Abstract
Investigation of the three-generation KE family, half of whose members are affected by a pronounced verbal dyspraxia, has led to identification of their core deficit as one involving sequential articulation and orofacial praxis. A positron emission tomography activation study revealed functional abnormalities in both cortical and subcortical motor-related areas of the frontal lobe, while quantitative analyses of magnetic resonance imaging scans revealed structural abnormalities in several of these same areas, particularly the caudate nucleus, which was found to be abnormally small bilaterally. A recent linkage study [Fisher, S., Vargha-Khadem, F., Watkins, K. E., Monaco, A. P. & Pembry, M. E. (1998) Nat. Genet. 18, 168–170] localized the abnormal gene (SPCH1) to a 5.6-centiMorgan interval in the chromosomal band 7q31. The genetic mutation or deletion in this region has resulted in the abnormal development of several brain areas that appear to be critical for both orofacial movements and sequential articulation, leading to marked disruption of speech and expressive language.
Developmental disorders of speech and language are estimated to occur in as many as 7% of children who have no gross deficits in hearing, intelligence, or socioemotional stimulation (1). In at least some of these children, genetic factors have been implicated (2, 3). For example, in a study of a large cohort of twins, a high incidence of heritability was reported for two of the several subtypes of such disorders, namely, expressive language impairment without an articulation disorder, the most common subtype, and expressive language impairment with an articulation disorder (4). The neural basis of these developmental impairments is unclear. The limited results available (5) suggest the presence of widespread neuropathology, which is not wholly consistent with findings in adult patients with acquired aphasia and speech apraxia in whom the critical site of pathology is known to be located in the perisylvian and anterior insular cortices on the left (6). The inconsistency may arise in part because children with frank brain damage, whether congenital or acquired before puberty, are seriously impaired in speech and language only if there is bilateral pathology of the perisylvian areas or of the subcortical structures with which they are directly connected (7–10). Therefore, in developmental disorders where speech and language functions are selectively and permanently compromised, bilateral pathology of one or more components of the responsible neural system must be suspected.
Against this background, the large three-generation KE family, half of whose members are affected by a developmental verbal dyspraxia (11), offers a unique opportunity to identify the brain abnormalities associated with their particular form of impairment, namely, an expressive language deficit accompanied by an articulation disorder. The locus of the abnormal gene (SPCH1) in the affected members was recently mapped to a 5.6-centiMorgan interval in 7q31, confirming autosomal dominant inheritance with full penetrance (12). Importantly, there is complete concordance between inheritance of the mutant chromosomal region and presence of the overt speech disorder, thereby providing an independent genetic index of affected versus unaffected status. The most striking feature of the disorder is an impairment in sequential articulation (i.e., a verbal dyspraxia) so severe that the speech of the affected members is often rendered largely incomprehensible to the naive listener (13, 14). This articulatory disorder is likely to be due in part to an orofacial dyspraxia, which is reflected in a relative immobility of the affected members’ lower face and mouth, particularly the upper lip, and consequently in an impairment in nonspeech movements as well (14, 15). The disorder is not restricted to orofacial movements and articulation but also extends to both expressive and, to a lesser degree, receptive language abilities (14). Furthermore, the affected family members show significant deficits not only in verbal intelligence but also in nonverbal intelligence such that the full-scale intelligence quotients of the majority of the affected individuals (7 of the 13 tested) fall below the low average range (80–89), with two more scoring only 81. In contrast, none of the unaffected members tested had full-scale intelligence quotients below the low average range (12, 14). However, the developmental verbal and orofacial dyspraxias are the most prominent symptoms, and they suggest not only that the responsible neuropathology is bilateral (see above) but also that this bilateral pathology is located in one or more components of the motor system.
Here we report behavioral data that identify the core neuropsychological deficits associated with this genetic disorder. In addition, we present functional and structural brain imaging evidence suggesting that a key site of bilateral pathology is in the basal ganglia, and that this pathology in turn affects several frontal cortical motor areas important for speech and language.
METHODS
Word and Nonword Repetition (16).
Forty words ranging in length from two to five syllables and 40 nonwords ranging in length from one to four syllables were read to the subjects one at a time. The subjects were required to repeat each word or nonword immediately after it was spoken. Responses were recorded on audiotape and scored as correct or incorrect (maximum score for each test was 40). Comparison was between the performance of the affected and of the unaffected family members.
Simultaneous and Sequential Orofacial Movements to Command (15).
