Abstract
A semi-intact preparation of the chick basilar papilla was developed to study calcium-dependent neurotransmitter release by tall hair cells (avian equivalent of cochlear inner hair cells).
Tall hair cell depolarization resulted in changes in cell membrane capacitance (ΔCm) that reflected cell surface area increases following synaptic vesicle exocytosis and provided a surrogate measure of neurotransmitter release. Both calcium current (ICa) and ΔCm were reversibly blocked by cobalt, and exhibited a similar bell-shaped dependency on voltage with a peak response around −10 mV.
Pharmacological agents selective for L-type calcium channels were employed to assess the role of this channel type in neurotransmitter exocytosis. Nimodipine, a dihydropyridine (DHP) antagonist, suppressed ICa and blocked ΔCm. Conversely, the DHP agonist Bay K 8644 increased both ICa and ΔCm amplitude nearly 3-fold. These findings suggest that chick tall hair cell neurotransmitter release is mediated by calcium influx through L-type calcium channels.
Hair cells in auditory end organs convert the mechanical energy of acoustic waves into a neural code. The hair cell transduces stereocilia displacement into membrane depolarization (Hudspeth & Corey, 1977). Membrane depolarization is encoded into a discrete chemical signal following calcium influx via voltage-gated channels (Tucker & Fettiplace, 1995), and subsequent calcium-dependent exocytosis of neurotransmitter-containing synaptic vesicles (Parsons et al. 1994). The presumptive transmitter at this synapse is glutamate or another compound that can excite glutamate receptors (Kataoka & Ohmori, 1994). Surprisingly, little more is known about the details of neurotransmitter release by this ribbon-type synapse of hair cells. Although limited success has been achieved in other end organs (for example see Furukawa et al. 1978), the unusually small size of the afferent fibre endings and encasement of the synapse in the temporal bone have largely precluded its study with conventional postsynaptic recordings in the cochlea (Siegel & Dallos, 1986).
The release of classical or fast neurotransmitters by neurons at other synapses is mediated by calcium influx through subclasses of voltage-gated channels. These channels most often are high voltage activated (HVA), demonstrate some rapid form of voltage- or calcium-dependent inactivation, and can be classified as N-, or P/Q-type (for review see Dunlap et al. 1995). Neurotransmitter release by primary sensory receptors such as the hair cell is driven not only by phasic depolarizations, but also by tonic graded potentials. Thus, the fidelity of signal transmission through the hair cell afferent synapse requires that calcium channel kinetics faithfully follow both the phasic and tonic components of graded transduction potentials. Inactivating N- or P/Q-type calcium channels would seem poorly suited to meet the demands of neurotransmitter release by hair cells.
The most common calcium channel found in hair cells demonstrates characteristics that appear to preserve the timing, frequency and amplitude of auditory or vestibular stimuli. The calcium channel activates both quickly and at low voltages, does not inactivate, deactivates rapidly upon repolarization and is, at least partially, blocked by dihydropyridine (DHP) antagonists (Ohmori, 1984; Fuchs et al. 1990; Zidanic & Fuchs, 1995). The α1D calcium channel has been cloned from the sensory epithelium of the chick cochlea and is the most likely candidate for the special form of L-type calcium channel found in hair cells (Kollmar et al. 1997). Evidence also exists in hair cells for additional types of calcium channels with different conductances or kinetic properties (Rennie & Ashmore, 1991; Prigioni et al. 1992; Kimitsuki et al. 1994; Su et al. 1995). Despite the characterization of different calcium channel types, it is not yet clear what role these different calcium channels play in hair cell neurotransmitter release.
Here we demonstrate an example of calcium-triggered neurotransmitter release mediated by DHP-sensitive calcium channels. This conclusion was achieved by studying hair cell synaptic vesicle exocytosis in a semi-intact preparation of the avian equivalent of the mammalian cochlea, the sensory epithelium of the chick basilar papilla.
