Abstract
Recently, it has been demonstrated that the human immunodeficiency virus type 1 (HIV-1) Nef from laboratory strains down-modulates cell surface expression of mature major histocompatibility complex class II (MHC-II) molecules, while up-regulating surface expression of the invariant chain (Ii) associated with immature MHC-II (P. Stumptner-Cuvelette, S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch, Proc. Natl. Acad. Sci. USA 98:12144-12149, 2001). These Nef functions could contribute to impaired CD4+-T-helper-cell responses found in HIV-1-infected patients with progressive disease. However, it is currently unknown whether nef alleles derived from HIV-1-infected individuals or from other primate lentiviruses also modulate MHC-II and Ii. In the present study, we demonstrate that both activities are conserved among primary HIV-1 nef alleles, as well as among HIV-2 and simian immunodeficiency virus (SIV) nef alleles. Down-modulation of mature MHC-II required high levels of Nef expression. In contrast, surface expression of Ii was already strongly increased at low to medium levels of Nef expression. Notably, nef genes derived from two of four HIV-1-infected long-term nonprogressors did not up-regulate Ii, whereas nef alleles derived from 10 individuals with progressive disease were active in this assay. Unlike other in vitro Nef functions, the average activity of Nef in modulating MHC-II and Ii surface expression did not change significantly during the course of infection. Mutational analysis confirmed that MHC-II down- and Ii up-regulation are functionally separable from each other and from other Nef functions and identified acidic residues, located at the base of the flexible C-proximal loop of Nef, that are critical for increased Ii expression. Overall, our results suggest that the ability of Nef to interfere with MHC-II antigen presentation might play a role in AIDS pathogenesis.
A functional nef gene is critical for the full pathogenic potential of primate lentiviruses. Nef-defective mutants of simian immunodeficiency virus (SIV) persist inefficiently and usually do not cause disease in juvenile or adult rhesus macaques (36). Some human immunodeficiency virus type 1 (HIV-1)-infected individuals harbor attenuated, mutant forms of the virus from which nef has been deleted (14, 40). All of these individuals showed low viral loads and unusually slow disease progression (40, 41). Furthermore, the HIV-1 nef-gene enhances the pathogenicity of SIV (3, 37) and can cause immunodeficiency in transgenic mice (26). In vitro, Nef (i) down-modulates CD4 (1, 18), CD28 (65), and major histocompatibility complex class I (MHC-I) cell surface expression (23, 42, 58), (ii) alters cellular signal transduction pathways (50, 57), (iii) affects T-cell receptor signaling (5, 28, 31, 56), (iv) enhances virion infectivity (9), and (v) stimulates viral replication (4, 44, 61). The contributions of these in vitro Nef functions to AIDS pathogenesis are largely unknown. However, accumulating evidence suggests that a combination of multiple, functionally separable, Nef activities allows HIV-1 and SIVmac to maintain high viral loads and cause disease in the majority of infected hosts (8, 30, 45-47).
Nef appears to interfere with the antiviral immune response (32) and down-modulates MHC-I from the cell surface (23, 34, 42, 58). Although this function requires high levels of Nef expression, several findings suggest that it contributes to efficient viral persistence in the infected host. Nef down-regulates cell surface expression of HLA-A and HLA-B but not of HLA-C and protects HIV-infected cells from lysis by cytotoxic T lymphocytes (CTL) and natural killer cells in vitro (10, 11). Only nef alleles obtained during the asymptomatic phase of HIV-1 and SIV infection efficiently down-modulate MHC-I, indicating that a selective pressure exists in immunocompetent hosts only (8, 47). Furthermore, a point mutation in SIVmac Nef, which selectively disrupts MHC-I down-regulation, reverts rapidly in infected rhesus macaques, suggesting an important role of this Nef function in SIV replication in vivo (45).
In addition to reducing MHC-I-restricted lysis of HIV-infected cells by CTL, Nef might also affect the stimulation of CD4+ T helper cells by antigen-presenting cells (APCs) that requires interaction between the T-cell receptor and antigen presented in the context of MHC-II on the surface of infected cells. It is known that Nef impairs T-cell receptor signaling in infected CD4+ T cells by down-modulating the cell surface expression of CD4 (1, 18) and CD28 (65) and the expression or signaling of CD3 (28, 31, 56). Recently, it has been shown that Nef also affects MHC-II antigen presentation by two distinct mechanisms (62): (i) down-regulation of surface expression of mature MHC-II and (ii) up-regulation of the MHC-II-associated invariant chain (Ii, CD74). The importance of Ii in the regulation of MHC-II antigen presentation is well established (7, 13, 63). MHC-II associated with Ii represents a maturation intermediate of mature, peptide-loaded MHC-II molecules and is nonfunctional in stimulating CD4+ T cells (63). Stable surface expression of Ii prevents peptide presentation (52). APCs such as dendritic cells and macrophages, as well as activated CD4+ T cells, express MHC-II and are infected by HIV-1. Antigen-specific activation of T helper cells orchestrates the activities of CTL and B-cell responses and is crucial for an efficient antiviral immune response (48). Helper T-cell responses are impaired in HIV-1-infected individuals with progressive infection but not in long-term nonprogressors (LTNPs), suggesting an important role in the development of disease (6, 15, 16, 53).
Modulation of MHC-II-restricted antigen presentation by Nef might contribute to persistent viral replication in HIV-1-infected individuals. However, it is currently unclear whether this Nef function is conserved among primary HIV-1 isolates and other primate lentiviruses. Addressing this, we demonstrate that the abilities of Nef to down-regulate surface expression of MHC-II and up-regulate Ii are well conserved among patient-derived HIV-1 nef alleles. Similar observations were made with HIV-2 and SIV Nef proteins. These activities did not change significantly during late stages of infection, in contrast to our previous findings on MHC-I and CD4 down-modulation and stimulation of HIV-1 replication (8). The greatly increased cell surface expression of Ii in HIV-1-infected cells, which was not observed for some nef alleles derived from LTNPs, suggests that this function is physiologically relevant and contributes to viral immune evasion in vivo.
MATERIALS AND METHODS
Plasmids.
