Abstract
Adult inferior vagal ganglion neurons (nodose ganglion neurons, NGNs) were acutely isolated 4–6 days after section of their peripheral axons (vagotomy) and examined with the whole-cell patch-clamp technique. A subset (∼25 %) of vagotomized NGNs displayed depolarizing after-potentials (DAPs), not present in control NGNs. DAPs were inhibited by niflumic acid (125 μm) or cadmium (100 μm), and had a reversal potential near ECl, indicating that they were due to Ca2+-activated chloride current (ICl(Ca)). N-type, L-type, T-/R- and other types of voltage-dependent Ca2+ channels provided about 43, 2, 16 and 40 % of the trigger Ca2+ for DAP generation, respectively. Intracellular Ca2+ concentration ([Ca2+]i) was estimated using fura-2 fluorescence. Resting [Ca2+]i and peak [Ca2+]i elevation induced by activating Ca2+-induced Ca2+ release (CICR) stores with 10 mm caffeine were not significantly different among control NGNs, vagotomized NGNs with DAPs and vagotomized NGNs without DAPs, averaging 54 ± 7.9 (n = 19; P = 0.49) and 2022 ± 1059 nm (n = 19; P = 0.44), respectively. Blocking CICR with 10 μm ryanodine reduced DAP amplitude by ∼37 %. Ca2+ influx induced by action potential waveforms was increased by over 250 % in vagotomized NGNs with DAPs (19.0 ± 2.1 pC) compared to control NGNs (5.0 ± 0.8 pC) or vagotomized NGNs without DAPs (7.0 ± 0.8 pC). L-type, N-type, T-/R-type and other types of Ca2+ influx were increased proportionately in vagotomized NGNs with DAPs. In conclusion, a subset of vagotomized NGNs have increased Ca2+ currents and express ICl(Ca). These NGNs respond electrically to increases in [Ca2+]i during regeneration.
Nerve injury elicits an array of profound morphological, biochemical and physiological alterations within the nerve cell body or somata. The spectrum of neurophysiological changes occurring in the somata of primary sensory neurons following nerve section (axotomy) ranges from subthreshold membrane potential oscillations and spontaneous activity in somatic afferents (Kocsis & Devor, 2000) to hypoexcitability manifested by enhanced spike frequency adaptation in visceral, vagal afferents (Lancaster et al. 2001). The reduced excitability exhibited by axotomized vagal somata (nodose ganglion neurons; NGNs) appears to be due to a reorganization of sodium currents (Lancaster & Weinreich, 2001), though the contributions of other ionic currents to these axotomy-induced excitability changes have not yet been explored.
The effects of axotomy on calcium currents (ICa) are of particular importance because Ca2+ regulates critical cellular processes including neurotransmitter release, growth, synaptic plasticity, action potential (AP) discharge, Ca2+-dependent ionic currents, and gene expression. Reductions in ICa and calcium-dependent currents following axon injury have recently been demonstrated in somatic afferents and dorsal root ganglion (DRG) neurons (Baccei & Kocsis, 2000; Hogan et al. 2000; Abdulla & Smith, 2001a).
In the current work, we extend our studies of the electrophysiological changes produced by vagus nerve section (vagotomy) to examine ICa and calcium-dependent currents in NGNs. We observed that a subpopulation of NGNs have significantly augmented ICa compared to control or other vagotomized NGNs. These same NGNs have depolarizing after-potentials (DAPs) and calcium-activated chloride current (ICl(Ca)), electrophysiological properties not normally present in control NGNs. These results further highlight the fundamental differences in the retrograde response to nerve injury between vagal and somatic primary afferent neurons.
METHODS
Vagotomy
Vagus nerve transection, vagotomy, was elicited by unilaterally removing a section of the right or left cervical vagus nerve of adult (200–300 g), male Sprague-Dawley rats, as described previously (Lancaster et al. 2001) and approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore, MD, USA. Under ketamine (50 mg kg−1i.p.)-xylazine (10 mg kg−1i.p.) anaesthesia, a 5 mm section of the vagus nerve was removed approximately 1 cm distal to the right or left inferior vagal (nodose) ganglion. This operation severed the afferent processes of approximately 90 % of the NGNs on the operated side (with the remainder projecting their afferents via the superior laryngeal nerve, proximal to nerve transection). For some animals, a ‘sham’ vagotomy procedure was performed, which consisted of exposing, but not cutting, the vagus nerve. Except where otherwise specified, ganglia were maintained 4–6 days in vivo following vagotomy. Rats were then killed by CO2 inhalation and nodose ganglia were removed bilaterally. Previous work has revealed consistent changes in the excitability and sodium currents of vagotomized NGNs 5 days and longer following vagotomy (Lancaster et al. 2001, Lancaster & Weinreich 2001).
Nodose neuron dissociation
NGNs were dissociated enzymatically as described previously (Jafri et al. 1997). Briefly, ganglia were rapidly removed from animals, desheathed, and then incubated in enzyme solution (10 mg collagenase type 1A (Sigma, St Louis, MO, USA), 10 mg dispase II (Boehringer Mannheim, Mannheim, Germany), 10 ml Ca2+- and Mg2+-free Hanks' balanced salt solution) for 2 h at 37 °C. Neurons were dissociated by trituration, washed by centrifugation (three times at 700 g for 45 s), suspended in L15 medium (Gibco BRL, Rockville, MD, USA) containing 10 % fetal bovine serum (JRH Biosciences, Lenexa, KS, USA), then transferred onto circular 15 mm glass coverslips (Bellco Glass Inc., Vineland, NJ, USA) coated with poly-d-lysine (0.1 mg ml−1, Sigma). NGNs adhered to coverslips and were maintained in vitro for 2–9 h following plating at 37 °C.
Whole-cell patch-clamp and current-clamp recording
Whole-cell patch-clamp techniques (Hamill et al. 1981) were employed with an Axopatch 200B amplifier, Digidata 1200 interface, and pCLAMP7 software (all from Axon Instruments, Union City, CA, USA). Patch pipettes (1–4 MΩ) were fabricated from glass capillaries (MTW150F-4, World Precision Instruments, Sarasota, FL, USA). Pipettes were filled with a variant of a solution described previously (Ikeda et al. 1986) for rat NGNs, with a composition of (mm): 140 KCl; 2 MgCl2; 10 Hepes; 0.11 EGTA; 10 dextrose; adjusted to pH 7.3 with KOH; 314 mosmol l−1 adjusted with sucrose. Pipette voltage offset was neutralized prior to the formation of a gigaseal. Membrane input resistance (Rin), series resistance (Rs), and capacitance (Cm) were determined from current transients elicited by 5 mV depolarizing voltage steps from a holding potential of −60 mV, delivered using the membrane test application of pCLAMP7. Capacitance compensation and 80 % Rs compensation were used. Criteria for cell inclusion in the study were: Rs≤ 10 MΩ, Rin > 100 MΩ and stable recording with 80 % Rs compensation throughout the entire experiment. Coverslips were superfused (2–4 ml min−1) continuously during recording with room temperature (22–24 °C) Locke solution (composition in mm: 10 dextrose; 136 NaCl; 5.6 KCl; 1.2 MgCl2; 2.2 CaCl2; 1.2 NaH2PO4; 14.3 NaHCO3) equilibrated with 95 % O2− 5 % CO2 to a pH of between 7.3 and 7.5). The ‘low chloride’ external solution was composed of (mm): 150 sodium isothionate; 5.6 KCl; 10 Hepes; 2.4 CaCl2; 1.2 MgCl2; 10 dextrose. The pH of this solution was corrected to 7.4 with NaOH. The recording chamber was grounded via a 3 m KCl agar bridge. All chemicals for internal and external solutions were from Sigma, except NaCl, KCl, MgCl2, NaHCO3, NaH2PO4, dextrose, sucrose and CaCl2, which were from J.T. Baker (Phillipsburg, NJ, USA).
