Abstract
The vasodilatation resulting from prolonged square-wave monopolar current application as used in iontophoresis is assumed to rely on an axon reflex. Involvement of prostaglandins in the anodal current-induced vasodilatation remains unclear. We tested the hypothesis that prostaglandins participate in a sensitisation mechanism to current application rather than as direct vasodilators. In healthy volunteers, laser Doppler flowmetry (LDF) was recorded in the forearm during and following isolated or repeated 0.1 mA transcutaneous anodal current applications, using deionised water as a vehicle. Segmented current applications of 6 or 12 mC resulted in an LDF increase twice that observed following current applications of comparable total charge delivered all at once (P < 0.05). Following a 1 min anodal application, a slow and prolonged LDF drift occurred (slope: 0.3 ± 0.5 arbitrary units min−1). When the same current application was repeated after intervals of 5 and 20 min, an abrupt vasodilatation occurred, with maximal LDF amplitude of 53.5 ± 34.0 and 48.2 ± 19.1 arbitrary units, respectively. Pretreatment with 1 g oral aspirin abolished the abrupt vasodilatation to repeated current application but not the initial slow drift. We suggest that vasodilatation occurs through two parallel pathways: (1) a slow progressive drift of LDF of limited amplitude insensitive to aspirin pretreatment, and (2) an abrupt vasodilatation probably resulting from afferent fibre activation, appearing if a preliminary sensitisation by current application is performed. Sensitisation lasts for at least 20 min, and is blocked by aspirin, suggesting participation of prostanoids.
In vascular physiology, monopolar current applications are used to increase the migration of vasoactive drugs through the skin in humans and animals. This technique is called iontophoresis, and is often coupled with laser Doppler flowmetry (LDF). As a non-invasive technique, compared to intravascular administration of drugs, it is a useful tool for the study of physiology or physiopathology of the human cutaneous microcirculation. Unfortunately, it has been reported for years that in parallel to the ‘specific’ vasomotor physiological effect resulting from the diffused drug, a ‘non-specific’ vasodilatation occurs as a result of the current application itself (Grossmann et al. 1995; Berliner, 1997a; Asberg et al. 1999). The amplitude of this current-induced vasodilatation depends on the electrical charge expressed in millicoulomb (mC), defined as the product of current intensity (mA) by duration of application (s) (Grossmann et al. 1995). Although control probes, on which comparable current is applied using the vehicle alone, may estimate the relative participation of the ‘non-specific’ response to the total response observed with the diffused drug, underlying mechanisms activated by the current itself are likely to interfere with the physiological response to the drug (Hamdy et al. 2001). Thus a better understanding of these ‘non-specific’ effects of prolonged monopolar current application is of major interest in the study of vascular physiology and has not been adequately investigated.
Primary afferent nerves are assumed to play a major role in the current-induced vasodilatation observed during monopolar prolonged current application through the skin (Berliner, 1997a; Hamdy et al. 2001). Multiple neuropeptides, such as calcitonin gene-related peptide (CGRP) or substance P, are released by these afferents following noxious or non-noxious stimulation (Holzer, 1997). These neuropeptides can have direct vasodilator effects. In parallel, some of these neuropeptides are able to affect the neural response, both directly or indirectly (through the release of secondary mediators), and thus exert feedback modulation of the sensitivity of the nociceptors at nerve endings. Among these modulating mediators, PGE2 has been shown to sensitise the response of afferent endings to excitation. Previous studies showed no apparent involvement of prostaglandins in anodal current-induced vasodilatation (Morris & Shore, 1996; Berliner, 1997b).
We hypothesised that prostaglandins could be released as a result of current application, but would not act as direct vasodilators. However, they could play a role in anodal current-induced vasodilatation, specifically if current application is repeated by sensitising the response of afferent nerve endings to renewed monopolar current application and thus increasing the vascular response to a total defined electrical charge. To test this hypothesis, we used prolonged or segmented continuous anodal current applications of comparable cumulative charge to study whether these two modalities result in the same vasodilator responses. Due to the progressive catabolism of prostaglandin, sensitisation, if present, should decrease over time. Then, we studied the duration of the sensitisation phenomenon using two inter-application intervals for segmented current applications. Lastly, we analysed the influence of aspirin pretreatment on the responses observed to confirm the role of prostaglandins.
METHODS
Non-smoking healthy volunteers with no clinical signs of, or risk factors for, vascular disease participated in three different experimental protocols. Patients in protocol 1 and 2 were 26.1 ± 4.0 years old (mean ± s.d.), three females, five males; height, 172.0 ± 9.0 cm; weight, 62.6 ± 4.6 kg (n = 8). In protocol 3, patients were 25.1 ± 6.4 years old, one female, seven males; height, 174.0 ± 10.6 cm; weight, 68.1 ± 13.0 kg (n = 8). In protocol 4, patients were 26.1 ± 4.2 years old, two females, six males; height, 174.5 ± 3.5 cm; weight, 62.3 ± 4.9 kg (n = 8). A minimal period of 1 week elapsed between any two experiments on the same subject. Volunteers were not involved in regular competitive exercise training and had not been administered any drug in the 3 weeks prior to each experiment. Before their participation, all subjects were thoroughly informed of the methods and procedures and gave their written consent to participate in this institutionally approved study that was carried out in accordance with The Declaration of Helsinki. Experiments were performed with the subjects placed supine in a quiet room with the ambient temperature set at 23 ± 1 °C. They rested for 15 min before each trial.
