Abstract
The cytokine interleukin (IL) 18 (formerly interferon γ-inducing factor) induces the T helper type 1 response. In the present studies, IL-18 increased HIV type 1 (HIV-1) production from 5- to 30-fold in the chronically infected U1 monocytic cell line. Inhibition of tumor necrosis factor (TNF) activity by the addition of TNF-binding protein reduced IL-18-stimulated HIV-1 production by 48%. In the same cultures, IL-18-induced IL-8 was inhibited by 96%. Also, a neutralizing anti-IL-6 mAb reduced IL-18-induced HIV-1 by 63%. Stimulation of U1 cells with IL-18 resulted in increased production of IL-6, and exogenous IL-6 added to U1 cells increased HIV-1 production 4-fold over control. A specific inhibitor of the p38 mitogen-activated protein kinase reduced IL-18-induced HIV-1 by 73%, and a 50% inhibition was observed at 0.05 μM. In the same cultures, IL-8 was inhibited by 87%. By gel-shift and supershift analyses, increased binding activity of the transcription factor NF-κB was measured in nuclear extracts from U1 cells 1 h after exposure to IL-18. These results demonstrate induction of HIV-1 by IL-18 in a monocyte target associated with an intermediate role for TNF and IL-6, activation of p38 mitogen-activated protein kinase, and nuclear translocation of NF-κB.
Keywords: T cell helper-1 response/p38 mitogen-activated protein kinase/tumor necrosis factor/interferon γ
Host cytokines play an important role in HIV type 1 (HIV-1) infection by promoting a long-lasting T helper type 1 (Th1) lymphocyte response to viral antigens. A vigorous Th1 response against HIV-1 results in the generation of HIV-1-targeted cytotoxic T lymphocytes (1–4). Cytokines such as interleukin (IL) 2 and IL-12 and interferon γ (IFN-γ) are important to the development of cytotoxic T lymphocytes, and the administration of Th1 cytokines has been considered a therapeutic strategy in HIV-1 disease. IL-18 is a recently described cytokine that belongs to the family of Th1-associated cytokines. IL-18 was first identified as an IFN-γ-inducing factor (5–7). Although IL-12 is also an IFN-γ-inducing factor, mice deficient in IL-18 have markedly reduced IFN-γ production in response to endotoxin in the presence of IL-12 (8). Natural killer (NK) cells from IL-18-deficient mice exhibit an impaired cytotoxic T lymphocyte response in vitro, despite normal production of IL-12 (8). Thus, the role for IL-12 in IFN-γ production appears to be permissive for IL-18 activity (9, 10).
Advances in the molecular pathogenesis of HIV-1 infection have underscored the ability of host proinflammatory cytokines to induce virus expression (11, 12) and of host chemokines to suppress viral entry (13–15). In vitro experiments in the monocytic U1 cell line or in freshly obtained human peripheral blood mononuclear cells have demonstrated increased HIV-1 production after stimulation with proinflammatory cytokines, such as IL-1 and tumor necrosis factor α (TNF), or after exposure to IL-6 (16–20). A mechanism for the increased expression of HIV-1 because of stimulation with proinflammatory cytokines is via activation of NF-κB and subsequent binding to specific recognition domains in the long terminal repeat promoter region of HIV-1 (21, 22).
To date, T lymphocytes and NK cells appear to be primary targets for IL-18 (reviewed in ref. 23). For example, IL-18 directly stimulates production of TNF in human blood CD4+ and NK cells (24). It is likely that these biological effects of IL-18 are mediated, in part, through postreceptor activation of NF-κB (25). At least one component of the IL-18 receptor complex has been identified. A putative IL-18 receptor was purified by using a neutralizing mAb produced to cell surface proteins, and the N-terminal amino acid sequence matched that of the IL-1 receptor-related protein (IL-1Rrp) (26). The IL-1Rrp is an orphan receptor previously identified as a member of the IL-1 receptor family (27). IL-1Rrp appears to function as a signaling chain in the putative IL-18 receptor complex. After exposure to IL-18, IL-1Rrp recruits and activates the IL-1 receptor-associated kinase (10, 28). Engagement of IL-18 with the cell results in physical association of IL-1 receptor-associated kinase with TNF receptor-associated factor 6 (29), as well as activation of p56lck and p42 mitogen-activated protein kinase (MAPK) (30). In the present studies, we examined the activity of IL-18 on HIV-1 expression in the U1 monocytic cell line.
MATERIALS AND METHODS
Materials.