Conventional ‘sharp’ microelectrode current-clamp recording
For some experiments, dissociated nodose neurons were studied using standard intracellular recording techniques with conventional ‘sharp’ microelectrodes, as described in detail by Weinreich et al. (1997). Data acquisition and analysis were performed using an Axoclamp-2A amplifier, Digidata 1200 interface, and pCLAMP7 software (Axon Instruments).
Calcium current recording
For calcium current recording, the pipette (internal) solution was composed of (mm): 40 TEA-Cl (tetraethylammonium chloride); 100 CsCl; 2 MgCl2; 10 Hepes; 11 EGTA; 1 CaCl2. Osmolarity was corrected to 310 mosmol l−1 with sucrose, and pH was adjusted to 7.2 with CsOH. Criteria for cell acceptance included Rin > 50 MΩ, Ra < 10 MΩ and stable recording with 80 % series resistance compensation throughout the experiment. For calcium current recording, cells were continually superfused with room temperature external solution composed of (mm): 35 TEA-Cl; 100 Choline-Cl; 10 CsCl; 0.8 MgCl2; 10 Hepes; 5 CaCl2; 10 dextrose. Osmolarity was corrected to 325 mosmol l−1 with sucrose, and pH was adjusted to 7.4 with CsOH.
Calcium channel blockers
The L-type calcium channel antagonist nifedipine (Sigma) was diluted in external solution to a working concentration of 10 μm from 10 mm stock solutions prepared in distilled water and stored at −20 °C. The N type calcium channel antagonist ω-conotoxin GVIA, and the P-/Q-type antagonist ω-agatoxin GIVA (both from Alomone Labs, Jerusalem, Israel) were stored as lyophilized powders at −20 °C until the day of the experiment, and then dissolved in external solution to working concentrations of 500 nm and 50 nm, respectively. The T-/R-type calcium channel antagonist nickel chloride (Fisher Scientific, Fair Lawn, NJ, USA) and non-specific calcium channel blocker cadmium chloride (JT Baker) were prepared as 1 m stock solutions in distilled water, then diluted in external solution to working concentrations of 500 μm and 100 μm, respectively. Unless otherwise specified, calcium channel antagonists were added serially, without lowering the concentration of previously applied antagonists. All drugs were superfused for at least 2 min prior to assessing their effects.
Calcium measurement
To measure intracellular Ca2+, fura-2 (K+ salt, 50 μm, Tef Labs, Austin, TX, USA) was included in the intracellular solution described above for whole-cell current clamp recording. While whole-cell electrophysiological recordings were performed, fura-2 fluorescence measurements were made with a DeltaScan illumination system (Photon Technology International (PTI), South Brunswick, NJ, USA) coupled to the microscope through a fibre optic cable. Each NGN under study was alternately excited with 340 and 380 nm light, and the fluorescence emission, after passing through a 510 nm bandpass filter, was sampled by a photomultiplier tube (D-104 microscope photometer, PTI), the output from which was digitized and stored for subsequent analysis. Instrument control, data acquisition and analysis were performed using FELIX 1.1 software (PTI). Values of intracellular Ca2+ were derived using the ratio method of Grynkiewicz et al. (1985), as described previously (Weinreich et al. 1997).
Statistics
Parameters of vagotomized NGNs expressing DAPs (DAP(+) NGNs), were compared to parameters of vagotomized NGNs without DAPs (DAP(-) NGNs), and parameters of control NGNs using either ANOVA or Kruskal-Wallis one-way ANOVA on ranks, as appropriate. Pair-wise comparisons were obtained using Dunn's test. All tests were implemented with SigmaStat version 2.03 software (SPSS Inc., Chicago, IL, USA). For all tests, P < 0.05 was considered significant. Values are given as means ±s.e.m., unless otherwise specified.
RESULTS
Incidence of depolarizing after-potentials in vagotomized NGNs
Following the termination of depolarizing stimuli, the membrane potential of control NGNs invariably hyperpolarizes. However, in a subset (∼25 %) of NGNs vagotomized for 4–6 days a depolarizing after-potential (DAP) was observed after the cessation of depolarizing stimuli. In order to maximize our ability to detect and quantify DAPs we used several different stimulation protocols. These protocols, illustrated in Fig. 1, include: A, a 750 ms 900 pA depolarizing current; B, a single 3 ms 500 pA current; C, a 1 s 20 Hz burst of 3 ms 1 nA stimuli delivered at −55 mV; and D, a burst similar to C except the membrane potential was pre-set to −70 mV.
Figure 1. Various stimulation protocols used to elicit depolarizing after-potentials (DAPs).
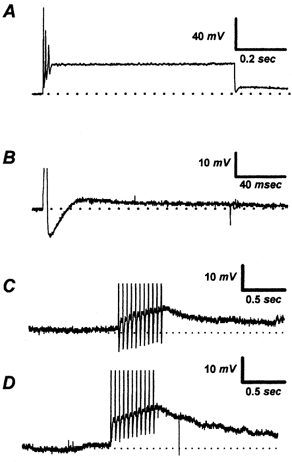
A, a 750 ms step injection of 0.9 nA depolarizing current evokes a long DAP at the end of the step; B, a 3 ms 500 pA current injection elicits a single AP and a DAP lasting ∼160 ms; C, a train of 3 ms 1 nA depolarizing pulses delivered at 20 Hz to the neuron at −55 mV results in a DAP lasting > 1.5 s; D, a similar protocol as in C, to the same NGN hyperpolarized to −70 mV, results in a larger DAP. The baseline membrane potential for experiments A-C was pre-set to −55 mV. The horizontal dotted line represents the pre-set membrane potential.
A 750 ms step injection of depolarizing current, which elicited at least one AP from all studied NGNs, evoked a DAP (16 ± 2 mV in peak amplitude) in 20 % (16/80) of vagotomized NGNs. Application of a 3 ms depolarizing current pulse, designed to evoke a single AP, elicited DAPs (7 ± 1 mV) from 31 % (25/80) of vagotomized NGNs. A 3 ms 500 pA current pulse was used to evoke the single AP; if this pulse failed to evoke an AP, a 3 ms 1000 pA current pulse was applied. A burst of APs, evoked in all studied NGNs by a 20 Hz train of 3 ms 1 nA inward current pulses, was the most effective stimulus for producing measurable DAPs when the baseline membrane potential was −55 mV (48 %, 38/80, 12 ± 1 mV). Triggering a burst of APs from a baseline membrane potential of −70 mV produced larger DAPs but did not increase the frequency of their occurrence (49 % of NGNs tested, 36/73, 19 ± 1 mV). The larger DAPs recorded at −70 mV may be due to the increased driving force for an inward DAP current, and/or decreased opposition from calcium- or voltage-activated potassium currents. Approximately 58 % (46/80) of vagotomized NGNs tested displayed a DAP in response to one or more of the four protocols; NGNs expressing a DAP are referred to as DAP(+). This estimate of DAP(+) NGNs is biased by our cell selection. By selecting larger diameter NGNs cells with clearly eccentric nuclei, we could increase DAP(+) cells to over 60 %. When vagotomized NGNs were randomly sampled, the incidence of DAPs was 25 % (4 of 16 cells). The randomly sampled neurons were selected by recording from whichever NGNs were closest to the center of the microscopic field after a new visual field was selected without visual guidance. DAPs were not observed in any of the 33 control NGNs tested by all four protocols. DAPs were also not observed following single APs or prolonged step depolarization in NGNs removed following a ‘sham’ vagotomy operation (n = 12).
