Abstract
Microneurography was used to search for primary afferents responsive to innocuous low temperature in human nerves supplying the hairy skin of the hand or foot. Eighteen units were identified as cold-specific units: they displayed a steady-state discharge at skin temperatures in the range 28–30 °C, they were sensitive to small changes in temperature, and they responded vigorously when a cool metal probe touched their receptive fields (RFs). They were insensitive to mechanical stimuli and sympathetic activation. Their RFs comprised one, or at most two, spots less than 5 mm in diameter.
Nine units were characterised in detail by a series of 10 s cooling and warming pulses from a holding temperature of 35 °C. The threshold temperature for activation by cooling was 29.4 ± 2.0 °C (mean ±s.d.). Adaptation of the responses to supra-threshold cooling pulses was partial: mean peak and plateau firing rates were maximal on steps to 15 °C (35.9 and 19.9 impulses s−1, respectively). Three of these units also displayed a paradoxical response to warming, with a mean threshold of 42.3 °C.
Sixteen of the eighteen cold-specific units were also studied by electrical stimulation of their RFs. They conducted in the velocity range 0.8-3.0 m s−1. When stimulated at 2 Hz, their latency increased according to a characteristic time course, reaching a plateau within 3 min (mean slowing (±s.d.) 5.2 ± 1.1 %) and recovering quickly (50 % recovery in 17.8 ± 4.5s).
To reconcile these findings with previous studies of reaction times and the effects of nerve compression on sensation, it is concluded that either human cold-specific afferent fibres are incompletely myelinated ‘BC’ fibres, or else there are C as well as Aδ cold fibres, with the C fibre group contributing little to sensation.
Specific cutaneous cold and warm receptors have been defined as slowly conducting units that exhibit a steady-state discharge at constant skin temperature, a dynamic response to temperature changes and insensitivity to mechanical stimuli (Hensel et al. 1960; Hensel, 1973). Cold-specific receptors can therefore be readily distinguished from nociceptors that respond to noxious low temperatures (< 20 °C) in addition to heat and/or strong mechanical stimuli (Torebjörk & Hallin, 1976; Campero et al. 1996), and also from thermosensitive mechanoreceptors (Hensel & Boman, 1960; Konietzny, 1984). Few direct recordings of human cold-specific units have been described. Hensel & Boman (1960) recorded one cold unit, of unspecified conduction velocity (CV), from the exposed superficial radial nerve of a healthy volunteer. Konietzny (1984) recorded 13 cold-specific units by microneurography in human volunteers, and measured the CVs of three, which were in the C fibre range (0.43-2.04 m s−1). We previously reported a number of spontaneously active fibres in microneurographic recordings from human cutaneous fascicles, which were sensitive to small temperature changes (Serra et al. 1999). These appeared to be cold-specific units, but all had CVs in the C fibre range (0.43-1.27 m s−1), whereas several current medical and neuroscience textbooks state that the cutaneous cold sense in man is mediated by Aδ fibres, and that Aδ fibres have CVs in the range 12-30 m s−1. According to the Handbook of Physiology, the CVs of primate cold fibres ‘identify them unequivocally as small-diameter (Aδ) myelinated fibers’ (Darian-Smith, 1984). This was based on a study of cold-specific receptors in macaque monkeys (Darian-Smith et al. 1973), in which 147 units with CVs in the range 5-30 m s−1 (mean 14.5 m s−1) were identified. In another extensive study of macaque cold fibres by Long (1977), 206 fibres were recorded, all with CVs ‘within the Aδ range’ (2.2-22.5 m s−1). A human study correlating cutaneous sensations with the electrically evoked compound neurogram in radial nerves also concluded that ‘only the Aδ fibre population in man signals discriminatory cold information’ (Mackenzie et al. 1975).
We have now searched for more human cold-specific units, and characterised their temperature responses in more detail. We have only found cold-specific afferents in human hairy skin conducting at less than 4 m s−1, which is much slower than those in the palm of the macaque. As judged by velocity, the fastest cold-specific fibres recorded appear to be myelinated, but in other respects they resemble unmyelinated cold fibres more closely than other Aδ fibres.
METHODS
Subjects and microneurographic recordings
Ninety-seven microneurographic recordings were obtained from 31 healthy volunteers. The subjects gave their written informed consent to participate and the local ethics committee approved the test protocol, which conformed to the Declaration of Helsinki. The subjects sat relaxed in a recliner with an arm or leg supported by a padded platform. Room temperature was maintained at 21 °C. The microneurographic recordings were obtained from the superficial peroneal nerve at the ankle level. A disposable lacquer-insulated tungsten microelectrode, 200 μm in diameter (FHC, Bowdoinham, ME, USA), was inserted manually through the skin into the nerve (Hagbarth & Vällbo, 1967). A subcutaneous reference electrode was inserted 1-2 cm away. The nerve signals were filtered, amplified, monitored by oscilloscope and loudspeaker, and digitised by a personal computer using Qtrac (Institute of Neurology, London) or Axoscope software.
The search for cold fibres was prompted by two clues: (1) the presence of low-frequency nerve impulse activity at room temperature, or (2) activity-dependent slowing of a single fibre reaching a plateau during repetitive electrical stimulation (a characteristic of cold fibres that was first described in the rat (Thalhammer et al. 1994) and confirmed in humans (Serra et al. 1999)). When sensory units fired continuously at baseline cutaneous temperature, and their discharge suddenly increased in frequency when lowering the skin temperature or was suppressed by warming, they were likely to be cold-sensitive afferents. The receptive fields (RFs) of these units were delineated with a chilled metal rod (3 mm in diameter). The units were tested for mechanical response with standard von Frey monofilaments (Stoelting) with sectional areas up to 0.554 mm2, exerting a bending force up to 1.18 N (21.4 bar).