Recombinant human 24-kDa precursor IL-18 was expressed as a histidine-tagged protein in Escherichia coli. Precursor IL-18 was purified by using Ni-affinity chromatography processed to the mature 18-kDa form after cleavage with recombinant IL-1β-converting enzyme (31). The p38 MAPK inhibitor SB 203580 was purchased from Calbiochem and was dissolved in dimethyl sulfoxide at a stock concentration of 40 mM. The TNF-binding protein (TNFbp) was a construct of two recombinant TNF receptor extracellular p55 chains linked by polyethylene glycol and was a kind gift from Carl K. Edwards (Amgen, Boulder, CO). RPMI medium 1640 tissue culture medium was purchased from Mediatech (Herndon, VA) and contained 2.5 mM l-glutamine, 25 mM Hepes, 100 units/ml penicillin, and 100 μg/ml streptomycin (GIBCO/BRL) with 10% (vol/vol) heat-inactivated fetal bovine serum (GIBCO). The monoclonal anti-IL-6 antibody used in these studies was previously described (32). Recombinant human IL-6 was purchased from R&D Systems. Anti-p50 NF-κB antibody was purchased from Santa Cruz Biotechnology.
U1 Cell Cultures.
U1 cells were obtained from the AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Cells were grown in T-75 polystyrene culture flasks (Corning) suspended in the medium described above. When cell density achieved approximately 1 × 106 per ml, cells were resuspended in fresh medium at 2 × 106 per ml. Cells were examined for viability by trypan blue exclusion (>95% for all experiments). A cell suspension (500 μl) was added to either 12- × 75-mm polypropylene tubes (Falcon) or 24-well polystyrene plates (Falcon). An additional 500 μl of medium alone or reagents diluted in medium was added to each culture. TNFbp, anti-IL-6, or the p38 MAPK inhibitor SB 203580 was mixed with cells 1 h before the addition of IL-18. After 24 or 48 h of incubation (37°C, 5% CO2), cultures were frozen (−70°C) and thawed (37°C) for three cycles before assay for HIV p24 antigen, IL-6, IL-8, or TNF.
For experiments in which IL-18-induced IL-6 was evaluated, U1 cells were resuspended in fresh medium at 2 × 106 per ml. A cell suspension (100 μl) was added to wells of 96-well microtiter flat-bottomed plates. An additional 100 μl of medium or IL-18 diluted in medium was added to each well. Cultures were incubated for 48 h and then were frozen and thawed for three cycles before being assayed as described above.
NF-κB Gel-Shift Assay.
Ten million U1 cells suspended in fresh medium were cultured in 50-ml polypropylene tubes at a density of 5 × 106 per ml. After 1.0 h of incubation with IL-18 at 37°C, cells were centrifuged at 4°C for 5 min at 350 × g. The cell pellet was resuspended in 300 μl of lysis buffer containing 10 mM KCl, 0.1 mM EGTA, 10 mM Hepes at pH 7.9, 1.0 mM DTT, and 4% (vol/vol) complete protease inhibitor mixture (Boehringer Mannheim). Samples were then transferred to 1.5-ml microcentrifuge tubes and incubated for 15 min on ice, after which 20 μl of 10% Nonidet P-40 was added, and each sample was subjected to mechanical disruption by 15 passages through a 25-gauge needle. Lysates were centrifuged at 600 × g for 5 min at 4°C. The nuclear pellets were then resuspended in 40 μl of nuclear extract buffer [0.4 M NaCl/0.1 M EGTA/20 mM Hepes, pH 7.9/1.0 mM DTT/4% (vol/vol) full protease inhibitor cocktail] and incubated for 15 min on ice. The samples were then microfuged at 4°C (12,400 × g), and the supernatants containing the nuclear protein extracts were collected. An aliquot of each nuclear extract was quantified by Coomassie Plus protein assay (Pierce).
A double-stranded oligonucleotide probe comprising an NF-κB-binding region of the mouse B cell κ-light chain enhancer (5′-AGT TGA GGG GAC TTT CCC AGG C-3′; Promega) was end labeled with [γ-32P]ATP following the manufacturer’s recommended protocol. The labeled probe was purified by using a Nuctrap column (Stratagene), and the activity of the purified probe was determined on a Beckman LS 6500 scintillation counter. A probe (200,000 cpm; approximately 1.0 μl) was combined with 3.0 μg of nuclear protein extract, poly-dI/dC (0.01 μg per 1.0 μg of nuclear protein), and electromobility shift assay buffer (50 mM NaCl/0.5 mM EDTA/10 mM Tris⋅HCl, pH 7.5/1.0 mM MgCl2/4% glycerol/0.5 mM DTT) to a final volume of 25 μl. For supershift or competition experiments, parallel conditions containing anti-p50 antibody (5.0 μl) or nonradiolabeled probe (100-fold molar excess) were performed, respectively. The reactions were incubated for 30 min at room temperature and 20 μl of each reaction was loaded on a 5% acrylamide, 0.5× TBE (45 mM Tris/44 mM boric acid/11 mM EDTA, pH 8.3), and 2.5% glycerol gel and run at 10 V/cm. The gel was removed after the probe had run three-quarters of the length of the gel, dried on Whatman paper, and exposed to x-ray film overnight at −70°C.
Immunoassays.
An assay for HIV p24 antigen was performed by using an ELISA kit (Immunotech, Westbrook, ME). IL-8 and TNF were measured by using the Origen analyzer (IGEN International, Inc., Gaithersburg, MD) for a liquid-phase electrochemiluminescence assay with a lower limit of detection of 40 pg/ml as described (24, 33). IL-6 was quantified by an ELISA kit (Endogen, Woburn, MA).
