Abstract
There is broad agreement that the awake human ventilatory response to a moderate inspiratory load consists of a prolongation of inspiratory time (TI) with a maintenance of tidal volume (VT) and end-tidal PCO2 (PET,CO2), the response being severely blunted in sleep. There is no agreement on the mechanisms underlying this ventilatory response. Six naive healthy males (aged 39–44) were studied supine with their heads in a positron emission tomography (PET) scanner to allow relative regional cerebral blood flow (rCBF) to be measured with H215O given intravenously. A linearised resistive load (24 cmH2O (l s−1)−1) could be added to the inspiratory limb of a breathing valve inserted into a tightly fitting facemask; inspiratory flow was measured with a pneumotachograph. The load was applied, without alerting the subject, when the radioactivity first reached the head. Six scans were performed with and without the load, in each subject. Relative rCBF contrasts between the loaded and unloaded breathing states showed significant activations in inferior parietal cortex, prefrontal cortex, midbrain, basal ganglia and multiple cerebellar sites. No activations were found in the primary sensorimotor cortex. The findings suggest that there is a pattern of motor behavioural response to the uncomfortable sensation that inspiration is impeded. This results in a prolongation of TI, the maintenance of VT and a reduction in the degree of discomfort, presumably because of the reduction of mean negative pressure in the airways.
The effects of loads on the pattern of breathing in man has been extensively studied since the early work of Zechman et al. (1957). These studies have shown that the response to moderate inspiratory resistive loading is characterised by an immediate increase in inspiratory time, maintenance of tidal volume and ventilation together with minimal changes in end-tidal PCO2 (PET,CO2). The ability of normal subjects to tolerate and respond to added inspiratory loads has been the subject of an international conference (Pengelly et al. 1974). The work presented at this conference demonstrated the complexity of the subject, particularly in relation to the problem of how the compensation is achieved (Von Euler, 1974), but also highlighted the severe blunting of the ventilatory response to an inspiratory load during light general barbiturate anaesthesia (Freedman, 1974; Read et al. 1974). This need to be conscious for full ventilatory load compensation was subsequently demonstrated by the severe blunting of the response during non-REM sleep (Santiago et al. 1981; Iber et al. 1982; Wilson et al. 1984; Wiegand et al. 1988; Badr et al. 1990; Henke et al. 1992) and mild blunting of the response in REM sleep (Wiegand et al. 1988; Morrell et al. 2000).
The significance of consciousness in the load compensation response has focused interest on the contribution, if any, of cerebral centres. Gozal et al. (1995) used functional magnetic resonance imaging to define activated regions within the brain of man, whilst awake. Images during unloaded breathing were contrasted with those images obtained whilst breathing with an inspiratory resistive load of 30 cmH2O (l s−1)−1. Imaging was confined to mid-sagittal and axial regions only. Significant areas activated were found in the ventral and dorsal pons, the basal forebrain, the putamen and multiple cerebellar regions. The authors concluded that the ventilatory response to a moderate inspiratory load was associated with consistent activation in these discrete brain locations. Motor cortical areas were not studied.
The aim of the present study was to extend the work of Gozal et al. (1995) to the entire brain, with a view to establishing whether there is a significant behavioural contribution to the load compensation response.
METHODS
Subjects and measurements
Six healthy right-handed men (age range 39–44 years) were studied. Subjects were naive to the purposes of the study and were recruited from outside the medical/scientific community. Local ethical committee approval (Hammersmith Hospitals’ Medical Ethics Committee) and permission to administer radioactivity (Administration of Radioactive Substances Advisory Committee of the Department of Health, UK) were obtained and subjects gave written informed consent. The work conformed with the Declaration of Helsinki.
Inspiratory resistive loading was applied via a tightly fitting mask covering the nose and mouth with subjects in the supine position. A two-way breathing valve was mounted on the mask to allow separation of inspiratory and expiratory flow (total additional deadspace = 215 ml). A 1 m length of incompressible tubing was connected to the inspiratory limb of the valve and terminated behind a screen. To this a pneumotachograph was attached (Fleisch Number 2 + Gould-Godart model 19117, BV, The Netherlands) to measure inspiratory flow. A large bore three-way tap was connected peripherally to the pneumotachograph to enable a linearised inspiratory resistance (24 cmH2O (l s−1)−1; Hans Rudolph model 7100R8 in series with 7100R16) to be added, without alerting the subject. The magnitude of the load was determined in preliminary studies, which revealed that a level of 24 cmH2O (l s−1)−1 was associated with a reproducible response and no excessive respiratory discomfort. The pressure in the inspiratory limb of the breathing circuit was measured using a differential pressure transducer (100 cmH2O; Validyne). PET, CO2 was monitored via a probe placed just inside the nares and led under the edge of the mask to a rapid response infrared CO2 analyser (response time = 120 ms; Morgan Capnograph, Morgan Medical, Kent, UK). Data were recorded onto a tape recorder (TEAC RD135-T DAT Recorder, Tokyo, Japan) for subsequent analysis.
Familiarisation
Subjects were brought into the laboratory a few days prior to the PET study to familiarise them with the protocol and experimental conditions to be experienced in the PET scanner. Each subject was exposed to the inspiratory load for approximately 2 min on four occasions separated by periods of 6 min of spontaneous breathing on the unloaded circuit. Care was taken to avoid giving the subjects any feedback about their breathing responses to the added load. At the end of the session, subjects were asked to volunteer comments on their subjective experiences during the session.
