Abstract
Inner hair cells of the mammalian cochlea translate acoustic stimuli into ‘phase-locked’ nerve impulses with frequencies of up to at least 1 kHz. Little is known about the intracellular Ca2+ signal that links transduction to the release of neurotransmitter at the afferent synapse. Here, we use confocal microscopy to provide evidence that Ca2+-induced Ca2+ release (CICR) may contribute to the mechanism. Line scan images (2 ms repetition rate) of neonatal mouse inner hair cells filled with the fluorescent indicator FLUO-3, revealed a transient increase in intracellular Ca2+ concentration ([Ca2+]i) during brief (5–50 ms) depolarizing commands under voltage clamp. The amplitude of the [Ca2+]i transient depended upon the Ca2+ concentration in the bathing medium in the range 0–1.3 mm. [Ca2+]i transients were confined to a region near the plasma membrane at the base of the cell in the vicinity of the afferent synapses. The change in [Ca2+]i appeared uniform throughout the entire basal sub-membrane space and we were unable to observe hotspots of activity. Both the amplitude and the rate of rise of the [Ca2+]i transient was reduced by external ryanodine (20 μm), an agent that blocks Ca2+ release from the endoplasmic reticulum. Intracellular Cs+, commonly used to record at presynaptic sites, produced a similar effect. We conclude that both ryanodine and intracellular Cs+ block CICR in inner hair cells. We discuss the contribution of CICR to the measured [Ca2+]i transient, the implications for synaptic transmission at the afferent synapse and the significance of its sensitivity to intracellular Cs+.
The translation of sound into coded signals in the auditory nerve is accomplished by the inner hair cells of the mammalian cochlea. At low frequencies, changes in the membrane potential of the inner hair cell reflect the sinusoidal form of the stimulus (Russell & Sellick, 1983; Kros et al. 1992) and this periodicity is preserved in the pattern of action potentials in the auditory nerve (Rose et al. 1967). Little is known about the intracellular Ca2+ signal that links transduction at the apex of the hair cell to the release of neurotransmitter at the base. Here, we use confocal microscopy to follow depolarization-induced Ca2+ transients in the presynaptic region of neonatal mouse inner hair cells and provide evidence for Ca2+-induced Ca2+ release (CICR) in the vicinity of inner hair cell synapses. Intracellular Cs+, which is commonly used for recording at presynaptic sites, inhibits the release mechanism. We discuss whether CICR contributes to the presynaptic rise in [Ca2+]i during transmission and whether it can provide partial compensation for factors that reduce the fidelity of the signal transmitted to the CNS (Kidd & Weiss, 1990).
METHODS
These experiments were conducted on CD-1 mice (development stages P8–P9; P0 is day of birth), killed by cervical dislocation as authorized under the Scientific Procedures Act. In some experiments, mice at earlier stages (P6–P7) were used with the same results. Apical inner hair cells were semi-isolated from dissected organs of Corti, transferred to a recording chamber and viewed through a coverslip using an inverted microscope (Diaphot TMD, Nikon, UK) with a × 50 water-immersion objective lens (Fluoreszenz; NA, 1.0; E. Leitz, Wetzlar, Germany). Cells were loaded with FLUO-3 (100 μm; Molecular Probes Inc., OR, USA) through a patch pipette during whole-cell recording. Confocal images were collected in line scan mode using a Bio-Rad MRC 600 confocal microscope (Bio-Rad Microscience Ltd, Hemel Hempstead, UK). FLUO-3 fluorescence was excited with 488 nm light from a 150 mW argon-ion laser (Model 5425, Ion Laser Technology, UT, USA) with transmission set at 1 % with a neutral density filter. Emitted light at wavelengths greater than 515 nm was detected by the photomultiplier of the confocal system. The optical zoom facility (× 10) increased the size of the image and each scan was digitized into 768 pixels so that each pixel was equal to 0.033 μm. The scan interval was 2 ms so that the 512 lines collected during a single scan amounted to 1.024 s recording time. The slit width was near its minimum and it was maintained at this position for all experiments.
Software written by Mr M. Rickard (Department of Physiology, University of Bristol) allowed us to average adjacent pixels along the scanned line. In some cases two consecutive lines were averaged and points reported at 4 ms intervals. An average of 30 adjacent pixels along the scanned line corresponded to a band of cytoplasm nominally 1 μm wide, although it also contained light scattered from further away. Curves calculated from the theoretical point spread function of the objective lens show that points further than 0.4 μm away provide a negligible amount of light, 0.3 μm being the distance required for the resolution of two points in a general optical system (Rayleigh criterion). This represented an oversampling by about 4-fold (Nyquist Sampling Theorem) but bleaching was not evident and the larger image was easier to view. The images had an optical section of about 1 μm so that the volume of cytoplasm represented by the band of 30 pixels was approximately 1 μm3.
