Abstract
The Saccharomyces cerevisiae genome encodes four MutL homologs. Of these, MLH1 and PMS1 are known to act in the MSH2-dependent pathway that repairs DNA mismatches. We have investigated the role of MLH3 in mismatch repair. Mutations in MLH3 increased the rate of reversion of the hom3–10 allele by increasing the rate of deletion of a single T in a run of 7 Ts. Combination of mutations in MLH3 and MSH6 caused a synergistic increase in the hom3–10 reversion rate, whereas the hom3–10 reversion rate in an mlh3 msh3 double mutant was the same as in the respective single mutants. Similar results were observed when the accumulation of mutations at frameshift hot spots in the LYS2 gene was analyzed, although mutation of MLH3 did not cause the same extent of affect at every LYS2 frameshift hot spot. MLH3 interacted with MLH1 in a two-hybrid system. These data are consistent with the idea that a proportion of the repair of specific insertion/deletion mispairs by the MSH3-dependent mismatch repair pathway uses a heterodimeric MLH1-MLH3 complex in place of the MLH1-PMS1 complex.
Mismatch repair safeguards the integrity of the genome by recognizing and correcting mispaired bases that arise because of misincorporation of nucleotides during DNA replication (reviewed in refs. 1 and 2) or as a result of chemical damage to DNA and DNA precursors (3–5). Mismatch repair recognizes mispaired bases present in heteroduplex recombination intermediates and it can prevent recombination between divergent DNAs (6–8). The crucial role of mismatch repair proteins in the stabilization of the genome is illustrated by the finding that inactivation of certain human mismatch repair genes that are related to the bacterial mutS and mutL genes underlie both inherited cancer susceptibility syndromes (reviewed in ref. 9) and sporadic cancers (10–12).
The best understood mismatch repair pathway involving MutS and MutL-type proteins is the Escherichia coli MutHLS mismatch repair pathway (reviewed in refs. 1 and 2). This reaction has been reconstituted in vitro by using hemimethylated DNA substrates containing mismatches, MutH, MutL, MutS, and UvrD (helicase II) proteins along with DNA polymerase III holoenzyme, DNA ligase, single-stranded DNA binding protein, and any one of the single-stranded DNA exonucleases-exo I, exo VII, or RecJ protein, or possibly other exonucleases (13). This basic mismatch repair system is present in eukaryotes, although less is known about the relevant eukaryotic proteins or how they function in mismatch repair.
In yeast, humans, and mice, several genes that encode homologs of MutS and MutL have been cloned. Saccharomyces cerevisiae has six genes encoding proteins related to MutS, MSH1–6, three of which function in mismatch repair in the nucleus (1, 14). Repair is carried out by two different MSH2-dependent pathways (15). One involves a complex of MSH2 and MSH6 that repairs base-base mispairs and small insertion/deletion mispairs. The second requires a complex of MSH2 and MSH3 and corrects insertion/deletion mispairs. Similarly, human MSH2 appears to function as a complex with either MSH6 or MSH3 (16–18). Homologs of MutL also are found in eukaryotes. In yeast, two MutL homologs, MLH1 and PMS1, exist as a heterodimer and this complex appears to function in an analogous manner to E. coli MutL (19, 20). In humans, two MutL homologs, MLH1 and PMS2 (the human homolog of yeast PMS1) also function in mismatch repair as a heterodimeric complex (21). A third human MutL homolog, PMS1, has been suggested to function in mismatch repair by the finding of a germ-line mutation in this gene in a patient with a history of colon cancer (22) but a biochemical role for this protein in mismatch repair has not been demonstrated.
Recently, two additional MutL homologs have been revealed by the S. cerevisiae genome sequence project, MLH2 (ORF YLR035c) and MLH3 (ORF YPL164c) (14). In addition, a fragment of MLH3 was identified in a two-hybrid screen using MLH1 as bait because the C-terminal region of MLH3 appears to contain a MLH1 interaction sequence (23). The role of these two MutL homologs in mismatch repair has not yet been elucidated; however, the ability of a fragment of MLH3 to interact with MLH1 suggests a role for MLH3 in some type of MLH1-dependent reaction.
MATERIALS AND METHODS
General Genetic Methods.
Yeast extract/peptone/dextrose media, sporulation media, synthetic drop-out media, 5-fluoroorotic acid, and canavanine-containing media were as described (24, 25).
Strains.
All strains used in this study are isogenic derivatives of RDKY3023, a strain derived from S288c (Table 1). Strains were constructed by gene disruption as described (26, 27) and verified by PCR. RDKY2402-mlh3 strain was constructed by replacing nucleotides +26 to +2,094 of the 2,119-nt MLH3 ORF with the HIS3 gene by using PCR-mediated gene disruption. The required PCR product was generated by amplification of the HIS3 gene present in pRS423 (28) with primers HFR021 5′-GTGAACTCGTCAACTCAAAAAGAAAATGAGCCAGCATATTAGGAAATTAGAgagcagattgtactgagagtgcacc and HFR022 5′-GTTCCAGGATTAAGGTTCTCTTTACTTCAATTCTGCAATGGGTACCATAGctccttacgcatctgtgcggtatttc (uppercase characters correspond to MLH3-specific sequence and the lowercase sequences flank the selective marker present in plasmid containing the HIS3 gene).
Table 1.
