Abstract
PR-1a is a salicylic acid-inducible defense gene of tobacco (Nicotiana tabacum). One-hybrid screens identified a novel tobacco WRKY transcription factor (NtWRKY12) with specific binding sites in the PR-1a promoter at positions −564 (box WK1) and −859 (box WK2). NtWRKY12 belongs to the class of transcription factors in which the WRKY sequence is followed by a GKK rather than a GQK sequence. The binding sequence of NtWRKY12 (WK box TTTTCCAC) deviated significantly from the consensus sequence (W box TTGAC[C/T]) shown to be recognized by WRKY factors with the GQK sequence. Mutation of the GKK sequence in NtWRKY12 into GQK or GEK abolished binding to the WK box. The WK1 box is in close proximity to binding sites in the PR-1a promoter for transcription factors TGA1a (as-1 box) and Myb1 (MBSII box). Expression studies with PR-1a promoter∷β-glucuronidase (GUS) genes in stably and transiently transformed tobacco indicated that NtWRKY12 and TGA1a act synergistically in PR-1a expression induced by salicylic acid and bacterial elicitors. Cotransfection of Arabidopsis thaliana protoplasts with 35S∷NtWRKY12 and PR-1a∷GUS promoter fusions showed that overexpression of NtWRKY12 resulted in a strong increase in GUS expression, which required functional WK boxes in the PR-1a promoter.
R-gene-mediated recognition of pathogens by plants typically results in a hypersensitive response (HR) mediated by generation of reactive oxygen species and the increased production of salicylic acid (SA). The HR is accompanied by the induction of local and systemic expression of numerous genes involved in defense. The N-gene-mediated resistance of tobacco (Nicotiana tabacum) to infection with Tobacco mosaic virus (TMV) represents a classical model to study expression of pathogenesis-related (PR) proteins and development of systemic acquired resistance (SAR) in plant-pathogen interactions (van Loon and van Strien, 1999). Tobacco PR proteins of classes 1 to 5 are subdivided into acidic, extracellular proteins and basic, vacuolar proteins. Generally, TMV-induced expression of acidic PR proteins is mediated by SA, whereas expression of basic PR proteins is mediated by ethylene (Bol et al., 1990; Brederode et al., 1991; Linthorst, 1991). Although the function of tobacco PR-1 proteins is not clear, these proteins are highly conserved in the plant kingdom and are widely used as markers in studies of signal transduction processes involved in plant pathogenesis and induced resistance.
Studies on expression of PR genes in Arabidopsis (Arabidopsis thaliana) and tobacco revealed the central role of protein NONEXPRESSER OF PR GENES1 (NPR1). NPR1 also mediates cross talk between the SA signaling pathway and the jasmonic acid and ethylene signaling pathways, and interacts with members of the TGA family of transcription factors that bind to activator sequence-1 (as-1) or as-1-like elements that have been identified in promoters of PR-1 genes (Durrant and Dong, 2004). Two as-1-like elements in the Arabidopsis PR-1 promoter were shown to bind several of the 10 TGA factors in Arabidopsis with different affinity (Lebel et al., 1998; Johnson et al., 2003). The two as-1-like elements in the promoter of the tobacco gene encoding the acidic PR-1a protein bind TGA1a. Mutation of these elements affected SA-induced expression of a GUS reporter gene in transgenic plants (Strompen et al., 1998; Grüner et al., 2003). In addition to TGA1a, the Myb1 protein has been shown to bind to the PR-1a promoter in tobacco. Expression of the Myb1 gene was enhanced by TMV infection and application of exogenous SA, and the Myb1 protein preferentially bound to the MBSII sequence in the PR-1a promoter (Yang and Klessig, 1996). Silencing of Myb1 gene expression attenuated N-gene-mediated resistance to TMV (Liu et al., 2004).
Accumulating evidence indicates that WRKY proteins are involved in differential responses to biotic stresses, either as transcriptional activators or as repressors in Arabidopsis (Asai et al., 2002; Dong et al., 2003; Journot-Catalino et al., 2006; Kim et al., 2006; Li et al., 2006; Wang et al., 2006; Eulgem and Somssich, 2007) and other plants (for review, see Ülker and Somssich, 2004). For instance, silencing of the Nicotiana benthamiana homologs of the tobacco WRKY factors NtWRKY1, NtWRKY2, and NtWRKY3 compromised N resistance. These WRKY proteins share highest similarity at the amino acid level with Arabidopsis WRKY20, WRKY4, and WRKY70, and particularly expression of NtWRKY3 is rapidly induced upon infection with TMV (Liu et al., 2004). WRKY proteins bind to the W box (TTGAC[C/T]) in promoters of various pathogen-responsive genes, including genes encoding the basic, ethylene-responsive tobacco PR-1, PR-2, PR-3, and PR-5 proteins (Eulgem et al., 2000; Kim and Zhang, 2004; Yamamoto et al., 2004).
We have shown that a fragment of 902 bp upstream of the transcription start site of the tobacco PR-1a gene confers inducibility to the GUS reporter gene by TMV infection and SA treatment. This inducibility involved multiple elements in the promoter fragment (van de Rhee and Bol, 1993). The PR-1a promoter was found to contain a number of sites that bind GT-1-like factors with different affinity. The observation that the level of GT-1 decreased after infection of tobacco with TMV suggested a negative role of GT-1 in regulation of PR-1a expression. However, mutation of the GT-1 binding sites did not affect promoter activity (Buchel et al., 1996). In this article, we used the yeast one-hybrid system to identify tobacco proteins interacting with fragments of the PR-1a promoter. One of the proteins obtained turned out to be a novel WRKY protein, named NtWRKY12. Similar to PR-1a, expression of the NtWRKY12 gene was strongly induced by TMV infection, SA treatment, or infiltration of tobacco leaves with a suspension of Agrobacterium tumefaciens. Two binding sites for NtWRKY12 were identified in the PR-1a promoter with a surprisingly low similarity to the consensus W box sequence. Wild-type and mutant PR-1a promoter sequences were fused to the GUS reporter gene and these fusions were expressed in transgenic tobacco to assay induction by SA and expressed from a T-DNA vector in agroinfiltrated leaves to assay induction by bacterial elicitors. The results indicated that NtWRKY12 acts synergistically with TGA1a in the SA-mediated and pathogen-associated molecular pattern (PAMP)-mediated expression of the PR-1a gene. In addition, transactivation assays in Arabidopsis protoplasts provided evidence that NtWRKY12 is a transcriptional activator of PR-1a gene expression.
RESULTS
A Novel WRKY Factor Binds to the PR-1a Promoter
Previous studies have indicated that elements in the 902-bp tobacco PR-1a promoter are important for SA and TMV-induced expression (van de Rhee et al., 1990; van de Rhee and Bol, 1993; Strompen et al., 1998). Here, we used the yeast one-hybrid system to identify transcription factors binding to the PR-1a promoter. Tetramers of various fragments of the 902-bp promoter sequence were inserted in front of the yeast (Saccharomyces cerevisiae) His reporter gene and integrated into the genome of his− yeast strain Y187. TMV-infected tobacco was used as a source for construction of a library of cDNAs fused to the GAL4 activation domain in vector pACT. This library was used to transform yeast strains harboring the various PR-1a promoter fragments. Screening of the cDNA library with fragment IV (bp −605 to −513 of the PR-1a promoter in yeast strain Y187-IV) yielded 37 independent transformants growing on His-free medium (pACT/IV clones). Of the cDNA inserts in these clones, 22 cross-hybridized with each other. Clone pACT/IV-80 was selected for further analysis.
Sequencing of the cDNA insert of pACT/IV-80 revealed that it corresponded to the 610 3′-terminal nucleotides of a mRNA, excluding a poly(A) track of 54 residues probably representing the 3′-terminal poly(A) tail. The cDNA corresponding to the missing 5′-part of the mRNA was obtained using RACE on total RNA from TMV-infected tobacco plants. This resulted in a stretch of 415 additional nucleotides at the 5′-end of the mRNA. The combined 5′- and 3′-sequences revealed an open reading frame for a protein of 220 amino acid residues. The insert in pACT/IV-80 encoded the C-terminal 107 amino acids of this protein. The presence of WRKY and zinc (Zn)-finger domains in the C-terminal half indicates that the protein is a member of the large group of DNA-binding WRKY proteins. Upstream of the WRKY domain, the amino acid sequence contains a stretch of basic residues, reminiscent of nuclear targeting signals. The N-terminal region is relatively rich in acidic residues and has low similarity to WRKY51 from Arabidopsis. Based on the criteria described by Eulgem et al. (2000), the novel tobacco WRKY protein appears to be a member of subgroup 2c of the WRKY superfamily of plant transcription factors. Currently, 11 different tobacco WRKY genes are described in the EMBL/GenBank database. In line with the tobacco WRKY nomenclature, the novel protein identified in our study was named NtWRKY12. The accession number of the full-length cDNA is DQ460475. DNA-blot analyses of restriction enzyme digests of genomic DNA using a probe corresponding to the cDNA insert from pACT/IV-80 showed that the amphidiploid tobacco varieties Samsun NN and Samsun nn contain two to four NtWRKY12-related genes (Supplemental Fig. S1).
