Abstract
Airway administration of synthetic cationic proteins, poly-l-lysine (PLL) and poly-l-arginine (PLA), is known to induce bronchial hyper-responsiveness, and an involvement of bronchopulmonary C fibre activation has been suggested. In this study we investigated the effects of PLL and PLA on single-unit pulmonary vagal C fibre afferents in anaesthetized, open-chest rats.
Intratracheal (i.t.) instillation of PLL or PLA activated C fibre endings in a dose-dependent manner; for example, a high dose of PLL (50 μg in 0.1 ml) had a sporadic but intense stimulatory effect on these afferents. The augmented C fibre activity slowly declined but remained elevated even after 120 min.
Intratracheal instillation of PLL or PLA greatly enhanced the sensitivities of pulmonary C fibres to both lung inflation and chemical stimuli (e.g. capsaicin); for example, the change in fibre activity in response to constant-pressure lung inflation (tracheal pressure (Pt) = 30 cmH2O; 10 s duration) increased by ∼6-fold after PLL instillation.
When administered by intravenous injection or instilled into a different region of the lung, PLL or PLA, even at a higher dose, failed to have any effect on the C fibre endings.
The stimulatory and sensitizing effects of PLL or PLA were completely nullified when their cationic charges were neutralized with low molecule weight heparin.
In conclusion, i.t. instillation of synthetic cationic proteins causes an intense stimulatory effect on pulmonary C fibres and potentiates their sensitivities to both lung inflation and chemical stimuli. These effects are probably generated by an interaction between the cationic charges carried by these proteins and the airway mucosa.
Infiltration of inflammatory cells such as eosinophils and neutrophils into the airway lumen is a characteristic of various airway inflammatory diseases such as asthma (Durham & Kay, 1985; Gleich, 1990). These cells are known to secrete a number of cationic proteins, including major basic protein and eosinophil cationic protein released from eosinophils, and cathepsin G and neutrophil cationic proteins released from neutrophils. These cationic proteins are believed to play a key role in the development of the pathophysiological conditions associated with airway inflammation (Flavahan et al. 1988; Wardlaw et al. 1988; Hamann et al. 1991). For example, previous studies have demonstrated that intratracheal (i.t.) instillation of major basic protein induces airway inflammation and bronchial hyper-responsiveness in a number of animal species (Gundel et al. 1991; Coyle et al. 1993; Uchida et al. 1993). Furthermore, similar effects can be also induced by i.t. administration of synthetic cationic proteins such as poly-l-lysine (PLL) (Coyle et al. 1993; Uchida et al. 1993). The airway hyper-responsiveness and plasma protein extravasation resulting from the i.t. challenge with PLL in the rat both involve the release of tachykinins, suggesting a possible involvement of stimulation of C fibre sensory terminals in the airways and lungs (Coyle et al. 1994). However, direct electrophysiological evidence that these synthetic cationic proteins stimulate C fibre sensory endings is still lacking.
Non-myelinated (C) vagal afferent nerves innervate the entire respiratory tract (Coleridge & Coleridge, 1984), and represent 75 % of the vagal afferents arising from the lungs (Agostoni et al. 1957). Indeed, the importance of bronchopulmonary C fibre afferents in regulating the cardiorespiratory functions under both normal and abnormal physiological conditions has been well documented (Paintal, 1973; Coleridge & Coleridge, 1984; Lee & Pisarri, 2001). Furthermore, increasing evidence indicates that pulmonary C fibre afferents play an important role in the manifestation of airway hyper-responsiveness associated with mucosal injury and inflammation (Spina, 1996; Lee & Pisarri, 2001). In the light of the existing knowledge described above and the information that is currently lacking, the present study was carried out to investigate: (1) whether vagal pulmonary C fibres are activated by these cationic proteins delivered into the lung by i.t. instillation; (2) whether the sensitivity of pulmonary C fibre afferents to chemical and mechanical stimuli is enhanced by i.t. instillation of these proteins; and (3) if so, whether the effects on pulmonary C fibres are generated by the cationic charges carried by these proteins. In addition, we sought to determine whether the effects of these cationic proteins are dependent on the route of delivery.
METHODS
Animal preparation
The procedures described below were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and were also approved by the University of Kentucky Institutional Animal Care and Use Committee.
Sprague-Dawley rats (340-435 g) were initially anaesthetized with an intraperitoneal injection of α-chloralose (100 mg kg−1) and urethane (500 mg kg−1) dissolved in a 2 % borax solution; supplemental doses of the same anaesthetics were given, whenever necessary, to maintain abolition of the pain reflex elicited by paw pinch. The right femoral artery and left jugular vein were cannulated for recording arterial blood pressure (BP) and for injections, respectively; the venous catheter was advanced until its tip was positioned slightly above the right atrium. The trachea was cannulated just below the larynx, and the lungs were artificially ventilated using a respirator (UGO Basile 7025, Comerio-Varese, Italy). Tidal volume (VT) and respiratory frequency were set at 8-10 ml kg−1 and 44 breaths min−1, respectively, to mimic those of a unilaterally vagotomized rat (see explanation below). A midline thoracotomy was performed, and the expiratory outlet of the respirator was placed under 3 cmH2O pressure to maintain a near-normal functional residual capacity. Body temperature was maintained at 36-37 °C throughout the experiment by a heating pad placed under the animal, which lay in a supine position. Animals were killed at the end of the experiments by an intravenous (i.v.) injection of an overdose of pentobarbitone.
Recording of single pulmonary C fibres
Single-unit pulmonary C fibre activity was recorded as described previously (Ho & Lee, 1998). Briefly, the right cervical vagus nerve was separated from the carotid artery and sectioned rostrally. The caudal end of the cut vagus nerve was placed on a small dissection platform, desheathed and a thin filament teased away from the nerve trunk and placed on a platinum-iridium hook electrode. Action potentials were amplified (Grass P5-11K), monitored with an audio monitor (Grass AM8RS) and displayed on an oscilloscope (Tektronix 2211). The thin filament was further split until the afferent activity from a single unit was isolated. Both vagi were ligated just above the diaphragm to eliminate the electrical signals arising from the abdominal viscera. The afferent activity of a single unit was first searched for by hyperinflation (3-4 times VT), and then identified by the immediate (delay < 1 s) response to bolus injection of capsaicin (0.5-1 μg kg−1) into the right atrium. The conduction velocity of the afferent fibre was measured as described previously (Ho & Lee, 1998). Finally, the general locations of pulmonary C fibres were identified by their responses to the gentle pressing of the lungs with a blunt-ended glass rod. The signals of the afferent activities, tracheal pressure (Pt) (Validyne MP 45-28) and BP (Statham P23AA) were recorded on a Gould Thermal Writer (TW11) and a videocassette recorder (Vetter 500H). Fibre activity (FA) was analysed later by computer for each 0.5 s interval.
