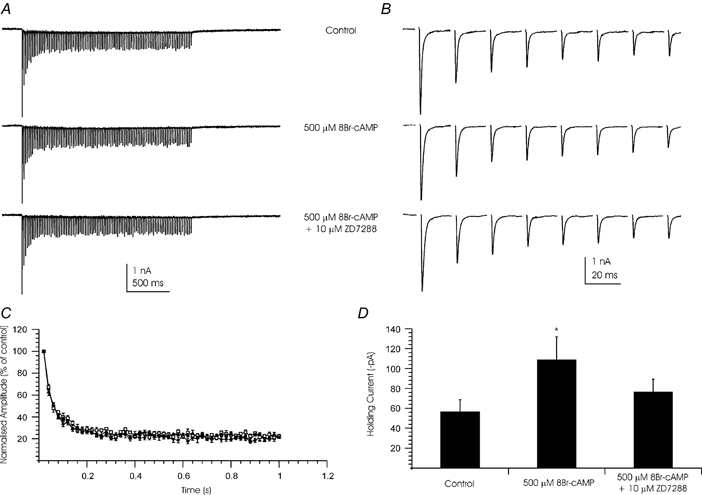
Whole-cell patch clamp recordings were made from MNTB neurones. EPSCs were generated by the calyx of Held in response to a 2 s train of stimuli at 50 Hz. A, traces show mean of 5 sweeps before (top), during 500 μm 8-Br-cAMP (middle) and after perfusion of 500 μm 8-Br-cAMP and 10 μm ZD7288 (lower). B, data trace showing the first 8 EPSCs of the trains shown in A. Steady-state depression is reached by the 6th EPSC of the train. C, plot of EPSC amplitude during the train. Amplitudes were normalised to the peak of the first EPSC of the train. ♦, control; ▪, 500 μm 8Br-cAMP; ▴, 500 μm 8-Br-cAMP and 10 μm ZD7288. Data are plotted as means ±s.e.m. for 4 cells. D, 8-Br-cAMP (500 μm) increased the holding current of the same postsynaptic neurones and this was blocked by ZD7288. Data are presented as mean holding current under each condition (±s.e.m., n = 4). * Statistical difference from control and ZD7288 values (Student's 2-tailed, paired t test; P < 0.05). (Postsynaptic holding potential was -60 mV. EPSC recordings were made in the presence of 1 μm strychnine, 10 μm bicuculline, 50 μmd-AP5 and 10 μm MK-801. Stimulus artifacts have been blanked.)
We also considered whether Ih could influence the timing of the presynaptic action potential rather than the probability of release. The variability of EPSC amplitude and latency were examined at two time points: the first EPSC (Fig. 7A and C) and the 50th EPSC (Fig. 7B and D) of each train. The mean normalised response for four cells is plotted against time in minutes, with drug applications indicated by the black bars. The mean EPSC latency of 1.53 ± 0.08 ms changed to 1.47 ± 0.02 ms in the presence of 8-Br-cAMP and 1.50 ± 0.03 ms after perfusion of ZD7288. The mean latencies for the 50th EPSCs under the same conditions were 1.57 ± 0.02 ms, 1.83 ± 0.03 ms and 1.96 ± 0.02 ms, respectively. In other experiments where a CsCl-based patch solution was employed, identical results were observed in an additional four cases (data not shown).
Figure 7. Summary of Ih effects on calyx of Held EPSCs.
