Abstract
The aim of this study was to investigate some of the cellular mechanisms involved in the effects caused by changes in extracellular Ca2+ concentration ([Ca2+]o).
Current and voltage-clamp experiments were carried out on acutely isolated thalamic neurons of rats.
Increasing [Ca2+]o alone induced a transition of the discharge from single spike to burst mode in isolated current-clamped neurons.
Increasing [Ca2+]o caused the voltage-dependent characteristics of the low voltage-activated (LVA) transient Ca2+ currents to shift towards positive values on the voltage axis. Changing [Ca2+]o from 0.5 to 5 mM caused the inactivation curve to shift by 21 mV.
Extracellular Ca2+ blocked a steady cationic current. This current reversed at −35 mV, was scarcely affected by Mg2+ and was completely blocked by the non-selective cation channel inhibitor gadolinium (10 μM). The effect of [Ca2+]o was mimicked by 500 μM spermine, a polyamine which acts as an agonist for the Ca2+-sensing receptor, and was modulated by intracellular GTP-γ-S.
At the resting potential, both the voltage shift and the block of the inward current removed the inactivation of LVA calcium channels and, together with the increase in the Ca2+ driving force, favoured a rise in the low threshold Ca2+ spikes, causing the thalamic firing to change to the oscillatory mode.
Our data indicate that [Ca2+]o is involved in multiple mechanisms of control of the thalamic relay and pacemaker activity. These findings shed light on the correlation between hypercalcaemia, low frequency EEG activity and symptoms such as sleepiness and lethargy described in many clinical papers.
The role of internal Ca2+ in neuronal processing has been widely studied but less is known about Ca2+ from an extracellular point of view. In pathological conditions involving persistent hypercalcaemia, various neuropsychiatric symptoms can be present, including a state ranging from drowsiness to lethargy, and there is low frequency electroencephalographic (EEG) activity (Evaldsson et al. 1969; Allen et al. 1970; Guisado et al. 1975; Joborn et al. 1991). The thalamic neurons play an important role in the electro-cortical rhythms. They fire with single spikes during wakefulness (relay mode), and oscillate and synchronise their firing, producing bursts of action potentials, during slow-wave sleep (oscillatory mode). The rhythms arise as a consequence of the intrinsic pacemaker property of the neurons and the network of excitatory and inhibitory synaptic connections within the thalamus and between the thalamus and the cortex (Steriade & Llinas, 1988; McCormick & Bal, 1997).
The transition from relay mode to oscillatory mode is caused by a resting membrane hyperpolarisation of the thalamo-cortical neurons (Jahnsen & Llinas, 1984; Steriade & Llinas, 1988; Von Krosigk et al. 1993; McCormick & Bal, 1997). The hyperpolarisation de-inactivates the LVA Ca2+ currents. Activation of the LVA Ca2+ current depolarises the membrane towards the threshold for a burst of Na+ and K+-dependent action potentials. Ca2+ also enters the cell during the burst through a high voltage-activated (HVA) Ca2+ conductance, leading to the activation of a Ca2+-dependent K+ current. Combined with the inactivation of LVA Ca2+ channels this causes membrane hyperpolarisation, which subsequently de-inactivates LVA Ca2+ channels and activates a hyperpolarisation-activated cation current (Ih), which slowly depolarises the membrane towards the threshold for another Ca2+ spike (McCormick & Bal, 1997). Resting membrane hyperpolarisation therefore leads to the interplay between the low threshold Ca2+ spike and the Ih that results in low frequency oscillations.
According to the surface potential theory, Ca2+ ions should modify the cellular transmembrane potential, masking the negative fixed charges on the surface, hyperpolarising the membrane (for review see Green & Andersen, 1991; Hille, 1991) and causing the thalamic pacemaker and cortical neurons to shift from the relay to the oscillatory mode.
Here we show that in current-clamped isolated thalamic neurons an increase in extracellular Ca2+ alone causes the neuronal firing to change from single spikes to the bursting oscillatory mode. Whole-cell patch-clamp recordings indicate that this transition is caused by a voltage shift to positive values of the voltage-dependent characteristics of the channels, and by an increase in the Ca2+ driving force, but unexpectedly also by membrane hyperpolarisation due to a decrease in a novel membrane cationic inward current coupled through G-proteins to a Ca2+-sensing receptor. Preliminary reports of our results have already been presented (Formenti et al. 1996,Formenti 1998a; De Simoni et al. 2000).
METHODS
Cell preparation
The experiments were done on primary rat thalamic cell cultures. The neurons were prepared as previously described (Formenti et al. 1995; Formenti & De Simoni, 2000). Briefly, 14 to 18-day-old Wistar rats were anaesthetised with ether, decapitated and the brain was removed and placed in cold Pipes-buffered saline (4 °C) containing (mm): NaCl 120, KCl 5, MgCl2 4, glucose 25 and Pipes 20; pH was adjusted to 7.4 with NaOH. The brain was then dissected in the frontal plane using a vibroslice (Campden Instruments Ltd, UK). Frontal slices (500 μm thick) containing the thalamus were incubated at room temperature for 75 min in Pipes-buffered saline containing 0.8 mg ml−1 trypsin (Sigma type XI), 1 mm kynurenic acid and 50 μm 2-amino-5-phosphonovalerate (APV), saturated with O2. After the enzymatic treatment, the thalamus was identified, carefully isolated, and gently minced in Pipes-buffered solution using a Pasteur pipette. The isolated neurons were plated on Petri dishes and stored at room temperature. All procedures were in accordance with local and national laws and ethical guidelines (municipal act no. 143650.400/16754/90; decree 36/94-A Ministry of Health).
Isolated cell recordings
Current and voltage-clamp recordings were carried out a few hours after plating at 22 °C, using the whole-cell patch-clamp technique (Hamill et al. 1981). The methods and instrumentation used in this study were similar to those previously described (Formenti et al. 1998b). Patch pipettes of about 6 MΩ resistance were used, with a NPI Sec1L switching amplifier (Tamm, Germany; sampling rate 15-30 kHz), interfaced with an IBM-compatible computer. Stimulation and data acquisition were done using pCLAMP programs (Axon Instruments, Foster City, CA, USA) and the data were digitised at 50 μs sampling times using a 12-bit A/D Tecmar Master board. Statistical data are presented as means ± s.e.m.
Solutions for recordings
The solutions used for whole-cell patch-clamp recordings are summarised in Table 1. To reduce the run-down of calcium current, an ATP regenerating system (Forscher & Oxford, 1985) consisting of 20 mm creatine phosphate, 4 mm Na2-ATP and 50 IU ml−1 creatine phosphokinase (bovine heart, Sigma Type III) was added to the patch pipette solution. All the drugs were supplied by Sigma (St Louis, MO, USA).
Table 1.