The subjects, who were seated facing the experimenter, were required to perform two types of movements in response to the experimenter’s verbal instructions. One type required three simultaneous movements: for each of 11 such sets, 3 single movements were carried out at the same time: e.g., “open your mouth wide, stick out your tongue, and make an ‘ah’ sound.” A second type required three sequential movements; for each of 11 such sets, 3 single movements were carried out one after the other; e.g., “first open your mouth wide, then close your lips tightly together, then make an ‘ah’ sound.” Each subject’s movements were recorded on video for later analysis and scoring, with partial credit given for partial performance. Comparison was between the performance of the affected family members and that of a group of 52 age-matched normal control subjects.
Positron Emission Tomography (PET).
PET imaging was performed by using H2O15 and a dedicated head scanner (Siemens 953B, CTI, Knoxville, TN). During the scans the subjects listened via headphones to real words or reversed words at a rate of 40 stimuli/min. The subjects were instructed to repeat aloud the real words and to say one specified word repeatedly when presented with reversed words. Each subject received 6 scans under each of the two conditions presented in alternating order, for a total of 48 scans across the four normal control subjects and 24 scans in the two affected family members. Both conditions require acoustic processing and motor output, but repeating heard words requires, in addition, phonological analysis and reformulation of speech sounds into articulation plans.
The reconstructed images had a transaxial resolution of 8.5 mm full width at half maximum, and contained 128 × 128 pixels, each 2.05 mm × 2.05 mm × 4 mm thick. The data were preprocessed in terms of anatomical normalization, motion correction, and smoothing to improve the signal to noise ratio and analyzed by using statistical parametric mapping [Statistical Parametric Mapping (SPM) 96, Wellcome Department of Cognitive Neurology, London]. The anatomical coordinates of activations are given in standard Talairach and Tournoux space (17). All anatomical normalization was performed automatically, without user interaction, and employed the Montreal Neurological Institute standard data set as a template (18). The main effects of condition and the interactions of pathology by condition were assessed by using analysis of covariance and linear contrasts as described elsewhere (19).
Magnetic Resonance Imaging (MRI).
Family members and age-matched controls were scanned by using a 1.5 T Siemens system with a standard quadrature head coil. Three-dimensional data sets of the whole head were collected by using a T1-weighted magnetization prepared rapid acquisition gradient echo (20) sequence (repetition time = 10 ms, echo time = 4 ms, inversion time = 200 ms, flip angle = 12°, matrix size = 256 × 256, field of view = 250 mm, partition thickness = 1.25 mm, 128 sagittal partitions in the third dimension, acquisition time = 8.3 min). The data sets were analyzed by a procedure similar to that described by Wright et al. (21), implemented in SPM96 software running in matlab (Mathworks, Sherborn, MA). Thirty-four three-dimensional data sets (from the 17 family members, 10 affected and 7 unaffected, and 17 age-matched controls) were spatially normalized by minimizing the sum of squared differences between them and a template image according to the basis-function approach (18), and the mean image of these spatially normalized scans was smoothed to 8 mm full width at half maximum to generate a second template. The original three-dimensional MRI data sets of the 13 third-generation family members (6 affected and 7 unaffected), who were more closely matched in age than the full group of 17 family members, were then spatially normalized by matching to the second template. These images were resampled to produce voxels of 1.5 × 1.5 × 1.5 mm. The normalized images were next partitioned into gray, white, cerebro-spinal fluid, and scalp images (22). The resulting gray matter images were smoothed by using a 12 mm full width at half maximum isotropic Gaussian kernel and analyzed by using SPM96 to compare affected and unaffected family members. The resulting t maps were generated for two contrasts, one comparing areas in which the affected family members had more gray matter than the unaffected members, and the other comparing areas in which they had less gray matter. The t maps were transformed to the unit normal distribution (Z) and thresholded at 3.09 (P = 0.001). The significance of each region was estimated by using distributional approximations from the theory of random Gaussian fields. This characterization is in terms of the probability that the peak height observed (or higher) could have occurred by chance [P(Zmax > u)] over the entire volume analyzed (i.e., a corrected P value of <0.05). An uncorrected P value of <0.001 was used for regions that had been predicted in advance (i.e., showed functional abnormality in the PET study or were in known anterior language or motor regions).