PET scanning protocol
PET scanning was performed to measure relative regional cerebral blood flow (rCBF) by recording the regional distribution of cerebral radioactivity after i.v. slow bolus injection (Silbersweig et al. 1993) of radiolabelled water (H215O); 15O is a positron emitter with a half-life of 2.1 min. Because no arterial blood samples were taken (Mazziotta et al. 1985), calibration was not possible and the term ‘relative’ rCBF implies that there was no absolute quantification of rCBF. Twelve successive scans were performed on each subject at 8 min intervals (allowing decay of radioactivity) with rCBF data being acquired over a 90 s period. Six of these scans had the load in place during the scan and six did not; the order was randomised. Following injection of H215O, the appearance of radioactivity in the head provided the signal to switch in the resistive load obeying the following rules: if the signal occurred in inspiration or late expiration then the load was switched in during the next expiration; if the signal was in early expiration, the load was switched in immediately. The load was removed following acquisition of the PET data.
Data acquisition
Subjects lay supine with eyes closed in a dimmed room with low ambient noise. They were positioned on an adjustable scanner table attached to the PET camera (ECAT Exact 2+, model 966, Siemens CTI). The head was positioned to minimise movement during the tasks and was supported in a stable position. PET data were acquired in three-dimensional mode with interdetector collimating septa removed (Townsend et al. 1991); these data were corrected for effects of tissue attenuation by use of measurements from a transmission scan (137Cs source) carried out before the first PET scan.
For each measurement of relative rCBF, 185 MBq of H215O were given via an indwelling cannula placed in an antecubital vein. Emission data were collected sequentially over 150 s comprising a 30 s background scan (scan A), a 30 s delay and a 90 s scan after tracer arrival in the brain (scan B). The integrated counts during scan B were corrected for background activity (from scan A); this gave an estimate of rCBF. The resolution of the scanner at the centre of the field of view was 4.8 mm transaxially and 5.6 mm axially (Spinks et al. 2000). The corrected PET scan data were then reconstructed with the use of filtered back-projection and a Hanning filter (cut-off frequency 0.5 cycles per pixel). For further processing, image planes were displayed in a 128 pixel × 128 pixel format, with a pixel size of 2 mm × 2 mm.
Image processing and data analysis
Image manipulations and calculations were performed on SPARC computers (SUN Computers Europe) with the use of MATLAB (the Mathworks Inc., Natick, MA, USA) software. SPM99 software (Wellcome Department of Cognitive Neurology, Institute of Neurology, London) was used to create statistical parametric maps (SPMs) of significant relative rCBF changes (Friston et al. 1995a,b; see Appendix). Within each area of activation, the local maxima of significant relative rCBF changes were then derived in terms of x, y and z-coordinates specified by the Montreal Neurological Institute (MNI) (Evans et al. 1993). The anatomic locations of local maxima were defined with reference to the stereotactic atlas of Talairach & Tournoux (1988) after transfomation from MNI to Talairach coordinates (M. Brett; unpublished data: http://www.mrc-cbu.cam.ac.uk/Imagining/vol-corr.html). In addition a ‘region of interest’ analysis was performed on an area comprising the pons and midbrain where we hypothesised that activations existed (see Discussion in Gozal et al. 1995). To achieve this, a small volume correction was applied to the SPM analysis described above such that statistical corrections for multiple comparisons were confined to this smaller region (Worsley et al. 1996).
Statistics
Respiratory data were analysed using analysis of variance (ANOVA), Fisher's least significant difference (LSD) or a simple paired t test, as appropriate. A P value of < 0.05 was accepted as significant.
RESULTS
Subjects' comments
In the familiarisation session, all subjects reported being aware of a difficulty in inspiring and commonly expressed the view that they had deliberately put more effort into inspiration, lengthening the time for inspiration to minimise their discomfort. During the scanning protocol, subjects commented that the periods of inspiratory loading felt the same as in the familiarisation session; no other reports of particular discomfort were noted.
Respiratory response to inspiratory loading
A typical example of the respiratory response measured during a scan is shown in Fig. 1. Following application of the load, inspiratory time increased while tidal volume and PET,CO2 were maintained throughout the loading period with increased negative mask pressure indicating increased inspiratory effort. This preservation of tidal volume did not occur immediately; moreover, on load removal, tidal volume immediately increased (and PET,CO2 decreased) presumably as a result of sustained inspiratory effort. The on-load and off-load transient changes in breathing for the group are shown in Fig. 2. Load application was associated with a significant first breath increase in TI with a non-significant fall in VT; this pattern was still present in the last two breaths before the load was removed. On load removal, the TI of the first breath significantly fell back to the control level while VT became significantly elevated and remained so on the subsequent breath. For each condition (i.e. no load and load) the individual respiratory data were averaged over the 90 s scan period; these data are shown in Fig. 3. The significantly increased inspiratory time was associated with a significantly lower ventilation, albeit with no significant differences in expiratory time, total breath time, tidal volume and PET,CO2
Figure 1. Typical response to the addition of a 24 cmH2O (l s−1)−1 inspiratory resistive load.
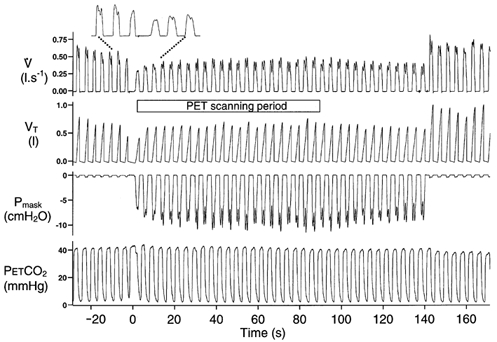
V̇, inspiratory flow; VT inspiratory tidal volume; Pmask, pressure in face mask; and PET,CO2, signal from the nose. The period over which the PET scan was taken is indicated. The increase in inspiratory time in response to the load is more clearly seen in the expanded section at the top of the figure. Note the immediate overshoot in VT following the removal of the load.