Statistical Analysis.
Data are presented as means ± SEM. Group means were compared by ANOVA by using Fisher’s least significant difference.
RESULTS
Mature but Not Pro-IL-18 Stimulates Production of HIV-1 in U1 Cell Cultures.
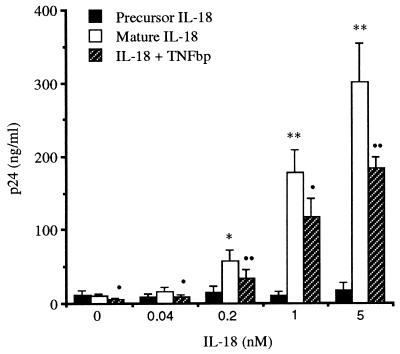
Mice deficient in IL-1β-converting enzyme (caspase-1) have markedly reduced IFN-γ production (34, 35) because the leaderless precursor of IL-18 requires processing by IL-1β-converting enzyme to an active, mature form. As shown in Fig. 1, mature IL-18 (0.04–5.0 nM) stimulated the production p24 antigen from U1 cells cultured in polystyrene plates after 48 h of incubation. In five separate experiments, control cultures (incubated with medium alone) contained 10.2 ± 2.8 ng/ml of p24 antigen. As shown, significant increases in p24 antigen production were observed with 0.2, 1.0, and 5.0 nM IL-18 (57.8 ± 14.2, 177.3 ± 2.2, and 300.4 ± 54 ng/ml, respectively). In parallel cultures with the same split of U1 cells, the precursor of IL-18 did not significantly induce p24 antigen at the concentrations depicted in Fig. 1.
Figure 1.
Mature, but not precursor, form of IL-18 stimulates p24 antigen production in U1 cells: effect of TNFbp. In five separate experiments, U1 cells were incubated in medium alone (control) or with increasing concentrations of precursor (solid bars) or mature IL-18 (open bars) as indicated. After 48 h, p24 antigen was measured. ∗, P < 0.05 compared with medium alone; ∗∗, P < 0.001 compared with medium alone. Cells were also cultured with mature IL-18 in the presence of 10 μg/ml TNFbp added 1 h before IL-18 (hatched bars). •, P < 0.05 compared with IL-18 alone; ••, P < 0.001 compared with IL-18 alone.
IL-18-Induced HIV-1 and IL-8 Depend on TNF Activity.
IL-18 induces production of TNF in human peripheral blood mononuclear cells in vitro (24), and it is well established that TNF stimulates the production of HIV-1 (11, 12, 16, 21). To assess an intermediate production of TNF in IL-18-induced HIV-1, U1 cells were also cultured for 48 h in polystyrene plates in the presence of 10 μg/ml TNFbp. TNFbp is the extracellular domain of the p55 TNF receptor that neutralizes TNF activity. As shown in Fig. 1, TNFbp significantly decreased IL-18-induced HIV-1 production. In control (unstimulated) cultures, spontaneous HIV-1 production was reduced by 47% in the presence of TNFbp. For IL-18 added at 0.04, 0.2, 1.0 and 5.0 nM, the presence of TNFbp inhibited HIV-1 production by 48, 40, 32, and 39%, respectively. However, we were unable to detect TNF in these IL-18-stimulated cultures, despite the use of a TNF electrochemiluminescence assay with a lower limit of detection of 40 pg/ml.
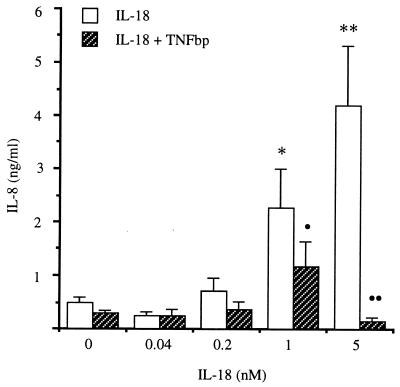
In the same cultures, IL-8 was measured and the data are presented in Fig. 2. Control cultures (incubated with medium alone) contained 0.5 ± 0.04 ng/ml IL-8. Increased IL-8 production was seen with 0.2, 1.0, and 5.0 nM IL-18 (0.7 ± 0.3, 2.3 ± 0.7, and 4.2 ± 1.1 ng/ml, respectively). In the presence of TNFbp, a significant inhibitory effect on IL-18-induced IL-8 was observed with 1.0 and 5.0 nM IL-18 (50 and 96% inhibition, respectively).
Figure 2.
Effect of TNFbp on IL-18-induced IL-8. In the same cultures shown in Fig. 1, cells were incubated with mature IL-18 at the concentrations shown (open bars) in the presence or absence of 10 μg/ml TNFbp added 1 h before IL-18 (hatched bars). IL-8 was measured as described in Materials and Methods. ∗, P < 0.05 compared with medium alone; ∗∗, P < 0.001 compared with medium alone; •, P < 0.05 compared with IL-18 alone; ••, P < 0.001 compared with IL-18 alone.