Experimental design and protocol
Eight series of experiments were carried out. (1) To determine whether pulmonary C fibres are activated by i.t. instillation of PLL in a dose-dependent manner, a small volume (0.1 ml) of PLL was delivered into the lungs via the tracheal cannula with a Hamilton microsyringe connected to a PE 50 tubing; the tip of the tubing was advanced just beyond the caudal end of the endotracheal tube. The baseline activities of pulmonary C fibres were determined at 10 min before and 2, 10, 30, 60 and 120 min after the instillation. Two doses of PLL were tested: 25 and 50 μg; only one dose was administered to each animal. (2) To investigate whether the pulmonary C fibre responses to mechanical stimuli are altered by i.t. PLL instillation, hyperinflation of the lung was generated at two different inflation pressures (Pt= 15 or 30 cmH2O), and maintained for 10 s. C fibre responses to lung inflations were determined at 10 min before and 10, 30, 60 and 120 min after PLL instillation. (3) To investigate whether the pulmonary C fibre responses to chemical stimulants are altered by PLL, three chemical agents known to stimulate pulmonary C fibre afferents were chosen: capsaicin (0.5 μg kg−1), phenyl biguanide (PBG, 4 μg kg−1) and adenosine (0.15 mg kg−1); only one stimulus was studied in each animal. To determine the time course of the PLL effect and to avoid potential cumulative effects of chemical agents, the same injection was repeated only at three selected time points: 10 min before, and 45 and 90 min after the PLL instillation. (4) To study whether the effect of PLL on pulmonary C fibres is dependent on the route of delivery, a higher dose of PLL (100 μg) was administered by i.v. injection. (5) To determine whether the stimulatory effect of PLL on a C fibre ending can also be generated when the delivery of PLL is confined to another region of the lung, we instilled PLL (50 μg in 0.1 ml) into the contralateral (left) lung or a single lobe of the right lung by advancing the catheter ∼5 mm beyond the carina; the distribution of PLL was determined by pre-mixing the solution with a dye (Chicago Sky Blue, 0.05 % or Trypan Blue, 0.08 %). The instillation site and distribution of PLL were determined by postmortem examination. (6) To examine whether pulmonary oedema is induced by i.t. instillation of PLL, the lungs were removed 10 min after the PLL challenge, bled, and then placed in an oven (80 °C, 24 h) to dry. Wet-weight to dry-weight ratios of the lungs (Lindqvist, 1964) were then determined. Control rats of matching body weight were treated with vehicle. (7) To investigate the role of cationic charge in producing the effect of PLL instillation, 0.5 mg ml−1 of PLL was mixed with an equal concentration and volume of low molecule weight heparin (LMWH), which carries negative charges, and 0.1 ml of the mixture was instilled into the airway. The same protocols were followed to study the C fibre responses before and after the challenges. (8) To determine whether the same effects of PLL on pulmonary C fibres can also be produced by other synthetic cationic proteins, poly-l-arginine (PLA) was administered in the same manner, and the same protocols as described above were followed in separate groups of animals.
Materials
A mixture of 2 % α-chloralose (Sigma) and 10 % urethane (Sigma) was dissolved in a 2 % borax (Sigma) solution. Stock solutions of capsaicin and PBG (both from Sigma) were made by dissolving capsaicin in a vehicle of 10 % Tween 80, 10 % ethanol and 80 % isotonic saline at 250 μg ml−1, and PBG in saline at 1 mg ml−1. A stock solution of the hemisulphate salt of adenosine (Sigma; at 10 mg ml−1) was prepared in saline. The volume of each bolus injection of these chemical agents was kept at 0.2 ml and a solution of each of these agents at the desired concentration was prepared daily by diluting the stock solution with saline on the basis of the animal's body weight. PLL (MW 30 000-70 000 or 70 000-150 000; Sigma), PLA (MW 15 000-70 000; Sigma) and LMWH (MW 6000; Sigma) were dissolved in saline at 2.5 mg ml−1, and were diluted in saline to the desired concentration before use.
Statistical analysis
A two-way repeated-measures analysis of variance (ANOVA) was used for the statistical analysis, unless otherwise indicated. When the two-way ANOVA showed a significant interaction, pairwise comparisons were made with a post hoc analysis (Newman-Keuls test). Data are expressed as means ±s.e.m. Responses were considered to be significantly different when P < 0.05.
RESULTS
A total of 117 pulmonary C fibres were studied in 74 anaesthetized, open-chest rats. The distribution of these C fibre endings was as follows: 38 in the upper lobe of the right lung, 37 in the right middle lobe, 31 in the right lower lobe and 11 in the right accessory lobe. The conduction velocity measured in 26 of these fibres was 1.10 ± 0.07 m s−1 (range, 0.78-1.75 m s−1), which was within the range described for non-myelinated fibres.
Study series 1: effect of i.t. instillation of PLL on baseline pulmonary C fibre activity
Intratracheal instillation of PLL activated pulmonary C fibre endings in a dose-dependent manner (Fig. 1 and 2). A single bolus of a high dose of PLL (50 μg in 0.1 ml) induced a sporadic but intense and long-lasting stimulatory effect on these afferents. FA (20 s average) increased from a control (10 min before) of 0.02 ± 0.01 impulses s−1 (n = 16) to a peak of 2.69 ± 0.12 impulses s−1∼10 min after the delivery, then gradually declined, but remained significantly higher than control after 120 min (0.38 ± 0.04 impulses s−1; Fig. 1 and 2). In contrast, i.t. instillation of vehicle in six experiments did not cause any significant change in baseline activity over the same 120 min period. A similar but less intense effect was generated by a lower dose of PLL (25 μg in 0.1 ml). Tracheal pressure increased only slightly and transiently after the instillation of both PLL (ΔPt= 14.3 % and 14.1 % after high and low doses, respectively) and vehicle (ΔPt= 13.3 %), and returned completely to the baseline within 10 min. Heart rate (HR) decreased significantly by 21 % and 25 % (n = 16) at 2 and 10 min, respectively, after the instillation of a high dose of PLL. A similar effect on HR was also elicited after the low dose of PLL, but no change in HR response was found after vehicle. Blood pressure was significantly reduced (ΔBP = 21.0 %) only by a high dose of PLL at 10 min after the instillation.