Since the patch-clamp technique can change the intracellular constituents of the cell, calcium buffering and ion concentrations may not be the same as those in intact NGNs. Therefore, in order to determine if DAPs could still be generated in NGNs with normal intracellular constituents, we used conventional ‘sharp’ microelectrode recording in dissociated NGNs. Under these recording conditions, DAPs were still elicited in a subset of NGNs (2/12) vagotomized (for 4–6 days) by the same four current clamp protocols effective at inducing DAPs with whole-cell patch pipettes (data not shown). DAPs were not observed in control NGNs in intact ganglia (n = 20) following a single AP, following the discharge of multiple APs, or following step depolarizing currents (data not shown).
Time course of DAP emergence following vagotomy
To determine the time course over which DAPs emerge following vagotomy, NGNs were isolated from two rats vagotomized 17 h previously. These neurons therefore remained in vivo for only 17 h following the vagotomy, and in vitro for only 2–9 h prior to recording. Vagotomized NGNs from these rats already displayed DAPs; 25 % (3/12) of vagotomized NGNs were DAP(+) (e.g. produced a DAP in response to one or more of the protocols described above). Since this incidence is similar to that in an unbiased selection of NGNs vagotomized for 4–6 days, it demonstrates that the current(s) responsible for producing DAPs is up-regulated within 1 day of vagotomy.
NGNs were also isolated from two rats vagotomized for 21 days. At this time, substantial regeneration of the vagus nerve had already occurred; upon dissection, the operated cervical vagus could not be grossly distinguished from the contra-lateral vagus. Of the vagotomized NGNs isolated from these animals, 8 % (1/12) were DAP(+), an incidence not significantly different from that in NGNs vagotomized for 4–6 days (P = 0.36, Fisher's exact test). The current underlying the DAP therefore persists for at least 21 days, though it may decrease in incidence as the vagus regenerates.
Sensitivity of DAPs to external divalent cations
To determine whether DAPs were calcium sensitive, we varied the concentration of divalent cations in the external solution (Fig. 2). The peak magnitude of DAPs in response to 20 Hz stimulation (10 APs, holding potential −70 mV, 3 ms, 500 pA pulses) was measured in five vagotomized NGNs displaying prominent DAPs. Under control conditions (e.g. with a Locke solution containing 2.2 mm Ca2+), DAPs averaged 11 ± 2 mV. Superfusion with an external solution in which Ca2+ was replaced with Mg2+ (e.g. containing nominally zero extracellular Ca2+) reduced the DAPs to 6 ± 2 % (range 0–10 %) of control values. Subsequent superfusion with an external solution with Ba2+ substituted for Ca2+ restored DAP magnitude to 38 ± 7 % (range 14–50 %) of control values. Superfusion with a Locke solution containing normal Ca2+, but with 100 μm CdCl2 added to block voltage-dependent calcium channels (VDCCs), reduced DAP amplitude to 2 ± 2 % (range 0–10 %) of control values. Following a 2 min superfusion with control Locke solution (to remove Cd2+), DAP magnitude returned to 64 ± 6 % of control values (n = 4); the remaining cell detached from the pipette during the final superfusion and could not be tested. The nearly complete abolition of DAPs by the replacement of permeant divalent cations with Mg2+ or block of VDCCs with Cd2+ indicate that DAP production in NGNs is dependent on extracellular divalent ions (normally Ca2+) entering via VDCCs. Additional evidence for this contention is presented in a later section showing the dependence of DAPs on Ca2+ entry through various sub-types of VDCCs.
Figure 2. Sensitivity of DAPs to divalent cations.
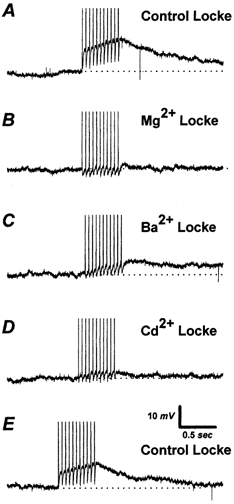
A, a single vagotomized NGN displayed a DAP elicited by a train of 1 nA pulses 3 ms in duration delivered at 20 Hz. B, the substitution of 2.2 mm magnesium for calcium in the Locke solution almost completely blocked the DAP. C, a partial recovery of the DAP occurred when 2.2 mm barium was substituted for magnesium. D, switching to a normal (calcium-containing) Locke solution with 100 μm cadmium blocked the DAP again. E, a 2 min wash with normal Locke solution resulted in DAP recovery. All recordings from the same NGN. The horizontal dotted line represents the preset membrane potential, −70 mV.
The ability of Ba2+ to activate DAPs, though less effectively than Ca2+, has two important implications. First, Ba2+ is known to support calcium-activated chloride currents (Dolphin et al. 1986; Scott et al. 1988), suggesting DAPs may be due to a chloride current. Secondly, Ba2+ is not thought to directly activate neuronal calcium-activated chloride channels, but rather indirectly activate these channels by stimulating Ca2+ release from intracellular stores, particularly calcium-induced calcium release (CICR) stores (Scott et al. 1988; Frings et al. 2000). Divalent cations entering through VDCCs may therefore, at least in part, activate calcium-activated chloride channels by triggering CICR. The relative contribution of Ca2+ from CICR and Ca2+ entering through calcium channels to triggering DAPs is further explored below.
DAP reversal potential
To estimate the reversal potential (Erev value) of the DAP current, a voltage-clamp protocol depicted in Fig. 3 was used to elicit DAP currents with depolarizing pulses (to 0 mV). Baseline current-voltage (I-V) curves, produced by voltage ramps (from −50 to −90 mV), were compared to I-V curves acquired near the peak of the DAP current to estimate the Erev value of the DAP current in seven DAP(+) NGNs. Voltage ramps from −50 to −90 mV were chosen to avoid activating voltage-gated sodium, potassium and calcium currents, which would interfere with the I-V curves, and, in the case of calcium, influence the DAP current during the measurement. The extrapolated Erev for these seven NGNs was −2 ± 3 mV. This value is similar to the estimated Nernst potential for chloride for the external and internal solutions (∼0 mV). While this experiment might have been disrupted by the presence of other slow currents triggered by depolarization, we generally did not observe slow hyperpolarizing or depolarizing after-potentials in DAP(+) NGNs in which the DAP was inhibited by niflumic acid (125 μm; see below).
Figure 3. Estimation of DAP reversal potential (Erev).
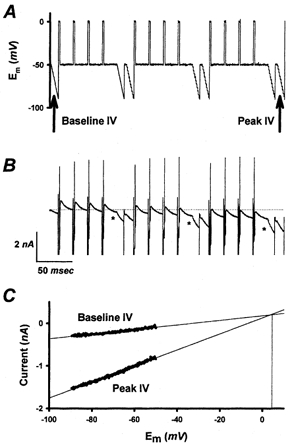
The DAP current was elicited by a voltage-clamp protocol shown in A. B, a series of brief (3 ms) depolarizing voltage steps (upward deflections) to 0 mV resulted in the gradual activation of an inward DAP current (*). A voltage ramp from −50 to −90 mV was used to generate a baseline I-V relationship and an I-V relationship during the DAP current to determine Erev for the DAP. The first ramp shown was used for the baseline I-V relationship, and the ramp immediately after the final set of depolarizing membrane voltage steps was used to generate the peak DAP I-V relationship. C, the baseline and peak I-V relationships are plotted to estimate the extrapolated Erev value for the DAP current, in this case +4 mV.
Neuronal chloride channels activated by Ca2+ are poorly selective among anions (Frings et al. 2000). Thus, extracellular substitution of the larger, less permeant anion isethionate for chloride and bicarbonate would be predicted to shift Erev in a more positive direction. In four cells, the substitution of a ‘low chloride’ extracellular solution (see Methods) for the normal extracellular solution shifted Erev to +16 ± 4 mV.
Inhibition of DAPs by blockers of calcium-activated chloride currents
Neuronal calcium-activated chloride currents (ICl(Ca)) are sensitive to (125 μm) niflumic acid (Scott et al. 1988; Sánchez-Vives & Gallego, 1994). Thus, if DAPs recorded in NGNs are due to ICl(Ca), this antagonist should inhibit them. Indeed, DAPs in vagotomized rat NGNs were inhibited by niflumic acid (125 μm, n = 5, 89 ± 5 %).
