Abstract
This study was carried out to determine the effect of intravenous injection of anandamide on pulmonary C-fibre afferents and the cardiorespiratory reflexes. In anaesthetized, spontaneously breathing rats, intravenous bolus injection of anandamide near the right atrium immediately elicited the pulmonary chemoreflex responses, characterized by apnoea, bradycardia and hypotension. After perineural treatment of both cervical vagi with capsaicin to block the conduction of C-fibres, anandamide no longer evoked these reflex responses. In open-chest, and artificially ventilated rats, anandamide injection evoked an abrupt and intense discharge in vagal pulmonary C-fibres in a dose-dependent manner. After injection of the high dose, the fibre discharge generally started within 1 s, reached a peak in ∼2 s, and returned to baseline within 7 s. The stimulation of C-fibres by anandamide was completely and reversibly blocked by pretreatment with capsazepine, a competitive antagonist of the vanilloid type 1 receptor. Anandamide (0.4 mg kg−1) stimulated ∼93 % of pulmonary C-fibres that were activated by capsaicin at a much lower dose (0.6 μg kg−1); the response to anandamide showed similar intensity, but had slightly longer latency and duration than that to capsaicin. In conclusion, intravenous bolus injection of anandamide evokes a consistent and distinct stimulatory effect on pulmonary C-fibre terminals, and this effect appears to be mediated through an activation of the vanilloid type 1 receptor.
Bronchopulmonary C-fibre afferents innervate all levels of the respiratory tract and play an important role in the regulation of various airway functions during both physiological and pathological conditions (Coleridge & Coleridge, 1984; Lee & Pisarri, 2001). Capsaicin, the pungent ingredient in hot peppers, is the chemical agent most commonly used in the studies of the physiological properties and functions of these afferents because of its specificity and potency in stimulating these afferents (Holzer, 1991; Ho et al. 2001). Bolus injection of capsaicin into the pulmonary circulation stimulates pulmonary C-fibre afferents and elicits pulmonary chemoreflex responses (apnoea, bradycardia and hypotension) (Coleridge & Coleridge, 1984; Lee & Pisarri, 2001), which are mediated through the activation of the vanilloid type 1 (VR1) receptor, a ligand-gated non-selective cation channel (Lee & Lundberg, 1994; Caterina et al. 1997). Despite the well-established background information, the endogenous ligand for the VR1 receptor remains to be identified.
Anandamide (arachidonylethanolamide), an arachidonate derivative, is released from macrophages, basophils, neurones and epithelium (Di Marzo et al. 1994, 1996; Deutsch et al. 1997) and was originally identified as an endogenous ligand of the cannabinoid (CB) receptor (Devane et al. 1992). Anandamide has been shown to inhibit nociception, in part, via activation of the CB1 receptor on capsaicin-sensitive sensory neurones (Richardson et al. 1998). In contrast to its inhibitory action, it has been reported recently that anandamide excites VR1-transfected cells (Zygmunt et al. 1999; Smart et al. 2000) and nociceptive neurones of dorsal root ganglia (DRG) (Tognetto et al. 2001) by activating the VR1 receptor. One recent study has shown that anandamide produces a contractile response in the isolated bronchus and that this effect is blocked either by pretreatment with the antagonist of the VR1 receptor or by desensitization of C-fibre afferents with capsaicin (Tucker et al. 2001), suggesting that activation of the VR1 receptor on bronchopulmonary C-fibres may be involved. However, whether anandamide has any direct effect on these afferents is not known.
In light of the existing information about the potential physiological role of anandamide, this study was carried out (1) to characterize the cardiorespiratory reflex responses to intravenous injection of anandamide in spontaneously breathing rats; (2) to evaluate the role of vagal C-fibre afferents in eliciting these responses by selectively blocking the conduction of these afferents; (3) to determine the stimulatory effect of anandamide on individual pulmonary C-fibres by using the single-fibre recording technique in artificially ventilated rats; (4) to study the relative contribution of the VR1 and CB1 receptors to the responses of pulmonary C-fibres to anandamide by pretreatment with the antagonists of these receptors; and (5) to compare the patterns of afferent responses to anandamide and capsaicin in the same pulmonary C-fibres.
METHODS
Animal preparation
The procedures described below were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health, USA, and were also approved by the University of Kentucky Institutional Animal Care and Use Committee.
Male Sprague-Dawley rats (360–445 g) were initially anaesthetized with an intraperitional injection of α-chloralose (∼100 mg kg−1; Sigma Chemical, St Louis, MO, USA) and urethane (∼500 mg kg−1; Sigma) dissolved in a borax solution (2 %; Sigma); supplemental doses of α-chloralose (∼20 mg kg−1 h−1) and urethane (∼100 mg kg−1 h−1) were injected intravenously to maintain abolition of pain reflexes elicited by paw-pinch. One femoral artery was cannulated for recording the arterial blood pressure (ABP) with a pressure transducer (Statham P23AA). For intravenous administration of pharmacological agents, the left jugular vein was cannulated and a catheter was advanced until its tip was positioned slightly above the right atrium. The volume of each bolus injection was 0.1 ml, which was first injected into the catheter (dead space, ∼0.2 ml) and then flushed into the circulation by an injection of 0.3 ml saline. A short tracheal cannula was inserted just below the larynx via a tracheostomy. Body temperature was maintained at ∼36 oC throughout the experiment by means of a heating pad placed under the animal lying in a supine position. At the end of the experiment, the animal was killed by an intravenous injection of KCl.
