Abstract
Whole-cell N-methyl-d-aspartate (NMDA)-activated currents were recorded from cultured rat cortical neurons. We report here a powerful effect of changing permeant ion concentrations on the voltage-dependent inhibition by external Mg2+ (Mg2+ () of these currents. Internal Cs+ () affected inhibition of the NMDA-activated currents in a voltage-dependent manner. A decrease in concentration ([Cs+]i) from 125 to 8 mm reduced IC50 by 1.4-fold at −105 mV and by 11.5-fold at – 15 mV. A decrease in external Na+ ( concentration ([Na+]o) also reduced IC50. This effect was voltage independent. A decrease in [Na+]o from 140 to 70 mm reduced IC50 by 1.4-fold at–105 mV and by 1.6-fold at–15 mV. Varying external Ca2+ () concentrations ([Ca2+]o) from 0.1 to1 mm did not affect inhibition, even though changing [Ca2+]o in the same range strongly influenced the magnitude of NMDA-activated currents in the absence of . However, increasing [Ca2+]o to higher concentrations (2–20 mm) greatly increased IC50 at hyperpolarized voltages. These data are consistent with a model in which and modulate inhibition of NMDA-activated currents by occupying external permeant ion binding sites. The IC50 values reported here are similar to KD values calculated from previous single-channel measurements of blocking kinetics. This similarity implies that does not affect gating while blocking the channel.
N-methyl-d-aspartate (NMDA) receptors are critically involved in physiological processes of both the developing and mature vertebrate central nervous system (CNS), including neuronal patterning formation (Iwasato et al. 2000) and learning and memory (Bliss & Collingridge, 1993; Tang et al. 1999). NMDA receptors are also implicated, directly or indirectly, in diseased states ranging from acute ischaemia-induced cell death to neurodegenerative disorders (Meldrum, 1992). Hence, regulation of NMDA receptors is crucial for proper functioning of the CNS.
Many substances modulate NMDA receptor function (McBain & Mayer, 1994; Dingledine et al. 1999). Magnesium is a particularly important modulator that exhibits a number of modes of action. It can act as a voltage-dependent channel blocker from both the external (Mayer et al. 1984; Nowak et al. 1984; Ascher & Nowak, 1988) and internal (Johnson & Ascher, 1990; Li-Smerin & Johnson, 1996) sides of the membrane. Magnesium can potentiate NMDA receptor activity by increasing the affinity of glycine for the NMDA receptor (Wang & MacDonald, 1995) or through a glycine-independent mechanism (Paoletti et al. 1995). Under certain conditions, Mg2+ also permeates the channel of NMDA receptors (Mayer & Westbrook, 1987; Stout et al. 1996; Antonov & Johnson, 1999; Zhu & Auerbach, 2001a, b).
The open-channel block of channels of NMDA receptors by has been extensively investigated. The widely accepted mechanism of external Mg2+ () block involves binding to a discrete site within the open channel of NMDA receptors, obstructing current flow, and then unbinding to the external solution under most circumstances. The binding site is thought to be deep in the channel of the NMDA receptor, within the region where transmembrane voltage drop occurs. As a result, the affinity of for the binding site changes as a function of membrane voltage. At hyperpolarized membrane voltages, the probability of occupying the site is higher, hence the block is more pronounced.
While occupies the pore, it may interact with channel gating transitions. There are many ways in which a blocker can interact with gating. Sequential blockers, examples of which are thought to include 9-aminoacridine (Costa & Albuquerque, 1994; Benveniste & Mayer, 1995), IEM-1857 (Antonov & Johnson, 1996) and tetrapentyl-ammonium (Sobolevsky et al. 1999), have the extreme effect of preventing the channel gate from closing during block. Other channel blocks allow the gate to close and can be subsequently ‘trapped’ in the channel when the agonist is removed. Examples of such blockers of the NMDA receptor are MK-801 (Huettner & Bean, 1988), memantine (Blanpied et al. 1997; Chen & Lipton, 1997) and ketamine (MacDonald et al. 1987). The trapped blocker may also perturb gating parameters by affecting binding of agonists or gating transitions (Blanpied et al. 1997; Sobolevsky et al. 1999). The action of on gating is not yet clear. Several pieces of evidence suggest that there is little or no effect of block by on channel gating: (1) single-channel burst analysis of block indicated a clear departure from predictions for a sequential blocker (Nowak et al. 1984); (2) the NMDA receptor channel can close from the blocked state (Jahr & Stevens, 1990; Sobolevsky & Yelshansky, 2000); and (3) does not prevent NMDA or glycine dissociation (Benveniste & Mayer, 1995; Sobolevsky & Yelshansky, 2000). On the other hand, a reduction in the burst duration and a decrease in the channel opening frequency may indicate that channel closure is accelerated during block by (Nowak et al. 1984; Ascher & Nowak, 1988).
Several molecular constituents of the channel of NMDA receptors have been identified that influence block. Notably, the N-site, located at the tip of the M2 region, and its neighbouring asparagine of NR2 subunits are most likely the key residues that co-ordinate during block (Burnashev et al. 1992; Mori et al. 1992; Kupper et al. 1996; Wollmuth et al. 1998). Kuner & Schoepfer (1996) found that many other regions of the receptor also influence block. These regions probably help form the external and internal vestibules and the pore region (Kuner et al. 1996; Beck et al. 1999) and thus it is unlikely that they all contribute to the Mg2+ binding site. This diffuse distribution of structural elements responsible for block appears inconsistent with the idea that blocks at a discrete site within the channel. This discrepancy echos the long-existing puzzle of how block acquires its unusually strong voltage dependence.
An explanation of this puzzle was provided by recent single-channel studies (Antonov & Johnson, 1999; Zhu & Auerbach, 2001a, b) which demonstrated that permeant monovalent ions have a powerful effect on the kinetics of channel block and unblock by . These studies suggest that voltage-dependent binding of permeant monovalent ions to the external channel vestibule of NMDA receptors accentuate the voltage dependence of block.
In the work presented here, we investigated the effect of permeant ions on inhibition of whole-cell NMDA-activated currents. The goal is twofold. First, we characterized the relevance of the interaction between permeant ions and to current inhibition. The results demonstrate that the equilibrium constant of inhibition of macroscopic currents is regulated dramatically by the ionic environment of a neuron. Second, we compare the whole-cell data with predictions of the previously proposed model based on single-channel measurements (Antonov & Johnson, 1999). This comparison tests whether the model, which was derived from kinetic measurements made at low concentrations ([Mg2+]o), is adequate to explain inhibition of macroscopic currents at higher, more physiologically relevant [Mg2+]o. The good agreement between whole-cell measurements of IC50 and single-channel measurements of KD also implies that block has no effect on channel gating. Preliminary versions of some of these results have been presented previously (Qian & Johnson, 1997, 1998).