Due to their Erev value near ECl, dependence on divalent cation influx, and inhibition by niflumic acid, the DAPs in vagotomized NGNs are likely to be due to a ICl(Ca) similar or identical to those reported in subsets of cultured rat DRG neurons (Mayer, 1985; Scott et al. 1988) or axotomized rat sympathetic neurons (Sánchez-Vives & Gallego, 1994).
Electrical membrane properties of DAP(+) and DAP(-) NGNs
We have previously characterized changes in passive membrane properties, AP characteristics and AP discharge patterns in vagotomized and control NGNs (Lancaster et al. 2001). Vagotomized NGNs have larger membrane capacitance, lower membrane resistance, decreased AP overshoot, attenuated peak hyperpolarization following an AP and decreased AP discharge in response to various depolarizing current protocols compared to control NGNs. In order to determine which of these changes are associated, causally or otherwise, with the emergence of the DAP current, we have repeated the same measurements in DAP(+) vagotomized NGNs, DAP(-) vagotomized NGNs, and control NGNs. For the purposes of these experiments, any vagotomized neuron displaying a DAP in response to any of the four protocols described above was considered DAP(+), and all other vagotomized NGNs DAP(-). All control neurons were, as indicated above, DAP(-).
The electrical membrane properties of the three groups of NGNs are summarized in Table 1. DAP(+) and DAP(-) vagotomized NGNs differ from each other only in that DAP(+) NGNs had significantly larger membrane capacitance (∼15 %) and higher membrane input resistance (∼30 %). Compared to control NGNs, both types of vagotomized NGNs had similar increases in rheobase, and reductions in APs discharged in response to various stimuli.
Table 1.
Electrophysiological membrane properties of DAP(+) and DAP(−) vagotomized NGNs
| Vagotomized NGNs | |||
|---|---|---|---|
| Parameter | Control NGNs (n = 36) | DAP(+) (n = 46) | DAP(−) (n = 36) |
| Em (mV) | −46 ± 1 | −51 ± 1* | −52 ± 1* |
| Cm (pF) | 37 ± 2 | 46 ± 2*† | 40 ± 2 |
| Rin (MΩ) | 389 ± 34 | 412 ± 30 † | 300 ± 26 |
| AP overshoot (mV) | 51 ± 2 | 46 ± 2 | 50 ± 2 |
| AP peak hyperpolarization (mV) | −70 ± 0 | −66 ± 3* | −68 ± 0* |
| AP duration (ms) | 1.6 ± 0.2 | 1.4 ± 0.1 | 1.5 ± 0.1 |
| Rheobase (pA) | 108 ± 23 | 193 ± 18* | 289 ± 26* |
| 1 × threshold (#APs) | 2 ± 1 | 2 ± 1* | 1 ± 0* |
| 2 × threshold (#APs) | 6 ± 1 | 3 ± 1* | 2 ± 0* |
| 3 × threshold (#APs) | 7 ± 1 | 3 ± 2* | 3 ± 0* |
| 0.1–0.9 nA (#APs) | 15 ± 3 | 4 ± 2* | 3 ± 1* |
Em (mV), membrane potential; Rin (MΩ), membrane input resistance; Cm (pF), membrane capacitance; AP overshoot (mV), most positive membrane potential during a single evoked AP; AP peak hyperpolarization (mV), most negative membrane potential during at single evoked AP; AP duration (ms), AP width at 0 mV; rheobase, minimum 750 ms current required to induce an AP; 1 × threshold (#APs), number of APs evoked by a 750 ms 1 × threshold depolarizing current; 2 × threshold (#APs), number of APs evoked by a 750 ms 2 × threshold depolarizing current; 3 × threshold (#APs), number of APs evoked by a 750 ms 3 × threshold depolarizing current; 0.1–0.9 nA (#APs), maximum number of APs evoked by a single 750 ms of magnitude 100–900 pA. Values are means ±s.e.m.
Significantly different from control.
Denotes significantly different from DAP(−) vagotomized NGNs.
Contribution of ICa subtypes to DAP generation
NGNs express both low-voltage-activated (LVA; T-type) and high-voltage-activated (HVA; L- and N-type) ICa (Ikeda et al. 1986; Mendelowitz and Kunze, 1992). In control NGN somata, N-type currents contribute 60-70 % of total ICa, while T-type and L-type ICa contribute 16 and 12 %, respectively. However, additional experiments are required to clarify which types of ICa trigger DAPs in NGNs for three reasons. First, the subtypes of ICa expressed in NGNs may change following vagotomy. Second, the subtypes of ICa in DAP(+) NGNs may not be the same as those in other vagotomized NGNs. Third, not all types of ICa are equally effective at triggering DAPs (Martínez-Pinna et al. 2000).
To quantify the contribution of various subtypes of ICa to DAP generation, we determined the peak amplitude of DAPs in eight NGNs as we serially applied various Ca2+ channel blockers. In this set of NGNs, DAPs were elicited by a train of 10 APs at 20 Hz from a holding potential of −70 mV and averaged 16 ± 5 mV. After the addition of nifedipine (10 μm) to the external solution, DAP amplitude was unaffected (16 ± 5 mV; 98 ± 8 % of control). Less than 3 % of DAP amplitude (2 ± 8 %) can therefore be attributed to L-type ICa. Subsequent addition of 500 μm NiCl2 (without removing the nifedipine) reduced the peak DAP amplitude to 13 ± 5 mV (82 ± 13 % of control values). Thus, approximately 16 ± 14 % of DAP magnitude was due to nickel-sensitive R- and T-type ICa. On addition of the N-type calcium channel blocker ω-conotoxin GVIA (0.5 μm), without removing nifedipine or nickel, DAP amplitude was further reduced to 6 ± 5 mV (40 ± 28 % of control). N-type ICa therefore is responsible for approximately 43 ± 20 % of DAP magnitude. The addition of 100 μm CdCl2 to the external solution reduced DAP magnitude to 0 ± 1 mV (1 ± 3 % of control values). Because DAPs persisted even in the presence of nifedipine, nickel, and ω-conotoxin GVIA, we attribute approximately 40 % of DAP magnitude to other (presumably Q- or P-type) voltage-gated calcium currents resistant to these reagents (Cordoba-Rodriguez et al. 1999).
In order to confirm the importance of N-type and P-/Q-type calcium currents in triggering DAPs, NGNs with large DAPs (20 ± 4 mV, n = 4) were first exposed to ω-conotoxin GVIA (0.5 μm), and then to both ω-conotoxin GVIA (0.5 μm) and ω-agatoxin GIVA (50 nm). In this group, ω-conotoxin alone reduced DAP magnitude by 66 ± 17 %. In the presence of both calcium channel antagonists, DAP magnitude was reduced to 20 ± 14 % of baseline. These experiments confirm the importance of N-type and P-/Q-type calcium currents in triggering DAPs in NGNs.
In conclusion, these data show AP discharge coupled to voltage-gated calcium currents generate DAPs in vagotomized NGNs. We find no evidence that L-type ICa contributes to DAP generation, and only a small role for T-/R-type ICa. N-type ICa contributes substantially to DAP generation, and the inhibition of N-type and P-/Q-type currents greatly reduces DAP magnitude. This situation contrasts markedly to that in mouse sympathetic neurons, where L- and P-type currents, but not N-type currents, are necessary to trigger DAPs (Martínez-Pinna et al. 2000). In DRG neurons, a similarly detailed analysis has not been reported, though L-type, N-type and, to a lesser extent, T-type currents may be involved in triggering ICl(Ca) (Scott et al. 1995).