Measurement of reflex responses
Rats breathed spontaneously via the tracheal cannula. Respiratory flow was measured with a heated pneumotachograph and a differential pressure transducer (Validyne MP45–14), and was integrated (Grass 7P10) to give the tidal volume (VT). Tracheal pressure (Pt) was measured (Validyne MP45–28) via a side-port of the trachea cannula. Ventilatory and cardiovascular signals were recorded on a polygraph (Grass model 7) and were also analysed by an on-line computer (CompuAdd model 433). Inspiratory and expiratory durations (TI and TE), VT, heart rate (HR) and ABP were analysed on a breath-by-breath basis; the respiratory frequency (f) of each breath was then calculated as (60/(TI + TE)). These measurements were made continuously for at least 10 breaths before (baseline) and 20 breaths after the injection of anandamide or capsaicin. The lungs were hyperinflated (Pt > 10 cmH2O) to establish a constant volume history 2 min before each injection because lung atelectasis is known to develop progressively in anaesthetized animals (Mead & Collier, 1959). Results obtained from the computer analysis were routinely compared with those obtained by hand calculation for accuracy.
Measurement of single C-fibre activity
The rats were artificially ventilated with a respirator (UGO Basile 7025); VT and f were set at 8–10 ml kg−1 and 44 breaths min−1, respectively, to mimic those of unilaterally vagotomized rats (see explanation below). After a midline thoracotomy, both vagus nerves were ligated just above the diaphragm to eliminate afferent signals arising from lower visceral organs. The opening of the thorax was covered by a sheet of polyethylene film to keep the lung moist, and the expiratory outlet of the respirator was placed under 3 cmH2O pressure to maintain a near-normal functional residual capacity. The right cervical vagus nerve was separated from the carotid artery and was sectioned as far rostrally as possible. The caudal end of the cut vagus nerve was placed on a small dissecting platform and immersed in a pool of mineral oil. A thin filament was teased away from the desheathed nerve trunk and placed on a platinum-iridium hook electrode. Action potentials were amplified (Grass P511K), monitored by an audio monitor (Grass AM8RS) and displayed on an oscilloscope (Tektronix 2211). The thin filament was further split until the afferent activity arising from a single unit was electrically isolated.
Because vagal pulmonary C-fibres usually have a sparse (< 0.5 impulses (imp) s−1) and irregular baseline discharge, hyperinflation of the lung (3–4 times VT) was used as the first step in searching for these fibres. Once the afferent activity of a single unit was identified by hyperinflation, capsaicin (1.0 μg kg−1) was injected via the jugular venous catheter with its tip positioned near the right atrium. The criteria used to identify pulmonary C-fibres were: (1) an abrupt and intense response to capsaicin within 2 s after the injection; (2) a weak response to lung inflation; and (3) the general locations of the receptors identified by their responses to gentle pressing of the lungs with a blunt-ended glass rod at the end of the experiment. These criteria were established in our recent study (Ho et al. 2001) for reliable identification of pulmonary C-fibres. The signals of the afferent activity, Pt and ABP were recorded on a thermal writer (Gould TW11) and on a VCR format data recorder (Vetter 4000A). Fibre activity (FA) was analysed by a computer in 0.5 s intervals. Increase in FA (ΔFA) was calculated as the difference between peak FA (5 s average) and baseline FA (10 s average). A C-fibre was considered to be activated when ΔFA exceeded 0.5 imp s−1.
Experimental design and protocol
Five series of experiments were carried out.
Study series 1
The ventilatory and cardiovascular responses elicited by intravenous bolus injections of the vehicle and increasing doses of anandamide (0.4, 0.8 and 1.2 mg kg−1) were determined in six rats; the sequence of these injections was alternated to achieve a balanced design. To avoid any accumulated effect, at least 15 min elapsed between two injections in this and subsequent protocols.
Study series 2
The role of vagal C-fibre afferents in anandamide-induced cardiorespiratory responses was evaluated in 12 rats. The responses to anandamide injections were studied in two matching groups of rats. Subsequently, studies were repeated 15 min after a bilateral perineural treatment (PNT) with either capsaicin or vehicle in the same rats; the former produces a selective and reversible blockade of the C-fibre conduction in the vagus nerves (Jancsó & Such, 1983; Lee et al. 1996). Briefly, cotton strips soaked in capsaicin solution (250 μg ml−1) or its vehicle (1 % Tween 80, 1 % ethanol and 98 % saline) were wrapped around a 2–3 mm segment of the isolated cervical vagi for 20 min and then removed. Our criterion for a successful capsaicin treatment was judged on the abolition of the reflex responses induced by the injection of capsaicin (0.8 μg kg−1).
Study series 3
The dose-response curve of pulmonary C-fibres to injected anandamide was determined with four different doses of anandamide (vehicle, 0.2, 0.4 and 0.6 mg kg−1) in eight C-fibres. These doses were chosen after the reproducibility of afferent responses to the same dose of anandamide was established in four additional C-fibres in our preliminary experiments; due to the difficulty of maintaining single-unit C-fibre recording for > 1 h, higher doses of anandamide were not included in this series.