METHODS
Tissue preparation
Seven- to twelve-day-old white leghorn chicks (Gallus domesticus) were killed by anaesthetic overdose (intracardiac injection of 0.5 ml of a 50 % ethyl carbamate solution; Sigma Chemical Company, St Louis, MO, USA) and the cochleae bilaterally harvested. All animal procedures followed a protocol approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. The soft tissue capsule of the cochlea was removed through the oval window, as described previously (Zidanic & Fuchs, 1995). The cochlear capsule was transferred to a dissection saline (mm): 146 NaCl, 3 KCl, 10 glucose, 15 Hepes, 7 MgCl2, 0.1 CaCl2, adjusted with NaOH to pH 7.4, supplemented with endopeptidase (0.01 % protease type XXVII, Sigma) and bovine serum albumin (BSA; 0.05 %, Sigma). After 2 min incubation in endopeptidase-containing solution to loosen the attachment of the tectorial membrane to the sensory epithelium, the cochlea was transferred to a solution comprising dissection saline plus BSA alone in order to deactivate any remaining endopeptidase.
The cochlea was then transferred to a Sylgard (Dow Chemical Company, Midland, MI, USA)-coated dish filled with ice-cold dissection saline and secured with insect pins. The tegmentum vasculosum, and in some cases the tectorial membrane, was removed and the sensory epithelium isolated from the cochlea. Either a section of the sensory epithelium approximately 400 μm long, starting 0.5 mm from the apical tip of the basilar papilla, or a larger 2 mm in length apical section was transferred to a recording chamber and placed with the hair bundles up. The neural edge of the sensory epithelium was then partially retracted and secured such that the basolateral surface of the individual tall hair cell could be visualized (Fig. 1A). The recording chamber contained control external saline (mm: 154 NaCl, 6 KCl, 5 Hepes, 8 glucose, 2 MgCl2, 5 CaCl2, pH balanced to 7.4 with NaOH), and the preparation was viewed with Nomarski optics using a Zeiss Axioskop or UEM microscope with a × 63 water-immersion objective.
Figure 1. Calcium influx and exocytosis recorded in semi-intact preparation of chick basilar papilla.
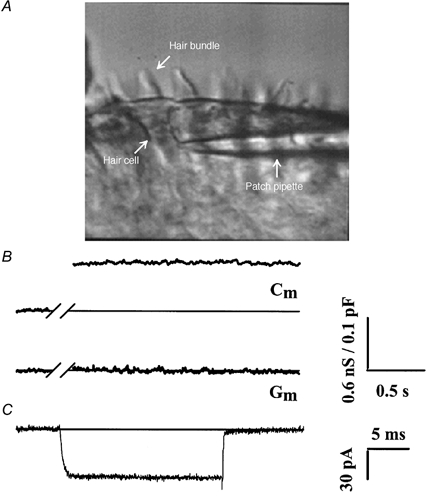
A, the semi-intact chick basilar papilla preparation. Individual tall hair cells within the epithelium can be visualized and are patch clamped on the basolateral surface. B, cell membrane capacitance increase as a measure of exocytosis. Example recording of cell membrane capacitance (Cm) and conductance (Gm) before and after a depolarization to -21 mV for 1 s (note that the trace has been abridged during the depolarizing pulse when the capacitance measurements are not interpretable). The ≈150 fF Cm jump (ΔCm) is interpreted as the increase in cell membrane surface area that results from vesicle fusion during exocytosis. Note that there is no change in Gm. C, isolated calcium current (ICa). Typical recording where the cell was held at -81 mV and depolarized to -21 mV for 20 ms. The inward current exhibited fast activation, little to no inactivation during the voltage pulse, and rapid deactivation following a return to the holding potential.