Changes in FLUO-3 fluorescence (ΔF) were used to estimate the effect of depolarizing voltage commands on [Ca2+]i. It is possible to correct such data for an uneven distribution of dye by dividing by the pre-stimulus (baseline) fluorescence (F0). Thus:
| (1) |
where Ft is the measured fluorescence at any time after the stimulus. This ratio gives an appropriate correction providing that there is no change in the FLUO-3 concentration during the course of the measurement. Judging by the pre-stimulus fluorescence, the FLUO-3 concentration reached a steady level 2–3 min after establishing the whole cell-clamp configuration and so all experiments were conducted after this period of time. All line scan images have been normalized. Up to 300 lines acquired before the stimulus were averaged to determine the resting FLUO-3 fluorescence profile (F0) and images were then subjected to a pixel-by-pixel correction to allow for uneven dye loading. However, when changes in external [Ca2+] caused changes in baseline fluorescence, as in Fig. 2a, raw fluorescence data are also presented. In some cases the peak change in fluorescence, ΔFpeak, is given in the text.
Figure 2. Effect of Ca2+- free bathing solution on the transient increase in [Ca2+]i.
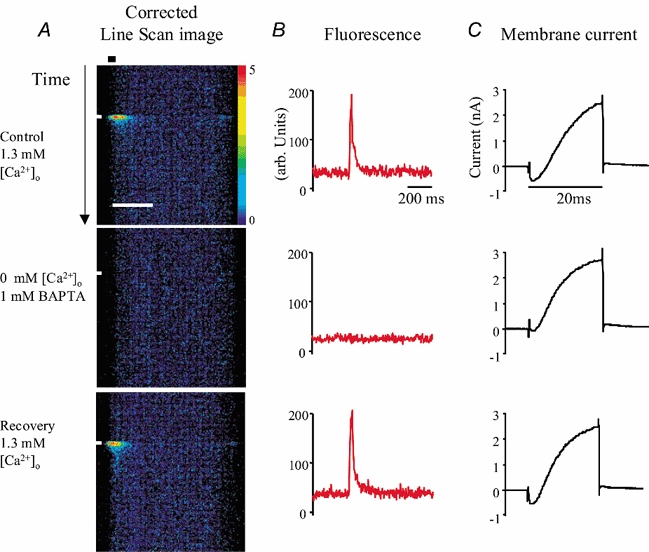
Hair cells were depolarized under voltage clamp and confocal images collected in line scan mode with the base of the cell towards the left. A, line scan traces show the [Ca2+]i-dependent increase in fluorescence in response to 20 ms command to −10 mV (white bar at left-hand side). Traces have been corrected for uneven dye distribution (see Methods; 3 × 3 median filter applied). Scale bar for line scan images, 5 μm; colour scale bar, 0 = black, 5 = red. Control responses (upper and lower traces) were recorded in the presence of 1.3 mm[Ca2+]o while a Ca2+-free bathing medium with 1 mm BAPTA was used to demonstrate (middle trace) the dependence of the response on [Ca2+]o. Black horizontal bar (upper line scan image) identifies the 1 μm near-membrane band used to estimate the average time course of the [Ca2+]i transient. B, average time course of the [Ca2+]i transient; raw data shows the change in baseline caused by the Ca2+-free bathing medium. C, membrane currents recorded during the depolarizing stimulus.
Membrane currents were recorded in voltage-clamp configuration using an Axopatch 200B (Axon Instruments, CA, USA). The ‘prediction-correction’ circuitry was adjusted during brief 10 mV pulses to provide partial compensation for the whole-cell capacitance. Patch pipette series resistance was 60 % compensated and all reported voltages were corrected for both the voltage drop across the uncompensated component, and for the calculated change in liquid junction potential (4 mV) in the whole cell configuration. The holding potential was −84 mV. Linear leak currents were subtracted during subsequent analysis. Data are expressed as means ± s.e.m. Experiments were carried out at 28–32 °C.
In addition to FLUO-3, patch pipettes normally contained (mm): KCl 140, EGTA-KOH 1, MgCl2 3.0, Na2ATP 5 and Hepes-KOH 5; pH 7.3, 290 mOsm. For experiments with Cs+, the pipettes contained (mm): CsCl 147, EGTA-KOH 1, MgCl2 3.0, Na2ATP 2.5 and Hepes-KOH 5; pH 7.3. For the experiment shown in Fig. 3B and C the pipette solution was Cs+-based and also contained 20 mm TEA chloride. The normal bathing solution contained (mm): NaCl 138, NaH2PO4 0.7, KCl 5.8, CaCl2 1.3, MgCl2 1.4, d-glucose 5.6 and Hepes-NaOH 10; pH 7.5. Vitamins and amino acids for Eagle's Minimal Essential Medium were added from a concentrate (Life Technologies, UK). NaCl was used to adjust the osmolarity of other bathing solutions. Ryanodine-containing solutions (Calbiochem, UK) were prepared from stock.
Figure 3. Membrane currents from inner hair cells in whole-cell configuration.