Study series 4
This series was carried out to determine the role of the VR1 receptor in the anandamide-induced stimulation in pulmonary C-fibres. The anandamide-induced afferent responses were studied before and 2 min after either a treatment of capsazepine (3 mg kg−1; n = 11), a competitive VR1 receptor antagonist, or its vehicle (n = 7). To examine the possible involvement of the CB1 receptor, responses were also determined before and 15 min after a treatment of AM281 (0.3 mg kg−1; n = 7), a selective CB1 receptor antagonist, in a different group of rats. These doses of capsazepine and AM281 have been shown to effectively block VR1 and CB1 receptors, respectively (Lee & Lundberg, 1994; Cosenza et al. 2000).
Study series 5
To study the correlation between the temporal patterns of the C-fibre discharge following the injection of the two substances, the afferent responses elicited by anandamide (0.4 mg kg−1) were compared with those elicited by capsaicin (0.6 μg kg−1) in the same C-fibres (n = 32); these doses were chosen because they evoked similar intensities of discharge in the same fibres. The sequence of these injections was alternated between fibres to achieve a balanced design.
Materials
A stock solution of anandamide (50 mg ml−1; Sigma) was prepared in a vehicle of 50 % ethanol and 50 % emulphor (EL-620; Rhodia, Cranbury, NJ, USA), and that of capsaicin (250 μg ml−1; Sigma) in 1 % Tween 80, 1 % ethanol and 98 % saline. Solutions of anandamide and capsaicin for injection at the desired concentrations were prepared daily by dilution with saline on the basis of the animal's body weight. Capsazepine (Sigma) was first dissolved in dimethyl sulfoxide (Sigma) at a concentration of 10−1m and further diluted with saline containing 10 % Tween 80 and 10 % ethanol to a final concentration of 3 mg ml−1. AM281 (Tocris, Ballwin, MO, USA) was first dissolved in dimethyl sulfoxide at a concentration of 1.2 mg ml−1 and further diluted with saline to a final concentration of 0.3 mg ml−1 before use.
Statistical analysis
Data of study series 2 and 4 were analysed with a two-way ANOVA and those of the other series with a one-way ANOVA, unless indicated otherwise. When the ANOVA showed a significant interaction, pair-wise comparisons were made with a post hoc analysis (Fisher's least significant difference). A P value of < 0.05 was considered significant. Data are means ± s.e.m.
RESULTS
Reflex responses
Anandamide at the higher dose (0.8 or 1.2 mg kg−1) consistently elicited apnoea, bradycardia and hypotension immediately (∼1 s) after a bolus intravenous injection near the right atrium (Fig. 1 and Fig. 2). Respiratory and cardiovascular responses both returned to baseline within ∼10 s. The reproducibility of the cardiorespiratory responses to anandamide was established in four rats; no tachyphylaxis was observed in these preliminary trials.
Figure 1. Experimental records illustrating the effect of perineural capsaicin treatment of both cervical vagi on cardiorespiratory responses to injections of capsaicin and anandamide in an anaesthetized rat.
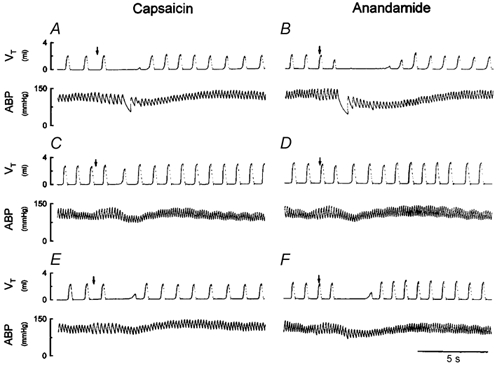
A, C and E, responses to intravenous injections of capsaicin (0.8 μg kg−1) near the right atrium before, and 5 and 60 min after perineural capsaicin treatment, respectively. B, D and F, responses to intravenous injections of anandamide (0.8 mg kg−1) before, and 15 and 75 min after perineural capsaicin treatment, respectively. Injectate (0.1 ml) was first slowly injected into the catheter (dead space, 0.2 ml) and then flushed (at arrow), as a bolus with saline (0.3 ml). Rat body weight, 405 g. VT, tidal volume; ABP, arterial blood pressure.
Figure 2. Effect of perineural capsaicin treatment of both cervical vagi on cardiorespiratory responses to intravenous injections of anandamide.
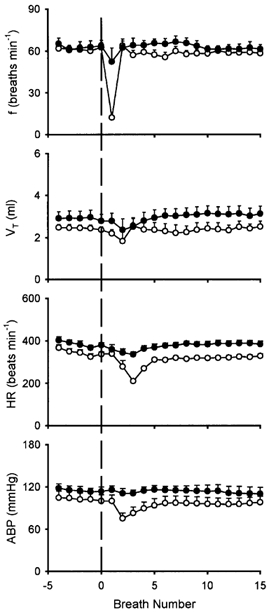
Ventilatory and cardiovascular responses to anandamide (0.8–1.2 mg kg−1) before (○) and 15 min after (•) perineural capsaicin treatment. f, respiratory frequency; VT, tidal volume; HR, heart rate; ABP, arterial blood pressure. The vertical dashed line depicts time of injection. Data are means ±s.e.m. of 6 rats.
