Abstract
Electrical stimulation of the hypothalamus, basal ganglia or pedunculopontine nucleus in decorticate animals results in locomotion and a cardiorespiratory response resembling that seen during exercise. This has led to the hypothesis that parallel activation of cardiorespiratory and locomotor systems from the midbrain could form part of the ‘central command’ mechanism of exercise. However, the degree to which subcortical structures play a role in cardiovascular activation in awake humans has not been established. We studied the effects on heart rate (HR) and mean arterial blood pressure (MAP) of electrically stimulating the thalamus and basal ganglia in awake humans undergoing neurosurgery for movement disorders (n = 13 Parkinson's disease, n = 1 myoclonic dystonia, n = 1 spasmodic torticollis). HR and MAP increased during high frequency (> 90 Hz) electrical stimulation of the thalamus (HR 5 ± 3 beats min−1, P = 0.002, MAP 4 ± 3 mmHg, P = 0.05, n = 9), subthalamic nucleus (HR 5 ± 3 beats min−1, P = 0.002, MAP 5 ± 3 mmHg, P = 0.006, n = 8) or substantia nigra (HR 6 ± 3 beats min−1, P = 0.001, MAP 5 ± 2 mmHg, P = 0.005, n = 8). This was accompanied by the facilitation of movement, but without the movement itself. Stimulation of the internal globus pallidus did not increase cardiovascular variables but did facilitate movement. Low frequency (< 20 Hz) stimulation of any site did not affect cardiovascular variables or movement. Electrical stimulation of the midbrain in awake humans can cause a modest increase in cardiovascular variables that is not dependent on movement feedback from exercising muscles. The relationship between this type of response and that occurring during actual exercise is unclear, but it indicates that subcortical command could be involved in ‘parallel activation’ of the locomotor and cardiovascular systems and thus contribute to the neurocircuitry of ‘central command’.
Exercise is accompanied by increases in heart rate (HR) and ventilation and a redistribution of blood flow towards exercising muscle. The ways in which these changes are brought about have not been firmly established, although both feedforward mechanisms (‘central command’) and feedback mechanisms have been shown to exist with a high degree of redundancy between mechanisms. The brain regions involved in ‘central command’ may include the motor and insular cortices (Fink et al. 1995; Williamson et al. 1997; Thornton et al. 2001), together with subcortical structures including the hypothalamus (Eldridge et al. 1981, 1985), basal ganglia (Angyan, 1991), mesencephalic locomotor region (MLR; Bedford et al. 1992), cerebellum (Fink et al. 1995, Thornton et al. 2001), pons and medulla (Gozal et al. 1994, 1995).
Whilst a positron emission tomography (PET) study of mild exercise and the post-exercise state revealed activation of the basal ganglia (Fink et al. 1995), the head movement associated with exercise limits the resolution of neuroimaging subcortical structures in exercising man. Consequently most evidence for midbrain involvement during exercise comes from animal studies. In decorticate animals, where descending central inhibition has been removed, electrical stimulation of the hypothalamus, thalamus, basal ganglia and MLR results in a locomotion together with increases in cardiorespiratory variables similar to those occurring during volitional exercise (Smith et al. 1960; Eldridge et al. 1981; Angyan, 1991; Bedford et al. 1992). The cardiorespiratory responses have been shown to persist following paralysis (‘fictive locomotion’), indicating that they are a genuine feedforward response and not related to peripheral feedback from exercising muscles.
Owing to a resurgence of surgical treatment for advanced Parkinson's disease and other movement disorders, it is now possible to perform a similar experiment in man to those performed in animals. Electrolytic lesioning or placement of electrodes for chronic electrical stimulation in the basal ganglia requires surgery in which electrical stimulation of the midbrain is performed in awake, unsedated patients (Limousin et al. 1998). Therefore the aim of this study was to establish whether subcortical structures can drive heart rate and arterial blood pressure during electrical stimulation of midbrain nuclei in awake humans.
METHODS
Subjects were patients undergoing stereotaxic neurosurgery (Papanastassiou et al. 1998) for either the placement of electrical stimulating electrodes or electrolytic lesioning of the subthalamic nucleus (STN), internal globus pallidus (Gpi), ventralis intermedius thalamus (VIM thalamus) or ventralis oralis posterior thalamus (VOP thalamus). Thirteen patients had Parkinson's disease, one had essential tremor and one had spasmodic torticollis. All experiments conformed to the Declaration of Helsinki and all patients gave written informed consent after receiving an account of the nature of the study. Experiments were carried out with local ethics committee approval (COREC 99.083).