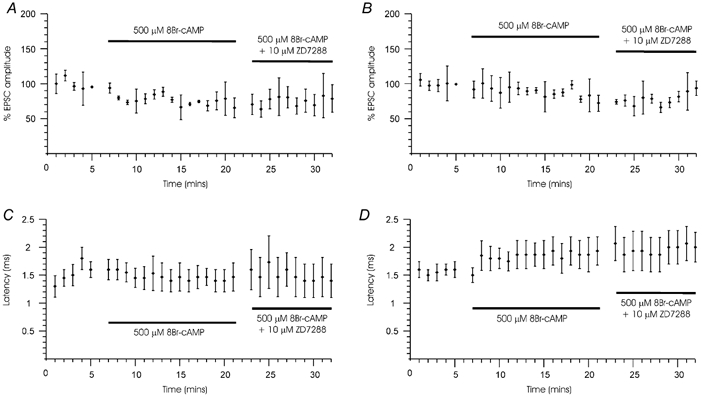
Presynaptic axons were stimulated for 2 s once a minute at a frequency of 50 Hz and the postsynaptic MNTB neurone was held at a potential of -60 mV. Means ±s.e.m. (n = 4) are plotted against time for the first (A and C) and 50th EPSCs (B and D) for two parameters: EPSC amplitude (A and B) and EPSC latency (C and D) during application of 8-Br-cAMP and 8-Br-cAMP + ZD7288. Horizontal bars indicate drug application periods. A, effects on the amplitude of the first EPSC in the train. Responses are normalised to the mean amplitude of the first EPSC of all 5 trains during the 5 min control period. B, effects on the amplitude of 50th EPSC (halfway through the train, see Fig. 6). EPSC amplitudes were normalized to the mean amplitude of the EPSC during the 5 control sweeps (minutes 1-5). C, there was no effect on the latency of the first EPSC in the train or (D) on the latency of the 50th EPSC.
Bushy cell Ih
The bushy cell somatic Ih was similar to that observed at the calyx of Held synaptic terminals. The activation curve was determined in either normal ACSF or in an ACSF which contained 1 μm TTX and 2 mm 4-AP; the results were similar so the data were pooled. The current-voltage relationship for one cell is shown in Fig. 8A and B and the response under current clamp is shown in Fig. 8D. Voltage-dependent activation was measured from tail currents generated at a potential of -60 mV. The normalised amplitudes were fitted to a Boltzmann distribution (eqn (1)) as shown for mean data from 15 bushy neurones (Fig. 8C). The mean half-activation voltage was -94 ± 6 mV with a slope factor of 13 ± 2 mV. The reversal potential was estimated as -33 ± 2 mV in five cells (data not shown) using similar methods to those used for the presynaptic Ih recordings. When bushy cell somata were hyperpolarized under current clamp (n = 7), the membrane potential showed a characteristic ‘sag’ early in the voltage trace (Fig. 8D) and an anodal break action potential was sometimes observed on termination of current injection.
Figure 8. Bushy cells of the aVCN also possess an Ih.
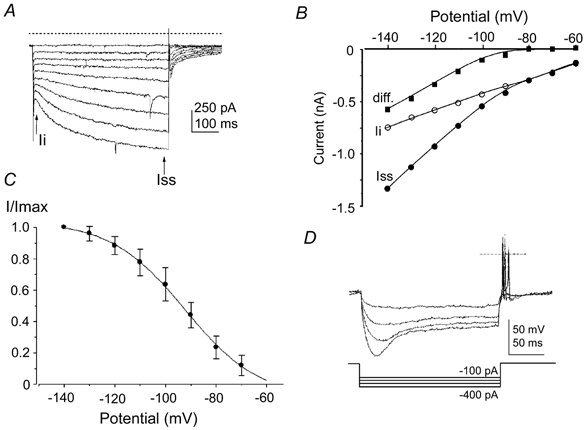
A, inward currents recorded from a bushy cell evoked by 500 ms hyperpolarizing voltage steps from -60 to -140 mV (in -10 mV increments, holding potential was -60 mV). Data are not leak subtracted; dashed line indicates the zero current level and arrows indicate latency of measurements for the current-voltage (I-V) relationship. Ii, instantaneous current; Iss, steady state at 500 ms. B, I-V relationship for the data in A, measured as the difference (▪) between the instantaneous current (Ii, ○) and the current measured at 500 ms (Iss, •). C, the mean Ih activation curve obtained from 15 bushy cells. The normalised tail current magnitude is plotted against the test potential and fitted to a Boltzmann function (continuous line) with half-activation (V1/2) of -94 mV and with a slope factor of 13 mV. Vertical bars are ±s.d. D, a different bushy neurone under current clamp, in response to a 200 ms hyperpolarizing current stimulus. Increasing hyperpolarizing current injection causes a slow time-dependent relaxation of the voltage trace, which can trigger action potentials at the end of the pulse. A dashed line indicates zero voltage level and current steps are plotted below. The resting membrane potential was -58 mV.