Study series 1: dose dependence of anandamide-induced pulmonary chemoreflex responses
Anandamide elicited apnoeic responses in a dose-dependent manner (Fig. 3A), with the higher dose producing the strongest inhibition. The apnoeic breaths induced by anandamide at doses of 0.8 and 1.2 mg kg−1 had a prolonged TE, reaching 624 ± 108 % and 941 ± 139 % of baseline TE, respectively, which resulted in marked decreases in f. However, 0.4 mg kg−1 anandamide only produced very mild and less consistent ventilatory and cardiovascular inhibitions. The injection of vehicle did not cause any detectable change in respiration. There was no significant difference between the responses induced by vehicle and 0.4 mg kg−1 anandamide.
Figure 3. Apnoeic responses to intravenous injections of anandamide.
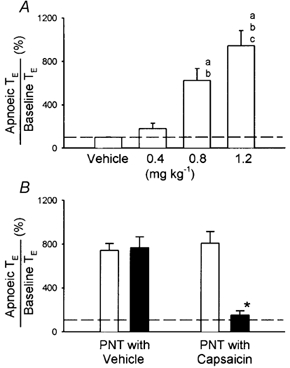
A, effect of increasing doses of anandamide on apnoeic responses. a,b,c Significantly different (P < 0.05) from the responses to vehicle, and 0.4 and 0.8 mg kg−1 anandamide, respectively. B, effect of perineural treatment (PNT) of both cervical vagi with capsaicin or vehicle on anandamide (0.8–1.2 mg kg−1)-induced apnoea. □, before PNT; ▪, after PNT. * Significantly different (P < 0.05) from the corresponding responses before PNT. Apnoeic TE, longest expiratory duration (TE) within 3 s after the injection. Baseline TE, average TE over 10 control breaths. Dashed lines, 100 % level. Data of each group are means ±s.e.m. of 6 rats.
Study series 2: effect of PNT of both vagi with capsaicin on anandamide-induced pulmonary chemoreflex responses
PNT of both cervical vagi with capsaicin caused a slight increase in baseline VT, but did not cause any significant change in baseline f, HR or ABP (Fig. 1 and Fig. 2). However, the capsaicin-induced respiratory and cardiovascular responses (apnoea, bradycardia and hypotension) were nearly abolished by the PNT with capsaicin (Fig. 1), verifying its effectiveness in blocking the vagal afferent C-fibre-mediated reflex responses. In the same animals, the anandamide-induced respiratory and cardiovascular inhibitions were similarly abolished after this treatment (Figs 1, 2 and 3B); the responses recovered in ∼1 h in the three animals tested (e.g. Fig. 1F). In comparison, these reflex responses to anandamide were not affected by the PNT with vehicle (Fig. 3B).
Single-fibre afferent responses
A total of 38 pulmonary C-fibres were studied in 24 rats. The majority of the receptors were tested in no more than two study series. The reproducibility of the anandamide effect was determined in preliminary studies; for example, in four C-fibres from three rats, the second injection of anandamide (0.4 mg kg−1) elicited a response (ΔFA, 1.55 ± 0.68 imp s−1) similar to that after the first injection (ΔFA, 1.68 ± 0.44 imp s−1; P > 0.05, paired t test). The locations of the C-fibre endings were as follows: 5, 17, 11 and 2 in the upper, middle, lower and accessory lobes of the right lung, respectively. The locations of the remaining three fibres could not be identified.
Study series 3: dose dependence of anandamide-induced C-fibre activities
Anandamide stimulated pulmonary C-fibre afferents in a dose-dependent manner (Fig. 4 and Fig. 5). Injections of anandamide at the high dose of 0.6 mg kg−1 activated all C-fibres tested (ΔFA, 5.35 ± 1.01 imp s−1); most of the fibres started to discharge within 1 s, and the activity reached a peak in ∼2 s and returned to baseline within 7 s after the injection. In five of eight C-fibres, this high dose caused a second burst of discharge of lower intensity after the initial burst had subsided (e.g. Fig. 4D). A low dose of anandamide (0.2 mg kg−1) activated only two C-fibres (ΔFA, 0.17 ± 0.14 imp s−1) and the average response was not significantly different from that to vehicle. Vehicle did not activate any of the C-fibres tested (Fig. 4 and Fig. 5). In addition, the effects of anandamide (0.6 mg kg−1) were studied in the same manner in nine slowly adapting pulmonary receptors (SARs) and nine rapidly adapting pulmonary receptors (RARs); SARs and RARs were identified by the conventional method based upon the adaptation index (Widdicombe, 1954). No stimulatory effect was found either in SARs (baseline FA, 28.46 ± 3.39 imp s−1; peak FA after anandamide, 29.97 ± 3.86 imp s−1; P > 0.5, paired t test) or RARs (baseline FA, 16.35 ± 3.15 imp s−1; peak FA after anandamide, 11.17 ± 3.83 imp s−1; P > 0.5, paired t test).
Figure 4. Experimental records illustrating responses of a pulmonary C-fibre arising from an ending in the right accessory lobe to intravenous injections of anandamide in an anaesthetized and open-chest rat.
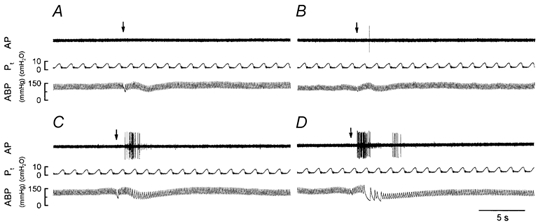
A, B, C and D, responses to injections (arrows) of vehicle, and 0.2, 0.4 and 0.6 mg kg−1 anandamide, respectively. An interval of at least 15 min elapsed between two injections. Rat body weight, 385 g. AP, action potential; Pt, tracheal pressure; ABP, arterial blood pressure.
