Abstract
Epidemiological and animal studies suggest that tea may be protective towards cancers of the GI tract. White tea, the least processed form of tea, contains high levels of polyphenols and, like green tea, is chemopreventive towards heterocyclic amine-initiated colonic aberrant crypt formation in male F344 rats. We examined for the first time the relative effectiveness of white and green tea in suppressing intestinal tumorigenesis in C57BL/6J-ApcMin/+ (Apcmin) mice. Each tea was also compared with sulindac, a nonsteroidal anti-inflammatory drug known to be highly effective in Apcmin mice. Male C57BL/6J+/+ (wild-type) and Apcmin mice were treated in the drinking water with white tea or green tea (1.5% w/v, 2 min brew-time), 80 p.p.m. sulindac, a combination of 80 p.p.m. sulindac in 1.5% white tea, or pH buffered water. After 12 weeks of treatment, Apcmin mice given white tea, green tea, or sulindac had significantly fewer tumors than controls (P < 0.05). The protection provided by 1.5% green or white tea was comparable to that provided by 80 p.p.m. sulindac. Mice treated with a combination of white tea plus sulindac had significantly fewer tumors than either treatment alone (P < 0.05). β-catenin and β-catenin/Tcf-4 regulated proteins Cyclin D1 and c-Jun were readily detected in polyps, but markedly reduced in normal-looking intestines of mice treated with both tea and sulindac. This research provides evidence that teas, particularly when administered in combination with sulindac, are highly effective at inhibiting intestinal neoplasia in male Apcmin mice via direct or indirect effects on the β-catenin/APC pathway.
Introduction
Tea, prepared from the dried leaves of Camellia sinensis, is one of the most widely consumed beverages in the world. Recent upswings in the sales of green tea in the United States can be attributed to reports of potential health benefits against cancer and other chronic diseases. Indeed teas exert significant protective effects in experimental animal models of skin, lung, esophageal, gastric, hepatic, small intestinal, pancreatic, colon, bladder, and mammary cancer (reviewed in ref. 1).
Previously, our laboratory reported that green and black teas were protective towards heterocyclic-amine induced colonic aberrant crypt formation in the F344 rat (2,3). Green tea was more effective than black tea, presumably because green tea undergoes less processing than does black tea and, therefore, contains higher levels of polyphenols such as epigallocatechin-3-gallate (EGCG). White tea is a rare form of tea that undergoes the least amount of processing of any of the teas. We reported recently that white tea contains high levels of EGCG and related polyphenols. White tea was more potent than green tea in inhibiting heterocyclic amine-induced mutagenicity in vitro (4), and protected against aberrant crypt formation in the F344 rat (5).
Some of the most promising pharmaceutical agents described to date for the prevention of intestinal cancer are the nonsteroidal anti-inflammatory drugs (NSAIDs). NSAIDs, such as aspirin and sulindac, are highly effective in both genetically predisposed and chemically induced animal models of intestinal cancer (6–8). Sulindac slows the formation and causes regression of polyps in humans with familial adenomatous polyposis (FAP) (9). High aspirin intake is associated with a 40–50% decrease in colon cancer mortality (10) suggesting that the effectiveness of NSAIDs in humans is not limited to hereditary cancer. Although sulindac and other NSAIDs are potent cancer preventative agents, these pharmaceuticals can cause severe side effects including GI bleeding, GI perforation, hepatotoxicity, and even death (11,12). These effects are generally dose-dependent, with higher doses more likely to cause toxicity. Thus, serious side effects limit the potential of NSAIDs as cancer preventative agents.
One strategy to minimize the toxicity of NSAIDs is to use lower doses in combination with other chemopreventive agents having complementary modes of action. This approach was recently tested by Torrance et al. (13) who found that low-dose sulindac combined with the pharmaceutical agent EKB-569 effectively prevented intestinal neoplasia in a mouse model of FAP. EKB-569 was engineered to strongly inhibit tyrosine phosphorylation of epidermal growth factor receptors (EGFRs). The combination of a NSAID with an EGFR kinase inhibitor provided far greater protection than either agent alone, and indicated that a combination approach may be highly effective in cancer prevention. However, natural products such as tea also may be effective in combined chemoprevention and offer some advantages over pharmaceuticals. Like EKB-569, tea extracts or tea components inhibit tyrosine phosphorylation of the EGFR (14–17). However tea or individual tea constituents have been reported to have many additional cancer-preventive mechanisms (reviewed in ref. 18). These mechanisms include antioxidant properties, induction of cell-cycle arrest, apoptosis induction, inhibition of oncogene expression, inhibition of telomerase activity, inhibition of urokinase, inhibition of p38 mitogen-activated kinase activity, blockage of nitric oxide synthase, inhibition of angiogenesis and inhibition of tumor necrosis factor-α (TNFα) expression and release (1,15,17,19–22). Each of these mechanisms, in principle, has the potential to complement sulindac and provide for enhanced chemo-prevention. Indeed, evidence has been reported that tea or tea constituents plus sulindac may interact synergistically to induce apoptosis in human lung cancer cells in vitro (23) and to inhibit aberrant crypt formation in azoxymethane-initiated rats (24). Therefore, in addition to testing white tea and green tea separately, we also tested a combination of tea plus low-dose sulindac in the Apcmin mouse, an animal model that is genetically predisposed to the development of intestinal polyps (25).