The activation time constant was fitted to a single exponential function over the first 300 ms of each current trace. Activation of Ih accelerated with more negative voltage steps, with a time constant of 84.3 ± 2.9 ms at -130 mV (n = 19); data for a range of voltages are shown in Fig. 3 for comparison with the data for presynaptic Ih. The voltage dependence of activation was similar for both somatic (open circles) and presynaptic (filled circles) Ih, with the time constant changing e-fold over 32 mV, as shown by the continuous line in Fig. 3. External application of CsCl (1 mm) largely blocked the bushy cell somatic Ih (mean block at -140 mV was 93.7 ± 0.4 %; n = 4). Somatic Ih was partially sensitive to BaCl2 (1 mm, mean block at -140 mV was 31 ± 8 %; n = 4, data not shown) in contrast to the presynaptic current, which was insensitive to barium.
Ih increased in magnitude with animal age. In order to control for the possibility that this was simply due to increasing neuronal size we normalised Ih amplitudes to cell capacitance. In Fig. 9 normalised values of Ih (pA pF−1, measured at -140 mV) for each bushy cell are plotted against animal age. Although the scatter of the individual points was large, the trend is clear: between 5 and 14 days old the current density of Ih increased linearly (r= -0.68, P = 0.00007 measured at -140 mV). There was a statistically significant increase (P < 0.05) in the magnitude of Ih pooled from ages under 10 days compared to those of 11 days or older.
Figure 9. Bushy cell Ih density increases with animal age.
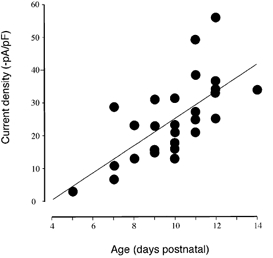
The current density of Ih (-pA pF−1, measured on stepping to a potential of -140 mV) for 27 bushy cells are plotted as a function of animal age. The thin line shows the linear regression of the individual values.
DISCUSSION
We have characterised the Ih currents at bushy cell bodies and their giant synaptic terminals in the MNTB (known as the calyx of Held). Ih is a voltage-dependent conductance acting at around resting membrane potentials and on hyperpolarization of this excitatory synapse. It activates in a voltage-dependent manner, with time constants around 100 ms at -120 mV. Activation is fitted by a Boltzmann distribution with V1/2 of -94 mV and accumulation of intracellular cAMP shifts V1/2 to membrane potentials close to resting levels (around -75 mV), suggesting that regulation of presynaptic cAMP can influence the resting conductance of synaptic terminals. Activation of Ih at resting potentials through perfusion of membrane-permeant cAMP analogues had little effect on transmitter release at this mammalian excitatory synapse. The presynaptic Ih had similar properties to that present in bushy cell bodies (which give rise to the calyx of Held). Somatic Ih showed a significant increase in current density with increasing animal age. Small differences in the slope of the activation curve and a higher sensitivity to Ba2+ may indicate that the somatic Ih is more heterogeneous than that at the presynaptic terminal.
Four members of a hyperpolarization-activated cation channel gene family have been cloned (HCN1-4). HCN1 activates rapidly (around 100 ms at potentials negative to -100 mV) and shows a relatively small shift in the voltage dependence of activation with cAMP (Ludwig et al. 1998; Santoro et al. 1998). HCN2 activates more slowly than HCN1 (time constants greater than 250 ms) and exhibits a large positive shift in the activation curve in the presence of cAMP (Ludwig et al. 1998). HCN3 is expressed widely, including the thalamus, hippocampus and neocortex (Franz et al. 2000). HCN4 also exhibits slow activation and is sensitive to cAMP (Seifert et al. 1999). In situ hybridisation studies show that HCN1 and HCN2 are highly expressed in the cortex, hippocampus and brainstem, including the ventral cochlear nucleus (Moosmang et al. 1999; Santoro et al. 2000). HCN4 is highly expressed in the thalamus (along with HCN2, although the thalamus has low levels of HCN1), but it is expressed only at low levels in the brainstem and cochlear nucleus. Single cell PCR from hippocampal, thalamocortical and substantia nigral neurones suggests that expression of HCN1 correlates with the fast-gating Ih phenotype (Franz et al. 2000). Our evidence shows that both the somatic and presynaptic Ih have fast gating, suggesting that HCN1 subunits are present in Ih channels at both locations. The distribution of HCN1 and the large shift in half-activation of the presynaptic Ih with cAMP suggest the possibility that the bushy cell Ih channels could be heteromultimeric channels of HCN1 and HCN2 or 3 subunits.