Figure 1. Experimental records illustrating the effect of i.t. instillation of PLL on the baseline activities of two pulmonary C fibres.
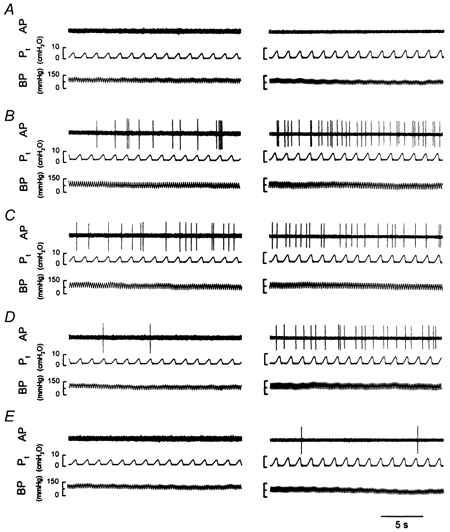
Left panels: low dose of PLL (25 μg in 0.1 ml); receptor location, upper lobe of right lung; rat body weight, 375 g. Right panels: high dose of PLL (50 μg in 0.1 ml); receptor location, middle lobe of right lung; rat body weight, 410 g. A–E, baseline activities at 10 min before (A), and 10 min (B), 30 min (C), 60 min (D) and 120 min (E) after PLL instillation. AP, action potential; Pt, tracheal pressure; BP, arterial blood pressure.
Figure 2. Time course of the effect of i.t. instillation of PLL on baseline pulmonary C fibre activities.
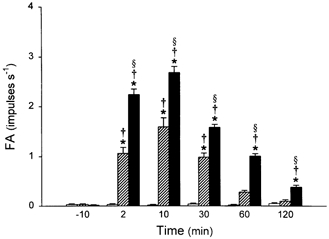
Baseline fibre activities (FA) were determined at 10 min before, and 2, 10, 30, 60 and 120 min after i.t. instillation of a low dose of PLL (25 μg in 0.1 ml,  ; n = 8), a high dose of PLL (50 μg in 0.1 ml, ▪; n = 16) and vehicle control (□; n = 6). FA at each time point was averaged over a 20 s interval for each fibre. Only one dose of PLL or vehicle was administered in each animal. Data are means ±s.e.m. *P < 0.05, significant difference between vehicle and 25 or 50 μg of PLL at any time point; §P < 0.05, significant difference between 25 and 50 μg of PLL at any time point; †P < 0.05, significant difference between before and any time point after instillation.
; n = 8), a high dose of PLL (50 μg in 0.1 ml, ▪; n = 16) and vehicle control (□; n = 6). FA at each time point was averaged over a 20 s interval for each fibre. Only one dose of PLL or vehicle was administered in each animal. Data are means ±s.e.m. *P < 0.05, significant difference between vehicle and 25 or 50 μg of PLL at any time point; §P < 0.05, significant difference between 25 and 50 μg of PLL at any time point; †P < 0.05, significant difference between before and any time point after instillation.
Study series 2: effect of i.t. instillation of PLL on pulmonary C fibre response to lung inflation
All pulmonary C fibres showed a very weak response to constant-pressure lung inflation at control, which was consistent with our previous observations (Ho et al. 2000). However, after instillation of PLL, the response to lung inflation was greatly elevated. This potentiating effect of PLL was dose dependent and extremely long lasting (Fig. 3). For example, the ΔFA generated by lung inflation at Pt= 30 cmH2O (the difference between the 10 s average during lung inflation and the 10 s average at baseline) increased from 1.08 ± 0.12 impulses s−1 (n = 16) at control (10 min before) to a peak of 7.64 ± 1.36 impulses s−1 at 10 min after instillation of a high dose of PLL (50 μg in 0.1 ml), and remained elevated (4.38 ± 0.32 impulses s−1) even after 2 h (Fig. 4). PLL also enhanced the C fibre response to lung inflation at lower pressure (15 cmH2O) in a similar manner, but the effect appeared to last for a shorter time (Fig. 4). In contrast, i.t. instillation of vehicle had no significant effect on the C fibre response to lung inflation (Fig. 4).
Figure 3. Experimental records illustrating the effect of i.t. instillation of PLL on the responses of two pulmonary C fibres to lung inflation.
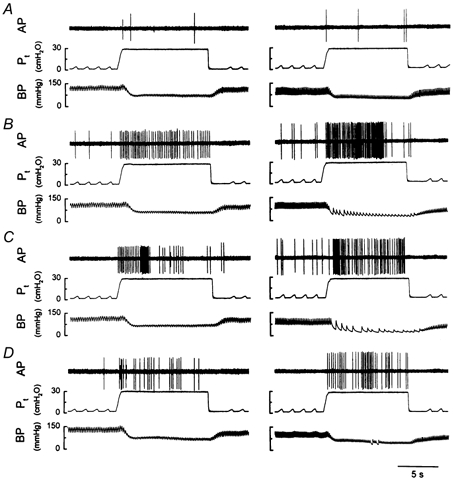
Left panels: low dose of PLL (25 μg in 0.1 ml); receptor location, upper lobe of right lung; rat body weight, 390 g. Right panels: high dose of PLL (50 μg in 0.1 ml); receptor location, middle lobe of right lung; rat body weight, 385 g. A–D, responses to lung inflation (Pt= 30 cmH2O) at 10 min before (A), and 10 min (B), 60 min (C) and 120 min (D) after PLL instillation. See legend to Fig. 1 for further explanation.
Figure 4. Time course of the effect of i.t. instillation of PLL on pulmonary C fibre responses to lung inflation.