METHODS
Cell culture
Primary cultures of cortical neurons were prepared as described by Li-Smerin & Johnson (1996). The procedure was approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Briefly, 16-days-old pregnant Sprague-Dawley rats were killed by CO2 inhalation. Brains from the embryos were removed and cerebral cortices were harvested. The cortical cells were dissociated enzymatically and plated at a density of 2 × 105 to 2.5 × 105 cells ml−1 onto 35 mm diameter plastic Petri dishes that contained glass coverslips, which either had been coated with poly-l-lysine or contained a glial cell feeder layer. Cells were used from 10 to 42 days after plating.
Solutions
Solutions were delivered through a five- or seven-barrel fast perfusion system, similar to the one described in Blanpied et al. (1997). Solution changes were accomplished by movements of barrels. Each barrel was connected to a gravity-fed reservoir of solution. At the output of the reservoir, a solenoid valve was used to turn solution flow on or off. The speed of flow was adjusted by varying the height of the reservoirs to ensure rapid and complete change of solution following movement of the barrels. With this system, a 98–99% complete solution exchange typically occurs within 120 ms (Blanpied et al. 1997).
Solutions were prepared daily from frozen stocks. For external solutions, 0.2 μm tetrodotoxin was added. Currents were activated by 10 μm NMDA + 30 μm glycine (identified on Figures as NMDA). Strychnine (1 μm) was also included to prevent activation of the strychnine-sensitive glycine receptor. Magnesium concentrations from 1 μm to 3 mm were added to external solutions. To determine whether the contaminating [Mg2+] was sufficiently great to affect the final [Mg2+] of external solutions, we used an atomic absorption spectrophotometer (Perkin-Elmer 2380; Shelton, CT, USA) to measure [Mg2+]. Contaminating [Mg2+] in the control external solution + 10 μm NMDA + 30 μm glycine was too low to be measured accurately, but we established that it must be less than 0.31 μm. Based on this low maximal level of contaminating Mg2+, we did not use Mg2+ buffers (which in some cases may yield inaccurate final-free [Mg2+]; Li-Smerin et al. 2001) or make corrections to Mg2+ concentrations. The abbreviations and contents of external bath solutions are (mm): ‘140 ’ solution (where represents external Na+), 140 NaCl, 1 CaCl2, 2.8 KCl and 10 Hepes; ‘70 ’ solution, 70 NaCl, 140 sucrose, 0.5 CaCl2, 2.8 KCl and 10 Hepes. The pH of external solutions was adjusted to between 7.1 and 7.2 using NaOH. In experiments in which external Ca2+ () concentration ([Ca2+]o) was changed to 0.2, 0.5 or 2 mm (in 140 solution) or to 0.1 mm (in 70 solution), no other changes in solute concentration were made. When [Ca2+]o was raised to 5 or 20 mm (in 70 solution), the sucrose concentration was reduced to 125 or 80 mm, respectively.
Caesium was used as the principal intracellular permeant cation because it inhibits K+ conductance in neurons, but is about as permeant through the channel of NMDA receptors as Na+ and K+ (Tsuzuki et al. 1994). The abbreviations and contents of the internal solutions are (mm): ‘125 ’ solution, where represents internal Cs+, 125 CsCl, 10 EGTA and 10 Hepes; ‘8 ’ solution, 8 CsCl, 117 N-methyl-d-glucamine (NMDG), 10 EGTA and 10 Hepes. The pH of the internal solutions was adjusted to between 7.1 and 7.2 using CsOH or HCl.
NMDG was used to adjust the osmolality of the internal solutions because it maintains ionic strength while neither blocking nor permeating the channel (Villarroel et al. 1995). Sucrose was used for external solutions because no ionic substitute for Na+ could be found that did not either block or permeate the channel from the external solution at the necessary concentration (Antonov et al. 1998). The junction potentials between the pipette and bath solution were measured and found to be 5 mV for the 140 /125 solution, −3 mV for the 140 /8 solution, and −7 mV for the 70 /8 solution. All holding potentials were corrected for junction potentials. Ultra-pure salts were used when available. Tetrodotoxin was purchased from Alomone Labs (Jerusalem, Israel); all other chemicals were from Sigma Chemical Co. (St Louis, MO, USA).
Whole-cell recordings and analysis
Whole-cell patch-clamp recordings were performed at room temperature according to standard methods (Hamill et al. 1981). Pipettes (resistance of 2–5 MΩ) were pulled from borosilicate thin-walled glass capillaries with filaments (Warner Instrument Corp., Portland, OR, USA). Access resistance was compensated at 60–80%. Currents were recorded with an Axopatch-1D or −200 amplifier, low-pass filtered at 10 kHz, digitized at 44 kHz with a Neuro-Corder (Cygnus Technology, Delaware Water Gap, PA, USA) and stored on videotape for later analysis. A continuous printout of the current trace on a chart recorder was used to monitor the quality of the recording and for later analysis. We imposed a delay of at least 5 min between breaking into the whole-cell configuration and the start of recording to allow for adequate exchange of the cytoplasm with the pipette solution. NMDA-activated currents in the absence and presence of were used to estimate IC50 from −105 to −15 mV at 10 mV increments. During each measurement, agonists were applied to obtain a steady-state current in the absence of (Icontrol). One or several different [Mg2+]o were applied in the presence of agonists to obtain currents in the presence of (IMg); application was followed by a second measurement of Icontrol. Recordings were discarded if the amplitudes of the first and second steady-state Icontrol differed by more than 12%.
The steady-state whole-cell current amplitudes were measured either directly from the chart recorder output or using pCLAMP 6 (Axon Instruments, Union City, CA, USA). Similar results were obtained with either approach. Normalized current in the presence of at each voltage was calculated as a ratio of IMg to Icontrol (represented as a percentage).
Millimolar can potentiate NMDA responses in a glycine- and voltage-independent manner (Paoletti et al. 1995). To accurately measure inhibition of NMDA-activated current by , IMg/Icontrol was corrected for potentiation. We tested the potentiation only when [Mg2+]o was 1 mm or higher because initial experiments indicated that 300 μm (the next highest [Mg2+]o to 1 mm) showed negligible potentiation. Potentiation by was quantified by measuring IMg/Icontrol at +35 or +55 mV. Then, IMg/Icontrol measured at negative voltages was corrected for potentiation using the equation:
where corrected fractional current is expressed as a percentage and A is the value of IMg/Icontrol measured at +35 or +55 mV in the same cell. Figure 1A shows a recording where potentiation by 1 mm was observed. At +55 mV, IMg/Icontrol was 116%; at −25 mV, IMg/Icontrol was 48% in the presence of 1 mm , with the corrected IMg/Icontrol being 42%. potentiation interfered with accurate measurements of IC50, as shown in Fig. 1B, where corrected and uncorrected IC50 values are compared from −15 mV to −45 mV.