Surgical procedures
Patients underwent an MRI scan 2 days prior to surgery (T1 weighted volume scan, 3 mm axial slices, Siemens Vision 1.5 Tesla scanner). Anti-Parkinsonian medication was withdrawn 12 h prior to surgery. On the morning of surgery patients were given a general anaesthetic (propofol i.v.), an endotracheal tube was inserted and artificial ventilation commenced. Venous and arterial cannulae were inserted for fluid and drug administration and recording of mean arterial blood pressure (MAP). A three-lead ECG was used to monitor cardiac electrical activity. A stereotactic CT scan was then performed (3 mm axial slices, Siemens ART scanner). Since metallic stereotaxic localisers cannot be used within MR scanners and CT scans lack anatomical detail, a computerised neurosurgical planning system (ImageFusion and Stereoplan, Radionics, Burlington, MA, USA) was used to volumetrically fuse CT and MR images. This allows for the surgical target to be identified using the superior anatomical detail of the MRI within stereotactic space (Alexander et al. 1995). Anaesthesia was reversed and through a scalp incision under local anaesthesia (lignocaine), a posterior frontal twist drill was used to make a small craniostomy (2.7 mm burr hole). To minimise risk of cerebral haemorrhage anti-hypertensive drugs (nifedipine or clonidine i.v.) were administered if arterial blood pressure exceeded ca 140/80. Patients were breathing freely without mechanical ventilation during the experimental protocols.
Protocols
A Radionics electrode, 1.8 mm diameter, 2.0 mm exposed tip length, was introduced into the brain and connected to a combined stimulator/lesion generator (RFG 3CF, Radionics). In patients undergoing surgery on the STN (n = 8), the electrode trajectory passed through the VIM thalamus, into the STN and through to the SN (Fig. 1 top panel). In patients undergoing surgery on the GPi (n = 6), a more lateral trajectory was used, the electrode passing through the external then internal globus pallidus. In the patient undergoing surgery on the VOP thalamus this was the only structure stimulated. Electrical stimulation began 6 mm above the surgical target and was repeated whilst proceeding along the electrode trajectory in steps of 3 mm. Stimulation parameters (pulse width, frequency and voltage) were chosen by the surgeon according to the changes in motor function observed and were in the range of 0.1-1 ms, 2-130 Hz, 0-7 V and 1-3 mA. Motor function was assessed by rapid finger tapping to test for bradykinesia and arm flexion against a load to test for power. Tremor was also assessed. Impaired speech and visual function may result from stimulation in these brain regions (Rowe et al. 1999) and so were assessed to avoid permanent adverse side effects from lesions. Three days post-operatively, patients were given a CT scan (chronic stimulator implanted patients) or MRI scan (electrolytic lesion patients) which was fused with the preoperative MRI to confirm electrode location/lesion site. This postoperative anatomical information could then be used to inform the intraoperative data.
Figure 1. Cardiovascular response to midbrain stimulation in an awake patient.
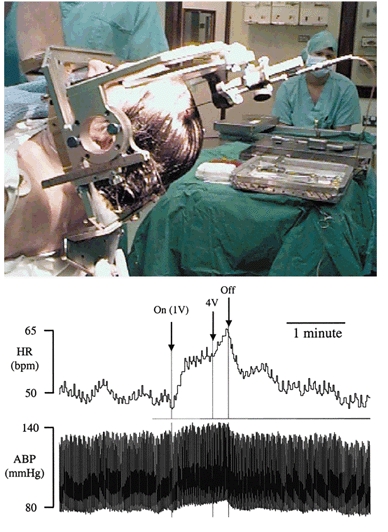
Top panel illustrates stereotaxic placement of electrode in patient. Bottom panel: raw data trace showing the cardiovascular effects of electrical stimulation of the subthalamic nucleus (frequency 100 Hz, voltage 1 V then increased to 4 V, pulse width 1 ms) in an awake Parkinson's patient. Electrical stimulation results in an increase in heart rate (HR) and mean arterial pressure (MAP) that is maintained for the duration of the stimulation.