Bicistronic cytomegalovirus-based pCG expression vector coexpressing the green fluorescent protein (GFP) and consensus, individual, or pooled HIV-1 nef alleles were generated as described elsewhere (8). For most HIV-1 nef alleles used in the present study, sequence analysis and functional activity in down-modulation of CD4 and MHC-I cell surface expression and enhancement of viral infectivity and replication has been described (8, 39). Briefly, nef alleles were derived from HIV-1-infected individuals enrolled into a United Kingdom cohort of HIV-1-infected individuals based in London (17) and from patients with severe hemophilia A monitored by the New England Area Hemophilia Center at the University of Massachusetts Memorial Hospital, Worcester (24). The definitions of non-, slow, and rapid progressors of HIV-1 infection were as described previously (39). Individual participants with earlier designations in the literature are as follows: LTNP2, AD (2, 8, 39, 43); LTNP4, HP (2, 8, 25, 39, 43); SP7, MB (8, 39, 43); and P2, FA (8, 39, 43). The NPex, NPcon, Pcon, Pex, and PexP consensus nef alleles were generated based on the analysis of nef sequences derived from 91 HIV-1-infected individuals at different stages of disease (8, 39). Vectors expressing HIV-2 Ben (38) or Rod (54) nef alleles and the NL4-3nef* control vector containing inactivating mutations of ATGGGTGGCAAGTGGTCC to gTGGGTGGCtAGTGaTCA at the 5′ end of nef′ were generated by standard PCR and cloning techniques (lowercase letters specify mutated positions). Site-specific mutagenesis of NA7 Nef, a natural HIV-1 nef allele, has been described elsewhere (31).
Transfections and cell culture.
Transfection of Jurkat T cells was performed by using the DMRIE-C reagent (Gibco-BRL, Karlsruhe, Germany) according to the manufacturer's instructions. HeLa CIITA cells were transfected with Metafectene (Biontex, Munich, Germany). Briefly, 2.5 μg of DNA in 100 μl of optimized minimum essential medium (Invitrogen, Karsruhe, Germany) was mixed with 12 μl of Metafectene in 100 μl of optimized minimum essential medium, followed by incubation for 30 min at room temperature. Subsequently, the mixture was added to 2 × 105 cells, followed by incubation for 6 h at 37°C. Thereafter, the medium was changed, and cells were analyzed by fluorescence-activated cell sorting (FACS) on the following day. Transfection efficiencies varied between 20 and 35%. HeLa CIITA, Jurkat T, and 221-B7 cells were cultured as described previously (8, 10, 62).
Transduction with VSV-G pseudotypes.
To generate pseudotyped viral particles, 293T cells were cotransfected with NL4-3 proviral constructs carrying either nef open or nef-defective reading frames, followed by an internal ribsosome entry site and the GFP gene, and a plasmid (pHIT-G) expressing the Env protein of the vesicular stomatitis virus (VSV-G) as described previously (27). Viral stocks were divided into aliquots and frozen at −80°C. The p24 antigen concentrations were quantified by using an HIV-1 enzyme-linked immunosorbent assay provided by the NIH AIDS Research and Reference Program. 221-B7 cells, peripheral blood mononuclear cells (PBMC), or HeLa CIITA cells were transduced with the pseudotyped virus particles, and cell surface expression of MHC-I, MHC-II, and Ii on GFP-expressing cells was analyzed by FACS 2 days later.
Flow cytometry.
CD4, MHC-I, CD28, and GFP reporter molecules in Jurkat T cells transfected with a bicistronic vector coexpressing Nef and GFP were measured as described previously (8). Down-modulation of MHC-II and up-regulation of Ii was measured on transfected HeLa CIITA cells. The following phycoerythrin-conjugated antibodies were used: anti-human CD4, anti-human CD3, and anti-Leu-28 (BD Biosciences Pharmingen); anti-CD74/R-PE M-B741 (Ancell); anti-HLA-ABC antigen/RPE (Dako); mouse anti-human HLA-DR TU36 (Caltag Laboratories); and L243 (BD Biosciences Pharmingen). Staining with both TU36 or L243 gave similar results. The levels of MHC-II and Ii expression (red fluorescence) were measured from aliquots of the same transfection as a function of green GFP fluorescence. For the quantitation of Nef-mediated MHC-II and Ii down- or up-regulation, the mean channel numbers of red fluorescence were determined for cells expressing no, low, medium, or high levels of GFP (Fig. 1). Cells were defined by either background levels of GFP expression (i.e., no expression) or by their different ranges of green fluorescence (low, medium, and high) and hence Nef expression (Fig. 1). The mean channel numbers of red fluorescence obtained for cells transfected with the NL4-3nef* construct expressing GFP only were divided by the corresponding numbers obtained for cells coexpressing Nef and GFP to calculate the values for X-fold down- or up-modulation, respectively. The same regions of GFP expression were used in all calculations.
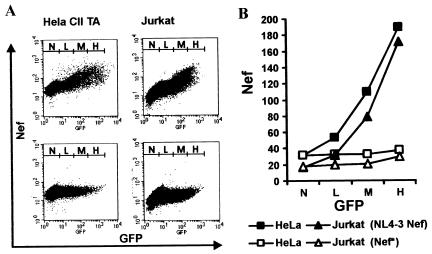
FIG. 1.
Correlation between Nef and GFP expression. (A) HeLa-CIITA cells and Jurkat cells were transfected with a bicistronic vector coexpressing GFP and NL4-3 Nef (upper panel) or GFP alone (lower panel). As described in Materials and Methods, cells were permeabilized, and Nef and GFP expression was detected simultaneously by two-color flow cytometric analysis. Ranges for green fluorescence on cells defined as expressing no (N), low (L), medium (M), or high (H) levels of GFP are indicated. (B) Quantitation of Nef expression in the transiently transfected cells shown in panel A. The ordinate gives the mean channel numbers of red Nef fluorescence for the four different ranges of GFP expression.
Intracellular Nef staining.
HeLa CIITA or Jurkat cells were fixed in 2% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min. For permeabilization, cells were treated for 10 min with 1% saponin in PBS and subsequently incubated in 10% fetal calf serum in PBS for 30 min. Intracellular Nef expression was detected with a mouse monoclonal antibody directed against amino acid residues 31 to 50 of NL4-3 Nef (Applied Biosystems) and a secondary phycoerythrin-conjugated goat anti-mouse antibody (Jackson Immunoresearch). Two-color flow cytometric analysis for Nef and GFP expression was performed as described above.
RESULTS
Up-regulation of Ii and down-modulation of MHC-II are conserved properties of primate immunodeficiency virus nef alleles.
It has been previously shown that nef alleles derived from the HIV-1 NL4-3, LAI, and HXB2 molecular clones modulate Ii and MHC-II cell surface expression (62). However, it remained unknown whether these in vitro Nef functions are conserved among different groups of primate lentiviruses. SIVmac and HIV-2 Nef perform functions similar to those of HIV-1 Nef, but the various in vitro activities are often mediated by different domains and/or involve different mechanisms (5, 28, 31, 64). We used transient transfection of HeLa CIITA cells with a bicistronic vector (8) to assess these in vitro Nef activities. Transfected cells coexpressed Nef and GFP at correlating levels, allowing a quantitative evaluation of Nef function (Fig. 1).