We studied cutaneous blood flow on the volar aspect of the forearm using laser Doppler flowmetry (LDF). We used three laser Doppler probes. Two of the laser Doppler multifibre probes used (probe 481–1, Perimed, Sweden) were specially designed to allow for simultaneous cutaneous blood flow recording, current application and local heating. These ‘active’ probes were 22 mm in diameter and comprised a small optic fibre placed at 90 ° to the skin surface, centred in a cylindrical thermostatic holder. The thermostatic holder had a circular chamber of ∼1 cm allowing for the positioning of a specially designed disposable sponge. A hole in the middle of the sponge allowed for the recording of cutaneous blood flow through the optic fibre. Each sponge was wet with 0.2 ml of deionised water before each experiment, and the probe was fixed to the skin with double-sided adhesive rings. A third probe (PF408, Perimed, Sweden) was used as a reference to confirm the absence of response to the current application at an adjacent unstimulated site. The three LDF probes were positioned to form an equilateral triangle, each area measured being at a distance of 5 cm from each of the others. Probes were connected to laser Doppler flowmeters (Periflux PF4001, Perimed, Sweden). The two ‘active’ probes were also connected to temperature-regulated heating systems (Peritemp PF4005, Perimed, Sweden) and to the anode of two regulated 9 V current suppliers (Periiont, Micropharmacology System, PF 382 Perimed, Sweden), allowing for the delivery of constant continuous currents for programmable durations. The cathode was positioned on disposable Ag-AgCl adhesive electrodes (Care 610, Kendall, Neustadt, Germany) 5 cm apart from the laser probes. The sites and order of current application were chosen randomly. The current application consisted of the transcutaneous delivery of a 0.1 mA current, and was never felt as painful by the subjects. To confirm that the responses between two sites and two experiments can be compared, temperature for local heating was set to 44 °C to abolish resistance vessel tone (Taylor et al. 1984) and thus to cause maximal vasodilatation (Taylor et al. 1984; Johnson et al. 1986; Saumet et al. 1998), which is a reproducible parameter (Savage & Brengelmann, 1994).
Local cutaneous temperature at an unstimulated site was measured using a surface thermocouple probe positioned 5 cm from two of the three laser probes. The thermocouple was connected to an electronic thermometer (BAT-12, Physitemp Instruments, Inc., Clifton, NJ, USA).
Systemic blood pressure was monitored using a Finapres 2350 (Ohmeda, Englewood, CO, USA) positioned on the second or third finger of the hand contra-lateral to the sites of LDF measurements.
Procedures
The different procedures are summarised in Table 1.
Table 1.
Summary of the durations (mim) of the different procedures in the protocols
| Protocol | Pretreatment | Resting period | Current application | Interstimulation interval | Current application | Recovery | Local heating |
|---|---|---|---|---|---|---|---|
| 1 | — | — | 25 | ||||
| 1 | None | 2 | 0.5 | 5 | 0.5 | 20 | 24 |
| 2 | — | — | 25 | ||||
| 2 | None | 2 | 1 | 5 | 1 | 20 | 24 |
| 5 | 35 | ||||||
| 3 | None | 2 | 1 | 20 | 1 | 20 | 24 |
| 5 | 35 | ||||||
| 4 | Aspirin/placebo | 2 | 1 | 20 | 1 | 20 | 24 |
Protocol 1. Response to 6 mC current application delivered all at once or in two consecutive half-duration applications
A reference period of 2 min was recorded in resting conditions. After the reference period, on one of the two ‘active’ probes, current was applied for 1 min. On the other probe, the current was delivered for 30 s and a subsequent period of current application of the same duration was started 5 min following the end of the first current application period. Recovery periods of 26 and 20 min, respectively, on the probe where the current was delivered all at once and on the other active probe, were recorded to study the long-lasting effects of the current. At the end of the recovery period, local warming to 44 °C was performed simultaneously on the ‘active’ probes for 24 min.
Protocol 2. Response to 12 mC current application delivered all at once or in two consecutive half-duration applications
A reference period of 2 min was recorded in resting conditions. After the reference period, on one of the two ‘active’ probes, current was applied for 2 min. On the other probe, the current was delivered for 1 min and a subsequent period of current application of the same duration was started 5 min following the end of the first current application period. Recovery periods and local warming were as in protocol 1.
Protocol 3. Influence of the duration of the inter-stimulation interval on the response to a 12 mC anodal current application delivered as two consecutive 1 min applications
Following a 2 min period in resting conditions, a total 12 mC current application was delivered through two consecutive 6 mC applications. A first 1 min 0.1 mA anodal current application was performed on the two active probes. Thereafter a second 1 min current delivery was performed on the two active probes at 5 or 20 min interstimulation intervals. A recovery period was observed for 35 and 20 min, respectively. Finally, local heating was started simultaneously on the two probes and prolonged for 24 min as previously described.
Protocol 4. Influence of aspirin pretreatment on the response to 12 mC anodal current application delivered as two consecutive 1 min applications
The subjects in this protocol were submitted to two experiments separated by a minimum period of 3 weeks. Each subject underwent both aspirin and placebo pretreatment in a random order. Aspirin (Catalgine 1 g, Lipha Santé, Lyon, France) was dissolved in 125 ml glass of orange juice in order to disguise the taste and appearance of aspirin, whereas nothing was added to the orange juice in the placebo experiments. Two hours before each experiment, subjects were given the 125 ml of orange juice, blinded as to the presence or not of aspirin in the glass. Two hours after an oral dose of 1 g aspirin, vasodilatation to intra-arterial infusion of arachidonic acid in humans has been shown to be efficiently decreased about 15 % of the normal response, while platelet aggregation was almost abolished (Bhagat et al. 1995). Thus we assume that local generation of prostanoids was efficiently inhibited in our subjects. The protocol was the same as protocol 3, with 5 and 20 min interstimulation intervals.