Figure 2. Means ± s.d. for TI and VT across subjects for the two breaths before the application of the load (pre), the first two breaths after the application of the load (on), the last two breaths before the removal of the load (on), and the first two breaths after removal of the load (off).
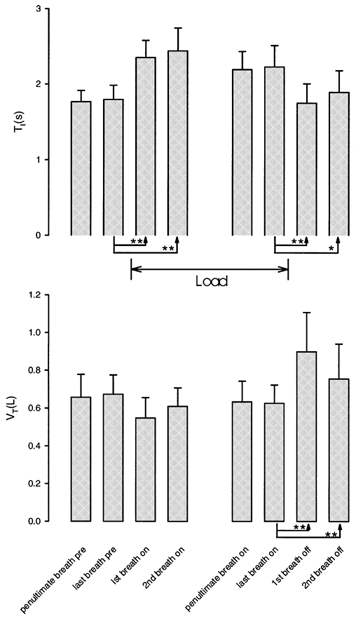
Significant differences (using Fisher's LSD) are shown as *P < 0.05 and **P < 0.01; the first two breaths after load application are not significantly different from the preceding breath. Results derived from ANOVA.
Figure 3. Indicated respiratory variables averaged across runs for each subject for the 90 s period at the start of the scan.
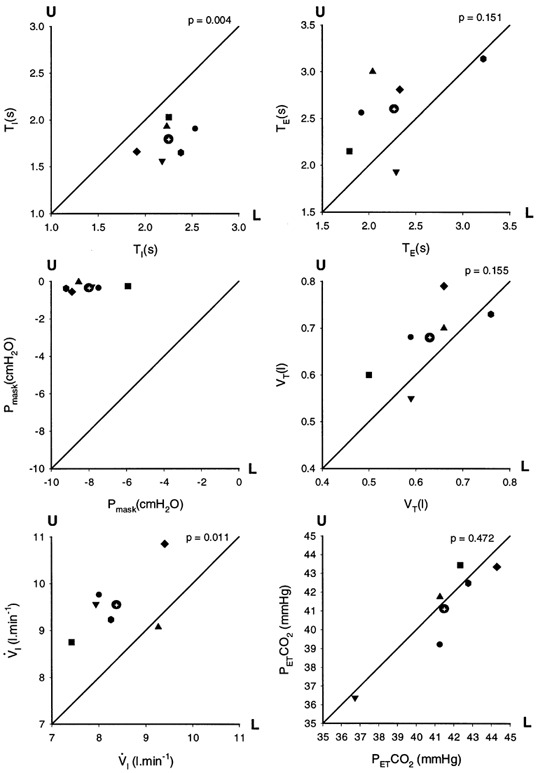
Inspiratory time (TI), expiratory time (TE), tidal volume (VT), ventilation (VI) end-tidal PCO2 (PET,CO2) and the peak inspiratory mask pressure (Pmask). The mean values are shown with different symbols for each subject and are plotted in the ‘no-load’ condition (U) on the Y-axis and the ‘loaded’ condition (L) on the X-axis; the ‘line of identity’ is shown. The mean value across all subjects for each variable is shown as a circle with a cross. The P value (paired t test) is shown above each graph and compares unloaded with loaded breathing.
Brain areas activated with inspiratory loading
The results of the relative rCBF contrast between loaded and unloaded breathing are shown as ‘through-projection’ images in Fig. 4. This reveals significant activations in parietal cortex, frontal cortex, midbrain, basal ganglia and multiple sites in the cerebellum. The stereotactic coordinates of the activation maxima within these regions and their levels of significance are shown in Table 1. A further anatomical representation of the load-related activations is depicted in Fig. 5 with significant relative rCBF increases superimposed on a representative structural magnetic resonance image of the brain (Evans et al. 1993). A sagittal and coronal image of the midbrain activation (extending into the thalamus) is shown in Fig. 6A. The region of interest analysis confined to the pons and midbrain revealed that this midbrain activation resolved into two statistically significant activations (P = 0.003). The stereotactic coordinates of the maxima of these activations were: (i) X +2, Y −28, Z −10 relating to a region close to the aqueduct of Sylvius (Fig. 6B; Talairach and Tournoux, 1988, Fig. 85); (ii) X −2, Y −12, Z −10 relating to a region close to the red nucleus and extending into the thalamus (Fig. 6C; Talairach & Tournoux, 1988, Fig. 81). This analysis did not show any significant activations in the pons.
Figure 4. Group results of relative rCBF increases associated with inspiratory resistive loading are shown as through projections onto representations of stereotactic space in sagittal view (A), coronal view from behind (B) and transverse view from above (C), anterior to the right.

The areas of activation reaching statistical significance (P < 0.0001; T > 3.93) without correction for multiple comparisons are shown. Increasing significance is shown by an arbitrary scale ranging from light gray to black.
Table 1.