For measurement of the caudate nucleus volumes (CNVs), 1-mm-thick contiguous slices parallel to the transverse plane through the anterior and posterior commissures were reformatted from each three-dimensional data set. The cross-sectional areas of the caudate nuclei were measured on each slice, from the superior surface of the nucleus, where it appears lateral to the lateral ventricles, to its inferior limit, at about the level of the anterior commissure, where it merges into the nucleus accumbens. Although the head and body of the caudate nucleus were easily seen on all slices, the tail was often indistinguishable from the nearby ventricle and hippocampus/amygdala and so was not measured. The volumes were calculated by summing the cross-sectional areas and multiplying by the slice thickness (1 mm). Measurements were made by the same researcher blind to the classification of family members as affected or unaffected. Volumes were measured twice, and good measure–remeasure reliability (r = 0.98, P < 0.0005) was obtained. The mean of the two volume measurements was then used in the analysis. Intracranial volumes (ICVs) were estimated on sagittal slices from the three-dimensional data set (23). The caudate volumes were then divided by the ICVs to produce a percentage. The left and right hemisphere CNVs, ICVs, and percentages for left and right measurements were then compared in affected and unaffected family members by independent t-tests (see Table 3).
Table 3.
CNV and ICV in affected and unaffected family members
| Side | Affected
|
Unaffected
|
t (df = 11) | P (two-tail) | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| CNV, cm3 | L | 4.09 | 0.42 | 5.11 | 0.64 | 3.30 | 0.007 |
| R | 4.19 | 0.48 | 5.28 | 0.60 | 3.58 | 0.004 | |
| ICV, cm3 | 1402 | 49 | 1431 | 139 | 0.49 | NS | |
| CNV/ICV, % | L | 0.29 | 0.03 | 0.36 | 0.03 | 3.88 | 0.003 |
| R | 0.30 | 0.03 | 0.37 | 0.04 | 3.51 | 0.005 | |
Results of analysis comparing third-generation family members only (i.e. six affected and seven unaffected), closely matched in age. An analysis that included all 10 of the affected family members for whom MRI data were available yielded similar results. L, left; R, right; NS, not significant.
RESULTS
Behavioral Phenotype.
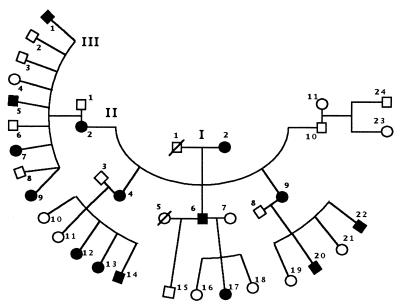
The pedigree of the KE family, consisting of 15 affected members, 16 unaffected members (including founding grandfather), and 6 unaffected spouses who married into the family, is shown in Fig. 1.
Figure 1.
Pedigree of KE family. Roman numerals indicate the generation, and Arabic numerals, the member’s pedigree number within a generation. Affected members, filled shapes; unaffected members, open shapes; females, circles; males, squares; /, deceased; ∧, twins.
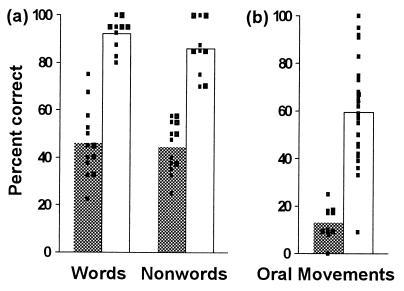
Identification of the core deficit in this genetic disorder has been a subject of considerable interest. A core deficit, or a characterizing phenotype, is one shown by every affected family member but not by any who are unaffected. Although the mean scores of the affected members taken as a group fall significantly below those of the group of unaffected members on nearly every test used thus far to assess an aspect of their speech and language function and orofacial praxis,‡‡ every one of the affected members is impaired individually on just three tests, namely, word repetition, nonword repetition, and simultaneous and sequential orofacial movements (refs. 15 and 16; see Methods). On none of these three tests do the individual scores of the affected members overlap with those of the comparison groups (unaffected family members in the case of word and nonword repetition, and age-matched normal controls in the case of orofacial movements) (Fig. 2). Thus, whether or not an individual family member has the disorder can be determined on the basis of these scores alone.
Figure 2.
(a) Word and nonword repetition. Bars indicate mean percent correct for the groups of affected and unaffected family members (n = 13 and 10, respectively). Filled bars, affected group; open bars, unaffected group; small squares, scores of individual family members. Note the absence of overlap between the scores of the two groups on both tests. (b) Simultaneous and sequential orofacial movements to command. Bars indicate mean percent correct for the group of affected family members and the normal control group (n = 11 and 52, respectively). Filled bar, affected group; open bar, control group; small squares, scores of individuals (for clarity, the same score obtained by two or more control subjects is marked by a single square). Again note the absence of overlap between the scores of the two groups, except for one statistical outlier (a 45-year-old male) in the control group.