Cardiovascular recordings and data analysis
HR and MAP were obtained from the three-lead ECG and the arterial cannula respectively. All data were recorded on an anaesthetic monitor (AS/3, Datex-Engstrom) whose analog outputs were sampled at 500 Hz, digitised (BIOPAC MP100WS) and recorded on a Power Macintosh computer using Acqknowledge software (Biopac Systems, Santa Barbara, CA, USA) for subsequent analysis. Control values for HR and MAP were taken as the mean value for the 5 s preceding the onset of electrical stimulation. Stimulation values were taken from the mean of a 5 s period at the maximum voltage occurring during that stimulation. Data for electrical stimulation were pooled for an individual subject according to the nucleus in which the electrode was situated (determined according to the number of millimetres above or below the surgical target), and the frequency of stimulation (‘high’ frequency > 90 Hz, ‘low’ frequency < 20 Hz). The mean response was calculated for each patient at each nucleus for both high and low frequency stimulation and then averaged across all patients. All data are expressed as means ± s.e.m. Control and electrical stimulation data were compared using Student's paired t test.
RESULTS
Fifteen patients (7 male) aged 57 ± 2 years with a disease duration of 13 ± 1 years were studied (surgical targets; n = 1 VOP thalamus, n = 8 STN, n = 6 GPi). Patient characteristics are given in Table 1.
Table 1.
Subject characteristics
| Patient | Age (years) | Sex | Disease duration | Surgery | Disease | Previous surgery | Drug therapy |
|---|---|---|---|---|---|---|---|
| 01 | 58 | F | 9 | L STN stim. | PD | R VIM les. | Sinemet, selegiline. |
| 02 | 55 | M | 12 | Bilateral STN stim. | PD | L STN les. | Madopar, clonazepam. |
| 03 | 60 | F | 19 | L STN stim. | PD | R STN les. | Pergolide, sinemet. |
| 04 | 54 | F | 12 | Bilat STN stim. | PD | — | Pergolide, madopar. |
| 05 | 40 | M | 13 | L GPi les. | PD | — | Sinemet, selegiline. |
| 06 | 54 | M | 9 | L GPi les. | PD | — | Sinemet, pergolide, selegiline. |
| 07 | 78 | F | 11 | R GPi les. | PD | — | Madopar, sinemet. |
| 08 | 47 | F | 15 | R STN les. | PD | — | Sinemet, selegiline, orphenadrine, bromocriptine. |
| 09 | 67 | M | 6 | L STN les. | PD | — | Madopar, Clonazepam, Propranolol. |
| 10 | 63 | M | 8 | L GPi les. | PD | R GPi les. | — |
| 11 | 67 | F | 7 | Bilateral GPi stim. | ST | — | Diazepam, fluoxcetine. |
| 12 | 50 | M | 24 | R STN les. | PD | L GPi les. | Apomorphine, sinemet. |
| 13 | 64 | M | 17 | L GPi les. | PD | — | Sinemet, pergolide. |
| 14 | 64 | F | 9 | Bilat STN stim. | PD | — | Sinemet, baclofen, pergolide, domperidone, apomorphine |
| 15 | 32 | F | 20 | L VOP les. | ET | — | Propranolol |
Only drugs related to treatment of movement disorders are listed (other drugs included anti-depressants, anti-emetics, analgesics and sedatives). Abbreviations: STN, subthalamic nucleus; GPi, internal globus pallidus; VOP, ventralis oralis posterior thalamus; VIM, ventralis intermedius thalamus; PD, Parkinson's disease; ST, spasmodic torticollis; ET, essential tremor; les., lesion; stim., stimulator implantation.