Characterisation of thermal sensitivity
For units with an adequate signal-to-noise ratio, sequences of low- or high-temperature stimuli were delivered to the skin through a Peltier thermode with a contact area of 1 cm2 placed over the RF of the unit. Electronic circuitry maintained the stimulus temperature within ±0.1 °C of the desired level via feedback from a thermocouple located at the interface between the thermode and skin. The baseline temperature of the thermode was maintained constant at 35 °C. The receptor threshold was determined by delivering 10 s pulses of low temperature in 1 °C steps in descending order. The rate of temperature change at the beginning and end of each temperature pulse was limited by the feedback circuitry to 10 °C s−1. Once the threshold was obtained, 10 s pulses to 30, 25, 20, 15, 10 and 5 °C were delivered at 2 min intervals. The nerve signals and temperature changes were recorded with Axoscope, and the number of action potentials elicited every second by the low-temperature stimulus was counted. Following low-temperature stimuli, the units were tested for their response to high temperature using 5 s pulses of temperatures up to 47 °C. High-temperature thresholds were determined by delivering sequences of thermal stimuli, ascending in 1 °C steps from a baseline of 35 °C. The temperature threshold in either direction was defined by the weakest temperature pulse that consistently elicited at least one action potential.
Measurements of latency and activity-dependent slowing
Nerve impulse conduction latency was measured using electrical stimulation with a pair of disc or needle electrodes placed on the RF. Square-wave electrical pulses (0.25 ms duration) were delivered using a Grass S48 stimulator with a stimulus isolation unit (SIU5). The RF was stimulated at 0.25 Hz until a steady baseline latency was established. The latencies of all units excited were displayed in a raster plot (equivalent to the compressed latency display of Torebjörk & Hallin, 1973) using Qtrac. Units sensitive to temperature or other stimuli could be identified by a sharp increase in baseline latency following natural receptor activation. Activity-dependent slowing was assessed by increasing the rate of electrical stimulation to 2 Hz for 3 min, followed by a recovery period of 6 min at 0.25 Hz. In some early experiments, a more extensive repetitive stimulation protocol was used, with 1, 2 and 4 Hz stimulus trains, as described previously (Serra et al. 1999). As a result of that study, the 2 Hz train alone was considered adequate in later experiments for distinguishing between probable cold units and nociceptors. The distance between stimulating and recording electrodes was measured to estimate the CV.
RESULTS
Eighteen units were identified as cold-specific units, as defined by Hensel (1973; see Introduction), on the basis of a temperature-sensitive resting discharge at normal skin temperature, and/or activation by innocuous low temperatures, and by their insensitivity to mechanical stimulation. Nine of these units with a favourable signal-to-noise ratio were characterised fully by the thermode with a sequence of low- and high-temperature pulses. Apart from the 18 cold-specific units, 9 units were classified as ‘probable’ cold-specific units purely on the basis of their activity-dependent slowing, which resembled that of the identified cold units and was clearly distinct from that of nociceptors.
General characteristics of cold units
The CVs of the fully characterised units were all within the C fibre range (< 2 m s−1), but cold-sensitive units conducting as fast as 3.0 m s−1 were identified off-line in raster plots (see below). The RF was a single punctate area of skin less than 5 mm in diameter in all but two units, which had two separate punctate RFs that were a few millimetres apart.
A conspicuous feature of these units was their resting discharge at room temperature (21 °C), firing at low frequencies (≈1 Hz) at irregular intervals, but never in bursts. This steady-state activity was suppressed by sudden warming of the RF, whereas the discharge frequency was increased or a discharge was initiated by cooling the RF (Fig. 1). The resting activity of cold fibres could also be documented indirectly by small variations in the CV of single afferents while stimulating their RF electrically at low frequency (0.25 Hz; Fig. 2A). On warming the skin with radiant heat, these latency fluctuations would disappear, leaving a clean line in the raster display (Fig. 2B). After equilibration at this higher temperature (≈35 °C), activation by a low-temperature stimulus would produce an abrupt increase in latency (e.g. Figs 3, 7 (lower unit) and 8A), followed by a gradual return to baseline, a typical indication of physiological activation of a C fibre (Torebjörk & Hallin, 1973). This gradual return to baseline latency after a burst of activity is probably due mainly to the reduction to resting level of the activity of the electrogenic sodium pump (Serra et al. 1999), whereas we attribute the pronounced latency fluctuations observed during the resting discharge, as shown in Fig. 2A, to the regular test stimuli falling at random within the refractory and supernormal periods after the last naturally activated impulse.
Figure 1. Resting discharge of C cold fibre at room temperature.
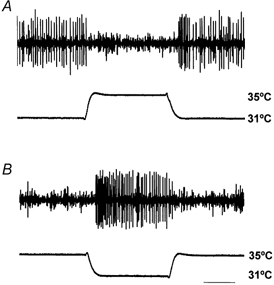
A, the resting discharge is temporarily suppressed by sudden warming of the receptive field (RF) from 31 °C to 35 °C (the Peltier device was initially set to 31 °C, since at that temperature the discharge was the same as with the device removed). B, from a holding temperature of 35 °C, at which the unit is silent, activity is initiated by cooling the RF to 31 °C. Time bar: 5 s (NB the nerve signal in this figure and in Figs 4 and 6 has been subjected to a ‘grass-cutting’ filter to reduce noise, so that spike amplitudes appear to be more variable than they actually are).