Solutions used for whole-cell patch-clamp recordings
| CaCl2 | MgCl2 | NaCl | KCl | NaH2PO4 | NaHCO3 | Glucose | pH | |
|---|---|---|---|---|---|---|---|---|
| Ext 1 | 0.5–5 | 2 | 125–117.5 | 3 | 1.2 | 26 | 10 | 7.4 |
| Ext 2 | 0.5–4.5 | 4.5–0.5 | 122 | 3 | 1.2 | 26 | 10 | 7.4 |
| CaCl2 | MgCl2 | NaCl | TEA | Hepes | Choline chloride | Glucose | pH | |
| Ext 3 | 0.5–5 | 1 | — | 20 | 10 | 100.5–105 | 10 | 7.4 |
| CaCl2 | MgCl2 | NaCl | KCl | Hepes | Choline chloride | Glucose | pH | |
| Ext 4 | 4.5–0 | 0–4.5 | 141 | 3 | 10 | — | 10 | 7.4 |
| Ext 5 | 0.01–0.1 | — | 141 | 3 | 10 | — | 10 | 7.4 |
| 0.5–1–5 | ||||||||
| 10 | ||||||||
| Ext 6 | 4.5–0 | 0–4.5 | — | — | 10 | 144 | 1 | 7.4 |
| Ext 7 | 4.5–0 | 0–4.5 | — | 20 | 10 | 124 | 10 | 7.4 |
| Ext 8 | 4.5–0 | 0–4.5 | — | 144 | 10 | — | 10 | 7.4 |
| Ext 9 | 4.5–0 | 0–4.5 | 20 | — | 10 | 124 | 10 | 7.4 |
| Ext 10 | 4.5–0 | 0–4.5 | 144 | — | 10 | — | 10 | 7.4 |
| Ext 11 | 0.5–4.5 | — | 141 | 3 | 10 | — | 10 | 7.4 |
| Ext 12 | — | 0.5–4.5 | 141 | 3 | 10 | — | 10 | 7.4 |
| Ext 13 | 0.5–4.5 | 4.5–0.5 | 141 | 3 | 10 | — | 10 | 7.4 |
| CaCl2 | MgCl2 | Potassium gluconate | KCl | Hepes | EGTA | — | pH | |
| Int 1 | 1 | 1 | 135 | — | 10 | 11 | — | 7.3 |
| Int 2 | 1 | 2 | — | 140 | 10 | 11 | — | 7.3 |
| CsCl | MgCl2 | TEA | — | Hepes | EGTA | Glucose | pH | |
| Int 3 | 110 | 0.8 | 30 | — | 10 | 10 | 8 | 7.3 |
Ext, extracellular solution; Int, intracellular solution. Concentrations are millimolar. Solutions Ext 3, 6, 7 and 8 and Int 3 were titrated with CsOH.
RESULTS
An increase of [Ca2+]o caused the discharge to change to burst mode in current-clamped isolated thalamic neurons
To study the effects of [Ca2+]o on the neuronal firing mode, current-clamp experiments were carried out on isolated thalamic neurons. Figure 1A shows the discharge induced by a depolarising current stimulus at different extracellular calcium concentrations. Extracellular and intracellular solutions Ext 1 and Int 1 (Table 1) were used in these experiments. Cellular firing could change from the single spike mode (relay mode) to the burst mode (oscillatory mode), usually with variations of about 1 mm in the [Ca2+]o, especially in the concentration range of 1 to 2 mm, and the effect was completely reversible. Figure 1B shows the appearance of a low threshold calcium spike after an ‘anode break’ stimulus, when changing the [Ca2+]o from 1.5 to 2 mm. The increase in [Ca2+]o was accompanied by measurable membrane hyperpolarisation in all neurons (n = 22; Fig. 1A and Fig. 3).
Figure 1. Single spikes and burst mode discharge in isolated thalamic neurons in vitro as a function of [Ca2+]o.
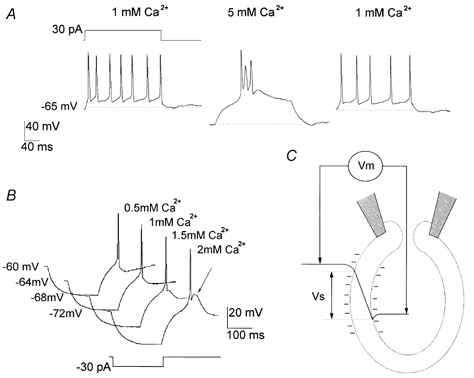
A, in a current-clamped cell, a depolarising current stimulus (upper trace) evoked cell firing. Altering [Ca2+]o from 1 to 5 mm caused the discharge to turn into burst mode (oscillatory mode), which returned to the single spike discharge (relay mode) when the [Ca2+]o was returned to 1 mm. Note the hyperpolarisation of the resting potential induced by the increase in [Ca2+]o. B, scaled current traces from a neuron with an ‘anode-break’ stimulus (duration, 200 ms; amplitude,-30 pA) from a resting potential near −60 mV, during superfusion with [Ca2+]o at the values indicated beside each trace. Note the appearance of a low threshold calcium spike on changing from 1.5 to 2 mm Ca2+ (arrow). C, the profile of the electrical potential across the cell membrane. According to the surface potential hypothesis, the potential difference between the bulk solutions (Vm) recorded in the whole-cell patch-clamp configuration is different from the potential between the two membrane surfaces (Vs), which is sensed by the channels.
Figure 3. Effects of changes in [Ca2+]o on the membrane resistance and on the resting potential in whole cell current-clamp experiments.
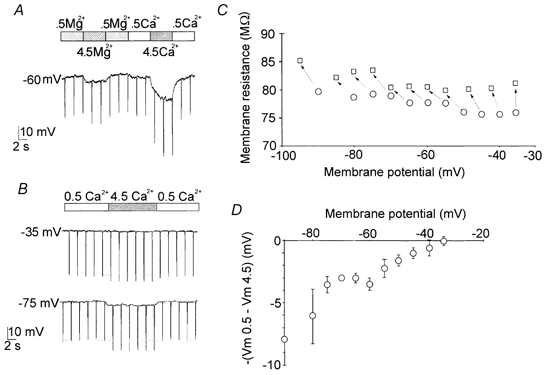
A, increasing extracellular Mg2+ from 0.5 to 4.5 mm caused a weak hyperpolarisation compared to that caused by the same change in Ca2+ (Mg2+,-6.6 ± 0.7 mV; Ca2+,-22.8 ± 2.5 mV; n = 4; Int 2 and Ext 11/12 in Table 1). The membrane hyperpolarisation was accompanied by a proportional increase in membrane resistance, tested by hyperpolarising current steps (amplitude, 500 pA; duration, 50 ms). B, the divalent cations in all the extracellular solutions were balanced to 5 mm by adding Mg2+, to minimise the induction of a voltage shift of the voltage-dependent characteristics of the channels. Raising [Ca2+]o from 0.5 to 4.5 mm increased membrane resistance from 78.5 ± 0.3 to 82 ± 0.7 MΩ, n = 11, when evaluated between -80 and −60 mV. This effect was accompanied by persistent hyperpolarisation when the resting membrane potential was maintained below −35 mV (Int 1 and Ext 13 in Table 1). In A and B the bars at the top indicate the external solution changes. C, the resistance and voltage changes seen on raising [Ca2+]o from 0.5 mm (○) to 4.5 mm (□). Same cell as shown in B; each arrow represents one trial. D, the amplitude of the membrane hyperpolarisation on raising [Ca2+] from 0.5 to 4.5 mm as a function of the resting potential in 0.5 mm Ca2+. Each point represents the mean of 11 recordings from different cells.
In a preliminary study we used the cell-attached configuration of the patch-clamp technique to quantify the effects of changes in [Ca2+]o on seal resistance, in order to exclude the possibility that the calcium-induced hyperpolarisation was an artifact caused by increased seal resistance (Formenti & De Simoni, 2000). The seal resistance rose significantly as a function of [Ca2+]o only for seal resistances lower than 1 GΩ; with better seal resistances changes in [Ca2+]o had a negligible effect on seal resistance.