IL-18-Induced HIV-1 Depends on IL-6 Induction.
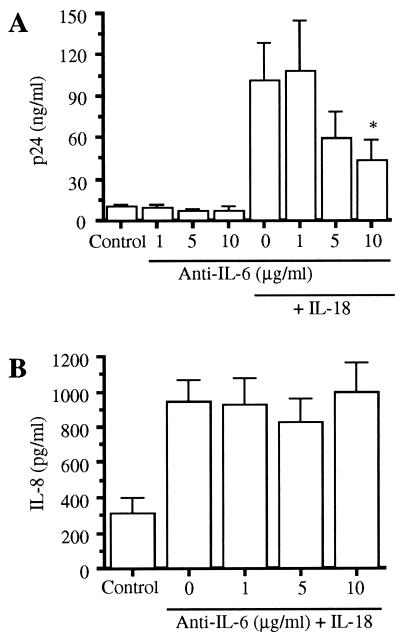
IL-6 has been shown to stimulate HIV-1 expression (18). We used a specific neutralizing mAb directed against IL-6 to assess the role of IL-6 in IL-18-induced HIV-1 production in 48-h cultures conducted in polystyrene plates. As shown in Fig. 3A, the addition of 1, 5, or 10 μg/ml anti-IL-6 alone to U1 cell cultures had no significant effect on unstimulated (basal) p24 antigen production. The addition of 1.0 nM IL-18 significantly increased p24 antigen production, from 9.7 ± 1.4 (control) to 101 ± 28 ng/ml, a 10.4-fold increase (P < 0.001). Preincubation of U1 cells with anti-IL-6 antibody for 1 h significantly reduced IL-18-induced p24 antigen when added at 10 μg/ml. Compared with stimulation with IL-18 alone, the percentage reduction observed in the presence of anti-IL-6 antibody at 5 and 10 μg/ml was 45 and 63%, respectively. Fig. 3B depicts the effect of anti-IL-6 on production of IL-8 in the same cultures. Control U1 cells (medium alone) produced 311 ± 92 pg/ml IL-8, whereas IL-18-stimulated cultures (1.0 nM) contained 944 ± 124 pg/ml IL-8 (P < 0.001). However, in contrast to IL-18-induced p24 antigen, anti-IL-6 did not reduce IL-18-induced IL-8 synthesis. This observation is consistent with those from other studies that indicate that cytokines that activate the gp130-signaling apparatus inhibit, rather than augment, IL-8 synthesis (36, 37).
Figure 3.
Effect of neutralizing anti-IL-6 antibody on IL-18-induced p24 antigen in U1 cells. (A) In three separate experiments, cells were incubated with medium alone (control), with 1.0 nM IL-18, or with IL-18 in the presence of anti-IL-6 mAb (1–10 μg/ml) added 1 h before IL-18. p24 antigen was measured after 48 h of incubation. (B) In the same cultures shown in A, IL-8 was measured. No significant effect of anti-IL-6 (1–10 μg/ml) was observed for IL-18-induction of IL-8. ∗, P < 0.05 compared with cells stimulated with IL-18 in the absence of anti-IL-6 mAb.
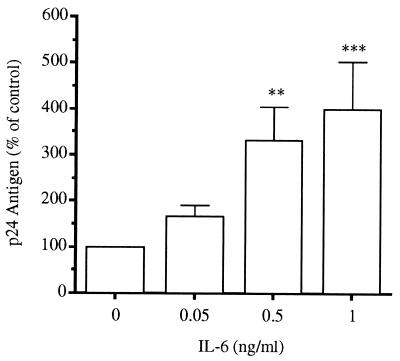
Consistent with the effect of anti-IL-6 on IL-18-induced HIV-1, the addition of exogenous IL-6 increased p24 antigen production (Fig. 4). The addition of 0.05, 0.5, or 1 ng/ml IL-6 resulted in p24 antigen increases of 166, 332, and 399% compared with the control, respectively. IL-6 levels in U1 cultures stimulated with IL-18 were also measured. In three separate experiments, U1 cells cultured in medium alone (control) produced no measurable IL-6 (limit of detection, 4.0 pg/ml). At IL-18 concentrations of 1.0 or 5.0 nM, IL-6 levels of 21.6 ± 10.6 and 35.1 ± 14.3 pg/ml were induced, respectively (P < 0.05, n = 3).
Figure 4.
Stimulation of p24 antigen by IL-6. In six separate experiments, U1 cells were stimulated with recombinant human IL-6 at the concentrations indicated. After 48 h, p24 antigen was assayed in the cultures. The baseline (medium alone) value for each experiment was set at 100% and the mean percentage increase ± SEM is shown for the different concentrations of IL-6. ∗∗, P < 0.01; ∗∗∗, P < 0.001 compared with unstimulated cultures.
IL-18-Induced HIV-1 and IL-8 Depend on p38 MAPK Activity.