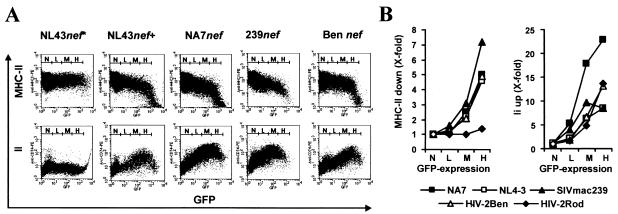
The nef alleles of HIV-1 NL4-3, HIV-1 NA7, SIVmac239 (35, 49), and HIV-2 Ben (38) were functionally active in down-modulating MHC-II and up-regulating Ii cell surface expression (Fig. 2A). None of these effects was observed in HeLa CIITA cells transiently transfected with the vector NL43nef*, where the nef allele was inactivated by point mutations in the ATG initiation codon and premature in frame stop codons. Notably, the effect of Nef on MHC-II down-modulation did not saturate at high levels of Nef expression. In contrast, maximum Ii up-regulation was observed at medium to high levels of Nef expression and became less efficient above an optimum (Fig. 2A). The ideal concentration of Nef was allele dependent: for example, higher amounts of HIV-2 Ben than SIVmac239 Nef were required for efficient Ii up-regulation (Fig. 2A). Modulation of MHC-II and Ii cell surface expression were both measured from the same transfection. Therefore, these results were not biased by different transfection efficiencies. For a more quantitative evaluation the mean channel numbers of red MHC-II or Ii fluorescence obtained for cells expressing GFP only were divided by the corresponding numbers obtained for cells coexpressing Nef and GFP to calculate X-fold down- or up-modulation, respectively (Fig. 2B). Of the nef alleles studied, SIVmac239 was most active in down-regulating MHC-II. In comparison, HIV-1 NA7, HIV-1 NL4-3, and HIV-2 Ben gave similar activities and the HIV-2 Rod molecular clone (54) lacked this function (Fig. 2B, left). For enhancement of Ii surface expression, HIV-1 NA7 was most efficient, giving >20-fold-enhanced cell surface expression levels, with the other four nef alleles showing lower but similar activities (Fig. 2B, right). In agreement with a previous study (61), we found that Nef up-regulates Ii at low expression levels, whereas efficient down-modulation of MHC-II requires higher concentrations. These results demonstrate that Nef-driven up-regulation of Ii and down-modulation of MHC-II is conserved among different groups of primate lentiviruses.
FIG. 2.
Modulation of MHC-II and Ii cell surface expression by Nef is conserved between different groups of primate immunodeficiency viruses. (A) Flow cytometric analysis of HeLa-CIITA cells transfected with a bicistronic vector expressing GFP alone (NL4-3nef*) or GFP with either NA7, NL4-3, SIVmac239, or HIV-2 Ben Nef. Ranges for green fluorescence are indicated. (B) Quantitative effect of HIV-1 NA7, HIV-1 NL4-3, SIVmac239, HIV-2 Ben, and HIV-2 Rod Nef on MHC-II and Ii surface expression. Values were determined as described in Materials and Methods. Shown are data derived from one representative transfection. Similar results were obtained in three independent experiments.
Modulation of MHC-II and Ii surface expression by Nef is conserved in primary HIV-1 isolates.
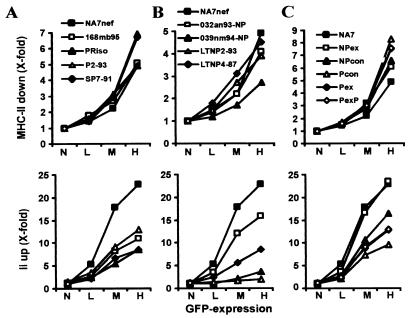
Next, we analyzed the functional activities of primary nef alleles derived from four HIV-1-infected individuals with progressive disease (Fig. 3A) and four LTNPs (Fig. 3B). nef alleles from both immunodeficient subjects and LTNPs down-modulated MHC-II cell surface expression. Those from the progressors P2-93 and SP7-91 showed the highest efficiencies (Fig. 3A, upper panel), while that from a LTNP (039nm94) showed ∼2-fold-reduced functional activity (Fig. 3B, upper panel). Functional activity of these primary nef alleles in up-regulating Ii showed more variation. Those derived from the four progressors and the asymptomatic individuals 032an93 and LTNP4-87 increased Ii surface expression, albeit with lower efficiency than NA7 Nef (Fig. 3A and B, lower panel). However, nef alleles derived from 039nm94 and LTNP2-93 did not up-regulate Ii surface expression, although they were functionally active in other in vitro assays for Nef function, such as down-regulation of MHC-I, MHC-II, and CD4 and enhancement of viral infectivity (Fig. 3B, lower panel, and data not shown). From previous nef sequencing studies we generated consensus nef alleles for large numbers of nonprogressors (NPcon) and immunodeficient individuals (Pcon), as well as alleles containing changes most commonly seen in either nonprogressors (NPex) or progressors (Pex) and an additional variant with an N-terminal PxxP motif (PexP) (8). All of these have been analyzed for their ability to down-modulate MHC-I and CD4 and to enhance HIV-1 NL4-3 infectivity and replication (8). The five consensus nef alleles were more active in down-modulating MHC-II than NA7 Nef with the Pcon, Pex, and PexP Nefs being slightly more active than those of NPex and NPcon (Fig. 3C, upper panel). In contrast, the nef alleles representative of progressing individuals (Pcon, Pex, and PexP) were approximately twofold less active in up-regulating Ii than those (NPcon and NPex) representing nonprogressing individuals (Fig. 3C, lower panel). These functional differences were consistently observed in three independent experiments (data not shown). Thus, Nef amino acid substitutions representative of HIV-1-infected nonprogressors and progressors might have differential effects on down-regulation of MHC-II and up-modulation of Ii cell surface expression.
FIG. 3.
Modulation of MHC-II and Ii cell surface expression is a conserved property of primary and consensus HIV-1 nef alleles. Quantitative presentation of MHC-II down-modulation (upper panel) and up-regulation of Ii (lower panel) by nef alleles obtained from progressors of HIV-1 infection (A), LTNPs (B), and consensus Nef sequences derived from a large number of primary nef alleles (C) (8, 38). Parameters were determined and reproduced in independent experiments, as described in the legend to Fig. 2.
Taken together, our results demonstrate that most primary HIV-1 nef alleles modulate MHC-II and Ii cell surface expression. However, the functional activity in Ii up-regulation varied considerably, and nef alleles derived from two nonprogressors showed little activity in this assay. In comparison, down-modulation of MHC-II was well conserved but, similar to MHC-I down-regulation, required high Nef expression levels.
Nef activity in modulating Ii and MHC-II expression does not change significantly during AIDS progression.