According to the surface potential theory, Ca2+ ions should modify the cellular transmembrane potential (Vs), masking the negative fixed charges on the surface and hyperpolarising the membrane. This effect is not directly measurable, since in our experimental conditions the electric potential (Vm) is measured not between the inner and outer membrane surfaces, but between the intracellular and extracellular bulk solutions (Fig. 1C); however, the transmembrane voltage change should be sensed by the voltage-activated channels in the membrane, and it may appear as a shift of the voltage-dependent characteristics along the voltage axis. On the other hand, this theory can hardly account for the hyperpolarisation observed directly in current-clamp recordings. [Ca2+]o may have some effect on membrane conductance.
External changes in [Ca2+] shifted the voltage-dependent characteristics of LVA Ca2+ channels
To evaluate the voltage shift expected when the [Ca2+]o increased, whole-cell voltage-clamp recordings were made on cultured thalamic neurons. The effects were studied on LVA calcium channels, because this membrane conductance is involved in generating the low threshold spikes and oscillatory behaviour.
LVA Ca2+ currents were evoked by a 200 ms voltage ramp from a holding potential of −140 mV to +20 mV. These experiments were done using solutions Ext 3 and Int 2 in Table 1. Increasing [Ca2+]o from 0.5 to 5 mm raised the Ca2+ current peak amplitude, with a shift of the LVA Ca2+ current peak and of the relative activation curve of 18.7 ± 1.6 mV, n = 8; (Fig. 2A). The voltage-dependent characteristics of HVA Ca2+ currents showed a shift that did not significantly differ from the LVA currents (22.5 ± 2.3 mV, n = 6; data not shown). Figure 2B shows the voltage shift of the inactivation curve of the LVA Ca2+ currents. As the [Ca2+]o increased, the inactivation curve shifted towards positive potentials, and the channels were de-inactivated.
Figure 2. Voltage-dependent characteristics of LVA Ca2+ currents measured at different [Ca2+]o.
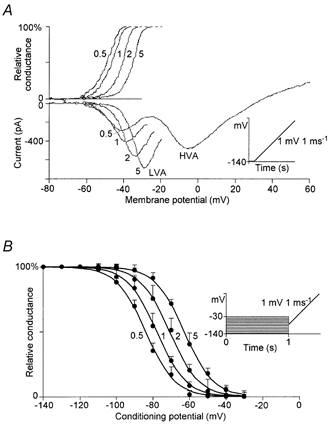
The effects were tested by whole-cell voltage-clamp recordings in acutely dissociated thalamic neurons. A, lower panel, the current-voltage relationship (I-V) of LVA Ca2+ currents was obtained using voltage ramp stimuli from a holding potential of −140 mV to +20 mV (inset) at different [Ca2+]o; the concentration (mm) is indicated on each curve. The activation curves (upper panel) were calculated from the experimental I-V traces: relative conductance = ICa/(VmECa), normalised to 100 % (where ECa is the Ca2+ equilibrium potential). An increase in [Ca2+]o caused an augmentation of the peak Ca2+ current amplitude and shifted the current activation and current peak towards positive values. B, the inactivation curve measured at different [Ca2+]o. LVA Ca2+ currents (not shown) were obtained using a voltage ramp stimulus repeated after conditioning potentials scaled between -140 and −30 mV (inset). The ramp stimulus was adopted in order to identify and measure the peak LVA calcium current and to distinguish it clearly from other currents during the voltage changes induced by different [Ca2+]o. For each concentration and each cell recorded, peak currents were normalised as a percentage of the maximum current. The means ± s.e.m. from six cells were plotted in the figure as a function of the conditioning potential. The values were fitted with Boltzmann curves: 1/[1 + exp(VV0.5)/k] where V0.5, the midpoint, changed from -84 to-78,-71 and −63 mV,when the [Ca2+]o was 0.5, 1, 2 and 5 mm, respectively; k (8 mV) is the slope factor.
An increase in [Ca2+]o hyperpolarised the isolated thalamic neurons, blocking an inward current
The possibility that the Ca2+-induced hyperpolarisation was caused by a voltage shift in the voltage-dependent characteristics of the membrane was considered. Testing different divalent cations in the extracellular solution, we noted that Ba2+ mimicked the hyperpolarising effects of Ca2+ (data not shown), whereas Mg2+ caused only a small negative change in membrane potential in current-clamped thalamic neurons (n = 11, Fig. 3A). The screening effect of Mg2+ on the negative fixed charges of the membrane was similar to that induced by Ca2+ ions since switching from 4.5 mm Ca2+ to 4.5 mm Mg2+ (solution Ext 13 in Table 1) did not change the voltage at the peak of the Na+ current evoked in voltage-clamped neurons (Fig. 4, n = 15). The voltage-dependent Na+ current was measured in place of the LVA Ca2+ current because the change in LVA Ca2+ current intensity, due to the variation in [Ca2+]o, tended to modify the slope of the voltage ramp to which the neuron was subjected, even in well-clamped cells. This would cause a small artefact, shifting the current peak on the voltage axis. In view of its properties, Mg2+ was used to set the divalent cations of the extracellular solutions to a constant concentration, to minimise the induction of a voltage shift while changing from one Ca2+ concentration to another. In these experimental conditions too, Ca2+ had a similar but weaker hyperpolarising effect (Fig. 3B). An increase in [Ca2+]o raised membrane resistance. This was observed in current-clamp experiments using hyperpolarising current steps (Fig. 3B). The increase in membrane resistance was accompanied by persistent hyperpolarisation when the resting membrane potential was below −35 mV (n = 11; Fig. 3C). The hyperpolarisation increased at more negative resting potentials (Fig. 3D). These data suggest that an inward current is blocked or modulated by Ca2+.
Figure 4. External Ca2+ and Mg2+ had similar effects on the surface potential.
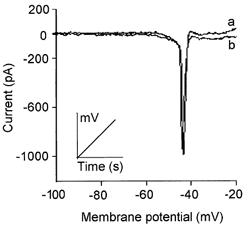
To study the effect of changes in [Ca2+]o on membrane current, avoiding the voltage shift due to the non-specific screening effect of Ca2+ ions, the solutions were balanced with Mg2+. This figure shows that the voltage at the peak of the Na+ current evoked by a voltage ramp (0.66 mV ms−1; inset) was not affected by changing the external solution from 4.5 mm Ca2+ and 0.5 mm Mg2+ (trace a) to 0.5 mm Ca2+ and 4.5 mm Mg2+ (trace b; Int 1 and Ext 13 in Table 1). The voltage at the peak was -43 ± 0.5 and -45 ± 3.4 mV with 4.5 mm Ca2+ and 4.5 mm Mg2+, respectively (n = 15). The difference was not significant (P > 0.05). This indicates that Ca2+ and Mg2+ ions exert the same screening effect on the negative fixed charges at the mouth of the Na+ channels.