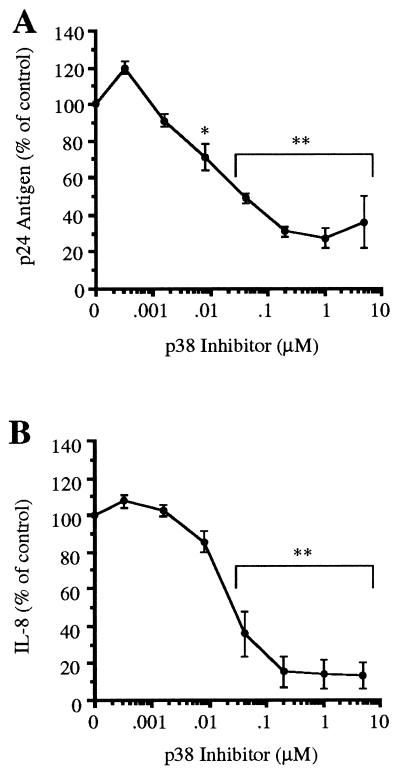
In IL-1-stimulated U1 cells and human peripheral blood mononuclear cells freshly infected with HIV-1, a specific inhibitor of p38 MAPK suppresses HIV-1 production (38). The pyridinyl imidazole compound SB 203580 specifically binds to and inactivates p38 MAPK (39, 40); therefore, U1 cells were preincubated with the p38 MAPK inhibitor (0.00032–5.0 μM) for 1 h and then stimulated with IL-18. Cultures were conducted in polypropylene tubes for 24 h. As shown in Fig. 5A, significant inhibition of p24 antigen production was observed, starting at an inhibitor concentration of 0.008 μM (29% reduction, P < 0.01). The maximum effect was observed at a concentration of 1.0 μM (73% reduction, P < 0.001). In the same cultures (Fig. 5B), the effect of p38 MAPK inhibition on IL-8 production was determined. At a p38 MAPK inhibitor concentration of 0.04 μM, there was a reduction of 65% in IL-18-induced IL-8. At 1.0 μM, IL-8 synthesis was reduced by 86% (P < 0.001), whereas maximum inhibition was seen at 5.0 μM (87%).
Figure 5.
Effect of p38 MAPK inhibitor on IL-18-induced p24 antigen and IL-8 in U1 cells. (A) Cells were cultured in polypropylene tubes with IL-18 (1.0 nM) alone or in the presence of the p38 MAPK inhibitor at the concentrations indicated. Production of p24 antigen was measured after 24 h. For each experiment, the baseline (unstimulated) level was subtracted from each condition, and the level of stimulation with IL-18 was set at 100%. The results are expressed as percentage change (±SEM) compared with IL-18 only. (B) In the same cultures shown in A, IL-8 was measured as described in Materials and Methods. ∗, P < 0.01 compared with IL-18 alone (100%); ∗∗, P < 0.001 compared with IL-18 alone (100%). n = 3 separate experiments.
Translocation of NF-κB.
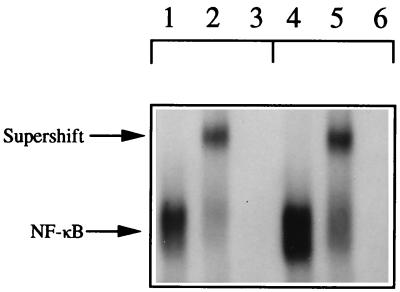
The HIV-1 long terminal repeat contains two consensus binding regions for NF-κB, and cytokines capable of activating NF-κB are associated with HIV-1 expression in the U1 cell. As shown in Fig. 6, U1 cells were exposed to 5 nM IL-18 for 1 h, and nuclear extracts were assessed for NF-κB-binding activity. Lane 1 depicts binding of the labeled probe to nuclear extracts from unstimulated U1 cells, whereas the nuclear extract from U1 cells exposed to IL-18 showed a marked increase in NF-κB binding (lane 4). Coincubation with an antibody against the p50 subunit of NF-κB confirmed the IL-18-stimulated increase in binding resulted from NF-κB activity in the nuclear extracts of control and IL-18-stimulated U1 cells (supershift, lanes 2 and 5, respectively). Added excess unlabeled probe resulted in loss of binding of the nuclear extracts to confirm the specificity of the supershifted band (lanes 3 and 6, respectively).
Figure 6.
Nuclear translocation of NF-κB in IL-18-stimulated U1 cells. U1 cells (5.0 × 106 per ml) were cultured in medium for 1 h in the absence (control, lanes 1–3) or presence (5.0 nM, lanes 4–6) of IL-18. Nuclear proteins were isolated and hybridized to an oligonucleotide probe containing the NF-κB-binding region as described in Materials and Methods (the unbound probe is not shown). Lanes: 1 and 4, gel shift for control cells and cells exposed to IL-18, respectively; 2 and 5, a supershift by using anti-p50 subunit antibody for control and stimulated cells, respectively; 3 and 6, incubation of nuclear proteins with radiolabeled probe in the presence of excess unlabeled probe.