Modulation of Ih by cAMP acting either directly on the channel or via a PKA-mediated mechanism has been demonstrated (McCormick & Pape, 1990a;Banks et al. 1993; DiFrancesco, 1993) and we confirm that cAMP is a highly effective modulator at the presynaptic Ih. Our data were recorded with conventional patch methods where the terminal is dialysed with the pipette solution (containing no ATP or GTP), suggesting a direct action by cAMP, similar to that reported in sino-atrial node myocytes (DiFrancesco & Tortora, 1991) and dorsal root ganglion cells (Ingram & Williams, 1996). The +20 mV shift in half-activation at the calyx is similar to that reported in the postsynaptic target cell in the MNTB (Banks et al. 1993). Modulation of Ih by cAMP, calcium (Khakh & Henderson, 1988; Hagiwara & Irisawa, 1989; Luthi & McCormick, 1998), nitric oxide (Pape & Mager, 1992) and pH (Munsch & Pape, 1999) suggests that voltage-dependent activation is not the only mechanism by which Ih may influence neuronal excitability.
Somatodendritic Ih in the auditory pathway
We observed an age-related increase in somatic Ih amplitude over the postnatal period (from 5 to 14 days) but found no difference in the steady-state activation properties of these neurones over the same age range. Because of technical difficulties in presynaptic recording from older animals we did not record from a sufficient range of ages to make a comparison with presynaptic Ih. Developmental changes of neuronal membrane properties (including changes of the input resistance and rheobase) have been noted in the hypoglossal nucleus (Haddad et al. 1990; Viana et al. 1994), where the density of Ih increased by around 10 times from neonatal to adult rats (Bayliss et al. 1994). Other examples include ciliary and trigeminal ganglion neurones of the quail (Schlichter et al. 1991) where Ih was absent at early embryonic ages (before E10) but the number of neurones expressing Ih increased with older animals.
Hyperpolarization-activated currents are present along the length of the brainstem auditory pathway from spiral ganglion cells (Mo & Davis, 1997), bushy cells, their terminals, octopus cells (Golding et al. 1999; Bal & Oertel, 2000), MNTB neurones (Banks et al.1993), neurones of the medial superior olive (Owens et al. 1999) and dorsal nucleus of the lateral lemniscus (DNLL) (Fu et al. 1997). The function of Ih need not be identical at each site, since its activation will depend upon both voltage and intracellular second messengers. For example, Ih contributes to the resting membrane potential and membrane properties at VCN bushy cells, at the calyx of Held and particularly at octopus cells (Bal & Oertel, 2000), but is largely deactivated under normal resting conditions in the DNLL (Fu et al. 1997). Multiple adaptations help maintain high-fidelity transmission in the binaural pathway. These include large rapid time-course excitatory postsynaptic potentials (Barnes-Davies & Forsythe, 1995; Isaacson & Walmsley, 1995), high voltage-activated potassium channels, such as Kv3.1, which minimise action potential duration, and low voltage-activated K+ currents (Shaker-related potassium channels), which shunt the generation of multiple postsynaptic APs (Brew & Forsythe, 1995; Grigg et al. 2000). One possible postsynaptic function of Ih is to limit the duration of inhibitory drive and provide a rebound excitatory mechanism following hyperpolarization which might aid synchronous firing (Luo & Perkel, 1999). Bushy cells possess transient, low-voltage-activated calcium channels as well as Ih (Doughty et al. 1998). Similar suites of somatodendritic channels are associated with rhythmic bursting (Crunelli et al. 1989; McCormick & Pape, 1990a; Hughes et al. 1998) and may contribute to improvement in timing of action potentials during propagation to more central nuclei (See Joris et al. 1994; Doughty et al. 1998) by reducing inter-spike latency fluctuation during action potential trains in the auditory pathway.
Presynaptic Ih
Ih channels are differentially localised within individual cells; apical dendrites of CA1 pyramidal cells can express up to 6 times more channels than the cell body (Magee, 1998, 1999). Here we report that Ih is present at excitatory glutamatergic synaptic terminals, and previously Southan et al. (2000) have demonstrated that Ih is present at inhibitory synapses in the cerebellum. We have shown that activation of presynaptic Ih at resting membrane potentials is dramatically increased on raising intracellular cAMP. This could depolarise the resting membrane potential and increase calcium entry into the terminal by slowing calcium channel deactivation during action potential repolarization (Forsythe et al. 1998). However, we found no significant change in transmitter release on activation or on blocking Ih.