It should be noted that the items on the test of orofacial movements were designed to resemble some of the movements that underlie the sequential articulation of speech sounds (15). Nevertheless, whereas the scores of the affected family members on word and nonword repetition were correlated with each other (ρ = 0.56, P = 0.044), neither of these scores correlated significantly with their scores on simultaneous and sequential orofacial movements. The results indicate that even though both the orofacial movement and speech repetition tests are probably tapping into the same core deficit—i.e., both are measures of oromotor coordination and every affected individual is impaired on both—there are also important differences between the two, with speech repetition requiring a far greater oromotor range, complexity, and precision than the other.
Functional Brain Abnormalities.
To test for the presence of functional brain abnormalities, we compared brain activation patterns with PET in two affected family members (II-2 and II-9; see Fig. 1)†† and four normal control subjects. During scanning, the participants repeated words heard over earphones, and, in the baseline condition, they repeated a single specified word in response to hearing words that were reversed. Comparison of these two conditions in normal right-handed volunteers (24) reveals the major speech and language areas of the left hemisphere. Although their articulation was not as clear as that of the control subjects, the two affected family members uttered all the words that were presented.
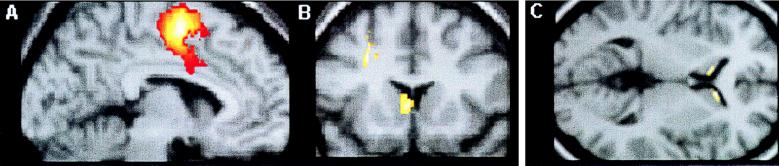
Statistical analyses were performed to determine whether the change in activation patterns between the two conditions differed in the affected members from that in the normal controls (i.e., interaction between pathology and condition). The results revealed several brain regions that were less active (relative to baseline levels) in the two affected family members than in the control subjects and other areas that were more active in the family members (Table 1). The areas identified as being either more or less active than normal satisfied the statistical threshold criteria, which were chosen a priori (25). The underactive regions included the left supplementary motor area (SMA), the subjacent cingulate cortex on the left, and the left preSMA/cingulate cortex (Fig. 3A), all of which were activated relative to the baseline condition in the normal subjects but not in either of the affected family members. The left sensorimotor face and mouth region was also less active than normal in the two affected family members, although in this case it was significantly activated in each of them relative to baseline levels. The regions that were overactive in the affected family members included the head (Fig. 3B) and tail of the left caudate nucleus, the left premotor cortex (Fig. 3B) with a ventral extension into Broca’s area (area 44), and a left ventral prefrontal area (area 47/45).
Table 1.
Results of SPM analysis of PET data
| Area | Coordinate | Z | Size | ||
|---|---|---|---|---|---|
| Underactive | |||||
| L. preSMA/cingulate cortex | −10 | 12 | 48 | ||
| L. SMA | −6 | −2 | 56 | 6.05 | 692 |
| L. SMA/cingulate cortex | −2 | −4 | 48 | ||
| L. sensorimotor cortex (face, lips) | −50 | −20 | 36 | 4.77 | 148 |
| L. middle temporal cortex | −56 | −54 | 4 | 3.12 | 58 |
| Overactive | |||||
| L. ventral prefrontal cortex (47/45) | −46 | 32 | −4 | 3.27 | 20 |
| L. premotor cortex | −26 | 18 | 36 | 3.49 | 149 |
| −24 | 18 | 48 | 3.32 | ||
| L. caudate nucleus (head) | −2 | 14 | 8 | 3.77 | 140 |
| L. Broca’s area (44) | −46 | 10 | 20 | 3.27 | 149 |
| L. caudate nucleus (tail) | −18 | −32 | 16 | 3.01 | 153 |
| −28 | −38 | 16 | 3.23 | ||
| −22 | −42 | 12 | 3.96 | ||
| L. angular gyrus (39/19) | −42 | −72 | 32 | 3.37 | 91 |
| −36 | −76 | 36 | 3.40 | ||
L, left; Z, no. of SDs away from the mean; Size, no. of activated voxels (2.05 × 2.05 × 4.0 mm).
Figure 3.
Results of SPM analysis of PET data (A and B; see also Table 1) and MRI data (C; see also Table 2). (A) Parasagittal section through left hemisphere, 6 mm from midline. Colored area, encompassing parts of SMA, preSMA, and cingulate cortices, indicates a region that was less active in the affected family members than in the controls. (B) Coronal section, 14 mm in front of the coronal plane through the anterior commissure. Colored areas, located in the head of the left caudate nucleus and left premotor cortex, indicate regions that were more active in the affected family members than in the controls. (C) Transverse section, 2 mm above the transverse plane through the anterior and posterior commissures. Colored areas, located in the head of the caudate nucleus bilaterally, indicate areas that had less gray matter in the group of affected than in the group of unaffected family members (n = 6 and 7, respectively).