Figure 1. Correction for potentiation by .
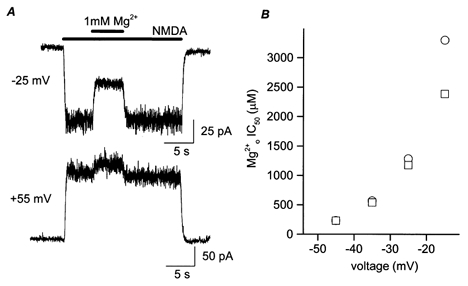
A, example of a cell in which the NMDA-activated current was potentiated by . Bars above the traces indicate times of agonist and application. At −25 mV, the inward current was inhibited by 1 mm ; at +55 mV, 1 mm potentiated the outward current. B, calculated IC50 values before (○) and after (□) correction for potentiation are plotted here for comparison. At depolarized voltages, where IC50 reaches the millimolar range, potentiation leads to overestimation of IC50 values and exaggeration of the voltage dependence of inhibition.
The IC50 of at each voltage was obtained by fitting IMg/Icontrol at various concentrations using the following equation:
IC50 and nh (Hill coefficient) were free parameters in fitting and the result is expressed as a percentage. Curve fitting was performed using Origin 3.0 or 4.0 (Microcal Software, Northampton, MA, USA). All data points were used to obtain the IC50, although the means were plotted for clarity in Fig. 2B and Fig. 3B. Each IC50 value was based on IMg/Icontrol measurements at 3–8 different [Mg2+]o from 20–112 cells. Data are expressed as means ± s.e.m. Student's t test was used for statistical comparisons.
Figure 2. Effect of on inhibition by of NMDA-activated current.
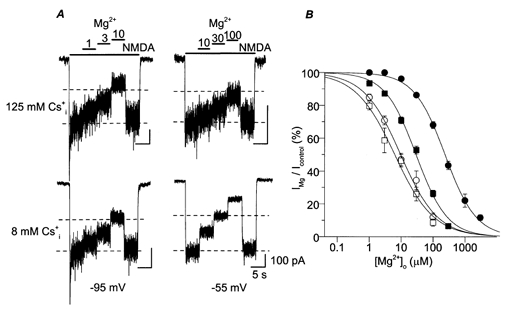
A, whole-cell NMDA-activated currents were inhibited by the indicated [Mg2+]o (in μm). The [Mg2+]o used were the same for upper and lower current traces. Bars above the traces show times of application of each solution. Each of the four current traces is from a different cell. At −95 mV (left), the values of IMg/Icontrol were 80, 69 and 38% for 125 (upper trace) and 85, 71 and 47% for 8 (lower trace) in 1, 3 and 10 μm , respectively. At −55 mV (right), the values of IMg/Icontrol were 91, 77 and 59% for 125 (upper trace) and 73, 50 and 27% for 8 (lower trace) in 10, 30 and 100 μm , respectively. The dashed lines indicate 50 and 100% of Icontrol to assist visual comparison among traces. B, examples of concentration-inhibition curves are shown. The symbol, solution, voltage and IC50 for each trace are: ○, 140 /125 , −95 mV, 9.79 μm; □, 140 /8 , −95 mV, 6.46 μm; •, 140 /125 , −45 mV, 232 μm; ▪, 140 /8 , −45 mV, 30.8 μm.
Figure 3. Effect of on inhibition by of NMDA-activated currents.
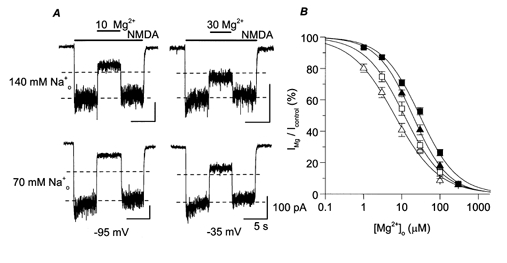
A, whole-cell NMDA-activated currents were inhibited by the indicated [Mg2+]o (in μm). The upper traces are from two different cells and the lower traces are from a third cell. At −95 mV (left), the values of IMg/Icontrol were 36% for 140 (upper trace) and 19% for 70 (lower trace). At −35 mV (right), the values of IMg/Icontrol were 60% for 140 (upper trace) and 38% for 70 (lower trace). Dashed lines, 50 and 100% of Icontrol. B, examples of concentration-inhibition curves are shown. The symbol, solution, voltage and IC50 for each trace are: ▵, 70 /8 , −85 mV, 3.72 μm; □, 140 /8 , −85 mV, 6.55 μm; ▴, 70 /8 , −45 mV, 19.4 μm; ▪, 140 /8 , −45 mV, 30.8 μm.
RESULTS
Effect of internal permeant ion on inhibition of NMDA-activated currents
We first investigated the effect of changing concentration ([Cs+]i) on inhibition of NMDA-activated whole-cell current. We compared inhibition of NMDA-activated currents in two sets of solutions, 140 /125 and 140 /8 solutions. Figure 2A shows current traces recorded at −95 mV (left) and −55 mV (right) for 125 (upper traces) and 8 (lower traces). At −95 mV, the two traces (with 125 and 8 ) exhibited similar values of IMg/Icontrol at each [Mg2+]o. At −55 mV, inhibited NMDA-activated currents more effectively with 8 .
To further quantify inhibition of NMDA-activated current with the 125 and 8 solutions, concentration-inhibition curves were constructed at each voltage tested. The IC50 of at each voltage was obtained by curve fitting of IMg/Icontrol at various [Mg2+]o as described in Methods. Examples of concentration-inhibition curves are shown in Fig. 2B. These examples demonstrate a general trend: IC50 was lower with 8 than 125 at each voltage, suggesting that reduces inhibition of the NMDA receptor-mediated currents. In addition, this effect of changing [Cs+]i was voltage dependent, since at −95 mV, had a much weaker effect on IC50 than at −45 mV.
Effect of external permeant monovalent ion on inhibition of NMDA-activated currents
We next investigated how changing concentration ([Na+]o) affects inhibition of NMDA receptors. To minimize potential competition between and (Antonov & Johnson, 1999), low [Cs+]i was used in this series of experiments. inhibition of NMDA-activated currents was quantified with 70 /8 and with 140 /8 solutions. Figure 3A shows sample traces recorded at −95 mV (left) and −35 mV (right) with 140 (upper traces) and 70 (lower traces). At each voltage, increasing [Na+]o reduced inhibition by . Voltage dependence of the effect of changing [Na+]o on inhibition of NMDA-activated currents was not apparent.