Whole-cell patch-clamp recording
Individual tall hair cells were patch clamped with capillary glass (Kimax 51, World Precision Instruments) microelectrodes pulled to a tip resistance between 2 and 4 MΩ. Electrodes were coated with purple ski wax (SWIX, Norway) to reduce their capacitance. Tight seal recordings were made in whole-cell configuration (Hamill et al. 1981) (see Fig. 1A), and cells were voltage clamped at a holding potential of -81 mV with either an Axopatch 200B (Axon Instruments, Foster City, CA, USA) or an EPC9 patch-clamp amplifier (HEKA Electronics GmbH, Germany). The EPC9 was controlled by Pulse software (HEKA) run on Power Macintosh computer. A mean resting membrane capacitance of 5.9 ± 0.1 pF (mean ±s.e.m., n = 105) was recorded from tall hair cells via a mean access resistance 8.0 ± 0.3 MΩ (n = 105). Pipettes were filled with control internal solution (mm: 115 glutamic acid, 115 CsOH, 20 tetraethylammonium (TEA) chloride, 13 NaCl, 10 Hepes, 3 MgCl, 5 Na-ATP, 0.3 GTP, 0.2 EGTA, pH balanced with CsOH to between 7.3 and 7.4). The caesium glutamate internal solution resulted in a liquid junction potential of 11 mV and was similar to that reported for internal solutions of similar composition (Neher, 1992). Holding potentials and test potentials were corrected for this liquid junction potential. TEA was added to block outward currents that persisted in the presence of internal caesium, and in some experiments 6 mm CsCl2 replaced KCl in the external solution to isolate further calcium currents. Calcium influx was blocked by equimolar replacement of calcium chloride with cobalt chloride. Bay K 8644, a DHP agonist (Research Biochemicals International, Natick, MA, USA), and nimodipine, a DHP antagonist (Calbiochem, La Jolla, CA, USA), were prepared as either a 5 or 10 mm stock solution in ethanol. Unless otherwise noted other chemicals were obtained from Sigma. Solution exchanges were accomplished by changing the bath volume (either 1.0 or 1.5 ml) three times. A 2 min equilibration period was observed prior to any additional data collection. All experiments were done at 20-22 °C, and all measurements are given as mean values ± one standard error of the mean (s.e.m.) unless otherwise stated.
Calcium current recording
Hair cell currents activated by 10-20 ms voltage-clamp steps from a holding potential of -81 mV to various potentials were low-pass filtered at 5 kHz. Calcium currents (ICa) were isolated via ionic substitution and then leak subtracted with P/N = 4. Traces were digitized on-line at up to 25 kHz and sometimes averaged for four or more trials. Calcium current magnitude was measured by averaging 2 ms current amplitude taken 2 ms after the pulse onset.
Capacitance recordings
Changes in cell membrane capacitance (ΔCm) were used to monitor fusion of neurotransmitter-containing vesicles during exocytosis, and were measured with the ‘piecewise linear’ technique described thoroughly elsewhere (Gillis, 1995). A dual lock-in amplifier (H. Meyer, Goettingen, Germany) generated an 800 Hz sinusoidal command voltage (40 mV peak to peak) that was superimposed on the holding potential of -81 mV. ΔCm were elicited by depolarizing voltage-clamp steps of 1 s duration. Phase lags due to electronics and resistance of the electrode-cell junction were cancelled by placing a 1 MΩ resistor in series with the bath and adjusting the phase of the sinusoid until adding or withdrawing the resistor resulted in no changes in the resting capacitance trace. In order to calibrate capacitance measurements, a 100 fF capacitor was transiently added into the circuit prior to each voltage pulse and recorded for off-line analysis of ΔCm. Both phase check and capacitance calibration were performed within 1 s of each depolarizing pulse. Capacitance and conductance were low-pass filtered at 33 Hz, recorded with a personal computer using pCLAMP software and a TL-1 DMA interface (Axon Instruments), and stored on the hard disk. Brief capacitive transients immediately after the voltage step were not included in the analysis or display of recordings. Digitized data were analysed using macros written for Excel software (Microsoft Corporation, Redmond, WA, USA). In other experiments, capacitance measurements were made in ‘sine + DC’ mode of the EPC-9 software lock-in amplifier, with 1.25 kHz sinusoidal command voltage (25 mV peak to peak) that was superimposed on the holding potential of -81 mV. ΔCm were elicited by depolarizing voltage-clamp steps of 250 ms to 1 s duration. Currents were sampled at 50 μs intervals, filtered at 2.9 kHz, and off-line filtered at 1 kHz. Capacitance data were filtered and analysed off-line using IgorPro software (Wavemetrics).
RESULTS
Calcium dependence of hair cell exocytosis
Brief depolarizing voltage-clamp pulses resulted in step changes in membrane capacitance (ΔCm) that occurred in the absence of changes in membrane conductance (Fig. 1B). Following a 1 s depolarizing voltage step to -21 mV cell membrane capacitance increased on average 147 ± 18 fF (n = 41) above resting levels of membrane capacitance or ≈2.5 % of the resting cell surface area. The same amplitude depolarizing voltage-clamp pulses also activated an inward current that turned on both quickly and at low voltages, deactivated rapidly upon repolarization, and did not inactivate during 20 ms voltage steps (Fig. 1C). The mean ICa amplitude for cells depolarized from -81 mV to -21 mV was 57.1 ± 2.0 pA (n = 95 cells). This is similar to that reported by others in isolated chick hair cells (Ohmori, 1984; Fuchs et al. 1990) and the frog crista ampularis (Prigioni et al. 1992).