We have previously shown that the functional activity in MHC-I down-modulation decreases in immunocompromised individuals (8). Similarly, the Pcon and Pex nef alleles had lower functional activity in Ii modulation compared to the NPcon and NPex nef alleles (Fig. 3C). Next, we evaluated whether the ability of Nef to modulate MHC-II or Ii expression also changes during or after AIDS progression. Representative mixtures of primary nef alleles for each patient and time point derived from six individuals prior to, during, and after AIDS progression, characterized for activity in other in vitro assays of Nef function (8), were assessed for modulation of Ii and MHC-II cell surface expression. As summarized in Table 1, all pooled HIV-1 nef alleles were functionally active in modulating MHC-II and Ii surface expression. In contrast to our findings on MHC-I down-modulation with the same set of nef alleles (8), the functional activity in down-modulation of MHC-II did not decrease during late stages of infection. On the contrary, nef alleles obtained during the asymptomatic stage of infection were slightly less active (7.1 ± 0.9 [n = 8]) than those obtained after disease progression (10.1 ± 2.6 [n = 6]) (Table 1). Our results also demonstrate that, on average, the activity of Nef in inducing Ii up-regulation does not change significantly during the course of HIV-1 infection (Table 1). Some exceptions existed, however. nef alleles derived from P9 showed increased Ii up-regulation after AIDS progression, whereas a twofold drop was observed with late-stage nef alleles derived from P10 (Table 1). Most remarkably, all pools of primary nef alleles were highly active and typically resulted in ∼10-fold-enhanced levels of Ii surface expression even at medium levels of Nef expression.
TABLE 1.
Modulation of MHC-II and Ii surface expression by nef alleles obtained at different stages of HIV-1 infection
| Patient or sample group | Yr of PBMC sampling | CD4+ cells/mm3 | Regulation (n-fold)a
|
|||||
|---|---|---|---|---|---|---|---|---|
| MHC-II down-regulation
|
Ii up-regulation
|
|||||||
| L | M | H | L | M | H | |||
| SP8 | 1984 | 616 | 1.5 | 2.8 | 6.7 | 2.7 | 10.5 | 19.3 |
| 1987 | 601 | 1.5 | 2.8 | 7.3 | 2.2 | 9.0 | 16.1 | |
| 1996 | 39 | 1.9 | 4.6 | 14.6 | 3.8 | 12.9 | 15.3 | |
| P5 | 1983 | 616 | 1.6 | 3.2 | 6.8 | 2.7 | 11.4 | 21.7 |
| 1991 | 121 | 1.9 | 3.8 | 8.5 | 4.3 | 17.5 | 24.0 | |
| 1995 | 5 | 1.5 | 2.9 | 7.0 | 3.5 | 16.8 | 22.3 | |
| P7 | 1982 | NDc | 1.7 | 3.6 | 8.9 | 2.6 | 10.1 | 15.5 |
| 1985 | 822 | 1.8 | 3.2 | 7.6 | 2.5 | 9.9 | 17.6 | |
| 1993 | 7 | 1.7 | 3.5 | 9.6 | 2.9 | 11.1 | 13.8 | |
| P8 | 1983 | 526 | 1.8 | 3.9 | 11.2 | 2.7 | 10.5 | 15.2 |
| 1987 | 126 | 2.0 | 4.1 | 9.6 | 2.5 | 9.9 | 16.8 | |
| 1989 | 12 | 1.8 | 3.6 | 8.0 | 2.4 | 7.9 | 14.5 | |
| P9 | 1984 | 636 | 1.5 | 2.7 | 6.3 | 1.6 | 3.4 | 7.1 |
| 1989 | 118 | 1.7 | 4.1 | 10.0 | 2.7 | 9.2 | 14.4 | |
| 1998 | 95 | 2.0 | 4.4 | 10.4 | 2.7 | 9.4 | 16.1 | |
| P10 | 1984 | 1,021 | 1.6 | 2.8 | 7.3 | 3.9 | 18.4 | 29.6 |
| 1986 | 1,357 | 1.5 | 2.8 | 6.1 | 3.2 | 13.9 | 22.9 | |
| 1996 | 9 | 1.6 | 3.6 | 10.8 | 3.7 | 14.4 | 17.8 | |
| Group 1 (n = 8)b | >600 | 1.6 ± 0.1 | 3.0 ± 0.3 | 7.1 ± 0.9 | 2.7 ± 0.7 | 10.8 ± 4.3 | 18.7 ± 6.5 | |
| Group 2 (n = 4) | 100-600 | 1.9 ± 0.1 | 4.0 ± 0.2 | 9.8 ± 1.1 | 3.1 ± 0.0 | 11.8 ± 3.9 | 17.6 ± 4.4 | |
| Group 3 (n = 6) | <100 | 1.8 ± 0.2 | 3.8 ± 0.6 | 10.1 ± 2.6 | 3.2 ± 0.6 | 12.1 ± 3.3 | 16.6 ± 3.1 | |
Values for down-regulation (MHC-II) or up-regulation (Ii) (n-fold) of cell surface expression levels were determined for HeLa CIITA cells expressing low (L), medium (M), and high (H) levels of GFP as described in Materials and Methods. The results were confirmed in two additional experiments and with three individual nef alleles derived from each patient and time point. Means ± the standard deviation are given for sample groups.
n = number of samples with CD4+-cell counts in the ranges indicated.
ND, not determined.
These findings show that alterations in Nef-driven MHC-II and Ii modulation are observed in some individuals, but usually both Nef functions are well conserved throughout the course of infection, whereas late-stage nef alleles were more active in CD4 down-modulation and enhancing viral replication but impaired in MHC-I down-modulation (8).
Modulation of MHC-II and Ii surface expression in HIV-1-infected cells.
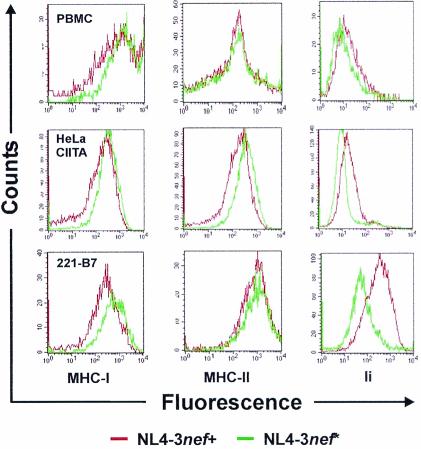
To evalute whether Nef affects MHC-II and Ii expression in infected cells, VSV-G-pseudotyped nef-open and nef-defective HIV-1 NL4-3 particles were used to transduce human PBMC, HeLa CIITA cells (62), and the B-lymphoblastoid 221-B7 cell line (10) coexpressing CD4, defined MHC-I proteins (HLA-B702) and high levels of MHC-II. As a control, we measured MHC-I downregulation, a function known to require relatively high Nef expression levels. All cell types transduced with proviral NL4-3 constructs containing a functional nef gene showed a significant reduction in surface levels of MHC-I (Fig. 4, left panel) and increased surface expression of Ii (Fig. 4, right panel). The effect of Nef on Ii expression on PBMC was only moderate, probably because only a subset of this mixed cell population can express this marker. MHC-II surface expression was clearly reduced on HeLa CIITA cells but remained normal on PBMC and 221-B7 cells (Fig. 4, middle panel). For all three surface antigens, mock-transduced cells gave results comparable to cells transduced with nef-defective constructs. These results suggest Ii is up-regulated in HIV-1-infected cells and might alter antigen presentation. However, it remains to be elucidated whether mature MHC-II is significantly reduced in HIV-1-infected human APCs.