S. cerevisiae strains used in this study
| RDKY3023: wild type* |
| RDKY2402: mlh3∷HIS3 |
| RDKY2419: msh6∷hisG |
| RDKY2431: msh3∷hisG |
| RDKY2407: mlh1∷hisG-URA3-his G |
| RDKY2750: pms1∷hisG |
| RDKY2405: mlh3∷HIS3, msh6∷TRP1 |
| RDKY2408: mlh1∷hisG-URA3-hisG, mlh3∷HIS3 |
| RDKY2412: mlh3∷TRP1, pms1∷hisG |
| RDKY2403: mlh3∷HIS3, msh3∷TRP1 |
| RDKY2404: mlh3∷HIS3, msh2∷hisG |
| RDKY2707: msh2∷hisG |
| RDKY3535: msh3∷TRP1, msh6∷hisG |
All strains containing the following markers: MAT a, ura3-52, leu2Δ1, trp1Δ63, his3Δ200, lys2ΔBgl, hom3-10, ade2Δ1, ade8.
RDKY2419-msh6∷hisG was generated by using the EcoRI/SphI disruption fragment from plasmid pEAI108 that results in the replacement of nucleotides +108 to +3,000 of the 3,725-nt MSH6 ORF with a hisG-URA3-hisG cassette. Excision of the URA3 marker by recombination between the hisG repeats resulting in the msh6∷hisG derivative was selected for on minimal drop-out plates containing 5-fluoroorotic acid. RDKY2431-msh3∷hisG was similarly constructed by using an EcoRI fragment containing a hisG-URA3-hisG cassette replacing nucleotides +638 to +3,135 of the 3141-nt MSH3 ORF derived from plasmid pEN33. RDKY2750-pms1∷hisG was constructed by replacing nucleotides +168 to +2,231 of the 2,712-nt PMS1 ORF with the hisG-URA3-hisG containing SalI fragment of plasmid pEAI100. The MLH1 gene was disrupted with hisG in the isogenic strain RDKY2669 (α, ura3–52, leu2Δ1, trp1Δ63, his3Δ200, lys2ΔBgl, hom3–10, ade2Δ1, ade8) using the hisG-URA3-hisG cassette present in the SphI/KpnI fragment of plasmid pEAI105, which removes nucleotides +34 to +2,303 of the 2,305-nt MLH1 ORF generating strain RDKY2407-mlh1∷hisG.
Strain RDKY2408-mlh3 mlh1 was generated by crossing strains RDKY2402-mlh3 and RDKY2407-mlh1 followed by tetrad dissection and genotyping. The strain RDKY2412-mlh3 pms1 was generated by disrupting the MLH3 gene in strain RDKY2750-pms1 as described for RDKY2402, except that the TRP1 gene present in plasmid pRS424 was amplified by using oligonucleotides HFR021 and HFR022 and used as the disruption marker. Strain RDKY2403-mlh3 msh3 was generated by replacing nucleotides +45 to +3,160 of the 3,140-nt MSH3 ORF in RDKY2402-mlh3 using a PCR fragment obtained by amplifying the TRP1 gene of pRS424 using oligonucleotides HFR307 5′-GAACAATGGTGATAGGTAATGAACCTAAACTGGTACTTTTGAGAGCCAAAAgagcagattgtactgagagtgcacc and HFR308 5′-GCTGCATTTAGAACATACGTACCATCCGCATCAGTGGATATCCAATGATAGctccttacgcatctgtgcggtatttc. Strain RDKY2405-mlh3 msh6 was generated by transforming strain RDKY2402-mlh3 with a PCR product containing the TRP1 gene amplified from plasmid pRS424 with oligonucleotides HFR011 5′-CTCCAAAATGGCCCCAGCTACCCCTAAAACTTCTAAGACTGCACACTTCGAAgagcagattgtactgagagtgcacc and HFR012 5′-CGTAAATGAAAATACTTAGGATTGTAAATCATCAATTATACTAAATAGACTctccttacgcatctgtgcggtatttc which replaces nucleotides +34 to +3,692 of the 3,725-nt MSH6 ORF with TRP1. The MSH2 ORF was disrupted in strain RDKY2402-mlh3 by transforming with a PvuII/AatII restriction fragment of plasmid pEAI98 that carries the msh2∷hisG-URA3-hisG cassette. The resulting strain, RDKY2404-mlh3 msh2, has nucleotides +1 to +2,694 of the 2,895-nt MSH2 ORF replaced by hisG sequences. Strain RDKY3535-msh6∷hisG msh3∷TRP1 was generated by replacing the MSH3 ORF in RDKY2419-msh6 as described above for strain RDKY2403. Strain RDKY2707-msh2∷hisG has been previously described (25).
Mutation Analysis.
Mutation rates were determined by fluctuation analysis using at least five independent colonies (15, 25, 29). Each fluctuation test was repeated at least twice. Spectrum analysis was carried out by selecting revertants (Lys+ or Thr+) or mutants (Canr) on selective minimum media drop-out plates. Chromosomal DNA was isolated from the revertants (30) and the relevant regions of LYS2, HOM3, and CAN1 were amplified by PCR and sequenced (15, 25).
DNA Sequence Analysis.
All DNA sequencing was performed by using an Applied Biosystems 377 DNA sequencer and standard chemistry. Analysis of the sequence chromatograms was carried out by using Sequencher (Gene Codes, Ann Arbor, MI), and sequence comparisons, alignments, and phylogenetic trees were generated by using DNAStar software (Madison, WI). Database searches were performed by using the blast algorithm (http://genome-www2.stanford.edu/cgi-bin/SGD/nph-blast2sgd). The determination of sequence identity between two homologs was performed by using the fasta algorithm available at http://genome.eerie.fr/bin/align-guess.cgi.
Plasmids.