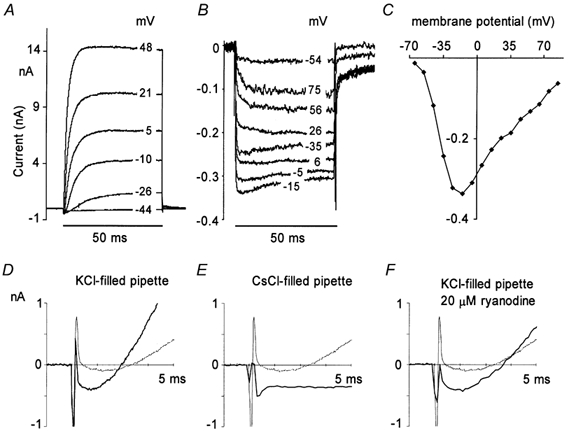
A, superimposed whole cell currents recorded using KCl-filled pipette; 50 ms depolarizing commands to different membrane potentials (shown on right-hand side). Holding potential, −84 mV. B, as A but with CsCl-filled pipette containing 20 mm TEA. C, peak inward current-voltage relationship obtained in B. D-F, representative records from inner hair cells under different recording conditions; membrane currents shown on an expanded time scale. Command step to −10 mV imposed at 1 ms; holding potential, −84 mV. Thick traces obtained with different pipette filling solutions; KCl (D), CsCl, no TEA (E), KCl, plus 20 μm external ryanodine (F). Thin traces superimposed on each record obtained with KCl-filled pipette in nominally Ca2+-free external solution.
RESULTS
When filled with fluorescent Ca2+ indicator (FLUO-3) and depolarized under voltage clamp, mouse inner hair cells showed a transient increase in intracellular Ca2+ concentration ([Ca2+]i). [Ca2+]i transients at near-membrane sites were measurable with 5 ms depolarizing commands to −10 mV and were maximal with commands lasting about 50 ms. The following account is a summary of experiments conducted on 80 hair cells.
Confocal images collected in line scan mode are shown in Fig. 1 (scan orientation indicated on left-hand side). In Fig. 1A an axial scan permitted simultaneous imaging of both basal and apical regions of the cell. The most rapid change in [Ca2+]i was in the 1 μm band of cytoplasm adjacent to the plasma membrane at the base of the cell (see time course in red on right-hand side). The recovery phase, which is independent of external Na+ (Kennedy et al. 2000), was complete within about 0.4 s. There was no measurable change in [Ca2+]i(n = 7) in a corresponding band of cytoplasm towards the cell apex (see time course in blue on right-hand side). Thus the [Ca2+]i transients were confined to the region of the cell with the greatest concentration of afferent synapses (Spoendlin, 1973). This confirms findings in lower vertebrate hair cells where Ca2+ entry is restricted to regions with presynaptic contacts (Issa & Hudspeth, 1994). However Ca2+ entry in the mouse was less sharply localized and we did not observe hotspots like those recorded in frog and turtle (Tucker & Fettiplace, 1995) in any of the 80 cells we examined. In Fig. 1B, where the line scan was located across the basal half of the cell, the [Ca2+]i transient on each side had a uniform amplitude and time course. When the line scan was positioned close to the plasma membrane at the very base of the cell as in Fig. 1C, similar transients were recorded in bands of cytoplasm selected from right, left and central regions and the [Ca2+]i transient was apparent throughout the entire basal area.
Figure 1. Transient increase in [Ca2+]i in semi-isolated, apical inner hair cells from neonatal mice.
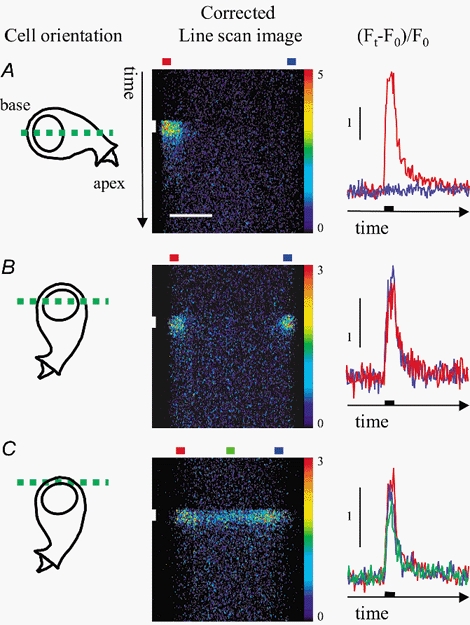
FLUO-3 loaded cells in whole-cell configuration were depolarized under voltage clamp and confocal images collected in line scan mode. Left, cartoons show the orientation of the cell; the dashed green line gives the location of the confocal section. Middle, line scan traces show the [Ca2+]i-dependent increase in fluorescence in response to 50 ms command to −10 mV (vertical white bar). Horizontal colour coded bars (top) identify bands used to estimate average time course in different regions of the cell. Traces have been corrected for uneven dye distribution (see Methods; 3 × 3 median filter applied). Scale bar for line scan images shown in A, 5 μm; colour scale bar, 0 = black, 5 = red (A); 0 = black, 3 = red (B,C). Right, change in fluorescence, (Ft - F0)/F0), with time in different regions of the cell (see Methods). Black bar on the time axis shows the duration of the depolarizing command. Cells were orientated either for simultaneous fluorescence measurements at the base and towards the apex of the cell (A), or for fluorescence measurements through the base of the cell (B and C).