Previous reports have suggested a rectifying, Ih-like current is present at the chick ciliary ganglion presynaptic terminal (Fletcher & Chiappenelli, 1992) and in rat vagus nerve (Takigawa et al. 1998). Ih is also present at the crustacean neuromuscular junction (Beaumont & Zucker, 2000), where sustained activation of Ih by strong hyperpolarization leads to a long-lasting potentiation of transmitter release. We used an alternative strategy to activate Ih (at resting potentials), by perfusion of a membrane-permeant analogue of cAMP. Ih activation did not increase EPSC amplitude at the calyx of Held, nor was there any change in the mean response amplitude over the next 10-20 min. The most obvious difference between these two studies is in the use of vertebrate versus invertebrate preparations, but both studies also used differing means of activating Ih. We were unable to apply a strong hyperpolarization protocol to activate Ih at the calyx of Held, because sustained hyperpolarization leads to loss of the patch recording (I. D. Forsythe & M. F. Cuttle, unpublished observations). Sustained hyperpolarization lasting several seconds generates a significant Na+ influx; a possible volume increase may compromise membrane or seal integrity, thereby leading to loss of our patch clamp recording under these conditions. Use of 8-Br-cAMP to shift the voltage-dependence of Ih to resting membrane potentials reduced this problem and since 8-Br-cAMP is membrane permeant, it also avoided dialysis of the presynaptic terminal. In case Ih was already maximally activated in the presynaptic terminal, we followed the application of 8-Br-cAMP with the specific Ih antagonist ZD7288 (blocking the Ih current). Synaptic transmission in the presence of ZD7288 was unchanged (Fig. 7) confirming that the relatively modest changes observed on activation of Ih by 8-Br-cAMP were not due to maximal activation of Ih. A reduction in spontaneous inhibitory transmitter release has been observed in cerebellar Purkinje neurones on application of ZD7288 (Southan et al. 2000). However this effect was blocked by tetrodotoxin, implying that the action of Ih on spontaneous synaptic currents was an indirect action, via interneurone excitability and spontaneous firing.
The structure of the mature calyx of Held shows many finger-like processes (Forsythe, 1994) rather than the single spoon-like structure seen at immature terminals. One way of thinking about this giant synapse is that it constitutes a ‘minimal’ axon terminal field that enfolds the soma of a single neurone. Such a structure may risk branch point failure during orthodromic propagation of an invading action potential. By analogy with the recent work on propagation of EPSPs into the soma from remote dendrites (Magee, 1998, 1999), one possible function for Ih could be to boost AP propagation into the fine branches of the axon terminal.
Acknowledgments
Thanks to the Mechanical and Electronics Workshops for excellent technical assistance and to Brian Billups for reading the manuscript. S.O. is an MRC PhD scholar. This work was supported by the Wellcome Trust.
References
- Bader CR, Bertrand D, Schwartz EA. Voltage-activated and Ca2+-activated currents studied in solitary rod inner segments from the salamander retina. Journal of Physiology. 1982;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal R, Oertel D. Hyperpolarization-activated, mixed-cation current (Ih) in octopus cells of the mammalian cochlear nucleus. Journal of Neurophysiology. 2000;84:806–817. doi: 10.1152/jn.2000.84.2.806. [DOI] [PubMed] [Google Scholar]
- Bal T, McCormick DA. Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current Ih. Journal of Neurophysiology. 1997;77:3145–3156. doi: 10.1152/jn.1997.77.6.3145. [DOI] [PubMed] [Google Scholar]
- Banks MI, Pearce RA, Smith PH. Hyperpolarization-activated cation current Ih in neurons of the medial nucleus of the trapezoid body: Voltage-clamp analysis and enhancement by norepinephrine and cAMP suggest a modulatory mechanism in the auditory brain stem. Journal of Neurophysiology. 1993;70:1420–1432. doi: 10.1152/jn.1993.70.4.1420. [DOI] [PubMed] [Google Scholar]