The yeast MLH3 gene was amplified from genomic DNA by PCR using oligonucleotides HFR300 5′-CGGTTAGCACGAATTCACCATGAGCCAGCATATTAGGAAATTAG and HFR302 5′-GGTCGACGCGTAAGCTTTACTTCAATTCTGCAATGGGTACCATAG, and cloned into pYX243 (Novagen) to yield pRDK742. The entire MLH3 ORF was excised from pRDK742 as an EcoRI/XhoI fragment and cloned into pEG202 (31) to generate MLH3-bait plasmid pRDK743. The MLH1 prey construct, pRDK744, was generated by cloning a PCR fragment obtained by amplifying MLH1 from pEN71 (20, 32) using oligonucleotides HFR601 5′-ATCGAATTCGGCCTAGGGATGTCTCTCAGAATAAAAGCACTT and HFR602 5′-TCTCACGCTCGCTCGAGTTAACACCTCTCAAAAACTTTGTAT into the AvrII and XhoI sites of pJG4–5* [pJG4–5 (31) modified in this laboratory by Pascale Bertrand]. The PMS1 prey plasmid (pRDK745) was constructed by amplifying PMS1 from pRDK433 (constructed in this laboratory by Ruchira Das Gupta) using oligonucleotides HFR603 5′-ATCGAATTCGGCCTAGGGATGTTTCACCACATCGAAAACTTA and HFR602, and inserting the resulting fragment between the AvrII and XhoI sites of pJG4–5*. The presence of the relevant wild-type sequence in all plasmids was confirmed by DNA sequencing.
Interaction Experiments.
Yeast strain RDKY2926 [strain EGY48 harboring reporter plasmid pSH18–34 (33, 34)] was simultaneously transformed with a LexA-fusion bait construct (either pEG202 or pRDK743-MLH3) and an acid activation-tagged prey construct (pJG4–5*, pRDK745-PMS1, or pRDK744-MLH1) and selected on Ura−His−Trp− minimal drop-out plates. Isolates were tested for interaction by replica plating onto minimal media drop-out plates containing galactose and 5-bromo-4-chloro-3-indolyl β-d-galactoside (31, 35).
RESULTS
Identification of MLH3.
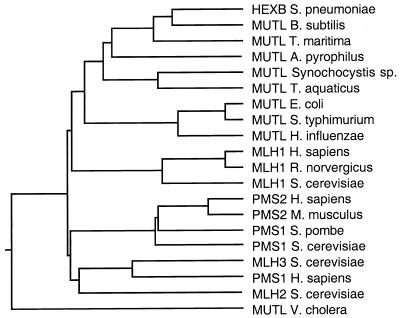
In addition to MLH1 and PMS1, two ORFs with homology to bacterial MutL can be identified in the S. cerevisiae Genome Database using blast searches, ORF YLR035c, which has been previously termed MLH2 (1, 14), and ORF YPL164c called MLH3 (14, 23). The molecular weight of the predicted MLH3 protein is 81,997. In comparison to different MutL homologs, MLH3 appears to be most closely related to human PMS1 (Fig. 1). The observed sequence identity (20%) and similarity (47%) between yeast MLH3 and human PMS1 is comparable to that observed for other yeast and human MSH and MLH proteins known to be functional homologs.
Figure 1.
Phylogenetic tree of MutL-related proteins. The dendrogram was generated with Megalign (DNAStar) by the clustal method with the weight table set to identities, a gap penalty of 10, and a gap length penalty of 10. All sequences used are full length and were retrieved from GenBank.
Strains Deficient in MLH3 Display a Mutator Phenotype.
To investigate a possible role of MLH3 in mismatch repair, we constructed a series of isogenic mutant strains deleted for MLH3 and other mismatch repair genes (Table 1). The mlh3 mutant strain was viable, had no growth phenotype, exhibited the same sensitivity to alkylating agents (methyl methane sulfonate) and UV irradiation as the wild type, and was able to grow in media containing nonfermentable sugars (data not shown). The mlh3 mutant strain also was examined for mutator phenotypes by determining the rate of reversion of the hom3–10 and lys2-Bgl alleles and the rate of accumulation of Canr mutations.
Compared with the wild type, an increase in the rate of reversion of hom3–10 and lys2-Bgl, two assays that are particularly sensitive to defects in mismatch repair, was observed in the mlh3 strain (3.3- and 2.2-fold, respectively). There was no significant increase in the rate of accumulation of Canr mutants (Table 2). This phenotype closely resembles that exhibited by msh3 strains (Table 2) (15, 36), suggesting that MLH3 and MSH3 could function in the same pathway. Consistent with this finding, the mlh3 msh3 double mutant had essentially the same rate of reversion of lys-2Bgl and hom3–10 and the same rate of accumulation of Canr mutations as either single mutant strain (Table 2). A msh6 mutant showed a substantial increase in the rate of accumulation of Canr mutations but only had a modest increase in the reversion rate of hom3–10 and lys2-Bgl. This finding agrees with previous results indicating that msh6 mutants are defective in repair of base-base mispairs but only show small defects in the repair of insertion/deletion mispairs (15, 37). The reversion rate for the hom3–10 allele was greatly increased in a msh6 mlh3 double mutant compared with either single mutant (Table 2). Reversion of the lys-2Bgl allele was increased to a lesser, but significant, extent in the msh6 mlh3 double mutant compared with each single mutant. In contrast, the rate of accumulation of Canr mutations was the same in the msh6 mlh3 strain compared with the msh6 single mutant. This genetic interaction between mlh3 and msh6 mutations is consistent with the idea that MLH3 participates in the repair of some insertion/deletion mispairs by the MSH3 pathway.
Table 2.