Effect of external [Ca2+]
The amplitude of the [Ca2+]i transient depended upon the concentration of Ca2+ in the external saline ([Ca2+]o). In Fig. 2A line scan images show that the [Ca2+]i transient, which was generated by a 20 ms depolarization to −10 mV, was reversibly abolished in Ca2+-free saline (no added Ca2+, 1 mm BAPTA). There was a reduction in the baseline fluorescence in Ca2+-free saline and a small increase upon returning to solution containing 1.3 mm [Ca2+]o. These changes together with the time course of the [Ca2+]i transient are shown in Fig. 2B. The leak-subtracted membrane currents recorded during the stimulus are shown in Fig. 2C. In Ca2+-free saline there was a small residual inward current (50–100 pA) which we attribute to Ca2+ trapped in unstirred layers between the cells and the base of the recording chamber. Under control conditions (1.3 mm [Ca2+]o) the mean amplitude of the [Ca2+]i transient measured as ΔFpeak/F0 (see Methods), was 3.0 ± 0.5 (n = 7) whereas in 0.5 mm [Ca2+]o the amplitude was 1.3 ± 0.17 (n = 4). We conclude that the [Ca2+]i transient depended upon Ca2+ influx across the plasma membrane and that its amplitude increased with increasing [Ca2+]o in the range 0–1.3 mm.
Inward current measured with Cs+ in the patch pipette
Figure 2C shows the large whole cell currents observed during commands to −10 mV under our standard recording conditions. Although the inward current activates within 1 ms, the outward potassium current develops relatively slowly throughout the 20 ms pulse as is characteristic of currents in these cells (Kros et al. 1998). This is seen most clearly in Ca2+-free saline (middle) because there is little inward current under these conditions in this preparation (see also Beutner & Moser, 2001; Fig. 1B). Typical membrane currents recorded over a range of command potentials are shown in Fig. 3A. When 147 mm CsCl was included in the patch pipette much, but not all, of the outward current was blocked (Ohmori, 1984). To compare the amplitude of the inward current recorded using KCl- and CsCl-filled pipettes we measured the inward current 1 ms from the start of the depolarizing command pulse, a point at which the effect of the capacitive transient appeared complete and the outward current contamination appeared minimal. Figure 3D shows the typical membrane currents recorded with KCl-filled pipettes during the first 4 ms after a command to −10 mV. Currents recorded under nominally Ca2+-free conditions are superimposed (thinner trace), to give a better idea of the duration of the semi-compensated capacitive transient seen at the onset of the command step (see Methods). Typical currents recorded with CsCl-filled pipettes are shown in Fig. 3E. At early times after the onset of the depolarizing command there appeared to be a negligible contribution from Cs+-resistant outward current. During commands to −4 mV the inward ionic current recorded using CsCl-filled pipettes was 0.37 ± 0.02 nA (n = 9), a value not significantly different to the mean amplitude of the peak inward ionic current recorded with KCl-filled pipettes at the same voltage (0.41 ± 0.02 nA; n = 13).
In order to reveal the full time course of the inward current the experiment was repeated using patch pipettes containing 122 mm CsCl and 20 mm TEA (Fig. 3B). A full inward current-voltage relationship obtained under these conditions is shown in Fig. 3C. The maximum inward current was observed with pulses to about −10 mV.
Effect of internal Cs+ on [Ca2+]i transient
Although CsCl had no significant effect on the inward current or the baseline fluorescence, it markedly depressed the [Ca2+]i transient. Figure 4A shows average [Ca2+]i transients recorded during 50 ms pulses to −10 mV using either a KCl- (7 cells) or a CsCl-filled recording pipette (9 cells). The mean amplitude of the near-membrane [Ca2+]i transient with CsCl in the recording pipette was about 55 % of that obtained with KCl. Figure 4B shows that the Cs+-based pipette solution markedly reduced the amplitude of the response over a wide range of voltage commands. However with or without Cs+ in the pipette the maximum [Ca2+]i transient was obtained with pulses to about −10 mV. Note that in Fig. 4B the data points have been normalized to the value obtained at −44 mV.
Figure 4. Effect of internal Cs+ or external ryanodine on depolarization-induced [Ca2+]i transient in inner hair cells.