Figure 5. Dose dependence of anandamide-induced stimulatory effect on 8 pulmonary C-fibres in 6 anaesthetized and open-chest rats.
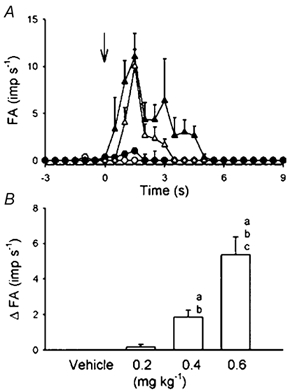
A, average responses to intravenous injections (arrow) of vehicle (○), and 0.2 (•), 0.4 (▵) and 0.6 mg kg−1 (▴) of anandamide. Fibre activity (FA) was measured in 0.5 s intervals. B, ΔFA, difference between peak FA (5 s average) after the injection and baseline FA (10 s average). a, b, c Significantly different (P < 0.05) from the responses to vehicle, and 0.2 and 0.4 mg kg−1 anandamide, respectively. Data are means ±s.e.m.
Study series 4: effect of capsazepine or AM281 on anandamide-induced C-fibre activation and cardiovascular responses
After the capsazepine treatment, there was no change in the baseline FA of C-fibre (before treatment, 0.036 ± 0.043 imp s−1; after treatment, 0.027 ± 0.020 imp s−1; P > 0.5, paired t test), but ΔFA induced by anandamide (0.4–0.6 mg kg−1) was almost completely abolished (ΔFA: before capsazepine, 3.63 ± 0.61 imp s−1; after capsazepine, 0.04 ± 0.04 imp s−1; P < 0.05). The responses to the same dose of anandamide returned to the pre-capsazepine control ∼20 min after capsazepine treatment (3.18 ± 0.46 imp s−1) (Fig. 6 and Fig. 7A). Capsazepine also nearly blocked the anandamide-induced changes in HR (ΔHR (% of baseline): control, −30.4 ± 6.5 %; after capsazepine, −4.8 ± 0.6 %; P < 0.05, paired t test) and ABP (ΔABP (% of baseline): control, −16.7 ± 3.3 %; after capsazepine, −2.1 ± 0.2 %; P < 0.05, paired t test; e.g. Fig. 6C). In contrast, the vehicle of capsazepine did not have any significant effect on anandamide-induced C-fibre discharge or the reflex bradycardia and hypotension (Fig. 7A). Pretreatment with AM281, a selective antagonist of the CB1 receptor, also did not have any effect on the C-fibre response to anandamide (Fig. 7B).
Figure 6. Experimental records illustrating the responses of a pulmonary C-fibre arising from the right middle lobe to capsaicin or anandamide before and after pretreatment with capsazepine in an anaesthetized and open-chest rat.
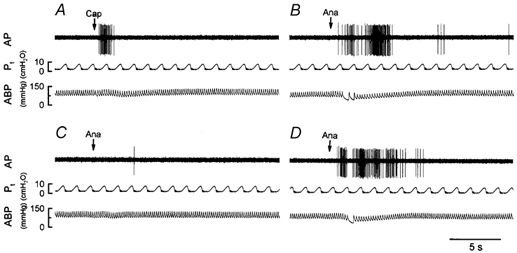
A, response to intravenous injection of capsaicin (Cap; 0.6 μg kg−1). B, C and D, responses to intravenous injections of anandamide (Ana; 0.4 mg kg−1) before, and 2 and ∼20 min after pretreatment with capsazepine (3 mg kg−1), respectively. See legend to Fig. 4 for further explanation. Rat body weight, 398 g.
Figure 7. Effect of pretreatment with capsazepine or AM281 on pulmonary C-fibre responses to anandamide.
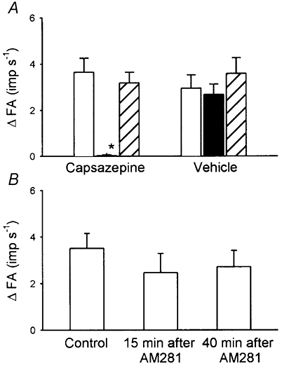
A, afferent responses to injections of anandamide (0.4–0.6 mg kg−1) before (□), and 2 (▪) and ∼20 min (recovery;  ) after pretreatment with capsazepine (3 mg kg−1; n = 11) or vehicle (n = 7). B, afferent responses to injections of anandamide (0.4–0.6 mg kg−1) before, and 15 and 40 min after pretreatment with AM281 (0.3 mg kg−1; n = 7). *P < 0.05, compared with the corresponding control. Data of each group are means ±s.e.m. See legend to Fig. 5 for further explanation.
) after pretreatment with capsazepine (3 mg kg−1; n = 11) or vehicle (n = 7). B, afferent responses to injections of anandamide (0.4–0.6 mg kg−1) before, and 15 and 40 min after pretreatment with AM281 (0.3 mg kg−1; n = 7). *P < 0.05, compared with the corresponding control. Data of each group are means ±s.e.m. See legend to Fig. 5 for further explanation.