Mutation rate analysis of mlh3 and other mismatch repair-deficient strains
| Strain | Mutation rate
|
||
|---|---|---|---|
| Thr+ | Lys+ | Canr | |
| Wild type | 1.2 × 10−8 (1.0) | 1.0 × 10−8 (1.0) | 2.4 × 10−7 (1.0) |
| mlh3 | 3.9 × 10−8 (3.3) | 2.2 × 10−8 (2.2) | 2.8 × 10−7 (1.2) |
| msh3 | 5.5 × 10−8 (4.6) | 2.1 × 10−8 (2.1) | 3.1 × 10−7 (1.3) |
| mlh3 msh3 | 5.9 × 10−8 (4.9) | 2.9 × 10−8 (2.9) | 4.0 × 10−7 (1.6) |
| msh6 | 9.1 × 10−8 (7.6) | 2.3 × 10−8 (2.3) | 2.9 × 10−6 (12) |
| mlh3 msh6 | 8.2 × 10−7 (68) | 4.6 × 10−8 (4.6) | 3.1 × 10−6 (13) |
| msh2 | 6.7 × 10−6 (558) | 4.8 × 10−7 (48) | 2.9 × 10−6 (12) |
| mlh3 msh2 | 5.1 × 10−6 (425) | 4.3 × 10−7 (43) | 2.9 × 10−6 (12) |
| msh3 msh6 | 5.7 × 10−6 (475) | 5.9 × 10−7 (59) | 2.7 × 10−6 (11) |
| mlh1 | 5.5 × 10−6 (458) | 4.8 × 10−7 (48) | 3.9 × 10−6 (16) |
| mlh3 mlh1 | 6.6 × 10−6 (545) | 7.9 × 10−7 (79) | 3.9 × 10−6 (16) |
| pms1 | 3.6 × 10−6 (300) | 5.1 × 10−7 (51) | 3.1 × 10−6 (13) |
| mlh3 pms1 | 9.7 × 10−6 (804) | 5.0 × 10−7 (50) | 5.8 × 10−6 (21) |
Mutation rates were calculated by using the method of the median using five independent colonies per experiment. The average of several experiments is presented. The number of tests carried out with each strain is as follows: wild type, five times; mlh3, six times; msh6, mlh3 msh6, and msh3 msh6, three times each; msh3, mlh3 msh3, msh2, and mlh3 msh2, twice each. For mlh1, mlh3 mlh1, pms1, and mlh3 pms1, the test was repeated twice. The fold increase in the mutation rate compared to the wild-type strain is shown in parentheses.
Analysis of the Spectrum of Mutations in mlh3 Mutants.
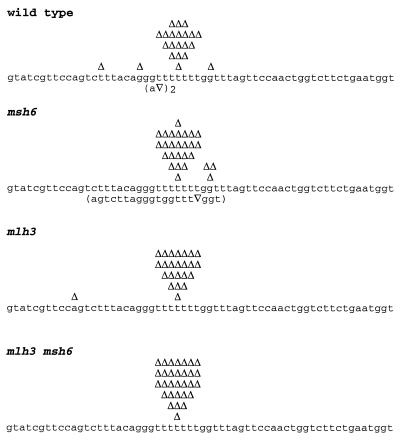
To further characterize the possible role of MLH3 in mismatch repair, we analyzed the spectrum of mutations causing the increased reversion of the hom3–10 and lys2-Bgl alleles seen in mlh3 mutants. As previously described, the hom3–10 allele carries an additional T in a run of six Ts, resulting in a +1 frameshift (15). As shown in Fig. 2, reversion occurs predominantly by deletion of the extra T in the mononucleotide run. In the wild-type strain, 21% (5/24) of the reversions occurred at sites different from the T tract. In the mlh3 and msh6 single mutants, the number of mutations at sites other than the run of Ts was 4% (1/24) and 14% (4/28), respectively, whereas in the msh6 mlh3 double mutant, reversion occurred exclusively at the T tract (30/30).
Figure 2.
Spectra of mutations reverting the hom3–10 allele in wild-type and different mutant strains. Nucleotides 623–682 of the sequenced region is shown. Each independent frameshift event detected is indicated over the sequence with a Δ. Complex mutations that include base changes are shown below the sequence in parentheses. In the wild–type strain, two identical complex events were observed. The total number of isolates analyzed was: wild type, 24; msh6, 28; mlh3, 24, and mlh3 msh6, 30.
When the mutation rates presented in Table 2 are recalculated by using the mutation spectra data to obtain rates for mutation at a specific site (Table 3) (38), we find that reversions arising exclusively at the T tract in hom3–10 occur at a rate of 9.5 × 10−9 in the wild-type strain. For mlh3, the rate is 3.9-fold higher than that of wild-type cells. The rate for msh6 is 8.6-fold higher than wild type, whereas the reversion rate at this site in the mlh3 msh6 double mutant is 86-fold higher than that of wild-type cells. This latter 86-fold increase is about 15% of the events seen at this site in a msh2 mutant strain. These results suggest that mlh3 mutations cause a defect in repair of insertion/deletion mispairs that result because of replication errors at mononucleotide runs and that this defect is most pronounced when MSH6-dependent mismatch repair has been inactivated.
Table 3.