FIG. 4.
Effect of Nef on MHC-II and Ii cell surface expression in HIV-1-infected cells. Human PBMC (upper panel), HeLa CIITA cells (middle panel), and 221-B7 cells (lower panel) were transduced with GFP-expressing HIV-1 NL4-3 particles containing an intact (nef+) or disrupted (nef*) nef gene pseudotyped with the VSV-G glycoprotein. FACS profiles were determined as described in Materials and Methods. The results were generated from the same pool of transduced cells. Similar results were obtained in independent experiments and when either the L243 or the TÜ36 antibody was used for MHC-II detection.
Charged residues at the base of the C-proximal flexible loop in HIV-1 Nef are important for Ii up-regulation.
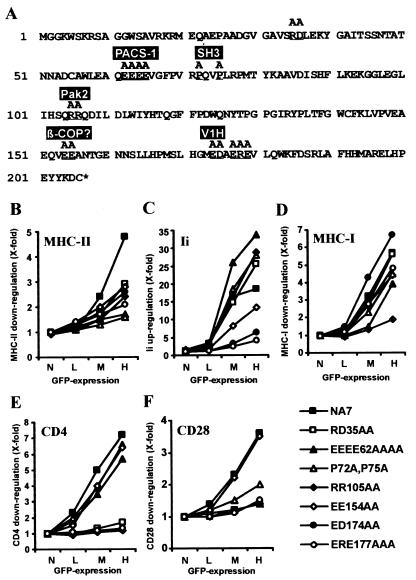
We analyzed a set of 22 HIV-1 NA7 Nef mutants to investigate which domains are relevant for the modulation of Ii and MHC-II cell surface expression and to compare these regions with those involved in other in vitro Nef functions. Seven of these NA7 Nef mutants (Fig. 5A) were defective in at least one of the in vitro functions investigated. Western blot analysis revealed that all of these Nef mutants were expressed at similar levels (data not shown). Several changes reduced the ability of NA7 Nef to down-regulate MHC-II, with alterations in the acidic domain (EEEE62AAAA) and in the PxxP motif (P72A and P75A; representing an SH3-binding domain) having the strongest disruptive effects (Fig. 5B). In contrast, these mutations enhanced Ii up-regulation (Fig. 5B). Mutations of the EE motif near the N terminus of the loop (EE154AA) and the acidic residues near the C terminus of the loop (ED174AA and ERE177AAA) reduced and disrupted Ii up-regulation, respectively (Fig. 5C).
FIG. 5.
Functional analysis of HIV-1 NA7 Nef mutants. (A) The NA7 Nef amino acid sequence is given in single-letter code. Mutated positions are underlined and sites interacting with some previously defined Nef-binding proteins (20, 55) are indicated. HeLa-CIITA cells (B and C) or Jurkat T cells (D to F) were transfected with the indicated HIV-1 NA7 Nef mutants and assayed for surface expression of MHC-II (B), Ii (C), MHC-I (D), CD4 (E), and CD28 (F). Quantification was performed as described in Materials and Methods. The results were confirmed in two independent experiments.
These seven Nef mutants had differential effects on the five in vitro Nef activities analyzed. For example, mutation of EK35AA near the N terminus impaired down-modulation of CD4 and CD28 having no effect on up-regulation of Ii and down-modulation of MHC-I, respectively (Fig. 5). The acidic region (EEEE62AAAA) was important for efficient down-regulation of MHC-II and CD28 but dispensable for up-regulation of Ii or down-modulation of CD4. The PxxP motif (P72A, P75A) proved to be relevant for modulation of MHC-II and to some extent MHC-I and CD28 cell surface expression but was dispensable for up-regulation of Ii and down-modulation of CD4. The mutation RR105AA, affecting the PAK2 binding site (51), impaired all Nef functions but up-regulation of Ii (Fig. 5C). In comparison, mutation of EE154AA reduced functional Nef activity in altering MHC-I and -II and Ii expression but had little effect on CD4 and CD28 down-regulation. Finally, mutation of the charged residues near the C terminus of the flexible loop (ED174AA and ERE177AAA) impaired or disrupted Nef-mediated modulation of Ii, MHC-II, CD4, and CD28 cells surface expression but not down-regulation of MHC-I (Fig. 5D). Thus, Nef-mediated modulation of these different cell surface markers is mediated by overlapping but not identical surfaces of the molecule.
DISCUSSION
This study demonstrates that primary HIV-1, HIV-2, and SIVmac nef alleles modulate MHC-II and Ii cell surface expression, indicating that the ability of Nef to impair MHC-II-mediated antigen presentation and, hence, CD4+-T-helper-cell responses are conserved among different groups of primate lentiviruses. Usually, virus-specific T helper responses are low or absent in most hosts infected with pathogenic HIV-1 or SIV (19) but strong in HIV-1-infected individuals harboring nef-defective forms of the virus and in monkeys experimentally infected with attenuated SIVmac239 variants from which nef has been deleted (15, 16, 19). However, it remains to be clarified whether these differences result from the lack of Nef function or are just due to the lower viral loads and asymptomatic status of hosts infected with attenuated virus variants. Vigorous and sustained CD4+-T-helper responses have been observed in HIV-1-infected long-term nonprogressors (53). To further elucidate whether Nef specifically impairs CD4+-T-helper responses in vivo, it would be interesting to compare the T-helper and CTL responses in macaques infected with either nef-defective or vpx/vpr-defective SIVmac variants showing similar degrees of attenuation (21).
Surface expression of Ii was strongly increased in transfected cells expressing low to moderate levels of Nef and in HIV-1-infected cells. In contrast, down-modulation of mature MHC-II was only observed at high levels of Nef expression. Extending a previous study, we demonstrated that the levels of MHC-II on the surface of Nef-expressing HIV-1-infected HeLa CIITA cells are slightly reduced (62). However, we did not observe a significant decrease in MHC-II surface levels on 221-B7 cells or human PBMC transduced with proviral HIV-1 constructs (Fig. 4). It has been demonstrated recently that Nef down-modulates MHC-I cell surface expression much more efficiently on primary T cells compared to HeLa cells (34). The influence of Nef on MHC-II surface expression might also be cell type dependent, and it will be important to investigate this in HIV-1-infected primary APCs.