The contribution of intracellular calcium stores to DAP generation
The release of Ca2+ from intracellular stores has been shown to activate ICl(Ca) in sensory neurons, and CICR has been shown to contribute to DAP magnitude in these neurons (Currie et al. 1995; Scott et al. 1995; Ayar & Scott, 1999). In order to determine if Ca2+ from intracellular stores is sufficient to activate ICl(Ca) in NGNs, we applied caffeine (10 mm) by superfusion to control NGNs, DAP(+) and DAP(-) vagotomized NGNs voltage clamped at −60 mV. Large inward currents (1850 ± 790 pA) were generated by the application of caffeine in 100 % (8/8) of the DAP(+) vagotomized NGNs tested. The caffeine-induced currents in DAP(+) NGNs had Erev values (0 ± 4 mV, n = 7) similar to those of the DAP current (−2 ± 3 mV). Normally, caffeine-induced currents in DAP(+) NGNs could be repeatedly evoked with little decrement (data not shown). However, during a 10 min bath application of ryanodine (10 μm, n = 6), a pharmacologic blocker of CICR, the addition of caffeine still produced an inward current, but only once or twice (Fig. 4). The first reapplication of caffeine in the presence of ryanodine invariably produced a current (55 ± 8 % of original magnitude, n = 6). The third application of caffeine in the presence of ryanodine invariably failed to produce a current (n = 6). These experiments indicate that caffeine-induced currents in DAP(+) NGNs were dependent upon Ca2+ release from ryanodine-sensitive stores. Control NGNs (n = 5) and DAP(-) vagotomized NGNs (n = 4) did not show measurable caffeine-induced inward currents, although some produced small (< 100 pA) outward currents. The failure of caffeine to evoke inward currents in DAP(-) vagotomized NGNs and control NGNs indicates that they could have smaller CICR pools than DAP(+) cells and/or that they lack functional calcium-activated chloride channels, an issue which is addressed below.
Figure 4. Contribution of Ca2+ from Ca2+-induced Ca2+ release (CICR) stores to DAP magnitude.
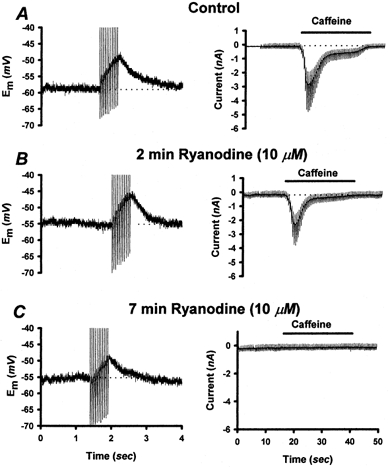
A, left panel: vagotomized NGN displaying a DAP evoked by a 20 Hz (1 nA, 3 ms) train of current pulses. Right panel: 10 mm caffeine application (horizontal bar) to the same NGN voltage clamped at −60 mV induced an inward current. Deflections on the current trace are due to voltage ramps delivered during caffeine application to permit the estimation of reversal potential. B, following a 2 min application of 10 μm ryanodine, the DAP was slightly reduced (by ∼13 %); the caffeine response was reduced by ∼21 %. C, after 7 min of ryanodine application, the caffeine response had totally disappeared, but the DAP was still ∼60 % of its original magnitude. The horizontal dotted lines represent baseline membrane potential or membrane current prior to AP-train application AP-train application or caffeine application, respectively.
In order to quantify the contribution of CICR to the production of AP-induced DAPs, we measured the amplitude of DAP in NGNs whose CICR stores were rendered non-functional by ryanodine application. In these experiments, we first verified that the CICR pool was depleted by repeated caffeine application. Once caffeine application failed to evoke a measurable inward current, AP-induced DAP magnitude was measured again. This experiment is illustrated in Fig. 4. In the six vagotomized DAP(+) NGNs studied in this series of experiments, control DAPs averaged 19 ± 3 mV (range 6–26 mV) in response to a 20 Hz 10 AP train of supra-threshold 3 ms current pulses. These same NGNs also responded to the bath application of 10 mm caffeine with transient inward currents (peak current 1100 ± 300 pA, range 300 − 2600 pA). Two minutes after switching to a superfusate containing 10 μm ryanodine, caffeine was reapplied. In the presence of ryanodine, only one or, at most, two measurable responses to caffeine could be obtained. Once the caffeine-induced inward current was blocked, DAP amplitudes were measured again using the 20 Hz AP train. CICR depletion reduced DAP magnitude (to 13 ± 5 mV or 63 ± 15 % of original magnitude, n = 6). While the effect of ryanodine on caffeine-induced currents was unambiguous (eliminating them completely in all neurons tested), the reduction of DAP magnitude was variable, ranging from 0 to 95 %. We therefore conclude that CICR contributes roughly 37 % of DAP magnitude, though its contribution varies greatly from neuron to neuron. Additional evidence in support of this conclusion is that when Ba2+ was substituted for Ca2+ in the external solution, the amplitude of DAPs was reduced to ∼38 % of control values, as discussed in a previous section. Ba2+ is thought to activate CICR, but it is ineffective at directly activating ICl(Ca) (Scott et al. 1988; Frings et al. 2000).
Calcium currents in vagotomized NGNs
Conventional methods for measuring calcium currents use large, depolarizing voltage-clamp steps under calcium-current recording conditions. While this method could be readily applied to control NGNs, and many vagotomized NGNs, in a subset of NGNs, calcium influx resulted in production of large, slow tail currents, often lasting seconds (Fig. 5). The relatively high level of calcium buffering (11 mm EGTA/1 mm Ca2+) in the internal solution did not prevent these tail currents, nor did the substitution of extracellular Ca2+ with Ba2+. These slow inward tail currents were inhibited by niflumic acid (125 μm), suggesting they may reflect ICl(Ca). Similar slow Ca2+- or Ba2+-dependent tail currents in neurons expressing ICl(Ca) and DAPs have been reported previously (Scott et al. 1988; Sánchez-Vives & Gallego, 1994).
Figure 5. Voltage step method for measuring calcium currents.
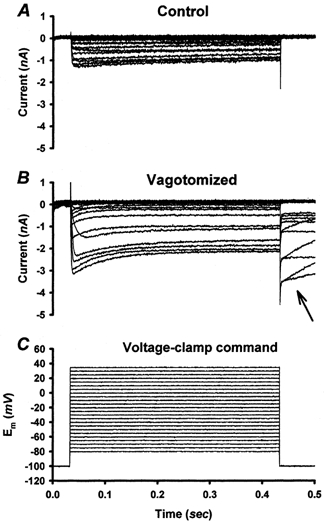
A, voltage-clamp protocol illustrated in C elicited calcium currents recorded from a control NGN. In all control NGNs and many vagotomized NGNs, calcium currents terminated rapidly, with a tail current lasting less than 3 ms, when the membrane potential was returned to −100 mV. B, in a subset (∼25 %) of vagotomized NGNs, slow ‘tail’ currents (arrow) were observed which often lasted > 1 s. Calcium current internal and external solutions are composed as described in Methods.
The magnitude of calcium current, and the voltage-dependent activation of calcium current were similar in control NGNs and DAP(-) NGNs.