Materials and methods
Materials
Sulindac and various tea standards, including EGCG, were purchased from Sigma Chemical Company (St Louis, MO). Teas were a gift of the Stash Tea Company (Portland, OR) and included Exotica China White Tea and Dragonwell Green Tea (referred to hereafter as ‘white’ and ‘green’ tea). Due to limited solubility of sulindac at low pH, sulindac and teas were administered in buffered drinking water (4 mM sodium phosphate, pH 7.6). Teas were prepared in a French press by adding 1.5 g tea leaves per 100 ml buffered water (1.5% w/v), brewing for 2 min, and then filtering (pH of final tea solution was 7.4). Teas were brewed fresh every other day and were routinely analyzed by high performance liquid chromatography (HPLC) as previously described (4). Table I compares the levels of the nine primary tea constituents in freshly brewed teas and after 48 h.
Table I.
| White tea (mg/mlc)
|
Green tea (mg/mlc)
|
|||
|---|---|---|---|---|
| 0 h | 48 h | 0 h | 48 h | |
| GA | 0.030 ± 0.003 | 0.015 ± 0.005 | 0.026 ± 0.003 | 0.022 ± 0.004 |
| TB | 0.013 ± 0.003 | 0.013 ± 0.012 | 0.025 ± 0.003 | 0.025 ± 0.023 |
| TP | 0.001 ± 0.001 | 0.001 ± 0.001 | 0.001 ± 0.001 | 0.001 ± 0.001 |
| EGC | 0.061 ± 0.029 | 0.015 ± 0.033 | 0.046 ± 0.019 | 0.053 ± 0.042 |
| CAT | 0.083 ± 0.011 | 0.038 ± 0.044 | 0.150 ± 0.039 | 0.038 ± 0.005 |
| CAF | 0.807 ± 0.030 | 0.547 ± 0.124 | 0.558 ± 0.041 | 0.362 ± 0.122 |
| EGCG | 0.335 ± 0.103 | 0.044 ± 0.088 | 0.280 ± 0.120 | 0.123 ± 0.085 |
| EC | 0.100 ± 0.076 | 0.002 ± 0.003 | 0.065 ± 0.033 | 0.003 ± 0.001 |
| ECG | 0.215 ± 0.125 | 0.077 ± 0.032 | 0.125 ± 0.061 | 0.086 ± 0.012 |
Analysis was by HPLC as previously described (4).
GA, gallic acid; TB, theobromine; TP, theophylline; EGC, (−)-epigallocatechin; CAT, (+)-catechin; CAF, caffeine; EGCG, epigallocatechin-3-gallate; EC, (−)-epicatechin; ECG, (−) epicatechin gallate.
Mean ± standard deviation.
Animals
Forty-two male Apcmin mice and 24 C57BL/6J+/+ (wild-type) mice were obtained at 5 weeks of age from the Jackson Laboratory (Bar Harbor, Maine). Mice were given 1.5% white tea, 1.5% green tea, 80 mg/ml sulindac, 80 mg/ml sulindac plus 1.5% white tea, or buffered water. Animals were given water and pelleted AIN-93G diet (Dyets, Bethlehem, PA) ad libitum. Mice were housed 2–3 per cage in filter-topped shoebox cages with CareFRESH bedding (Absorbtion, Bellingham, WA). Nestlets (Ancare, Bellmore, NY) and cardboard tubes were provided for environmental enrichment. Room temperature was maintained at 72° (±4°) with a relative humidity of 40–60%. Tea and water were changed every other day, and diet was replaced twice weekly.
Schedule of events
During the first 8 days of the experiment the tea and sulindac concentrations were gradually increased from ¼ to full strength. Animals remained on the experimental treatments for 12 weeks, then after a 12 h fast they were killed by an overdose of CO2. Blood was withdrawn by cardiac puncture, livers and spleens were removed and weighed, and livers were snap-frozen in liquid nitrogen. The GI tract was excised and cleaned. The intestinal tract was divided sequentially into sections that were opened longitudinally and examined under a dissecting microscope by individuals blinded to the genotype and treatment status of the animal. The number, size, and locations of polyps along the GI tract were recorded. A section of normal-looking tissue (from jejunum) and up to six polyps were removed and snap frozen, then intestinal sections were pinned flat and fixed mucosa side up in 10% neutral buffered formalin.