- Barnes-Davies M, Forsythe ID. Pre- and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brainstem slices. Journal of Physiology. 1995;488:387–406. doi: 10.1113/jphysiol.1995.sp020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Viana F, Bellingham MC, Berger AJ. Characteristics and postnatal development of a hyperpolarization-activated inward current in rat hypoglossal motoneurons in vitro. Journal of Neurophysiology. 1994;71:119–128. doi: 10.1152/jn.1994.71.1.119. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nature Neuroscience. 2000;3:133–141. doi: 10.1038/72072. [DOI] [PubMed] [Google Scholar]
- BoSmith RE, Briggs I, Sturgess NC. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current If in guinea-pig dissociated sinoatrial node cells. British Journal of Pharmacology. 1993;110:343–349. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew HM, Forsythe ID. Two voltage-dependent K+ conductances with complementary functions in postsynaptic integration at a central auditory synapse. Journal of Neuroscience. 1995;15:8011–8022. doi: 10.1523/JNEUROSCI.15-12-08011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HF, DiFrancesco D. Voltage clamp investigations of currents underlying pacemaker activity in rabbit sino-atrial node. Journal of Physiology. 1980;308:331–351. doi: 10.1113/jphysiol.1980.sp013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Not so funny any more: Pacing channels are cloned. Neuron. 1998;21:5–7. doi: 10.1016/s0896-6273(00)80508-5. [DOI] [PubMed] [Google Scholar]
- Crepel F, Penit-Soria J. Inward rectification and low threshold calcium conductance in rat cerebellar Purkinje cells. An in vitro study. Journal of Physiology. 1986;372:1–23. doi: 10.1113/jphysiol.1986.sp015993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Lightowler S, Pollard CE. A T-type Ca2+ current underlies low threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. Journal of Physiology. 1989;413:543–561. doi: 10.1113/jphysiol.1989.sp017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annual Review of Physiology. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic-AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Tromba C. Inhibition of the hyperpolarization-activated current If induced by acetylcholine in rabbit sinoatrial node myocytes. Journal of Physiology. 1988;405:477–491. doi: 10.1113/jphysiol.1988.sp017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty JM, Barnes-Davies M, Rusznák Z, Harasztosi C, Forsythe ID. Contrasting Ca2+ channel subtypes at cell bodies and synaptic terminals of rat anteroventral cochlear bushy neurones. Journal of Physiology. 1998;512:365–376. doi: 10.1111/j.1469-7793.1998.365be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher GH, Chiappenelli VA. An inward rectifier is present in presynaptic nerve terminals in the chick ciliary ganglion. Brain Research. 1992;575:103–112. doi: 10.1016/0006-8993(92)90429-d. [DOI] [PubMed] [Google Scholar]
- Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. Journal of Physiology. 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID, Barnes-Davies M. The binaural auditory pathway: excitatory amino acid receptors mediate dual time course excitatory postsynaptic currents in the rat medial nucleus of the trapezoid body. Proceedings of the Royal Society B. 1993a;251:151–157. doi: 10.1098/rspb.1993.0022. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Barnes-Davies M. The binaural auditory pathway: membrane currents limiting multiple action potential generation in the rat medial nucleus of the trapezoid body. Proceedings of the Royal Society B. 1993b;251:143–150. doi: 10.1098/rspb.1993.0021. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- Franz O, Liss B, Neu A, Roeper J. Single-cell mRNA expression of HCN1 correlates with a fast gating phenotype of hyperpolarization-activated cyclic nucleotide-gated ion channels (Ih) in central neurons. European Journal of Neuroscience. 2000;12:2685–2693. doi: 10.1046/j.1460-9568.2000.00151.x. [DOI] [PubMed] [Google Scholar]
- Friauf F, Ostwald J. Divergent projections of physiologically characterized rat cochlear nucleus neurons as shown by intra-axonal injection of horseradish peroxidase. Experimental Brain Research. 1988;73:263–284. doi: 10.1007/BF00248219. [DOI] [PubMed] [Google Scholar]
- Fu XW, Brezden BL, Wu SH. Hyperpolarization-activated inward current in neurons of the rat's dorsal nucleus of the lateral lemniscus in vitro. Journal of Neurophysiology. 1997;78:2235–2245. doi: 10.1152/jn.1997.78.5.2235. [DOI] [PubMed] [Google Scholar]
- Gauss R, Seifert R, Kaupp UB. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature. 1998;393:583–587. doi: 10.1038/31248. [DOI] [PubMed] [Google Scholar]