Anatomical locations and stereotactic coordinates of local maxima within areas of significant relative rCBF increases associated with the addition of an inspiratory resistive load during spontaneous breathing
| Left | Right | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | X | Y | Z | T | Pcorr | X | Y | Z | T | Pcorr | ||
| Parietal | Supramarginal gyrus (40) | −56 | −40 | +30 | 4.9 | 0.042 | — | — | — | — | — | |
| Inferior parietal lobule (40) | — | — | — | — | — | +66 | −30 | +26 | 7.17 | 0.000 | ||
| Frontal | Inferolateral precentral gyrus (6) | — | — | — | — | — | +62 | +2 | +12 | 5.66 | 0.004 | |
| Cerebellum | Dentate nucleus | — | — | — | — | — | +12 | −58 | −28 | 5.34 | 0.011 | |
| Anterior lobe | Culmen (IV, V) | −26 | −52 | −30 | 5.72 | 0.003 | — | — | — | — | — | |
| Posterior lobe | Declive (VI) | −6 | −74 | −30 | 5.05 | 0.027 | — | — | — | — | — | |
| Pyramis (VIII) | −30 | −68 | −42 | 4.88 | 0.045 | — | — | — | — | — | ||
| Uvula (IX) | — | — | — | — | — | +24 | −68 | −32 | 5.22 | 0.016 | ||
| Tonsil (IX) | — | — | — | — | — | +24 | −44 | −48 | 4.93 | 0.039 | ||
| Basal ganglia | Putamen | −28 | −10 | −2 | 5.24 | 0.015 | — | — | — | — | — | |
| Putamen/claustrum | −26 | +8 | −8 | 5.16 | 0.019 | — | — | — | — | — | ||
| Globus pallidus | — | — | — | — | — | +16 | −10 | 0 | 5.36 | 0.01 | ||
| Midbrain | Red nucleus* | — | — | — | — | — | +2 | −18 | −2 | 5.42 | 0.008 | |
Coordinates in standard MNI stereotactic space (Evans, et al. 1993) refer to local maxima as indicated by the highest T value within areas of significant relative regional cerebral blood flow (rCBF) increases. X, distance (mm) to right (+) or left (−) of mid sagittal hemispheric plane; Y, distance anterior (+) or posterior (−) to vertical plane through anterior commissure; Z, distance above (+) or below (−) the plane through the anterior and postior commissures. Numbers in parenthesis; Brodmann areas of the cerebral cortex, in the cerebellum the roman numbers refer to the classification of Jansen & Brodal (1958). T is the ‘t’ statistic. Pcorr indicates the level of statistical significance after correction for multiple comparisons.
Activation extends into the thalamus.
Figure 5. Group results of relative rCBF increases associated with inspiratory resistive loading, shown on a surface-rendered representative MRI of the brain (Evans et al. 1993).

The rendering process follows the true brain surface and shows activations up to 3 cm below this surface, The colour scale indicates the statistical level (T) of the activations. Sagittal section in mid-line: A, left hemisphere showing both cerebella and midbrain activations; B, right hemisphere, showing both cerebella and midbrain activations. C, posterior view showing activations in the cerebellum and both parietal cortices; D, anterior view showing activation in the right parietal cortex. E, right lateral brain surface showing activations in the cerebellum, inferolateral precentral gyrus and inferior parietal lobule; F, left brain surface showing activations in the inferolateral precentral gyrus and the parietal supramarginal gyrus. G, inferior surface, right anterior, showing widespread activations in the cerebellum and probably thalamus; H, superior surface, right anterior, showing activations in the right and left parietal cortex and the right inferolateral precentral gyrus.
Figure 6. Group results of relative rCBF increases within the midbrain associated with inspiratory resistive loading are shown on sagittal and coronal sections of a T1-weighted magnetic resonance image of a representative brain (Evans et al. 1993).
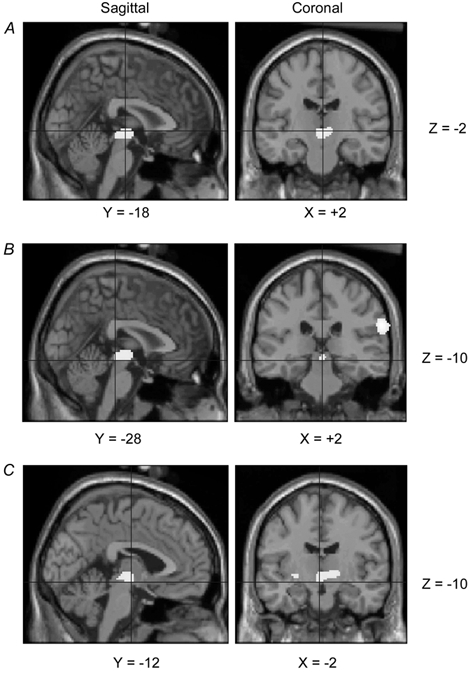
The cross hairs are centred on the most significantly activated voxel; X, Y and Z are the standard MNI coordinates in stereotatic space (Evans et al. 1993). The images are displayed (P < 0.01) after correction for multiple comparisons. A, mid-brain activation as in Table 1; B, activation near aqueduct of Sylvius; C, activation close to red nucleus, extending into the right thalamus.
DISCUSSION
Subjects
The subjects were chosen for their lack of knowledge of biology, because of the fear that any behavioural response to an added inspiratory load, influenced by prior knowledge, might dominate the pattern of brain activation and hence ventilatory responses.
Perception of load and ventilatory response
The uniformity of the description by the subjects was that the perception of difficulty in breathing in immediately led them to slow their inspiration; this was consistent with the reduction in inspiratory flow rate observed on the first breath. Most of the subjects said that they had deliberately done this in order to minimise this difficulty of breathing in. The use of the term ‘deliberate’, while not conclusive evidence, does point to the possibility that we are dealing with a behavioural ventilatory response, at least in part. Furthermore, the overshoot in tidal volume in the two breaths after removal of the load, in the face of a return of inspiratory time to control levels within the first breath (Fig. 2), implies that subjects continued to exert a larger inspiratory effort than required at this time; this would also be in keeping with a behavioural component of the ventilatory response.