Western blotting
Normal-appearing intestinal mucosa and polyps (pool of 2–3 polyps for each individual animal in a group) were homogenized in ice-cold RIPA buffer (1X PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) using five presses of a glass/Teflon homogenizer. Samples were left on ice for at least 30 min, then centrifuged at 4°C at 13 000 r.p.m. for 15 min. The supernatant was saved and considered the whole cell lysate. Protein quantification was performed using bovine serum albumin as standard (26). Proteins were separated on 4–12% bis–tris gels (Novex, San Diego, CA) and transferred to nitrocellulose membranes. Equal loading and transfer were confirmed by staining blots with amido black. Primary and secondary antibodies were as follows: β-catenin, 1:600 dilution of mouse monoclonal (Transduction Laboratories, Lexington, KY); peroxisome proliferator-activated receptor delta (PPAR-δ), 1:500 dilution of rabbit polyclonal (Affinity bioreagents, Golden, CO); cyclin D1, 1:200 dilution of rabbit polyclonal (Neomarker, Fremont, CA); c-Jun (Transduction Laboratories), 1:1000 dilution of mouse monoclonal; goat anti-mouse HRPx, 1:25 000 dilution (BioRad, Hercules, CA); goat anti-rabbit HRPx, 1:25 000 dilution (BioRad). Detection was by chemiluminescence (NEN, Boston, MA), with detection onto film (Amersham, Piscataway, NJ) and blots were quantified using NIH Image 1.58.
Statistics
Hematocrits, animal weights, tea and water consumption, and tumor size were compared by ANOVA followed by Fisher’s PLSD. Tumor multiplicity was compared by the non-parametric Kruskal–Wallis test. The correlation between the hematocrits and tumor multiplicity was compared by linear regression. All statistics were performed using Stat View 5.0.1 (SAS Institute, Cary, NC).
Results
There were no statistically significant treatment-related differences in final body weights or in water/or tea consumption (not shown). Based on the average daily water consumption, animals treated with sulindac or sulindac plus tea received 9.92–11.05 mg/kg sulindac/day. Table I shows the concentration of the nine primary tea constituents quantified by HPLC in freshly brewed teas and after 48 h. The composition of the two freshly brewed teas was similar to that reported previously (4) except that in this study a shorter brew time (1.5 min rather than 5 min) was utilized. This resulted in a higher proportion of the rapidly extracted tea constituents (i.e. caffeine) to those less rapidly extracted with hot water (EGCG). The addition of sulindac to white tea did not alter the levels of the nine primary tea constituents (not shown). However, because sulindac is insoluble at low pH, chemopreventive agents were prepared in a phosphate buffer (final pH of 7.4). The stability of green tea catechins has been reported to be pH dependent with greater stability at lower pHs (27), and in this study certain tea constituents were much lower after 48 h in the buffered solution than in freshly brewed tea (Table I). This suggests that similar studies in the future should replace the tea solutions on a frequent basis, and no later than 48 h (as in this report). Alternatively, the tea and sulindac might be administered in water and AIN diet, respectively.
By experimental design, and in agreement with previous studies (28), the dose of 80 p.p.m. sulindac produced approximately a 50% decrease in intestinal tumor multiplicity (Figure 1); Apcmin controls had an average of 30.88 ± 3.38 tumors per intestine (small and large intestine combined) and those treated with 80 p.p.m. sulindac had 16.67 ± 2.30 tumors per animal (P < 0.0004). Apcmin mice treated with green tea had 17.00 ± 4.22 tumors per animal (P < 0.0004), those treated with white tea had 13.11 ± 0.10. (P < 0.0001), and those treated with the combination of white tea and sulindac had 7.67 ± 1.49 tumors per animal (P < 0.0001). Thus, sulindac, green tea, and white tea were equally effective under these conditions at suppressing polyp formation, and the combination of white tea plus sulindac was significantly more effective than either treatment alone (P < 0.025). As expected, wild-type mice had no macroscopically visible tumors.
Fig. 1.
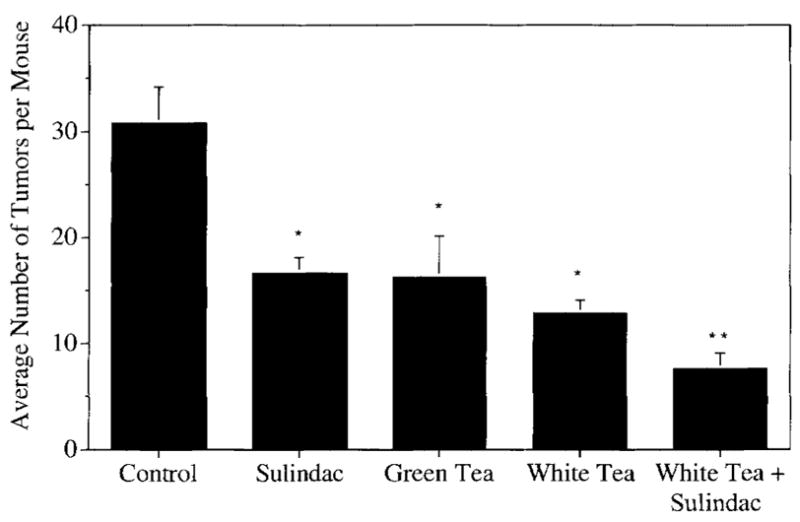
Inhibition of intestinal polyp formation in Apcmin mice by sulindac, white or green tea, or by a combination of white tea plus sulindac. Data are mean ± SE of eight or nine animals per treatment. Bars with different symbols are significantly different from one another (P < 0.05).