Figure 2. Resting discharge indicated by latency fluctuations during electrical stimulation of the RF.
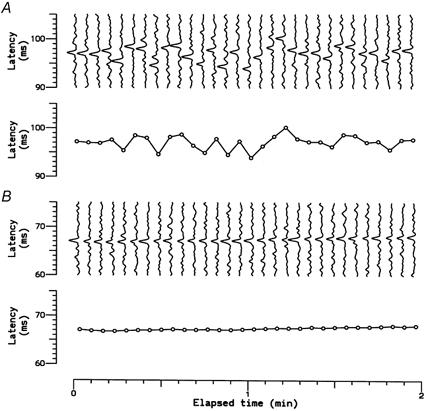
A, sequence of 30 successive recordings of a single C cold fibre at room temperature (top) and measured latencies, showing latency fluctuations due to the resting discharge (bottom). B, similar recordings from the same unit recorded 10 min later, after the skin had been warmed by radiant heat (to ≈35 °C) to suppress resting discharge.
Figure 3. Latency changes during thermal testing.
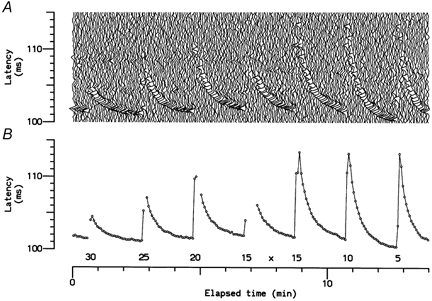
A, successive recordings from a single C cold fibre electrically stimulated at 0.25 Hz during testing with 10 s temperature pulses. B, measured latencies, showing activity-dependent slowing. Gaps indicate sweeps in which the response of the unit could not be identified. Numbers indicate the plateau level of temperature pulses (in °C). ‘x’ indicates the point when the electrical stimulus was increased, to overcome activity-dependent failure to excite.
Figure 7. Comparison of responses of two C units to electrical and natural stimulation.
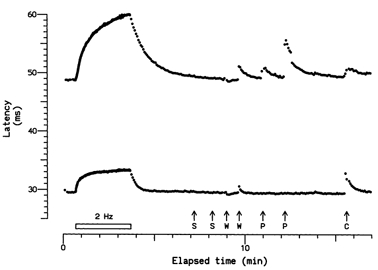
Activity-dependent slowing of two units recorded at the same time in response to electrical stimulation at 2 Hz for 3 min, and to a sequence of natural stimuli: S, sympathetic activation by the subject taking a deep breath and by a loud, unexpected noise; W, warm metal probe applied to the RF (note slight reduction in latency in response to the first application, due to warming of the axons, and abrupt latency increases due to activity caused by the second application); P, pressure from von Frey monofilaments (6.69 and 11.61 bar), which excited only the upper unit; and C, cool metal probe applied to the RF (note the slow onset of slowing of the upper unit, probably caused only by cooling of the axon, in contrast to the abrupt onset of slowing of the lower unit, caused by activity). The upper unit was classified as ‘type 1′ from its response to 2 Hz stimulation, and as a polymodal nociceptor from its responses to heat and pressure, while the lower unit was classified as ‘type 2′ (plateau unit), from its response to electrical stimulation, and as a cold unit (with a paradoxical response) from its responses to natural stimulation.
Figure 8. Type 2 units conducting at Aδ velocities.
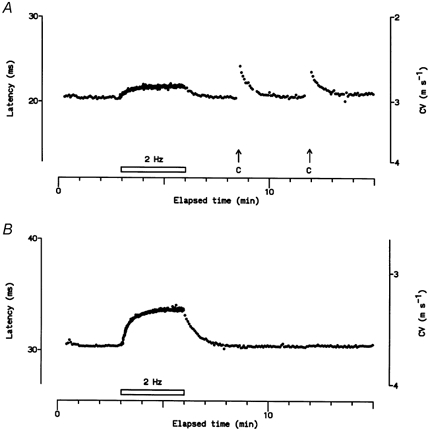
A, responses of a unit conducting at nearly 3 m s−1 to repetitive electrical and natural stimulation. C, application of a cold metal rod to the RF. B, response of an unidentified unit conducting at 3.6 m s−1 to repetitive electrical stimulation. This unit was considered to be a probable cold-specific receptor from its latency profile (cf. lower unit in Fig. 7). The latency and conduction velocity (CV) scales are logarithmic.
Neither noxious nor non-noxious mechanical stimulation excited any of the cold fibres of this sample.
Responses to low temperature
The nine cold-specific fibres characterised by the thermode applied to their RFs responded to 10 s low-temperature pulses at an average threshold of 29.4 ± 2.0 °C (mean ±s.d.). For one unit, the thermal stimulation was combined with electrical stimulation at 0.25 Hz (Fig. 3). This recording indicates that the 2 min recovery period allowed between thermal stimuli was the minimum required for nerve excitability to return to baseline. The discharge patterns for another cold unit in response to the sequence of temperature pulses are illustrated in Fig. 4. This unit had a threshold for continuous discharge close to 30 °C and a CV of 1.2 m s−1. On cooling, it discharged more vigorously than the other eight units, achieving a maximum peak frequency at 15 °C of 74 impulses s−1.