DISCUSSION
These studies demonstrate that the Th1-associated cytokine IL-18 stimulates the monocytic U1 cell to express HIV-1 as measured by p24 antigen production. This was an unexpected finding because target cells for IL-18 have been primarily T lymphocytes and NK cells. The myelomonocytic leukemia cell line, KG-1, responds to IL-18 with production of IFN-γ (41). Primary murine macrophages also synthesize IFN-γ in response to a combined stimulation with IL-18 and IL-12 (42). These reports are consistent with the present results establishing activity of IL-18 for monocytic cells. However, unlike the requirement for costimualtion in murine macrophages, IL-18 is a direct stimulant for HIV-1 production. The parent of the U1 cell line is the U937 cell line, which was derived from a patient with histiocytic lymphoma. It expresses several characteristics of human monocytes/macrophages and is a rich source of proinflammatory cytokines such as IL-1, TNF, and IL-8. The IL-18-induced stimulation of HIV-1 was as high as 30-fold over unstimulated cells. In contrast, the unprocessed precursor form of IL-18 had no detectable activity in these cells (Fig. 1). Several reports have noted that costimulation with IL-12, mitogens, or microorganisms was required to observe biological activity of IL-18. Here, we demonstrate that costimulation was not necessary for IL-18-induced HIV-1 production. When added to freshly infected human peripheral blood mononuclear cells (38), IL-18 also enhanced HIV-1 production after 4 days of culture (data not shown).
The proinflammatory cytokine TNF increases HIV-1 production in U1 cells (16, 17), and IL-18 alone induces TNF in CD4+ and in NK cells from human peripheral blood mononuclear cells (24). To investigate a possible intermediate role for TNF in IL-18-induced HIV-1, TNF activity was neutralized by using excess TNFbp. Under these conditions, IL-18-induced p24 antigen production was reduced by approximately 50% for each concentration of IL-18 (Fig. 1). However, in these cultures we could not detect soluble TNF by electrochemiluminescence assay (detection limit, 40 pg/ml). Therefore, IL-18-induced TNF activity in U1 cells may be caused by cell-surface-associated TNF. Increased bioactive cell-surface TNF expression in IL-10-stimulated U1 cells has been documented by flow cytometry (43).
Although not a classic proinflammatory cytokine, IL-6 stimulates HIV-1 production in blood monocyte-derived macrophages and in U1 cells (18, 44). We observed that inhibition of IL-6 biological activity with a neutralizing anti-IL-6 mAb also reduced the amount of IL-18-induced HIV-1 as measured by p24 antigen production (Fig. 3A). We also observed an increase in HIV-1 production in U1 cells exposed to exogenous IL-6 at a concentration as low as 50 pg/ml (Fig. 4). Moreover, U1 cells produced significant amounts of IL-6 in response to stimulation with IL-18. A 5-fold increase in IL-6 production with 1.0 nM IL-18 compared with cells in medium alone was observed. Thus, from three different experimental approaches, the findings from these studies support the concept that there is a significant role for intermediate production of IL-6 in IL-18-stimulated HIV-1 production by the U1 cell. Because IL-6 does not induce TNF (45, 46) but TNF induces IL-6, the cascade may be IL-18-induced TNF, followed by TNF-induced IL-6. There may be a role for additional IL-18-induced cytokines in the control of HIV-1 expression in U1 cells because excess TNFbp or anti-IL-6 did not result in complete HIV-1 suppression (50–60%).
Activation of the p38 MAPK is associated with HIV-1 production in U1 cells and primary infected human T cells (38, 47). Inhibition of p38 MAPK activity by a p38 antisense or a pharmacological p38 inhibitor significantly blocked HIV-1 production in these studies. Other investigators confirmed that activation of an HIV-1 long terminal repeat reporter construct required activation of p38 MAPK in transfected HeLa cells (48). Preincubation of IL-18-stimulated U1 cell cultures with a specific p38 inhibitor (the pyridinyl imidazole SB 203580) resulted in a dose-dependent and significant decrease in HIV-1 induction in 24-h cultures. Compared with cultures stimulated with IL-18 (1.0 nM) in the absence of inhibitor, addition of 1.0 μM inhibitor decreased HIV-1 production by 73% (Fig. 5A). These data indicate a requirement for p38 MAPK activation in signaling events leading from IL-18 receptor activation to HIV-1 production in the U1 cell model.
We also measured synthesis of the α-chemokine IL-8 in the same experimental conditions used to study HIV-1 production. Like HIV-1 p24 antigen, the levels of IL-8 induced by IL-18 were significantly inhibited by the presence of TNFbp, indicating a role for TNF in the induction of IL-8 by IL-18 (Fig. 2). Also, IL-18-induced IL-8 required activation of the p38 MAPK (Fig. 5B). These data are similar to those obtained for IL-1-induced HIV-1 and IL-8 in the presence of p38 inhibition, as previously reported (38). However, as shown in Fig. 3B, neutralization of IL-6 bioactivity with a specific mAb had no significant effect on IL-18-induced IL-8. These results contrast with the significant reduction in IL-18-induced HIV-1 observed in the same cultures (Fig. 3A). Specific induction of HIV-1 by IL-6 suggests different postreceptor events involved in production of HIV-1 compared with IL-8 after stimulation with IL-18. However, like HIV-1, IL-8 transcription is highly sensitive to activation by NF-κB.