The distribution of polyps (Figure 2) along the intestinal tract in control animals was in accordance with previous reports (29,30), most of the tumors being found in the ileum. This was also the portion of the intestine in which the treatment-related tumor suppression was most marked. Apcmin mice treated with water, green tea, white tea, sulindac, or tea plus sulindac had on average 20, 12, 7, 10, and 2 polyps in the ileum respectively. In this region of the intestine, white tea was significantly more effective than green tea in reducing tumor number (P = 0.048) and the combination treatment was significantly more effective than tea or sulindac alone (P < 0.002). The number of polyps in the jejunum was also reduced significantly by each of the treatments relative to the controls, although there was no significant difference between the different tea and/or sulindac groups. Due to the small number of tumors in the other sections (duodenum, colon) suppression by the various treatments was not statistically significant.
Fig. 2.

Tumor distribution in the intestines of Apcmin mice. Intestines were divided into sections, examined under a stereomicroscope, and the number, size, and location of polyps was determined. Data are given as mean ± SE of eight or nine animals per treatment.
Apcmin mice reportedly develop a tumor-associated anemia with enlarged spleens (25,31). In this study, the average packed cell volume (PCV) or hematocrit in control Apcmin mice was just 22.25 ± 3.08 compared with 54.5 ± 0.86 in the wild-type control (P < 0.0001). Treatment with green tea, sulindac, white tea, or a combination of white tea plus sulindac resulted in PCVs of 32.63 ± 3.26, 33.56 ± 2.12, 40.44 ± 1.63, and 45.88 ± 2.10. There was a strong inverse correlation between the tumor multiplicity and hematocrits (P < 0.001) (Figure 3). The improvement in the severity of the tumor associated anemia was also reflected by the spleen size (Figure 4), with spleens of tea and sulindac-treated animals being much closer in size to those of the wild-type controls. This paper is to our knowledge the first to demonstrate the utility of using hematocrits and spleen size as markers of chemoprevention in the Apcmin mouse.
Fig. 3.
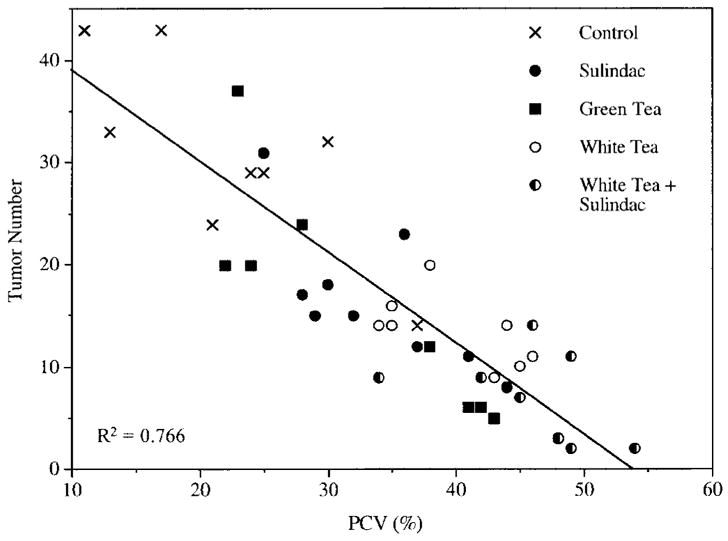
Inverse correlation between tumor number and hematocrits. Data points are intestinal tumor numbers plotted against the packed red blood cell volumes (PCV) for each individual animal. By linear regression analysis, R2 = 0.766.
Fig. 4.