- Golding NL, Ferragamo MJ, Oertel D. Role of intrinsic conductances underlying responses to transients in octopus cells of the cochlear nucleus. Journal of Neuroscience. 1999;19:2897–2905. doi: 10.1523/JNEUROSCI.19-08-02897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg JJ, Brew HM, Tempel BL. Differential expression of voltage-gated potassium channel genes in auditory nuclei of the mouse brainstem. Hearing Research. 2000;140:77–90. doi: 10.1016/s0378-5955(99)00187-2. [DOI] [PubMed] [Google Scholar]
- Haddad GG, Donnelly DF, Getting PA. Biophysical properties of hypoglossal neurons in vitro: intracellular studies in adult and neonatal rats. Journal of Applied Physiology. 1990;69:1509–1517. doi: 10.1152/jappl.1990.69.4.1509. [DOI] [PubMed] [Google Scholar]
- Hagiwara N, Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit sino-atrial node cells. Journal of Physiology. 1989;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NC, Constanti A. Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. Journal of Neurophysiology. 1995;74:2366–2378. doi: 10.1152/jn.1995.74.6.2366. [DOI] [PubMed] [Google Scholar]
- Held H. Die centrale Gehorleitung. Arch. f Anat. u. Physiol. Anat Abtheil. 1893;17:201–248. [Google Scholar]
- Hughes SW, Cope DW, Crunelli V. Dynamic clamp study of Ih modulation of burst firing and delta oscillations in thalamocortical neurons in vitro. Neuroscience. 1998;87:541–550. doi: 10.1016/s0306-4522(98)00170-5. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Williams JT. Modulation of the hyperpolarization-activated current Ih by cyclic nucleotides in guinea-pig primary afferent neurons. Journal of Physiology. 1996;492:97–106. doi: 10.1113/jphysiol.1996.sp021292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Receptors underlying excitatory synaptic transmission in slices of the rat anteroventral cochlear nucleus. Journal of Neurophysiology. 1995;73:964–973. doi: 10.1152/jn.1995.73.3.964. [DOI] [PubMed] [Google Scholar]
- Ishii TM, Takano M, Xie LH, Noma A, Ohmori H. Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sino-atrial node. Journal of Biological Chemistry. 1999;274:12835–12839. doi: 10.1074/jbc.274.18.12835. [DOI] [PubMed] [Google Scholar]
- Joris PX, Smith PH, Yin TCT. Enhancement of neural synchronization in the antero-ventral cochlear nucleus. II. Responses in the tuning curve tail. Journal of Neurophysiology. 1994;71:1037–1051. doi: 10.1152/jn.1994.71.3.1037. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Henderson G. Hyperpolarization-activated cationic currents (Ih) in neurones of the trigreminal mesencephalic nucleus of the rat. Journal of Physiology. 1998;510:695–704. doi: 10.1111/j.1469-7793.1998.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Stieber J, Hullin R, Hofmann F, Biel M. Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO Journal. 1999;18:2323–2329. doi: 10.1093/emboj/18.9.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Perkel DJ. A GABAergic, strongly inhibitory projection to a thalamic nucleus in the zebra finch song system. Journal of Neuroscience. 1999;19:6700–6711. doi: 10.1523/JNEUROSCI.19-15-06700.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, Mccormick DA. Periodicity of thalamic synchronized oscillations: The role of Ca2+-mediated up-regulation of Ih. Neuron. 1998;20:553–563. doi: 10.1016/s0896-6273(00)80994-0. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Mangoni M, Lazzari A, DiFrancesco D. Properties of the hyperpolarization-activated current in rat hippocampal CA1 pyramidal cells. Journal of Neurophysiology. 1993;69:2129–2136. doi: 10.1152/jn.1993.69.6.2129. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. Journal of Physiology. 1990a;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. Journal of Physiology. 1990b;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. Journal of Neuroscience. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nature Neuroscience. 1999;2:508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- Manis PB, Marx SD. Outward currents in isolated ventral cochlear nucleus neurons. Journal of Neuroscience. 1991;11:2865–2880. doi: 10.1523/JNEUROSCI.11-09-02865.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo ZL, Davis RL. Heterogeneous voltage dependence of inward rectifier currents in spiral ganglion neurons. Journal of Neurophysiology. 1997;78:3019–3027. doi: 10.1152/jn.1997.78.6.3019. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Biel M, Hofmann F, Ludwig A. Differential distribution of four hyperpolarization-activated cation channels in mouse brain. Biological Chemistry. 1999;380:975–980. doi: 10.1515/BC.1999.121. [DOI] [PubMed] [Google Scholar]