Concentration-inhibition curves were constructed at each voltage tested with the 140 and the 70 solutions. Examples of these curves are shown in Fig. 3B. The IC50 of was lower when [Na+]o was reduced, indicating that block becomes more pronounced in low [Na+]o. The shift of concentration-inhibition curves was comparable at both voltages.
Comparison of the effects of internal and external permeant monovalent ions on inhibition of NMDA-activated currents
External magnesium IC50 values measured as described above are listed in Table 1 and plotted in Fig. 4 for all three solution combinations. The data demonstrate that changing permeant monovalent ion concentrations have a profound effect on the IC50. For example, at −15 mV, a decrease in permeant ion concentration resulted in reduction of IC50 from 2390 μm (in 140 /125 ) to 208 μm (in 140 /8 ), and further to 132 μm (in 70 /8 ). The large variation in IC50 values in different solutions gradually diminished with hyperpolarization such that at −105 mV, the same decrease in permeant ion concentrations resulted in reduction of IC50 from 4.60 μm (in 140 /125 ) to 3.43 μm (in 140 /8 ), and further to 2.36 μm (in 70 /8 ). Changing permeant ion concentrations not only affected the magnitude of inhibition, it also altered the voltage dependence of inhibition, which was shallower in 140 /8 and 70 /8 than in 140 /125 . The lines plotted in Fig. 4 are quantitative predictions from the kinetic model of Antonov & Johnson (1999). In this model, binding of permeant monovalent ions to sites in the external vestibule of the channel of NMDA receptors prevents block and unblock of the channel (see Discussion).
Table 1.
Measured IC50 values
| Voltage (mV) | IC50 140 /125 (μM) | IC50 140 /8 (μM) | IC50 70 /8 (μM) |
|---|---|---|---|
| −15 | 2390 | 208 | 132 |
| −25 | 1180 | 70.3 | 63.2 |
| −35 | 541 | 56.3 | 35.8 |
| −45 | 232 | 30.8 | 19.4 |
| −55 | 116 | 24.9 | 12.3 |
| −65 | 55.5 | 12.1 | 7.06 |
| −75 | 41.5 | 12.1 | 5.89 |
| −85 | 17.2 | 6.55 | 3.72 |
| −95 | 9.79 | 6.46 | 2.98 |
| −105 | 4.60 | 3.43 | 2.36 |
IC50 values are shown here for the three indicated solution combinations. Because each IC50 value was derived from a single fit to population data, no standard deviation or error is shown.
Figure 4. Voltage and permeant monovalent ion concentration dependence of IC50.
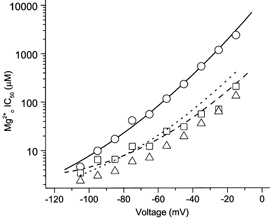
IC50 values (symbols); measured as shown in Figs 3 and 4, and quantitative predictions of KD values (lines) made with the model of Antonov & Johnson (1999; see Fig. 8); are plotted as follows: 140 /125 , ○ and —; 140 /8 , □ and—;70 /8 , ▵ and ……. The following equations were used with no free parameters to calculate the predictions of the model (Vm is membrane potential in mV; kNa and kCs are in mm; k-,o and k-,i are in s−1; and k+ is in m−1 s−1.
KD (apparent dissociation constant for ) = k-,app/k+,app,
k-,app (apparent unbinding rate) = k-,o/[(1 + [Na+]o/KNa)]2+k-,i,
k+,app (apparent binding rate) = k+/((1 + [Na+]o/KNa)(1 + [Na+]o/KNa+ [Cs+]i/KCs)),
k-,o (intrinsic unbinding rate to external solution) = 1.10 × 105 exp(Vm/52.7),
k-,i (intrinsic unbinding rate to internal solution) = 61.8 exp(-Vm/50.0),
k+ (intrinsic binding rate) = 1.10 × 109 exp(-Vm/55.0),
KNa ([Na+] at which each external site half occupied by Na+) = 34.4,
KCs ([Cs+] at which external site half occupied by Cs+) = 0.279 exp(-Vm/21.0).
The effects on IC50 of changing [Cs+]i and [Na+]o exhibit a striking difference in voltage dependence. To illustrate this difference, the ratio of IC50 values measured in normal and low concentrations of each permeant monovalent ion is plotted as a function of voltage in Fig. 5 (see legend). Decreasing either [Cs+]i or [Na+]o reduced the IC50, since all the ratios were greater than 1. At −105 mV, the effects of changing [Cs+]i and [Na+]o were comparable, with both ratios at 1.4. With depolarization, the effect of changing [Cs+]i on IC50 became more pronounced. At −15 mV, decreasing [Cs+]i caused an 11.5-fold reduction in IC50 value, while decreasing [Na+]o resulted in a 1.6-fold reduction in the IC50 value.
Figure 5. Comparison of voltage dependence of internal and external permeant monovalent ion effects on IC50.
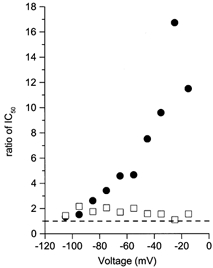
Ratios of IC50 values measured in normal and low permeant ion concentrations are plotted as a function of voltage. •, IC50 (in 140 /125 )/IC50 (in 140 /8 ); □, IC50 (in 140 /8 )/IC50 (in 70 /8 ). The dashed line marks a ratio of 1. The ratio for a change in [Cs+]i increases with depolarization, while the ratio for a change in [Na+]o shows no apparent voltage dependence.
Effect of [Ca2+]o on inhibition of NMDA-activated currents
In the whole-cell experiments described so far, external solutions contained a [Ca2+]o of 1 mm when 140 mm was used and 0.5 mm when 70 mm was used. As the channel of NMDA receptors is highly permeable to (MacDermott et al. 1986; Mayer & Westbrook, 1987; Ascher & Nowak, 1988; Burnashev et al. 1995), even these low [Ca2+]o might have interfered with our measurements of the effects of and on inhibition. Therefore, we next examined the effects on inhibition of variations of [Ca2+]o around 1 mm.
Representative current traces recorded at several [Ca2+]o are shown in Fig. 6A. The left panel shows current traces recorded in the same cell in 140 /125 at −65 mV, in normal [Ca2+]o (top, 1 mm ) and in low [Ca2+]o (bottom, 0.2 mm ). The right panel shows an example recorded in a second cell in 70 /8 at −45 mV, in normal [Ca2+]o (top, 0.5 mm ) and in low [Ca2+]o (bottom, 0.1 mm ). Within each trace, the [Ca2+]o was the same in each solution (external solutions without agonists or , with only agonists, and with agonists and ). When [Ca2+]o was decreased fivefold, the steady-state Icontrol became larger, as expected (Mayer & Westbrook, 1987; Ascher & Nowak, 1988), while inhibition by (reflected by IMg/Icontrol) was not noticeably affected.