Expression of the full-length NtWRKY12 protein in yeast strain Y187-IV rendered the strain independent of exogenous His (data not shown). This indicates that NtWRKY12 contains an activation domain that is able to replace the GAL4 activation domain fused to the DNA binding region of NtWRKY12 in pACT/IV-80 and to activate transcription of the His reporter gene in yeast. This strongly supports a role for NtWRKY12 as a transcription factor in tobacco. Expression of an NtWRKY12/GFP fusion construct using an alfalfa mosaic virus-based expression system (Sánchez-Navarro et al., 2001) resulted in specific fluorescence of tobacco nuclei. Similar expression of nonfused GFP showed a more diffuse fluorescence of the cytoplasm and nuclei (Supplemental Fig. S2). This indicates that the NtWRKY12 sequence contains a nuclear localization signal, which targets the fusion protein to the nucleus.
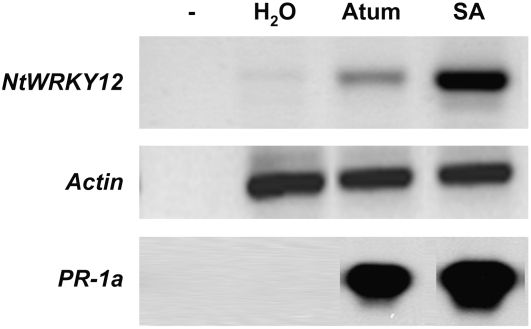
The reverse transcription (RT)-PCR results shown in Figure 1 indicate that, like PR-1a, expression of the NtWRKY12 gene was induced in tobacco leaves by salicylate treatment and by infiltration of leaf tissue with A. tumefaciens strain LBA4404. The last type of induction probably corresponds to a PAMP-type response, similar to responses triggered by peptide patterns of conserved elicitors like bacterial flagellins or elongation factor (EF)-Tu (Felix et al., 1999; Kunze et al., 2004). Indeed, also infiltration with Escherichia coli resulted in induced expression of NtWRKY12 and PR-1a (data not shown).
Figure 1.
Induction of expression of NtWRKY12 and PR-1a by A. tumefaciens elicitors and SA treatment of tobacco. Leaves were infiltrated with water (H2O), a suspension of A. tumefaciens (Atum), or a solution of salicylic acid (SA), and RNA was extracted 2 d after infiltration. RNA from infiltrated leaves was analyzed by RT-PCR with primers corresponding to NtWRKY12, Actin, or PR-1a. As a control for genomic DNA contamination, the first lane (−) was loaded with the product of a PCR on RNA from untreated leaf without initial RT reaction. The PCR products were electrophoresed and stained with ethidium bromide.
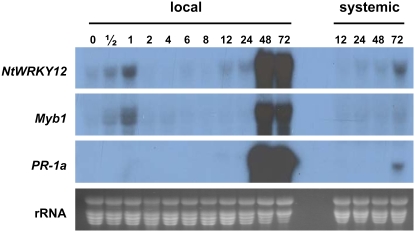
The time course of expression of the NtWRKY12 gene was studied in TMV-infected Samsun NN tobacco plants. Figure 2 shows northern blots with RNA isolated at various time points after inoculation. It is evident that in noninfected plants the gene was expressed at relatively low levels (top, lane 0). After infection with TMV, expression increased in the inoculated leaves (local) and reached a transient maximum after 1 h. At 2 h postinoculation (hpi), expression was back to the low basal level and remained low until 8 hpi. Subsequently, NtWRKY12 mRNA accumulation was slightly increased at 12 and 24 hpi and became very high at 48 h and later. The strong increase in NtWRKY12 expression coincided with the development of local lesions that first appeared at 36 hpi. Also, in the noninoculated leaves, expression increased, although with some delay and to lower levels. We have not investigated mRNA accumulation in the systemic tissues at later time points. The second image shows the TMV-induced expression pattern of the gene encoding transcription factor Myb1 (Yang and Klessig, 1996). Like NtWRKY12, the Myb1 gene is transiently expressed until 1 hpi and at high levels at 48 and 72 hpi. The timing of NtWRKY12 and Myb1 expression corresponded to that of the PR-1a gene (middle), although PR-1a was not transiently expressed immediately after inoculation.
Figure 2.
Time course of the expression of NtWRKY12, Myb1, and PR-1a genes after inoculation of Samsun NN tobacco with TMV. Total RNA was extracted from inoculated (local) leaves and uninoculated (systemic) upper leaves at the indicated time points (hpi). After electrophoresis and blotting, the membranes were hybridized to cDNA probes corresponding to NtWRKY12, Myb1, and PR-1a as indicated in the left margin. A photograph of an ethidium bromide-stained gel is included as a loading control (rRNA). [See online article for color version of this figure.]
Characterization of NtWRKY12 Binding Sites in the PR-1a Promoter
The results of the yeast one-hybrid screening indicated that NtWRKY12 specifically bound to a PR-1a promoter sequence ranging from positions −605 to −513 upstream of the transcription start site. To delineate the binding site in the DNA, this region was further divided into four overlapping subfragments A to D (Fig. 3). With a similar approach as was used above, tetramerized versions of subfragments B and C were able to confer His independence in the yeast one-hybrid system, whereas fragments A and D were not (data not shown). This suggested that the overlap region of fragments B and C contains the NtWRKY12 binding site. This was confirmed in the one-hybrid system with mutants of subfragments B and C of which either the left halves (mutants Blm and Clm) or the right halves (mutants Brm and Crm) were mutated by changing each G to A, A to G, C to T, and T to C (e.g. compare the sequences of B and Blm in Fig. 3).
Figure 3.
Wild-type (WT) and mutant PR-1a promoter fragments analyzed in yeast one-hybrid assays and EMSAs. Promoter fragment IV (nucleotides −603 to −513) is subdivided into overlapping fragments A, B, C, and D. In Blm, Brm, Clm, and Crm, either the left or the right halves of fragments B and C were mutated ( ). Plus and minus signs indicate binding and no binding in the yeast one-hybrid assays and EMSAs, respectively. Mutants Blm-m1 to Blm-m10 contain additional mutations in the right half of mutant Blm. In the wild-type sequence, the position of the as-1, WK2, and MBSII boxes are italic and underlined.
). Plus and minus signs indicate binding and no binding in the yeast one-hybrid assays and EMSAs, respectively. Mutants Blm-m1 to Blm-m10 contain additional mutations in the right half of mutant Blm. In the wild-type sequence, the position of the as-1, WK2, and MBSII boxes are italic and underlined.