Measurements
The data were expressed in arbitrary units (AU) and recorded on a computer via an analog to digital converter (Biopac Systems, Inc., Santa Barbara, CA, USA) with a sample rate of 3 Hz, on 16 bits. Due to instantaneous variability of the LDF signal, results were averaged on 15-s periods for future analysis.
For data analysis and to discriminate between slow and rapid components of the response to current application, the following points were defined (an example showing the different defined points is provided in Fig. 1). The resting value (LDFrest) was defined as the last 15 s of the resting period. A5 represents the maximal value observed on averaged responses within 5 min following the end of current application, A20 represents the value observed at 20 min following current application and Apeak represents the peak value observed on averaged data in the recovery period following a current application. When two consecutive current application periods were applied, Aend was the value observed in the last 15 s before the start of the second current application. Consistently, B5, B20 and Bpeak represent, respectively, the maximal value observed on averaged responses within 5 min, the value at 20 min, and the peak value observed on averaged data, in the recovery period following the end of the second current application. For each experiment, vasodilatation to local heating (LDFheat) was represented by the last 15 s of the heating period. Note that for a 5 min interstimulation interval A5 and Apeak are equivalent, whereas for a 20 min interstimulation interval A20 and Aend are the same.
Figure 1. Analysis of LDF signals.
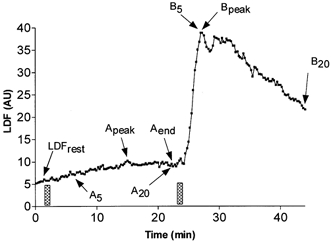
Illustration of the different points analysed on the laser Doppler flowmetry signal (LDF) in arbitrary units on averaged data as defined in Methods, during a repeated 1 min, 0.1 mA anodal current application (bars), with 20 min interstimulation intervals. The heating period is not presented to simplify the figure. In this particular situation A20 is equal to Aend and B5 is equal to Bpeak.
The slopes of the LDF changes (SLDF) were calculated by iterative subtraction of averaged LDF values (mean of the eight subjects) over 1 min. This method was chosen in preference to the average of individual slopes due to vasomotion and eventual transient instability of the signal, which increased the signal-to-noise ratio, specifically that of derivative values in individual recordings. All values are expressed as means ± s.d. in the text and presented as means ± s.e.m. in the figures. Differences between groups were performed with ANOVA and Student's unpaired t test and within an experiment with a paired t test. For all statistical analyses, a P value < 0.05 was considered significant. Non-significant results are reported as ‘NS’.
RESULTS
In all experiments, compared with starting values, no significant changes were observed for skin blood flow at the reference probe, mean arterial pressure and local skin temperature. A typical recording of the vascular response to a single or segmented delivery of 12 mC is presented in Fig. 2.
Figure 2. LDF response to a 12 mC monopolar anodal current application.
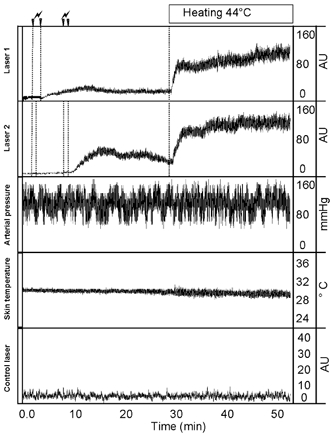
Typical recording of cutaneous laser Doppler flowmetry (LDF) response during and following a 12 mC monopolar anodal current application delivered all at once or in segmented periods of application and during the 24 min of local heating. From top to bottom, recordings are: Laser 1, LDF in arbitrary unit (AU) for a single 2 min current application; Laser 2, LDF for a twice-repeated 1 min current application; Arterial pressure; Skin temperature, local skin temperature 5 cm from the heated areas; Control laser, LDF on an unstimulated probe. Whereas vasodilatation in response to a 2 min current application is of limited amplitude, two 1 min periods result in an increased response following the second application rather than the first once the current has been stopped. This leads to higher LDF values than those achieved at sites where the current was applied in a single application.
Average LDF values for protocol 1 and 2 are presented in Fig. 3 and LDF values of the important time points of protocol 1 and 2 are summarised in Table 2.
Figure 3. LDF response to a 0.1 mA current of 6 mC.