A novel Ca2+-sensitive conductance in thalamic neurons
Whole-cell voltage-clamp experiments were performed to test the blocking effect of Ca2+ on steady-state total currents at different test potentials. Solutions containing divalent cations (Ca2+ plus Mg2+) at a constant concentration were used. The voltage-clamp protocol used to study how [Ca2+]o affected the whole-cell persistent ionic currents consisted of a positive depolarising step or ramp, followed by scaled steps of 10 s duration from -20 to −80 mV. The first depolarisation was intended to check and to inactivate the transient current components. During the next set of steps, changing [Ca2+]o from 4.5 mm to 0 mm caused the ionic currents to increase (Fig. 5A). The current amplitude was determined as the mean during the period from 2 s after the beginning of the superfusion till the end. The difference at 0 and 4.5 mm[Ca2+]o plotted as a function of the potential represents the current-voltage relationship of the Ca2+-sensitive current. The reversal potential was about −35 mV (n = 17; Fig. 5B). The I-V relationship was almost linear over the whole potential range tested, suggesting that this conductance is not voltage dependent.
Figure 5. Effects of external Ca2+ on the total persistent ionic currents.
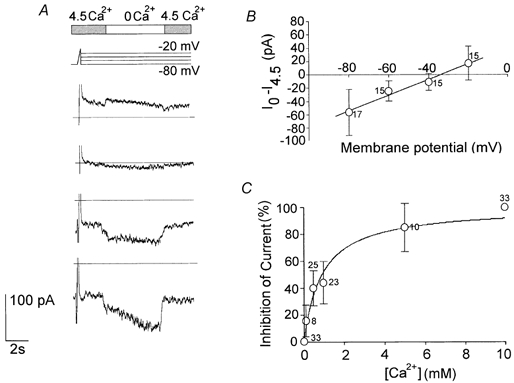
The effects of different [Ca2+]o on the total persistent ionic currents were investigated in whole-cell voltage-clamp experiments using solutions balanced with Mg2+ (Ext 4, Int 2 in Table 1). A, to study the effects of [Ca2+]o (top bar) on the whole-cell currents (traces below) a voltage-clamp protocol was used, consisting of a 100 ms depolarising ramp from -80 to −20 mV (to check and inactivate the transient current components), followed by 10 s scaled step potentials from -20 to −80 mV. Removing the extracellular Ca2+ increased the ionic currents. B, the current-voltage relationship shows the fraction of the currents activated by changing from 4.5 to 0 mm Ca2+, as a function of the test potential (○, ± s.e.m.). Note that the reversal potential is nearly the same as in current-clamp recordings (Fig. 3D). C, the percentage inhibition of the current as a function of [Ca2+]o (Int 2 and Ext 5 in Table 1). Data were fitted with a saturation binding curve: y = [Ca2+]/Kd+[Ca2+], where Kd, the equilibrium dissociation constant, is 0.91 ± 0.15 mm (n = 19). All measurements were taken at −80 mV. The number of observations is indicated beside each data point in B and C.
Various [Ca2+]o were tested and the dose-response curve is shown in Fig. 5C. The current was recorded at −80 mV at different Ca2+ concentrations (0.01, 0.1, 0.5, 1, 5 and 10 mm), then it was normalised and plotted as a percentage of the total current. The data points were fitted using a saturation binding curve. The thalamic current was maximal at [Ca2+]o below 10 μm. The concentration that blocked the current by 50 % was 0.9 ± 0.15 mm (n = 19), and above 5 mm the current was reduced to less than 20 % of its maximum amplitude.
The Ca2+-sensitive current is carried by sodium and potassium ions and blocked by gadolinium
The current evoked by lowering [Ca2+]o from 4.5 to 0 mm was completely abolished in a Na+-free, K+-free solution in all neurons (n = 4; holding potential,−80 mV). This therefore excludes any permeability to Cl− since this species was present in the Na+-free, K+-free solution with a reversal potential of about -2mV (solutions Ext 6 and Int 3 in Table 1; Fig. 6A). We tested the relative permeability of the two ions in 13 neurons. The Ca2+-sensitive current was present in Na+-free solutions, its intensity proportional to the external K+ concentration. The amplitude of the current in the presence of 144 and 20 mm[K+]o was 49.6 ± 3.2 and 17.7 ± 1.2 pA (n = 6), respectively (solutions Ext 7/8 and Int 3 in Table 1; Fig. 6B). Similarly, the amplitude of the currents in the presence of 144 and 20 mm[Na+]o as a charge carrier, using K+-free solutions, was 114.1 ± 9.2 and 35.6 ± 7 pA (n = 7), respectively (solutions Ext 9/10 and Int 3; Fig. 6C).
Figure 6. Effects of the external concentration of Na+ and K+ on the selectivity of the Ca2+-sensitive channels.
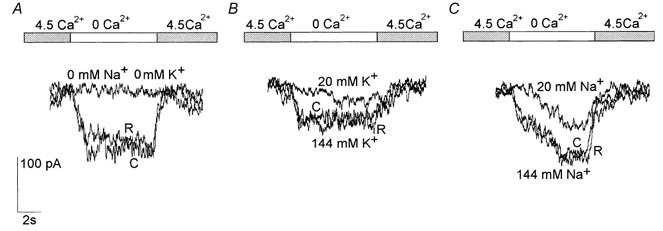
The current activated by decreasing the [Ca2+]o to zero (top bar) was completely abolished when the external Na+ and K+ (Ext 4 in Table 1) were replaced with choline (A, Ext 6 in Table 1). The Ca2+-sensitive current was partially preserved in the presence of external K+ at 144 and 20 mm (B, Ext 8 and 7 in Table 1) or 144 and 20 mm Na+ (C, Ext 10 and 9 in Table 1). Note that Na+ is more permeant than K+. All the recordings were taken at −80 mV. C, control before changing to 0 Ca2+; R, recovery on return to 4.5 mm Ca2+.
We used blockers with the aim of pharmacologically identifying the Ca2+-sensitive conductance. Gadolinium added to the external solution had a powerful blocking effect: at 10 μm it completely blocked the current evoked by lowering [Ca2+]o from 4.5 to 0 mm, and also reduced the residual current at 4.5 mm Ca2+ (n = 14; Fig. 7). External Cs+ (1-3 mm) or the Na+ channel blocker TTX (1 μm), and internal Cs+ (110 mm) or TEA (30 mm) did not affect the Ca2+-sensitive current in the neurons (n = 25).
Figure 7. Gadolinium was a powerful blocker of the Ca2+-sensitive cation current.
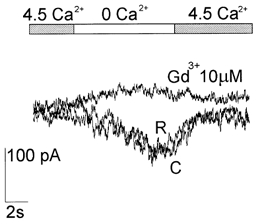
Gd3+ (10 μm) added to the external solutions completely abolished the current activated by lowering [Ca2+]o from 4.5 to 0 mm (top bar), recorded at −80 mV (Int 2 and Ext 4 in Table 1). The effect persisted even when the [Ca2+]o was raised again to 4.5 mm. This suggests that it blocks a residual Ca2+-sensitive current present at that Ca2+ concentration. C, control before changing to 0 Ca2+; R, recovery on return to 4.5 mm Ca2+.
The Ca2+-sensitive current is modulated by GTP-γ-S
We tested whether these channels were controlled by G-proteins, utilising the non-hydrolysable GTP analogue guanosine 5′-O-(3-thiotriphosphate) (GTP-γ-S; 500 μm) in the recording pipette. GTP-γ-S caused a marked transient increase in the conductance followed by a complete block in about 8 min of whole-cell current recorded in all the neurons tested (n = 15; Fig. 8). This biphasic action suggests that multiple G-protein mechanisms are involved.