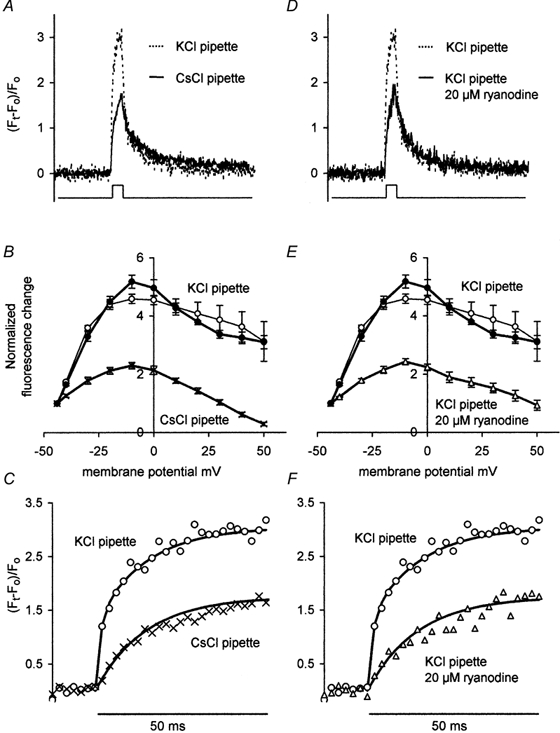
Average [Ca2+]i change in 1 μm band adjacent to the plasma membrane. Responses obtained with either K+- or Cs+-based pipette solutions (A-C) or with a K+-based pipette solution ± 20 μM external ryanodine (D-F). Controls with KCl-based pipette solution repeated. A and D, averages of [Ca2+]i transients associated with depolarization to −10 mV (50 ms pulse; see lower trace). KCl-filled pipette, n = 7 cells; CsCl-filled pipette, n = 9 cells; KCl-filled pipette plus 20 μM external ryanodine, n = 9 cells. B and E, mean amplitude of the [Ca2+]i transient plotted against membrane voltage (interpolated data); data normalized to the value obtained at −44 mV (same cells as in A and D). Cs+-based pipette solution (×) or K+-based pipette solution (•; ○) plus 20 μM extracellular ryanodine (▵). Pulse duration, 8 ms (•) and 50 ms (○, × and ▵). C and F, rise time of averaged responses shown in A and D on an expanded time scale. Data points fitted with continuous lines having one (× and ▵; τ= 14 ms) or two (○; τ= 1.3 and 14 ms) exponential components. Key for symbols as B and E.
The changes in [Ca2+]i obtained with KCl in the recording pipette were so large that we were concerned that the somewhat flattened relationship shown (Fig. 4B; ○) might have been brought about by saturation of the fluorescent indicator. We therefore repeated the experiment using 8 ms depolarizing commands (Fig. 4B; •). Generally there was little difference between the two sets of data recorded in the presence of KCl although the small increase in the maximum [Ca2+]i transient obtained with an 8 ms command to −10 mV was significant.
In Fig. 4C the rise time of the two [Ca2+]i transients shown in Fig. 4A are plotted on an expanded time scale. The lines through the points are fitted by eye and are drawn with either a double exponential time course (τ = 1.3 and 14 ms; KCl-based pipette solution) or a single exponent (τ = 14 ms; CsCl-based pipette solution). Although the 2 ms collection interval precludes a precise measure of its rise time, Cs+ blocks a fast component of the Ca2+ signal whose time constant is 1.3 ms or less.
Effect of 20 μm ryanodine on [Ca2+]i transient
In Cs+-dialysed ventricular myocytes, depolarization-induced [Ca2+]i transients are reduced by 50 % when compared with K+-dialysed controls and it is possible that Cs+ inhibits intracellular Ca2+ release in these cells (Han et al. 1994; Levi et al. 1996). The plant alkaloid, ryanodine promotes CICR at 10–100 nm and inhibits it at concentrations above 10 μm (Meissner, 1986). We therefore tested the effect of 20 μm ryanodine to determine whether such a mechanism could explain this inhibitory action of Cs+. Figure 4D shows that the average peak amplitude of the [Ca2+]i transient in ryanodine-treated hair cells (data from 7 cells) matched that obtained with Cs+-filled pipettes. Figure 4E shows that ryanodine, like Cs+, suppressed the [Ca2+]i transient over a wide range of command potentials. For pulses to −10 mV the amplitude of the near-membrane [Ca2+]i transient was about 60 % of the control value. As with Cs+, it was the fast component of the [Ca2+]i transient that was affected (Fig. 4F). None of these changes can be attributed to an effect on the inward Ca2+ current. In these experiments KCl was used as the pipette filling solution and although ryanodine reduced the rate of rise of the outward current (see Fig. 3F) the inward current appeared unaffected. Measurement of the inward current at 1 ms after the start of the depolarizing command to −4 mV gave a mean value of 0.39 ± 0.02 nA (n = 9), which was not significantly different from the mean values obtained under control conditions (see above).
DISCUSSION
In this study we found that membrane depolarization increased [Ca2+]i at the base of inner hair cells taken from P8–P9 mice. The change in [Ca2+]i appeared uniform throughout the entire basal sub-membrane space and we were unable to observe hotspots even when we used long duration depolarizing pulses. The [Ca2+]i transient depended on Ca2+ influx across the plasma membrane but was also markedly reduced by 20 μm external ryanodine. We conclude that ryanodine-sensitive Ca2+-induced Ca2+ release (CICR) (Endo et al. 1970), contributes to the depolarization-dependent change in [Ca2+]i.
The time course of the [Ca2+]i transient reflects the change in [Ca2+]i in a 1 μm3 volume of cytoplasm near the plasma membrane. Its rising phase has two components, the fastest of which is sensitive to ryanodine (see Fig. 4F). We suggest that the time course of the slower, ryanodine-insensitive component arises from the influx of Ca2+ through voltage-dependent Ca2+ channels in the plasma membrane. Its rise-time does not directly reflect the activation kinetics of the calcium current, but is a measure of the increase in [Ca2+]i that develops in the sub-membrane volume defined above. It rises relatively slowly in the face of the highly effective Ca2+ regulation that creates the compartmentalization essential for multi-tasking in this cell type. The ryanodine-sensitive component, although also opposed by these same regulatory mechanisms, rises more rapidly because CICR, although only semi-regenerative, increases the apparent diffusion coefficient for Ca2+.