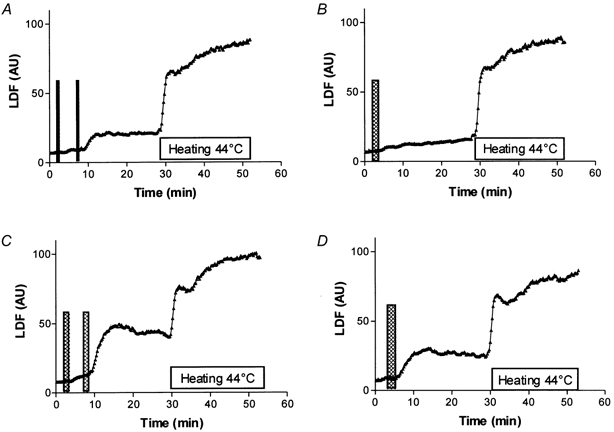
Average laser Doppler flowmetry (LDF) responses expressed in arbitrary unit (AU) to a 0.1 mA anodal current application of 6 mC delivered as: A, two half-charge applications at 5 min intervals or B, as a single application and 12 mC delivered as: C, two half-charge applications at 5 min intervals or D, as a single application. A recovery period of 20 min is recorded following the completion of current delivery, then local heating to 44 °C is carried out. Vertical bars symbolise the period of current application. The horizontal bar represents the heating period. Segmented application leads to an abrupt response of higher amplitude than a single delivery of comparable total charge.
Table 2.
Result of 0.1 mA anodal transcutaneous current application of 6 and 12 mC total charge, delivered all at once or in two consecutive half-duration periods of application
| 6 mC | 12 mC | |||
|---|---|---|---|---|
| t30/30 | t60 | t60/60 | t120 | |
| LDFrest | 7.8 ± 3.0 | 7.4 ± 3.3 | 8.1 ± 2.4 | 8.0 ± 3.3 |
| A5 | 10.1 ± 3.5 (NS) | 10.9 ± 5.2* | 12.7 ± 6.2* | 22.7 ± 17.2* |
| Aend | 9.1 ± 3.6 (NS) | X | 12.7 ± 6.6* | X |
| B5 | 21.2 ± 12.5* | X | 46.6 ± 23.6* | X |
| A20 and B20 | 22.4 ± 14.8* | 14.8 ± 8.7* | 49.4 ± 25.9* | 30.3 ± 25.4* |
| LDFheat | 94.6 ± 31.2* | 85.7 ± 20.1* | 97.2 ± 30.3* | 86.3 ± 30.7* |
t30/30, repeated current applications of 30 s each with a 5 min interval; t60, single current application of 60 s; t60/60, repeated current application of 60 s each with a 5 min interval; t120, single current application of 2 min. Laser Doppler flow (LDF; mean ± s.d.) is expressed in a arbitrary units. Indices for LDF are defined in the text, X, no apparent event.
P < 0.05; NS, not significantly different from resting value.
Protocol 1
Following a 1 min single current application of 6 mC total charge, a slow progressive vasodilatation was found and was prolonged over the whole recovery period. SLDF during this period was on the average 0.3 ± 0.5 AU min−1 and never exceeded 1.8 AU min−1. Apeak was 16.1 ± 8.2 AU and reached this value at the end of the 26 min following current application just before heat stress (P < 0.05 vs. LDFrest). Following a segmented application of comparable total charge (6 mC), a slow increase occurred following the first 3 mC current application (SLDF = 0.3 ± 0.7 AU min−1, never exceeding 1.5 AU min−1 over the 5 min interstimulation interval). Thus Aend was not significantly increased compared to the resting value. After the second period of current application, an abrupt vasodilatation was found (SLDF = 2.3 ± 1.7 AU min−1 during the 5 min post current application, maximum SLDF = 5.4 AU min−1). This vasodilatation was prolonged over the whole recovery period (B5 and B20 vs. Aend, P < 0.05 and B5 vs. B20, NS). The delivery of the 6 mC electrical charge in two distinct periods rather than all at once leads to significantly greater values of B5, B20 and Bpeak (Fig. 4) as compared to the values for A5, A20 and Apeak of the once-delivered comparable charge. No significant difference was found on LDFrest and LDFheat between current delivered in a single application and that delivered in several applications but amounting to the same total charge.
Figure 4. Maximal LDF values observed within the 5 min following the final current application of 6 and 12 mC.
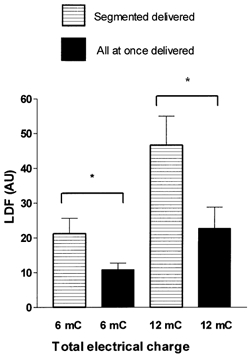
Maximal value in arbitrary units (AU) (mean ± s.e.m.), observed within the 5 min following the final current application of 6 and 12 mC total anodal charge delivered all at once (filled bars) or in two consecutive intervals separated by 5 min (horizontal lines) (*P < 0.05). For a comparable total charge, the peak laser Doppler flowmetry (LDF) response is increased if the current is delivered in two distinct periods, rather that all at once.
Protocol 2
Following a single 2 min current application of 12 mC, LDF increased rapidly (SLDF = 2.5 ± 2.2 AU min−1 over the 5 min post-current application; maximal SLDF = 6.1 AU min−1) and then reached a plateau. (A5 vs. LDFrest, P < 0.05 and A5 vs. A20: NS.). Following a segmented application of comparable total charge (12 mC), the initial 6 mC current application was followed by a slow vasodilatation (SLDF 0.8 ± 0.7 AU min−1, never exceeding 1.8 AU min−1). At the end of the 5 min interstimulation interval Aend was not significantly increased compared to LDFrest. The subsequent 6 mC current application, resulted in an abrupt increase of LDF (SLDF = 6.2 ± 3.2 AU min−1 during the 5 min post second current application, maximal SLDF = 11.9 AU min−1). This vasodilatation was prolonged over the whole recovery period (B5 and B20 vs. Aend: P < 0.05 and B5 vs. B20: NS). As observed in protocol 1, the segmented delivery of the charge led to significantly greater B5 (Fig. 4), B20 and Bpeak values as compared to A5, A20 and Apeak of comparable charge delivered all at once. As in protocol 1, no difference was observed for LDFrest and LDFpeak between the two methods of current application (Table 2).