Figure 4. Responses of a single C cold fibre to 10 s temperature pulses.
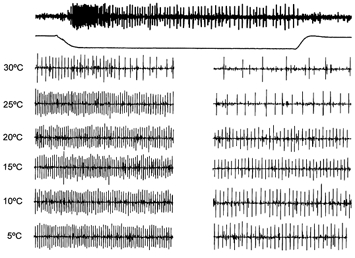
The top trace shows the full response to a temperature pulse from the baseline skin temperature of 35 °C to 30 °C, with the temperature profile shown below. The set of traces beneath these represent expanded 1 s sections of responses showing peak phasic (left) and tonic (right) discharges, for temperature steps from 35 °C to the temperatures indicated. The rate of temperature change at the start and end of the pulses was 10 °C s−1. The unit was silent between temperature pulses. These responses were filtered as in Fig. 1.
All nine fully characterised units displayed an initial phasic response to low temperature lasting 2-3 s, which decayed towards a slowly adapting, lower-frequency tonic response. On average, the number of impulses during each second of the low-temperature pulses increased rapidly for temperatures from 30 to 15 °C (Fig. 5A), but lower temperatures did not evoke further increases in either the phasic or tonic discharge frequency. The average firing rates, shown in Fig. 5A, conceal a considerable range among the nine units, and Fig. 5B shows the tonic firing rates (averaged over the last 4 s) of each unit as a function of temperature. Most units fired maximally at 15-20 °C, but the two with the lowest firing rates showed increasing activity down to 5-10 °C. The number of units was too small to tell whether there were different populations, with response maxima at different temperatures, as proposed by Konietzny (1984) for both cold and warm receptors in human skin. Bursts of action potentials were not observed at any temperature between 30 and 5 °C.
Figure 5. Responses of C cold fibres to 10 s temperature pulses.
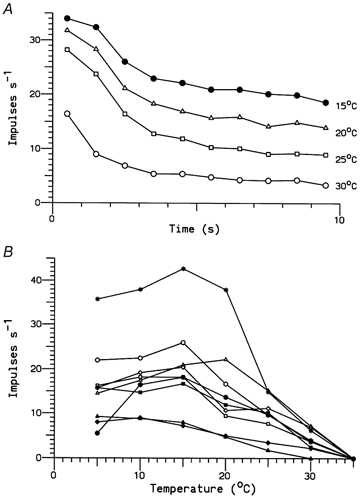
A, mean discharge frequencies when the temperature was lowered from 35 °C to 30, 25, 20 and 15 °C for 10 s. Each point represents the number of impulses in 1 s averaged over nine units. B, tonic discharge frequencies (averaged over the final 4 s) plotted as a function of temperature for all nine units. Different symbols represent different temperatures in A and different fibres in B.
Paradoxical responses
Three out of the nine fully characterised units displayed an additional response to high-temperature stimuli. The threshold for a high-temperature response was 42.3 ± 3.4 °C (mean ±s.d.). The ‘cold’ fibre with the lowest heat threshold (38 °C) responded similarly to heat and cold, in that it displayed a phasic and a tonic stage (Fig. 6). However, at both 40 and 45 °C this unit fired in short bursts of action potentials during the tonic stage. Another unit responded in bursts without an initial increase in discharge frequency.
Figure 6. Paradoxical response to heat of a single C cold fibre.
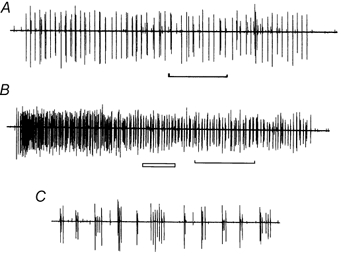
A, activity provoked by warming the skin from 35 °C to 40 °C for 10 s. B, response to a 10 s pulse to 45 °C. C, expansion of the period marked by the open bar in B, showing the tendency of the unit to discharge in bursts. The time marker for A and B indicates 2 s. The responses were filtered as in Fig. 1.
CVs and responses to repetitive stimulation
It has been reported previously that when stimulation frequency was increased from 0.25 Hz to 2 Hz, different patterns of slowing were observed among human C fibres (Serra et al. 1999): ‘type 1′ fibres slowed progressively (average latency increase at 3 min = 28.3 %), whereas ‘type 2′ fibres slowed to reach a plateau (average latency increase 5.2 %) within 1 min. The former were identified as polymodal nociceptors, whereas the latter were tentatively identified as cold-specific receptors.
Our results have confirmed fully the identification of type 2 or ‘plateau’ units as cold-specific receptors. Of the 18 cold-specific units, 16 were tested with repetitive stimulation at 2 Hz and all exhibited slowing of conduction that reached a plateau within about 1 min. The degree of slowing after 3 min averaged 5.2 ± 1.1 % (mean ±s.d., range 3.3-6.4 %). This group included one unit conducting faster than 2 m s−1, which is illustrated in Fig. 8A. In no case have we found a type 2 unit that did not respond to natural stimuli as a cold-specific receptor, or a unit responding to non-noxious cold stimuli that did not exhibit a type 2 latency profile on repetitive stimulation. For this reason we regard it as highly probable that the 10 additional type 2 units that were not characterised with natural stimuli were also cold-specific receptors. The fastest conducting fibre of this type (3.6 m s−1) is illustrated in Fig. 8B, and the profile of activity-dependent slowing is very similar to that of the cold fibre shown in Fig. 7B (for which the CV is not known).