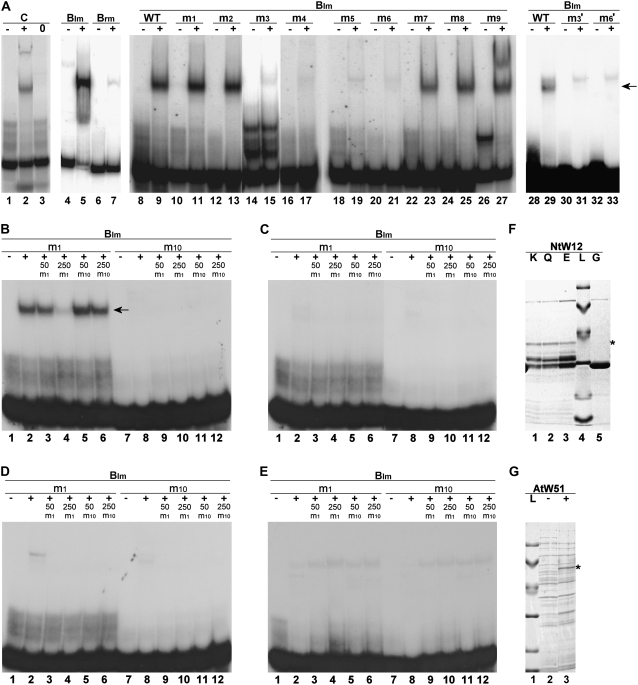
The results of the yeast one-hybrid assays were confirmed in vitro using electrophoretic mobility shift assays (EMSAs) with complementary oligonucleotides corresponding to regions C and B and a glutathione S-transferase (GST)/NtWRKY12-binding domain (BD) fusion protein expressed in E. coli. This fusion protein contained the C-terminal 111 amino acids of NtWRKY12 and was purified by glutathione-Sepharose 4B column chromatography. In the EMSA, the complementary oligonucleotides could anneal to double-stranded structures. Figure 4A, lane 1, shows a band corresponding to the labeled fragment C probe. After incubation with the GST/NtWRKY12-BD protein, part of the probe is shifted to a higher position in the gel (Fig. 4A, lane 2). When only GST protein is used, no band shift is observed (Fig. 4A, lane 3). This indicates that the NtWRKY12-BD is able to form a protein-DNA complex with fragment C. Similarly, lane 5 shows the formation of a complex of GST/NtWRKY12-BD with fragment Blm, but not with Brm (Fig. 4A, lane 7). To determine the exact location of the NtWRKY12 binding site in subfragment Blm, a scanning analysis was performed with a series of complementary oligonucleotides based on Blm, in which two adjacent base pair were changed (Fig. 3; Blm-m1–Blm-m9). The results of EMSAs with these fragments are shown in Figure 4A, lanes 8 to 27. It is evident that the lanes with mutants Blm-m3 to Blm-m6 lack a band shift and neither did the single mutants Blm-m3′ and Blm-m6′ (Figs. 3 and 4, lanes 29–33). This suggests that the corresponding sequence TTTTCCAC is essential for binding to the NtWRKY12-BD. Complementary oligonucleotides corresponding to fragment Blm-m1, but with the central TTTCCA sequence of the binding site changed into the consensus WRKY box TTGACC (Fig. 3; Blm-m10), were not able to compete with fragment Blm-m1 for binding of GST/NtWRKY12-BD in EMSAs (Fig. 4B, lanes 5 and 6), whereas fragment Blm-m10 alone showed no binding to GST/NtWRKY12-BD (Fig. 4B, lane 8). This indicates that NtWRKY12 does not bind to the consensus WRKY binding site. As discussed in more detail below (see “Discussion”), in NtWRKY12 the WRKY sequence is followed by the sequence GKK rather than by the sequence GQK found in WRKY factors that have been shown to bind to the consensus W box. We have expressed GST/NtWRKY12-BD with the GKK sequence mutated into GQK or GEK in E. coli (Fig. 4F), but the purified mutant proteins showed no binding in band shift assays to either the WK box in the PR-1a promoter or the consensus W box sequence (Fig. 4, C and D). Apparently, the central Lys in the GKK sequence is essential for binding of NtWRKY12 to the WK box sequence.
Figure 4.
Binding of NtWRKY12 to wild-type (WT) and mutant PR-1a promoter fragments. EMSAs were done with the promoter fragments shown in Figure 3: wild-type fragment C (A, lanes 1, 2, and 3), Blm (A, lanes 4, 5, 8, 9, 28, and 29), Brm (A, lanes 6 and 7), and the indicated Blm mutants (A, lanes 10–27 and 30–33; B, C, D, and E, lanes 1–12). In A, B, C, D, and E, plus signs indicate binding mixtures containing 0.5 μg recombinant GST fusion protein purified from E. coli transformed using a pGEX-KG vector with wild-type NtWRKY12-BD (A and B), mutant NtWRKY12-BD with the amino acids GKK mutated to GQK (C), mutant NtWRKY12-BD with the amino acids GKK mutated to GEK (D), and AtWRKY51 (E). In these sections, minus signs above the lanes indicate binding mixtures without recombinant protein. In A and B, the position of the protein-DNA complexes is indicated by an arrow. In B, C, D, and E, lanes 3 to 6 and 9 to 12, a 50- or 250-fold excess of unlabeled fragment Blm-m1 (m1) or Blm-m10 (m10) was added as competitor to the EMSA incubation mixtures. Zero sign (A, lane 3), Control with recombinant GST protein purified from E. coli. F, SDS-PAGE gel containing purified GST fusion proteins of wild-type NtWRKY12-BD with WRKYGKK (K, lane 1) and mutants NtWRKY12-BDs with WRKYGQK (Q, lane 2) and WRKYGEK (E, lane 3), respectively, which were used in the EMSAs of A to E. Lane 5 of F was loaded with a purified extract from empty GST expression vector (G). G, SDS-PAGE gel of extract from uninduced (minus sign, lane 2) or induced (plus sign, lane 3) E. coli containing pGEX-KG vector with AtWRKY51-GST fusion protein. In F and G, the position of the full-length induced fusion proteins is indicated by asterisks, whereas lanes labeled L were loaded with size markers of 94, 67, 43, 30, 20, and 14 kD. NtW12, NtWRKY12; AtW51, AtWRKY51.
We have investigated whether binding to the WK box is a general feature of WRKY proteins with a GKK sequence. Therefore, the full-length GKK-containing AtWRKY51 coding sequence was expressed as a GST fusion protein in E. coli (Fig. 4G). However, this Arabidopsis WRKY was not able to bind to either the WK or the W box sequence (Fig. 4E). The faint bands visible at higher positions in the gel (Fig. 4E, lanes 2–6 and 8–12) are the result of aspecific binding because they cannot be competed by an excess of either unlabeled WK or W box. The same results were obtained with a full-length GST/AtWRKY59 fusion protein (data not shown). These results suggest that the WK box is not a general consensus binding site for GKK WRKYs.
The synthetic oligonucleotides that were used for the above band shift assays contained nonpaired GTAC extensions at the 5′ termini. These sticky ends allowed transient base pairing and formation of multimerized fragments, which greatly facilitated DNA-protein interaction during incubation. Annealed oligonucleotides that did not contain sticky ends at best showed only weak band shifts. Apparently, the multimers remained at least partly intact during electrophoresis and are visible as faint bands above the positions of the monomeric free probes. In several lanes, these oligomers were apparently stable enough to produce stable multiple free probe bands (Fig. 4A, lanes 14/15, 26) and even double band shifts (Fig. 4A, lane 27).
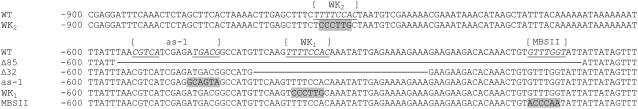
The sequence TTTTCCAC also occurs at the far upstream position −859 in the PR-1a promoter (Fig. 5). EMSAs with annealed oligonucleotides corresponding to the region −871 to −839 confirmed also that this region of the promoter is able to bind NtWRKY12 (data not shown). The two TTTTCCAC sequences in the PR-1a promoter were named box WK1 (−564) and box WK2 (−859).
Figure 5.
Partial sequence of the wild-type (WT) and mutant PR-1a promoters. The sequence of the wild-type PR-1a promoter is shown from nucleotides −900 to −801 (first row) and from −600 to −501 (second row), taking the transcription start site as +1. Binding sites for transcription factors NtWRKY12 (WK2 and WK1), TGA1a (as-1), and Myb1 (MBSII) are underlined and mutant sequences (blocked) are shown below the wild-type sequence. The lines in mutants Δ32 and Δ85 represent deleted nucleotides.
Role of NtWRKY12 and TGA1a in SA-Induced PR-1a Expression
To determine whether the NtWRKY12 binding site has functional significance for SA-induced expression of PR-1a, stably transformed transgenic tobacco plants were made containing a series of mutant PR-1a promoter∷GUS constructs. In close proximity to the WK1 box (−564 to −558), the PR-1a promoter contains binding sites for transcription factors TGA1a (box as-1, −592 to −577) and Myb1 (box MBSII, −520 to −514; Fig. 5; Yang and Klessig, 1996; Strompen et al., 1998; Grüner et al., 2003). Mutations affecting single transcription factor binding sites in mutants WK2, as-1, and WK1 are shown in Figure 5. The double mutant WK2/WK1 contains both the WK2 and WK1 mutations. In addition, promoter deletions of 85 bp (mutant Δ85) and 32 bp (mutant Δ32) were made. As outlined in Figure 5, the 85-bp deletion removed binding sites as-1, WK1, and MBSII, whereas the 32-bp deletion removed the WK1 binding site only. The double mutant WK2/Δ85 contained both the WK2 and the Δ85 mutations.