AP-associated calcium influx in vagotomized NGNs
Due to contamination of their ICa with ICl(Ca), ICa in DAP(+) vagotomized NGNs could not easily be quantified with traditional long duration voltage-clamp steps. While we could have measured ICa in the presence of niflumic acid (to block ICl(Ca)), 125 μm niflumic acid was not totally effective at inhibiting ICl(Ca) in our experiments, and higher concentrations of niflumic acid may inhibit some calcium conductances (Yao & Tsien, 1997). We therefore applied action potential (AP) waveforms, recorded originally from a control NGN, as a voltage-clamp command while using calcium-current recording solutions. Each AP command results in the activation of voltage-gated calcium currents, and an associated calcium influx (Fig. 6). While the kinetics of the current components cannot be studied in detail by this method, it does provide an assay of the primary function of voltage-dependent ICa: coupling calcium influx to AP discharge. The currents evoked under these conditions were blocked by extracellular Cd2+ (100 μm), confirming that they are due to voltage-gated calcium channels, as discussed in the next section. The size of the calcium influx (QCa) associated with each AP command was estimated by integrating the AP-produced ICa. This method has the advantages of being physiologically relevant (indicating the amount of calcium influx expected to result during AP discharge), and by its short duration, not activating the DAP current. In order to determine whether either DAP(+) NGNs or DAP(-) NGNs had changes in their calcium currents, all cells were first tested with the standard voltage-clamp protocols described above to test for the presence of slow tail currents (as shown in Fig. 5). Cells displaying this slow tail current were considered DAP(+), and all others DAP(-). Then the AP-associated calcium influx (QCa) was compared in the three groups of cells: control NGNs, DAP(+) NGNs, and DAP(-) NGNs. Of the vagotomized NGNs tested, 17 of 41 cells (41 %) were DAP(+). Only one of 19 control cells tested displayed the DAP current.
Figure 6. Calcium currents evoked by action-potential waveforms.
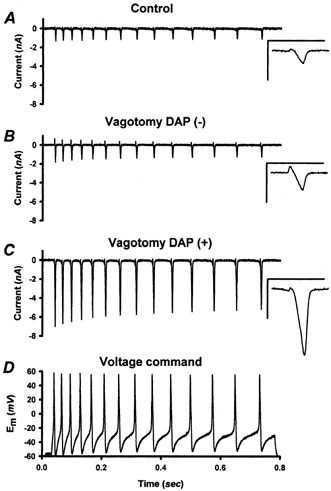
A-C, under calcium current recording conditions (see Methods), a train of action potential (AP) waveforms (D) was used as a voltage-clamp command. An inward calcium current was produced by each AP waveform. The calcium currents were integrated to measure the amount of calcium charge entering the cell in association with each AP waveform in the series (see text). The calcium currents and calcium influx of vagotomized NGNs with DAPs (C) were significantly larger than those of vagotomized DAP(-) NGNs (B) or control NGNs (A). Insets: the Ca2+ current associated with the first AP for each NGN is shown at higher temporal resolution. Inset scale: 15 ms and 4 nA. The small outward currents at the beginning of each AP-command (best seen in the insets) may represent a transient efflux of intracellular ions via voltage gated calcium channels prior to the establishment of calcium influx. These small outward currents, like the larger inward calcium currents, were inhibited by 100 μm CdCl2 (data not shown).
AP-produced QCa was much larger in DAP(+) NGNs (19.0 ± 2.1 pC, n = 17) than in either control NGNs (5.0 ± 0.8 pC, n = 18) or DAP(-) NGNs (7.0 ± 0.8 pC, n = 24). ANOVA on Ranks with Dunn's pair-wise comparisons revealed that the differences between DAP(+) NGNs and either DAP(-) NGNs or control NGNs were statistically significant (P < 0.05). QCa in DAP(-) NGNs was not significantly different from QCa recorded in control NGNs.
Membrane surface area (estimated by membrane capacitance) was significantly (P < 0.05) larger in the DAP(+) NGNs (48 ± 3 pF) than in either DAP(-) NGNs (37 ± 2 pF) or control NGNs (39 ± 4 pF). However, larger membrane area did not entirely account for the increased QCa recorded in DAP(+) NGNs. The AP-produced Ca2+ influx, normalized by membrane capacitance (QCa/Cm), was significantly (P < 0.05) larger in DAP(+) NGNs (0.39 ± 0.03 pC pF−1) than in either DAP(-) NGNs (0.21 ± 0.03 pC pF−1) or control NGNs (0.15 ± 0.03 pC pF−1).
Therefore DAP(+) NGNs have an AP-evoked ICa approximately 3.8 times that of control NGNs, and an ICa density approximately 2.6 times that of controls. This could theoretically be due to either a selective expression of DAPs in NGNs which previously had large QCa and/or an up-regulation of QCa in DAP(+) NGNs. Because QCa in DAP(+) NGNs is so far outside the normal range of QCa in control NGNs (Fig. 6), it is likely that DAP(+) NGNs drastically up-regulate both the absolute numbers and density of functional voltage-gated calcium channels in their soma membranes after vagotomy. By contrast, vagotomized NGNs without ICl(Ca) do not show any significant differences from control NGNs in their calcium currents.
ICa subtypes in vagotomized NGNs
To determine which types of ICa are augmented in DAP(+) NGNs, we quantified the fraction of the AP-produced calcium influx (QCa) in five control NGNs, six DAP(+) NGNs and six DAP(-) NGNs. We measured QCa in these three groups of cells, as described above, during the sequential addition of various VDCC blockers to the external solution.
The total QCa in the DAP(+) NGNs studied in this series (23.1 ± 5.3 pC) was significantly (P < 0.05) greater than that in control NGNs (6.3 ± 1.9 pC) and tended (P = 0.07) to be greater than QCa in DAP(-) NGNs (10.7 ± 1.1 pC). The density of QCa per unit cell membrane capacitance (QCa/Cm) for this series also tended (P = 0.05) to be larger in DAP(+) NGNs (0.42 ± 0.07 pC pF−1) than in DAP(-) NGNs (0.33 ± 0.04 pC pF−1) or control NGNs (0.18 ± 0.07 pC pF−1). The addition of the L-type antagonist nifedipine (10 μm) to the external solution reduced QCa similarly (P > 0.05) in DAP(+) (by 8 ± 6 %), DAP(-) (by 10 ± 6 %) and control NGNs (by 19 ± 4 %). Thus, in all three groups of cells L-type ICa accounted for only a small fraction of AP-produced Ca2+ influx.
Upon further addition of the T/R calcium channel blocker NiCl2 (500 μm), without removing nifedipine, QCa was similarly reduced in DAP(+) (by an additional 38 ± 4 % of original value), DAP(-) (by an additional 37 ± 3 % of original value) and control NGNs (by an additional 37 ± 7 % of original value).
Adding the N-type calcium channel blocker ω-conotoxin GVIA (0.5 μm) to the external solution (without removing nifedipine or nickel) blocked additional QCa similarly in DAP(+) (33 ± 3 %), DAP(-) (32 ± 7 %) and control NGNs (31 ± 6 %).
In the presence of all three antagonists (nickel, ω-conotoxin GVIA and nifedipine), a fraction of the original QCa invariably remained in DAP(+) (22 ± 3 %), DAP(-) (21 ± 3 %) and control NGNs (13 ± 4 %). This final fraction QCa was therefore due to calcium current though VDCCs resistant to nickel, nifedipine and ω-conotoxin GVIA, presumably P-/Q-type current (Cordoba-Rodriguez et al. 1999). QCa was reduced to near zero by the bath application of CdCl2 (100 μm) in DAP(+) (2 ± 1 % of original value), DAP(-) (0 ± 2 % of original value) and control NGNs (3 ± 3 % of original value).
Taken together, this series of experiments indicates that there is no obvious change in the fraction of AP-associated Ca2+ influx carried by various types of calcium channels, in either DAP(+) or DAP(-) vagotomized NGNs. Vagotomy therefore most probably induces an up-regulation in functional numbers of several types of VDCCs, including N-type, T-/R-type, and at least one other type resistant to the blockers tested above.
The relative amount of each type of QCa is compared to its relative efficacy in triggering DAPs in Fig. 7.
Figure 7. Relative contribution of calcium current subtypes to action potential-evoked calcium influx and to DAP amplitude.