Figure 6. Effect of low [Ca2+]o on NMDA-activated current and its inhibition by .
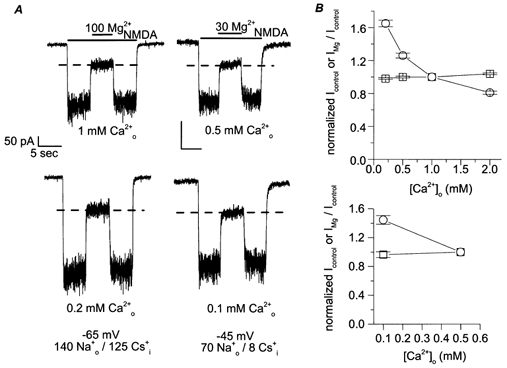
A, sample traces are from recordings in the [Ca2+]o indicated below each trace. The effect of is shown for experiments using 140 /125 at −65 mV (left two traces) and with 70 /8 at −45 mV (right two traces). Top traces are in normal [Ca2+]o and bottom traces are in fivefold reduced [Ca2+]o. [Mg2+]o was in μm. To assist visual data comparison, dashed lines are plotted at the value of IMg/Icontrol measured in normal [Ca2+]o: 34.5% in 140 /125 (left, top) and 36.8% in 70 /8 (right, top) in A. The measured values of IMg/Icontrol in low [Ca2+]o are 35.1% in 140 /125 (left, bottom) and 36.6% in 70 /8 (right, bottom). B, Icontrol (○) and IMg/Icontrol (□) were measured in various [Ca2+]o; all data were normalized to values in normal [Ca2+]o from the same cell (top, 1 mm ; bottom, 0.5 mm ) and were plotted as a function of [Ca2+]o for 140 /125 (top) and for 70 /8 (bottom). Icontrol depended on [Ca2+]o, but IMg/Icontrol was not affected by lowering [Ca2+]o, except for a small decrease in inhibition by at 2 mm . Plotted data are pooled from recordings performed at −45 and −65 mV in 140 /125 and at −45, −65 and −95 mV in 70 /8 . At least three cells were used under each , , and voltage condition.
Measurement of Icontrol and IMg/Icontrol at various [Ca2+]o were normalized to values measured in normal [Ca2+]o within the same cell. The mean normalized values are plotted as a function of [Ca2+]o in Fig. 6B. The majority of the experiments were performed at −45 or −65 mV; for experiments in 70 /8 , recordings were also made at −95 mV to rule out a voltage-dependent effect of changing [Ca2+]o on IMg/Icontrol measurements. None of the normalized IMg/Icontrol values at any voltage was statistically different from 1 at [Ca2+]o from 0.1 to 1 mm (the P values ranged from 0.12 to 0.96). Normalized Icontrol values, on the other hand, increased with reduction of [Ca2+]o. Therefore, under the conditions used here, did not competitively influence the effects of permeant monovalent ions on inhibition.
We noticed a slight increase in IMg/Icontrol value in 2 mm compared with normal [Ca2+]o (ratio of 1.04 ± 0.01, P < 0.005). This observation, and the previous work of Mayer & Westbrook (1987) on relief of inhibition by , led us to examine the effects of higher [Ca2+]o. We tested whether 5 or 20 mm affected the IC50 of NMDA-activated currents. We used a hyperpolarized voltage (−95 mV) because, if the effect of is voltage dependent, its effect should be greater at more negative voltages (Mayer & Westbrook, 1987). 70 mm and 8 mm were used to minimize potential competition from other permeant ions. Figure 7A shows current traces recorded in 5 mm (left) and 20 mm (right). inhibited NMDA-activated currents more effectively in 5 than in 20 mm
Figure 7. Effect of high [Ca2+]o on inhibition by of NMDA-activated current.
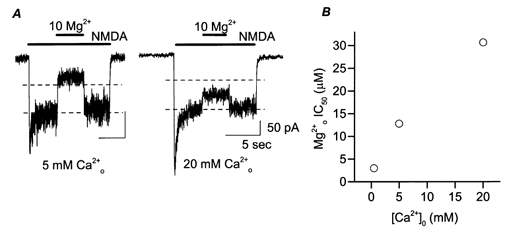
A, whole-cell NMDA-activated currents were inhibited by the indicated [Mg2+]o (in μm) at −95 mV. The traces are from different cells. The values of IMg/Icontrol were 32% in 5 mm (left) and 71% in 20 mm (right). Dashed lines indicate 50 and 100% of Icontrol. B, whole-cell measurements of IC50 are plotted for three [Ca2+]o (0.5, 5 and 20 mm ). Data for 0.5 mm are replotted from Fig. 4. The IC50 values (in μm) are: 2.98, 12.8 and 30.7 in 0.5, 5 and 20 mm .
Figure 7B shows IC50 measured at in 0.5, 5 and 20 mm . Increasing [Ca2+]o greatly weakened inhibition. Changing [Ca2+]o from 0.5 to 5 mm increased the IC50 by 4.3-fold, and further increasing [Ca2+]o to 20 mm increased IC50 by an additional factor of 2.4.
DISCUSSION
In this study, the effects of the permeant ions , and on inhibition by of whole-cell NMDA-activated currents were investigated. The main findings are as follows. (1) inhibition of NMDA-activated currents can be profoundly modulated by changing permeant ion concentrations. (2) Decreasing [Cs+]i decreased IC50 in a voltage-dependent manner. At hyperpolarized voltages, the effect was weak; at depolarized voltages, the effect was powerful. (3) Decreasing [Na+]o weakly decreased the IC50 in a voltage-independent manner. (4) In contrast to the permeant monovalent ions, at concentrations up to those normally present (0.5 or 1 mm) had no effect on inhibition of NMDA-activated currents, while higher [Ca2+]o appeared to greatly reduce inhibition.
Model of permeant monovalent ion effects on IC50
Mechanistic interpretation of these results can be aided by a previous model based on a single-channel study of the effects of permeant monovalent ions on blocking kinetics (Antonov & Johnson, 1999). The characteristics of this model (schematized in Fig. 8) are as follows. (1) There are two binding sites for permeant monovalent ions in the external vestibule of the NMDA receptor. can bind to one or both of these sites, while can occupy only one of the sites. (2) When either site is occupied by or , cannot enter to block the pore. (3) When is blocking the pore, can occupy one or both of the external permeant monovalent ion sites and prevent from exiting to the external solution until dissociates from the binding site. This aspect of the model is reminiscent of the ability of external K+ to slow Ba2+ unblock by binding to the external lock-in site of Ca2+-activated K+ channels (Neyton & Miller, 1988; Jiang & MacKinnon, 2000). (4) can permeate the channel at a low, voltage-dependent rate. An alternative kinetic model (Zhu & Auerbach, 2001a, b) of permeant monovalent ion effects on block will be discussed below.