The magnitude of both calcium current (ICa) and ΔCm varied with the voltage of the depolarizing step (Fig. 2A and B). In general, depolarizations to potentials around -10 mV yielded the largest ΔCm, while considerably smaller or larger depolarizations resulted in smaller ΔCm. However, a hysteresis was observed in the ΔCm-voltage relationship. When voltage-clamp protocols progressed from -60 to +80 mV, the maximum exocytosis occurred at -20 mV (Fig. 2C); conversely, if the protocols progressed from +80 to -60 mV, the peak exocytosis was at 0 mV (Fig. 2D). The hysteresis in the exocytotic response may result from depletion of a releasable pool of synaptic vesicles during the repeated steps of the stimulation protocol. Linear summation of the mean values from eight cells subjected to both ascending and descending voltage-clamp protocols resulted in a bell-shaped relationship between ΔCm and voltage with a peak at -10 mV (Fig. 2E). The magnitude of the calcium current (ICa) demonstrated a similar bell-shaped relationship as ΔCm to membrane voltage (Fig. 2E), suggesting a dependence of ΔCm on calcium influx (Parsons et al. 1994; Moser & Beutner, 2000).
Figure 2. Voltage dependence of calcium influx and exocytosis.
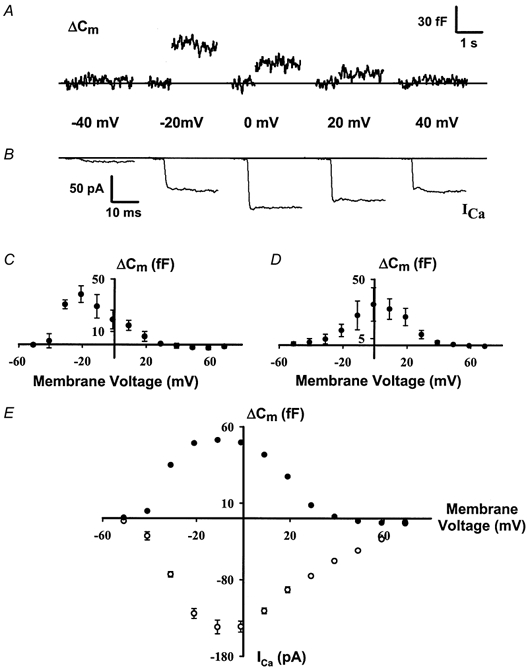
A and B, cell membrane capacitance increases and calcium current depends on voltage. Step increases in ΔCm and ICa following voltage-clamp pulses to different depolarizing potentials from a holding potential of -81 mV (note that the capacitance trace has been abridged during the 250 ms depolarizing pulses when the measurements are not interpretable). C and D, hysteresis in ΔCm-voltage relationship. Magnitude of capacitance changes for each voltage step applied was quantified and plotted. ΔCm responses evoked by ascending voltage-clamp steps (C) and those evoked by descending voltage-clamp steps (D) are shown. Peak response shifts from -20 mV to 0 mV. E, summed ΔCm-voltage and ICa-voltage relationships. Simple addition of the ΔCm-voltage relationships in C and D yields a bell-shaped relationship that is similar to the ICa-voltage relationship. Both curves exhibit maximum response at -10 mV. Data from 8 cells are presented here where ascending ΔCm-voltage, descending ΔCm-voltage and ICa-voltage relationships were all recorded from each cell.
The dependence of ΔCm on calcium influx was addressed by blocking ICa. Replacing extracellular calcium with 5 mm cobalt suppressed ICa (Fig. 3A) by 99 ± 1 % (n = 3 cells) and blocked ΔCm (Fig. 3B) by 98 ± 3 % (n = 5 cells). Inhibition of both ICa and ΔCm reversed to 90 ± 9 % and 80 ± 27 % of control values, respectively, following washout of the cobalt-containing solution and a return of 5 mm Ca2+ (Fig. 3C). Cadmium (100 μm), which also blocks voltage-gated calcium channels, inhibited both ICa and ΔCm (data not shown). No attempt was made to reverse the Cd2+ inhibition. These experiments demonstrate the calcium dependence of exocytosis in the tall hair cell.