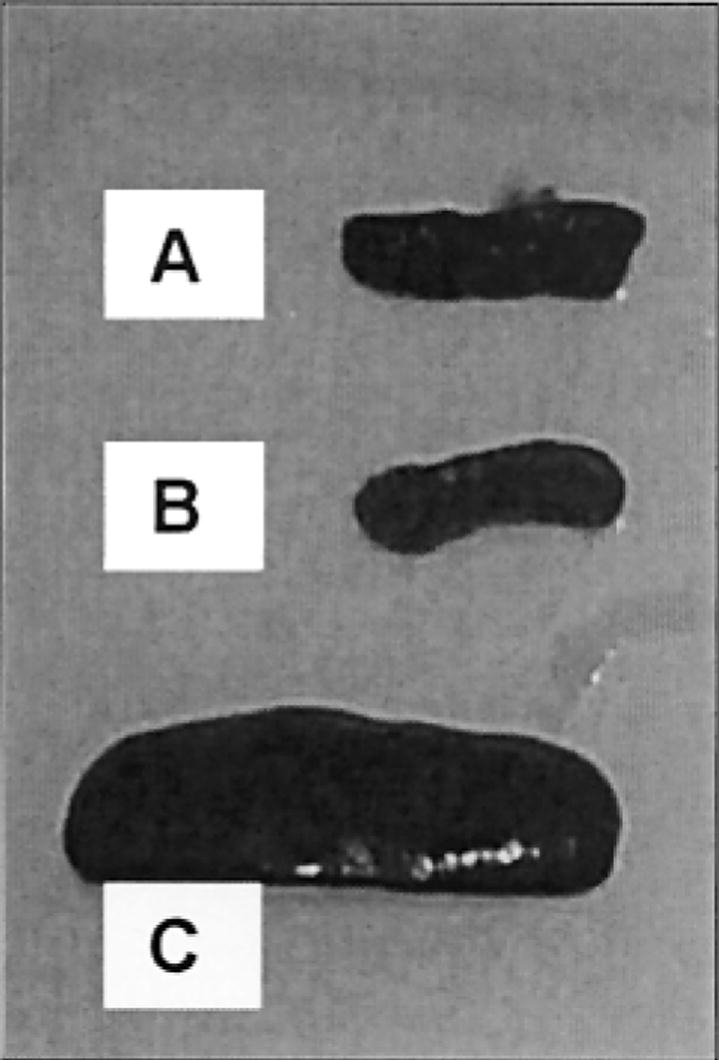
Representative spleens from (A) Apcmin mouse treated with a combination of tea plus sulindac, (B) untreated Apc wild-type mouse and (C) untreated Apcmin mouse.
Figure 5A shows representative western blots of polyps versus normal-appearing intestinal mucosa probed with antibodies to β-catenin, cyclin D1, c-Jun, PPAR-δ, and β-actin. Levels of β-catenin and two of the downstream signaling targets, c-Jun and Cyclin D1, were strongly reduced in the normal intestinal mucosa of animals treated with the combination of tea plus sulindac. In these animals, levels of c-Jun and Cyclin D1 in the jejunum were almost undetectable. However, the levels of these proteins were not reduced noticeably in the polyps. Surprisingly, the levels of PPAR-δ, another reported target of β-catenin/Tcf signaling (32) were not elevated in polyps relative to normal tissue, nor were they altered by tea and/or sulindac treatment. Levels of β-actin, a loading control, were also unaffected by these treatments. Densitometry measurements (Figure 5B) confirmed that normal-looking intestines from mice given tea plus sulindac had >5-fold lower levels of β-catenin, Cyclin D1 and c-Jun versus polyps from the same mice, or versus normal-looking intestines of control mice given drinking water alone (no tea or sulindac).
Fig. 5.
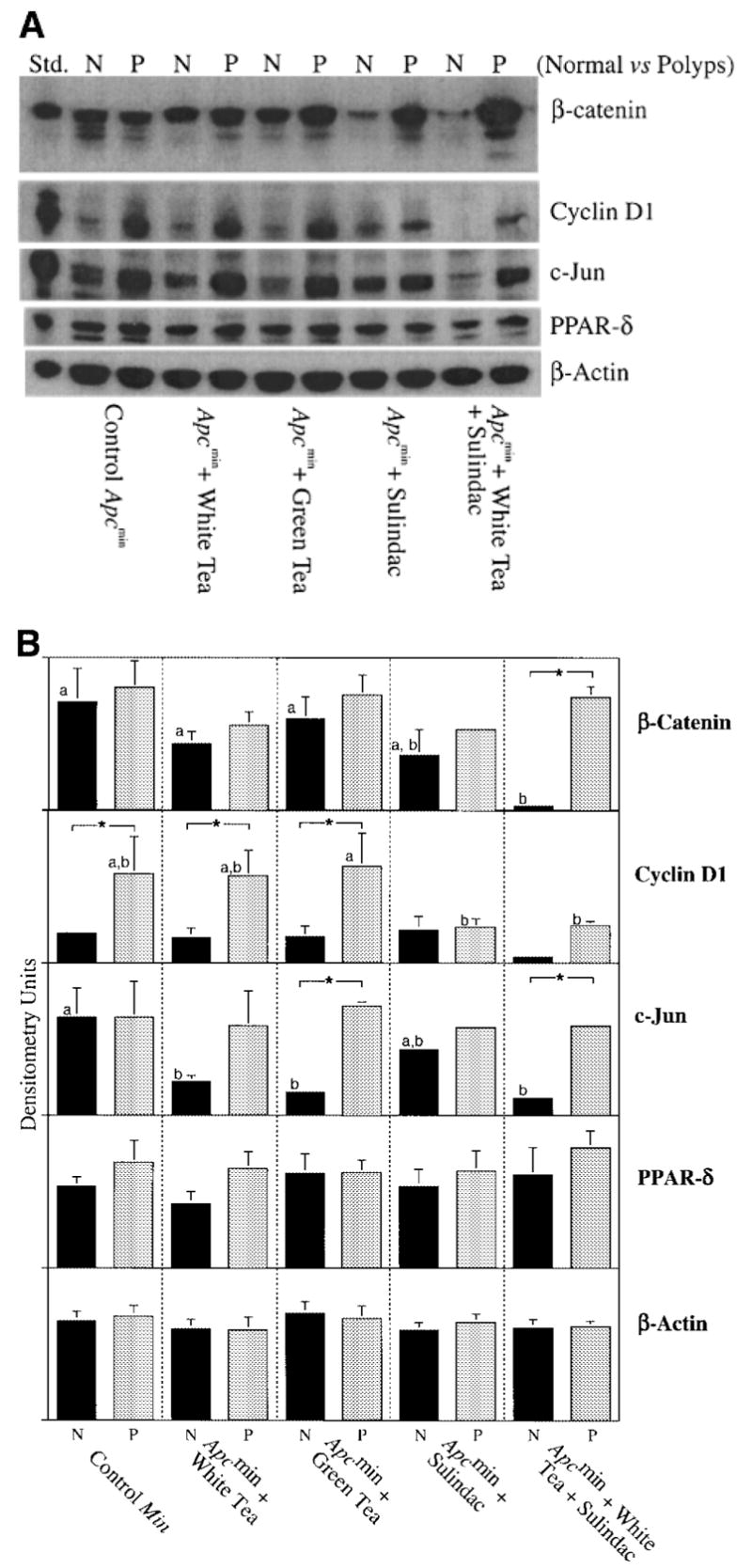
(A) Expression of β-catenin, Cyclin D1, c-Jun, and PPAR-δ proteins in normal intestinal mucosa (N) and polyps (P) of Apcmin mice treated with buffered water (controls), white tea, green tea, sulindac, or white tea plus sulindac. Twenty μg of protein were loaded into each lane. For each treatment group, a minimum of three polyps and three pieces of normal looking mucosa were analyzed by western blotting. The blots shown in (A) are representative examples for each test agent; three such experiments were performed in order to enable densitometry of three individual blots (mean ± SD) for each protein in (B). Black bars are of normal intestinal mucosa; gray bars are of polyps. Bars with different superscripts are significantly different from one another (within normal or polyp tissue). Pairs marked with * are significantly different when normal versus polyp tissue from the same animal were compared.
Discussion
The NSAIDs are promising agents for the inhibition of intestinal cancer. However, their utility is limited by gastrointestinal, hepatic, and renal toxicity (12). This study found that green and white teas at concentrations comparable with those consumed by humans provided similar protection against spontaneous intestinal polyp formation in male Apcmin mice and that both teas were as effective as 80 p.p.m. sulindac. Therefore, this widely consumed beverage may be useful in the prevention of intestinal cancer in genetically predisposed individuals, such as FAP patients.