Protocol 3
Figure 1 shows the vasodilatation resulting from repeated current application with a 20 min interstimulation interval and Fig. 5 represents the mean ± s.e.m. LDF values of the important time points of the protocol. LDFrest was 7.0 ± 2.9 and 6.5 ± 3.3 AU for interstimulation intervals of 5 and 20 min, respectively. Consistent with what was observed in protocol 1, the initial 1 min current application was followed by a slow vasodilatation for 5 and 20 min interstimulation intervals (SLDF was 0.4 ± 0.5 and 0.4 ± 0.6 AU min−1, respectively, over the whole interstimulation interval). As a consequence of the slow LDF increases following the first current application, Aend was 9.8 ± 4.1 AU for the 5 min interstimulation intervals (NS vs. LDFrest) but significantly increased compared to LDFrest for the 20 min interstimulation intervals (14.1 ± 9.3 AU). Following the second 1 min current application LDF abruptly increased in both cases and Bpeak was 53.5 ± 34.0 and 48.2 ± 19.1 AU for interstimulation intervals of 5 and 20 min, respectively. SLDF was 8.2 ± 6.2 and 5.9 ± 5.4 AU min−1 in the first 5 min following the second current application, with the maximal value for SLDF being 17.7 and 15.2 AU min−1 for interstimulation intervals of 5 and 20 min, respectively. Finally, LDFheat was not significantly different for interstimulation intervals of 5 and 20 min: 112.4 ± 29.3 and 110.9 ± 23.5 AU, respectively.
Figure 5. LDF values for two consecutive 1 min 0.1 mA current applications delivered at 5 and 20 min intervals.
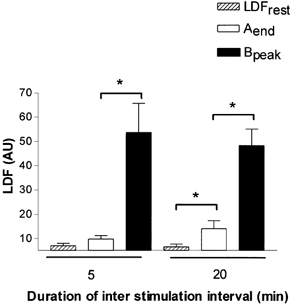
Laser Doppler flowmetry (LDF) values (mean ± s.e.m.), in arbitrary units (AU) observed for two consecutive 1 min, 0.1 mA anodal current application at 5 and 20 min intervals: hatched bars represent resting LDF values (LDFrest), open bars represent LDF before the second current application (Aend) and filled bars represent peak LDF following the second current application (Bpeak). All values are significantly increased from rest. (*P < 0.05). Following a single current application a slow vasodilatation results in a significant increase of LDF at minute 20. Renewed comparable current application at 5 and 20 min interstimulation intervals results in an amplified response.
Protocol 4
Average LDF responses are presented in Fig. 6 and LDF values of the important time points of the protocol are presented in Fig. 7. The LDF response under placebo pretreatment mimicked the responses observed in protocols 2 and 3 for comparable interstimulation intervals. Following aspirin pretreatment, the first current application resulted in a slow LDF increase (SLDF = 0.2 ± 0.3 and 0.1 ± 0.3 AU min−1 for 5 and 20 min intervals respectively), but the abrupt vasodilatation following the second current application was abolished. In contrast to the values of SLDF of ∼6–8 AU min−1 found after placebo pretreatment as in protocol 3 for comparable durations of the interstimulation intervals, following the second current application LDF continued to increase slowly. SLDF was 0.3 ± 0.6 and 0.2 ± 0.4 AU min−1 during the first 5 min of recovery for 5 and 20 min interstimulation intervals, respectively. These increases were in the range of those observed before the second current application of this protocol, and were comparable to those observed before the second current application in protocol 3. As a result of this slow vasodilatation Bpeak was observed just before heating and was significantly increased compared to resting values. Thus aspirin did not abolish the slow vasodilatation observed after electrical current application. Last, LDFheat was not significantly different for interstimulation intervals of 5 and 20 min under placebo pretreatment: 123.6 ± 35.9 and 122.6 ± 30.7 AU, respectively; under aspirin pretreatment: 101.9 ± 55.2 and 101.5 ± 62.8 AU, respectively, and between aspirin and placebo pretreatment (P < 0.05 in all cases).
Figure 6. LDF response after aspirin or placebo pretreatment.
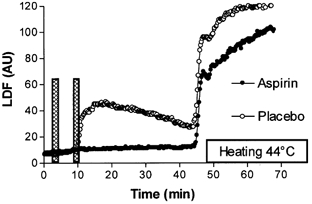
Average laser Doppler flowmetry (LDF) responses expressed in arbitrary units (AU) to a twice repeated 1 min, 0.1 mA anodal current application with 5 min interstimulation intervals (represented by the columns), after aspirin or placebo pretreatment. A 35 min period of recovery is allowed before local heating is started in order to estimate maximal vasodilation. Aspirin pretreatment abolished the abrupt response to the second current application observed in the placebo experiment.
Figure 7. Peak and rest values of LDF after aspirin or placebo pretreatment.