Figure 3. Pharmacology of calcium influx and exocytosis.
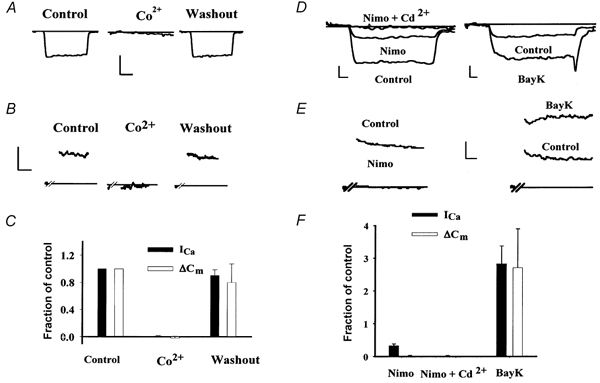
A and B, calcium currents (ICa; A) and capacitance changes (ΔCm; B) evoked by depolarizations to -21 mV are shown for two different cells before, during, and after substitution of 5 mm Co2+ for Ca2+ in the external medium. C, summary data for cells reversibly exposed to 5 mm Co2+. D and E, calcium currents (ICa; D) and capacitance changes (ΔCm; E) evoked by depolarizations to -21 mV are shown for another two cells before and after exchanging the bath solution for one containing either nimodipine (Nimo) or Bay K 8644. (Note as in B the capacitance trace in E has been abridged during the 1 s control and 3 s test depolarizing pulses when measurements are not interpretable.) ICa was elicited with 15 ms depolarizations to -21 mV. Residual ICa following nimodipine exposure was blocked by the addition of 200 μm Cd2+. F, summary data for cells exposed to either nimodipine, nimodipine + Cd2+ or Bay K 8644. In both C and F, the magnitude of ΔCm (□) and ICa (▪) is expressed as a fraction of the control value ±s.e.m. Calibration bars: A: 40 pA, 5 ms; B: 40 fF, 500 ms; D: left trace 20 pA, right trace 50 pA, 2 ms; E: 40 fF, 300 ms.
The putative role of the α1D calcium channel in tall hair cell neurotransmitter release was examined by applying pharmacological agents selective for L-type calcium channels. Derivatives of DHPs were used to assess both ICa and ΔCm. The DHP antagonist nimodipine (10 μm) partially reduced the magnitude of ICa (Fig. 3D) by 69 ± 5 % (n = 5 cells) similar to previous reports (Fuchs et al. 1990; Zidanic & Fuchs, 1995), but wholly blocked ΔCm (Fig. 3E; 98 ± 2 % reduction). Residual DHP-resistant inward current was blocked by 100 μm Cd2+ (98 ± 1 % reduction, n = 4 cells). Conversely, the DHP agonist Bay K 8644 (5 μm) increased both the ICa and ΔCm amplitude nearly 3-fold (282 ± 55 % and 270 ± 120 % increase, respectively, n = 3 cells; Fig. 3F). Detailed analysis of the effect of Bay K 8644 on ICa demonstrated a -10 mV shift in the current-voltage relationship (data not shown) as described for L-type calcium channels in other tissues and barium currents in these cells (Fuchs et al. 1990). These findings suggest that chick tall hair cell neurotransmitter release is mediated by calcium influx through L-type calcium channels.
DISCUSSION
In this study, we have utilized a semi-intact preparation of chick basilar papilla to examine the role of calcium influx in cochlear hair cell exocytosis. Both ICa and ΔCm share a similar voltage dependency having a peak near -10 mV, and are blocked by divalent cations such as cobalt and cadmium, consistent with hair cell exocytosis being triggered by the influx of calcium via voltage-gated calcium channels. Modulation of both ICa and ΔCm by both a DHP agonist and antagonist suggests that tall hair cell exocytosis is mediated specifically by the L-type calcium channel, as recently reported at another ribbon-type synapse (von Gersdorff et al. 1998).