The importance of both Nef functions for MHC-II antigen presentation and the pathogenicity of HIV-1 remains to be defined. However, since surface expression of Ii was usually dramatically increased and MHC-II associated with Ii cannot stimulate CD4+ T cells, it is likely that this Nef function interferes with MHC-II-restricted peptide presentation. Furthermore, Ii up-regulation was seen for nef alleles derived from all progressing HIV-1-infected individuals studied except for two of four LTNPs only, suggesting that this Nef function might be relevant for progressive infection. nef alleles from patients LTNP2 and 039nm94 did not up-regulate Ii; however, they maintained low viral loads and stable CD4+-T-cell counts despite more than 15 and 12 years, respectively, of documented HIV-1 infection. LTNP2 is among seven individuals of 128 HIV-1-infected participants monitored by the New England Hemophilia Center of the University of Massachusetts/Memorial Health Care System who met the criteria for long-term nonprogression (24). Notably, LTNP2 was the only individual with nonprogressive infection in this cohort in whom no unusual, difficult-to-revert polymorphisms in HIV-1, which might attenuate viral replication, could be detected (2). Furthermore, this individual's class I HLA alleles (HLA-B49 and HLA-A29) are typically associated with more rapid progression to AIDS (24, 33). Clearly, a larger number of LTNPs must be analyzed, but the preliminary results suggest that the inability of Nef to up-regulate Ii might contribute to the lack of disease progression in LTNP2. nef alleles derived from another nonprogressor, LTNP4, could modulate MHC-II and Ii surface expression (Fig. 3), but neither down-modulated CD4 or enhanced viral replication (8, 43). Thus, in addition to grossly defective nef genes, more-subtle differences in Nef function might contribute to nonprogressive HIV-1 infection.
Nef affects multiple aspects of the interactions between CD4+ T cells and MHC-II-expressing APCs (5, 22, 28, 29, 31). The concerted modulation of CD4, CD28, Ii, MHC-II, and/or CD3 cell surface expression should impair both the adhesion between T cells and APCs, as well as the duration and strength of the antigen-specific signal in the T cell. Recent studies suggest that Nef prevents antigen-specific T-cell activation while activating downstream effectors in signaling pathways that mediate cellular activation and lead to efficient virus production (30, 60, 64). SIVmac variants containing mutations that selectively disrupt specific in vitro functions of Nef are useful in studying the relative contribution of different Nef activities to viral replication and pathogenesis in vivo (30, 45). However, we have not been able to identify motifs in SIV Nef exclusively required for down-modulation of MHC-II or up-regulation of Ii. Thus, it will not be easy to determine to what extent these Nef functions contribute to viral pathogenesis.
We demonstrated previously that nef alleles obtained during late stages of HIV-1 infection did not down-modulate MHC-I efficiently, but they were highly active in stimulating viral replication and down-regulating CD4 (8). The progressor consensus nef alleles (Pcon, Pex, and PexP) and the late stage P2-93 or SP7-93 nef genes were less active in up-regulating Ii than the NPcon, NPex, P2-87, and SP7-88 nef alleles (Fig. 3). However, the average functional activity of Nef in modulating Ii and MHC-II surface expression did not change significantly during or after progression to AIDS in six progressing HIV-1-infected individuals. One possibility is that these Nef functions are relevant for efficient viral replication throughout the course of infection. However, it should be noted that mutations in the C-proximal flexible loop disrupted Ii up-regulation and impaired down-regulation of CD4 (Fig. 5). Thus, whereas some mutations in Nef selectively affect either CD4 or Ii surface modulation, both functions are apparently mediated by overlapping domains. Accordingly, changes reducing the ability of Nef to modulate Ii or MHC-II surface expression might frequently also impair other Nef functions, which are important for efficient viral replication during late stages of HIV-1 infection. This might explain why a loss of Nef function in MHC-II down- and Ii up-regulation is not observed in most AIDS patients.
In agreement with Stumptner-Cuvelette and Benaroch (62), we found that most, if not all, in vitro Nef functions investigated are genetically separable. The acidic domain of Nef was shown in both studies to be involved in down-modulation of MHC-II but dispensable for up-regulation of the Ii chain. Mutations in the C-proximal flexible loop consistently abolished the ability of Nef to modulate Ii surface expression but had little effect on down-regulation of MHC-II. Nef-induced down-regulation of CD4 and up-regulation of Ii chain required the presence of the dileucine and the five charged residues at positions 174, 175, and 177 to 179. However, they had opposite dependencies toward the dibasic motif (residues 105 and 106) and the diacidic motif (residues 154 and 155). These genetic differences probably reflect differences in the mechanisms underlying these effects. The dileucine motif of Nef has been implicated in the capacity of Nef to associate with clathrin adaptor complexes, including AP2, thus contributing to the Nef-induced accelerated endocytosis of CD4 (12). However, the effect of Nef on Ii surface expression does not seem to result from AP2 titration by the viral protein because other AP2-dependent proteins such as the transferrin receptor are not up-regulated. Mutation of prolines 72 and 75 moderately affected MHC-I down-regulation and disrupted the ability of Nef to modulate MHC-II but not Ii surface expression (Fig. 5). In comparison, mutation of prolines 75 and 78 totally impaired MHC-I down-regulation but had only a moderate effect on MHC II and Ii modulation (62). Of note, it has recently been shown that of the four prolines at positions 69, 72, 75, and 78 in Nef, only position 78 is critical for MHC-I down-regulation (66). Taken together, these data suggest that proline 72 of Nef is involved in MHC-II down-modulation. However, an intact SH3-binding domain in Nef is not required for the modulation of Ii and MHC-I cell surface expression.
Overall, our results indicate that Nef-mediated up-regulation of Ii might play a relevant role in the pathogenesis of HIV-1. Other viruses might use a similar strategy to impair MHC-II antigen presentation (59, 67). However, much remains to be done to elucidate the mechanisms by which Nef modulates MHC-II and Ii cell surface expression and to clarify their roles in determining viral pathogenicity in vivo. It will be important to evaluate the effects of Nef on MHC-II antigen presentation in primary cells and to use Nef mutants, selectively impaired for modulation of CD3, CD4, CD28, Ii, and/or MHC-II surface expression, to clarify which activities are critical for the interaction between T cells and APCs. Such SIV and HIV-1 Nef mutants would also be useful for investigating the importance of these in vitro Nef activities to viral pathogenicity in the SIV/macaque and Nef-SHIV models (3, 36, 37). Currently, we are analyzing nef alleles derived from a larger panel of LTNPs to clarify whether long-term nonprogressive HIV-1 infection is frequently associated with impaired functional activity in Ii up-regulation or other Nef functions.
Acknowledgments
We thank Thomas Mertens for constant support and encouragement, Nadja Auer and Nicola Bailer for excellent technical assistance, Ingrid Bennett for critical reading of the manuscript, George B. Cohen for 221-B7 cells, Klaus Strebel for the HIV-2 Rod molecular clone, and John Iafrate and Jacek Skowronski for providing the HIV-1 NA7 Nef mutants. We are also indebted to all of the individuals who participated in the study, and we thank Ann Forsberg and Pat Forand for their efforts in the clinic. We appreciate the excellent technical support of laboratory members in the Division of Pediatric Immunology.