The literature describes many variations in the ventilatory response to an inspiratory load. The load used in the present study was close to a load used by Zechman et al. (1957) (R2, 22 cmH2O (l s−1)−1) applied during inspiration only via a mouthpiece, and the ventilatory response in the present study was similar with an increase in inspiratory time and no fall in PET,CO2. Gozal et al. (1995), using a slightly greater inspiratory load (30 cmH2O (l s−1)−1) again applied via a mouthpiece, reported the same increase in inspiratory time, but with slower, deeper breathing, resulting in a fall in PET,CO2 of approximately 5 mmHg. We think that these and many other variations reported in the ventilatory response may well be due to the size of the load and the manner in which that load was applied. Cherniack & Altose (1981), have reviewed many factors that could contribute to variations in the ventilatory response to loaded breathing, including the probable role of higher brain centres. There is general agreement among authors that inspiratory time increases in conscious man with inspiratory loading (Freedman & Weinstein, 1965; Zin et al. 1986; Milic-Emili & Zin, 1986; Daubenspeck & Rhodes, 1995; Calabrese et al. 1998).
Imaging the brain
Image collection was optimised for sensitivity, by the timing of the application of the load with the rising phase of radioactivity within the brain occuring in the first 20–30 s of the 90 s scan (Raichle et al. 1983). The images obtained must therefore have been dominated by the initial phase of the ventilatory response.
Parietal cortex
The very strong activation in the inferior parietal lobule on the right (Brodmann area 40), together with activation of the supramarginal gyrus (an anterior extension of the inferior parietal lobule) on the left (Brodmann area 40), presumably occurred because these sites are somatosensory association areas, particularly concerned with vigilance (Roland, 1993). These areas are in receipt of a large input, at least in the monkey, from sensory association cortex (SII) and perhaps also from primary sensory cortex (SI) (Andersen et al. 1990). Complex movements of the shoulder in man (Colebatch et al. 1991) have been shown to activate a contralateral parietal area (Brodmann area 40) with coordinates similar to those shown in the present study (Table 1). There is increasing support in the literature for the view that Brodmann area 40 in man and the anterior part of area 7 (7b) in the monkey are in fact similar in terms of function (somatic sensation; Cavada & Goldman Rakic, 1989) and cytoarchitecture (Hyvarinen, 1982; Eidelberg & Galaburda, 1984; Passingham, 1998).
The absence of other activations within the parietal cortex (e.g. SI and SII) in the present study, in spite of the fact there must have been afferent traffic from lower and upper airways, the mouth, chest wall and diaphragm, is surprising. We hypothesise that the effects of these afferent signals in the parietal cortex were not at a level that our methodology was able to detect.
Frontal lobe
The highly significant activation in the ventrolateral premotor area is the best evidence that the cerebral cortex is concerned with the change in ventilatory pattern with inspiratory loading. Primate studies have shown that this particular area of the premotor cortex receives input from the parietal cortex area 7b (Petrides & Pandya, 1988; Cavada & Goldman Rakic, 1989). There is evidence, again in primates, that many cells in the ventrolateral premotor area respond to tactile stimuli to the face (Rizzolatti et al. 1981). Passingham (1993, chapter 3, Fig. 3.15) has suggested that the activity in these cells may be related to the motor response to the tactile stimulus; this may be relevant to the negative mouth and face pressures seen during loading in the present study. It is thought that activations in the ventral premotor area particularly occur when a motor response happens on the basis of sensory information (Roland et al. 1980), in contrast to activation in the supplementary motor area (SMA), typically activated during self-generated motor tasks (Roland & Friberg, 1985). Imaging studies of volitional inspiration (Colebatch et al. 1991; Fink et al. 1996) show activation in the SMA together with activation in the right premotor cortex superolaterally, as contrasted with the inferolateral activation in the right premotor cortex of the present study.
The major projections from the premotor areas are to the motor cortex (Brodmann area 4); however, pyramidal projections from premotor areas to the spinal cord do exist. We might have expected to see activations in the motor cortex in the present study, particularly in the representation of the facial areas ventrally and the breathing areas dorsolaterally (Foerster, 1936; Colebatch et al. 1991). Surprisingly, we did not find such activations and presume that the physiological changes in response to the load were associated with motor cortical activation too small to be detected by our scanning methodology. The imaging studies that have demonstrated activation in the superolateral motor cortex with inspiration (Colebatch, et al. 1991; Fink, et al. 1996) have used tidal volumes of the order of 1 to 1.6 l.
Cerebellum
The widespread activations in the cerebellum in the present study presumably reflect its role in the coordination of volitional movement originating from the cerebral cortex (Schmahmann & Pandya, 1997). The cerebellum receives sensory information from the spinal cord via the dorsal and ventral spinocerebellar tracts. The projections to the cerebellar cortex are somatotopically organised in both the anterior and posterior lobes. There is evidence from studies in the rat (Shambes, et al. 1978) that the chest and upper abdomen are mapped to the culmen (IV, V) while the head and upper face are mapped to the declive (VI) together with the area VII between the declive and the pyramis (VIII) posteriorly. All these areas of the body could be affected by the loading paradigm and these cerebellar areas were activated in the present study (Table 1). By contrast, the somatotopic representation in the anterior pyramis, uvula and tonsil is unclear (Brodal, 1981; Berry et al. 1995); we therefore cannot interpret the activations seen in these areas in the present study.