Electrical stimulation of the thalamus, STN and substantia nigra
HR increased during high frequency electrical stimulation of the thalamus (5 ± 3 beats min−1, P = 0.002, n = 9), STN (5 ± 3 beats min−1, P = 0.002, n = 8) or SN (6 ± 3 beats min−1, P = 0.001, n = 8) (Fig. 1 and Fig. 2). MAP also increased significantly during high frequency stimulation of the thalamus (4 ± 3 mmHg, P = 0.05), STN (5 ± 3 mmHg, P = 0.006) or SN (5 ± 2 mmHg, P = 0.005) (Fig. 1 and Fig. 2). Stimulation parameters were 0.5 ± 0.2 ms pulse width, frequency 100 Hz, 1.3 ± 0.2 V (≈1.62 mA), 72 ± 12 s stimulus duration (thalamus), 0.5 ± 0.1 ms, 100 ± 3 Hz, 1.5 ± 0.2 V (≈1.87 mA), 80 ± 14s (STN), 0.4 ± 0.2 ms, 101 ± 1 Hz, 1.9 ± 0.4 V (≈2.37 mA), 67 ± 15 s (SN). Electrical stimulation resulted in improved motor signs including improved rigidity, a lessening of bradykinesia and decreased tremor. Low frequency electrical stimulation of the thalamus, STN or SN did not affect either HR or MAP (Fig. 3) and did not improve motor signs. Stimulation parameters during low frequency stimulation were 0.5 ± 0.3 ms pulse width, frequency 3 ± 1 Hz, 2.2 ± 0.2 V (≈2.75 mA), 80 ± 14 s stimulus duration (thalamus), 0.5 ± 0.2 ms pulse width, frequency 14 ± 9 Hz, 2.1 ± 0.3 V (≈2.62 mA), 75 ± 19 s (STN), 0.5 ± 0.2 ms pulse width, frequency 18 ± 12 Hz, 1.6 ± 0.3 V (≈2.0mA), 61 ± 21 s (SN). The interval between electrical stimulations varied, but always exceeded 30 s.
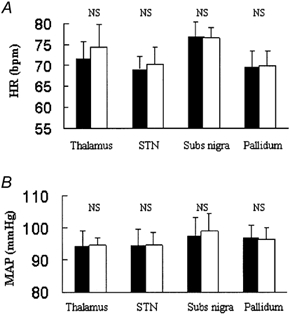
Figure 2. Group data (n = 9 thalamus, n = 8 STN and substantia nigra, n = 8 GPi) for the effects of high frequency electrical stimulation on HR (A) and MAP (B).

Filled bars are control, open are during stimulation. Thalamus refers to either the ventralis intermedius (n = 8) or ventralis oralis posterior nuclei (n = 1). Stimulation parameters were 0.5 ± 0.2 ms pulse width, frequency 100 Hz, 1.3 ± 0.2 V (thalamus), 0.5 ± 0.1 ms, 100 ± 3 Hz, 1.5 ± 0.2 V (STN), 0.4 ± 0.2 ms, 101 ± 1 Hz, 1.9 ± 0.4 V (SN). All data are means ± s.e.m. STN, subthalamic nucleus. * P < 0.05, ** P < 0.01; NS, not significant; paired t test.
Figure 3. Group data (n = 3 thalamus, n = 5 STN, n = 4 SN, n = 7 GPi) for the effects of low frequency electrical stimulation on HR (A) and MAP (B).
Filled bars are control, open are during stimulation. Thalamus refers to either the ventralis intermedius (n = 2) or ventralis oralis posterior nuclei (n = 1). STN, subthalamic nucleus. There was no significant change in HR or MAP during low frequency stimulation of any nucleus. Stimulation parameters were 0.5 ± 0.3 ms pulse width, frequency 3 ± 1 Hz, 2.2 ± 0.2 V (thalamus), 0.5 ± 0.2 ms pulse width, frequency 14 ± 9 Hz, 2.1 ± 0.3 V (STN), 0.5 ± 0.2 ms pulse width, frequency 18 ± 12 Hz, 1.6 ± 0.3 V (SN). All data are means ± s.e.m.
Electrical stimulation of the globus pallidus
Neither high nor low frequency electrical stimulation of the GPi had any effect on HR or MAP (Fig. 2 and Fig. 3). Stimulation parameters were 0.4 ± 0.2 ms, 100 Hz, 1.9 ± 0.2 V (≈2.37mA), 94 ± 21 s stimulus duration (high frequency stimulation) and 0.4 ± 0.2 ms, 2.4 ± 0.4 Hz, 2.2 ± 0.4 V (≈2.75 mA), 56 ± 10s (low frequency stimulation). Electrical stimulation of the GPi was accompanied by improvements in rigidity and akinesia.