Mutation rates at the hom3-10 and lys2-Bgl hotspots
| Strain | Mutation rate
|
|||
|---|---|---|---|---|
|
hom3-10
|
lys2-Bgl
|
|||
| T646–652 | A364–369 | C401–404 | T417–419 | |
| Wild type | 9.5 × 10−9 (1.0) | 0.2 × 10−8 (1.0) | 0.13 × 10−8 (1.0) | 0.05 × 10−8 (1.0) |
| mlh3 | 3.7 × 10−8 (3.9) | 1.1 × 10−8 (5.5) | 0.44 × 10−8 (3.4) | 0.04 × 10−8 (0.9) |
| msh3 | N.D. | 1.1 × 10−8 (5.5) | 0.18 × 10−8 (1.4) | <0.04 × 10−8 (<1) |
| mlh3 msh3 | N.D. | 1.4 × 10−8 (7.0) | 0.5 × 10−8 (3.8) | 0.15 × 10−8 (3.0) |
| msh6 | 9.1 × 10−8 (8.6) | 0.23 × 10−8 (1.2) | 0.23 × 10−8 (1.8) | 0.76 × 10−6 (15.2) |
| mlh3 msh6 | 8.2 × 10−7 (86) | 2.9 × 10−8 (14.5) | 0.6 × 10−8 (4.6) | 0.23 × 10−6 (4.6) |
Mutation rates were recalculated based on the frequency of occurrence of frameshifts at the indicated sites. N.D., not determined (see Table 2 for gross mutation rates at this site).
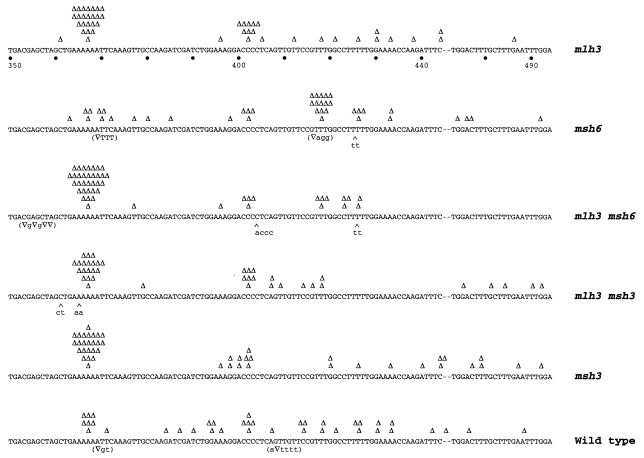
The analysis of the spectra of the lys2-Bgl revertants indicates that reversion can occur by mutations within a window of approximately 150 bp (Fig. 3) (15, 25, 39). In contrast to the wild-type strain, the distribution of revertants in the mismatch repair-deficient strains appears to be nonrandom and three mutation hot spots account for most of the reversion events (Fig. 3). Mutations were found to occur at significantly higher rates at a run of six As (A364–369), a run of four Cs (C401–404), and a run of three Ts (T417–419). Furthermore, there were significant differences between the mutation spectra observed in the different mutant strains analyzed. As shown in Fig. 3, the most notable mutation hot spot in the msh6 strain was the T417–419 site where 33% (14/42) of the mutations were found versus 5% (2/40) in the wild-type strain. The distribution of mutations at the other sites was 10% (4/42) at A364–369 and 10% (4/42) at C401–404 for the msh6 mutant strain and 20% (8/40) at A364–369 and 13% (5/40) at C401–404 in the wild-type strain, respectively. The spectra of mutations seen in the mlh3 and msh3 mutants had similar patterns with 50% (23/46) and 52% (24/46) of the frameshifts occurring at the A364–369 site in the mlh3 and msh3 mutant strains, respectively. The mlh3 msh3 double mutant had the same spectrum of the frameshifts (49%, 20/41 at A364–369) as observed in the respective single mutants. The proportion of frameshifts at the A364–369 site observed in the mlh3 msh6 double mutant (63%, 32/51) was quite different from that seen in the msh6 single mutant.
Figure 3.
Spectra of mutations reverting the lys-2Bgl allele in wild-type and different mutant strains. The region comprising nucleotides 350–444 and 473–494 is shown. Each frameshift reversion is indicated on top of the sequence depicted by a Δ. Reversion events involving single basepair deletions and base changes are shown in parentheses below the sequence and insertions are indicated by a ∧. The deletion of an A occurring at position 467 in a revertant of the wild-type strain is indicated over the –. The number of revertants analyzed was: wild type, 40; msh6, 42; mlh3, 46; mlh3 msh6, 51; msh3, 46; mlh3 msh3, 41.
When the mutation rates at individual sites were calculated (Table 3), we found that the rate of reversion at the A364–369 site in the mlh3 strain was 5.5-fold greater than that of wild type and similar to that of msh3 (5.5-fold greater than wild type) and mlh3 msh3 mutants (7-fold greater than wild type). The msh6 strain did not show an increase in the mutation rate at this site compared with the wild type. In contrast, a 14.5-fold increase in the reversion rate at this site was observed for the mlh3 msh6 strain. These data demonstrate (i) a synergistic effect on the rate of mutation at the A364–369 site in the mlh3 msh6 double mutant and (ii) that the mlh3 msh3 double mutant shows a mutation rate at this site that is not significantly different to that seen for the respective single mutants. These observations are consistent with the results obtained with the hom3–10 reversion assay and suggest that MLH3 functions in the MSH3 pathway and not in the MSH6 pathway.
A similar analysis of the effect of mlh3 mutations on mutation rates at the other lys2-Bgl reversion hot spots also was performed (Table 3). The mutation rate at the C401–404 site in the mlh3 strain was 3.4-fold greater than wild type and was similar to that of msh3 (1.4-fold greater than wild type) and mlh3 msh3 strains (3.8-fold greater than wild type). For msh6, the mutation rate was 1.8-fold greater than wild type, whereas for the mlh3 msh6 strain a 4.6-fold increase was observed. The mutation rate at the T417–419 site in mlh3 mutants was the same as in the wild type and, similarly, no significant increase in the reversion rate at this site was found for msh3 (<1-fold greater than wild type). However, in msh6, 33% of the reversion events occurred at the T417–419 site, resulting in a rate increase that was 15.2-fold greater than wild type. Modest increases were obtained for mlh3 msh3 (3.0-fold greater than wild type) and mlh3 msh6 strains (4.6-fold greater than wild type). These data suggest that there is little, if any, effect of mutations in MLH3 on the accumulation of frameshift mutations at the C401–404 and T417–419 sites, and that the effect at these sites is not as large as at the lys2-Bgl A364–369 site or the hom3–10 site.