There is general agreement about the existence of a cerebro-cerebellar circuit consisting of a feed-forward limb composed of the corticopontine projection followed by the ponto-cerebellar cortical projections. The feedback loop consists of cerebellar cortex projections to the deep cerebellum especially the interpositus and the dentate nucleii. One of the projections from the interpositus nucleus goes via the red nucleus in the midbrain to the ventral posterolateral thalamic nucleus and thence to the motor cortex. The dentate nucleus projects to the ventral premotor, motor and prefrontal cortical areas via other ventrolateral nucleii in the thalamus (Middleton & Strick, 1997; Ramnani & Miall, 2001; Middleton & Strick, 2001). We presume that the activation seen in the dentate nucleus in the present study (Table 1) resulted from activity within this feedback loop; other elements of this loop were also activated, and are discussed below.
The cerebellum is thought to predict the sensory consequences of movement thereby detecting mismatches between the sensation predicted and that obtained; this could result in correction of motor behaviour until the sensation matches the prediction (Miall & Wolpert, 1996; Wolpert et al. 1998). In the context of the present study, the sensation when inspiring with no load could be regarded as the ‘predicted sensation’ and what is felt when inspiring with a load, as the ‘sensation obtained’. This mismatch could generate a cerebellar ‘correction’ signal to prolong inspiration which would reduce both airflow and negative airway pressure (Fig. 1). This respiratory behaviour is likely to be responsible for minimising the sensation accompanying loading as reported in the subjects’ comments (see Results).
Midbrain
The structure adjacent to the highly significant local maximum activation within the midbrain is the red nucleus (Fig. 6A, Table 1). However, the activation extends, albeit without local maxima, into the ventral part of the thalamus across the midline (Fig. 4 and 6C). We interpret these activations as resulting from activity in the cerebellar feedback loop described above. The activation seen in the peri-aquedutal grey matter may result from activation of the cardiovascular control centres in this region (Barman, 1990). We did not measure blood pressure or heart rate during these studies. However, measuring the effect of the same inspiratory load on blood pressure and heart rate in two further individuals showed no changes. We cannot interpret the activation seen near the aqueduct of Sylvius, in physiological terms.
Basal ganglia
The function of the basal ganglia in normal man is poorly understood. The review by Alexander et al. (1986) makes a case for the basal ganglia being the site of multiple parallel cortico-striato-thalamo-cortical loops with a great deal of functional heterogeneity. Many simple motor tasks fail to induce activation (Colebatch et al. 1991) in these structures. The lack of a comprehensive understanding of the function of these structures makes it impossible to interpret the activations in the putamen, claustrum and globus pallidus found in the present study (Table 1, Fig. 4).
Conclusion
The present study has shown that in healthy naive volunteers, the unannounced imposition of a moderate resistive inspiratory load during spontaneous inspiration leads to a pattern of neuronal activation which is consistent with a motor behavioural response to an awareness that that inspiration is impeded; this response reduces the unpleasantness of the sensation due to the load. Our findings do not support the view that ventilatory compensation to an added resistive load in awake man could be explained solely on the basis of respiratory reflexes activated from the chest wall, diaphragm, lungs or airways.
During the course of the present study, Peiffer et al. (2001) have reported on the results of an elegant brain imaging study in which both inspiration and expiration were loaded with the aim of producing breathlessness. Significant activations were found in the cerebellar vermis, right anterior insula and left medial pons when the loaded and unloaded states were compared. Differences between our results and those of Peiffer et al. (2001) may have arisen because of (a) the difference in the way the load was applied (continuous as opposed to intermittent); (b) the level of the load as judged by the mask pressure change (14 cmH2O vs. 8 cmH2O); (c) the resulting difference in the nature and degree of respiratory discomfort; none of our subjects reported ‘breathlessness’. Peiffer et al. (2001) report that when using a moderate load (8 cmH2O) of magnitude similar to that used in the present study, no significant activations were seen.
The present study has extended the work of Gozal et al. (1995). It gives substance to the early predictions of von Euler (1974) concerning the ‘corticalisation’ of the control of respiratory movements - behavioural control system - in man along the lines that have developed for the motor control of the forelimb muscles.
Acknowledgments
G.I. was supported by a travelling research fellowship from the Wellcome Trust and also the Royal Society. We would like to thank Dr Dick Passingham for his advice.
APPENDIX
Image processing
First, all scans of each individual were realigned to the first scan of the series, thus ensuring spatial congruency. All PET scans were transformed to standard stereotactic space to account for individual differences in brain size and shape. The images were further filtered in three dimensions by use of a 16 mm full-width half-maximum (FWHM) isotropic Gaussian kernel filter (a) to improve signal to noise ratio (b), to reduce inter-subject variability caused by differences in local anatomy and (c) to condition the data so that it conforms more closely to the Gaussian field theory; this forms the basis for the model from which the statistical inferences are drawn when correcting for multiple comparisons. Subsequently, a pixel-based analysis of covariance (ANCOVA), treating ‘global’ activity (reflecting global blood flow) as the covariate, controlled for state-dependent differences in global blood flow associated with the different conditions. A simple general linear model was used to model relative rCBF as a linear combination of condition, subject and global effects. In this analysis each scan was treated as an independent observation. Thereafter, contrasts of condition effects were assessed using the ‘t’ statistics (at each voxel). The resulting sets of spatially distributed T values constitute statistical parametric maps (SPM{T}) showing regions of significant condition-associated relative rCBF changes; these regions were displayed with a statistical threshold at P < 0.0001 and T ≥ 3.93 without correction for multiple comparisons (Friston et al. 1995a, b).