Figure 8. The Ca2+-sensitive current is modulated by GTP-γ-S.
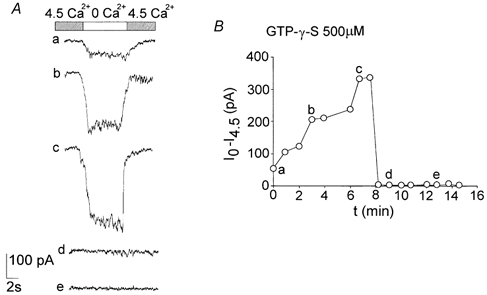
GTP-γ-S (500 μm) added to the intracellular solution in the recording pipette had a biphasic effect. It increased the Ca2+-sensitive current, which reached a peak about 6-7 min after the establishment of the whole-cell configuration. The Ca2+-sensitive current then showed rapid decay until it was almost completely abolished in all the neurons (B). The current traces shown in A relate to the labelled points in B. All the recordings were taken at −80 mV (Int 2 and Ext 4 in Table 1).
Spermine mimics Ca2+ in blocking the cationic current
Since the Ca2+-sensitive current found in thalamic neurons was modulated by GTP-γ-S, the non-selective cationic channels may have been G-protein coupled to a Ca2+-sensing receptor. To test this, spermine, a Ca2+-sensing receptor agonist, was added to the extracellular solution (Ext 4, with 0.5 mm Ca2+ and 50 μm APV) at a concentration of 500 μm. This drug partially and reversibly inhibited the whole-cell persistent ionic currents evoked at scaled step potentials from -80 to −20 mV. The current-voltage relationship of the inhibited component showed a reversal potential at about −28 mV (Fig. 9) very close to that for the Ca2+-sensitive current component (Fig. 5B).
Figure 9. Spermine mimics Ca2+ in blocking the cationic current.
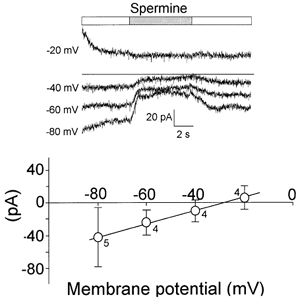
The effects of spermine (500 μm; top bar) on the total persistent ionic currents (traces below) were tested in whole-cell voltage-clamp experiments. The stimulus protocol was similar to that shown in Fig. 5. The potential at which each current was recorded is indicated (Ext 4 with 0.5 mm Ca2+, Int 2 in Table 1). Spermine perfused extracellularly partially inhibited the persistent ionic currents. The effect was reversible. The graph below shows the spermine-sensitive current plotted against the membrane potential (○, ± s.e.m.). The current reversed at about −28 mV.
DISCUSSION
A relatively small increase in [Ca2+]o alone is enough to induce the transition of neuronal firing from single spikes to burst mode in current-clamped isolated thalamic neurons. The potential sensed by the ionic channels is what controls the firing mode of thalamic and other central neurons. We investigated, in whole-cell patch-clamp experiments, the mechanisms by which calcium affects the neuronal discharge.
The rise in [Ca2+]o hyperpolarises the neuronal membrane, shifting the conductance-voltage relationship of LVA Ca2+ channels to the right (see Fig. 2). The block of or changes in the Ca2+-sensitive cation current also seem to be important in controlling the transmembrane potential.
The membrane hyperpolarisation induced by a rise in [Ca2+]o through the above-mentioned effects, and the increase in the Ca2+ driving force at the resting potential, which favours the appearance of low threshold Ca2+ spikes, cause the neuronal firing to shift from the relay to the oscillatory mode.
Changes in external Ca2+ concentration affect the voltage-dependent properties of ionic channels
As predicted by the surface potential theory (Green & Andersen, 1991; Hille, 1991), there was a shift in the voltage-dependent characteristics of LVA Ca2+ channels. On increasing the [Ca2+]o, the inactivation curve of the LVA Ca2+ currents shifted to positive values on the voltage axis. This was consistent with de-inactivation of the LVA Ca2+ channels, which are necessary to give a low threshold Ca2+ spike; in our experimental conditions, it is likely that the LVA Ca2+ current was less than in vivo since some of these channels are presumably lost with the distal dendrites. However, the effects of Ca2+ are not only based on this cellular mechanism. This is particularly evident if we compare the effects of Ca2+ and Mg2+. Mg2+ shared with Ca2+ similar screening effects on the negative fixed charges of the membrane (Fig. 4), but induced a smaller hyperpolarisation at the resting potential in current-clamped neurons (Fig. 3A). Mg2+, in place of Ca2+, prevented the generation of low threshold spikes in guinea-pig nucleus reticularis thalami neurons (Bal & McCormick, 1993). Similarly, experiments from our laboratory indicated that Mg2+ did not cause the neuronal discharge to shift to the burst mode (authors’ unpublished results).
External Ca2+ blocks an inward non-selective cation current in isolated thalamic neurons
We constantly observed a directly measurable hyperpolarising effect of the increase in [Ca2+]o. Since this effect persists during the perfusion, and is accompanied by a steady increase in the membrane resistance at all potentials tested, we assume that extracellular Ca2+ blocks channels that underlie a steady depolarising current. This block could hardly be attributed to a side effect of the voltage shift, since Mg2+ was less effective in inducing hyperpolarisation (Fig. 3A) though, as discussed above, its screening properties were comparable to Ca2+. However, in order to completely rule out the effect of the voltage shift, Mg2+ was added to the external solutions to a final concentration of 5 mm divalent cations (Ca2+ and Mg2+ together). The hyperpolarising effect was seen in these experimental conditions too, confirming that it was due to a specific block by extracellular Ca2+ of an inward current. The effect of Ca2+ was mimicked by Ba2+. The reversal potential of −35 mV implies that more than one ion is involved as a charge carrier.
Various currents, such as the Ih (McCormick & Pape, 1990; Pape, 1996), the persistent Na+ current (INa,P) (Jahnsen & Llinas, 1984; Crill, 1996), and the Ca2+-activated non-specific cation currents (McCormick & Bal, 1997), were observed in these thalamic cells, and they can all be considered as possible targets for the Ca2+ ions.
Although a weak blocking effect of extracellular Ca2+ on the inwardly rectifying cation current (If, present in heart and similar to Ih) has been reported (Ono et al. 1994), we can exclude the possibility that a block of Ih could account for the hyperpolarisation we observed in vitro, since in these acutely dissociated thalamic neurons Ih is hardly detectable in the hours that follow cell dissociation (data not shown), in agreement with a previous observation (Budde et al. 1994). In addition, 1-3 mm Cs+ added to the external solution, which is supposed to block If (Pape, 1996), did not prevent the blocking effect of an increase in [Ca2+]o, and Ca2+ exerts its blocking effect even at potentials of −20 mV, at which Ih should not be activated (Pape, 1996). Nevertheless, in vivo there is likely to be a partial block of Ih by extracellular Ca2+, and this should result in further hyperpolarisation of the thalamic neurons in the negative voltage range at which this current is activated.