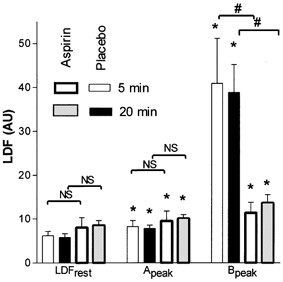
Mean ± s.e.m. values of laser Doppler flowmetry (LDF) in arbitrary units (AU) at rest and peak values observed between the first and the second (Apeak), and after the second (Bpeak) period of current application (1 min, 0.1 mA) observed with placebo (thin lines) or aspirin (thick lines) pretreatment, with 5 min (open bars) and 20 min (grey and black bars) intervals between the periods of current application. (*P < 0.05 from rest, #P < 0.05 between aspirin and placebo pretreatment). Oral aspirin did not affect the slow vasodilatation in response to a single current application as compared to the placebo but almost abolished the response to renewed current delivery.
DISCUSSION
Multiple microvascular studies have been performed using iontophoresis with various protocols, but this technique confronts the problem of a ‘non-specific’ vasodilatation due to the use of the current (Grossmann et al. 1995; Hamdy et al. 2001).
The amplitude of the vasodilator response during monopolar current application is directly proportional to the total charge (Grossmann et al. 1995). In protocols 1 and 2, segmented current application resulted in a peak vasodilatation superior to the one observed following a current of comparable total charge delivered all at once (Fig. 4). Since a pure additive effect should not provide a comparable result, this suggests an increased sensitivity to the electrical current induced by the first period of current application. Small sensory afferents appear to be the principal fibres involved in the vascular response to monopolar transcutaneous constant current application through an axon reflex resulting from the excitation of cutaneous afferent endings (Berliner, 1997a; Hamdy et al. 2001). Pulse stimuli in the range 1–10 Hz are known to readily activate primary afferents (Wardell et al. 1993; Schmelz et al. 2000), but as reviewed by Holzer, current application with frequencies as low as 0.025 Hz are sufficient to elicit vasodilatation (Holzer, 1997). These frequencies are still more than 10 times higher than those used in the present study during repeated current application experiments in the range 10−3-10−4 Hz. We think that both the duration of each square wave stimulus (30 s or 1 min) and the frequency of current application advocate against the assimilation of the prolonged repeated square current applications in this study in comparison to the short pulse stimulations reported in the literature. Thus, the hypothesis of sensitisation to electrical current application seems more likely.
Much evidence exists in the literature that some of the neuropeptides released from afferent nerve endings can activate mast cells and/or leukocytes, resulting in a secondary liberation of vasoactive substances (Hagermark et al. 1978). This cell-mediated pathway is the first step of what is usually referred to as neurogenic inflammation. Some of these secondary mediators (which include histamine and prostaglandins) act as positive feedback modulators of the excitability of afferent endings (see for review Holzer, 1997). The positive modulation of the excitability of afferent endings is described as a sensitisation mechanism. We speculate that the amplified response observed in our experiments (Figs 2–4) may result from sensitisation of afferent endings following the first current application. Should a sensitisation of nerve endings be considered as the underlying mechanism of the amplified vascular response to segmented current application in our experiments, this is a long-lasting phenomenon, as might be expected from a cell-mediated pathway.
As previously discussed, prostaglandins may participate in the vasodilatation mechanisms resulting from the release of neuropeptides by afferent nerves following excitation. They may be involved both as direct and indirect mediators of vasodilatation. PGE2 and PGI2 are powerful vasodilators on their own. Prostaglandin are also able to sensitise the response of afferent unmyelinated fibres in animals (Martin et al. 1987; Rueff & Dray, 1993; Lopshire & Nicol, 1997, Minami et al. 1999). PGE2 increases bradykinin-evoked pain from human skin and veins in a dose-dependent manner (Kindgen Milles, 1995). Assuming that the increased sensitivity to current application leading to the abrupt vasodilatation following renewed anodal current delivery results from neural sensitisation, this sensitisation may rely on prostaglandin-dependent mediation. Furthermore, prostaglandins could also play a key role as direct vasodilators, both in the moderate slow vasodilatation observed following the first current application and in the amplified abrupt vasodilatation resulting from renewed current delivery. Although a direct blockade of vanilloid receptors by aspirin has been described (Szallasi & Blumberg, 1999), the major effect of aspirin is the inhibition of prostaglandin synthesis (Vane, 1971). Aspirin pretreatment abolished the abrupt responses to repeated current application in our experiments (Fig. 6 and Fig. 7) suggesting that prostaglandins play a key role in the underlying mechanisms of this phenomenon. Since various neuropeptides released at nerve endings may exert direct vasodilator effects independent of the prostaglandin pathway (Herbert et al. 1993; Wallengren, 1997; Holzer, 1997), it is improbable that the blockade of prostaglandin synthesis alone could totally abolish the vasodilatation resulting from readily current-excited fibres. Thus, we hypothesise that the amplified abrupt response to a repeated anodal current application relies on a sensitisation of afferent endings that can be abolished by the blockade of prostaglandin synthesis with aspirin. On the contrary, the slow vasodilatation of limited amplitude that follows a single current application is mainly insensitive to aspirin pretreatment, as suggested by protocol 4 (Fig. 6), and renewed current application does not increase the slope of LDF slow changes to current application. Therefore, we hypothesise that a 1 min, 0.1 mA anodal current application allows for a slow progressive aspirin-insensitive vasodilatation, whereas sensitisation of afferent endings may be required before the same current application can induce a trigger-type response leading to an abrupt aspirin-dependent vasodilatation. Whether the slow vasodilatation results from the effects of other neuropeptides, such as CGRP and neurokinins, released by sub-trigger moderately excited afferent endings or whether it may result from a different pathway of non-neural origin remains to be studied.