TNF-induced HIV-1 production occurs through activation of NF-κB, which in turn binds to the long terminal repeat region of the HIV-1 genome and initiates transcription (21, 22). In murine T cell clones, IL-18 activates NF-κB (25). As shown in the present studies, IL-18 induces NF-κB translocation to the nucleus in the U1 cell line. Therefore, receptor-mediated events characterized on T cells in response to IL-18 appear to also occur in macrophages. At least one component of the IL-18 receptor complex has been characterized (26). Monoclonal antibody affinity chromatography was used to obtain a purified IL-18 receptor, of which the N-terminal amino acid sequence matched that of the IL-1Rrp. The IL-1Rrp is a previously described orphan receptor (27). However, the relatively low affinity of IL-1Rrp for IL-18 (25–45 nM) and the demonstration of a high and a low dissociation constant (9) implies the existence of one or more additional component(s) of the IL-18 receptor. Signaling events after engagement of IL-18 with the IL-1Rrp involve complexing of IL-1Rrp with IL-1 receptor-associated kinase (10). Activation of IL-1 receptor-associated kinase by IL-18 leads to phosphorylation of TNF receptor-associated factor 6 (reviewed in ref. 29); hence, it was not surprising that IL-18 stimulation resulted in translocation of NF-κB to the nucleus in U1 cells.
These studies suggest a role for IL-18 in HIV-1 pathogenesis because of the ability IL-18 shares with the proinflammatory cytokines IL-1 and TNF to stimulate HIV-1 production in vitro. For full biological activity, IL-18 requires cleavage of the precursor form by IL-1β-converting enzyme, which may represent a target for therapeutic intervention in HIV-1 infection. On the other hand, reducing the biological activity of IL-18 may suppress an efficient Th1 response and reduce cytotoxic T lymphocytes targeted to HIV-1-infected cells.
Acknowledgments
We thank Drs. Edward Abraham and Brian Shames for advice concerning gel-shift assays. We are also grateful to Dr. Carl K. Edwards for providing TNFbp, Endogen for IL-6 ELISA kits, and IGEN International for providing the Origen analyzer. These studies were supported by National Institutes of Health Grant AI 15614 (to C.A.D.).
ABBREVIATIONS
- HIV-1
HIV type 1
- IL
interleukin
- IL-1Rrp
IL-1 receptor-related protein
- IFN
interferon
- NK
natural killer
- MAPK
mitogen-activated protein kinase
- Th1
T helper type 1
- TNF
tumor necrosis factor α
- TNFbp
TNF-binding protein
References
- 1. Walker, B. D. (1994) J. Acquired Immune Defic. Syndr. 7, Suppl. 1, S6–S13. [PubMed]
- 2.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 3.Jassoy C, Harrer T, Rosenthal T, Navia B A, Worth J, Johnson R P, Walker B D. J Virol. 1993;67:2844–2852. doi: 10.1128/jvi.67.5.2844-2852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrer T, Harrer E, Kalams S A, Elbeik T, Staprans S I, Feinberg M B, Cao Y, Ho D D, Yilma T, Caliendo A M, et al. AIDS Res Hum Retroviruses. 1996;12:585–592. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura K, Okamura H, Wada M, Nagata K, Tamura T. Infect Immun. 1989;57:590–595. doi: 10.1128/iai.57.2.590-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura K, Okamura H, Nagata K, Komatsu T, Tamura T. Infect Immun. 1993;61:64–70. doi: 10.1128/iai.61.1.64-70.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Toridoe K, Okura T, Nukada Y, K, H, Akita K, et al. Nature (London) 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto K, Okamura H, Nakanishi K, Akira S. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi K, Yoshimoto T, Ohkushu K, Takeda K, Kashiwamura S-I, Torigoe K, Kurimoto M, Akira S, Okamura H. Cytokine. 1997;9:947. (abstr.). [Google Scholar]
- 10.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley S B, Menon S, Kastelein R, Bazan F, et al. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 11.Fauci A S. Nature (London) 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 12.Poli G, Kinter A L, Vicenzi E, Fauci A S. Res Immunol. 1994;145:578–582. doi: 10.1016/s0923-2494(05)80036-7. [DOI] [PubMed] [Google Scholar]