Study series 5: comparison between afferent responses induced by capsaicin and anandamide
Capsaicin at a dose of 0.6 μg kg−1 immediately evoked an abrupt and intense discharge (ΔFA, 3.55 ± 0.70 imp s−1) in 28 of 32 pulmonary C-fibres studied (Fig. 6 and Fig. 8). Anandamide (0.4 mg kg−1) evoked a discharge of similar intensity in 93 % (29/32) of these receptors (ΔFA, 2.96 ± 0.56 imp s−1); 26 of the 32 receptors were stimulated by both capsaicin and anandamide at these median doses. In comparison, the peak response to anandamide showed a slightly longer latency (capsaicin: 0.86 ± 0.04 s; anandamide: 1.54 ± 0.07 s; P < 0.05, paired t test) and duration than that to capsaicin. In addition, at 1 s after the injection, FA evoked by anandamide (2.10 ± 0.58 imp s−1) was significantly smaller than that evoked by capsaicin (11.64 ± 3.06 imp s−1; P < 0.05, paired t test), whereas the order was reversed 2 s later (FA after anandamide, 3.50 ± 0.82 imp s−1; after capsaicin, 0.78 ± 0.30 imp s−1; P < 0.05, paired t test). In 41 % (13/32) of these C-fibres, the response to anandamide, similar to that seen in study series 3, showed a second burst of action potentials 3–7 s after the injection (Fig. 8).
Figure 8. Comparison between responses to anandamide and capsaicin in 32 pulmonary C-fibres from 19 anaesthetized and open-chest rats.
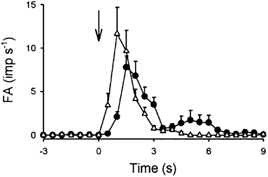
Average responses to anandamide (•; 0.4 mg kg−1) and capsaicin (▵; 0.6 μg kg−1) injected (arrow) near the right atrium. Data are means ±s.e.m.
DISCUSSION
The present study demonstrated that intravenous injection of anandamide near the right atrium immediately elicited the classic pattern of pulmonary chemoreflex responses, characterized by apnoea, bradycardia and hypotension. These reflex responses were nearly abolished by perineural treatment of both cervical vagi with capsaicin, suggesting that vagal C-fibres are involved in eliciting these anandamide-induced responses. Indeed, our electrophysiological experiments have further demonstrated that anandamide stimulated the individual pulmonary C-fibres in a dose-dependent manner. In addition, our results suggest that the stimulatory effect of anandamide on these afferents is mediated through the activation of the VR1 receptor, since the stimulation was totally eliminated by pretreatment with a competitive antagonist of the VR1 receptor but not by pretreatment with its vehicle or an antagonist of the CB1 receptor.
The present study provides direct evidence of a distinct stimulatory effect of anandamide on pulmonary C-fibre afferents. This stimulatory effect appears to be limited to the C-fibre afferents because anandamide failed to activate either RARs or SARs in the lungs. These results are consistent with our finding that pulmonary chemoreflex responses elicited by anandamide were completely abolished by perineural capsaicin treatment of both vagi. In addition, our contention is also supported by a recent study of isolated DRG neurones that showed that VR1 receptors are present almost exclusively in the small-diameter nociceptive neurones, most of which are C-neurones (Helliwell et al. 1998). We cannot totally rule out the possibility that this stimulation resulted from an indirect effect of anandamide on the C-fibre terminals. However, the short latency (< 1 s at the high dose) for the onset of this action suggests that a secondary effect of release of other mediators by anandamide is less likely to account for the stimulation.
Morphological evidence shows that ∼75 % of the afferent fibres in the vagal branches innervating the respiratory tract are C-fibres (Agostoni et al. 1957). Stimulation of pulmonary C-fibre afferents is known to elicit a number of important defence reflex responses, in addition to the pulmonary chemoreflex, which are mediated through the central nervous system and/or autonomic nervous system, such as bronchoconstriction, mucus hypersecretion and coughing (Coleridge & Coleridge, 1984; Lee & Pisarri, 2001). In addition, several sensory neuropeptides such as tachykinins (e.g. substance P, neurokinin A) are synthesized in the cell bodies of pulmonary C-fibres and are released locally from the sensory terminals upon stimulation. These peptides are known to act on a number of effector cells (e.g. airway smooth muscles, cholinergic ganglia, inflammatory cells, mucous glands) and produce potent local effects such as bronchoconstriction, plasma extravasation and oedema of airway mucosa in various species including humans (Solway & Leff, 1991). Although these reflexes and local responses resulting from the C-fibre activation were not determined in this study, anandamide can, presumably, evoke these airway responses to varying degrees of intensity, depending on the dose administered. For example, a bronchoconstrictive effect of anandamide caused by endogenous tachykinins has been demonstrated recently (Tucker et al. 2001).