Interaction Between MLH1 and MLH3.
In eukaryotes, MutL homologs have been shown to function as heterodimers (19, 21, 23, and reviewed in refs. 1 and 2). To examine the possibility that MLH3 also may function as part of a heterodimer with another MutL homolog, a series of strains containing different combinations of mutations in genes encoding MutL homologs were constructed (Table 1). Strains containing deletion mutations in MLH1 and PMS1 displayed high mutation rates similar to those observed in msh2 strains and msh3 msh6 double mutant strains (Table 2). No significant difference was observed between strains carrying a deletion of MLH1 and strains deleted for both MLH1 and MLH3, consistent with MLH1 being epistatic to MLH3. On the other hand, the rate of reversion of the hom3–10 allele in the pms1 mlh3 double mutant was significantly higher than that observed in the pms1 single mutant (P < 0.05, Mann–Whitney U test) but not significantly higher than observed in the msh2 single mutant (P < 0.05, Mann–Whitney U test, Table 2), suggesting that MLH3 and PMS1 may function in different repair pathways.
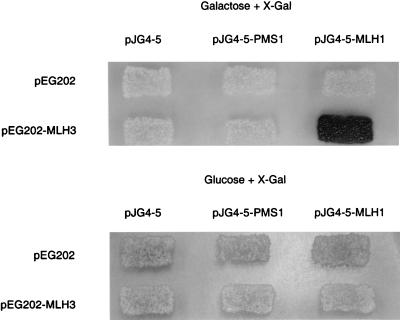
Pang et al. (23) identified a C-terminal fragment of MLH3 by virtue of its ability to interact with MLH1 in a two-hybrid screen and further demonstrated that MLH3 has significant homology to PMS1 in the C-terminal region required for interaction with MLH1. To extend these results and demonstrate that full-length MLH3 can interact with MLH1, the interaction between MLH3 and MLH1 was analyzed using a two-hybrid–based system. A bait construct expressing MLH3 fused to the LexA DNA-binding domain was cotransformed with plasmids carrying either PMS1 or MLH1 fused to an acid activation domain, or a control plasmid carrying only the activation domain, into a strain carrying the LacZ reporter gene in a plasmid, under the control of a GAL1 promoter (31, 35). Activation of the reporter gene was only observed only when MLH3 and MLH1 were coexpressed in the same cell, suggesting an interaction between MLH3 and MLH1 (Fig. 4). There was no activation of the reporter gene when the MLH1 or PMS1 prey plasmids were cotransformed with plasmids containing only the LexA DNA-binding domain, indicating that the observed interaction of MLH3 and MLH1 required the expression of both proteins.
Figure 4.
Interaction of MLH3 and MLH1 by two-hybrid analysis. Yeast strain RDKY2926 was cotransformed with the indicated combination of one bait plasmid and one prey plasmid, and the transformants were replica plated onto Ura−His−Trp− 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) glucose and Ura−His−Trp− X-Gal galactose indicator plates and incubated at 30°C for 2 days.
DISCUSSION
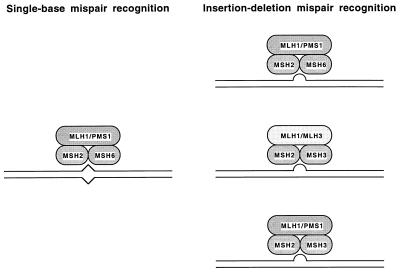
A model (reviewed in ref. 1) has been proposed for the role of MutS and MutL homologs in eukaryotic mismatch repair (Fig. 5). Mismatch recognition involves two different heterodimeric complexes of MutS-related proteins, MSH2–MSH6 and MSH2—MSH3. These two complexes are partially redundant with regard to their mispair recognition specificity. In S. cerevisiae, the MSH2–MSH6 complex functions in the repair of base-base and small insertion/deletion mispairs but probably plays only a limited role in the repair of larger insertion/deletion mispairs (larger than two unpaired bases) (15, 37). In contrast, the MSH2–MSH3 complex does not likely recognize base-base mispairs but rather only functions in the repair of insertion/deletion mispairs (1, 15, 40) and is most uniquely important for the repair of insertion/deletion mispairs larger than two unpaired bases (37). These two complexes are most redundant for the recognition of single-base insertion/deletion mispairs (1, 15, 37). Similarly, a heterodimer of two MutL-related proteins, MLH1 and PMS1 (PMS2 in humans), functions as the MutL equivalent (1, 2, 19, 21).
Figure 5.
Model of action of MLH3 in mismatch repair. Note that specific contacts between individual MLH and MSH proteins are not meant to be implied by this model as no information is presently available about such contacts.