REFERENCES
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:9357–9381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. Journal of Comparative Neurology. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- Badr MS, Skatrud JB, Dempsey JA, Begle RL. Effect of mechanical loading on expiratory and inspiratory muscle activity during NREM sleep. Journal of Applied Physiology. 1990;68:1195–1202. doi: 10.1152/jappl.1990.68.3.1195. [DOI] [PubMed] [Google Scholar]
- Barman SM. Brainstem control of cardiovascular function. In: Klemm WR, Vertes RP, editors. Brainstem Mechanisms of Behavior. New York: John Wiley & Sons; 1990. [Google Scholar]
- Berry M, Bannister LH, Standing SM. Gray's Anatomy. 38. New York: Churchill Livingston; 1995. Cerebellum; pp. 1027–1065. [Google Scholar]
- Brodal A. Neurological Anatomy in Relation to Clinical Medicine. Oxford: Oxford University Press; 1981. [Google Scholar]
- Calabrese P, Dinh TP, Eberhard A, Bachy JP, Benchetrit G. Effects of resistive loading on the pattern of breathing. Respiration Physiology. 1998;113:167–179. doi: 10.1016/s0034-5687(98)00063-2. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. Journal of Comparative Neurology. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cherniack N, Altose M. Respiratory response to ventilatory loading. In: Hornbein TF, editor. Regulation of Breathing. Lung Biology in Health and Disease. Vol. 17. New York: Marcel-Dekker; 1981. pp. 905–964. part 2. [Google Scholar]
- Colebatch JG, Adams L, Murphy K, Martin AJ, Lammertsma AA, Tochon Danguy HJ, Clark JC, Friston KJ, Guz A. Regional cerebral blood flow during volitional breathing in man. Journal of Physiology. 1991;443:91–103. doi: 10.1113/jphysiol.1991.sp018824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, Deiber MP, Passingham RE, Friston KJ, Frackowiak RS. Regional cerebral blood flow during voluntary arm and hand movements in human subjects. Journal of Neurophysiology. 1991;65:1392–1401. doi: 10.1152/jn.1991.65.6.1392. [DOI] [PubMed] [Google Scholar]
- Daubenspeck JA, Rhodes ES. Effect of perception of mechanical loading on human respiratory pattern regulation. Journal of Applied Physiology. 1995;79:83–93. doi: 10.1152/jappl.1995.79.1.83. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Galaburda AM. Inferior parietal lobule. Divergent architectonic asymmetries in the human brain. Archives of Neurology. 1984;41:843–852. doi: 10.1001/archneur.1984.04050190049013. [DOI] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TL. Proceedings of the IEEE Nuclear Science Symposium and Medical Imaging Conference. 1993. 3D statistical neuroanatomical models from 305 MRI volumes; pp. 1813–1817. [Google Scholar]
- Fink GR, Corfield DR, Murphy K, Kobayashi I, Dettmers C, Adams L, Frackowiak RS, Guz A. Human cerebral activity with increasing inspiratory force: a study using positron emission tomography. Journal of Applied Physiology. 1996;81:1295–305. doi: 10.1152/jappl.1996.81.3.1295. [DOI] [PubMed] [Google Scholar]
- Foerster O. Motorische Felder und Bahen. In: Bumke OF, Foerster O, editors. Handbuch der Neurologie. Berlin: Springer; 1936. pp. 50–51. [Google Scholar]
- Freedman S. The effects of added loads in man; conscious and anaesthetized. In: Pengelly LD, Rebuck AS, Campbell EJM, editors. Loaded Breathing. Canada: Longman; 1974. pp. 22–25. [Google Scholar]
- Freedman S, Weinstein SA. Effects of external elastic and threshold loading on breathing in man. Journal of Applied Physiology. 1965;20:469–472. doi: 10.1152/jappl.1965.20.3.469. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995a;3:165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Heather JD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995b;2:1–25. [Google Scholar]
- Gozal D, Omidvar O, Kirlew KA, Hathout GM, Hamilton R, Lufkin RB, Harper RM. Identification of human brain regions underlying responses to resistive inspiratory loading with functional magnetic resonance imaging. Proceedings of the National Academy of Sciences of the USA. 1995;92:6607–6611. doi: 10.1073/pnas.92.14.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke KG, Badr MS, Skatrud JB, Dempsey JA. Load compensation and respiratory muscle function during sleep. Journal of Applied Physiology. 1992;72:1221–1234. doi: 10.1152/jappl.1992.72.4.1221. [DOI] [PubMed] [Google Scholar]
- Hyvarinen J. Posterior parietal lobe of the primate brain. Physiological Reviews. 1982;62:1060–1129. doi: 10.1152/physrev.1982.62.3.1060. [DOI] [PubMed] [Google Scholar]
- Iber C, Berssenbrugge A, Skatrud JB, Dempsey JA. Ventilatory adaptations to resistive loading during wakefulness and non-REM sleep. Journal of Applied Physiology. 1982;52:607–614. doi: 10.1152/jappl.1982.52.3.607. [DOI] [PubMed] [Google Scholar]
- Jansen J, Brodal A. Möllendorff's Handbuch der mikroskopischen Anatomie des Menschen. IV/8. Berlin: Springer-Verlag; 1958. Das Klienhirn. [Google Scholar]
- Mazziotta JC, Huang SC, Phelps ME, Carson RE, Macdonald NS, Mahoney K. A noninvasive positron computed tomography technique using oxygen-15-labeled water for the evaluation of neurobehavioral task batteries. Journal of Cerebral Blood Flow and Metabolism. 1985;5:70–78. doi: 10.1038/jcbfm.1985.10. [DOI] [PubMed] [Google Scholar]