Non-specific cation currents with a different sensitivity to Ca2+ ions have been described in various cells. Calcium-activated non-specific cation (CAN) channels are found in the reticular thalamic neurons and it is proposed that they induce a slow depolarising afterpotential and the generation of a tonic discharge at the end of the oscillatory burst (Bal & McCormick, 1993). The probability of their opening increases as the cytoplasmic Ca2+ concentration rises (Partridge et al. 1994), whereas, to our knowledge, no effects of the [Ca2+]o on these channels have been described. Furthermore, Ca2+ had a hyperpolarising effect in almost all the cells tested, and in our cell preparations there are reticular neurons, but also relay neurons, whereas ICAN was found only in the former (McCormick & Bal, 1997).
A non-selective cation conductance has been observed in the midbrain dopaminergic neurons of the rat, which is blocked by extracellular Ca2+ (Farkas et al. 1996). However, in that study, unlike in ours, Mg2+applied externally had the same blocking effect as Ca2+.
The INa,P presents some interesting features (Crill, 1996). It is non-inactivating and is affected by [Ca2+]o. High [Ca2+]o shifted the activation curve to positive potentials, and reduced the current amplitude (Li & Hatton, 1996); however, this cannot account for the effects at very negative potentials, where INa,P is deactivated. In addition, 1 μm TTX added to the external solution did not prevent the blocking effect induced by increasing [Ca2+]o, and this channel cannot account for the permeability to K+ (Fig. 6B).
Recently, the inhibitory effect of gadolinium on the Na+-Ca2+ exchanger in guinea-pig ventricular myocytes has been described (Zhang & Hancox 2000). The effect observed differs from that described here, since it is necessary to use a 10-fold higher concentration of Gd3+ to fully block the Na+-Ca2+ exchanger and the time from Gd3+ application to steady-state effect was very slow in their experiments.
The current described here has some characteristics similar to those observed in Xenopus oocytes (Arellano et al. 1995), in mouse hyppocampal neurons (Xiong et al. 1997), and in rat cardiac muscle (Mubagwa et al. 1997). All these currents are activated by lowering the [Ca2+]o, are permeable to Na+ and K+, and are blocked by Gd3+ at micromolar concentrations. However, the current described by Xiong et al. (1997) in hippocampal neurons was not affected by non-hydrolysable GTP analogues, the current observed by Arellano et al. (1995) had very slow activation kinetics and limited permeability to calcium ions, and that observed by Mubagwa et al. (1997) was also blocked by Mg2+ and Ba2+. The thalamic current was half-maximal at 0.9 mm[Ca2+]o. This concentration is higher than other Ca2+-sensitive currents and closer to the physiological Ca2+ concentration range in the intraventricular fluid.
Ca2+-sensitive cation conductance is G-protein linked to a Ca2+-sensing receptor
The extracellular, G-protein-linked Ca2+-sensing receptor, first identified in the parathyroid gland (Brown et al. 1993), has been found in various regions of the brain (Ruat et al. 1995; Rogers et al. 1997). In some cases this receptor is functionally coupled to ionic channels (Ye et al. 1996a; b; McGehee et al. 1997; Chattopadhyay et al. 1999; Washburn et al. 2000). Since the Ca2+-sensitive current described here was modulated by GTP-γ-S; Ye et al. 1998b the possibility that non-selective cationic channels are G-protein-coupled to a Ca2+-sensing receptor, as observed in pancreatic β cells (Straub et al. 2000) and monocytes (Yamaguchi et al. 2000), was taken into account. Spermine, an endogenous polyamine that agonises the Ca2+-sensing receptor (Quinn et al. 1997), mimicked the effects of external Ca2+ when added to the extracellular solution. This confirms that the Ca2+-sensing receptor is G-protein coupled to a non-selective cationic channel at the postsynaptic level in thalamic neurons too.
Physiopathological considerations
Calcium is exchanged between blood and cerebrospinal fluid (CSF) through the blood-brain barrier and the Ca2+ influx to the CSF and brain seems to be linearly related to the plasma ionised calcium concentration (Tai et al. 1986). Effects on EEG rhythms are seen in many pathological states primarily involving calcium metabolism, such as hyperparathyroidism and vitamin D poisoning (Evaldsson et al. 1969). They may also occur in other conditions in which the [Ca2+]o is secondarily influenced, such as malignancies (Moure & Houston, 1967; Allen et al. 1970), renal failure (Guisado et al. 1975), and some disorders of the endocrine system (Evaldsson et al. 1969; Allen et al. 1970). As mentioned in the Introduction, during persistent hypercalcaemia a state ranging from drowsiness to lethargy can be present, and there is low frequency EEG activity (Evaldsson et al. 1969; Allen et al. 1970; Guisado et al. 1975; Joborn et al. 1991).
In vivo experiments from our laboratory showed that intraventricular CaCl2 injections in freely moving adult rats tripled the EEG amplitude and increased the relative power of the low EEG frequencies, with a decrease of the fast activity (Formenti et al. 1996). It is reported in the literature that intracerebral injections of CaCl2 induced EEG slow wave electrical activity, synchronisation and a sleep-like state in cats, rabbits and other animals (Cloetta & Fischer, 1930; Stern & Chvoles, 1933).
There is a strong correlation between the thalamic mode of discharge and the EEG rhythms. Some mechanisms have been proposed to account for this correlation (Jahnsen & Llinas, 1984; Steriade & Llinas, 1988; Von Krosigk et al. 1993; McCormick & Bal, 1997); however, it is likely that other parts of the nervous system are involved in the effects of Ca2+. In vivo[Ca2+]o may also affect other neurons, for instance neocortical cells exhibit LVA Ca2+ currents and can produce a burst discharge.
Although highly synchronous EEG and epileptic seizures may appear at the highest calcium concentrations both in vivo and in vitro (Bragdon et al. 1992), seizures arising from different structures in the brain are mainly observed at low [Ca2+]o (Heinemann et al. 1986; Dichter & Ayala, 1987; Jefferys, 1995) and tend to disappear when the [Ca2+]o returns to normal. Here again, the electrical potential sensed by the channels presumably controls the mode of discharge. This is corroborated by the observation that seizures are also induced by an increase in the external K+ concentration or other agents that can also induce depolarisation (for review see Dichter & Ayala, 1987). Many anti-epileptic drugs, on the other hand, have inhibitory and hyperpolarising effects, and can induce drowsiness (Macdonald & Kelly, 1995). Thus, whereas the hyperpolarising effects lead to EEG synchronisation induced by a neuronal burst discharge dependent on the LVA Ca2+ channel, the depolarising effects of low [Ca2+]o, or an increase in external K+, lead to a lower frequency pathological burst discharge and epileptic seizures. This latter activity may, at least in some cases, involve enhanced Na+ influx through INa,P rather than LVA Ca2+ currents (Li & Hatton, 1996). In addition, the reduction in [Ca2+]o that may occur during intense neuronal activity can act as a positive feedback, activating the Ca2+-sensitive depolarising current, leading to further depolarisation and hyperexcitability.
[Ca2+]o influences many behavioural aspects, depending on the brain structures involved. By increasing [Ca2+]o in the caudal portion of the hypothalamus, Veale & Myers (1971) induced sleep and a catatonic-like state, while changing [Ca2+]o within the posterior hypothalamus caused changes in body temperature (Myers & Veale, 1970), and changes in body temperature were accompanied by changes in the ventricular Ca2+ concentration (Myers & Tytell, 1972). Cytokines are involved in the immune response (Krueger et al. 1995) and drowsiness is correlated with their presence in some organic diseases. It is worth recalling that cytokines stimulate bone resorption (Gowen et al. 1983; Greenfield et al. 1995). [Ca2+]o within the ventro-medial or lateral areas of the hypothalamus modifies the set-point for the hunger drive (Myers & Veale, 1971). These observations have led to the interesting suggestion that the mechanism of the set-point for various functions, such as body temperature, feeding behaviour and steady-state level of arousal, may be controlled by the inherent ratio of Na+ to Ca2+ ions in specific brain regions (Veale & Myers, 1971; Myers & Tytell, 1972).