Changes in cell membrane capacitance and neurotransmitter release
Electrical measurements of cell surface area via membrane capacitance recording previously have been applied to both frog saccular hair cells (Parsons et al. 1994) and mouse cochlear inner hair cells (Moser & Beutner, 2000). In the absence of conventional postsynaptic recordings, such changes in membrane capacitance have been shown to be a reasonable surrogate for neurotransmitter release (Haller et al. 1998; von Gersdorff et al. 1998). This position is also supported for chick tall hair cells as similar stimulation protocols to ours resulted in the detection of glutamate release from isolated chick tall hair cells by exogenous glutamate receptors (Katakoa & Ohmori, 1994). However, signal-to-noise considerations do limit our resolution to about 10 fF or the release of ≈270 vesicles (assuming 37 aF per vesicle; Lenzi et al. 1999). Conventional postsynaptic recordings, when possible to perform, are usually more sensitive as the exocytosis of single vesicles can be observed. Thus in our experiments, neurotransmitter release possibly may have gone undetected under conditions of minimal calcium influx (membrane potentials more negative than -40 mV or in the presence of DHP antagonists).
Concomitant endocytosis in theory could confound our measurements of exocytosis as ΔCm reflects only the net change in membrane capacitance. Our previous work in the frog saccular hair cells (Parsons et al. 1994), however, suggests this to be unlikely. First, whole-cell dialysis washed out a small molecular weight compound essential for endocytosis; and second, when endocytosis was maintained with perforated patch-clamp recordings its time course (τ = 12 s) was slow compared to exocytosis. More recent recordings from mouse inner hair cells have demonstrated endocytosis with whole-cell patch-clamp recordings (Moser & Beutner, 2000). However, both their mean cell size (resting Cm) and access resistance were greater than that reported here, suggesting slower whole-cell dialysis than in our experiments (Pusch & Neher, 1988). Like saccular cells, the endocytosis observed in inner hair cells that followed exocytosis triggered by calcium influx was also slow (τ = 7.5 s). Thus, while we cannot unequivocally exclude endocytosis as a confounder in our experiments, evidence from other hair cells suggests that its role is probably minimal, and if present would serve only to underestimate tall hair cell exocytosis.
Semi-intact preparation of chick tall hair cells
Our findings on tall hair cells in sensory epithelium of the basilar papilla parallel previous observations made on dissociated hair cells. Calcium channel current amplitude and kinetics (Fuchs et al. 1990; Zidanic & Fuchs, 1995), and calcium dependence of exocytosis (Parsons et al. 1994) were very similar to what we report. Our observations confirm that these previously described electrophysiological features of dissociated hair cells do indeed reflect the behaviour of hair cells in situ.
Similar measures of exocytosis have also been reported on two other hair cell preparations: frog saccular hair cells enzymatically dissociated from the sensory epithelium (Parsons et al. 1994); and mouse inner hair cells from the acutely dissected organ of Corti (Moser & Beutner, 2000). Table 1 summarizes some relevant parameters from experiments on these three different hair cell types. Despite variation between cell types in size, vesicles released, and external calcium concentration, the normalized exocytotic properties of hair cells are quite similar. When accounting for cell size (resting Cm) or number of synapses (number of dense bodies), the mean amount or rate of exocytosis for a 1 s stimulus is nearly identical between chick tall and mouse inner hair cells. A larger difference is noted between these two hair cell types and the frog saccular hair cells. Such a difference may be in part related to different sized dense bodies between hair cell types. A common relationship across several hair cell types has been noted between the number of calcium channels and release area, a metric that considers both dense body number and size (Martinez-Dunst et al. 1997). Unfortunately, a lack of specific morphometric data on mouse hair cell dense bodies precludes such analysis of exocytosis here.
Table 1.
ΔCm measurements on different types of hair cells
| Frog saccular(Parsons et al. 1994) | Mouse inner (Moser & Beutner, 2000) | Chick tall | |
|---|---|---|---|
| Resting Cm (pF) | 9.4 | 8.2 | 5.9 |
| Dense bodies per hair cell | 20 | 25 | 15 |
| ΔCm (fF) (1 s depolarization) | 305 | 223 | 147 |
| Vesicles released (1 s depolarization) | 8243 | 6027 | 3973 |
| Percentage change in cell surface area | 3.3 | 2.7 | 2.5 |
| Exocytotic rate (fF s−1 (dense body)−1) | 15.3 | 8.9 | 9.8 |
| Exocytotic rate (vesicles s−1 (dense body)−1) | 412 | 241 | 265 |
| [Ca2+]o (mM) | 4 | 10 | 5 |
Summary of some parameters from different types of hair cells in which calcium-dependent exocytosis has been studied. Estimates of vesicle release are based on the assumption of an individual vesicle size of 37 aF (Lenzi et al. 1999). Parameters not taken from this work came from the cited papers or references therein with the one exception for the number of dense bodies in the chick tall hair cell which was obtained from Martinez-Dunst et al. (1997).