DISCUSSION
The major new findings of this study are as follows. (1) High frequency (> 90 Hz) electrical stimulation of the thalamus, subthalamic nucleus and substantia nigra increases HR and MAP accompanied by a facilitation of movement in awake humans. (2) High frequency electrical stimulation of the globus pallidus did not affect cardiovascular variables but did facilitate movement. (3) Low frequency (< 20 Hz) stimulation of these nuclei did not increase HR or MAP and had no effect on movement.
Parkinson's disease is a degenerative movement disorder in which the main symptoms are rigidity, bradykinesia and tremor. These symptoms are thought to be a result of the loss of dopaminergic neurones from the nigrostriatal pathway of the basal ganglia (Greenfield, 1997). The mainstay of drug treatment is levodopa (a dopamine precursor) which often has extremely disabling side effects including dyskinesias and rapid fluctuations between almost total symptom relief and complete disablement. Electrolytic lesioning or implantation of electrodes for chronic electrical stimulation of the thalamus or basal ganglia has also been used to treat Parkinson's disease that fails to respond to drug therapy and has also been used for dystonia and essential tremor (Obeso et al. 2001). Surgery results in decreased bradykinesia, tremor and rigidity, effects which are believed to be due to a decrease in the output of the basal ganglia (which are pathologically overactive in this disorder). The frequency of electrical stimulation used during the surgical procedure is based on the reported firing rate of the neurones in the basal ganglia (40 Hz for the STN; DeLong et al. 1985). High frequency electrical stimulation (defined in this study as > 90 Hz) causes a reversible inactivation of the region (via overdrive suppression resulting in Na+ channels being held in their refractory state), thus indicating to the surgeon what effect a lesion would have prior to actually performing one. In contrast, low frequency electrical stimulation (defined in this study as < 20 Hz) increases basal ganglia output. The surgical procedure of electrically stimulating the thalamus, STN and GP in awake man therefore provides a human model in which to study electrical stimulation of the midbrain, and the hypothesis that the midbrain can activate locomotion and the cardiovascular systems in parallel.
Electrical stimulation of the thalamus and basal ganglia
It seems likely that the cardiovascular changes seen in the current study were a direct consequence of electrical stimulation on the neurones within the region of the electrode since (1) the patient was not aware of whether or not the stimulating electrode was actually turned on or what stimulation parameters were being used, (2) electrical stimulation of the GPi (albeit in a separate group of patients) had no effect on cardiovascular variables yet resulted in improved motor activity, (3) responses were voltage dependent, maintained for the duration of stimulation and fell rapidly on cessation of stimulation (Fig. 1), and (4) there was no cardiovascular response to low frequency electrical stimulation of any site.
Since this study was performed on awake unsedated patients, ‘descending inhibition’ from superior brain regions was intact in contrast to animal studies on electrical stimulation which have generally used decorticate or anaesthetised preparations. This difference is exemplified by the fact that in many decorticate preparations spontaneous locomotion (Eldridge et al. 1981; Millhorn et al. 1987) and a high resting breathing frequency occur (DiMarco et al. 1983). The degree of descending inhibition will presumably influence the stimulation parameters required to elicit a response and might explain the more modest increases in HR and MAP in the current study compared to those in animal studies.
Electrical or chemical stimulation of a number of midbrain nuclei has been shown to increase cardiovascular variables in awake, decorticated and anaesthetised animals (Angyan, 1978; Tan et al. 1983; Dampney et al. 1984; Ward 1988; Angyan, 1991, 1994, 1996; Lin & Yang 1994). Many of these studies have shown that electrical stimulation of these regions is accompanied by movement in addition to their effects on cardiovascular variables. Following paralysis to eliminate peripheral feedback resulting from motor activity, cardiovascular variables still increase.