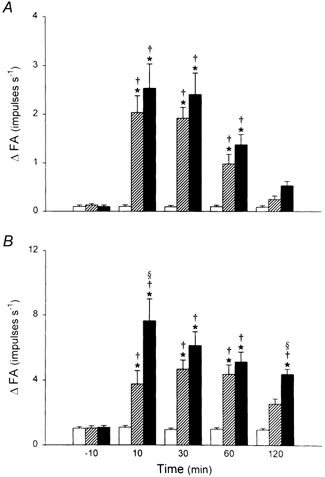
A, responses to lung inflation at low pressure (Pt= 15 cmH2O); B, responses to lung inflation at high pressure (Pt= 30 cmH2O). ΔFA was measured as the difference between the 10 s average during lung inflation and the 10 s average at baseline in each fibre. The responses were determined at 10 min before, and 10, 30, 60 and 120 min after i.t. instillation of a low dose of PLL (25 μg in 0.1 ml,  ; n = 8), a high dose of PLL (50 μg in 0.1 ml, ▪; n = 16) and vehicle control (□; n = 6). Data are means ±s.e.m. *P < 0.05, significant difference between vehicle and 25 or 50 μg of PLL at any time point; §P < 0.05, significant difference between 25 and 50 μg of PLL at any time point; †P < 0.05, significant difference between before and any time point after instillation of PLL or vehicle.
; n = 8), a high dose of PLL (50 μg in 0.1 ml, ▪; n = 16) and vehicle control (□; n = 6). Data are means ±s.e.m. *P < 0.05, significant difference between vehicle and 25 or 50 μg of PLL at any time point; §P < 0.05, significant difference between 25 and 50 μg of PLL at any time point; †P < 0.05, significant difference between before and any time point after instillation of PLL or vehicle.
Study series 3: effect of i.t. instillation of PLL on pulmonary C fibre response to chemical stimuli
Prior to PLL challenge, a bolus injection of capsaicin at a near-threshold dose of 0.5 μg kg−1 elicited either no or only a mild response in pulmonary C fibres. However, the stimulatory effect of the same dose of capsaicin on these fibres was markedly enhanced after i.t. instillation of PLL (50 μg in 0.1 ml; Figs 5 and 6). Both the peak activity and duration of firing of the fibres increased. The potentiating effect of instillation of PLL lasted for > 90 min in all fibres tested, and was not limited only to the response to capsaicin. The responses of pulmonary C fibres to injections of PBG (4 μg kg−1) and adenosine (0.15 mg kg−1) were also markedly elevated after instillation of PLL (Fig. 5 and 6). The concomitant decreases in HR and BP after injection of capsaicin or other chemical stimulants were also potentiated by PLL (e.g. Fig. 5). Presumably, these enhanced cardiovascular reflex responses were elicited by increased C fibre activity in the intact left vagus nerve (Ho & Lee, 1998). Instillation of vehicle did not cause any significant change of the pulmonary C fibre response to these three chemical stimulants (Fig. 6).
Figure 5. Experimental records illustrating the effect of i.t. instillation of PLL on pulmonary C fibre responses to three chemical stimuli.
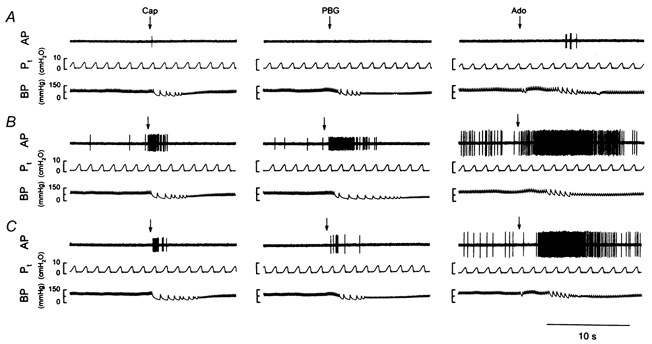
Left panels: responses to right arterial injection of capsaicin (Cap, 0.5 μg kg−1); receptor location, lower lobe of right lung; rat body weight, 371 g. Middle panels: responses to phenyl biguanide (PBG, 4 μg kg−1); receptor location, lower lobe of right lung; rat body weight, 379 g. Right panels: responses to adenosine (Ado, 0.15 mg kg−1); receptor location, upper lobe of right lung; rat body weight, 388 g. A–C, responses to chemical stimuli at 10 min before (A), and 45 min (B) and 90 min (C) after the delivery of PLL (50 μg in 0.1 ml). See legend to Fig. 1 for further explanation.
Figure 6. Time course of the effect of i.t. instillation of PLL on the response of pulmonary C fibres to three chemical stimuli.
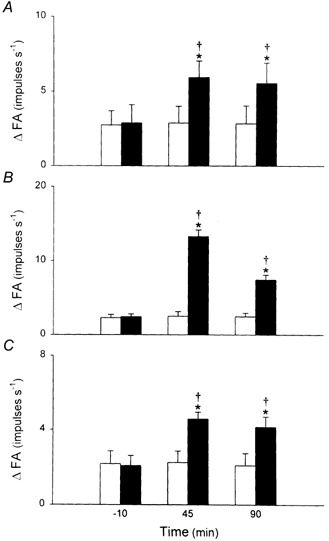
A, responses to right arterial injection of capsaicin (0.5 μg kg−1). B, responses to phenyl biguanide (4 μg kg−1). C, responses to adenosine (0.15 mg kg−1). ΔFA was measured as the difference between the 2 s average at peak and the 10 s average at baseline in each fibre. The responses were determined at 10 min before, and 45 and 90 min after i.t. instillation of PLL (50 μg in 0.1 ml, ▪; n = 8) and vehicle (□; n = 6). Data are means ±s.e.m. *P < 0.05, significant difference between vehicle and 50 μg of PLL at any time point; †P < 0.05, significant difference between before and any time point after instillation of PLL or vehicle.
Study series 4: effect of i.v. injection of PLL on sensitivity of pulmonary C fibres
Intravenous injection of PLL at a double dose (100 μg in 0.2 ml) failed to stimulate any pulmonary C fibres tested (FA (20 s average) = 0.02 ± 0.01 impulses s−1 at control (10 min before) and 0.02 ± 0.01 impulses s−1 at 10 min after injection; n = 10, P > 0.05), nor did it affect these C fibre afferent responses to either lung inflation or chemical stimulants (data not shown).