This work was supported in part by NIH grants HL42257 and AI39400, by University of Massachusetts CFAR grant AI42845, and by grants from the Deutsche Forschungsgemeinschaft and the Wilhelm-Sander-Stiftung. T.C.G. was also supported by the NIH K08 grant AI01382 and is currently supported by the Campbell Foundation.
REFERENCES
- 1.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, L., E. Weiskopf, T. C. Greenough, N. C. Gaddis, M. R. Auerbach, M. H. Malim, S. J. O'Brien, B. D. Walker, J. L. Sullivan, and R. C. Desrosiers. 2000. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J. Virol. 74:4361-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander, L., Z. Du, A. Y. Howe, S. Czajak, and R. C. Desrosiers. 1999. Induction of AIDS in rhesus monkeys by a recombinant simian immunodeficiency virus expressing nef of human immunodeficiency virus type 1. J. Virol. 73:5814-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander, L., Z. Du, M. Rosenzweig, J. U. Jung, and R. C. Desrosiers. 1997. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J. Virol. 71:6094-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell, I., C. Ashman, J. Maughan, E. Hooker, F. Cook, and T. A. Reinhart. 1998. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J. Gen. Virol. 79:2717-2727. [DOI] [PubMed] [Google Scholar]
- 6.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertolino, P., C. Rabourdin-Combe. 1996. The MHC class II-associated invariant chain: a molecule with multiple roles in MHC class II biosynthesis and antigen presentation to CD4+ T cells. Crit. Rev. Immunol. 16:359. [PubMed] [Google Scholar]
- 8.Carl, S., T. C. Greenough, M. Krumbiegel, M. Greenberg, J. Skowronski, J. L. Sullivan, and F. Kirchhoff. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 75:3657-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661-671. [DOI] [PubMed] [Google Scholar]
- 11.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 12.Craig, H. M., M. W. Pandori, and J. C. Guatelli. 1998. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl. Acad. Sci. USA 95:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cresswell, P. 1996. Invariant chain structure and MHC class II function. Cell 84:505-507. [DOI] [PubMed] [Google Scholar]
- 14.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, and C. Chatfield. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 15.Dyer, W. B., G. S. Ogg, M. A. Demoitie, X. Jin, A. F. Geczy, S. L. Rowland-Jones, A. J. McMichael, D. F. Nixon, and J. S. Sullivan. 1999. Strong human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte activity in Sydney Blood Bank Cohort patients infected with nef-defective HIV type 1. J. Virol. 73:436-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyer, W. B., A. F. Geczy, S. J. Kent, L. B. McIntyre, S. A. Blasdall, J. C. Learmont, and J. S. Sullivan. 1997. Lymphoproliferative immune function in the Sydney Blood Bank Cohort, infected with natural nef/long terminal repeat mutants, and in other long-term survivors of transfusion-acquired HIV-1 infection. AIDS 11:1565-1574. [DOI] [PubMed] [Google Scholar]
- 17.Easterbrook, P. J., T. Rostron, N. Ives, M. Troop, B. G. Gazzard, and S. L. Rowland-Jones. 1999. Chemokine receptor polymorphisms and human immunodeficiency virus disease progression. J. Infect. Dis. 180:1096-1105. [DOI] [PubMed] [Google Scholar]
- 18.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent down-regulation of cell-surface CD4 by nef. Nature 350:508-519. [DOI] [PubMed] [Google Scholar]
- 19.Gauduin, M. C., R. L. Glickman, S. Ahmad, T. Yilma, and R. P. Johnson. 1999. Immunization with live attenuated simian immunodeficiency virus induces strong type 1 T helper responses and beta-chemokine production. Proc. Natl. Acad. Sci. USA 96:14031-14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geyer, M., O. T. Fackler, and B. M. Peterlin. 2001. Structure-function relationships in HIV-1 Nef. EMBO Rep. 2:580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbs, J. S., A. A. Lackner, S. M. Lang, M. A. Simon, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1995. Progression to AIDS in the absence of genes for vpr or vpx. J. Virol. 69:2378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grakoui, A., S. K. Bromley, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 1999. The immunological synapse: a molecular machine controlling T-cell activation. Science 285:221-227. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenough, T. C., D. B. Brettler, F. Kirchhoff, L. Alexander, R. C. Desrosiers, S. J. O'Brien, M. Somasundaran, K. Luzuriaga, and J. L. Sullivan. 1999. Long-term nonprogressive infection with human immunodeficiency virus type 1 in a hemophilia cohort. J. Infect. Dis. 180:1790-1802. [DOI] [PubMed]
- 25.Greenough, T. C., M. Somasundaran, D. B. Brettler, R. M. Hesselton, A. Alimenti, F. Kirchhoff, D. Panicali, and J. L. Sullivan. 1994. Normal immune function and inability to isolate virus in culture in an individual with long-term human immunodeficiency virus infection. AIDS Res. Hum. Retrovir. 10:395-403. [DOI] [PubMed] [Google Scholar]
- 26.Hanna, Z., D. G. Kay, N. Rebai, A. Guimond, S. Jothy, and P. Jolicoeur. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163-175. [DOI] [PubMed] [Google Scholar]
- 27.Hiebenthal-Millow, K., and F. Kirchhoff. 2002. The most frequent naturally occurring length polymorphism in the HIV-1 LTR has little effect on proviral transcription and viral replication. Virology 292:169-175. [DOI] [PubMed] [Google Scholar]
- 28.Howe, A. Y., J. U. Jung, and R. C. Desrosiers. 1998. ζ chain of the T-cell receptor interacts with nef of simian immunodeficiency virus and human immunodeficiency virus type 2. J. Virol. 72:9827-9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang, I., J.-F. Huang, H. Kishimoto, A. Brunmark, P. A. Peterson, M. R. Jackson, C. D. Surh, Z. Cai, and J. Sprent. 2000. T cells can use either T-cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen presenting cells. J. Exp. Med. 191:1137-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iafrate, A. J., S. Carl, S. Bronson, C. Stahl-Hennig, T. Swigut, J. Skowronski, and F. Kirchhoff. 2000. Disrupting surfaces of Nef required for down-regulation of CD4 and for enhancement of virion infectivity attenuates simian immunodeficiency virus replication in vivo. J. Virol. 74:9836-9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iafrate, A. J., S. Bronson, and J. Skowronski. 1997. Separable functions of Nef disrupt two aspects of T-cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 16:673-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson, W. E., and R. C. Desrosiers. 2002. Viral persistance: HIV's strategies of immune system evasion. Annu. Rev. Med. 53:499-518. [DOI] [PubMed] [Google Scholar]
- 33.Kaslow, R. A., M. Carrington, R. Apple, L. Park, A. Munoz, A. J. Saah, J. J. Goedert, C. Winkler, S. J. O'Brien, C. Rinaldo, R. Detels, W. Blattner, J. Phair, H. Erlich, and D. L. Mann. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2:405-411. [DOI] [PubMed] [Google Scholar]