Potential mechanisms
Since we did not perform electrophysiological recordings from midbrain nuclei, it is not possible to define the relationship between stimulation parameters and the actual effect on basal ganglia output. Consequently it is not possible to clearly define a mechanism by which the observed effects were occurring. It is also unclear whether the effects were due to stimulation of neurones with cell bodies in the region of the electrode or stimulation of fibres of passage. The basal ganglia project to a number of nuclei which have been shown to affect cardiovascular function in addition to their projections to the thalamus via which they control movement. These projections include the hypothalamus, parabrachial nucleus, raphe nucleus, red nucleus, ventral tegmentum, locus ceruleus and the periaqueductal grey (reviewed by Dampney et al. 1984; Verberne & Owens, 1998), all areas involved in autonomic responses. Indeed, studies in rats found that the increase in HR elicited by substantia nigra stimulation was abolished by vagotomy, whilst the increase in MAP elicited by stimulation was abolished by spinal transection (Lin & Yang, 1994). The basal ganglia also project to the pedunculopontine nucleus (Lee et al. 1988; Lavoie & Parent, 1994). This is part of the mesencephalic locomotor region, stimulation of which increases HR and MAP and elicits locomotion in decerebrate or anaesthetised rats (Bedford et al. 1992; Chong & Bedford, 1997). It is therefore possible that electrical stimulation in the current study could be influencing this pathway. Interestingly, the GPi has opposite effects on the pedunculopontine nucleus compared to the STN and SN (Kodsi & Swerdlow, 1997); this could potentially explain why in our study stimulation of the thalamus, STN and SN resulted in increases in cardiovascular variables whereas pallidal stimulation did not.
Study limitations
Parkinsonian symptoms do not develop until ca 90 % of the dopaminergic cells of the nigrostriatal pathway are lost (Greenfield, 1997), so the pathology in these patients could potentially alter the neural circuitry of subcortical command structures making it difficult to draw inferences regarding normal exercising man. Indeed, since poor autonomic function is common in Parkinson's (Netten et al. 1995), and the structures stimulated have been shown to affect cardiovascular autonomic function (Lin & Yang, 1994), larger responses might well have been observed in subjects with intact autonomic function. Clearly this is an invasive study that cannot be performed with a group of normal humans so we are unable to exclude the possibility that defective basal ganglia are a prerequisite for these responses. However, the responses do not appear to be a unique feature of Parkinson's disease; patients with spasmodic torticollis and essential tremor yielded comparable results in the current study.
Owing to the relatively small number of patients available for this type of study it was not possible to exclude patients on the basis of previous lesional surgery (Table 1), but qualitatively similar results were observed in patients who had not undergone previous surgery. Whilst anti-Parkinsonian medication can have cardiovascular effects (Pollak et al. 1987), it was withdrawn ca 14 h prior to surgery. In addition, residual effects of the general anaesthetic could affect cardiovascular function, as could the fact that six patients had anti-hypertensives during surgery.
We were unable to measure respiratory changes during electrical stimulation since the patient is required to speak to the surgeon during the procedure. We are therefore unable to comment on the role of midbrain nuclei in generating a ventilatory ‘central command’.
Relationship to ‘central command’ in exercise
Much has been made in the literature of studies involving electrical stimulation of the midbrain and the potential role of this region in generating cardiovascular responses during exercise, the so called ‘central command’. This terminology is misleading since activation of midbrain nuclei in these studies involves a subcortical command. These structures may well form part of the relay station to cardiovascular brainstem nuclei, but their role must be viewed in context with the higher centres that are responsible for initiating the exercise response. The basal ganglia possess the neural connections to influence both motor and autonomic centres and the current study indicates that decreasing basal ganglia output may increase both HR and MAP in parallel with a facilitation of movement in man.
Although electrical stimulation did not drive movement per se, the result is compatible with the classical ‘parallel activation’ of locomotor and cardiovascular systems seen in the animal studies of Eldridge et al. (1981, 1985), since the akinesia of Parkinson's disease was relieved by electrical stimulation (as judged by improved performance of motor tasks such as finger tapping). Increases in cardiovascular variables without overt movement has been observed in animal studies at low stimulation currents (Eldridge et al. 1981, 1985). It was proposed that the neural networks controlling the cardiovascular system were above threshold at rest, while locomotor networks were below threshold. Consequently a small activation of a common driving mechanism would have a demonstrable effect on systems above threshold without any noticeable effect on systems below threshold. It is possible that increasing voltage in the current study may have resulted in a larger cardiovascular response and observable movement, but this was not attempted as it results in increased spread of the electrical current and a less specific neural stimulation. Indeed the voltages used in this study were necessarily low (mean < 2 V); higher voltages are perceived as unpleasant by the patient. This might indicate that the voltages used in animal studies particularly those in decorticated or anaesthetised animals have been excessive.
The increases in cardiovascular variables in this study were modest and are probably not of functional significance for the patients in whom the stimulation was performed; nevertheless they demonstrate an important principle based on animal studies that until now had not been studied in man. The fact that movement was not driven by electrical stimulation in the current study indicates that the subcortically driven cardiovascular response was not dependent upon peripheral feedback from exercising muscles.