Study series 5: effect of PLL delivered into contralateral lung or another lobe on the C fibre ending
During the preliminary trials, we discovered that it was difficult to advance the catheter containing PLL into the left lung, probably because the trachea was pushed slightly to the left in our set-up for nerve recording; we were successful in only one of the five animals in which this procedure was attempted. Consequently, in the later experiments we decided to advance the tip of the catheter blindly until it was wedged into one of the lobar bronchi in the right lung. In four of the nine rats studied, PLL was confined to lobes other than the one containing the receptor (as judged from the dye distribution and receptor localization after the experiment); in one of these four rats, PLL was instilled into the left lung. PLL failed to have any detectable stimulatory effect on any of the C fibre receptors in these four animals (FA (20 s average) = 0.01 ± 0.01 impulses s−1 at control (10 min before) and 0.01 ± 0.01 impulses s−1 at 10 min after PLL; P > 0.05). In sharp contrast, PLL caused an intense stimulation of the remaining five receptors whose locations were identified in the same lobes into which PLL was delivered (FA (20 s average) = 0.02 ± 0.01 impulses s−1 at control (10 min before) and 1.45 ± 0.22 impulses s−1 at 10 min after PLL; P < 0.05).
Study series 6: possibility of pulmonary oedema induced by PLL
The wet/dry ratio of the rats’ lungs was 4.64 ± 0.06 (n = 6) after i.t. instillation of PLL; this value was not significantly different from that obtained from control rats (4.74 ± 0.05; n = 6, P > 0.05, Student's paired t test). Thus, there was no sign of pulmonary oedema.
Study series 7: effect of LMWH on the responses to i.t. instillation of PLL
Intratracheal instillation of LMWH (50 μg in 0.1 ml) alone did not have any detectable effect on the baseline activities of pulmonary C fibres (Fig. 7). However, when mixed with an equal concentration of PLL (50 μg in 0.1 ml), LMWH completely prevented the stimulatory and sensitizing effects of PLL on pulmonary C fibres (Fig. 7), suggesting an important role of cationic charge in the development of pulmonary C fibre activation and hyper-responsiveness to lung inflation and various chemical stimuli.
Figure 7. The effect of LMWH on the sensitizing effect of i.t. instillation of PLL.
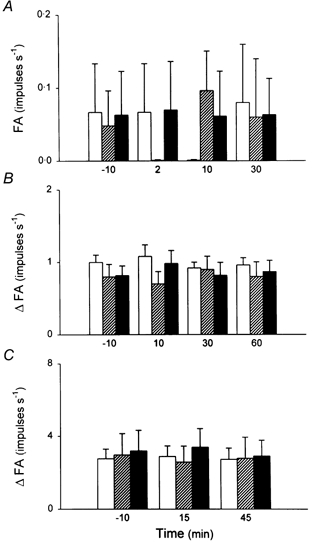
A, averaged baseline activities before and after i.t. instillation. FA at each time point was averaged over a 20 s interval for each fibre. B, responses to lung inflation (Pt= 30 cmH2O) before and after instillation. ΔFA was measured as the difference between the 10 s average during lung inflation and the 10 s average at baseline in each fibre. C, responses to capsaicin (0.5 μg kg−1) before and after instillation. ΔFA was measured as the difference between the 2 s average at peak and the 10 s average at baseline in each fibre. □, vehicle control; LMWH (50 μg in 0.1 ml); ▪, mixture of PLL (50 μg in 0.1 ml) and LMWH (50 μg in 0.1 ml). Data are means ±s.e.m. from six animals in each group.
LMWH (50 μg in 0.1 ml); ▪, mixture of PLL (50 μg in 0.1 ml) and LMWH (50 μg in 0.1 ml). Data are means ±s.e.m. from six animals in each group.
Study series 8: effect of PLA on the sensitivities of pulmonary C fibres
Intratracheal instillation of PLA (50 μg in 0.1 ml) had similar stimulatory effects on pulmonary C fibres in a separate group of rats (Fig. 8). PLA markedly elevated the baseline FA, which increased from a control (10 min before) of 0.01 ± 0.01 impulses s−1 to a peak of 2.29 ± 0.16 impulses s−1 (20 s average; n = 8, P < 0.05) at ∼10 min after the instillation. Instillation of PLA also significantly enhanced the sensitivity of pulmonary C fibre afferents to lung inflation (Pt= 30 cmH2O) and to right atrial injection of capsaicin (0.5 μg kg−1; Fig. 8). These stimulatory effects were also prevented when PLA was neutralized with LMWH before instillation (data not shown).
Figure 8. The effect of PLA on the activities of pulmonary C fibres.
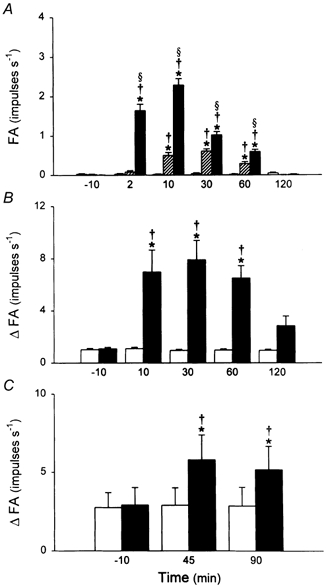
A, averaged baseline activities before and after i.t. instillation of a low dose of PLA (25 μg in 0.1 ml; ), a high dose of PLA (50 μg in 0.1 ml; ▪) and vehicle control (□). FA at each time point was averaged over a 20 s interval for each fibre. B, responses to lung inflation (Pt= 30 cmH2O) before and after PLA (50 μg in 0.1 ml; ▪) and vehicle (□). ΔFA was measured as the difference between the 10 s average during lung inflation and the 10 s average at baseline in each fibre. C, responses to capsaicin (0.5 μg kg−1) before and after PLA (50 μg in 0.1 ml; ▪) and vehicle (□). ΔFA was measured as the difference between the 2 s average at peak and the 10 s average at baseline in each fibre. Data are means ±s.e.m. of six to eight animals in each group. *P < 0.05, significant difference between vehicle and 25 or 50 μg of PLA at any time point; §P < 0.05, significant difference between 25 and 50 μg of PLA at any time point; †P < 0.05, significant difference between before and any time point after PLA or vehicle.
), a high dose of PLA (50 μg in 0.1 ml; ▪) and vehicle control (□). FA at each time point was averaged over a 20 s interval for each fibre. B, responses to lung inflation (Pt= 30 cmH2O) before and after PLA (50 μg in 0.1 ml; ▪) and vehicle (□). ΔFA was measured as the difference between the 10 s average during lung inflation and the 10 s average at baseline in each fibre. C, responses to capsaicin (0.5 μg kg−1) before and after PLA (50 μg in 0.1 ml; ▪) and vehicle (□). ΔFA was measured as the difference between the 2 s average at peak and the 10 s average at baseline in each fibre. Data are means ±s.e.m. of six to eight animals in each group. *P < 0.05, significant difference between vehicle and 25 or 50 μg of PLA at any time point; §P < 0.05, significant difference between 25 and 50 μg of PLA at any time point; †P < 0.05, significant difference between before and any time point after PLA or vehicle.