Figure 8. Diagram of model of block of NMDA receptors.
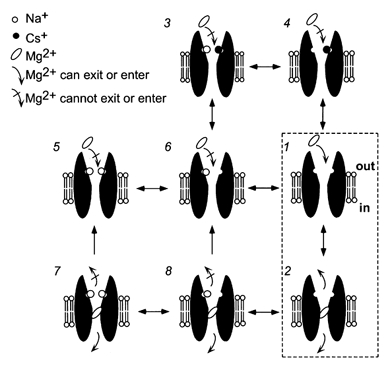
The model based on single-channel measurement of block (Antonov & Johnson, 1999) is schematized here. States 1 and 2 illustrate block and unblock of the open channel of the NMDA receptor when permeant ions are not bound. States 3–6 illustrate states in which and/or occupy the external permeant monovalent ion sites and prevent from entering the channel. States 7–8 illustrate that occupancy by of the external permeant monovalent ion site(s) prevents bound from exiting to the external solution, although permeation to the internal solution is still permitted.
The model above provides an explanation for the high voltage dependence of inhibition of NMDA receptors. reduces inhibition in a voltage-dependent manner by binding at the external permeant monovalent ion site. Since must cross the entire membrane field to reach its binding site, binding and its subsequent effect on inhibition is stronger at depolarized voltages. This voltage-dependent effect of on inhibition exaggerates the voltage dependence of inhibition that results from the location of the blocking site in the membrane field (Woodhull, 1973).
Effect of
To determine whether the model shown in Fig. 8 can explain the powerful effects of on IC50, we compared predicted values of KD derived from the model to the IC50 values measured here. KD values were predicted from the model as described in the legend of Fig. 4. Predictions were made with all parameters fixed at the values determined from previous single-channel experiments (Antonov & Johnson, 1999). Figure 4 compares the results of whole-cell experiments (symbols) to the model predictions (lines). The agreement was excellent for both 140 /125 and 140 /8 , supporting the accuracy of the model's description of the effect of on inhibition. This agreement between data and model predictions also suggests that the model, which was derived from measurements made in a low [Mg2+]o range (1–100 μm), is adequate to explain the blocking phenomenon at a more physiologically relevant (millimolar) [Mg2+]o range.
Effect of
The effect of changing [Na+]o on inhibition measured in whole-cell experiments and predicted by the model are also compared in Fig. 4. Three observations can be made. First, as the model predicts, changing [Na+]o did affect inhibition of NMDA-activated currents. Second, the effect of changing [Na+]o was small, which conforms with the model's prediction that the effect of [Na+]o on unbinding rate (Fig. 8, states 7 and 8) should partially compensate for the effect on binding rate. Third, there were some differences between the model predictions and the whole-cell data. The model predicts that the effect of changing [Na+]o on IC50 should be weakly voltage dependent, but there is no evidence of voltage dependency based on the whole-cell data. Despite this relatively small discrepancy between the model predictions and the measured IC50 values with 70 /8 , the whole-cell data are consistent with the basic aspects of the model.
The weak voltage dependence of the [Na+]o effect predicted by the model stems from competition between and for one of the external permeant monovalent ion sites. At depolarized voltages, the principal effect of increasing [Na+]o is to decrease the unblocking rate to the outside. This is because the probability of binding is high, and hence increasing [Na+]o has very little additional effect on the blocking rate. At hyperpolarized voltages, since occupies the binding site only infrequently, will the addition of reduce substantially the blocking as well as the unblocking rates of .
The reason for the small discrepancy is unclear. One possibility is that in 70 /8 , the channel of NMDA receptors adopts a modified conformation that blocks with slightly different kinetics. Similarly, a [K+]-dependent change in the conformation of the outer vestibule of K+ channels that strongly affects block by TEA has been reported (Immke et al. 1999). Consistent with this hypothesis, model predictions fit the 70 /8 data better when we used a different set of parameter values, those of Zhu & Auerbach (2001b). Both 140 data sets, however, are better described using parameter values shown in the legend of Fig. 4.
Another possible explanation for the discrepancy with 70 /8 is that lowering [Na+]o results in a change in the permeation rate. A previous study suggested that at 0 [Na+]o, permeation was greatly accelerated (Stout et al. 1996). Increased permeation could occur if, at low [Na+]o, a second can enter the channel vestibule, destabilize the blocking , and increase its rate of unblock to the internal solution.
We also attempted to explain our 70 /8 data by incorporating into our model an internal permeant ion binding site, as in the model of Zhu & Auerbach (2001a, b). Their model is based on single-channel studies of the effects of [Na+] and [K+] on the kinetics of block of recombinant NMDA receptors. Although there are many similarities between their model and the model summarized in Fig. 8, a prominent difference is that Zhu & Auerbach (2001b) proposed an additional internal ion binding site located 16% into the voltage field from the internal side of the membrane. Occupancy of this site by K+ was found to increase the unblocking rate of .
We did not expect that addition of an internal binding site to the model used here would yield better fits to the 70 /8 data because: (1) relatively little occupancy of the internal site would be expected at this low [Cs+]i; and (2) the internal site may selectively bind K+, since Na+ does not measurably interact with the site (Zhu & Auerbach, 2001b). The observation that changes in [Cs+]i do not affect unbinding rate (Antonov & Johnson, 1999) suggests that Cs+ may also have a low affinity for the site. To test the model with an internal permeant monovalent ion site, we fixed the electrical position of the internal site, and the unbinding rate with the site occupied, to the values specified in Zhu & Auerbach (2001a, b). The Cs+ affinity for the internal site was left as a free parameter. When the other parameters were fixed at the values reported by Antonov & Johnson (1999; see Fig. 4 legend), no significant improvement in fits was observed, despite the addition of a free parameter. When the other parameters were fixed at the values reported by Zhu & Auerbach (2001a, b), an improvement in the fit of the 70 /8 data was observed. This improvement, however, was also observed when the Zhu & Auerbach (2001a, b) parameters were used without addition of an internal site, as described above. The fit to the 140 /8 data set remained much worse than that shown in Fig. 4, despite addition of a free parameter. Thus, the data in Fig. 4 do not provide clear support for an internal binding site for .
It is possible that Cs+ can bind to an internal site, but does not measurably affect block or unblock while bound. Previous work with organic channel blockers suggested the presence of an internal Cs+ binding site located outside the voltage field (Antonov et al. 1998). It is not possible at present to determine whether this internal Cs+ site is distinct from the internal K+ site reported by Zhu & Auerbach (2001b).