The number of independent, phenotypically normal transformants obtained with wild-type and mutant PR-1a promoter∷GUS constructs ranged between 2 and 16. Primary transformants were analyzed at the six- to eight-leaf stage for noninduced GUS expression and for GUS activity after floating leaf discs on water and SA. The results are presented in Figure 6. As expected, the reporter gene was constitutively expressed in 35S∷GUS plants, whereas the wild-type PR-1a promoter conferred strong SA inducibility to the GUS gene. In agreement with the results of Strompen et al., (1998), we noticed that mutation of the as-1 box resulted in a modest reduction of SA inducibility of the PR-1a promoter. Mutation of the upstream binding site for NtWRKY12 (mutant WK2) did not reduce SA-inducible reporter gene expression. However, mutation (mutant WK1) or deletion (mutant Δ32) of the downstream NtWRKY12 binding site reduced the SA inducibility of the PR-1a promoter by approximately 60% to 70%. Although the number of transgenic lines with the WK1 mutation (two lines) or the Δ32 mutation (three lines) were relatively low, the results with these two mutants demonstrate that mutation or deletion of the WK1 box only partially affects PR-1a promoter activity. The combined mutation of both the WK2 and the WK1 binding site (mutant WK2/WK1; six lines) further reduced expression. Mutant Δ85 (16 lines) lacks the WK1, as-1, and MBSII boxes and showed no significant SA-inducible expression. Probably, NtWRKY12 is able to bind to the WK2 box of mutant Δ85, but this binding is not sufficient for SA-inducible expression. Mutation of the WK2 box in mutant Δ85 (mutant WK2/Δ85; seven lines)) did not affect the phenotype of mutant Δ85. Binding of TGA1a and/or Myb1 factors to the PR-1a promoter may be responsible for the approximately 20% level of SA inducibility observed with mutant WK2/WK1. Together, the results indicate that full SA inducibility of the PR-1a promoter requires synergistic interactions between NtWRKY12 and TGA1a or Myb1 factors.
Figure 6.
SA-induced expression of PR-1a∷GUS fusions in transgenic tobacco. Plants were transformed with constructs encoding a CaMV 35S promoter∷GUS fusion (35S), a wild-type (WT) 902-bp PR-1a promoter∷GUS fusion, and 902-bp PR-1a promoter∷GUS fusions containing the mutations shown in Figure 5. Four leaf discs from each plant were floated on water (H2O) or 1 mm SA for 48 h before GUS activity was measured, or four leaf discs were taken from the untreated transgenic plants (Leaf). The number of transgenic lines used is indicated (n). The bars represent the GUS activity of all transgenic plants per construct relative to that of wild type treated with 1 mm SA (100%). Error bars represent the sem.
Role of NtWRKY12, TGA1a, and Myb1 Factors in Elicitor-Induced PR-1a Expression
As shown in Figure 1, infiltration of tobacco leaves with A. tumefaciens results in induction of NtWRKY12 and PR-1a gene expression. Probably this expression is induced by bacterial elicitors (see “Discussion”). To study a possible role of NtWRKY12 in elicitor-induced expression of the PR-1a gene, tobacco leaves were agroinfiltrated with A. tumefaciens suspensions harboring PR-1a promoter∷GUS fusions in the T-DNA vector. For these experiments, the collection of promoter mutants used in the plant transformation experiments was extended with a mutant containing an altered Myb1 binding site (mutant MBSII; see Fig. 5) and a series of double and triple mutants. In double mutants as-1/WK1, as-1/MBSII, and WK1/MBSII, two of the boxes as-1, WK1, and MBSII contain the point mutations specified in Figure 5. In the triple mutant as-1/WK1/MBSII, all three boxes are mutated.
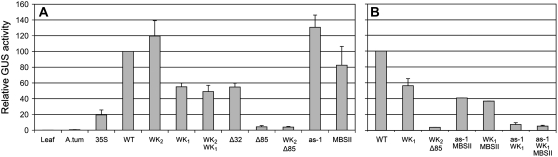
The results are shown in Figure 7, A and B. The relatively low GUS expression of the 35S∷GUS constructs can be ascribed to the much lower density of the 35S∷GUS Agrobacterium inoculum obtained in comparison to that of the PR-1a∷GUS strains (approximately A600 = 0.2 versus A600 = 1, respectively). To enable a comparison of different experiments, GUS activity in leaves expressing the wild-type PR-1a∷GUS construct was taken as 100%. The effects of mutations WK2, WK1, WK2/WK1, Δ32, Δ85, and WK2/Δ85 on elicitor-mediated GUS expression in tobacco plants (Fig. 7A) were largely similar to their effects on SA-mediated expression of GUS in plants with PR-1a∷GUS transgenes (Fig. 6). Expression of mutants WK1, WK2/WK1, and Δ32 was reduced by 40% to 60%, whereas mutants Δ85 and WK2/Δ85 did not support significant levels of elicitor-mediated GUS expression (Fig. 7A).
Figure 7.
Transient expression of PR-1a∷GUS fusions, induced by bacterial elicitors in agroinfiltrated tobacco leaves. Leaves were infiltrated with suspensions of A. tumefaciens harboring T-DNA vectors with promoter∷GUS fusions. 35S, Constitutive promoter from CaMV; WT, PR-1a promoter from base −1 to −902. Mutations shown in Figure 5 were engineered in the WT promoter. A.tum, A. tumefaciens with a promoterless GUS gene in the T-DNA vector. Leaf, Leaf material collected before agroinfiltration. The first series of experiments covered single mutations or combinations with WK2 (A). Additional agroinfiltration experiments of B show GUS expression levels of double and triple mutants in the Δ85 region. For each construct, GUS activity was determined in homogenates of 10 leaf discs from five infiltrated plants. The bars represent the GUS activity per construct relative to that of the wild type (100%). The sem was calculated for four (A) and two to four (B) replicates of independent experiments.
In mutant Δ85, the as-1, WK1, and MBSII boxes are deleted. To analyze the role of these boxes in the Δ85 phenotype, we made mutants with two or all three boxes mutated. Figure 7B shows that elicitor-mediated expression of the double mutant as-1/WK1 is as low as that of the Δ85 mutant. The effect of the double mutation in mutant as-1/WK1 (<5% of wild-type induction) is much stronger than the combined effects of the two single mutations as-1 (no significant reduction of wild-type induction; Fig. 7A) and WK1 (40%–60% of wild-type induction; Fig. 7, A and B). This demonstrates that a synergistic action of factors binding to the as-1 and WK1 boxes is essential for elicitor-induced PR-1a promoter activity. The additional mutation of the MBSII box in triple mutant as-1/WK1/MBSII did not alter the phenotype of the as-1/WK1 mutant.
Elicitor-mediated induction of the double mutants as-1/MBSII and WK1/MBSII is about 40% of the induction driven by the wild-type PR-1a promoter (Fig. 7B). The observation that expression by these double mutants is modestly reduced when compared to the single mutants as-1, WK1, and MBSII indicates that MBSII contributes to some extent to the expression driven jointly by the as-1 and WK1 boxes.
NtWRKY12 Activates PR-1a∷GUS Gene Expression in Arabidopsis Protoplasts
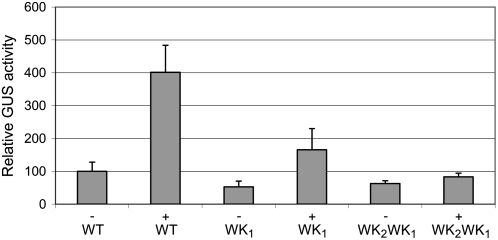
The above results indicate that NtWRKY12 plays a role in inducible PR-1a gene expression. To more directly demonstrate that NtWRKY12 functions as a positive transcriptional activator of PR-1a gene expression, Arabidopsis protoplasts were cotransfected with a plasmid containing the NtWRKY12 coding region under the control of the cauliflower mosaic virus (CaMV) 35S promoter together with a plasmid containing the GUS reporter gene cloned either behind the full-length (902 bp) wild-type PR-1a promoter or behind PR-1a promoters with mutations in the WK1 box or in both the WK1 and WK2 boxes. Similar cotransfections with a plasmid lacking the NtWRKY12 coding sequence were performed as controls. The results of these transactivation assays are shown in Figure 8. In the presence of the NtWRKY12 plasmid, PR-1a promoter-directed GUS expression was increased approximately 4-fold in comparison to the basal level obtained in protoplasts cotransfected with the empty vector. Apparently, NtWRKY12 produced in the protoplasts activates transcription of the GUS reporter gene by the Arabidopsis transcriptional machinery. Obviously, NtWRKY12 does so, at least partly, by binding to the WK1 box because mutation of the WK1 box resulted in a reduction of GUS activity to approximately 45% of that directed by the wild-type promoter. Upon mutation of both the WK1 and the WK2 box, NtWRKY12 no longer activated reporter gene expression.
Figure 8.
Transactivation of PR-1a∷GUS gene expression by NtWRKY12 in Arabidopsis protoplasts. Protoplasts were transfected with 2 μg of wild-type PR-1a promoter∷GUS (WT) construct or with PR-1a promoter∷GUS constructs containing the WK1 mutation (WK1) or the WK2/WK1 double mutant (WK2WK1) as shown in Figure 5. Plus signs, Cotransfection with 6 μg of expression vector pRT101 containing 35S∷NtWRKY12. Minus signs, Cotransfection with 6 μg of empty expression vector. The bars represent the percentage of GUS activity from triplicate experiments relative to that of the protoplasts cotransfected with the wild-type PR-1a∷GUS construct and the empty expression vector, which was set to 100%. Error bars represent the sem.