Figure 9A shows the range of CVs measured for the 26 type 2 units (comprising 16 cold-specific and 10 probable cold fibres). The CVs range from 0.8 to 3.6 m s−1, indicating that although most units were (at least mostly) unmyelinated, a few were (at least partly) myelinated. Figure 9B shows the extent of activity-dependent slowing after stimulation for 3 min at 2 Hz as a function of the CV, in comparison with the same data for 80 type 1 (nociceptor) C fibres. The cluster of unmyelinated type 2 units, with CVs of around 1 m s−1, showed much less activity-dependent slowing than the cluster of type 1 units. It might have been expected that the axons conducting faster than 2 m s−1, being myelinated, would exhibit less activity-dependent slowing than the C cold fibres (axons conducting in the 10-20 m s−1 velocity range in this study all showed less than 1 % slowing). Surprisingly, the slowing was comparable in the ‘myelinated’ group, but more variable, suggesting incomplete myelination (see Discussion). Among the type 1 fibres there was a negative correlation between activity-dependent slowing and CV (r = -0.33, P < 0.005, n = 80), consistent with the larger axons having a lower surface-to-volume ratio and proportionately less activity-dependent ion accumulation than the smaller axons. No such tendency was evident amongst the unmyelinated type 2 fibres (r = -0.04, n = 21).
Figure 9. CVs and activity-dependent slowing in cold and other type 2 units.
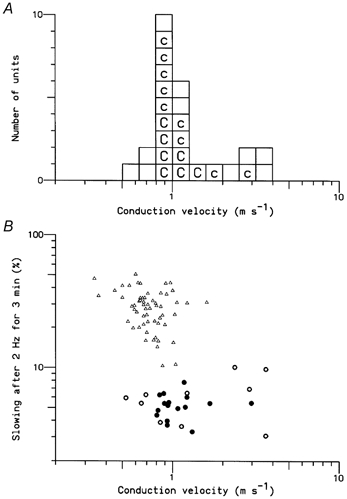
A, Distribution of CVs (logarithmic scale). Each box represents a single type 2 unit: ‘c’ denotes units identified as cold-specific receptors, ‘C’ indicates units that were fully characterised. B, relationship between CV and the degree of slowing after stimulation at 2 Hz for 3 min. The triangles represent the 80 type 1 units. The thick and filled circles represent the 26 type 2 units. The filled circles indicate cold-specific receptors.
In a previous study (Serra et al. 1999), the rate of recovery of C fibre latencies after repetitive stimulation was found to be significantly faster for type 2 than for type 1 fibres, although within each type there was no correlation between recovery time and the extent of slowing. These relationships were maintained for the additional units measured in the present study, although there was more overlap in the values, and a correlation between the time for recovery of latency by 50 % and the extent of slowing was evident for the type 1 fibres (Fig. 10). The mean (±s.d.) 50 % recovery time for the 26 type 2 units was 18.3 ± 4.8 s. There was no correlation among these fibres between recovery time and either CV (r = -0.03) or the extent of slowing (r = 0.01).
Figure 10. Rates of recovery following repetitive stimulation.
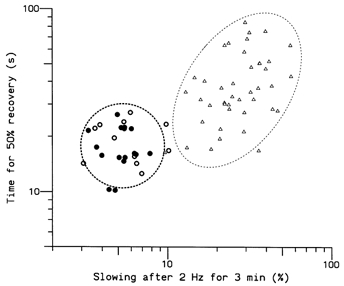
Times taken for the latency of a unit to recover half-way towards the resting value after stimulation at 2 Hz for 3 min. Triangles represent the 40 type 1 units. Circles represent the 29 type 2 units; filled circles indicate cold-specific receptors. Dashed ellipses are 95 % confidence limits for a single unit type.
DISCUSSION
Our results provide new information about cutaneous cold afferent fibres in humans. The present recordings from cold-specific units are entirely consistent with the previous limited data provided by Hensel & Boman (1960), Järvilehto & Hämäläinen (1979) and Konietzny (1984). However, our finding that the majority of such units conduct in the C fibre range appears to conflict with the commonly held view that human cold sensation is mediated wholly or mainly by Aδ fibres, and raises a number of questions. What is the range of CVs of cold-specific afferents in humans? Is CV uniform along the length of cold-specific afferents? Is CV related to skin type (hairy or glabrous)? What sensation results from impulses in C cold fibres? Before considering these questions, we will compare our new findings on the C fibre cold units with data on C and Aδ fibre cold units in other species.
Receptor characteristics of human C cold units
The nine fully characterised ‘C cold’ units found in the present study display several physiological features reported previously for cutaneous C cold afferents in mammals (Iggo, 1959; Hensel et al. 1960; Iriuchijima & Zotterman, 1960). They discharge continuously at normal skin temperature (≈30 °C), their nerve impulse activity is increased by lowering the temperature and decreased or suppressed by increasing the temperature, they are insensitive to mechanical stimuli and they have punctate RFs. Comparable features have been described for the more common cold afferents with Aδ fibres in animals (Hensel & Zotterman, 1951; Hensel, 1952; Iggo, 1969; Darian-Smith et al. 1973). The temperature at which the units fired maximally was appreciably lower than has been described for cold-specific afferents in monkey glabrous skin (≈15 °C, as against the value of 25 °C given by Darian-Smith et al. 1973), and the steady-state firing of the monkey units persisted at higher temperatures (40-45 °C). Konietzny (1984) also found that some human cold units reached peak firing rates at relatively low temperatures (range 10-27 °C). The present low-threshold cold receptors with C fibres are readily distinguished from human C nociceptors that are responsive to noxious low temperature by the higher threshold (< 20 °C), additional mechanosensitivity and lower firing rate of the latter (Torebjörk & Hallin, 1976; Campero et al. 1996).