DISCUSSION
Our results clearly demonstrate that i.t. instillation of synthetic cationic proteins, PLL and PLA, caused an intense and long-lasting stimulatory effect on pulmonary C fibres in anaesthetized rats. PLL and PLA instillation also markedly augmented the responses of these afferents to lung inflation and to various chemical stimuli, with a particularly strong effect on the former. Both the augmented baseline activity and sensitivity of these endings slowly declined but remained elevated at 2 h after the challenge. The delivery of these cationic proteins via instillation appears to be critical in evoking these effects, because intravenous injection of a much higher dose of PLL or PLA failed to have any detectable effect on pulmonary C fibre activity. Furthermore, our results suggest that the cationic charge carried by these proteins was important in the activation of pulmonary C fibres and the development of hypersensitivity to lung inflation and to chemical stimulation, because the effects could be completely eliminated when the cationic charges were nullified by LMWH.
It is well documented that low molecular weight, highly cationic, cysteine/arginine-rich proteins are synthesized and released by a number of inflammatory cells such as eosinophils, neutrophils, platelets and lymphocytes (Coyle et al. 1993). These cells are known to mediate chemotactic responses and infiltrate into the airways during inflammatory reaction and mucosal injury. For example, the number of eosinophils and the levels of eosinophil-derived cationic proteins are increased in bronchoalveolar fluid from asthmatic subjects, and are correlated with the severity of the disease (Wardlaw et al. 1988; Bousquet et al. 1990). In fact, airway instillation of these cationic proteins, either endogenous or synthetic, has been shown to induce bronchospasm, bronchial hyper-reactivity and mucosal injury in a variety of animal species, including non-human primates (Gundel et al. 1991; Coyle et al. 1993; Uchida et al. 1993). All this evidence strongly suggests that cationic proteins play an important role in the pathogenesis of airway disorder associated with granulocyte infiltration into the airways.
The importance of pulmonary C fibres in the regulation of airway functions under both physiological and pathophysiological conditions is well recognized (Paintal, 1973; Coleridge & Coleridge, 1984; Lee & Pissari, 2001). Stimulation of the C fibre endings is known to elicit cardiopulmonary responses mediated by both central reflex pathways and by local axon reflexes. The former include, but are not limited to, bronchoconstriction, airway hypersecretion, irregular breathing pattern, bradycardia and hypotension. The latter involve the release of tachykinins from C fibre endings; some of these sensory neuropeptides can act on a number of effector cells (e.g. airway and vascular smooth muscles, inflammatory cells, and mucous glands) and produce potent local effects such as bronchoconstriction, extravasation of macromolecules and oedema of airway mucosa (Lundberg & Saria, 1987; Solway & Leff, 1991). Taken together, it seems reasonable to suggest that the sustained stimulation of these afferent endings, as shown in this study, plays a critical role in the manifestation of the pathophysiological conditions induced by airway challenge with these cationic proteins (Coyle et al. 1993; Uchida et al. 1993).
The mechanism underlying the stimulatory and sensitizing effects of these synthetic cationic proteins on pulmonary C fibre endings is not fully understood, but several possibilities should be considered on the basis of the results obtained from this study in conjunction with the existing information in the literature. For example, previous investigators have demonstrated that administration of polycations such as protamine sulphate and PLL to isolated perfused rat lungs resulted in pulmonary vasoconstriction and oedema (Chang et al. 1987). Because pulmonary interstitial oedema is known to activate pulmonary C fibre endings (Paintal, 1973; Coleridge & Coleridge, 1984), we investigated its possible involvement in the PLL-induced stimulatory effect on these endings. However, our results showed no difference in the wet/dry ratios between control and treated groups. Therefore, it seems unlikely that pulmonary oedema was induced by PLL in this study. On the other hand, administration of these cationic proteins into the airways and pulmonary circulation has also been shown to increase the permeabilities of airway epithelium and vascular endothelium, respectively (Chang et al. 1987; Yu et al. 1994; Hulsmann et al. 1996). This action is believed to involve the interaction of the cationic charges carried by these proteins with the negatively charged glycocalyx of the luminal surface (Yu et al. 1994), and formation of transmembrane pores or functional channels that are neither voltage sensitive nor ion selective (Young et al. 1986). An increase in vascular permeability should enhance the accessibility of injected chemical agents to the C fibre terminals. Thus, the same dose of chemical stimulant, such as capsaicin, would evoke a greater response in these afferents. However, whether this mechanism is involved in our study is questionable because of the lack of any stimulatory or sensitizing effect on pulmonary C fibres of these proteins when they were injected into the systemic circulation at a much higher dose. Furthermore, this assumption cannot explain the striking increase in C fibre excitability to lung inflation induced by the PLL challenge.
The C fibre afferents in the lungs and airways are, in general, relatively insensitive to lung inflation (Ho et al. 2000). In the control condition, lung inflation at high pressure (Pt= 30 cmH2O) generated only mild responses and did not activate all the receptors tested in this study. However, after i.t. administration of PLL, the same C fibre afferents became exquisitely sensitive to lung inflation. Even the low-pressure lung inflation (Pt= 15 cmH2O) evoked clear and consistent responses from all 24 receptors tested, and the augmented sensitivity lasted for > 60 min (e.g. Fig. 4). The lower threshold of activation by lung inflation may have contributed, in part, to the elevated baseline activity of pulmonary C fibres following the PLL challenge; although the baseline discharge pattern of these afferents did not synchronize with the respirator cycles (e.g. Figs 1, 3 and 5), the phasic discharge might have been dampened by the slow responses (time constant > 1 s) of these afferents to volume change in the lungs (Ho et al. 2000). More importantly, the hypersensitivity of these sensory endings to lung inflation observed in this study may have particular implications because volume expansion in the lungs occurs commonly under normal physiological conditions, such as sighing, coughing or hyperventilation during exercise.