The studies presented here suggest there is a second heterodimeric complex of MutL-related proteins, MLH1 and MLH3, that can partially substitute for the MLH1–PMS1 complex in the repair of mispaired bases recognized by the MSH2–MSH3 complex (Fig. 5). A number of lines of evidence support this view. First, full-length MLH3 interacts with MLH1. This finding is consistent with previous studies demonstrating that MLH3 contains amino acid sequences that are conserved in the yeast PMS1 and human PMS2 proteins in a region that is crucial for the interaction of these proteins with MLH1 (23). Second, mutations in MLH3 cause a small but significant increase in the rate of accumulation of single-base frameshift mutations similar to that caused by mutations in MSH3; epistasis analysis consistent with the idea that these two genes are in the same pathway. Third, mutations in MLH3 significantly increase the rate of accumulation of frameshifts in strains lacking MSH6 consistent with the view that mutations in MLH3 cause defects in MSH3-dependent repair. And fourth, the mlh3 pms1 double mutant consistently had a greater mutation rate in the hom3–10 reversion assay than either single mutant, suggesting that at least for some mutation events, inactivation of both MLH3 and PMS1 is required to produce the greatest inactivation of mismatch repair.
There are several features of our data that should be commented on further. First, the mlh3 msh6 double mutant has at most 15% the mutation rate of a msh2 mutant or a msh3 msh6 double mutant. This finding indicates that only a portion of MSH3-dependent repair requires the MLH1–MLH3 complex. It is unlikely that any MLH3-independent repair involves the other MutL homolog MLH2 because mutations in MLH2 do not cause a mutator phenotype nor do they increase the mutator phenotype caused by mutations in MLH3 (A. Datta, H.F.-R. and R.D.K., unpublished results). Thus, the remainder of the repair most likely involves the MLH1–PMS1 complex. Second, in our analysis of the effects of msh3, msh6, and mlh3 single mutations and the mlh3 msh6 double mutation combination on the accumulation of frameshift mutations at specific frameshift hot spots, we have observed considerable hot spot to hot spot variation (compare the lys2-Bgl spectra presented in Fig. 3). The differences in mutation spectra and hot spot-specific mutation rates seen in msh3 and msh6 mutants in this and other studies most likely reflect differences in the specificity of the MSH2–MSH3 and MSH2–MSH6 complexes for different insertion/deletion mispairs (15, 39). In the case of the mlh3 msh6 double mutation combination, the increase in mutation rate compared with wild type ranged from 4.5- to 86-fold. This suggests that the ability of the MLH1–MLH3 complex to productively interact with a MSH2–MSH3-mispair complex depends on the mispair. Exactly what features of the MSH complex-mispair complex that allows productive interactions with other mismatch repair proteins and how these features vary depending on the mispair are unknown at present. An important implication of these results is that to study the biochemical role of the MLH1–MLH3 and MSH2–MSH3 complexes in mismatch repair, it will be important to identify mispair containing substrates whose repair is dependent on these complexes. It also should be pointed out that the source of the mispair, replication errors in our study, but possibly recombination or DNA-damaging agents in other circumstances, could affect which MSH and MLH complexes are targeted to the mispair to promote repair.
It is interesting to note that when compared with other known MutL homologs, MLH3 is most related to the PMS proteins (Fig. 1) and in particular to human PMS1. The homology between MLH3 and hPMS1 extends throughout the entire predicted amino acid sequences of these two proteins. The greatest region of identity is between residues 1–120 of MLH3 and hPMS1. Importantly, MLH3, hPMS1, and hPMS2 show high conservation of sequences present in the C-terminal region of yeast PMS1, a region which is thought to be crucial for interaction with MLH1 (23). A role for hPMS1 in mismatch repair has been suggested by the finding of a germ-line mutation in this gene in one patient with colon cancer (22). It is tempting to speculate that hPMS1 may carry out a similar role in human mismatch repair as MLH3 does in yeast. If this were the case, mutations in hPMS1 would be expected to cause only a weak mutator phenotype, possibly explaining why so few cases of cancer involving hPMS1 have been reported. This view has been substantiated in experiments with mice containing mutations in PMS1 (41). Furthermore, because hPMS1 would be expected to be partially redundant with hPMS2, this would help explain the reduced number of hereditary nonpolyposis colorectal carcinoma causing germ-line mutations reported in hPMS2 compared with the large number reported in hMLH1 (1, 9).
Acknowledgments
We thank Neelam Amin, Clark Chen, Ruchira Das Gupta, Abhijit Datta, Alex Shoemaker, and Dan Tishkoff for comments on the manuscript and John Weger and Jill Green for DNA sequencing. H. F.-R. is a fellow of The Jane-Coffin Childs Memorial Fund for Medical Research. This work also was supported by National Institutes of Health Grant GM50006 (to R.D.K.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Kolodner R. Genes Dev. 1996;10:1433–1442. doi: 10.1101/gad.10.12.1433. [DOI] [PubMed] [Google Scholar]