One feature that may distinguish our C cold units from Aδ units in primates is their discharge pattern. Iggo (1969) reported that primate cold receptors characteristically fire in bursts within the range 18-40 °C. Similarly, Long (1977) found that the typical pattern of discharge of macaque Aδ cold units at steady temperature consisted of short bursts separated by silent intervals. It has even been suggested that the number of impulses per burst encodes steady-state temperature, enabling cold receptors with a bell-shaped response curve to provide unambiguous temperature information (Dykes, 1975). In contrast, burst discharges are not typical for human cold units (Konietzny, 1984) and they were observed in the present sample of C cold units from hairy skin only while heating the RF of two out of nine units. Another feature that might reasonably have been expected to differ between myelinated and unmyelinated cold units is the maximum firing rate. However, the rate of 74 impulses s−1 achieved by one of our C cold units (Fig. 4) is comparable with the maximum rates found for monkey Aδ units (Darian-Smith et al. 1973; Kenshalo & Duclaux, 1977).
Von Frey (1906) observed that a paradoxical cold sensation was often evoked by heating a cold spot to 45 °C, and a corresponding ‘paradoxical’ response of cold-specific fibres in the lingual nerve of the cat was described by Dodt & Zotterman (1952). Such responses have since been reported regularly for a fraction of cold-specific receptors, whether connected to myelinated (e.g. Kenshalo & Duclaux, 1977) or unmyelinated axons, as in this study. This phenomenon has been described in detail in monkeys by Long (1977), and in humans by Järvilehto & Hämäläinen (1979).
CVs of human cold-specific fibres and the role of C cold fibres in sensation
Of the 16 cold-specific units tested with electrical stimulation, only one had a CV in the Aδ range (3.0 m s−1). The great majority of cold and probable cold units had a CV close to 1 m s−1 (Fig. 9A). These data appear to conflict not only with the evidence from some studies on monkeys that all cold-specific fibres conduct in the Aδ range (2.2-30 m s−1; see Introduction), but also with the evidence of Mackenzie et al. (1975) that only Aδ fibres signal discriminatory cold information in humans. Our data and those of Mackenzie et al. (1975) can be reconciled in two ways. Either the majority of cold-specific afferents that we recorded conducting in the C fibre velocity range do not signal discriminatory cold information and there is another population of faster-conducting Aδ cold fibres (which we presumably missed because of the selectivity of our electrodes), or cold-specific afferents in humans are mainly non-uniform in structure, predominantly unmyelinated for the most distal portion over which we recorded, but thinly myelinated more proximally. Fibres of this type, unmyelinated over their most distal 100-200 mm parts but conducting more proximally at 4-7 m s−1, were described by Iggo & Ogawa (1971) in primate saphenous nerve. Cat vagal afferents with similar properties, conducting at 1.5 m s−1 distally but 6 m s−1 proximally, were termed ‘BC’ fibres by Duclaux et al. (1976) because they had electrophysiological and morphological properties of C fibres in their distal part and B fibres in their proximal part. Our C cold fibres were all recorded over distances of less than 200 mm (range 30-170 mm, mean 92 mm), and the velocity ranges for the proximal segments of BC fibres correspond very well with the velocity estimates for human cold fibres obtained from reaction time and evoked response studies. Estimates include 2.1 m s−1 (Fowler et al. 1988), 2.6 m s−1 (Yarnitsky & Ochoa, 1991), 4.5 m s−1 (Fruhstorfer, 1976) and 7.7-8.0 m s−1 (Susser et al. 1999) from reaction times and 4.3 m s−1 from evoked responses (Fruhstorfer, 1976).
We have found no direct evidence for either of the above hypotheses. The reaction time data described above, and the absence of cold-specific fibres in the group of 140 Aδ human cutaneous afferents (CV 19.2 ± 7.2 m s−1, mean ±s.d.) recorded by Adriaensen et al. (1983) leave little doubt that humans do not possess cold-specific fibres with CVs comparable to those seen in monkeys (see Introduction), but it is quite possible that the fastest cold fibres were under-represented in our recordings. It is also conceivable that the unmyelinated cold fibres that we and Konietzny (1984) recorded have a thermoregulatory function that is distinct from the sensory function of myelinated cold fibres (Iriuchijima & Zotterman, 1960), but this remains a speculative hypothesis. In some cases the RF of a C cold unit appeared to correspond to a ‘cold spot’ (von Frey, 1906), but we have also recorded a vigorous discharge from a C cold unit without the subject being aware of a cold sensation. If human cold fibres became myelinated after 100-200 mm, then recordings over longer distances should give a higher range of CVs. A series of 96 C units recorded at knee level over distances of 220-510 mm by Weidner et al. (1999) did not include any cold-specific fibres, consistent with the cold fibres being myelinated at this distance, but positive evidence is lacking.