How does Cs+ inhibit CICR?
Intracellular Cs+ imitates ryanodine in reducing the amplitude of the [Ca2+]i transient (see Fig. 4A). Similar effects have been reported in depolarized ventricular myocytes (Han et al. 1994); Cs+ alters the voltage dependence of phasic contraction (Levi et al. 1996) and inhibits spontaneous Ca2+ release from the sarcoplasmic reticulum (Kawai et al. 1998). Two possible mechanisms to explain these effects are that Cs+ blocks K+ channels of the sarcoplasmic reticulum (SR) or that it blocks the Ca2+ release channel (ryanodine receptor, RyR).
K+ channel block
Cs+ is known to block K+ channels of the SR (Cukierman et al. 1985) so that an unfavourable electrochemical gradient might develop during Ca2+ release if K+ could not act as a counter ion (Williams, 1992).
RyR channel block
The CICR channel is poorly selective for Ca2+. When cardiac RyR channels are incorporated into planar lipid bilayers, monovalent cations compete with Ca2+ for occupancy of the RyR pore (Mejía-Alvarez et al. 1999). It is possible that Cs+ competes more effectively than K+ and markedly reduces the conductance of the RyR pore to Ca2+.
Depolarizing stimuli initiate large [Ca2+]i transients in frog skeletal muscle even when Cs+-loaded (Kovacs et al. 1983) and so there may be differences in Cs+ sensitivity among the different RyR isoforms. Changes in the large cytoplasmic ‘foot structure’ of the isoform RyR1 alter both the Ca2+ sensitivity and ion conduction properties (Bhat et al. 1997) of the channel so that differences in Cs+ sensitivity would not be unexpected.
Site of CICR in inner hair cells
In mice at developmental stage P9, inner hair cells make both afferent and efferent contacts (Sobkowicz et al. 1997). The afferent synapses are competent at P6 just prior to the onset of hearing at P12–14 (Moser & Beutner, 2000). Efferent endings average 1.2 μm in diameter and are characterized by classical postsynaptic cisternae. They are located between adjoining hair cells at the lower half of the receptors, close to their modiolar side (Sobkowicz & Slapnick, 1994). The changes in [Ca2+]i that we observe at the base of the hair cell are therefore most likely to be located near afferent synapses. However both afferent and efferent endings occur in the same plane in P12 cells (Sobkowicz et al. 1997) and so there is likely to be some overlap in the basolateral region.
The source of the released Ca2+ is not clear. Were it not for the changes we observe at the base of the cell, the postsynaptic cisternae at the efferent synapses might be considered as possible candidates, although release from such discrete sites might be expected to generate readily observable hot spots. A more distributed structure is present in osmium-ferrocyanide-treated sections of gerbil inner hair cells, and consists of small vesicles that ‘filled the subnuclear cytosol and congregated at synapses’ (Spicer et al. 1999). Such a distributed source of Ca2+ for CICR might significantly increase the rate of rise of [Ca2+]i in the immediate vicinity of the afferent synapses.
CICR at pre-synaptic sites
While the role of CICR in muscle excitation-contraction coupling is well-known, its contribution to exocytosis at nerve terminals is less well-established, although all three RyR isoforms are widely distributed in the CNS (Furuichi et al. 1994). The following reports of CICR in nerve terminals are of relatively recent origin. In adult guinea pig outer hair cells there is a report of CICR following acetylcholine-mediated Ca2+ influx (Evans et al. 2000). In the cerebellum, immunohistochemical evidence for RyRs at basket cell terminals is accompanied by ryanodine-sensitive [Ca2+]i transients at presumed transmitter release sites and ryanodine-sensitive miniature postsynaptic currents (Llano et al. 2000). At nerve terminals in the frog (Narita et al. 2000) and guinea pig (Smith & Cunnane, 1996), ryanodine-sensitive postsynaptic currents are seen after a prolonged priming or conditioning stimulus. It is possible that a presynaptic role for CICR has been overlooked in other synapses because in many instances Cs+ is used in patch pipette filling solutions.
Role of CICR at the inner hair cell synapse
Studies on many different cell types indicate that the multiple kinetic components of exocytosis may represent movements between morphologically distinct pools of vesicles. Vesicles closely associated with the plasma membrane may form an ‘ultrafast’ or ‘immediately releasable’ pool (Mennerick & Matthews, 1996) while the remaining vesicles, tethered to intracellular structures, are released more slowly (von Gersdorff et al. 1996). [Ca2+]i may regulate not just transmitter release but also the transfer of vesicles from pool to pool (Voets, 2000).