Our data reinforce the idea that functions dependent on [Ca2+]o are not confined to communication through the Ca2+ receptor in the parathyroid, kidney and other peripheral cells. [Ca2+]o is also an important extracellular factor in the central nervous system.
Acknowledgments
We thank Dr Ilaria Cino for assisting in the experiments with spermine and Ms Judy Baggott for her assistance in preparing the manuscript.
References
- Allen E M, Singer F R, Melamed D. Electroencephalographic abnormalities in hypercalcemia. Neurology. 1970;20:15–22. doi: 10.1212/wnl.20.1.15. [DOI] [PubMed] [Google Scholar]
- Arellano R O, Woodward R M, Miledi R. A monovalent cationic conductance that is blocked by extracellular divalent cations in Xenopus oocytes. Journal of Physiology. 1995;484:593–604. doi: 10.1113/jphysiol.1995.sp020689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, McCormick D A. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. Journal of Physiology. 1993;468:669–691. doi: 10.1113/jphysiol.1993.sp019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragdon A C, Kojima H, Wilson W A. Suppression of interictal bursting in hippocampus unleashes seizures in entorhinal cortex: a proepileptic effect of lowering [K+] and raising [Ca2+] Brain Research. 1992;590:128–135. doi: 10.1016/0006-8993(92)91088-v. [DOI] [PubMed] [Google Scholar]
- Brown E M, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger M A, Lytton J, Hebert S C. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- Budde T, White J A, Kay A R. Hyperpolarization-activated Na+-K+ current (Ih) in neocortical neurons is blocked by external proteolysis and internal TEA. Journal of Neurophysiology. 1994;72:2737–2742. doi: 10.1152/jn.1994.72.6.2737. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N, Ye C, Yamaguchi T, Nakai M, Kifor O, Vassilev P M, Nishimura R N, Brown E M. The extracellular calcium-sensing receptor is expressed in rat microglia and modulates an outward K+ channel. Journal of Neurochemistry. 1999;72:1915–1922. doi: 10.1046/j.1471-4159.1999.0721915.x. [DOI] [PubMed] [Google Scholar]
- Cloetta M, Fischer H. Uber die wirkung der kationen Ca2+, Mg2+, Sr2+, Ba2+, K+ and Na+ bei intrazerebraler injektion. Archiv fur experimentelle Pathologie und Pharmakologie. 1930;158:254–281. [Google Scholar]
- Crill W E. Persistent sodium current in mammalian central neurons. Annual Review of Physiology. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- De Simoni A, Mancia M, Formenti A. The calcium sensitive cation current in rat thalamic neurons is G protein-regulated. Pflügers Archiv. 2000;440:R24.19. [Google Scholar]
- Dichter M A, Ayala G F. Cellular mechanisms of epilepsy: a status report. Science. 1987;237:157–164. doi: 10.1126/science.3037700. [DOI] [PubMed] [Google Scholar]
- Evaldsson U, Ertekin C, Ingvar D H, Waldenstrom J G. Encephalopathia hypercalcemica. A clinical and electroencephalographic study in myeloma and other disorders. Journal of Chronic Diseases. 1969;22:431–449. doi: 10.1016/0021-9681(69)90006-x. [DOI] [PubMed] [Google Scholar]
- Farkas R H, Chien P Y, Nakajima S, Nakajima Y. Properties of a slow nonselective cation conductance modulated by neurotensin and other neurotransmitters in midbrain dopaminergic neurons. Journal of Neurophysiology. 1996;76:1968–1981. doi: 10.1152/jn.1996.76.3.1968. [DOI] [PubMed] [Google Scholar]
- Formenti A, Arrigoni E, Mancia M. Effects on the electroencephalogram induced by the changes in cerebrospinal-fluid Ca2+-concentration: cellular mechanisms. Society for Neuroscience Abstracts. 1996;22:64.16. [Google Scholar]
- Formenti A, Arrigoni E, Martina M, Taverna S, Avanzini G, Mancia M. Calcium influx in rat thalamic relay neurons through voltage-dependent calcium channels is inhibited by enkephalin. Neuroscience Letters. 1995;201:21–24. doi: 10.1016/0304-3940(95)12138-t. [DOI] [PubMed] [Google Scholar]
- Formenti A, De Simoni A. Effects of extracellular Ca2+ on membrane and seal resistance in patch-clamped rat thalamic and sensory ganglion neurons. Neuroscience Letters. 2000;279:49–52. doi: 10.1016/s0304-3940(99)00951-9. [DOI] [PubMed] [Google Scholar]
- Formenti A, Martina M, De Simoni A, Mancia M. An external Ca2+ increase hyperpolarizes rat thalamic neurons blocking an inward current. Society for Neuroscience Abstracts. 1998a;24:814.10. [Google Scholar]
- Formenti A, Martina M, Plebani A, Mancia M. Multiple modulatory effects of dopamine on calcium channel kinetics in adult rat sensory neurons. Journal of Physiology. 1998b;509:395–409. doi: 10.1111/j.1469-7793.1998.395bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P, Oxford G S. Modulation of calcium channels by norepinephrine in internally dialyzed avian sensory neurons. Journal of General Physiology. 1985;85:743–763. doi: 10.1085/jgp.85.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen M, Wood D D, Ihrie E J, McGuire M K B, Russell G G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983;306:378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Green W N, Andersen O S. Surface charges and ion channel function. Annual Review of Physiology. 1991;53:341–359. doi: 10.1146/annurev.ph.53.030191.002013. [DOI] [PubMed] [Google Scholar]
- Greenfield E M, Shaw S M, Gornik S A, Banks M A. Adenyl cyclase and interleukin 6 are downstream effectors of parathyroid hormone resulting in stimulation of bone resorption. Journal of Clinical Investigation. 1995;96:1238–1244. doi: 10.1172/JCI118157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisado R, Arieff A I, Massry S G. Changes in the electroencephalogram in acute uremia. Journal of Clinical Investigation. 1975;55:738–745. doi: 10.1172/JCI107984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O P, Marty A, Neher E, Sakman B, Sigworth F J. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Konnerth A, Pumain R, Wadman W J. Extracellular calcium and potassium concentration changes in chronic epileptic brain tissue. Advances in Neurology. 1986;44:641–661. [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland MA USA: Sinauer Associates Inc; 1991. Modifiers of gating; pp. 445–471. [Google Scholar]
- Jahnsen H, Llinas R. Ionic basis for electroresponsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. Journal of Physiology. 1984;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys J G. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiological Reviews. 1995;75:689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- Joborn C, Hetta J, Niklasson F, Rastad J, Wide L, Agren H, Akerstrom G, Ljunghall S. Cerebrospinal fluid calcium, parathyroid hormone, and monoamine and purine metabolites and the blood-brain barrier function in primary hyperparathyroidism. Psychoneuroendocrinology. 1991;16:311–322. doi: 10.1016/0306-4530(91)90017-n. [DOI] [PubMed] [Google Scholar]