Structural Brain Abnormalities.
In an attempt to identify a possible structural basis for the above functional abnormalities, as well as to test the hypothesis that affected members suffer from bilateral abnormality in some motor-related structure(s), we examined 17 family members, 10 affected (including the two who had undergone PET scans) and 7 unaffected, with structural MRI. The initial neuroradiological examination revealed no overt pathology, except in the scan of the affected female III-9, which showed gross hemispheric asymmetry, the left hemisphere being much larger than the right posteriorly. However, a statistical analysis of brain morphology based on maps of gray matter in a subgroup of the affected members (n = 6) matched in age to the unaffected members (n = 7) (see Methods) revealed several regions where the affected group had either significantly more or significantly less gray matter than the unaffected group. Among the regions with more gray matter, one, the lentiform nucleus, comprising the putamen and globus pallidus, is a motor-related structure; and another, the angular gyrus, had shown overactivation on the left in the PET study. The statistical analysis of the MRI scans revealed that both of these regions were abnormal bilaterally. Among the regions in which affected members had less gray matter than the unaffected were three—preSMA/cingulate cortex, Broca’s area, and the caudate nucleus—that had also been found to be functionally abnormal on the left in the PET study (the first was underactive, and the other two, overactive). However, although all three are motor-related structures, only one, the caudate nucleus, showed structural abnormality bilaterally (Table 2 and Fig. 3C); the others showed structural abnormality only on the left.
Table 2.
Results of SPM analysis of MRI data
| Area | Coordinate | Z | ||
|---|---|---|---|---|
| Less gray matter in affected members | ||||
| L. Broca’s area*† | −50 | 28 | 18 | 3.41 |
| −48 | 21 | 28 | 3.49 | |
| R. caudate nucleus (head)† | 8 | 22 | 2 | 3.64 |
| 18 | 21 | 8 | 3.53 | |
| L. caudate nucleus (head)*† | −9 | 15 | 9 | 3.44 |
| L. preSMA/cingulate cortex*† | −10 | 9 | 51 | 3.72 |
| More gray matter in affected members | ||||
| L. anterior insula cortex† | −36 | 24 | 6 | 3.93 |
| R. lentiform nucleus† | 28 | −4 | −6 | 3.61 |
| L. lentiform nucleus† | −30 | −15 | −3 | 4.74 |
| R. sensorimotor cortex† | 62 | −9 | 44 | 4.06 |
| 51 | −18 | 58 | 3.95 | |
| R. posterior temporal cortex‡ | 56 | −24 | 0 | 4.28 |
| L. posterior temporal cortex§ | −56 | −38 | 6 | 4.80 |
| R. posterior thalamus§ | 21 | −27 | 0 | 5.01 |
| L. posterior thalamus‡ | −22 | −32 | 3 | 3.48 |
| R. angular gyrus‡ | 40 | −72 | 24 | 3.70 |
| L. angular gyrus* | −36 | −80 | 36 | 3.34 |
L, left; R, right.
Uncorrected P value of <0.001: showed functional abnormality in the PET study.
Uncorrected P value of <0.001: known motor or anterior language region.
Uncorrected P value of <0.001: symmetrical with region labeled with ∗ or §.
Corrected P value of <0.05.
Because the caudate nucleus was thus the one motor-related region that had shown both functional abnormality (on the left) and bilateral structural abnormality, thereby meeting all of our criteria for a critical site of pathology, we performed a second quantitative analysis in which we focused on the caudate nucleus. This second analysis, which involved direct volumetric measurements (see Methods), revealed that the affected group had significantly smaller left and right caudate volumes than the unaffected group (Table 3). Direct volumetric measurements of other cortical and subcortical structures are needed to evaluate the other differences that were obtained by the statistical analyses of the MRI scans.
DISCUSSION
The results obtained on the tests of both word and nonword repetition (16) and simultaneous and sequential orofacial movements (15) identify the core deficit in this genetic disorder as one that affects the rapid and precise coordination of orofacial movements, including those required for the sequential articulation of speech sounds. Although the scores of the affected family members on the two types of test were not correlated with each other, every one of the affected individuals was impaired on both. The orofacial praxis tests provide a quantified measure of the nonarticulatory aspects of this core deficit, whereas the repetition tasks highlight the special burden that speech imposes on the impaired orofacial motor system. Further, the fact that the affected family members are nearly as impaired on word as on nonword repetition suggests that the abnormality is less likely to reside in the phonological loop component of short-term verbal memory (16), the system engaged during subvocal rehearsal of unfamiliar phonological strings, than in the system responsible for sequential articulation. These behavioral data, in particular the absence of overlap between the scores of the affected members versus controls on the above tests only, and not on those of syntax or inflections, where they were impaired only as a group, argue against the notion that the characterizing phenotype in the KE family is a selective deficit in grammar (26–28).