One factor contributing to the differences in CV between human and monkey cold fibres may be the type of skin innervated. In his studies of primate cold receptors, Iggo (1969) found that three out of nine cold receptors from hairy skin had unmyelinated axons, whereas all the glabrous cold units were myelinated. Long (1977) did not find any C cold fibres in either the glabrous or hairy skin of macaque monkeys, but he did note a velocity difference within the Aδ cold fibre population: 13.3 ± 2.8 m s−1 (mean ±s.d., n = 156) for fibres from glabrous skin, in good agreement with the figure of 14.5 m s−1 given by Darian-Smith et al. (1973), but 7.0 ± 2.8 m s−1 for fibres from hairy skin. However, it is unlikely that cold-specific afferents in human glabrous skin have much higher CVs than those in hairy skin, since the estimate of 2.6 m s−1 from reaction times by Yarnitsky & Ochoa (1991) was obtained from the thenar eminence, and was no faster than the other estimates made from hairy skin.
Activity-dependent slowing of cold fibres
Activity-dependent slowing was used by Torebjörk & Hallin (1973) to determine whether a C fibre was activated by a natural stimulus, and this ‘marking’ technique (Schmelz et al. 1995) has since been used regularly to help characterise C fibres. More recently, Thalhammer et al. (1994) and Gee et al. (1996) have shown in the rat that the amount and pattern of slowing induced by repetitive electrical stimulation can be used to separate fibres into functional type. Human C fibres behave similarly, and evidence is accumulating that activity-dependent slowing on electrical stimulation, both at 2 Hz (Serra et al. 1999) and at low frequencies (Weidner et al. 1999), can provide a means of classifying C fibres that relates to function. Our observations in this study, relating cold-specific fibres to those that quickly reach a latency plateau on stimulation at 2 Hz, provide further support for such a classification.
A surprising finding was that this property of cold-specific fibres extends to those with CVs > 2 m s−1, without a sharp drop due to myelination (Fig. 9B). This is difficult to explain on the assumption that cold fibres are either all myelinated or all unmyelinated, since activity-dependent slowing is much less pronounced in other myelinated axons (and in the rat Aδ cold fibres studied by Thalhammer et al. 1994). If much of the variation in CV was due to the recordings sampling different proportions of myelinated and unmyelinated axons, one would expect to find a positive correlation between CV and conduction distance. There was no significant correlation (r = 0.20, P = 0.4, n = 26), but this does not rule out the possibility that a few axons were partly myelinated.
In conclusion, we have recorded activity from afferent fibres innervating the hairy skin of the human foot with the properties of cold-specific fibres, and found that nearly all conducted in the C fibre velocity range. We therefore consider that the commonly expressed view that cold-specific afferents in humans are Aδ fibres is either misleading or incomplete. Either they are incompletely myelinated BC fibres, conducting partly in the C fibre range and always below 10 m s−1, or else there are two populations of cold-specific afferents, with a C fibre group that contributes little to sensation.
Acknowledgments
This work was supported by NIH grant RO1 NS-39761.
References
- Adriaensen H, Gybels J, Handwerker HO, Van Hees J. Response properties of thin myelinated (A-δ) fibers in human skin nerves. Journal of Neurophysiology. 1983;49:111–122. doi: 10.1152/jn.1983.49.1.111. [DOI] [PubMed] [Google Scholar]
- Campero M, Serra J, Ochoa JL. C-polymodal nociceptors activated by noxious low temperature in human skin. Journal of Physiology. 1996;497:565–572. doi: 10.1113/jphysiol.1996.sp021789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith I. Thermal sensibility. In: Darian-Smith I, editor. Handbook of Physiology, section 1, The Nervous System, Sensory Processes. Vol. 3. Bethesda, MD, USA: American Physiological Society; 1984. pp. 879–913. part 2. [Google Scholar]
- Darian-Smith I, Johnson KO, Dykes R. Cold’ fibre population innervating palmar and digital skin of the monkey: responses to cooling pulses. Journal of Neurophysiology. 1973;36:325–346. doi: 10.1152/jn.1973.36.2.325. [DOI] [PubMed] [Google Scholar]
- Dodt E, Zotterman Y. The discharge of specific cold fibres at high temperatures (the paradoxical cold) Acta Physiologica Scandinavica. 1952;26:358–365. doi: 10.1111/j.1748-1716.1952.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Duclaux R, Mei N, Ranieri F. Conduction velocity along afferent vagal dendrites: a new type of fibre. Journal of Physiology. 1976;260:487–495. doi: 10.1113/jphysiol.1976.sp011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes RW. Coding of steady and transient temperatures by cutaneous ‘cold’ fibres serving the hand of monkeys. Brain Research. 1975;98:485–500. doi: 10.1016/0006-8993(75)90368-6. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Sitzoglou K, Ali Z, Halonen P. The conduction velocities of peripheral nerve fibres conveying sensations of warming and cooling. Journal of Neurology, Neurosurgery and Psychiatry. 1988;51:1164–1170. doi: 10.1136/jnnp.51.9.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhstorfer H. Conduction in the afferent thermal pathways of man. In: Zotterman Y, editor. Sensory Functions of the Skin of Primates With Special Reference to Man. Oxford: Pergamon; 1976. pp. 355–365. [Google Scholar]