- Krueger J M, Takahashi S, Kapas L, Bredow S, Roky R, Fang J, Floyd R, Renegar K B, Guha-Thakurta N, Novitsky S, Obal J F. Cytokines in sleep regulation. Advances in Neuroimmunology. 1995;5:171–188. doi: 10.1016/0960-5428(95)00007-o. [DOI] [PubMed] [Google Scholar]
- Li Z, Hatton G I. Oscillatory bursting of phasically firing rat supraoptic neurones in low-Ca2+ medium: Na+ influx, cytosolic Ca2+ and gap junctions. Journal of Physiology. 1996;496:379–394. doi: 10.1113/jphysiol.1996.sp021692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D A, Bal T. Sleep and arousal: thalamocortical mechanisms. Annual Review of Neuroscience. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McCormick D A, Pape H C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. Journal of Physiology. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R L, Kelly K M. Antiepileptic drug mechanisms of action. Epilepsia. 1995;36(suppl. 2):S2–S12. doi: 10.1111/j.1528-1157.1995.tb05996.x. [DOI] [PubMed] [Google Scholar]
- McGehee D S, Aldersberg M, Liu K P, Hsuing S, Heath M J, Tamir H. Mechanism of extracellular Ca2+ receptor-stimulated hormone release from sheep thyroid parafollicular cells. Journal of Physiology. 1997;502:31–44. doi: 10.1111/j.1469-7793.1997.031bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moure J B, Houston M D. The electroencephalogram in hypercalcemia. Archives of Neurology. 1967;17:34–51. doi: 10.1001/archneur.1967.00470250038004. [DOI] [PubMed] [Google Scholar]
- Mubagwa K, Stengl M, Flameng W. Extracellular divalent cations block a cation non-selective conductance unrelated to calcium channels in rat cardiac muscle. Journal of Physiology. 1997;502:235–247. doi: 10.1111/j.1469-7793.1997.235bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R D, Tytell M. Fever: reciprocal shift in brain sodium to calcium ratio as the set-point temperature rises. Science. 1972;178:765–767. doi: 10.1126/science.178.4062.765. [DOI] [PubMed] [Google Scholar]
- Myers R D, Veale W L. Body temperature: possible ionic mechanism in the hypothalamus controlling the set point. Science. 1970;170:95–97. doi: 10.1126/science.170.3953.95. [DOI] [PubMed] [Google Scholar]
- Myers R D, Veale W L. Spontaneous feeding in the satiated cat evoked by sodium or calcium ions perfused within the hypothalamus. Physiology and Behavior. 1971;6:507–512. doi: 10.1016/0031-9384(71)90198-3. [DOI] [PubMed] [Google Scholar]
- Ono K, Maruoka F, Noma A. Voltage and time-dependent block of If by Sr2+ in rabbit sino-atrial node cells. Pflügers Archiv. 1994;427:437–443. doi: 10.1007/BF00374258. [DOI] [PubMed] [Google Scholar]
- Pape H C. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annual Review of Physiology. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Partridge L D, Muller T H, Swandulla D. Calcium-activated non-selective channels in the nervous system. Brain Research. 1994;19:319–325. doi: 10.1016/0165-0173(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Quinn S J, Ye C P, Diaz R, Kifor O, Bai M, Vassilev P, Brown E. The Ca2+-sensing receptor: a target for polyamines. American Journal of Physiology. 1997;273:C1315–1323. doi: 10.1152/ajpcell.1997.273.4.C1315. [DOI] [PubMed] [Google Scholar]
- Rogers K V, Dunn C K, Hebert S C, Brown E M. Localization of calcium receptor mRNA in the adult rat central nervous system by in situ hybridization. Brain Research. 1997;744:47–56. doi: 10.1016/s0006-8993(96)01070-0. [DOI] [PubMed] [Google Scholar]
- Ruat M, Molliver M E, Snowman A M, Snyder S H. Calcium sensing receptor: molecular cloning in rat and localization to nerve terminals. Proceedings of the National Academy of Sciences of the USA. 1995;92:3161–3165. doi: 10.1073/pnas.92.8.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Llinas R. The functional states of the thalamus and the associated neuronal interplay. Physiological Reviews. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Stern L, Chvoles G J. Effect de l'injection intraventriculaire des ions Ca2+ et K+ Comptes rendus hebdomadaires des seances et memoires de la societe de biologie et de ses filiales et associees. 1933;112:568–572. [Google Scholar]
- Straub S G, Kornreich B, Oswald R E, Nemeth E F, Sharp G W. The calcimimetic R-467 potentiates insulin secretion in pancreatic β-cells by activation of a non-specific cation channel. Journal of Biological Chemistry. 2000;275:18777–18884. doi: 10.1074/jbc.M000090200. [DOI] [PubMed] [Google Scholar]
- Tai C, Smith Q R, Rapoport S I. Calcium influxes into brain and cerebrospinal fluid are linearly related to plasma ionized calcium concentration. Brain Research. 1986;385:227–236. doi: 10.1016/0006-8993(86)91068-1. [DOI] [PubMed] [Google Scholar]
- Veale W L, Myers R D. Emotional behavior, arousal and sleep produced by sodium and calcium ions perfused within the hypothalamus of the cat. Physiology and Behavior. 1971;7:601–607. doi: 10.1016/0031-9384(71)90115-6. [DOI] [PubMed] [Google Scholar]
- Von Krosigk M, Bal T, McCormick D A. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- Washburn D L, Anderson J W, Ferguson A V. The calcium receptor modulates the hyperpolarization-activated current in subfornical organ neurons. NeuroReport. 2000;11:3231–3235. doi: 10.1097/00001756-200009280-00036. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Lu W, Macdonald J F. Extracellular calcium sensed by a novel cation channel in hippocampal neurons. Proceedings of the National Academy of Sciences of the USA. 1997;94:7012–7017. doi: 10.1073/pnas.94.13.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Chattopadhyay N, Sanders J L, Vassilev P M, Brown E M. Enhanced expression of extracellular calcium sensing receptor in monocyte-differentiated versus undifferentiated HL-60 cells: potential role in regulation of a nonselective cation channel. Calcified Tissue International. 2000;66:375–382. doi: 10.1007/s002230010076. [DOI] [PubMed] [Google Scholar]
- Ye C, Kanazirska M, Quinn S, Brown E M, Vassilev P M. Modulation by polycationic Ca2+-sensing receptor agonists of nonselective cation channels in rat hippocampal neurons. Biochemical and Biophysical Research Communications. 1996a;224:271–280. doi: 10.1006/bbrc.1996.1019. [DOI] [PubMed] [Google Scholar]
- Ye C, Rogers K, Bai M, Quinn S J, Brown E M, Vassilev P M. Agonists of the Ca2+-sensing receptor (CaR) activate nonselective cation channels in HEK293 cells stably transfected with the human CaR. Biochemical and Biophysical Research Communications. 1996b;226:572–579. doi: 10.1006/bbrc.1996.1396. [DOI] [PubMed] [Google Scholar]
- Zhang Y H, Hancox J C. Gadolinium inhibits Na+-Ca2+ exchanger current in guinea-pig isolated ventricular myocytes. British Journal of Pharmacology. 2000;130:485–488. doi: 10.1038/sj.bjp.0703353. [DOI] [PMC free article] [PubMed] [Google Scholar]