- 13.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P, E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 14.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 16.Folks T M, Clouse K A, Justement J, Rabson A, Duh E, Kehrl J H, Fauci A S. Proc Natl Acad Sci USA. 1989;86:2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard O M Z, Clouse K A, Smith C, Goodwin R G, Farrer W L. Proc Natl Acad Sci USA. 1993;90:2335–2339. doi: 10.1073/pnas.90.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poli G, Bressler P, Kinter A, Duh E, Timmer W C, Rabson A, Justement J S, Stanley S, Fauci A S. J Exp Med. 1990;172:151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poli G, Kinter A L, Fauci A S. Proc Natl Acad Sci USA. 1994;91:108–112. doi: 10.1073/pnas.91.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granowitz E V, Saget B M, Wang M Z, Dinarello C A, Skolnik P R. Mol Med. 1995;1:667–677. [PMC free article] [PubMed] [Google Scholar]
- 21.Duh E J, Maury W J, Folks T M, Fauci A S, Rabson A B. Proc Natl Acad Sci USA. 1989;86:5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborn L, Kunkel S, Nabel G J. Proc Natl Acad Sci USA. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohno K, Kurimoto M. Clin Immunol Immunopathol. 1998;86:11–15. doi: 10.1006/clin.1997.4475. [DOI] [PubMed] [Google Scholar]
- 24.Puren A J, Fantuzzi G, Gu Y, Su M S-S, Dinarello C A. J Clin Invest. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto S, Tsuji-Takayama K, Aizawa Y, Koide K, Takeuchi M, Ohta T, Kurimoto M. Biochem Biophys Res Commun. 1997;234:454–457. doi: 10.1006/bbrc.1997.6665. [DOI] [PubMed] [Google Scholar]
- 26.Torigoe K, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikate T, Murakami T, Sanou O, Kojima H, Fuji M, et al. J Biol Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- 27.Parnet P, Garka K E, Bonnert T P, Dower S K, Sims J E. J Biol Chem. 1996;271:3967–3970. doi: 10.1074/jbc.271.8.3967. [DOI] [PubMed] [Google Scholar]
- 28.Croston G E, Cao Z, Goeddel D V. J Biol Chem. 1995;270:16514–16517. doi: 10.1074/jbc.270.28.16514. [DOI] [PubMed] [Google Scholar]
- 29.Kojima H, Takeuchi M, Ohta T, Nishida Y, Arai N, Ikeda M, Ikegami H, Kurimoto M. Biochem Biophys Res Commun. 1998;244:183–186. doi: 10.1006/bbrc.1998.8236. [DOI] [PubMed] [Google Scholar]
- 30.Tsuji-Takayama K, Matsumoto S, Koide K, Takeuchi M, Ikedo M, Ohta T, Kurimoto M. Biochem Biophys Res Commun. 1997;237:126–130. doi: 10.1006/bbrc.1997.7099. [DOI] [PubMed] [Google Scholar]
- 31.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 32.Novick D, Eshhar Z, Revel M, Mory Y. Hybridoma. 1989;8:561–567. doi: 10.1089/hyb.1989.8.561. [DOI] [PubMed] [Google Scholar]
- 33.Deaver D R. Nature (London) 1995;377:758–760. doi: 10.1038/377758a0. [DOI] [PubMed] [Google Scholar]
- 34.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et al. Nature (London) 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 35.Fantuzzi G, Puren A J, Harding M W, Livingston D J, Dinarello C A. Blood. 1998;91:2118–2125. [PubMed] [Google Scholar]
- 36.Shapiro L, Panayotatos N, Meydani S N, Wu D, Dinarello C A. Exp Cell Res. 1994;215:51–56. doi: 10.1006/excr.1994.1313. [DOI] [PubMed] [Google Scholar]
- 37.Richards C D, Langdon C, Botelho F, Brown T J, Agro A. J Immunol. 1996;156:343–349. [PubMed] [Google Scholar]
- 38.Shapiro L, Heidenreich K A, Meintzer M K, Dinarello C A. Proc Natl Acad Sci USA. 1998;95:7422–7426. doi: 10.1073/pnas.95.13.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M-J, Heys J R, Landvatter S W, et al. Nature (London) 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 40.Lee J C, Laydon J T, White J R. Agents Actions. 1994;41:C191–C192. [Google Scholar]
- 41.Ohtsuki T, Micallef M J, Kohno K, Tanimoto T, Ikeda M, Kurimoto M. Anticancer Res. 1997;17:3253–3258. [PubMed] [Google Scholar]
- 42.Munder M, Mallo M, Eichmann K, Modolell M. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barcellini W, Rizzardi G P, Marriott J B, Fain C, Shattock R J, Meroni P L, Poli G, Dalgleish A G. AIDS. 1996;10:835–842. doi: 10.1097/00002030-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Kishimoto T. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 45.Aderka D, Le J M, Vilcek J. J Immunol. 1989;143:3517–3523. [PubMed] [Google Scholar]
- 46.Schindler R, Mancilla J, Endres S, Ghorbani R, Clark S C, Dinarello C A. Blood. 1990;75:40–47. [PubMed] [Google Scholar]
- 47.Cohen P S, Schmidtmayerova H, Dennis J, Dubrovsky L, Sherry B, Wang H, Bukrinsky M, Tracey K J. Mol Med. 1997;5:339–346. [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar S, Orsin M J, Lee J C, McDonnell P C, Debouck C, Young P R. J Biol Chem. 1996;271:30864–30869. doi: 10.1074/jbc.271.48.30864. [DOI] [PubMed] [Google Scholar]