How might this behavioral profile be accounted for by the brain imaging findings? The articulatory disturbance and orofacial dyspraxia are probably related at least in part to the abnormal activation of the left motor, supplementary motor, and premotor areas. The underactivation in the motor cortex is at the level of the face and mouth representation, a region that is activated when subjects repeat words (24). The premotor cortex and Broca’s area, which were overactive in the affected family members, are part of the distributed system that is activated when normal subjects are required to generate words fluently (29). What the overactivation represents in terms of neural processing is unclear. One possibility is that it is compensatory, reflecting more effortful speech by the impaired individuals. Alternatively, it could constitute a primary abnormality that interferes with their speech. Abnormal electroencephalographic activity in the centro-temporal and frontal regions was observed in a three-generation family with inherited Rolandic epilepsy, in which nine affected members, like those of the KE family, had both verbal and oral dyspraxias (30). Also, electrical stimulation in the region of Broca’s area and the ventral premotor cortex has been found to impair mimicry of orofacial movements (31).
The functional abnormalities observed in the several motor-related areas of the frontal cortex could be exerting their effects via the projections of these areas to the neostriatum (caudate nucleus and putamen), among other structures. We suspect, however, that the cortical areas are secondary sites and that the primary pathology is located in the neostriatum, for two reasons. First, this is the only motor-related region in which we found both functional (on the left) and bilateral structural abnormalities. And second, the functional imaging pattern shown by the affected family members resembles a pattern previously observed in some patients with familial or idiopathic spastic dystonia (32), a condition that is sometimes associated with neostriatal lesions (33). Thus, two patients who had acquired the syndrome after sustaining vascular damage involving the putamen (34) showed overactivation of the premotor and prefrontal cortical areas, regions whose activity is modulated by the putamen via a circuit coursing through the globus pallidus and substantia nigra and thence through the thalamus (35, 36). The findings in these patients with dystonia suggest the possibility that the premotor overactivation in the affected members of the KE family was likewise due to pathology in the neostriatum, particularly the caudate nucleus, which also projects to the frontal cortex indirectly via the globus pallidus, substantia nigra, and thalamus (33, 35).
The functional interactions among the structures of the basal ganglia circuits are known to be extremely complex (33), and so it is not surprising that the many abnormalities in these circuits uncovered in the affected family members do not yet permit an unambiguous interpretation. A more definitive interpretation will require further quantitative analyses of the MRI scans together with further functional imaging studies, including ones that probe right-hemisphere as well as left-hemisphere function. Nevertheless, the imaging results confirm a major prediction derived from the affected members’ phenotypic profile and its persistence into adult life, namely, the presence of bilateral pathology in at least one and possibly other components of the motor system. Thus, the bilateral reduction in the volume of the caudate nucleus provides a plausible explanation for their orofacial dyspraxia, which has persisted into maturity largely unchanged despite an origin in early development. Importantly, this same brain abnormality might also explain their verbal dyspraxia. Evidence gathered over the past decade indicates that not only frontal but also neostriatal pathology due to hemispheric strokes (36) or neurodegenerative diseases (37, 38), such as Parkinson’s and Huntington’s, can lead to severe disorders of speech and language, particularly of the expressive subtype that is accompanied by marked articulatory disturbances.
Our data suggest that development of the neural mechanisms mediating the acquisition of fine oromotor coordination (both vocal and nonvocal) and of speech and language are interdependent, such that abnormality in the one will be associated with abnormality in the other. The same developing neural network controlling oromotor coordination and expressive language may also be a prerequisite for the emergence of “inner speech” and the development of higher-order thought processes (39), in which case a central abnormality affecting speech production could have a cascading effect resulting in intellectual defects. According to this view, the multiple behavioral impairments of the affected family members might all be traceable to abnormality of a single neural network basic to speech production, with this abnormality resulting from the deletion or disruption of a single gene at 7q31. At this stage, however, we cannot discount the alternative possibility that the different components of the phenotypic profile are the consequence of abnormalities in several different neural networks resulting from disruption of either a single gene or even several contiguous genes.
In conclusion, our structural brain imaging data indicate that mutation at this locus has resulted in abnormal development of several brain areas, including bilateral reduction in the volume of the caudate nucleus. These structural abnormalities could account for the functional abnormalities revealed with PET in these same and other regions of the frontal lobe, and these, in turn, could help explain the affected members’ developmental syndrome, which is characterized by orofacial dyspraxia together with a marked disorder of expressive language accompanied by a pronounced articulatory disturbance. These findings open the way toward defining brain/behavior correlations in this and other forms of inherited speech and language disorders, thereby offering a powerful approach to uncovering the neural basis of normal speech and language development.