In evaluation of the potential role of anandamide as an endogenous ligand of the VR1 receptor, an important and inevitable question is whether the level of endogenously released anandamide is sufficient to activate the VR1 receptor (Smart & Jerman, 2000; Szolcsányi, 2000a, b; Zygmunt et al. 2000; Tucker et al. 2001). Capsazepine pretreatment alone did not induce any detectable change in the baseline breathing pattern (Lee & Lundberg, 1994) or the baseline activity of C-fibre afferents in the present study. These observations seem to suggest a relatively insignificant role for anandamide in regulating the C-fibre activity under normal physiological conditions. However, we cannot completely rule out the possibility of an involvement of anandamide in activation of pulmonary C-fibres under pathophysiological conditions. For example, during airway inflammation, leukocytes (such as macrophages and basophils), the primary cells that produce and release anandamide (Di Marzo et al. 1996), are known to infiltrate into the extravascular space in the airways and presumably elevate the concentration of anandamide in the local tissue to a much higher level than that found in the systemic circulation. Furthermore, the sensitivity of the VR1 receptor to anandamide may be augmented under pathophysiological conditions such as tissue acidification (Olah et al. 2001), which often occurs concomitantly with tissue inflammation and ischaemia (Stevens et al. 1991; Bevan & Geppetti, 1994).
The role of anandamide as an endogenous ligand for activation of the VR1 receptor has also been questioned on the grounds that a relatively high level of anandamide in the plasma is required for activation of the VR1 receptor by intravenous injection (Willoughby et al. 1997). However, it may be somewhat misleading to evaluate the role of anandamide as an endogenous activator of the VR1 receptor on the basis of its concentration in the extracellular fluid. Recent studies have clearly revealed that capsaicin activates the VR1 receptor located on the DRG nociceptive neuronal membrane by binding to the cytosolic domain of the VR1 receptor (Jung et al. 1999). Moreover, it is known that movement of anandamide from the extracelluar space through the neuronal membrane is primarily accomplished by facilitated transport via a carrier protein and only to a very small extent by diffusion (Giuffrida et al. 2001). Hence, if anandamide binds to the same intracellular site on the VR1 receptor as capsaicin, the low potency and efficacy of anandamide as an activator of the VR1 receptor may be related, at least in part, to its limited ability to enter the intracellular space of these nerve terminals.
We reasoned that, if the stimulatory effect of anandamide on pulmonary C-fibres is mediated exclusively through the ‘capsaicin receptors’, a similar pattern of response to these two stimulants, anandamide and capsaicin, would be expected in the same fibres. To test this possibility, we selected doses of these two substances that produced similar intensities of C-fibre responses; anandamide (0.4 mg kg−1) activated ∼93 % of the pulmonary C-fibres that were activated by capsaicin at the much lower dose of 0.6 μg kg−1. Although our results show an interesting resemblance between the afferent responses of pulmonary C-fibres to anandamide and capsaicin (Fig. 8), certain differences are also evident. First, there was a striking difference in the potency of these two substances in stimulating pulmonary C-fibres; the dose of anandamide required to produce a similar intensity of stimulation in the same pulmonary C-fibres was almost three orders of magnitude higher than that of capsaicin. This observation is in general agreement with that reported recently by other investigators (Szöke et al. 2000; Tognetto et al. 2001). In addition to the limited accessibility of anandamide to its intracellular binding site as discussed earlier, another possible factor contributing to its lower potency is the lower affinity of the VR1 receptor to anandamide than to capsaicin (Szallasi & Di Marzo, 2000). Second, the discharge pattern of pulmonary C-fibres following the anandamide injection, particularly at the high dose (0.6 mg kg−1), frequently consisted of more than one burst of action potentials; in addition to the abrupt and intensive discharge similar to that initially evoked by capsaicin, a second burst of much smaller magnitude emerged 3–7 s after the injection in the majority of C-fibres tested. The possible cause of this second burst of fibre activity could not be determined in this study, though possibilities such as a secondary stimulatory effect of anandamide resulting from local release of other mediators or a recovery from transient desensitization of the VR1 receptor should be considered. Third, the onset time of peak afferent responses induced by capsaicin appeared to be slightly shorter than that of responses induced by anandamide (see Results, study series 5); this could be related to the difference in the time required for these two substances to move across the cell membrane (Jung et al. 1999; Giuffrida et al. 2001).
In conclusion, results from the present study have clearly demonstrated a dose-dependent stimulatory effect of anandamide on pulmonary C-fibre terminals in anaesthetized rats. Although this effect appears to be mediated through an activation of the VR1 receptor, injected anandamide exhibits a distinctly lower potency than capsaicin in its excitatory action on pulmonary C-fibres. Whether endogenous anandamide and its stimulatory effect on pulmonary C-fibres play any part in the regulation of airway functions during lung inflammation remains to be determined.
Acknowledgments
The authors would like to thank Dr Qihai Gu and Mr Robert Morton for their technical assistance. The authors are also grateful to Dr Grace Sears for her editorial assistance. This study was supported by NIH grant HL58686.