The combination of sulindac plus tea appears to lower β-catenin protein levels and subsequent β-catenin/Tcf-activated transcriptional activity. Apcmin mice treated with sulindac or a combination of tea plus sulindac had significantly lower intestinal levels of β-catenin as well as reduced expression of two downstream signaling targets, namely cyclin D1 and c-Jun, but not of PPAR-δ. Several mechanisms might be postulated to explain the effects on β-catenin signaling, both direct and indirect. In the latter case, a reduction in the number of contaminating microadenomas in the ‘normal appearing’ intestinal sections by the chemopreventive agents could account for the reduction in β-catenin and of certain target genes (cyclin D1, c-Jun), but not others (PPAR-δ). Alternatively, the combination of tea plus sulindac may directly modify β-catenin signaling. Indeed, we recently found that white tea, green tea, or EGCG inhibited β-catenin/TCF-4 activity in reporter assays and lowered β-catenin protein expression in HEK293 cells (33). In the same experiments, sulindac at 10 μM had no effect on β-catenin levels, although higher concentrations were not tested. On the other hand, Hawcroft et al. reported that treatment of human colorectal cancer cells with the NSAID indomethacin (600 μM) resulted in lower expression of β-catenin and cyclin D1, but not of PPAR-δ (34) somewhat paralleling the data reported here (Figure 5). Collectively, the results suggest that tea and NSAIDs may regulate β-catenin signaling via direct and indirect mechanisms, although further work is necessary to clarify the relative significance of these various mechanisms.
In summary, we have shown that green tea and white tea suppress intestinal tumorigenesis in the Apcmin mouse. In the ileum, the major site of tumor formation in Apcmin mice, white tea was significantly more effective than green tea, thus extending previous findings in vitro showing white tea to exert a more potent antimutagenic effect than green tea against heterocyclic-induced mutagenicity (4). In Apcmin mice, white tea in combination with sulindac provided greater tumor suppression than tea or sulindac treatment alone. This suggests that a combination of tea plus low-dose sulindac might provide the same protection as higher doses of sulindac alone, while minimizing the toxicity associated with long-term NSAID treatment. The inverse correlation between polyp number and hematocrits suggests that the determination of PCV in future studies with chemopreventive agents is warranted and may provide an early, non-invasive indication of tumor burden.