Acknowledgments
We are deeply indebted to all the members of the KE family, whose continued cooperation has made this research possible. We thank Cheryl Johnson and Caroline Moore for help with data acquisition on MRI and PET, respectively, and John Mazziotta and Paula Tallal for valuable comments on the manuscript. Approval for the research was obtained from the Ethics Committee of Great Ormond Street Hospital for Children National Health Service Trust (642, November, 20, 1987, and updated November 24, 1992), and the consent of each participant was obtained after the procedures were explained. The investigation was supported in part by the Wellcome Trust. R.S.J.F., K.J.F., and C.J.P. are Wellcome Trust Principal Research Fellows; M.E.P. is funded by Mothercare UK.
ABBREVIATIONS
- PET
positron emission tomography
- MRI
magnetic resonance imaging
- CNV
caudate nucleus volume
- ICV
intracranial volume
- SMA
supplementary motor area
- SPM
statistical parametric mapping
Footnotes
Of 19 separate measures of speech and language function (14), only two (object naming and judgment of morphological markers–words) failed to discriminate between the group of affected and the group of unaffected family members. The tests yielding reliable deficits in the affected group included five measures of word, nonword, and sentence repetition; three of phonology and rhyming; five of reception and production of grammar; two of nonword reading and spelling; and one each of lexical decision and receptive vocabulary. Of nine separate measures of orofacial praxis (14, 15), five to command and four to imitation, only two (single movements either to command or to imitation) failed to discriminate reliably between the affected group and their controls. As indicated in the text, however, even on the measures that did yield reliable impairment in the affected family members as a group, their individual scores overlapped with those of the comparison groups on all the tests except the ones noted.
None of the 13 other affected family members (see Fig. 1) could be scanned, because, at the time of this study, they either were in poor health (I-2, II-6, and III-1), were unavailable (III-20), had refused consent (II-4), or were below age 18 (III-5, -7, -9, -12, -13, -14, -17, and -22), the starting age at which PET scanning solely for experimental purposes is permitted in the United Kingdom.
References
- 1. Tomblin J B, Records N L, Buckwalter P, Zhang X, Smith E, O’Brien M. J Speech Lang Hear Res. 1997;40:1245–1260. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis B A, Thompson L A. J Speech Hear Res. 1992;35:1086–1094. doi: 10.1044/jshr.3505.1086. [DOI] [PubMed] [Google Scholar]
- 3.Tomblin J B, Buckwalter P R. In: Specific Language Impairments in Children. Watkins R V, Rice M L, editors. Baltimore: Brookes; 1994. pp. 17–34. [Google Scholar]
- 4.Bishop D V M, North T, Donlan C. Dev Med Child Neurol. 1995;37:56–71. doi: 10.1111/j.1469-8749.1995.tb11932.x. [DOI] [PubMed] [Google Scholar]
- 5.Jernigan T L, Hesselink J R, Sowell E, Tallal P A. Arch Neurol. 1991;48:539–545. doi: 10.1001/archneur.1991.00530170103028. [DOI] [PubMed] [Google Scholar]
- 6.Dronkers N F. Nature (London) 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- 7.Landau W M, Kleffner F R. Neurology. 1957;7:523–530. doi: 10.1212/wnl.7.8.523. [DOI] [PubMed] [Google Scholar]
- 8.Vargha-Khadem F, Watters G V, O’Gorman A M. Brain Lang. 1985;25:167–183. doi: 10.1016/0093-934x(85)90128-2. [DOI] [PubMed] [Google Scholar]
- 9.Fusco L, Vigevano F. J Neurol Neurosurg Psychiatry. 1991;54:556–558. doi: 10.1136/jnnp.54.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vargha-Khadem F, Carr L J, Isaacs E, Brett E, Adams C, Mishkin M. Brain. 1997;120:159–182. doi: 10.1093/brain/120.1.159. [DOI] [PubMed] [Google Scholar]