These observations are of clinical interest. During iontophoresis, the local concentration of the drug attained depends on the total electrical charge applied. The higher the total electrical charge, the higher the local concentration of the drug attained (Sage & Riviere, 1992; Labhasetwar et al. 1995). On the other hand, the ability of a drug to actively migrate depends on molecular weight, lipophilicity or molecular electrical charge (Green, 1996). Thus depending on the drug used and local concentration expected, high total electrical charges may be necessary (Drummond, 1999; Grossmann et al. 1999). Unfortunately, as previously discussed the non-specific vasodilatation to current application is also proportional to the total charge (Grossmann et al. 1995). This phenomenon is a logistical challenge in the study of iontophoretically applied vasoactive drugs on the cutaneous microcirculation (Morris & Shore, 1996; Asberg et al. 1999).
Various approaches can decrease the interference of this ‘non-specific’ effect on the ‘specific’ vasodilatation resulting from the drug. Local anaesthesia can be used to abolish the response to electricity (Morris & Shore, 1996) but interferes with the physiological controls of the circulation, and thus with the normal physiological effects of a drug. The use of anodal currents also decreases this non-specific effect of electricity, compared to cathodal currents (Berliner, 1997a), but restricts the use of the technique to positively charged drugs. Lastly, in an attempt to further decrease the non-specific effect of the current, or to study the effect of increasing doses of vasoactive drugs, some authors deliver the total electrical charge in repeated short periods of current application (Grossmann et al. 1995; Morris & Shore, 1996). This approach is based on the assumption that segmented current application would have lower (or at least the same) ‘non-specific’ effects as all-at-once delivery of currents of the same total charge.
Although the duration of each current application in the present study was longer than those used in previous studies, our data suggest that segmented application may actually increase the ‘non-specific’ effect of anodal current application, as compared to all-at-once delivery of comparable total charge. In studies investigating the local control of the microcirculation with anodal iontophoresis, the ‘non specific’ effect of current application can be investigated using a control probe with the vehicle alone. This ‘non-specific’ vasodilatation is usually subtracted from the results observed with the diffused drugs. Is this really satisfactory and sufficient? For many drugs, the vasodilator mechanisms rely directly or indirectly on the function of afferent endings or prostaglandin synthesis. As a result, the current-induced aspirin-sensitive changes in the excitability of local afferent endings (probably through prostaglandin release) are likely able to interfere with the ‘specific’ effects of the drugs under study. Thus the subtraction of the apparent vascular effect of the current alone may not entirely control for this effect.
In conclusion, the results of the present study support the concept of a dual action of 0.1 mA anodal square current application on the local microcirculation. The first effect is a slowly developing vasodilatation insensitive to the inhibition of the prostaglandin pathway. The second, an increased sensitivity to current appears soon after the first current has been delivered and relies on aspirin-sensitive mechanisms. This second mechanism likely occurs via sensitisation of prostaglandin-sensitive afferent nerve endings and lasts for at least 20 min. These effects should be taken into account when studying microvascular responses to iontophoretically applied drugs with anodal currents.
Acknowledgments
The authors gratefully acknowledge Dr Nisha Charkoudian for reviewing the manuscript. Sylvain Durand received financial support from Conseil Régional and IRMS des Pays de la Loire, Direction Régionale et Départementale de la Jeunesse et des Sports.
Supplementary material
The online version of this paper (http://www.jphysiol.org/cgi/content/full/540/1/261) contains a page of supplementary material.
REFERENCES
- Asberg A, Holm T, Vassbotn T, Andreassen AK, Hartmann A. Nonspecific microvascular vasodilation during iontophoresis is attenuated by application of hyperosmolar saline. Microvascular Research. 1999;58:41–48. doi: 10.1006/mvre.1999.2153. [DOI] [PubMed] [Google Scholar]
- Berliner MN. Skin microcirculation during tapwater iontophoresis in humans: Cathode stimulates more than anode. Microvascular Research. 1997a;54:74–80. doi: 10.1006/mvre.1997.2025. [DOI] [PubMed] [Google Scholar]
- Berliner MN. Reduced skin hyperemia during tap water iontophoresis after intake of acetylsalicylic acid. American Journal of Physical Medicine and Rehabilitation. 1997b;76:482–487. doi: 10.1097/00002060-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Bhagat K, Collier J, Vallance P. Vasodilatation to arachidonic acid in humans. An insight into endogenous prostanoids and effects of aspirin. Circulation. 1995;92:2113–2118. doi: 10.1161/01.cir.92.8.2113. [DOI] [PubMed] [Google Scholar]
- Drummond PD. Nitroprusside inhibits thermal hyperalgesia induced by noradrenaline in capsaicin-treated skin. Pain. 1999;80:405–412. doi: 10.1016/s0304-3959(98)00236-x. [DOI] [PubMed] [Google Scholar]