Acknowledgments
J.M.T. was supported by an MRC studentship and T.Z.A. by the MRC. We are grateful to the patients, and the neurosurgical team at the Radcliffe Infirmary for their assistance, particularly Nicky Maartens, Simon Parkin and Carol Joint.
REFERENCES
- Alexander E III, Kooy HM, van Herk M, Schwartz M, Barnes PD, Tarbell N, Mulkern RV, Holupka EJ, Loeffler JS. Magnetic resonance image-directed stereotactic neurosurgery: use of image fusion with computerized tomography to enhance spatial accuracy. Journal of Neurosurgery. 1995;83:271–276. doi: 10.3171/jns.1995.83.2.0271. [DOI] [PubMed] [Google Scholar]
- Angyan L. Cardiovascular effects of septal, thalamic, hypothalamic and midbrain self-stimulation. Physiology and Behaviour. 1978;20:217–226. doi: 10.1016/0031-9384(78)90212-3. [DOI] [PubMed] [Google Scholar]
- Angyan L. Substantia nigra stimulation and blood pressure effects of locally applied kainic acid. Neuroreport. 1991;2:785–788. [PubMed] [Google Scholar]
- Angyan L. Somatomotor and cardiorespiratory responses to basal ganglia stimulation in cats. Physiology and Behaviour. 1994;56:167–173. doi: 10.1016/0031-9384(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Angyan L. Cardiorespiratory effects of electrical stimulation of the globus pallidus in cats. Physiology and Behaviour. 1996;59:455–459. doi: 10.1016/0031-9384(95)02082-9. [DOI] [PubMed] [Google Scholar]
- Bedford TG, Loi PK, Crandalll CC. A model of dynamic exercise: the decerebrate rat locomotor preparation. Journal of Applied Physiology. 1992;72:121–127. doi: 10.1152/jappl.1992.72.1.121. [DOI] [PubMed] [Google Scholar]
- Chong RK, Bedford TG. Heart rate, blood pressure, and running speed responses to mesencephalic locomotor region stimulation in anesthetized rats. Pflügers Archiv. 1997;434:280–284. doi: 10.1007/s004240050397. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Goodchild AK, Tan E. Identification of cardiovascular cell groups in the brain stem. Clinical and Experimental Hypertension. 1984;6:205–220. doi: 10.3109/10641968409062561. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Crutcher MD, Georgopoulos AP. Primate globus pallidus and subthalamic nucleus: functional organization. Journal of Neurophysiology. 1985;53:530–543. doi: 10.1152/jn.1985.53.2.530. [DOI] [PubMed] [Google Scholar]
- Dimarco AF, Romaniuk JR, Von Euler C, Yamamoto Y. Immediate changes in ventilation and respiratory pattern associated with onset and cessation of locomotion in the cat. Journal of Physiology. 1983;343:1–16. doi: 10.1113/jphysiol.1983.sp014878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respiration Physiology. 1985;59:313–337. doi: 10.1016/0034-5687(85)90136-7. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Waldrop TG. Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science. 1981;211:844–846. doi: 10.1126/science.7466362. [DOI] [PubMed] [Google Scholar]
- Fink GR, Adams L, Watson JD, Innes JA, Wuyam B, Kobayashi I, Corfield DR, Murphy K, Jones T, Frackowiak RS, Guz A. Hyperpnoea during and immediately after exercise in man: evidence of motor cortical involvement. Journal of Physiology. 1995;489:663–675. doi: 10.1113/jphysiol.1995.sp021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Hathout GM, Kirlew KA, Tang H, Woo MS, Zhang J, Lufkin RB, Harper RM. Localization of putative neural respiratory regions in the human by functional magnetic resonance imaging. Journal of Applied Physiology. 1994;76:2076–2083. doi: 10.1152/jappl.1994.76.5.2076. [DOI] [PubMed] [Google Scholar]
- Gozal D, Omidvar O, Kirlew KA, Hathout GM, Hamilton R, Lufkin RB, Harper RM. Identification of human brain regions underlying responses to resistive inspiratory loading with functional magnetic resonance imaging. Proceedings of the National Academy of Sciences of the USA. 1995;92:6607–6611. doi: 10.1073/pnas.92.14.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield JG. Greenfield's Neurophathology. London: Arnold; 1997. [Google Scholar]