Pharmacology of calcium-dependent exocytosis in the chick cochlear hair cell
Neurotransmitter release is triggered by calcium entry through presynaptic voltage-gated calcium channels. Classical fast synaptic transmission at the neuromuscular junction or in the central nervous system is mediated by at least one of the high-voltage-activated, partially inactivating N-type, P/Q-type or R-type calcium channels. However, in some cases, more than one type of calcium channel may work synergistically to mediate synaptic vesicle exocytosis (Luebke et al. 1993; Takahashi & Momiyama, 1993). Here we report that at a ribbon-type synapse in the chick tall hair cell synaptic vesicle exocytosis is mediated completely by a single type of low-voltage-activated, non-inactivating calcium channel.
The calcium channel that mediates hair cell exocytosis reported here can be further classified as an L-type channel based on its pharmacology. Evidence for heterogeneous calcium channel types in guinea-pigs (Rennie & Ashmore, 1991), bullfrog (Su et al. 1995), and chick tall (Kimitsuki et al. 1994) hair cells has been reported, but our experiments do not support a role for other calcium channel types in tall hair cell exocytosis. Exocytosis was enhanced by a DHP-sensitive calcium channel agonist, and was completely blocked by a DHP antagonist. It is interesting to note that while nimodipine completely inhibited exocytosis, only ≈70 % of the calcium influx was blocked. One possible interpretation of this finding is that the residual calcium current is carried by a non-L-type calcium channel not coupled to exocytosis. Alternatively, the residual current also could be through a DHP-resistant L-type calcium channel (Zidanic & Fuchs, 1995). This would suggest that either a calcium current threshold is required to trigger hair cell exocytosis (Seward et al. 1995;) or tall hair cell exocytosis exhibits a very steep calcium dependency resulting from a high-order co-operativity in calcium binding, as has been recently reported for mammalian inner hair cells (Beutner et al. 2001).
Other previous studies also support our notion that the L-type calcium channel is critical for hair cell neurotransmitter release. Consistent with a role for L-type channels in hair cell exocytosis, both ICa and ΔCm in mouse inner hair cells were reduced ≈50 % with another DHP antagonist, nifedipine (Moser & Beutner, 2000). Cochlear perfusion with nimodipine suppressed sound-evoked compound action potentials in the guinea-pig auditory nerve (Bobbin et al. 1990). α1D Calcium channel knockout mice were deaf despite normal cochlear morphology; however, their calcium currents were dramatically reduced, and any residual calcium current was not responsive to DHP agonists (Platzer et al. 2000). A similar dependency of neurotransmitter release on L-type channels was found in the goldfish retinal bipolar synapse, a ribbon synapse like those of the tall hair cell (von Gersdorff et al. 1998). These findings all support the hypothesis that L-type calcium channels function as the primary mediator of exocytosis at ribbon synapses.
Acknowledgments
This work was supported by NIH DC00710 (J.C.S.), NIH DC03763 (T.D.P.) and a Sloan Research Fellowship Award to T.D.P.