DISCUSSION
DNA Binding Site of NtWRKY12
Among the first WRKY-type DNA binding proteins that were identified was a parsley (Petroselinum crispum) transcription factor involved in expression of the Phytophthora megasperma-induced gene encoding protein PR1 (Rushton et al., 1996; Eulgem et al., 1999). As a PR protein of class 10, parsley PR1 is not related to the classical PR-1 proteins originally characterized in tobacco and conserved in many other plant species. Induction of parsley PR1 is not mediated by SA and the protein accumulates in the cytoplasm as opposed to the classical PRs that accumulate either extracellularly or in the vacuole.
A number of recent studies have suggested the involvement of Arabidopsis WRKY transcription factors in induced PR gene expression, although no direct evidence has been presented for specific WRKY-PR promoter interactions (Chen and Chen, 2002; Robatzek and Somssich, 2002; Kim et al., 2006). In a screen of genes coexpressed with the Arabidopsis PR-1 gene under SAR-inducing conditions, Maleck et al. (2000) found the consensus WRKY binding site TTGAC(C/T) to be present in the promoters at twice the statistically expected frequency, whereas the as-1 element TGACG—the consensus binding site of TGA transcription factors—occurred only at one-half the statistically expected frequency.
In this article, we have identified NtWRKY12 as a WRKY-type DNA binding protein that specifically recognizes the sequence TTTTCCAC. This DNA element is located at two positions in the upstream region of the tobacco PR-1a promoter that was previously found to be important for inducible gene expression (van de Rhee et al., 1990; van de Rhee and Bol, 1993; Grüner and Pfitzner, 1994; Strompen et al., 1998). The NtWRKY12 binding box at position −564 is located between binding sites for transcription factors TGA1a (−592) and Myb1 (−520), which have been implicated in SA- and TMV-induced gene expression (Yang and Klessig, 1996; Strompen et al., 1998).
NtWRKY12 Contains a Variant WRKY Domain
NtWRKY12 is the first WRKY protein to be identified that interacts with a DNA binding site different from the consensus WRKY binding site TTGAC(C/T). As far as the sequence of the conserved WRKY domain is concerned, NtWRKY12 is different from most other WRKY proteins in that it contains a Lys (K) residue instead of a Gln (Q) in the conserved domain (WRKYG[Q/K]K). This variation of the WRKY domain is conserved among other plant species. A BLASTP (http://www.ncbi.nlm.nih.gov/BLAST) search of all 796 eukaryote proteins containing one or two WRKY domains of which sequence data were present in the National Center for Biotechnology Information databases resulted in 131 sequences with high protein-protein similarity to NtWRKY12. Of these, the 28 proteins with highest similarity to NtWRKY12 all contained the WRKYGKK variant domain. The 10 most similar WRKYGKK proteins (61%–86% similarity) were from both dicotyledonous (Vitis vinifera, Brassica rapa, Glycine max) and monocotyledonous (rice [Oryza sativa]) plants. All WRKY factors shown to bind the W box element contain the GQK sequence.
Of all 72 Arabidopsis WRKY genes, the three closest homologs of NtWRKY12 are AtWRKY50, AtWRKY51, and AtWRKY59 (68%, 64%, and 59% similarity, respectively; Supplemental Fig. S3). Although the similarity between NtWRKY12 and these Arabidopsis WRKYs is mainly limited to the C-terminal halves of the proteins, they share the variant WRKYGKK domain, have approximately similar sizes, and are all induced by SA and pathogenesis (Dong et al., 2003). It was suggested that AtWRKY59's lack of W box binding activity might be due to the Q to K change (Dong et al., 2003). Although an Ala scanning study showed that mutation of the Q residue had only a minor effect on binding of NtWRKY9 to the consensus W box (Maeo et al., 2001), NMR spectroscopy measurements have revealed that the Q residue is one of the four amino acids in the WRKYGQK sequence of AtWRKY4 that contacts the bases in the major groove of the DNA and therefore is highly significant for sequence-specific recognition (Yamasaki et al., 2005). Recently, an extensive mutational analysis of the region containing the C-terminal WRKY domain of AtWRKY1 confirmed that the Q to K mutation affected its binding to the consensus W box (Duan et al., 2007). NtWRKY12 mutant proteins in which the GKK sequence was changed to GQK or GEK (another WRKY domain sequence variation occurring, for example, in WRKY proteins of rice) were not able to bind to either the WK box-containing Blm-m1 probe or the Blm-m10 probe with the consensus W box (Fig. 4B). This suggests that, in addition to the WRKYG[Q/K]K domain, other regions in the WRKY proteins are probably also involved in the specificity of DNA binding.
Role of NtWRKY12 in PR-1a Gene Expression
PAMPs are universally conserved in a class of microbes. As in animals, plants recognize elicitors derived from pathogens, such as viruses, bacteria, fungi, or oomycetes. Well-characterized elicitors that induce defense responses in plants are represented by bacterial flagellin and EF-Tu or peptides from these proteins. In Arabidopsis, the flagellin-derived peptide flg22 binds to a Leu-rich repeat-type receptor-like kinase (FLS2), which activates a mitogen-activated protein kinase (MAPK) pathway and expression of WRKY transcription factors (Gómez-Gómez, 2004; Boller, 2005). We observed that agroinfiltration of tobacco leaves with a suspension of A. tumefaciens induced the expression of NtWRKY12 and PR-1a. The components of A. tumefaciens responsible for this induction have not yet been identified (Felix et al., 1999; Kunze et al., 2004), although recent results by Djamei et al. (2007) show that PR-1 gene expression is indirectly regulated by Agrobacterium-induced MAPK MPK3.
SA plays an essential role in the expression of extracellular acidic PR proteins and development of SAR after TMV infection of NN tobacco. To analyze the role of the NtWRKY12 binding sites in SA- or PAMP-mediated expression, we investigated PR-1a∷GUS expression in stably or transiently transformed tobacco plants. Although mutation of the upstream WK box (WK2) had little effect on SA- or PAMP-induced GUS expression, mutation or deletion of the downstream WK box (WK1) reduced GUS expression by 50% to 60% (Figs. 6 and 7). This clearly demonstrates the important role of the NtWRKY12 binding site in SA- and PAMP-mediated PR-1a expression. The consensus WRKY binding site TTGAC(C/T) has been found in promoters of genes encoding basic vacuolar PR proteins from classes PR-1, PR-2, PR-3, and PR-5 of tobacco, but has not been identified in the PR-1a promoter. Overexpression of the tobacco MAPK kinase NtMEK2 resulted in the expression of WRKY factors binding to the consensus WRKY binding site and expression of defense genes, including those encoding basic PR proteins (Kim and Zhang, 2004).
PR-1a promoter fragment IV, which was used to select NtWRKY12 in the one-hybrid screen, also contains binding sites for TGA1a and Myb1 factors (Yang and Klessig, 1996; Strompen et al., 1998). Our as-1 mutant was impaired in SA-mediated expression of a reporter gene (Fig. 6), but the effect was less pronounced than that observed by Strompen et al. (1998) and Grüner et al. (2003). The PAMP-mediated expression of the as-1 mutant showed no repression in comparison to the wild-type promoter. This may be due to differences in mutations that were engineered in the as-1 box. Our observation that single mutations in the as-1 or WK1 box are insufficient to completely knock out SA-induced expression driven by the PR-1a promoter indicates that multiple factors are required for promoter activity. A complete knock out (<5% of the wild-type activity) was obtained with the Δ85 promoter deletion, which removes the as-1, WK1, and MBSII boxes. This mutational analysis of the PR-1a promoter revealed that similar elements are involved in SA-mediated and elicitor-mediated expression of the reporter gene (Figs. 6 and 7).
The finding that point mutations in the as-1 and WK1 boxes in double mutant as-1/WK1 fully knocked out elicitor-mediated expression (Fig. 7B) demonstrates that TGA1a and NtWRKY12 are the major players in the regulation of PR-1a promoter activity. A comparison of the activity of this double mutant with the single mutants as-1 and WK1 revealed that TGA1a and NtWRKY12-like factors act synergistically in PR-1a gene expression. In contrast to mutant as-1/WK1, the double mutants as-1/MBSII and WK1/MBSII showed significant levels of elicitor-mediated PR-1a promoter activity (Fig. 7B). A comparison of this activity with that of the single mutants as-1, WK1, and MBSII indicates that, in addition to the major effectors TGA1a and NtWRKY12, Myb1 plays a modest role in expression of the PR-1a gene. Recently, it was shown that several structurally related WRKY proteins are able to physically interact to form homologous and heterologous complexes (Xu et al., 2006). The synergistic effect of NtWRKY12 and TGA1a on PR-1a gene expression provokes a study of their possible direct or indirect interaction.