- 34.Kasper, M. R., and K. L. Collins. 2003. Nef-mediated disruption of HLA-A2 transport to the cell surface in T cells. J. Virol. 77:3041-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kestler, H. W., T. Kodama, D. J. Ringler, M. Marthas, N. Pedersen, A. Lackner, D. Regier, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1990. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248:1109-1112. [DOI] [PubMed] [Google Scholar]
- 36.Kestler, H. W., D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 37.Kirchhoff, F., J. Munch, S. Carl, N. Stolte, K. Matz-Rensing, D. Fuchs, P. T. Haaft, J. L. Heeney, T. Swigut, J. Skowronski, and C. Stahl-Hennig. 1999. The human immunodeficiency virus type 1 nef gene can to a large extent replace simian immunodeficiency virus nef in vivo. J. Virol. 73:8371-8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirchhoff, F., K. D. Jentsch, A. Stuke, J. Mous, and G. Hunsmann. 1990. Genomic divergence of an HIV-2 from a German AIDS patient probably infected in Mali. AIDS 4:847-857. [DOI] [PubMed] [Google Scholar]
- 39.Kirchhoff, F., P. J. Easterbrook, N. Douglas, M. Troop, T. C. Greenough, J. Weber, S. Carl, J. L. Sullivan, and R. S. Daniels. 1999. Sequence variations in human immunodeficiency virus type 1 Nef are associated with a different stages of disease. J. Virol. 73:5497-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Absence of intact nef sequences in a long-term, nonprogressing survivor of HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 41.Learmont, J. C., A. F. Geczy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, and J. S. Sullivan. 1999. Immunological and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. N. Engl. J. Med. 340:1715-1722. [DOI] [PubMed] [Google Scholar]
- 42.Le Gall, S., M. C. Prevost, J. M. Heard, and O. Schwartz. 1997. Human immunodeficiency virus type I Nef independently affects virion incorporation of major histocompatibility complex class I molecules and virus infectivity. Virology 229:295-301. [DOI] [PubMed] [Google Scholar]
- 43.Mariani, R., F. Kirchhoff, T. C. Greenough, J. L. Sullivan, R. C. Desrosiers, and J. Skowronski. 1996. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 70:7752-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179:101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Münch, J., N. Stolte, D. Fuchs, C. Stahl-Hennig, and F. Kirchhoff. 2001. Efficient class I major histocompatibility complex down-regulation by simian immunodeficiency virus Nef is associated with a strong selective advantage in infected rhesus macaques. J. Virol. 75:10532-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Münch, J., A. Janardhan, N. Stolte, C. Stahl-Hennig, P. Ten Haaft, J. L. Heeney, T. Swigut, F. Kirchhoff, and J. Skowronski. 2002. T-cell receptor:CD3 down-regulation is a selected in vivo function of simian immunodeficiency virus Nef but is not sufficient for effective viral replication in rhesus macaques. J. Virol. 76:12360-12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel, P. G., M. T. Yu Kimata, J. E. Biggins, J. M. Wilson, and J. T. Kimata. 2002. Highly pathogenic simian immunodeficiency virus mne variants that emerge during the course of infection evolve enhanced infectivity and the ability to downregulate CD4 but not class I major histocompatibility complex antigens. J. Virol. 76:6425-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pieters, J. 2000. MHC class II-restricted antigen processing and presentation. Adv. Immunol. 75:159-208. [DOI] [PubMed] [Google Scholar]
- 49.Regier, D. A., and R. C. Desrosiers. 1989. The complete nucleotide sequence of a pathogenic molecular clone of SIV. AIDS Res. Hum. Retrovir. 6:1221-1231. [DOI] [PubMed] [Google Scholar]
- 50.Renkema, G. H., and K. Saksela. 2000. Interactions of HIV-1 NEF with cellular signal transducing proteins. Front. Biosci. 5:268-283. [DOI] [PubMed] [Google Scholar]
- 51.Renkema, G. H., A. Manninen, D. A. Mann, M. Harris, and K. Saksela. 1999. Identification of the Nef-associated kinase as p21-activated kinase 2. Curr. Biol. 9:1407-1410. [DOI] [PubMed] [Google Scholar]
- 52.Roche, P. A., C. L. Teletski, D. R. Karp, V. Pinet, O. Bakke, and E. O. Long. 1992. Stable surface expression of invariant chain prevents peptide presentation by HLA-DR. EMBO J. 11:2841-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo. S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 54.Ryan-Graham, M. A., and K. Peden. 1995. Both virus and host components are important for the manifestation of a Nef− phenotype in HIV-1 and HIV-2. Virology 213:158-168. [DOI] [PubMed] [Google Scholar]
- 55.Saksela, K., G. Cheng, and D. Baltimore. 1995. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 14:484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaefer, T. M., I. Bell, B. A. Fallert, and T. A. Reinhart. 2000. The T-cell receptor ζ chain contains two homologous domains with which simian immunodeficiency virus Nef interacts and mediates down-modulation. J. Virol. 74:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schrager, J. A., and J. W. Marsh. 1999. HIV-1 Nef increases T-cell activation in a stimulus-dependent manner. Proc. Natl. Acad. Sci. USA 96:8167-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 59.Sievers, E., J. Neumann, M. Raftery, G. Schonrich, A. M. Eis-Hubinger, and N. Koch. 2002. Glycoprotein B from strain 17 of herpes simplex virus type I contains an invariant chain homologous sequence that binds to MHC class II. Immunology 107:129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simmons, A., V. Aluvihare, and A. McMichael. 2001. Nef triggers a transcriptional program in T cells imitating single-signal T-cell activation and inducing HIV virulence mediators. Immunity 14:763-777. [DOI] [PubMed] [Google Scholar]
- 61.Spina, C. A., T. J. Kwoh, M. Y. Chowers, J. C Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 98:12144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stumptner-Cuvelette, P., and P. Benaroch P. 2002. Multiple roles of the invariant chain in MHC class II function. Biochim. Biophys. Acta 1542:1-13. [DOI] [PubMed] [Google Scholar]
- 64.Swigut, T., A. J. Iafrate, J. Münch, F. Kirchhoff, and J. Skowronski. 2000. Simian and human immunodeficiency virus Nef proteins use different surfaces to downregulate class I major histocompatibility antigen expression. J. Virol. 74:5691-5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swigut, T., N. Shody, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 20:1593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamada, T., N. Kaji, T. Odawara, J. Chiba, A. Iwamoto, and Y. Kitamura. 2003. Proline 78 is crucial for human immunodeficiency virus type 1 Nef to down-regulate class I human leukocyte antigen. J. Virol. 77:1589-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, B., P. Li, E. Wang, Z. Brahmi, K. W. Dunn, J. S. Blum, and A. Roman. 2003. The E5 protein of human papillomavirus type 16 perturbs MHC class II antigen maturation in human foreskin keratinocytes treated with interferon-gamma. Virology 310:100-108. [DOI] [PubMed] [Google Scholar]