- 2.Modrich P, Lahue R. Annu Rev Biochem. 1996;65:101–133. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- 3.Ceccotti S, Aquilina G, Macpherson P, Yamada M, Karran P, Bignami M. Curr Biol. 1996;6:1528–1531. doi: 10.1016/s0960-9822(96)00758-0. [DOI] [PubMed] [Google Scholar]
- 4.Fram R J, Cusick P S, Wilson J M, Marinus G M. Mol Pharmacol. 1985;28:51–55. [PubMed] [Google Scholar]
- 5.Karran P, Marinus M G. Nature (London) 1982;296:868–869. doi: 10.1038/296868a0. [DOI] [PubMed] [Google Scholar]
- 6.Selva E M, Maderazo A B, Lahue R S. Mol Gen Genet. 1997;257:71–82. doi: 10.1007/pl00008619. [DOI] [PubMed] [Google Scholar]
- 7.Rayssiguier C, Thaler D S, Radman M. Nature (London) 1989;342:396–401. doi: 10.1038/342396a0. [DOI] [PubMed] [Google Scholar]
- 8.Datta A, Hendrix M, Lipsitch M, Jinks-Robertson S. Proc Natl Acad Sci USA. 1997;94:9757–9762. doi: 10.1073/pnas.94.18.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peltomaki P, Vasen H F. Gastroenterology. 1997;113:1146–1158. doi: 10.1053/gast.1997.v113.pm9322509. [DOI] [PubMed] [Google Scholar]
- 10.Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Ruschoff J. Cancer Res. 1997;57:4749–4756. [PubMed] [Google Scholar]
- 11.Kane M F, Loda M, Gaida G M, Lipman J, Mishra R, Goldman H, Jessup J M, Kolodner R. Cancer Res. 1997;57:808–811. [PubMed] [Google Scholar]
- 12.Thibodeau S N, French A J, Cunningham J M, Tester D, Burgart L J, Roche P C, McDonnell S K, Schaid D J, Vockley C W, Michels V V, et al. Cancer Res. 1998;58:1713–1718. [PubMed] [Google Scholar]
- 13.Viswanathan M, Lovett S T. Genetics. 1998;149:7–16. doi: 10.1093/genetics/149.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crouse G F. In: DNA Repair in Prokaryotes and Lower Eukaryotes. Nickoloff J A, Hoekstra M F, editors. Vol. 1. Totowa, NJ: Humana; 1998. pp. 411–448. [Google Scholar]
- 15.Marsischky G T, Filosi N, Kane M F, Kolodner R. Genes Dev. 1996;10:407–420. doi: 10.1101/gad.10.4.407. [DOI] [PubMed] [Google Scholar]
- 16.Drummond J T, Li G M, Longley M J, Modrich P. Science. 1995;268:1909–1912. doi: 10.1126/science.7604264. [DOI] [PubMed] [Google Scholar]
- 17.Acharya S, Wilson T, Gradia S, Kane M F, Guerrette S, Marsischky G T, Kolodner R, Fishel R. Proc Natl Acad Sci USA. 1996;93:13629–13634. doi: 10.1073/pnas.93.24.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palombo F, Gallinari P, Iaccarino I, Lettieri T, D’arrigo A, Truong J, Hsuan J, Jiricny J. Science. 1995;268:1912–1914. doi: 10.1126/science.7604265. [DOI] [PubMed] [Google Scholar]
- 19.Prolla T A, Pang Q, Alani E, Kolodner R D, Liskay R M. Science. 1994;265:1091–1093. doi: 10.1126/science.8066446. [DOI] [PubMed] [Google Scholar]
- 20.Prolla T A, Christie D M, Liskay R M. Mol Cell Biol. 1994;14:407–415. doi: 10.1128/mcb.14.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G-M, Modrich P. Proc Natl Acad Sci USA. 1995;92:1950–1954. doi: 10.1073/pnas.92.6.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolaides N C, Papadopoulos N, Liu B, Wei Y, Carter K C, Ruben S M, Rosen C A, Haseltine W A, Fleischmann R D, Fraser C M, et al. Nature (London) 1994;371:75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- 23.Pang Q, Prolla T A, Liskay R M. Mol Cell Biol. 1997;17:4465–4473. doi: 10.1128/mcb.17.8.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alani E, Reenan R A G, Kolodner R. Genetics. 1994;137:19–39. doi: 10.1093/genetics/137.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tishkoff D X, Filosi N, Gaida G M, Kolodner R D. Cell. 1997;88:253–263. doi: 10.1016/s0092-8674(00)81846-2. [DOI] [PubMed] [Google Scholar]
- 26.Manivasakam P, Weber S C, McElver J, Schiestl R H. Nucleic Acids Res. 1995;23:2799–2800. doi: 10.1093/nar/23.14.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothstein R. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- 28.Christianson T W, Sikorski R S, Dante M, Shero J H, Hieter P. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 29.Lea D E, Coulson C A. J Genet. 1948;49:264–268. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman C S, Winston F. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 31.Gyuris J, Golemis E, Chertkov H, Brent R. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 32.Jeyaprakash A, Das Gupta R, Kolodner R. Mol Cell Biol. 1996;16:3008–3011. doi: 10.1128/mcb.16.6.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golemis E A, Brent R. Mol Cell Biol. 1992;12:3006–3014. doi: 10.1128/mcb.12.7.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Estojak J, Brent R, Golemis E A. Mol Cell Biol. 1995;15:5820–5829. doi: 10.1128/mcb.15.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finley R L, Brent R. Proc Natl Acad Sci USA. 1994;91:12980–12984. doi: 10.1073/pnas.91.26.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.New L, Liu K, Crouse G F. Mol Gen Genet. 1993;239:97–108. doi: 10.1007/BF00281607. [DOI] [PubMed] [Google Scholar]
- 37.Sia E A, Kokoska R J, Dominska M, Greenwell P, Petes T D. Mol Cell Biol. 1997;17:2851–2858. doi: 10.1128/mcb.17.5.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mo J Y, Maki H, Sekiguchi M. J Mol Biol. 1991;222:925–936. doi: 10.1016/0022-2836(91)90586-u. [DOI] [PubMed] [Google Scholar]
- 39.Greene C N, Jinks-Robertson S. Mol Cell Biol. 1997;17:2844–2850. doi: 10.1128/mcb.17.5.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strand M, Earley M C, Crouse G F, Petes T D. Proc Natl Acad Sci USA. 1995;92:10418–10421. doi: 10.1073/pnas.92.22.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prolla T A, Baker S M, Harris A C, Tsao J L, Yao X, Bronner C E, Zheng B, Gordon M, Reneker J, Arnheim N, et al. Nat Genet. 1998;18:276–279. doi: 10.1038/ng0398-276. [DOI] [PubMed] [Google Scholar]