NtWRKY12 Is a Transcriptional Activator of PR-1a Gene Expression
The effect of NtWRKY12 overexpression on PR-1a promoter activity was studied by transactivation experiments in Arabidopsis protoplasts. These clearly demonstrated that NtWRKY12 acts as a transcriptional activator of PR-1a gene expression in vivo. GUS activity resulting from the expression of the wild-type PR-1a promoter∷GUS gene was greatly enhanced in the presence of NtWRKY12 (Fig. 8). When the WK1 box in the promoter was mutated, GUS expression was reduced, albeit still higher than in the absence of NtWRKY12. The results presented in Figures 6 and 7 indicated that the WK2 box was less important for induction of the PR-1a promoter than the WK1 box. However, in the transactivation assay (Fig. 8), the difference in GUS expression obtained with the WK1 and WK2/WK1 mutants clearly points to a role of WK2 in NtWRKY12-mediated expression.
In nonstressed tobacco, the PR-1a gene is not expressed (Figs. 1 and 2). The basal level of GUS expressed in the absence of NtWRKY12 in transfected Arabidopsis protoplasts (Fig. 8) indicates that the tobacco PR-1a promoter is recognized by the Arabidopsis transcriptional machinery. It is arguable that protoplast preparation and transfection result in a stress response that triggers a certain level of expression of stress-inducible genes, including the transfected tobacco PR-1a∷GUS gene. The observation that mutation of the WK1 box results in reduced GUS expression in the absence of NtWRKY12 (Fig. 8) suggests that the WK1 box is also involved in stress-induced expression by Arabidopsis transcription factors.
Whether in Arabidopsis protoplasts NtWRKY12 activates expression of the tobacco PR-1a gene alone or in combination with Arabidopsis TGA, Myb, or other transcription factors is presently unknown. Experiments are under way to further investigate this.
Occurrence of the WK Box in Other Promoters
The NtWRKY12 binding site TTTTCCAC is remarkably similar to that of the E. coli protein DnaA (TTTTCCACA; Weigel et al., 1997). DnaA is involved in DNA replication and binds to single-stranded DNA. Our band shift results were not caused by contaminating DnaA from the E. coli extract because no band shift was produced with a similarly isolated unfused GST protein preparation from E. coli (Fig. 4A, lane 3).
In tobacco, a TTTTCCAC box is also found 249 bp upstream of the transcription start site in the SA-inducible PR-2d gene (EMBL/GenBank accession no. X69794) and 1,012 bp upstream of the initiation codon in Sar8.2b (U64816). We have checked the occurrence of the WK box in the Arabidopsis genome. Whereas Maleck et al. (2000) found the W box to be overrepresented at 2.5-fold the statistically expected level in the promoters of a set of 25 PR-1 coregulated genes, we found the WK box to be overrepresented 3.3-fold in this set. Moreover, in the 1,000-bp upstream promoter regions of a set of 372 BTH-induced genes (Bülow et al., 2007), the WK box is found at twice the expected level, whereas the W box is present at 1.4-fold. Interestingly, in both sets the as-1 element is present at exactly the statistically expected level.
Recently, Sun et al. (2003) characterized the region of the promoter of the sugar-responsive iso1 gene from barley (Hordeum vulgare) that bound to barley transcription factor SUSIBA2. The 573-amino acid protein SUSIBA2 contains two WRKY and Zn-finger domains, which classifies it as a member of group 1 of the WRKY superfamily. Interestingly, SUSIBA2 bound to a region of the iso1 promoter lacking the consensus TTGAC(C/T) W box. Although the authors have not further delineated the exact SUSIBA2 binding box, we noticed that the region contains the sequence TTTTCCA and that mutations in this sequence affected the formation of band shifts with SUSIBA2 protein. Our results with NtWRKY12 suggest that it could be this sequence that determines the SUSIBA2 binding site. If so, the occurrence of two such similar WRKY binding sequences in promoters of genes involved in different physiological processes and in different plant species would indicate that the consensus TTGAC(C/T) WRKY box is not the only conserved cis-element involved in binding WRKY transcription factors. However, it must be noted that neither of SUSIBA2's WRKY domains contains the WRKYGKK sequence present in NtWRKY12.
CONCLUSION
In WRKY transcription factors, the WRKY consensus sequence is followed by the amino acid sequences GQK, GKK, or GEK. Factors of the GQK type have been shown to bind to the W box element (TTGAC[C/T]). We identified a tobacco WRKY factor (NtWRKY12) of the GKK type, which specifically recognized two WK boxes (WK1 and WK2; TTTTCCAC) in the promoter of the SA-inducible tobacco PR-1a gene, but failed to bind to the W box element. The central K residue in the GKK sequence was crucial for binding of NtWRKY12 to the WK box. Overexpression of NtWRKY12 in protoplasts strongly stimulated PR-1a promoter activity via functional WK1 and WK2 boxes. Synergistic interactions between NtWRKY12 and other transcription factors, particularly TGA1a, appeared to be required for maximal induction of the PR-1a promoter in planta by SA or bacterial elicitors.
MATERIALS AND METHODS
Plants and Plant Treatments
Tobacco (Nicotiana tabacum ‘Samsun NN’) plants were grown in growth chambers at 25°C, 60% relative humidity, with a 16/8-h photoperiod.
For cDNA library cloning, 8-week-old plants were inoculated with 0.1 mL per leaf of an inoculum of 18 ng TMV/mL by rubbing the inoculum on three lightly carborundum-dusted leaves per plant after which the plants were immediately placed in a growth room at 33°C with a 16-h day/8-h night regime. After 2 d, the plants were returned to the 25°C growth room and inoculated leaves were collected after 5 h. For gene expression studies, three leaves of 8-week-old tobacco plants were inoculated with 3 ng TMV/mL and kept at 25°C. Inoculated and noninoculated leaves were sampled at different time points and immediately frozen in liquid nitrogen and stored at −80°C.
Discs of 24 mm were punched out of new, fully expanded leaves of wild-type and transgenic plants and floated on water or on 1 mm sodium salicylate, pH 6.8. After 2 d, the discs were blotted dry and four 12-mm discs were punched out, transferred to Eppendorf tubes, frozen in liquid nitrogen, and stored at −80°C.
Transgenic tobacco plants containing 35S∷GUS and PR-1a∷GUS reporter genes were obtained through Agrobacterium tumefaciens-mediated leaf disc transformation with transgene constructs cloned into the pMOG800 transformation vector and regeneration of kanamycin-resistant shoots (Linthorst et al., 1989). The number of transgenic plants obtained were 14 (35S), 14 (wild type), two (WK2), two (WK1), six (WK2/WK1), three (Δ32), 16 (Δ85), seven (WK2/Δ85), and three (as-1).
Tiny punctures were made with a scalpel in the bottom epidermis of new, fully expanded leaves of 8-week-old tobacco plants, through which Agrobacterium infiltration mixtures (A600 = 1 for the PR-1a∷GUS strains and A600 = 0.2 for the 35S∷GUS strain) were supplied to the intercellular spaces by gentle pressure using a syringe without needle. After 2 d, 12-mm leaf discs were sampled from fully infiltrated areas adjacent to the puncture hole, transferred to Eppendorf tubes, frozen in liquid nitrogen, and stored at −80°C.
One-Hybrid Screening
mRNA was isolated from TMV-infected tobacco 5 h after the plants were transferred from 33°C to 25°C using the PolyAtract mRNA isolation system (Promega). First-strand cDNA was synthesized on 5 μg poly(A) RNA using an XhoI-oligo(dT) linker primer and M-MLV reverse transcriptase (Promega), after which the second strand was synthesized using RNaseH and Pfu DNA polymerase (Stratagene). After ligation of EcoRI adapters and digestion with XhoI, 150 ng of the sized cDNA fraction longer than 500 bp was ligated into XhoI/EcoRI double-digested λACTII arms. After packaging, the λACTII cDNA library (1.4 × 106 independent transformants) was amplified in Escherichia coli XL-1 Blue MRF−. The phage library was subsequently obtained as a plasmid expression library in pACTII by in vivo excision using E. coli BNN132.
Fragments of the tobacco PR-1a promoter corresponding to the regions −701 to −612 (region III) and −605 to −513 (region IV) relative to the transcription start site and various mutants of region IV were obtained by PCR using forward primers extended with BamHI and reverse primers extended with BglII restriction sites. This allowed convenient cloning and concatamerization of the fragments in plasmid pIC19H. Collinear tetramers were cloned in front of the His-3 gene of plasmid pHIS3N/X and subsequently the PR-1a promoter tetramer/His-3 bait constructs were cloned into pINT1 for integration into the genome of yeast (Saccharomyces cerevisiae) strain Y187 containing an auxotrophic his3 mutation (Ouwerkerk and Meijer, 2001). This resulted in strains Y187-III and Y187-IV, respectively. Leakiness of the PR-1a promoter tetramer/His-3 genes of the respective strains was virtually absent.