Acknowledgments
Portions of this work were presented at the 8th International Conference on Carcinogenic/Mutagenic N-Substituted Aryl Compounds. The authors would like to thank Joy Edlund of The Stash Tea Company for providing Exotica China White tea and Ann Perez, Myron McMahan, Bill Amberg, and Lou-Anne Amberg for animal care. This work was supported in part by NIH grants CA65525 and CA80176 and by a Linus Pauling Institute pilot project. C.A.B. was supported by a toxicology training grant from the National Institute of Environmental Health Sciences (contract T32 ES0707060).
Abbreviations
- EGCG
epigallocatechin-3-gallate
- EGFRs
epidermal growth factor receptors
- FAP
familial adenomatous polyposis
- GI
gastrointestinal
- NSAIDs
non-steroidal anti-inflammatory drugs
- PPAR-δ
peroxisome proliferator-activated receptor delta
References
- 1.Katiyar SK, Mukhtar H. Tea antioxidants in cancer chemoprevention. J Cell Biochem. 1997;27:59–67. [PubMed] [Google Scholar]
- 2.Xu M, Bailey AC, Hernaez JF, Taoka CR, Schut HA, Dashwood RH. Protection by green tea, black tea and indole-3-carbinol against 2-amino-3-methylimidazo (4,5-f)quinoline-induced DNA adducts and colonic aberrant crypts in the F344 rat. Carcinogenesis. 1996;17:1429–1434. doi: 10.1093/carcin/17.7.1429. [DOI] [PubMed] [Google Scholar]
- 3.Xu M, Dashwood RH. Chemoprevention studies of heterocyclic amine-induced colon carcinogenesis. Cancer Lett. 1999;143:179–183. doi: 10.1016/s0304-3835(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 4.Santana-Rios G, Orner GA, Amantana A, Provost C, Wu SY, Dashwood RH. Potent antimutagenic activity of white tea in comparison with green tea in the Salmonella assay. Mutat Res. 2001;495:61–74. doi: 10.1016/s1383-5718(01)00200-5. [DOI] [PubMed] [Google Scholar]
- 5.Santana-Rios G, Orner GA, Xu M, Izquierdo-Pulido M, Dashwood RH. White tea as a potent inhibitor of 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine (PhIP)-induced colonic aberrant crypts in the F344 rat. Nutr Cancer. 2001;41:98–103. doi: 10.1080/01635581.2001.9680618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEntee MF, Chiu CH, Whelan J. Relationship of β-catenin and Bcl-2 expression to sulindac-induced regression of intestinal tumors in Min mice. Carcinogenesis. 1999;20:635–640. doi: 10.1093/carcin/20.4.635. [DOI] [PubMed] [Google Scholar]
- 7.Goluboff ET, Shabsigh A, Saidi JA, Weinstein IB, Mitra N, Heitjan D, Piazza GA, Pamukcu R, Buttyan R, Olsson CA. Exisulind (sulindac sulfone) suppresses growth of human prostate cancer in a nude mouse xenograft model by increasing apoptosis. Urology. 1999;53:440–445. doi: 10.1016/s0090-4295(98)00513-5. [DOI] [PubMed] [Google Scholar]
- 8.Piazza GA, Alberts DS, Hixson LJ, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57:2909–2915. [PubMed] [Google Scholar]
- 9.Waddell WR, Loughry RW. Sulindac for polyposis of the colon. J Surg Oncol. 1983;24:83–87. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- 10.Thun MJ. Aspirin and gastrointestinal cancer. Adv Exp Med Biol. 1997:395–402. doi: 10.1007/978-1-4615-5325-0_53. [DOI] [PubMed] [Google Scholar]
- 11.Bjorkman DJ. Current status of nonsteroidal anti-inflammatory drug (NSAID) use in the United States: risk factors and frequency of complications. Am J Med. 1999;107:3S–8S. doi: 10.1016/s0002-9343(99)00362-9. discussion 8S–10S. [DOI] [PubMed] [Google Scholar]
- 12.PDR Guide to Drug Interactions, Side Effects, Indications, Contraindications. Medical Economics; Montvale, NJ: 1997. [Google Scholar]
- 13.Torrance CJ, Jackson PE, Montgomery E, Kinzler KW, Vogelstein B, Wissner A, Nunes M, Frost P, Discafani CM. Combinatorial chemoprevention of intestinal neoplasia. Nat Med. 2000;6:1024–1028. doi: 10.1038/79534. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Jin X, Yaping E, et al. Photoprotective effect of black tea extracts against UVB-induced phototoxicity in skin. Photochem Photobiol. 1999;70:637–644. doi: 10.1562/0031-8655(1999)070<0637:peobte>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Lin JK, Liang YC, Lin-Shiau SY. Cancer chemoprevention by tea polyphenols through mitotic signal transduction blockade. Biochem Pharmacol. 1999;58:911–915. doi: 10.1016/s0006-2952(99)00112-4. [DOI] [PubMed] [Google Scholar]