- Kodsi MH, Swerdlow NR. Regulation of prepulse inhibition by ventral pallidal projections. Brain Research Bulletin. 1997;43:219–228. doi: 10.1016/s0361-9230(96)00440-6. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract-tracing methods. Journal of Comparative Neurology. 1994;344:210–231. doi: 10.1002/cne.903440204. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Rye DB, Hallanger AE, Levey AI, Wainer BH. Cholinergic vs. noncholinergic efferents from the mesopontine tegmentum to the extrapyramidal motor system nuclei. Journal of Comparative Neurology. 1988;275:469–492. doi: 10.1002/cne.902750402. [DOI] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. New England Journal of Medicine. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- Lin MT, Yang JJ. Stimulation of the nigrostriatal dopamine system produces hypertension and tachycardia in rats. American Journal of Physiology. 1994;266:H2489–2496. doi: 10.1152/ajpheart.1994.266.6.H2489. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG, Kiley JP. Diencephalic regulation of respiration and arterial pressure during actual and fictive locomotion in cat. Circulation Research. 1987;61:I53–59. [PubMed] [Google Scholar]
- Netten PM, De Vos K, Horstink MW, Hoefnagels WH. Autonomic dysfunction in Parkinson's disease, tested with a computerized method using a Finapres device. Clinical and Autonomic Research. 1995;5:85–89. doi: 10.1007/BF01827468. [DOI] [PubMed] [Google Scholar]
- Obeso JA. Deep brain stimulation of the subthalamic nucleus or pars interna of the globus pallidus in Parkinson's diease. New England Journal of Medicine. 2001;345:956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- Papanastassiou V, Rowe J, Scott R, Silburn P, Davies L, Aziz T. Use of radionics image fusion and steroplan programs for target localisation in functional neurosurgery. Journal of Clinical Neuroscience. 1998;5:28–32. doi: 10.1016/s0967-5868(98)90197-7. [DOI] [PubMed] [Google Scholar]
- Pollak P, Mallaret M, Gaio JM, Hommel M, Perret J. Blood pressure effects of apomorphine and domperidone in parkinsonism. Advances in Neurology. 1987;45:263–266. [PubMed] [Google Scholar]
- Rowe JG, Davies LE, Scott R, Gregory R, Aziz TZ. Surgical complications of functional neurosurgery treating movement disorders: results with anatomical localistion. Journal of Clinical Neuroscience. 1999;6:36–37. doi: 10.1054/jocn.1997.0213. [DOI] [PubMed] [Google Scholar]
- Smith OA, Rushmer RF, Lasher EP. Similarity of cardiovascular responses to exercise and to diencephalic stimulation. American Journal of Physiology. 1960;198:1139–1142. doi: 10.1152/ajplegacy.1960.198.6.1139. [DOI] [PubMed] [Google Scholar]
- Tan E, Goodchild AK, Dampney RA. Intense vasoconstriction and bradycardia evoked by stimulation of neurones within the midbrain ventral tegmentum of the rabbit. Clinical and Experimental Pharmacology and Physiology. 1983;10:305–309. doi: 10.1111/j.1440-1681.1983.tb00202.x. [DOI] [PubMed] [Google Scholar]
- Thornton JM, Guz A, Murphy K, Griffith AR, Pedersen DL, Kardos A, Leff A, Adams L, Casadei B, Paterson DJ. Identification of higher brain centres that may encode the cardiorespiratory response to exercise in humans. Journal of Physiology. 2001;533:823–836. doi: 10.1111/j.1469-7793.2001.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberne AJ, Owens NC. Cortical modulation of the cardiovascular system. Progress in Neurobiology. 1998;54:149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- Ward DG. Stimulation of the parabrachial nuclei with monosodium glutamate increases arterial pressure. Brain Research. 1988;462:383–390. doi: 10.1016/0006-8993(88)90570-7. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Nobrega AC, Mccoll R, Mathews D, Winchester P, Friberg L, Mitchell JH. Activation of the insular cortex during dynamic exercise in humans. Journal of Physiology. 1997;503:277–283. doi: 10.1111/j.1469-7793.1997.277bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