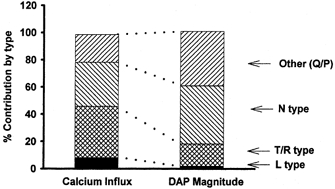
The relative contributions of N-type, L-type, T-/R-type, and P-/Q-type calcium channels to AP associated Ca2+ influx (measured in picocoulombs) and DAP magnitude (measured in millivolts) was assessed using calcium channel blockers, as described in the text. N-type and P-/Q-type calcium channels were disproportionately effective at inducing DAPs, while L-type and T-/R-type channels were disproportionately ineffective.
Calcium stores in vagotomized NGNs
CICR can contribute to DAP magnitude and the caffeine can activate ICl(Ca), as described above. This raises the question: are the calcium stores of DAP(+) vagotomized NGNs different from those of DAP(-) vagotomized NGNs or control NGNs? In order to address this issue, we performed simultaneous whole-cell patch-clamp recording and intracellular Ca2+ ([Ca2+]i) measurements with fura-2, introduced via the patch-pipette. NGNs were classified as DAP(+) or DAP(-) using the AP protocols described above during a 5 min time period in which fura-2 diffused into the NGN. We then measured membrane currents at a voltage-clamped membrane potential of −60 mV and simultaneously measured fura-2 fluorescence during a 30 s bath application of caffeine (10 mm) (Fig. 8).
Figure 8. Caffeine-induced Ca2+ transients in NGNs.
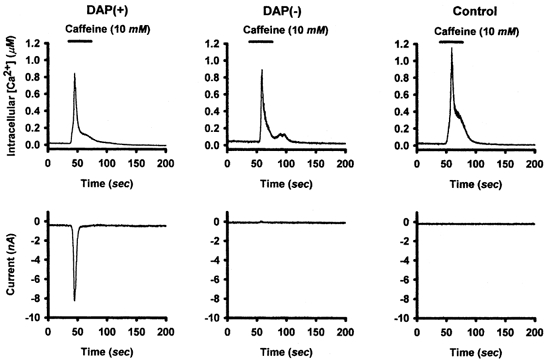
Calcium transients were evoked in DAP(+), DAP(-) and control NGNs by the bath application of caffeine (10 mm) while membrane current was recorded using whole cell voltage clamp at a membrane potential of −60 mV. While all NGNs tested responded to caffeine, the magnitude of the calcium transient varied greatly from NGN to NGN in all three groups. While caffeine-induced calcium elevation was similar in the three groups of NGNs, only DAP(+) NGNs produced inward currents. DAP(-) NGNs and control NGNs produced either no current or small (< 100 pA) outward currents in response to caffeine application.
Functional CICR was universal in both control and vagotomized NGNs; all control (5/5), DAP(+) (7/7) and DAP(-) (4/4) NGNs exposed to caffeine responded with an increase in [Ca2+]i. However, while the DAP(+) NGNs had large inward currents with a time course similar to the calcium transient, DAP(-) NGNs and control NGNs produced either small (< 100 pA) outward currents or no current. In order to determine if the CICR of DAP(+) NGNs was different than that of control NGNs or DAP(-) NGNs, the maximum increase in [Ca2+]i evoked by caffeine, the total area (elevation of [Ca2+]i integrated with respect to time) of the caffeine response, and its time to peak were quantified for each NGN tested. Baseline (resting) [Ca2+]i was similar (P = 0.49) in DAP(+) NGNs (65 ± 14 nm, n = 7), DAP(-) NGNs (42 ± 11 nm, n = 4) and control NGNs (48 ± 15 nm, n = 5). The maximum elevation of [Ca2+]i evoked by caffeine in DAP(+) NGNs (3607 ± 2361 nm, n = 7) tended to be larger (P = 0.44) than that in DAP(-) NGNs (829 ± 300 nm, n = 4) or control NGNs (760 ± 358 nm, n = 5). The time to peak elevation of [Ca2+]i was similar (P = 0.24) in DAP(+) NGNs (9 ± 2.3 s, n = 7), DAP(-) NGNs (7 ± 4.9 s, n = 4) and control NGNs (14 ± 4 s, n = 5). Taken together, these data indicate that, while a few DAP(+) NGNs had extremely large caffeine responses, there was considerable overlap between the responses of the three groups of NGNs and no significant differences were detected. Equivalent elevations in [Ca2+]i in the three groups of NGNs produced inward currents only in DAP(+) NGNs (Fig. 8). Electrophysiological responsiveness to caffeine in DAP(+) NGNs is therefore not due to augmented CICR in these NGNs, but rather due to the coupling of CICR to a calcium-activated chloride current. Similarly, caffeine does not induce depolarizing currents in control NGNs because they lack of functional ICl(Ca), not because of any deficit in CICR.
ICl(Ca) in DAP(+) NGNs followed very closely the time course of Ca2+ elevation (Fig. 8), showing no signs of inactivation. This supports the contention that ICl(Ca) may act as a sensor of Ca2+ levels near the membrane, activating when [Ca2+]i rises, deactivating when [Ca2+]i declines, but not inactivating (Scott et al. 1995). Our data also suggest the ICl(Ca) in NGNs is inactive at resting [Ca2+]i (less than 100 nm), but is activated by elevations of [Ca2+]i into the high nanomolar range (Fig. 8).
DISCUSSION
Following nerve transection (axotomy) there are significant changes in the electrical properties of sensory neuronal somata. Reorganization of voltage-gated sodium, potassium and calcium currents has been extensively documented in somatic afferents, DRG neurons (DRGNs) following axotomy; changes in these currents may support increased excitability in axotomized DRGNs (Cummins & Waxman, 1997; Everill & Kocsis, 1999; Baccei & Kocsis, 2000; Abdulla & Smith, 2001b; reviewed by Kocsis & Devor 2000). Far less information is available about electrophysiological changes that accompany nerve injury in vagal afferent somata (nodose ganglion neurons, NGNs), despite their importance in visceral sensory function. In our initial study of vagotomized NGNs, we observed that NGNs become profoundly less excitable within 5 days of vagus nerve transection (vagotomy; Lancaster et al. 2001). In the current work, our principal findings are that vagotomy induces DAPs in a subpopulation of NGNs, that these DAPs are produced by a calcium-activated chloride current (ICl(Ca)), and that these DAP(+) NGNs have massively augmented AP-associated calcium influx compared to control NGNs. The ICa of DAP(-) vagotomized NGNs was similar to the ICa of control NGNs. Both of these responses to vagus nerve injury are different from those of somatic afferents, where ICa was reduced (Baccei & Kocsis, 2000; Hogan et al. 2000; Abdulla & Smith, 2001a). Thus, the current result adds further evidence that fundamental differences in the response to nerve injury exist between vagal and somatic primary afferent neurons.
What properties of vagotomized NGNs support the appearance of DAPs and ICl(Ca)? Several possibilities exist, including: (1) the development of functional calcium-activated chloride channels; (2) enhanced Ca2+ influx to activate an existing ICl(Ca); and/or (3) augmented AP-associated elevation in intracellular Ca2+, perhaps due to larger stores, lower buffering, or slower clearance. Our results suggest that functional ICl(Ca) is probably absent from control NGNs, and that vagotomy up-regulates the channels responsible for this current. If ICl(Ca) were present in control NGNs, we would expect to observe this current (or DAPs) in control NGNs. However, DAPs were not detected in control NGNs, even after applying long trains of APs, and ICl(Ca) was not observed in control NGNs even after large and prolonged calcium currents. It could be that ICl(Ca) is present in control NGNs but its activation requires enhanced Ca2+ release from CICR pools. This possibility also seems unlikely because DAPs were elicited even when CICR was pharmacologically eliminated with ryanodine. DAP(+) NGNs have significantly increased AP-associated Ca2+ influx (0.39 ± 0.03 pC pF−1) compared to control NGNs (0.15 ± 0.03 pC pF−1), but reducing Ca2+ influx with calcium-channel blockers did not completely eliminate DAPs; even in the presence of nifedipine, nickel and ω-conotoxin GVIA, which together reduced AP-associated calcium influx by ∼75 %, DAPs generally persisted. Further, even when similar elevations of intracellular Ca2+ were produced by caffeine application in DAP(+) and control NGNs, only DAP(+) NGNs produced an associated inward current (Fig. 8). We therefore conclude that control NGNs do not express functional calcium-activated chloride channels. While it is most likely that control NGNs simply do not express calcium-activated chloride channels, we cannot, at this time, exclude the possibility that the channels are expressed but rendered non-functional by a highly efficient process.