- Miall RC, Wolpert DM. Forward models for physiological motor control. Neural Networks. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar output channels. International Review of Neurobiology. 1997;4:161–182. doi: 10.1016/s0074-7742(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. Journal of Neuroscience. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milic-Emili J, Zin WA. Breathing responses to imposed mechanical loads. In: Cherniak NS, Widdicome JG, editors. Handbook of Physiology, section 3, The Respiratory System, Control of Breathing. II. Bethesda, MD, USA: American Physiological Society; 1986. pp. 751–769. part 2. [Google Scholar]
- Morrell MJ, Browne HA, Adams L. The respiratory response to inspiratory resistive loading during rapid eye movement sleep in humans. Journal of Physiology. 2000;526:195–202. doi: 10.1111/j.1469-7793.2000.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham PE. The Frontal Lobes and Voluntary Action. Oxford: Oxford University Press; 1993. [Google Scholar]
- Passingham PE. The specializations of the human neocortex. In: Milner AD, editor. Comparative Neuropsychology. Oxford: Oxford University Press; 1998. pp. 271–298. [Google Scholar]
- Peiffer C, Poline JB, Thivard L, Aubier M, Samson Y. Neural substrates for the perception of acutely induced dyspnea. American Journal of Respiratory and Critical Care Medicine. 2001;163:951–957. doi: 10.1164/ajrccm.163.4.2005057. [DOI] [PubMed] [Google Scholar]
- Pengelly LD, Rebuck AS, Campbell EJM. Loaded Breathing. Canada: Longman; 1974. [Google Scholar]
- Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. Journal of Comparative Neurology. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Martin WR, Herscovitch P, Mintun MA, Markham J. Brain blood flow measured with intravenous H215O. II. Implementation and validation. Journal of Nuclear Medicine. 1983;24:790–798. [PubMed] [Google Scholar]
- Ramnani N, Miall RC. Expanding cerebellar horizons. Trends in Cognitive Sciences. 2001;5:135–136. doi: 10.1016/s1364-6613(00)01635-1. [DOI] [PubMed] [Google Scholar]
- Read DJ, Freedman S, Kafer ER. Pressures developed by loaded inspiratory muscles in conscious and anesthetized man. Journal of Applied Physiology. 1974;37:207–218. doi: 10.1152/jappl.1974.37.2.207. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Scandolara C, Gentilucci M, Camarda R. Response properties and behavioral modulation of ‘mouth’ neurons of the postarcuate cortex (area 6) in macaque monkeys. Brain Research. 1981;225:421–424. doi: 10.1016/0006-8993(81)90847-7. [DOI] [PubMed] [Google Scholar]
- Roland PE. Brain Activation. New York: Wiley-Liss; 1993. [Google Scholar]
- Roland PE, Friberg L. Localization of cortical areas activated by thinking. Journal of Neurophysiology. 1985;53:1219–1243. doi: 10.1152/jn.1985.53.5.1219. [DOI] [PubMed] [Google Scholar]
- Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. Journal of Neurophysiology. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- Santiago TV, Sinha AK, Edelman NH. Respiratory flow-resistive load compensation during sleep. American Review of Respiratory Disease. 1981;123:382–387. doi: 10.1164/arrd.1981.123.4.382. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. International Review of Neurobiology. 1997;41:4131–3160. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Shambes GM, Gibson JM, Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain, Behavior and Evolution. 1978;15:94–140. doi: 10.1159/000123774. [DOI] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith CD, Cahill C, Schnorr L, Grootoonk S, Spinks T, Clark J, Frackowiak R, Jones T. Detection of thirty-second cognitive activations in single subjects with positron emission tomography: a new low-dose H215O regional cerebral blood flow three-dimensional imaging technique. Journal of Cerebral Blood Flow and Metabolism. 1993;13:617–29. doi: 10.1038/jcbfm.1993.80. [DOI] [PubMed] [Google Scholar]
- Spinks TJ, Jones T, Bloomfield PM, Bailey DL, Miller M, Hogg D, Jones WF, Vaigneur K, Reed J, Young J, Newport D, Moyers C, Casey ME, Nutt R. Physical characteristics of the ECAT EXACT3D positron tomograph. Physics in Medicine and Biology. 2000;45:2601–2618. doi: 10.1088/0031-9155/45/9/313. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Townsend DW, Geissbuhler A, Defrise M, Hoffman EJ, Spinks TJ, Bailey DL, Gilardi MC, Jones T. Fully 3-dimensional reconstruction for a PET camera with retractable septa. IEEE Transactions on Medical Imaging. 1991;10:505–512. doi: 10.1109/42.108584. [DOI] [PubMed] [Google Scholar]
- Von Euler C. On the role of proprioceptors in perception and execution of motor acts with special refrerence to breathing. In: Pengelly LD, Rebuck AS, Campbell EJM, editors. Loaded Breathing. Canada: Longman; 1974. pp. 139–149. [Google Scholar]
- Wiegand L, Zwillich CW, White DP. Sleep and the ventilatory response to resistive loading in normal men. Journal of Applied Physiology. 1988;64:1186–1195. doi: 10.1152/jappl.1988.64.3.1186. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Skatrud JB, Dempsey JA. Effects of slow wave sleep on ventilatory compensation to inspiratory elastic loading. Respiration Physiology. 1984;55:103–120. doi: 10.1016/0034-5687(84)90120-8. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends in Cognitive sciences. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zechman FW, Hall FG, Hull WE. Effects of graded resistance to tracheal air flow in man. Journal of Applied Physiology. 1957;10:356. doi: 10.1152/jappl.1957.10.3.356. [DOI] [PubMed] [Google Scholar]
- Zin WA, Behrakis PK, Luijendijk SC, Higgs BD, Baydur A, Boddener A, Milic Emili J. Immediate response to resistive loading in anesthetized humans. Journal of Applied Physiology. 1986;60:506–512. doi: 10.1152/jappl.1986.60.2.506. [DOI] [PubMed] [Google Scholar]