REFERENCES
- Agostoni E, Chinnock JE, Daly M, De B, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lung and abdominal viscera in the cat. Journal of Physiology. 1957;135:182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends in Neurosciences. 1994;17:509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Reviews of Physiology Biochemistry and Pharmacology. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Cosenza M, Gifford AN, Gatley SJ, Pyatt B, Liu Q, Makriyannis A, Volkow ND. Locomotor activity and occupancy of brain cannabinoid CB1 receptors by the antagonist/inverse agonist AM281. Synapse. 2000;38:477–482. doi: 10.1002/1098-2396(20001215)38:4<477::AID-SYN13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, Dey SK, Arreaza G, Thorup C, Stefano G, Moore LC. Production and physiological actions of anandamide in the vasculature of the rat kidney. Journal of Clinical Investigation. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Sepe N, Buono A. Biosynthesis of anandamide and related acylethanolamides in mouse J774 macrophages and N18 neuroblastoma cells. Biochemical Journal. 1996;316:977–984. doi: 10.1042/bj3160977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz J-C, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation: biochemistry and pharmacology. Journal of Pharmacology and Experimental Therapeutics. 2001;290:7–14. [PubMed] [Google Scholar]
- Helliwell RJ, McLatchie LM, Clarke M, Winter J, Bevan S, McIntyre P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neuroscience Letters. 1998;250:177–180. doi: 10.1016/s0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- Ho C-Y, Gu Q, Lin YS, Lee L-Y. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respiration Physiology. 2001;127:113–124. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacological Reviews. 1991;43:143–201. [PubMed] [Google Scholar]
- Jancsó G, Such G. Effects of capsaicin applied perineurally to the vagus nerve on cardiovascular and respiratory functions in the cat. Journal of Physiology. 1983;341:359–370. doi: 10.1113/jphysiol.1983.sp014810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Hwang SW, Kwak J, Lee SY, Kang CJ, Kim WB, Kim D, Oh U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. Journal of Neuroscience. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L-Y, Lundberg JM. Capsazepine abolishes pulmonary chemoreflexes induced by capsaicin in anesthetized rats. Journal of Applied Physiology. 1994;76:1848–1855. doi: 10.1152/jappl.1994.76.5.1848. [DOI] [PubMed] [Google Scholar]
- Lee L-Y, Morton RF, Lundberg JM. Pulmonary chemoreflexes elicited by intravenous injection of lactic acid in anesthetized rats. Journal of Applied Physiology. 1996;81:2349–2357. doi: 10.1152/jappl.1996.81.6.2349. [DOI] [PubMed] [Google Scholar]
- Lee L-Y, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respiration Physiology. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- Mead J, Collier C. Relationship of volume history of lungs to respiratory mechanics in anesthetized dogs. Journal of Applied Physiology. 1959;14:669–678. [Google Scholar]
- Olah Z, Karai L, Ladarola MJ. Anandamide activates vanilloid receptor 1 at acidic pH in DRG neurones and cells ectopically expressing VR1. Journal of Biological Chemistry. 2001;276:31163–31170. doi: 10.1074/jbc.M101607200. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) British Journal of Pharmacology. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Jerman JC. Anandamide: an endogenous activator of the vanilloid receptor. Trends in Pharmacological Sciences. 2000;21:134. doi: 10.1016/s0165-6147(00)01459-0. [DOI] [PubMed] [Google Scholar]
- Solway J, Leff AR. Sensory neuropeptides and airway function. Journal of Applied Physiology. 1991;71:2077–2087. doi: 10.1152/jappl.1991.71.6.2077. [DOI] [PubMed] [Google Scholar]
- Stevens CR, Williams RB, Farrell AJ, Blake DR. Hypoxia and inflammatory synovitis: observations and speculation. Annals of the Rheumatic Disease. 1991;50:124–132. doi: 10.1136/ard.50.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Di Marzo V. New perspectives on enigmatic vanilloid receptors. Trends in Neurosciences. 2000;23:491–497. doi: 10.1016/s0166-2236(00)01630-1. [DOI] [PubMed] [Google Scholar]
- Szöke E, Balla Z, Csernoch L, Czeh G, Syröläinen J. Interacting effects of capsaicin and anandamide on intracellular calcium in sensory neurones. NeuroReport. 2000;11:1949–1952. doi: 10.1097/00001756-200006260-00028. [DOI] [PubMed] [Google Scholar]
- Syröläinen J. Are cannabinoids endogenous ligands for the VR1 capsaicin receptor. Trends in Pharmacological Sciences. 2000a;21:41–42. doi: 10.1016/s0165-6147(99)01436-4. [DOI] [PubMed] [Google Scholar]
- SzolcsZolcs ányi J. Anandamide and the question of its functional role for activation of capsaicin receptors. Trends in Pharmacological Sciences. 2000b;21:203–204. doi: 10.1016/s0165-6147(00)01484-x. [DOI] [PubMed] [Google Scholar]
- Tognetto M, Amadesi S, Harrison S, Creminon C, Trevisani M, Carreras M, Matera M, Geppetti P, Bianchi A. Anandamide excites central terminals of dorsal root ganglion neurones via vanilloid receptor-1 activation. Journal of Neuroscience. 2001;21:1104–1109. doi: 10.1523/JNEUROSCI.21-04-01104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RC, Kagaya M, Page CP, Spina D. The endogenous cannabinoid agonist, anandamide stimulates sensory nerves in guinea-pig airways. British Journal of Pharmacology. 2001;132:1127–1135. doi: 10.1038/sj.bjp.0703906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JG. Receptors on trachea and bronchi of the cat. Journal of Physiology. 1954;123:71–104. doi: 10.1113/jphysiol.1954.sp005034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby KA, Moore SF, Martin BR, Ellis EF. The biodisposition and metabolism of anandamide in mice. Journal of Pharmacology and Experimental Therapeutics. 1997;282:243–247. [PubMed] [Google Scholar]
- Zygmunt PM, Julius I, Di Marzo V, Hogestatt ED. Anandamide - the other side of the coin. Trends in Pharmacological Sciences. 2000;21:43–44. doi: 10.1016/s0165-6147(99)01430-3. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