- Gee MD, Lynn B, Cotsell B. Activity-dependent slowing of conduction velocity provides a method for identifying different functional classes of C-fibre in the rat saphenous nerve. Neuroscience. 1996;73:667–675. doi: 10.1016/0306-4522(96)00070-x. [DOI] [PubMed] [Google Scholar]
- Hagbarth K-E, Vällbo Å. Mechanoreceptor activity recorded percutaneously with semimicroelectrodes in human peripheral nerves. Acta Physiologica Scandinavica. 1967;69:121–122. doi: 10.1111/j.1748-1716.1967.tb03498.x. [DOI] [PubMed] [Google Scholar]
- Hensel H. Afferente impulse aus den Kältereceptoren der äußeren haut. Pflügers Archiv. 1952;256:195–211. doi: 10.1007/BF00364326. [DOI] [PubMed] [Google Scholar]
- Hensel H. Cutaneous thermoreceptors. In: Iggo A, editor. Somatosensory System. Berlin: Springer-Verlag; 1973. pp. 79–110. [Google Scholar]
- Hensel H, Boman KKA. Afferent impulses in cutaneous sensory nerves in human subjects. Journal of Neurophysiology. 1960;23:564–578. doi: 10.1152/jn.1960.23.5.564. [DOI] [PubMed] [Google Scholar]
- Hensel H, Iggo A, Witt I. A quantitative study of sensitive cutaneous thermoreceptors with C afferent fibres. Journal of Physiology. 1960;153:113–126. doi: 10.1113/jphysiol.1960.sp006522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel H, Zotterman Y. The effect of menthol on the thermoreceptors. Acta Physiologica Scandinavica. 1951;24:27–34. doi: 10.1111/j.1748-1716.1951.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Iggo A. Cutaneous heat and cold receptors with slowly conducting C afferent fibres. Quarterly Journal of Experimental Physiology. 1959;44:362–370. doi: 10.1113/expphysiol.1959.sp001417. [DOI] [PubMed] [Google Scholar]
- Iggo A. Cutaneous thermoreceptors in primates and sub-primates. Journal of Physiology. 1969;200:403–430. doi: 10.1113/jphysiol.1969.sp008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Ogawa H. Primate cutaneous thermal nociceptors. Journal of Physiology. 1971;143:47–48P. [PubMed] [Google Scholar]
- Iriuchijima J, Zotterman Y. The specificity of afferent cutaneous C fibres in mammals. Acta Physiologica Scandinavica. 1960;49:267–278. doi: 10.1111/j.1748-1716.1960.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Järvilehto T, Hämäläinen H. Touch and thermal sensations: psychophysical observations and unit activity in human skin nerves. In: Kenshalo DR, editor. Sensory Functions of the Skin of Humans. New York: Plenum; 1979. pp. 279–295. [Google Scholar]
- Kenshalo DR, Duclaux R. Response characteristics of cutaneous cold receptors in the monkey. Journal of Neurophysiology. 1977;40:319–332. doi: 10.1152/jn.1977.40.2.319. [DOI] [PubMed] [Google Scholar]
- Konietzny F. Peripheral neural correlates of temperature sensations in man. Human Neurobiology. 1984;3:21–32. [PubMed] [Google Scholar]
- Long RR. Sensitivity of cutaneous cold fibers to noxious heat: paradoxical cold discharge. Journal of Neurophysiology. 1977;40:489–502. doi: 10.1152/jn.1977.40.3.489. [DOI] [PubMed] [Google Scholar]
- Mackenzie RA, Burke D, Skuse NF, Lethlean AK. Fibre function and perception during cutaneous nerve block. Journal of Neurology, Neurosurgery and Psychiatry. 1975;38:865–873. doi: 10.1136/jnnp.38.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Forster C, Schmidt R, Ringkamp M, Handwerker HO, Torebjörk HE. Delayed responses to electrical stimuli reflect C-fiber responsiveness in human microneurography. Experimental Brain Research. 1995;104:331–336. doi: 10.1007/BF00242018. [DOI] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa JL, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. Journal of Physiology. 1999;515:799–811. doi: 10.1111/j.1469-7793.1999.799ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser E, Sprecher E, Yarnitsky D. Paradoxical heat sensation in healthy subjects: peripherally conducted by A delta or C fibres? Brain. 1999;122:239–246. doi: 10.1093/brain/122.2.239. [DOI] [PubMed] [Google Scholar]
- Torebjörk HE, Hallin RG. Perceptual changes accompanying controlled preferential blocking of A and C fibre responses in intact human skin nerves. Experimental Brain Research. 1973;16:321–332. doi: 10.1007/BF00233334. [DOI] [PubMed] [Google Scholar]
- Torebjörk HE, Hallin RG. A new method for classification of C-unit activity in intact human skin nerves. In: Bonica JJ, Albe-Fessard D, editors. Advances in Pain Research and Therapy. New York: Raven; 1976. pp. 29–34. [Google Scholar]
- Von Frey M. The distribution of afferent nerves in the skin. Journal of the American Medical Association. 1906;XLVII:645–651. [Google Scholar]
- Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjörk HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. Journal of Neuroscience. 1999;15:10184–10190. doi: 10.1523/JNEUROSCI.19-22-10184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitsky D, Ochoa JL. Warm and cold specific somatosensory systems: psychophysical thresholds, reaction times and peripheral conduction velocities. Brain. 1991;114:1819–1826. doi: 10.1093/brain/114.4.1819. [DOI] [PubMed] [Google Scholar]