At the squid giant synapse the insensitivity of synaptic transmission to injections of slower Ca2+ chelators such as EGTA, suggests that vesicle release sites and Ca2+ channels are closely coupled (Adler et al. 1991). However, at the goldfish hair cell synapse, an increase in sound intensity increases the amount of available transmitter rather than increasing the vesicle release probability (Furukawa, 1986). This could be accomplished if increasing the stimulus increased the spread of Ca2+ to more distant vesicle release sites. Such a mechanism, which should be sensitive to EGTA, is supported by evidence that endocytosis in saccular hair cells can occur at distant sites (see Lenzi et al. 1999).
In membrane capacitance studies on mouse inner hair cells both EGTA-sensitive and EGTA-insensitive vesicle release is evident (Moser & Beutner, 2000; Beutner et al. 2001) although the presence of intracellular Cs+ will have inhibited CICR in these experiments. It is unlikely that the sensor for the ‘ultrafast’ component of vesicle release is further from the voltage-gated Ca2+ entry than the CICR receptor. Consequently during a single brief pulse the fastest vesicle release is likely to be coupled directly to Ca2+ influx. However during a maintained stimulus, CICR may increase [Ca2+]i near vesicle release sites and promote the continuous mobilization of synaptic vesicles at the active zone.
Presynaptic CICR in inner hair cells could have at least four outcomes: first, during maintained stimuli it may contribute to a background increase in [Ca2+]i near vesicle release sites; second, it may increase the rate at which Ca2+ spreads to more distant release or mobilization sites; third, it may permit a lower affinity Ca2+ binding site, the weaker interactions with Ca2+ producing a more faithful response to brief changes in [Ca2+]i; fourth, it may reduce the need for a large presynaptic inward Ca2+ current during synaptic transmission and so reduce the tendency for the cell to generate ‘ringing’ oscillations of membrane potential upon depolarization.
Impulses in the auditory nerve may be ‘phase-locked’ to acoustic stimuli at frequencies of up to at least 1 kHz. At higher frequencies factors such as the time constant of the hair cell membrane and delays in synaptic transmission reduce the fidelity of the signal transmitted to the CNS (Kidd & Weiss, 1990). Future work will be directed towards determining whether pre-synaptic CICR may help compensate for the effect of this lowpass filtering.
Acknowledgments
We have benefited greatly from discussions with our colleagues: Nigel Cooper, Jules Hancox, Richard Helyer, Matthew Holley and Corné Kros and we thank them for their comments on the manuscript. We are indebted to Corné Kros for introducing us to the neonatal mouse preparation and to George Schofield and Lydia Henderson for introducing us to confocal microscopy. We thank Michael Rickard for computational help. This work was supported by the Wellcome Trust; Helen Kennedy is a Wellcome Trust Research Fellow
REFERENCES
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. Journal of Neuroscience. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Moser T. The presynaptic function of mouse cochlea inner hair cells during development of hearing. Journal of Neuroscience. 2001;21:4593–4599. doi: 10.1523/JNEUROSCI.21-13-04593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Bhat MB, Zhao J, Hayek S, Freeman EC, Takeshima H, Ma J. Deletion of amino acids 1641–2437 from the foot region of skeletal muscle ryanodine receptor alters the conduction properties of the Ca release channel. Biophysical Journal. 1997;73:1320–1328. doi: 10.1016/S0006-3495(97)78165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman S, Yellen G, Miller C. The K+ channel of sarcoplasmic reticulum. A new look at Cs+ block. Biophysical Journal. 1985;48:477–484. doi: 10.1016/S0006-3495(85)83803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Tanaka M, Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970;228:34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Evans MG, Lagostena L, Darbon P, Mammano F. Cholinergic control of membrane conductance and intracellular free Ca2+ in outer hair cells of the guinea pig cochlea. Cell Calcium. 2000;28:195–203. doi: 10.1054/ceca.2000.0145. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Furutama D, Hakamata Y, Nakai J, Takeshima H, Mikoshiba K. Multiple types of ryanodine receptor/Ca2+ release channels are differentially expressed in rabbit brain. Journal of Neuroscience. 1994;14:4794–4805. doi: 10.1523/JNEUROSCI.14-08-04794.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T. Sound reception and synaptic transmission in goldfish hair cells. Japanese Journal of Physiology. 1986;36:1059–1077. doi: 10.2170/jjphysiol.36.1059. [DOI] [PubMed] [Google Scholar]
- Han S, Schiefer A, Isenberg G. Ca2+ load of guinea-pig ventricular myocytes determines efficacy of brief Ca2+ currents as trigger for Ca2+ release. Journal of Physiology. 1994;480:411–421. doi: 10.1113/jphysiol.1994.sp020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa NP, Hudspeth AJ. Clustering of Ca2+ channels and Ca2+-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proceedings of the National Academy of Sciences of the USA. 1994;91:7578–7582. doi: 10.1073/pnas.91.16.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Hussain M, Orchard CH. Cs+ inhibits spontaneous Ca2+ release from sarcoplasmic reticulum of skinned cardiac myocytes. American Journal of Physiology. 1998;275:H422–430. doi: 10.1152/ajpheart.1998.275.2.H422. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, Kros CJ, Meech RW. Sodium independent regulation of intracellular calcium in inner hair cells from neonatal CD-1 mice. Journal of Physiology. 2000;523.P:94–95P. [Google Scholar]