Effect of [Ca2+]o
The powerful influence of and on inhibition of NMDA-activated currents suggests the possibility that may compete with permeant monovalent cations for their external sites, and thereby affect inhibition as well. If, under the conditions of these experiments, there was significant binding of to the external permeant monovalent ion sites, then lowering [Ca2+]o should have affected the IC50. However, we observed that lowering [Ca2+]o by a factor of 5 had no effect on IMg/Icontrol. This observation, in combination with previous single-channel observations (Antonov et al. 1998; Antonov & Johnson, 1999), suggests that does not compete with or for their external binding sites under normal (low [Ca2+]o) conditions. NMDA-activated current in 0 , in contrast, did increase as [Ca2+]o was reduced. This result is consistent with previous observations (Mayer & Westbrook, 1987; Ascher & Nowak, 1988) and indicates that the site at which binds with highest affinity during channel permeation is distinct from the external permeant monovalent ion sites.
At high [Ca2+]o, inhibition was strongly reduced at −95 mV, an observation consistent with the data of Mayer & Westbrook (1987). It is possible that high [Ca2+]o increases IC50 as a result of binding at a previously described binding site in the external portion of the NMDA-receptor channel (Premkumar & Auerbach, 1996; Sharma & Stevens, 1996). This external binding site might overlap with the external permeant monovalent ion sites. High may increase IC50 by slowing the apparent binding rate (similar to ), by binding while blocks and increasing the permeation rate (as proposed by Mayer & Westbrook, 1987), or by a combination of these effects.
interaction with channel gating
The similarity between IC50 measured in whole-cell experiments and KD (the ratio of unblocking and blocking rates) predicted from single-channel experiments also has implications for interaction with channel gating. IC50 and KD values should differ if channel gating or agonist binding equilibria are modified when occupies the channel (Johnson & Qian, 2002). For example, if binding prevents the channel from closing, channel burst duration would be increased by . As a result, reduction of current flow by channel block would be partially offset by the lengthening of channel bursts, and the macroscopic IC50 would be larger than the KD measured from single-channel experiments. The agreement between IC50 and KD values is consistent with the idea that binding has no effect on the gating of NMDA receptors or on agonist unbinding. This conclusion was also reached by Sobolevsky & Yelshansky (2000), who proposed that block does not affect channel desensitization, channel closure or agonist dissociation. They further suggested that can be trapped in the closed channel. This idea was strongly supported by Amar et al. (2001) using an NMDA receptor with a high affinity Zn2+ blocking site generated by mutating the N-site amino acid to cysteine. The slow unbinding of Zn2+ from the mutated receptor permitted a clear demonstration that Zn2+ is trapped by closure of the channel. The observation that blocks without perturbing gating suggests that the selectivity filter, which is very close to the blocking site (Burnashev et al. 1992; Mori et al. 1992; Wollmuth et al. 1996, 1998), is relatively insulated from conformational changes associated with gating transitions. The observation that many channel blockers, probably including , can be trapped points to conformational changes associated with gating in a region well external to the N-site.
Physiological or pathological implications
The profound effects of permeant ions on inhibition may indicate a role for permeant ions in modulating cellular excitability under physiological or pathological conditions. Ion concentrations fluctuate significantly as a result of neuronal activity. Rose & Konnerth (2001), using two-photon Na+ imaging, observed an increase of [Na+] in dendritic spines 100 mm in response to tetanic stimulation. More extreme fluctuations may occur in diseased states. Kager et al. (2000), for example, predicted an increase of over 50% in the total internal ion concentration during modelled seizure discharge. Based on the results presented here, changes of this magnitude in total internal permeant cation concentration would lead to a substantial decrease in inhibition by . The result could be a great exacerbation of neuronal damage.
Acknowledgments
The authors thank Juliann Jaumotte for her excellent skills in preparing the primary rat cortical culture and Dr Paul Rasmussen for his generous help with measurements of Mg2+ contamination. This work was supported by NIMH grants MH45817 and MH00944 to J. W. J. and Training Grant T32 MH18273 and Predoctoral NRSA MH12476 to A. Q.
REFERENCES
- Amar M, Perin-Dureau F, Neyton J. High-affinity Zn block in recombinant N-methyl-d-aspartate receptors with cysteine substitutions at the Q/R/N site. Biophysical Journal. 2001;81:107–116. doi: 10.1016/S0006-3495(01)75684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov SM, Gmiro VE, Johnson JW. Binding sites for permeant ions in the channel of NMDA receptors and their effects on channel block. Nature Neuroscience. 1998;1:451–461. doi: 10.1038/2167. [DOI] [PubMed] [Google Scholar]
- Antonov SM, Johnson JW. Voltage-dependent interaction of open-channel blocking molecules with gating of NMDA receptors in rat cortical neurons. Journal of Physiology. 1996;493:425–445. doi: 10.1113/jphysiol.1996.sp021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov SM, Johnson JW. Permeant ion regulation of N-methyl-d-aspartate receptor channel block by Mg2+ Proceedings of the National Academy of Sciences of the USA. 1999;96:14571–14576. doi: 10.1073/pnas.96.25.14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P, Nowak L. The role of divalent cations in the N-methyl-d-aspartate responses of mouse central neurones in culture. Journal of Physiology. 1988;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C, Wollmuth LP, Seeburg PH, Sakmann B, Kuner T. NMDAR channel segments forming the extracellular vestibule inferred from the accessibility of substituted cysteines. Neuron. 1999;22:559–570. doi: 10.1016/s0896-6273(00)80710-2. [DOI] [PubMed] [Google Scholar]
- Benveniste M, Mayer ML. Trapping of glutamate and glycine during open channel block of rat hippocampal neuron NMDA receptors by 9-aminoacridine. Journal of Physiology. 1995;483:367–384. doi: 10.1113/jphysiol.1995.sp020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied TA, Boeckman FA, Aizenman E, Johnson JW. Trapping channel block of NMDA-activated responses by amantadine and memantine. Journal of Neurophysiology. 1997;77:309–323. doi: 10.1152/jn.1997.77.1.309. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Schoepfer R, Monyer H, Ruppersberg J, Gunther W, Seeburg P, Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992;257:1415–1419. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. Journal of Physiology. 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HS, Lipton SA. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. Journal of Physiology. 1997;499:27–46. doi: 10.1113/jphysiol.1997.sp021909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa ACS, Albuquerque EX. Dynamics of the actions of tetrahydro-9-aminoacridine and 9-aminoacridine on glutamatergic currents: concentration-jump studies in cultured rat hippocampal neurons. Journal of Pharmacology and Experimental Therapeutics. 1994;268:503–514. [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacological Reviews. 1999;51:7–61. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-d-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proceedings of the National Academy of Sciences of the USA. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke D, Wood M, Kiss L, Korn SJ. Potassium-dependent changes in the conformation of the Kv2. 1 potassium channel pore. Journal of General Physiology. 1999;113:819–836. doi: 10.1085/jgp.113.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knopfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. A quantitative description of NMDA receptor-channel kinetic behavior. Journal of Neuroscience. 1990;10:1830–1837. doi: 10.1523/JNEUROSCI.10-06-01830.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, MacKinnon R. The barium site in a potassium channel by x-ray crystallography. Journal of General Physiology. 2000;115:269–272. doi: 10.1085/jgp.115.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Voltage-dependent block by intracellular Mg2+ of N-methyl-d-aspartate-activated channels. Biophysical Journal. 1990;57:1085–1090. doi: 10.1016/S0006-3495(90)82626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Qian A. Interaction between channel blockers and channel gating of NMDA receptors. Membrane and Cell Biology. 2002 (in the Press) [Google Scholar]
- Kager H, Wadman WJ, Somjen GG. Simulated seizures and spreading depression in a neuron model incorporating interstitial space and ion concentrations. Journal of Neurophysiology. 2000;84:495–512. doi: 10.1152/jn.2000.84.1.495. [DOI] [PubMed] [Google Scholar]
- Kuner T, Schoepfer R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. Journal of Neuroscience. 1996;16:3549–3558. doi: 10.1523/JNEUROSCI.16-11-03549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner T, Wollmuth LP, Karlin A, Seeburg PH, Sakmann B. Structure of the NMDA receptor channel M2 segment inferred from the accessibility of substituted cysteines. Neuron. 1996;17:343–352. doi: 10.1016/s0896-6273(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Kupper J, Ascher P, Neyton J. Probing the pore region of recombinant N-methyl-d-aspartate channels using external and internal magnesium block. Proceedings of the National Academy of Sciences of the USA. 1996;93:8648–8653. doi: 10.1073/pnas.93.16.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Smerin Y, Johnson JW. Kinetics of the block by intracellular Mg2+ of the NMDA-activated channel in cultured rat neurons. Journal of Physiology. 1996;491:121–135. doi: 10.1113/jphysiol.1996.sp021201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Smerin Y, Levitan ES, Johnson JW. Free intracellular Mg2+ concentration and inhibition of NMDA responses in cultured rat neurons. Journal of Physiology. 2001;533:729–743. doi: 10.1111/j.1469-7793.2001.t01-1-00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-d-aspartic acid receptor structure and function. Physiological Reviews. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321:519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Miljkovic Z, Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. Journal of Neurophysiology. 1987;58:251–266. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-d-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. Journal of Physiology. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Excitatory amino acid receptors and disease. Current Opinion in Neurology and Neurosurgery. 1992;5:508–513. [PubMed] [Google Scholar]
- Mori H, Masaki H, Yamakura T, Mishina M. Identification by mutagenesis of a Mg2+-block site of the NMDA receptor channel. Nature. 1992;358:673–675. doi: 10.1038/358673a0. [DOI] [PubMed] [Google Scholar]
- Neyton J, Miller C. Potassium blocks barium permeation through a calcium-activated potassium channel. Journal of General Physiology. 1988;92:549–567. doi: 10.1085/jgp.92.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J, Ascher P. Glycine-independent and subunit-specific potentiation of NMDA responses by extracellular Mg2+ Neuron. 1995;15:1109–1120. doi: 10.1016/0896-6273(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Auerbach A. Identification of a high affinity divalent cation binding site near the entrance of the NMDA receptor channel. Neuron. 1996;16:869–880. doi: 10.1016/s0896-6273(00)80107-5. [DOI] [PubMed] [Google Scholar]
- Qian A, Johnson JW. Internal permeant cations affect inhibition of NMDA-activated currents by external Mg2+ Society for Neuroscience Abstracts. 1997;23:1, 243. 375.2. [Google Scholar]
- Qian A, Johnson JW. External permeant cations affect inhibition of NMDA-activated currents by external Mg2+ Society for Neuroscience Abstracts. 1998;24:1, 343. [Google Scholar]
- Rose CR, Konnerth A. NMDA receptor-mediated Na+ signals in spines and dendrites. The Journal of Neuroscience. 2001;21:4207–4214. doi: 10.1523/JNEUROSCI.21-12-04207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Stevens CF. Interactions between two divalent ion binding sites in N-methyl-d-aspartate receptor channels. Proceedings of the National Academy of Sciences of the USA. 1996;93:14170–14175. doi: 10.1073/pnas.93.24.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Koshelev SG, Khodorov BI. Probing of NMDA channels with fast blockers. The Journal of Neuroscience. 1999;19:10611–10626. doi: 10.1523/JNEUROSCI.19-24-10611.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Yelshansky MV. The trapping block of NMDA receptor channels in acutely isolated rat hippocampal neurones. Journal of Physiology. 2000;526:493–506. doi: 10.1111/j.1469-7793.2000.t01-2-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout AK, Li-Smerin Y, Johnson JW, Reynolds IJ. Mechanisms of glutamate-stimulated Mg2+ influx and subsequent Mg2+ efflux in rat forebrain neurones in culture. Journal of Physiology. 1996;492:641–657. doi: 10.1113/jphysiol.1996.sp021334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tsuzuki K, Mochizuki S, Iino M, Mori H, Mishina M, Ozawa S. Ion permeation properties of the cloned mouse ɛ2/ζ1 NMDA receptor channel. Molecular Brain Research. 1994;26:37–46. doi: 10.1016/0169-328x(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Villarroel A, Burnashev N, Sakmann B. Dimensions of the narrow portion of a recombinant NMDA receptor channel. Biophysical Journal. 1995;68:866–875. doi: 10.1016/S0006-3495(95)80263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Macdonald JF. Modulation by magnesium of the affinity of NMDA receptors for glycine in murine hippocampal neurones. Journal of Physiology. 1995;486:83–95. doi: 10.1113/jphysiol.1995.sp020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth LP, Kuner T, Sakmann B. Adjacent asparagines in the NR2-subunit of the NMDA receptor channel control the voltage-dependent block by extracellular Mg2+ Journal of Physiology. 1998;506:13–32. doi: 10.1111/j.1469-7793.1998.013bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth LP, Kuner T, Seeburg PH, Sakmann B. Differential contribution of the NR1- and NR2A-subunits to the selectivity filter of recombinant NMDA receptor channels. Journal of Physiology. 1996;491:779–797. doi: 10.1113/jphysiol.1996.sp021257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. The Journal of General Physiology. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Auerbach A. Na+ occupancy and Mg2+ block of the N-methyl-d-aspartate receptor channel. Journal of General Physiology. 2001a;117:275–286. doi: 10.1085/jgp.117.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Auerbach A. K+ occupancy of the N-methyl- d-aspartate receptor channel probed by Mg2+ block. Journal of General Physiology. 2001b;117:287–298. doi: 10.1085/jgp.117.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]