It should be noted that the doses of PLL and PLA applied in this study (high dose, 50 μg) are considerably lower than those (100 μg or higher) administered in the same manner by previous investigators to induce airway hyper-responsiveness and mucosal injury in rat lungs (Uchida et al. 1993; Coyle et al. 1994). The bradycardia and hypotension following the challenge of PLL coincided with the peak change in the baseline activity of pulmonary C fibres and were probably reflex responses elicited by the PLL-induced stimulation of C fibre endings in the left lung (Coleridge & Coleridge 1984; Lee & Pissari, 2001); these reflex responses were presumably attenuated by the sectioning of the right vagus in our experiment. The transient and small increase in peak tracheal pressure after the instillation of PLL is most probably due to atelectasis of the lungs during the instillation procedure because a similar response was also observed after the instillation of vehicle and because the responses disappeared completely within 10 min. Since the PLL-induced stimulatory effect peaked at 10 min after the instillation, the increase in Pt cannot account for the action of PLL on pulmonary C fibres. Furthermore, to test whether local changes in mechanical properties of the lungs (e.g. bronchoconstriction) play a part in the stimulatory and sensitizing effects of PLL, we compared fibre activity before and after administration of methacholine (20 μg ml−1, 0.1 ml), a bronchoconstrictive agent. Intratracheal instillation of methacholine in the same manner as that for PLL caused an increase in Pt that was ∼290 % greater than that generated by the high dose of PLL at the same time (10 min) after the instillation. However, it did not affect either the baseline FA or the responses to lung inflation or capsaicin injection in these C fibres (Q. Gu & L.-Y. Lee, unpublished observation). These results further suggest that changes in lung mechanics play an insignificant role in the PLL-induced C fibre activation.
An alternative explanation for the PLL- and PLA-induced hypersensitivity of pulmonary C fibre afferents involves the local release of certain autacoids. For example, these cationic proteins have been shown to cause release of prostaglandins from epithelial cells (White et al. 1993) and histamine from mast cells (Zheutlin et al. 1984). These cationic proteins are also known to activate kallikrein and to generate bradykinin (Coyle et al. 1995). Some of these autacoids (e.g. PGE2) are known to possess potent sensitizing effects on pulmonary C fibre afferents, elevating their sensitivities to lung inflation and to chemical stimulants (Lee & Morton, 1993; Fox et al. 1996; Ho et al. 2000). Furthermore, the cationic charges carried by these proteins are expected to facilitate their binding to the anionic surfaces of epithelial cells and to gain their access to other potential target cells (e.g. mast cells) in the airway mucosa, triggering the subsequent release of these autacoids. The relatively longer latency (peaking at 10 min) and extremely long-lasting pattern of the C fibre responses to these cationic proteins appear to correspond to the time course of slow release of endogenous mediators. The fact that these C fibre endings are located in the mucosa, immediately below or between the airway epithelial cells (Baluk et al. 1992; Adriaensen et al. 1998), makes them more susceptible to the local release of autacoids evoked by cationic proteins administered via the airway lumen. This contention is consistent with our observation that the pronounced effects induced by i.t. instillation of PLL or PLA were absent when the much higher doses of these proteins were delivered via the i.v. route. Finally, administering these synthetic proteins via the airway lumen should simulate more closely the conditions in which the natural cationic proteins are secreted by the eosinophils and neutrophils after infiltration into the airways during airway inflammation.
Considering the fact that only a relatively small volume (0.1 ml) of the PLL solution was delivered into the trachea and that the locations of these C fibre endings were widely distributed in different regions of the right lung, we were somewhat surprised by the consistently positive responses to the i.t.instillation of PLL in almost all (> 95 %) of the C fibres tested in this study. We therefore suspected that the widespread effect of PLL might have resulted from the circulation or re-circulation of the endogenous mediators released by PLL in the lung. However, when PLL was delivered into lung lobes other than the one containing the receptor or the contralateral lung, PLL even at a higher dose failed to generate any stimulatory or sensitizing effects on the C fibre endings. These results suggest that the effect of PLL on these afferent endings was localized in the lung region exposed to PLL, and that the cationic protein was widely distributed in the lung after the i.t. instillation. Indeed, our unpublished observation (Q. Gu & L.-Y. Lee) using fluorescein (FITC)-labelled PLL clearly indicates a rather uniform distribution of PLL throughout the entire respiratory tract, from bronchi to alveoli.
In conclusion, we have demonstrated that intratracheal instillation of synthetic cationic proteins induces an intense and long-lasting stimulatory effect on pulmonary C fibres and markedly augments the response of these afferents to lung inflation and to various chemical stimuli. Our results also suggest that an interaction between the cationic charge carried by these proteins and the airway mucosa is probably involved in the observed effects. In view of the wide range of cationic proteins secreted by a variety of inflammatory cells and the potent cardiopulmonary effects caused by activation of pulmonary C fibres, the stimulatory and sensitizing effects of these cationic proteins on pulmonary C fibres may play an important part in the development of pathophysiological conditions associated with infiltration of inflammatory cells in the airways.
Acknowledgments
The authors thank Dr Mary K. Rayens for her statistical consultation and Dr You-Shuei Lin and Robert Morton for their excellent technical assistance. They also thank Dr Grace Sears for her critical reading of the manuscript. This study was supported by grant HL-58686 from the National Heart, Lung and Blood Institute.