- 11.Hurst J A, Baraitser M, Auger E, Graham F, Norell S. Dev Med Child Neurol. 1990;32:347–355. doi: 10.1111/j.1469-8749.1990.tb16948.x. [DOI] [PubMed] [Google Scholar]
- 12.Fisher S, Vargha-Khadem F, Watkins K E, Monaco A P, Pembry M E. Nat Genet. 1998;18:168–170. doi: 10.1038/ng0298-168. [DOI] [PubMed] [Google Scholar]
- 13.Vargha-Khadem F, Passingham R E. Nature (London) 1990;346:226. [Google Scholar]
- 14.Vargha-Khadem F, Watkins K, Alcock K, Fletcher P, Passingham R E. Proc Natl Acad Sci USA. 1995;92:930–933. doi: 10.1073/pnas.92.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alcock K J. D.Phil. Thesis. Oxford, U.K.: Univ. of Oxford; 1996. [Google Scholar]
- 16.Gathercole S E, Baddeley A B. J Memory Lang. 1989;28:200–213. [Google Scholar]
- 17.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- 18.Friston K J, Ashburner J, Frith C D, Poline J-B, Heather J D, Frackowiak R S J. Hum Brain Mapping. 1995;2:165–189. [Google Scholar]
- 19.Friston K J, Holmes A P, Worsley K J, Poline J-B, Frith C D, Frackowiak R S J. Hum Brain Mapping. 1995;2:189–210. doi: 10.1002/(SICI)1097-0193(1996)4:2<140::AID-HBM5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Mugler J P, Brookeman J R. Magn Reson Med. 1990;15:152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- 21.Wright I C, McGuire P K, Poline J-B, Travere J M, Murray R M, Frith C D, Frackowiak R S J, Friston K J. NeuroImage. 1995;2:244–252. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]
- 22.Ashburner J, Friston K. NeuroImage. 1997;6:209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- 23.Van Paesschen W, Connelly A, King M D, Jackson G D, Duncan J S. Ann Neurol. 1997;41:41–51. doi: 10.1002/ana.410410109. [DOI] [PubMed] [Google Scholar]
- 24.Price C J, Wise R J, Warburton E A, Moore C J, Howard D, Patterson K, Frackowiak R S, Friston K J. Brain. 1996;119:919–932. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- 25.Friston K J, Poline J B, Holmes A P, Price C J, Frith C D. NeuroImage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 26.Gopnik M. Nature (London) 1990;344:715. doi: 10.1038/344715a0. [DOI] [PubMed] [Google Scholar]
- 27.Gopnik M, Crago M B. Cognition. 1991;39:1–50. doi: 10.1016/0010-0277(91)90058-c. [DOI] [PubMed] [Google Scholar]
- 28.Pinker S. The Language Instinct. London: Allen Lane; 1994. [Google Scholar]
- 29.Warburton E, Wise R J, Price C J, Weiller C, Hadar U, Ramsay S, Frackowiak R S. Brain. 1996;119:159–179. doi: 10.1093/brain/119.1.159. [DOI] [PubMed] [Google Scholar]
- 30.Scheffer I E, Jones L, Pozzebon M, Howell R A, Saling M M, Berkovic S F. Ann Neurol. 1995;38:635–642. doi: 10.1002/ana.410380412. [DOI] [PubMed] [Google Scholar]
- 31.Ojemann G A. Behav Brain Sci. 1983;6:189–230. [Google Scholar]
- 32.Ceballos-Baumann A O, Passingham R E, Warner T, Playford E D, Marsden C D, Brooks D J. Ann Neurol. 1995;37:363–372. doi: 10.1002/ana.410370313. [DOI] [PubMed] [Google Scholar]
- 33.Alexander G E, DeLong M R, Strick P L. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 34.Ceballos-Baumann A O, Passingham R E, Marsden C D, Brooks D J. Ann Neurol. 1995;37:746–757. doi: 10.1002/ana.410370608. [DOI] [PubMed] [Google Scholar]
- 35.Saint-Cyr J A, Ungerleider L G, Desimone R. J Comp Neurol. 1990;298:129–156. doi: 10.1002/cne.902980202. [DOI] [PubMed] [Google Scholar]
- 36.Alexander M P, Naeser M A, Polumbo C L. Brain. 1987;110:961–991. doi: 10.1093/brain/110.4.961. [DOI] [PubMed] [Google Scholar]
- 37.Lieberman P, Kato E, Friedman J, Tajchman G, Feldman L S, Jimenez E. Brain Lang. 1992;43:169–189. doi: 10.1016/0093-934x(92)90127-z. [DOI] [PubMed] [Google Scholar]
- 38.Ullman M, Corkin S, Coppola M, Hickok G, Growdon J H, Koroshetz W J, Pinker S. J Cognit Neurosci. 1997;9:266–276. doi: 10.1162/jocn.1997.9.2.266. [DOI] [PubMed] [Google Scholar]
- 39.Vygotsky L S. Thought and Language. Cambridge, MA: MIT Press; 1962. [Google Scholar]