Screening of the cDNA library in the yeast one-hybrid system was performed essentially as described by Ouwerkerk and Meijer (2001). His-independent clones resulting from the transformations with the pACTII cDNA library were named pACT/IV-n.
Tetramerized subfragments and mutations thereof of promoter fragment IV were analyzed in the one-hybrid system for their ability to confer His-independent growth in one-hybrid assays with the NtWRKY12 DNA-BD of pACT/IV-80.
RACE
The cDNA region matching the 5′-part of the mRNA corresponding to the insert of pACT/IV-80 was obtained using RACE (Boehringer) on total RNA from TMV-infected tobacco plants using primer 5′-CCTTCATATGTTGTTATCAAATAGCTGG, which is complementary to an internal region starting at position 271 of the insert of clone pACT/IV-80. Resulting clones were characterized and the clone containing the longest insert was sequenced to confirm that it corresponded to pACT/IV-80. The insert was subsequently fused to the insert of pACT/IV-80 using a common BglII site to result in clone pNtWRKY12 containing the full-length coding region of NtWRK12.
Bacterial Expression of NtWRKY12 Fusion Proteins
The C-terminal partial open reading frame of pACT/IV-80 (NtWRKY12-BD), mutants in which the GKK sequence was changed into GQK or GEK, and the full-length coding sequence of AtWRKY51 and AtWRKY59 were cloned in frame behind the GST open reading frame of expression vector pGEX-KG (Guan and Dixon, 1991). These plasmids were transformed into E. coli BL21-DE3. For induction of protein expression, cultures were grown to mid-log phase at 37°C, after which isopropyl-β-thiogalactopyranoside was added to a final concentration of 0.1 mm and incubation continued for 3 h at 20°C. The cells were harvested by centrifugation, resuspended in 1/20th volume sonication buffer (1× phosphate-buffered saline containing 2% [v/v] Tween 20, 0.1% [v/v] Triton X-100, 5 mm dithiothreitol [DTT], and 1 mg mL−1 lysozyme) and lysed by sonication (Vibracell). The fusion proteins were purified using glutathione-Sepharose 4B columns (Amersham), which were eluted overnight at 4°C with 10 mm reduced glutathione, after which 1/50th volume Complete (Roche) protease inhibitors were added. Expressed fusion proteins were analyzed using 12% SDS-PAGE.
EMSA
EMSAs were performed essentially as described by Green et al. (1989). DNA probes for the EMSA assays were obtained by slowly cooling down mixtures of equimolar amounts of complementary oligonucleotides from 95°C to room temperature. Annealed oligonucleotides were subsequently labeled using T4-nucleotide kinase and [γ-32P]ATP, after which unincorporated label was removed by Autoseq G-50 column chromatography (Amersham-Pharmacia Biotech).
EMSA reaction mixtures contained 0.5 μg purified protein, 3 μL 5× gel shift binding buffer [20% glycerol, 5 mm MgCl2, 2.5 mm EDTA, 2.5 mm DTT, 250 mm NaCl, 50 mm Tris-HCl, pH 7.5, 0.25 mg mL−1 poly(dI-dC)×poly(dI-dC) (Promega)] in a total volume of 14 μL. After 10-min incubation at room temperature, 1 μL containing 60,000 cpm of labeled probe was added and incubation was continued for 20 min at room temperature. The total mixture was loaded onto a 5% polyacrylamide gel in Tris-borate buffer and electrophoresed at 4°C. After electrophoresis, the gel was dried, autoradiographed, and analyzed using a Bio-Rad Phosphoimager.
RT-PCR and RNA-Blot Analysis
Total RNA was isolated from pulverized frozen tobacco leaf tissue by phenol extraction and LiCl precipitation. Oligo(dT)-primed cDNA for PCR was obtained using M-MLV reverse transcriptase. Subsequently, PCR was performed during 25 cycles with primers corresponding to NtWRKY12 (AACACAGTTTAATCCTTAAACG, AGAACAAAGACCGAGCTTGAGATC), PR-1a (ATCCTCCATTGTTACACTGAAC, GCTTCCCAATTGGCTGCAG), and tobacco actin (TGCTAGGAGCCAGTGCAGTA, GTGATGGTGTCAGCCACACT). The products were analyzed on agarose gel.
For RNA-blot analysis, total RNA was denatured using formamide/formaldehyde, electrophoresed in 1.5% agarose gel, blotted to Hybond+ (Amersham), and hybridized to 32P-labeled cDNA probes as described previously (Brederode et al., 1991). After hybridization, the blots were washed at high stringency with a final wash step in 30 mm NaCl, 3 mm sodium citrate, 0.1% SDS at 50°C for 20 min.
Transactivation Experiments
Protoplasts were prepared from Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 cell suspensions according to Axelos et al. (1992), with some modifications. A 5-d-old cell suspension culture was diluted 5-fold in 50 mL medium (3.2 g/L Gamborg B5 basal medium with minimal organics [Sigma-Aldrich], 3% Suc, 1 μm naphthylacetic acid [NAA], pH 5.8) and incubated overnight at 25°C at 250 rpm. Cells were harvested and cell walls digested with 20 mL of enzyme mix (0.4% macerozyme R-10 [Yakult], 1.5% cellulose Onozuka R-10 [Yakult], 12% sorbitol, pH 5.8) for 3 h at 28°C. The protoplasts were filtered through a 63-μm steel sieve and washed twice in 50 mL of protomedium (Gamborg B5 basal medium, 0.1 m Glc, 0.25 m mannitol, 1 μm NAA, pH 5.8). The volume of the protoplast suspension was adjusted to 4 × 106 cells/mL. Protoplasts were cotransfected with 2 μg of plasmid carrying one of the PR-1a promoter∷GUS constructs (wild type, WK1, WK2WK1) and 6 μg of effector plasmid pRT101 (Töpfer et al., 1987) carrying 35S∷NtWRKY12. As a control, cotransformation of PR-1a promoter∷GUS fusions with the empty expression vector pRT101 was carried out. Protoplasts were transformed using polyethylene glycol as described previously (Schirawski et al., 2000). The protoplasts were harvested 16 h after transformation and frozen in liquid nitrogen.
Fluorometric GUS Assays
When the transgenic plants had reached a size of 15 to 20 cm for each transgenic plant for each treatment (untreated, water, and SA), four leaf discs were separately assayed for GUS activity, with each data point being the average of duplicate measurements. Each leaf disc was homogenized in 0.5 mL GUS extraction buffer (Jefferson, 1987), supplied with 20% methanol (Kosugu et al., 1990). After centrifugation for 5 min at 8,000g duplicate samples of 10 μL supernatant were incubated with 90 μL 1 mm 4-methylumbelliferyl-β-d-glucuronide at 37°C for 20 h. The reaction was terminated by adding 300 μL 0.2 m sodium carbonate and 460-nm fluorescence was measured using a Fluoroscan II (Titertek) at 355-nm excitation.
For transient GUS expression measurements, homogenates were made of 10 pooled 12-mm discs from infiltrated areas of leaves of five independently infiltrated plants. GUS activity, normalized against protein concentration, was determined from the average of duplicate measurements per sample.
For protoplast experiments, GUS activity was determined as described (van der Fits and Memelink, 1997), with minor modifications. GUS activities from triplicate experiments were normalized against total protein level.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number DQ460475.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Analysis of NtWRKY12 in the tobacco genome by Southern-blot hybridization.
Supplemental Figure S2. Nuclear localization of NtWRKY12.
Supplemental Figure S3. Amino acid alignment of NtWRKY12 and WRKYGKK variants of Arabidopsis.