References
- Adriaensen D, Timmermans JP, Brouns I, Berthoud HR, Neuhuber WL, Scheuermann DW. Pulmonary intraepithelial vagal nodose afferent nerve terminals are confined to neuroepithelial bodies: an anterograde tracing and confocal microscopy study in adult rats. Cell and Tissue Research. 1998;293:395–405. doi: 10.1007/s004410051131. [DOI] [PubMed] [Google Scholar]
- Agostoni E, Chinnock JE, Daly M, De Burgh, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. Journal of Physiology. 1957;135:182–x205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Nadel JA, McDonald DM. Substance P-immunoreactive sensory axons in the rat respiratory tract: a quantitative study of their distribution and role in neurogenic inflammation. Journal of Comparative Neurology. 1992;319:586–598. doi: 10.1002/cne.903190408. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Michel F. Eosinophilic inflammation in asthma. New England Journal of Medicine. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- Chang SW, Westcott JY, Henson JE, Voelkel NF. Pulmonary vascular injury by polycations in perfused rat lungs. Journal of Applied Physiology. 1987;62:1932–1943. doi: 10.1152/jappl.1987.62.5.1932. [DOI] [PubMed] [Google Scholar]
- Coleridge JCG, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Reviews of Physiology, Biochemistry and Pharmacology. 1984;99:1–100. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Coyle AJ, Ackerman SJ, Burch R, Proud D, Irvin CG. Human eosinophil-granule major basic protein and synthetic polycations induce airway hyperreponsiveness in vivo dependent on bradykinin generation. Journal of Clinical Investigation. 1995;95:1735–1740. doi: 10.1172/JCI117850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle AJ, Ackerman SJ, Irvin CG. Cationic proteins induce airway hyperresponsiveness dependent on charge interactions. American Review of Respiratory Disease. 1993;147:896–900. doi: 10.1164/ajrccm/147.4.896. [DOI] [PubMed] [Google Scholar]
- Coyle AJ, Perretti F, Manzini S, Irvin CG. Cationic protein-induced sensory nerve activation: role of substance P in airway hyperresponsiveness and plasma protein extravasation. Journal of Clinical Investigation. 1994;94:2301–2306. doi: 10.1172/JCI117594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham SR, Kay AB. Eosinophils, bronchial hyperreactivity and late-phase asthmatic reactions. Clinical Allergy. 1985;15:411–418. doi: 10.1111/j.1365-2222.1985.tb02290.x. [DOI] [PubMed] [Google Scholar]
- Flavahan NA, Slifman NR, Gleich GJ, Vanhoutte PM. Human eosinophil major basic protein causes hyperreactivity of respiratory smooth muscle. Role of the epithelium. American Review of Respiratory Disease. 1988;138:685–688. doi: 10.1164/ajrccm/138.3.685. [DOI] [PubMed] [Google Scholar]
- Fox AJ, Lalloo UG, Belvisi MG, Bernareggi M, Chung KF, Barnes PJ. Bradykinin-evoked sensitization of airway sensory nerves: a mechanism for ACE-inhibitor cough. Nature Medicine. 1996;2:814–817. doi: 10.1038/nm0796-814. [DOI] [PubMed] [Google Scholar]
- Gleich GJ. The eosinophil and bronchial asthma: current understanding. Journal of Allergy and Clinical Immunology. 1990;85:422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- Gundel RH, Letts LG, Gleich GJ. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. Journal of Clinical Investigation. 1991;87:1470–1473. doi: 10.1172/JCI115155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann KJ, Gleich GJ, Gundel RH, White AR. Interaction between respiratory epithelium and eosinophil granule proteins in asthma: the eosinophil hypothesis. In: Farmer SG, Hay DWP, editors. The Airway Epithelium: Physiology, Pathophysiology, and Pharmacology. Lung Biology in Health and Disease Series. New York: Marcel Dekker; 1991. pp. 255–300. [Google Scholar]
- Ho C-Y, Gu Q, Hong J-L, Lee L-Y. Prostaglandin E2 enhances chemical and mechanical sensitivities of pulmonary C fibers in the rat. American Journal of Respiratory and Critical Care Medicine. 2000;162:528–533. doi: 10.1164/ajrccm.162.2.9910059. [DOI] [PubMed] [Google Scholar]
- Ho C-Y, Lee L-Y. Ozone enhances excitabilities of pulmonary C fibers to chemical and mechanical stimuli in anesthetized rats. Journal of Applied Physiology. 1998;85:1509–1515. doi: 10.1152/jappl.1998.85.4.1509. [DOI] [PubMed] [Google Scholar]
- Hulsmann AR, Raatgeep HR, Den Hollander JC, Bakker WH, Saxena PR, De Jongste JC. Permeability of human isolated airways increases after hydrogen peroxide and poly-l-arginine. American Journal of Respiratory and Critical Care Medicine. 1996;153:841–846. doi: 10.1164/ajrccm.153.2.8564141. [DOI] [PubMed] [Google Scholar]
- Lee L-Y, Morton RF. Histamine enhances vagal pulmonary C-fiber responses to capsaicin and lung inflation. Respiration Physiology. 1993;93:83–96. doi: 10.1016/0034-5687(93)90070-q. [DOI] [PubMed] [Google Scholar]
- Lee L-Y, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C fibers. Respiration Physiology. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. Experimental uraemic pulmonary oedema. Acta Medica Scandinavica. 1964;418(suppl.):1–170. [PubMed] [Google Scholar]
- Lundberg JM, Saria A. Polypeptide-containing neurons in airway smooth muscle. Annual Review of Physiology. 1987;49:557–572. doi: 10.1146/annurev.ph.49.030187.003013. [DOI] [PubMed] [Google Scholar]
- Paintal AS. Vagal sensory receptors and their reflex effects. Physiological Reviews. 1973;53:159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- Solway J, Leff AR. Sensory neuropeptides and airway function. Journal of Applied Physiology. 1991;71:2077–2087. doi: 10.1152/jappl.1991.71.6.2077. [DOI] [PubMed] [Google Scholar]
- Spina D. Airway sensory nerves: a burning issue in asthma? Thorax. 1996;51:335–337. doi: 10.1136/thx.51.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida DA, Ackerman SJ, Coyle AJ, Larsen GL, Weller PF, Freed J, Irvin CG. The effect of human eosinophil granule major basic protein on airway responsiveness in the rat in vivo. A comparison with polycations. American Review of Respiratory Disease. 1993;147:982–988. doi: 10.1164/ajrccm/147.4.982. [DOI] [PubMed] [Google Scholar]
- Wardlaw AJ, Dunnette S, Gleich GJ, Collins JV, Kay AB. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. American Review of Respiratory Disease. 1988;137:62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- White SR, Sigrist KS, Spaethe SM. Prostaglandin secretion by guinea pig tracheal epithelial cells caused by eosinophil major basic protein. American Journal of Physiology. 1993;265:L234–242. doi: 10.1152/ajplung.1993.265.3.L234. [DOI] [PubMed] [Google Scholar]
- Young JD, Peterson CG, Venge P, Cohn ZA. Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature. 1986;321:613–616. doi: 10.1038/321613a0. [DOI] [PubMed] [Google Scholar]
- Yu XY, Schofield BH, Croxton T, Takahashi N, Gabrielson EW, Spannhake EW. Physiologic modulation of bronchial epithelial cell barrier function by polycationic exposure. American Journal of Respiratory Cell and Molecular Biology. 1994;11:188–198. doi: 10.1165/ajrcmb.11.2.8049079. [DOI] [PubMed] [Google Scholar]
- Zheutlin LM, Ackerman SJ, Gleich GJ, Thomas LL. Stimulation of basophil and rat mast cell histamine release by eosinophil granule-derived cationic proteins. Journal of Immunology. 1984;133:2180–2185. [PubMed] [Google Scholar]