- Kidd RC, Weiss TF. Mechanisms that degrade timing information in the cochlea. Hearing Research. 1990;49:181–207. doi: 10.1016/0378-5955(90)90104-w. [DOI] [PubMed] [Google Scholar]
- Kirkwood NK, Kros CJ. Rapid photorelease of calcium augments the fast potassium conductance of guinea-pig inner hair cells. Journal of Physiology. 1997;504.P:127P. [Google Scholar]
- Kovacs L, Rios E, Schneider MF. Measurement and modification of free calcium transients in frog skeletal muscle fibres by a metallochromic indicator dye. Journal of Physiology. 1983;343:161–196. doi: 10.1113/jphysiol.1983.sp014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rüsch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–284. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Rüsch A, Richardson GP. Mechano-electrical transducer currents in hair cells of the cultured neonatal mouse cochlea. Proceedings of the Royal Society B. 1992;249:185–193. doi: 10.1098/rspb.1992.0102. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Runyeon JW, Crum J, Ellisman MH, Roberts WM. Synaptic vesicle populations in saccular hair cells reconstructed by electron tomography. Journal of Neuroscience. 1999;19:119–132. doi: 10.1523/JNEUROSCI.19-01-00119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi AJ, Mitcheson JS, Hancox JC. The effect of internal sodium and caesium on phasic contraction of patch-clamped rabbit ventricular myocytes. Journal of Physiology. 1996;492:1–19. doi: 10.1113/jphysiol.1996.sp021284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Morphological differences among radial afferent fibers in the cat cochlea: an electron-microscopic study of serial sections. Hearing Research. 1980;3:45–63. doi: 10.1016/0378-5955(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nature Neuroscience. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. Journal of Biological Chemistry. 1986;261:6300–6306. [PubMed] [Google Scholar]
- Mejía-Alvarez R, Kettlun C, Ríos E, Stern M, Fill M. Unitary Ca2+ current through cardiac ryanodine receptor channels under quasi-physiological ionic conditions. Journal of General Physiology. 1999;113:177–186. doi: 10.1085/jgp.113.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron. 1996;17:1241–1249. doi: 10.1016/s0896-6273(00)80254-8. [DOI] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proceedings of the National Academy of Sciences of the USA. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K, Akita T, Hachisuka J, Huang S, Ochi K, Kuba K. Functional coupling of Ca2+ channels to ryanodine receptors at presynaptic terminals. Amplification of exocytosis and plasticity. Journal of General Physiology. 2000;115:519–532. doi: 10.1085/jgp.115.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Studies of ionic currents in the isolated vestibular hair cell of the chick. Journal of Physiology. 1984;350:561–581. doi: 10.1113/jphysiol.1984.sp015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Brugge JF, Anderson DJ, Hind JE. Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. Journal of Neurophysiology. 1967;30:769–793. doi: 10.1152/jn.1967.30.4.769. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Sellick PM. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. Journal of Physiology. 1983;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Cunnane TC. Ryanodine-sensitive calcium stores involved in neurotransmitter release from sympathetic nerve terminals of the guinea-pig. Journal of Physiology. 1996;497:657–664. doi: 10.1113/jphysiol.1996.sp021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz HM, Slapnick SM. The efferents interconnecting auditory inner hair cells. Hearing Research. 1994;75:81–92. doi: 10.1016/0378-5955(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Slapnick SM, Nitecka LM, August BK. Compound synapses within the GABAergic innervation of the auditory inner hair cells in the adolescent mouse. Journal of Comparative Neurology. 1997;377:423–442. doi: 10.1002/(sici)1096-9861(19970120)377:3<423::aid-cne9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Thomopoulos GN, Schulte BA. Novel membranous structures in apical and basal compartments of inner hair cells. Journal of Comparative Neurology. 1999;409:424–437. [PubMed] [Google Scholar]
- Spoendlin H. The innervation of the cochlear receptor. In: Moller AR, editor. Basic Mechanisms in Hearing. Orlando: Academic Press; 1973. pp. 185–230. [Google Scholar]
- Tucker T, Fettiplace R. Confocal imaging of calcium microdomains and calcium extrusion in turtle hair cells. Neuron. 1995;15:1323–1335. doi: 10.1016/0896-6273(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Voets T. Dissection of three Ca2+-dependent steps leading to secretion in chromaffin cells from mouse adrenal slices. Neuron. 2000;28:537–545. doi: 10.1016/s0896-6273(00)00131-8. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Vardi E, Matthews G, Sterling P. Evidence that vesicles on the synaptic ribbon of retinal bipolar neurons can be rapidly released. Neuron. 1996;16:1221–1227. doi: 10.1016/s0896-6273(00)80148-8. [DOI] [PubMed] [Google Scholar]
- Williams AJ. Ion conduction and discrimination in the sarcoplasmic reticulum ryanodine receptor/calcium-release channel. Journal of Muscle Research and Cell Motility. 1992;13:7–26. doi: 10.1007/BF01738423. [DOI] [PubMed] [Google Scholar]