- 16.Liang YC, Lin-shiau SY, Chen CF, Lin JK. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (−)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J Cell Biochem. 1997;67:55–65. doi: 10.1002/(sici)1097-4644(19971001)67:1<55::aid-jcb6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Liang YC, Chen YC, Lin YL, Lin-Shiau SY, Ho CT, Lin JK. Suppression of extracellular signals and cell proliferation by the black tea polyphenol, theaflavin-3,3′-digallate. Carcinogenesis. 1999;20:733–736. doi: 10.1093/carcin/20.4.733. [DOI] [PubMed] [Google Scholar]
- 18.Fujiki H. Two stages of cancer prevention with green tea. J Cancer Res Clin Oncol. 1999;125:589–597. doi: 10.1007/s004320050321. [DOI] [PubMed] [Google Scholar]
- 19.Jankun J, Selman SH, Swiercz R, Skrzypczak-Jankun E. Why drinking green tea could prevent cancer. Nature. 1997;387:561. doi: 10.1038/42381. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Dong Z, Valcic S, Timmermann BN, Bowden GT. Inhibition of ultraviolet B–induced c-fos gene expression and p38 mitogen-activated protein kinase activation by (−)-epigallocatechin gallate in a human keratinocyte cell line. Mol Carcinog. 1999;24:79–84. doi: 10.1002/(sici)1098-2744(199902)24:2<79::aid-mc1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Naasani I, Seimiya H, Tsuruo T. Telomerase inhibition, telomere shortening and senescence of cancer cells by tea catechins. Biochem Biophys Res Commun. 1998;249:391–396. doi: 10.1006/bbrc.1998.9075. [DOI] [PubMed] [Google Scholar]
- 22.Swiercz R, Skrzypczak-Jankun E, Merrell MM, Selman SH, Jankun J. Angiostatic activity of synthetic inhibitors of urokinase type plasminogen activator. Oncol Rep. 1999;6:523–526. doi: 10.3892/or.6.3.523. [DOI] [PubMed] [Google Scholar]
- 23.Suganuma M, Okabe S, Kai Y, Sueoka N, Sueoka E, Fujiki H. Synergistic effects of (−)-epigallocatechin gallate with (−)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Res. 1999;59:44–47. [PubMed] [Google Scholar]
- 24.Ohishi T, Kishimoto Y, Miura N, Shiota G, Kohri T, Hara Y, Hasegawa J, Isemura M. Synergistic effects of (−)-epigallocatechin gallate with sulindac against colon carcinogenesis of rats treated with azoxymethane. Cancer Lett. 2002;177:49–56. doi: 10.1016/s0304-3835(01)00767-4. [DOI] [PubMed] [Google Scholar]
- 25.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 26.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Chen Z, Zhu QY, Tsang D, Huang Y. Degradation of green tea catechins in tea drinks. J Agric Food Chem. 2001;49:477–482. doi: 10.1021/jf000877h. [DOI] [PubMed] [Google Scholar]
- 28.Beazer-Barclay Y, Levy DB, Moser AR, Dove WF, Hamilton SR, Vogelstein B, Kinzler KW. Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis. 1996;17:1757–1760. doi: 10.1093/carcin/17.8.1757. [DOI] [PubMed] [Google Scholar]
- 29.Ritland SR, Leighton JA, Hirsch RE, Morrow JD, Weaver AL, Gendler SJ. Evaluation of 5-aminosalicylic acid (5-ASA) for cancer chemoprevention: lack of efficacy against nascent adenomatous polyps in the Apc (Min) mouse. Clin Cancer Res. 1999;5:855–863. [PubMed] [Google Scholar]
- 30.Sorensen IK, Kristiansen E, Mortensen A, Nicolaisen GM, Wijnands JA, van Kranen HJ, van Kreijl CF. The effect of soy isoflavones on the development of intestinal neoplasia in ApcMin mouse. Cancer Lett. 1998;130:217–225. doi: 10.1016/s0304-3835(98)00139-6. [DOI] [PubMed] [Google Scholar]
- 31.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 32.He TC, Chan TA, Vogelstein B, Kinzler KW. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dashwood WM, Orner GA, Dashwood RH. Inhibition of β-catenin/Tcf activity by white tea, green tea and epigallocatechin-3-gallate (EGCG): minor contribution of H2O2 at physiologically relevant EGCG concentrations. Biochem Biophys Res Commun. 2002;296:584–588. doi: 10.1016/s0006-291x(02)00914-2. [DOI] [PubMed] [Google Scholar]
- 34.Hawcroft G, D’Amico M, Albanese C, Markham AF, Pestell RG, Hull MA. Indomethacin induces differential expression of beta-catenin, gamma-catenin and T-cell factor target genes in human colorectal cancer cells. Carcinogenesis. 2002;23:107–114. doi: 10.1093/carcin/23.1.107. [DOI] [PubMed] [Google Scholar]