Supplementary Material
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Huub J.M. Linthorst (h.j.m.linthorst@biology.leidenuniv.nl).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gómez-Gómez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 415 977–983 [DOI] [PubMed] [Google Scholar]
- Axelos M, Curie C, Mazzolini L, Bardet C, Lescure B (1992) A protocol for transient gene expression in Arabidopsis thaliana protoplasts isolated from cell suspension cultures. Plant Physiol Biochem 30 123–128 [Google Scholar]
- Bol JF, Linthorst HJM, Cornelissen BJC (1990) Plant pathogenesis-related proteins induced by virus infection. Annu Rev Phytopathol 28 113–138 [Google Scholar]
- Boller T (2005) Peptide signalling in plant development and self/non-self perception. Curr Opin Cell Biol 17 116–122 [DOI] [PubMed] [Google Scholar]
- Brederode FTh, Linthorst HJM, Bol JF (1991) Differential induction of acquired resistance and PR gene expression in tobacco by virus infection, ethephon treatment, UV light and wounding. Plant Mol Biol 17 1117–1125 [DOI] [PubMed] [Google Scholar]
- Buchel AS, Molenkamp R, Bol JF, Linthorst HJM (1996) The PR-1a promoter contains a number of elements that bind GT-1-like nuclear factors with different affinity. Plant Mol Biol 30 493–504 [DOI] [PubMed] [Google Scholar]
- Bülow L, Schindler M, Hehl R (2007) PathoPlant: a platform for microarray expression data to analyze co-regulated genes involved in plant defense responses. Nucleic Acids Res 34 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen Z (2002) Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol 129 706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djamei A, Pitzschke A, Nakagami H, Rajh I, Hirt H (2007) Trojan horse strategy in Agrobacterium transformation: abusing MAPK defense signaling. Science 318 453–456 [DOI] [PubMed] [Google Scholar]
- Dong J, Chen C, Chen Z (2003) Expression profile of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51 21–37 [DOI] [PubMed] [Google Scholar]
- Duan MR, Nan J, Liang YH, Mao P, Lu L, Li L, Wei C, Lai L, Li Y, Su XD (2007) DNA binding mechanism revealed by high resolution crystal structure of Arabidopsis thaliana WRKY1 protein. Nucleic Acids Res 35 1145–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant WD, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42 185–209 [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of transcription factors. Trends Plant Sci 5 199–206 [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Schmelzer E, Hahlbrock K, Somssich IE (1999) Early nuclear events in plant defence signalling: rapid gene activation by WRKY transcription factors. EMBO J 18 4689–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10 366–371 [DOI] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18 265–276 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L (2004) Plant perception systems for pathogen recognition and defence. Mol Immunol 41 1055–1062 [DOI] [PubMed] [Google Scholar]
- Green PJ, Kay SA, Lam E, Chua NH (1989) In vitro DNA footprinting. In SB Gelvin, RA Schilperoort, eds, Plant Molecular Biology Manual B11. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 1–22
- Grüner R, Pfitzner UM (1994) The upstream region of the gene for the pathogenesis-related protein 1a from tobacco responds to environmental as well as to developmental signals in transgenic plants. Eur J Biochem 220 247–255 [DOI] [PubMed] [Google Scholar]
- Grüner R, Strompen G, Pfitzner AJP, Pfitzner UM (2003) Salicylic acid and the hypersensitive response initiate distinct signal transduction pathways in tobacco that converge on the as-1-like element of the PR-1a promoter. Eur J Biochem 270 4876–4886 [DOI] [PubMed] [Google Scholar]
- Guan KL, Dixon JE (1991) Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem 192 262–267 [DOI] [PubMed] [Google Scholar]
- Jefferson R (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5 387–405 [Google Scholar]
- Johnson C, Boden E, Arias J (2003) Salicylic acid and NPR1 induce the recruitment of trans-activating TGA factors to a defense gene promoter in Arabidopsis. Plant Cell 15 1846–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journot-Catalino N, Somssich IE, Roby D, Kroj T (2006) The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana. Plant Cell 18 3289–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Zhang S (2004) Activation of a mitogen-activated protein kinase cascade induces WRKY family of transcription factors and defense genes in tobacco. Plant J 38 142–151 [DOI] [PubMed] [Google Scholar]
- Kim KC, Fan B, Chen Z (2006) Pathogen-induced Arabidopsis WRKY7 is a transcriptional repressor and enhances plant susceptibility to Pseudomonas syringae. Plant Physiol 142 1180–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G (2004) The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16 3496–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugu S, Ohashi Y, Nakajima K, Arai Y (1990) An improved assay for β-glucuronidase in transformed cells: methanol almost completely suppresses a putative endogenous β-glucuronidase activity. Plant Sci 70 133–140 [Google Scholar]
- Lebel E, Heifetz P, Thorne L, Uknes S, Ryals J, Ward E (1998) Functional analysis of regulatory sequences controlling PR-1 gene expression in Arabidopsis. Plant J 16 223–33 [DOI] [PubMed] [Google Scholar]
- Li J, Brader G, Kariola T, Palva ET (2006) WRKY70 modulates the selection of signaling pathways in plant defense. Plant J 46 477–491 [DOI] [PubMed] [Google Scholar]
- Linthorst HJM (1991) Pathogenesis-related proteins of plants. Crit Rev Plant Sci 10 123–150 [Google Scholar]
- Linthorst HJM, Meuwissen RLJ, Kauffman S, Bol JF (1989) Constitutive expression of pathogenesis-related proteins PR-1, GRP, and PR-S in tobacco has no effect on virus infection. Plant Cell 1 285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP (2004) Involvement of MEK1 MAPKK, NTF6 MAPK, WRKY/MYB transcription factors, COI1 and CTR1 in N-mediated resistance to tobacco mosaic virus. Plant J 38 800–809 [DOI] [PubMed] [Google Scholar]
- Maeo K, Hayashi S, Kojima-Suzuki H, Morikami A, Nakamura K (2001) Role of conserved residues of the WRKY domain in the DNA-binding of tobacco WRKY family proteins. Biosci Biotechnol Biochem 65 2428–2436 [DOI] [PubMed] [Google Scholar]
- Maleck K, Levine A, Eulgem T, Morgan A, Schmid J, Lawton KA, Dangl JL, Dietrich RA (2000) The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet 26 403–410 [DOI] [PubMed] [Google Scholar]
- Ouwerkerk BFO, Meijer AH (2001) Yeast one-hybrid screening for DNA-protein interactions. In FM Ausubel, R Brent, RE Kingston, DD Moore, JG Seidman, JA Smith, K Struhl, eds, Current Protocols in Molecular Biology. John Wiley & Sons, Chichester, UK, pp 12.12.1–12.12.22 [DOI] [PubMed]
- Robatzek S, Somssich IE (2002) Targets of AtWRKY6 regulation during plant senescense and pathogen defense. Genes Dev 16 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton PJ, Torres JT, Parniske M, Wernert P, Hahlbrock K, Somssich IE (1996) Interaction of elicitor-induced DNA binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J 15 5690–5700 [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Navarro J, Miglino R, Ragozzino A, Bol JF (2001) Engineering alfalfa mosaic virus RNA 3 into an expression vector. Arch Virol 146 923–939 [DOI] [PubMed] [Google Scholar]
- Schirawski J, Planchais S, Haenni AL (2000) An improved protocol for the preparation of protoplasts from an established Arabidopsis thaliana cell suspension culture and infection with RNA of a turnip yellow mosaic tymovirus: a simple and reliable method. J Virol 86 85–94 [DOI] [PubMed] [Google Scholar]
- Strompen G, Grüner R, Pfitzner UM (1998) An as-1-like motif controls the level of expression of the gene for the pathogenesis-related protein 1a from tobacco. Plant Mol Biol 37 871–883 [DOI] [PubMed] [Google Scholar]
- Sun C, Palmqvist S, Olsson H, Ahlandsberg S, Jansson C (2003) A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell 15 2076–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpfer R, Matzeit V, Gronenborn B, Schell J, Steinbiss HH (1987) A set of plant expression vectors for transcriptional and translational fusions. Nucleic Acids Res 15 5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ülker B, Somssich IE (2004) WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol 7 491–498 [DOI] [PubMed] [Google Scholar]
- van de Rhee MD, Bol JF (1993) Induction of the tobacco PR-1a gene by virus infection and salicylate treatment involves an interaction of multiple regulatory elements. Plant J 3 71–82 [Google Scholar]
- van de Rhee MD, van Kan JAL, González Jaén MT, Bol JF (1990) Analysis of regulatory elements involved in the induction of two tobacco genes by salicylate treatment and virus infection. Plant Cell 2 357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L, Memelink J (1997) Comparison of the activities of CaMV 35S and FMV 34S promoter derivatives in Catharanthus roseus cells transiently and stably transformed by particle bombardment. Plant Mol Biol 33 943–946 [DOI] [PubMed] [Google Scholar]
- van Loon LC, van Strien EA (1999) The families of pathogenesis-related proteins, their activities and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55 85–97 [Google Scholar]
- Wang D, Amornsiripanitch N, Dong X (2006) A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog 2 e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel C, Schmidt A, Rückert B, Lurz R, Messer W (1997) DnaA protein binding to individual DnaA boxes in the Escherichia coli replication origin, oriC. EMBO J 16 6574–6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chen C, Fan B, Chen Z (2006) Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 18 1310–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Nakano T, Suzuki K, Shinshi H (2004) Elicitor-induced activation of transcription via W box-related cis-acting elements from a basic chitinase gene by WRKY transcription factors in tobacco. Biochim Biophys Acta 1679 279–287 [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Kigawa T, Inoue M, Tateno M, Yamasaki T, Yabuki T, Aoki M, Seki E, Matsuda T, Tomo Y, et al (2005) Solution structure of an Arabidopsis WRKY DNA binding domain. Plant Cell 17 944–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Klessig DF (1996) Isolation and characterization of a tobacco mosaic virus-inducible myb oncogene homolog from tobacco. Proc Natl Acad Sci USA 93 14972–14977 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.