- Munsch T, Pape H-C. Modulation of the hyperpolarization-activated cation current of rat thalamic relay neurons by intracellular pH. Journal of Physiology. 1999;519:493–504. doi: 10.1111/j.1469-7793.1999.0493m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D. The role of timing in the brain stem auditory nuclei of vertebrates. Annual Review of Physiology. 1999;61:497–519. doi: 10.1146/annurev.physiol.61.1.497. [DOI] [PubMed] [Google Scholar]
- Okabe K, Inoue Y, Kawarabayashi T, Kajiya H, Okamoto F, Soeda H. Physiological significance of hyperpolarization-activated inward currents Ih in smooth muscle cells from the circular layers of pregnant rat myometrium. Pflügers Archiv. 1999;439:76–85. doi: 10.1007/s004249900140. [DOI] [PubMed] [Google Scholar]
- Owens S, Cuttle MF, Rusznák Z, Forsythe ID. Pre- and Postsynaptic inward rectifier current Ih in consecutive nuclei of the rat auditory brainstem. British Neuroscience Association Abstracts. 1999;15:P110. [Google Scholar]
- Pape H-C, Mager R. Nitric oxide controls oscillatory activity in thalamo-cortical neurons. Neuron. 1992;9:441–448. doi: 10.1016/0896-6273(92)90182-d. [DOI] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: The hyperpolarization-activated cation current in neurons. Annual Review of Physiology. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Rusznák Z, Forsythe ID, Brew HM, Stanfield PR. Membrane currents influencing action potential latency in granule neurons of the rat cochlear nucleus. European Journal of Neuroscience. 1997;9:2348–2358. doi: 10.1111/j.1460-9568.1997.tb01652.x. [DOI] [PubMed] [Google Scholar]
- Rusznák Z, Forsythe ID, Stanfield PR. Characterization of the hyperpolarization activated nonspecific cation current (Ih) of bushy neurones from the rat anteroventral cochlear nucleus studied in a thin brain slice preparation. Neurobiology. 1996;4:275–276. [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. Journal of Neuroscience. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Grant SGN, Bartsch D, Kandel ER. Interactive cloning with the SH3 domain of N-src identifies a new brain specific ion channel protein, with homology to Eag and cyclic nucleotide-gated channels. Proceedings of the National Academy of Sciences of the USA. 1997;94:14815–14820. doi: 10.1073/pnas.94.26.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- Schlichter R, Bader CR, Bernheim L. Development of anomalous rectification Ih and of a tetrodotoxin-resistant sodium current in embryonic quail neurones. Journal of Physiology. 1991;442:127–145. doi: 10.1113/jphysiol.1991.sp018786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R, Scholten A, Gauss R, Mincheva A, Lichter P, Kaupp UB. Molecular characterization of a slowly gating human hyperpolarization- activated channel predominantly expressed in thalamus, heart, and testis. Proceedings of the National Academy of Sciences of the USA. 1999;96:9391–9396. doi: 10.1073/pnas.96.16.9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan AP, Morris NP, Stephens GJ, Robertson B. Hyperpolarization-activated currents in presynaptic terminals of mouse cerebellar basket cells. Journal of Physiology. 2000;526:91–97. doi: 10.1111/j.1469-7793.2000.t01-1-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C, Quasthoff S, Grafe P. A specific blocker reveals the presence and function of the hyperpolarization-activated cation current Ih in peripheral mammalian nerve fibres. Neuroscience. 1998;82:631–634. doi: 10.1016/s0306-4522(97)00383-7. [DOI] [PubMed] [Google Scholar]
- Tolbert LP, Morest DK, Yurgeluntood DA. The neuronal architecture of the anteroventral cochlear nucleus of the cat in the region of the cochlear nerve root: Horseradish peroxidase labeling of identified cell types. Neuroscience. 1982;7:3031–3052. doi: 10.1016/0306-4522(82)90228-7. [DOI] [PubMed] [Google Scholar]
- Viana F, Bayliss DA, Berger AJ. Postnatal changes in rat hypoglossal motoneuron membrane properties. Neuroscience. 1994;59:131–48. doi: 10.1016/0306-4522(94)90105-8. [DOI] [PubMed] [Google Scholar]