- Green PG. Iontophoretic delivery of peptide drugs. Journal of Controlled Release. 1996;41:33–48. [Google Scholar]
- Grossmann MG, Jamieson MJ, Kellogg DL, Jr, Kosiba WA, Pergola PE, Crandall CG, Shepherd AMM. The effect of iontophoresis on the cutaneous vasculature: evidence for current-induced hyperemia. Microvascular Research. 1995;50:444–452. doi: 10.1006/mvre.1995.1070. [DOI] [PubMed] [Google Scholar]
- Grossmann MG, Jamieson MJ, Kirch W. The local effects of systemic digoxin on the cutaneous microcirculation. European Journal of Clinical Pharmacology. 1999;55:263–268. doi: 10.1007/s002280050627. [DOI] [PubMed] [Google Scholar]
- Hagermark O, Hökfelt T, Pernow B. Flare and itch induced by substance P in human skin. Journal of Investigative Dermatology. 1978;71:233–235. doi: 10.1111/1523-1747.ep12515092. [DOI] [PubMed] [Google Scholar]
- Hamdy O, Abouelenin K, Logerfo FW, Horton ES, Veves A. Contribution of nerve-axon reflex-related vasodilation to the total skin vasodilation in diabetic patients with and without neuropathy. Diabetes Care. 2001;24:344–349. doi: 10.2337/diacare.24.2.344. [DOI] [PubMed] [Google Scholar]
- Herbert MK, Tafler R, Schmidt RF, Weis KH. Cyclooxygenase inhibitors acetylsalicylic acid and indomethacin do not affect capsaicin-induced neurogenic inflammation in human skin. Agents Actions. 1993;38:C25–C27. doi: 10.1007/BF01991126. [DOI] [PubMed] [Google Scholar]
- Holzer P. Control of the cutaneous vascular system by afferent neurons. In: Morris JL, Gibbins IL, editors. Autonomic Innervation of the Skin. Amsterdam, The Netherlands: Harwood Academic Publishers; 1997. pp. 213–267. [Google Scholar]
- Johnson JM, O'leary D, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clinical Physiology. 1986;6:337–346. doi: 10.1111/j.1475-097x.1986.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Kindgen Milles DA. Effects of prostaglandin E2 on the intensity of bradykinin-evoked pain from skin and veins of humans. European Journal of Pharmacology. 1995;294:491–496. doi: 10.1016/0014-2999(95)00575-7. [DOI] [PubMed] [Google Scholar]
- Labhasetwar V, Underwood T, Schwendeman S, Levy R. Iontophoresis for modulation of cardiac drug delivery in dogs. Proceedings of the National Academy of Sciences of the USA. 1995;92:2612–2616. doi: 10.1073/pnas.92.7.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopshire JC, Nicol GD. Activation and recovery of the PGE2-mediated sensitization of the capsaicin response in rat sensory neurons. Journal of Neurophysiology. 1997;78:3154–3164. doi: 10.1152/jn.1997.78.6.3154. [DOI] [PubMed] [Google Scholar]
- Martin HA, Basbaum AL, Kwiat GC, Goetzl EJ, Levine JD. Leukotriene and prostaglandin sensitization of cutaneous high-threshold C- and A-delta mechanonociceptors in the hairy skin of rat hindlimbs. Neuroscience. 1987;22:651–659. doi: 10.1016/0306-4522(87)90360-5. [DOI] [PubMed] [Google Scholar]
- Minami T, Okuda-Ashitaka E, Hori Y, Sakuma S, Sugimoto T, Sakimura K, Mishina M, Ito S. Involvement of primary afferent C-fibres in touch evoked pain (allodynia) induced by prostaglandin E2. European Journal of Neuroscience. 1999;11:1849–1856. doi: 10.1046/j.1460-9568.1999.00602.x. [DOI] [PubMed] [Google Scholar]
- Morris SJ, Shore AC. Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanisms. Journal of Physiology. 1996;496:531–542. doi: 10.1113/jphysiol.1996.sp021704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueff A, Dray A. Sensitization of peripheral afferent fibres in the in-vitro neonatal rat spinal cord-tail by bradykinin and prostaglandins. Neuroscience. 1993;52:527–535. doi: 10.1016/0306-4522(93)90272-h. [DOI] [PubMed] [Google Scholar]
- Sage BH, Riviere JE. Model system in iontophoresis-transport efficacy. Advanced Drug Delivery Review. 1992;9:265–287. [Google Scholar]
- Saumet JL, Abraham P, Jardel A. Cutaneous vasodilation induced by local warming, sodium nitroprusside, and bretylium iontophoresis on the hand. Microvascular Research. 1998;56:212–217. doi: 10.1006/mvre.1998.2099. [DOI] [PubMed] [Google Scholar]
- Savage MV, Brengelmann GL. Reproducibility of the vascular response to heating in human skin. Journal of Applied Physiology. 1994;76:1759–1763. doi: 10.1152/jappl.1994.76.4.1759. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Michael K, Weidner C, Schmidt R, Torebjork HE, Handwerker HO. Which nerve fibers mediate the axon reflex flare in the human skin? Neuroreport. 2000;11:645–648. doi: 10.1097/00001756-200002280-00041. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacological Reviews. 1999;51:159–211. [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, O'leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. Journal of Applied Physiology. 1984;57:191–196. doi: 10.1152/jappl.1984.57.1.191. [DOI] [PubMed] [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for the aspirin-like drugs. Nature New Biology. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Wallengren J. Vasoactive peptides in the skin. Journal of Investigative Dermatology Symposium Proceedings. 1997;2:49–55. doi: 10.1038/jidsymp.1997.11. [DOI] [PubMed] [Google Scholar]
- Wardell K, Naver HK, Nilsson GE, Wallin BG. The cutaneous vascular axon reflex in humans characterized by laser Doppler perfusion imaging. Journal of Physiology. 1993;460:185–199. doi: 10.1113/jphysiol.1993.sp019466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