References
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Bobbin RP, Jastreboff PJ, Fallon M, Littman T. Nimodipine, an L-channel Ca2+ antagonist, reverses the negative summating potential recorded from the guinea pig cochlea. Hearing Research. 1990;46:277–287. doi: 10.1016/0378-5955(90)90009-e. [DOI] [PubMed] [Google Scholar]
- Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends in Neurosciences. 1995;18:89–98. [PubMed] [Google Scholar]
- Fuchs PA, Evans MG, Murrow BW. Calcium currents in hair cells isolated from the cochlea of the chick. Journal of Physiology. 1990;429:553–568. doi: 10.1113/jphysiol.1990.sp018272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Hayashida Y, Matsuura S. Quantal analysis of the size of excitatory post-synaptic potentials at synapses between hair cells and afferent nerve fibres in goldfish. Journal of Physiology. 1978;276:211–226. doi: 10.1113/jphysiol.1978.sp012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis K. Techniques for membrane capacitance measurements. In: Sakmann B, Neher E, editors. Single-Channel Recording. New York: Plenum; 1995. pp. 155–198. [Google Scholar]
- Haller M, Heinemann C, Chow RH, Heidelberger R, Neher E. Comparison of secretory responses as measured by membrane capacitance and by amperometry. Biophysical Journal. 1998;74:2100–2113. doi: 10.1016/S0006-3495(98)77917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ, Corey DP. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proceedings of the National Academy of Sciences of the USA. 1977;74:2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Ohmori H. Activation of glutamate receptors in response to membrane depolarization of hair cells isolated from chick cochlea. Journal of Physiology. 1994;477:403–414. doi: 10.1113/jphysiol.1994.sp020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimitsuki T, Nakagawa T, Hisashi K, Komune S, Komiyama S. Single channel recordings of calcium currents in chick cochlear hair cells. Acta Otolaryngologica. 1994;114:144–148. doi: 10.3109/00016489409126033. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Montgomery LG, Fak J, Henry LJ, Hudspeth AJ. Predominance of the alpha1D subunit in L-type voltage-gated Ca2+ channels of hair cells in the chicken's cochlea. Proceedings of the National Academy of Sciences of the USA. 1997;94:14883–14888. doi: 10.1073/pnas.94.26.14883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Runyeon JW, Crum JW, Ellisman MH, Roberts WM. Synaptic vesicle populations in saccular hair cells reconstructed by electron tomography. Journal of Neuroscience. 1999;19:119–132. doi: 10.1523/JNEUROSCI.19-01-00119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, Dunlap K, Turner TJ. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993;11:895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- Martinez-Dunst C, Michaels RL, Fuchs PA. Release sites and calcium channels in hair cells of the chick's cochlea. Journal of Neuroscience. 1997;17:9133–9144. doi: 10.1523/JNEUROSCI.17-23-09133.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proceedings of the National Academy of Sciences of the USA. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patchclamp experiments. Methods in Enzymology. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Studies of ionic currents in the isolated vestibular hair cell of the chick. Journal of Physiology. 1984;350:561–581. doi: 10.1113/jphysiol.1984.sp015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Lenzi D, Almers W, Roberts WM. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: capacitance measurements in saccular hair cells. Neuron. 1994;13:875–883. doi: 10.1016/0896-6273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Prigioni I, Masetto S, Russo G, Taglietti V. Calcium currents in solitary hair cells isolated from frog crista ampullaris. Journal of Vestibular Research. 1992;2:31–39. [PubMed] [Google Scholar]
- Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflügers Archiv. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Rennie KJ, Ashmore JF. Ionic currents in isolated vestibular hair cells from the guinea-pig crista ampullaris. Hearing Research. 1991;51:279–292. doi: 10.1016/0378-5955(91)90044-a. [DOI] [PubMed] [Google Scholar]
- Seward EP, Chernevskaya NI, Nowycky MC. Exocytosis in peptidergic nerve terminals exhibits two calcium-sensitive phases during pulsatile calcium entry. Journal of Neuroscience. 1995;15:3390–3399. doi: 10.1523/JNEUROSCI.15-05-03390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JH, Dallos P. Spike activity recorded from the organ of Corti. Hearing Research. 1986;22:245–248. doi: 10.1016/0378-5955(86)90101-2. [DOI] [PubMed] [Google Scholar]
- Su ZL, Jiang SC, Gu R, Yang WP. Two types of calcium channels in bullfrog saccular hair cells. Hearing Research. 1995;87:62–68. doi: 10.1016/0378-5955(95)00079-j. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Tucker T, Fettiplace R. Confocal imaging of calcium microdomains and calcium extrusion in turtle hair cells. Neuron. 1995;15:1323–1335. doi: 10.1016/0896-6273(95)90011-x. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Sakaba T, Berglund K, Tachibana M. Submillisecond kinetics of glutamate release from a sensory synapse. Neuron. 1998;21:1177–1188. doi: 10.1016/s0896-6273(00)80634-0. [DOI] [PubMed] [Google Scholar]
- Zidanic M, Fuchs PA. Kinetic analysis of barium currents in chick cochlear hair cells. Biophysical Journal. 1995;68:1323–1336. doi: 10.1016/S0006-3495(95)80305-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