Vagotomized NGNs have an increased spike threshold (∼200 % higher rheobase) and reductions of up to 80 % in the numbers of APs evoked by strongly depolarizing stimuli (Lancaster et al. 2001). The question therefore arises whether DAP(+) NGNs are also less excitable. Using measures of excitability similar to Lancaster et al. (2001), we have observed that both DAP(+) and DAP(-) vagotomized NGNs are less excitable than control NGNs, but highly similar to each other in most electrophysiological characteristics (Table 1). This suggests that the emergence of ICl(Ca) and enhancement of ICa are not important determinants of the AP discharge characteristics of vagotomized NGNs. Alterations in sodium current (INa) in vagotomized NGNs may underlie their reduced AP discharge (Lancaster & Weinreich, 2001). Because of the similarities in AP discharge properties between DAP(+) and DAP(-) vagotomized NGNs, it is likely that INa is similarly affected in both groups of NGNs.
Other than in olfactory neurons, where ICl(Ca) is important for signal transduction (for review, see Scott et al. 1995), it is unclear what roles ICl(Ca) plays in neurons, particularly in injured NGNs. ICl(Ca) might mediate post-axotomy changes in cell volume, influence intracellular pH (by passing HCO3− ions), or modulate electrical excitability (Sánchez-Vives & Gallego, 1994; Scott et al. 1995). However, vagotomized NGNs with and without this current have similar electrical excitability (Table 1), and there is no obvious reason why only a subset of vagotomized NGNs would handle intracellular pH or volume differently. DAP(+) NGNs differ most markedly from other NGNs in that they produce large inward currents when intracellular Ca2+ is elevated, while control NGNs do not. A plausible role for ICl(Ca) may therefore be to endow a subset of NGNs with electrophysiological responsiveness to agents which raise intracellular Ca2+. Calcium signals are important in neuronal growth and regeneration (Petersen & Cancela, 2000) and ICl(Ca) may be involved in growth cone signalling (Strittmatter et al. 1993; Nakamura et al. 1998). Additional work will be required to determine which signals are transmitted by ICl(Ca) in NGNs.
DAP(+) vagotomized NGNs display a dramatic up-regulation in AP-associated calcium influx compared to control NGNs or DAP(-) vagotomized NGNs. The enhancement of several types of ICa in these cells may be due to either an increase in the numbers of functional calcium channels and/or changes in their kinetics. In axotomized DRG neurons, changes in sodium currents have been correlated with alterations in the amount of mRNA for various sodium channel proteins (Waxman et al. 1999). Similar techniques might be employed on nodose ganglia neurons in order to determine if the augmentation of ICa in a subset of NGNs is due to an increase in the mRNA for one or more calcium channel proteins. Interestingly, there is a 17-fold increase in at least one calcium channel protein in the DRG following nerve injury (Luo et al. 2001), though this has yet to be reconciled with the decrease in DRGN ICa following axon injury (Baccei & Kocsis, 2000; Hogan et al. 2000; Abdulla & Smith, 2001a). Single-cell PCR coupled with the patch-clamp technique (Chen et al. 2000) may reveal whether mRNAs for calcium channel proteins are selectively up-regulated in DAP(+) vagotomized NGNs.
There are several plausible consequences of increased AP-associated Ca2+ influx observed in DAP(+) vagotomized NGNs. Each AP may have a greater impact on Ca2+-dependent processes such as protein phosphorylation by Ca2+-dependent kinases, gene expression, and the activation of Ca2+-dependent ionic currents. A larger calcium influx per AP may also allow intracellular calcium stores to be restored and maintained in vagotomized NGNs, which may be discharging AP infrequently due to a loss of normal inputs and a decrease in intrinsic excitability (Lancaster et al. 2001). A subset of vagotomized NGNs is paradoxically less able to discharge APs, yet permits the entry of much more calcium with each AP. Determining the net effect of these two phenomena on calcium homeostasis and calcium-dependent processes will require additional exploration.
Acknowledgments
The authors would like to thank Dr R. Cordoba-Rodriguez for her advice on the use of calcium-channel antagonists, and Drs M. S. Gold, D. R. Matteson, J. P. Y. Kao, and B. Undem for reviewing an earlier version of this manuscript. This work was supported by NIH grant NS-22069.
REFERENCES
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+and K+ channel currents of rat dorsal root ganglion neurons. Journal of Neurophysiology. 2001a;85:644–658. doi: 10.1152/jn.2001.85.2.644. [DOI] [PubMed] [Google Scholar]
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. Journal of Neurophysiology. 2001b;85:630–643. doi: 10.1152/jn.2001.85.2.630. [DOI] [PubMed] [Google Scholar]
- Ayar A, Scott RH. The actions of ryanodine on Ca2+-activated conductances in rat cultured DRG neurones; evidence for Ca2+-induced Ca2+ release. Naunyn-Schmeideberg's Archives of Pharmacology. 1999;359:81–91. doi: 10.1007/pl00005335. [DOI] [PubMed] [Google Scholar]
- Baccei ML, Kocsis JD. Voltage-gated calcium currents in axotomized adult rat cutaneous afferent neurons. Journal of Neurophysiology. 2000;83:2227–2238. doi: 10.1152/jn.2000.83.4.2227. [DOI] [PubMed] [Google Scholar]
- Chen KC, Blalock EM, Thibault O, Kaminker P, Landfield PW. Expression of α1D subunit mRNA is correlated with L-type Ca2+ channel activity in single neurons of hippocampal ‘zipper’ slices. Proceedings of the National Academy of Sciences of the USA. 2000;97:4357–4362. doi: 10.1073/pnas.070056097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba-Rodriguez R, Moore KA, Kao JP, Weinreich D. Calcium regulation of a slow post-spike hyperpolarization in vagal afferent neurons. Proceedings of the National Academy of Sciences of the USA. 1999;96:7650–7657. doi: 10.1073/pnas.96.14.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Waxman SG. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. Journal of Neuroscience. 1997;17:3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie KP, Wootton JF, Scott RH. Activation of Ca2+-dependent Cl− currents in cultured rat sensory neurones by flash photolysis of DM-nitrophen. Journal of Physiology. 1995;482:291–307. doi: 10.1113/jphysiol.1995.sp020518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC, McGuirk S, Scott RH. Calcium-dependent chloride currents in cultured rat dorsal root ganglion neurones are inhibited by an adenosine analogue. Journal of Physiology. 1986;390:47–61. doi: 10.1113/jphysiol.1986.sp016034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. Journal of Neurophysiology. 1999;82:700–708. doi: 10.1152/jn.1999.82.2.700. [DOI] [PubMed] [Google Scholar]
- Frings S, Reuter D, Kleene SJ. Neuronal Ca2+-activated Cl− channels - homing in on an elusive channel species. Progress in Neurobiology. 2000;60:247–289. doi: 10.1016/s0301-0082(99)00027-1. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hogan QH, McCallum JB, Sarantopoulos C, Aason M, Mynlieff M, Kwok W-M, Bosnjak ZJ. Painful neuropathy decreases membrane calcium current in mammalian primary afferent neurons. Pain. 2000;86:43–53. doi: 10.1016/s0304-3959(99)00313-9. [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Schofield GG, Weight FF. Na+ and Ca2+ currents of acutely isolated adult rat nodose ganglion cells. Journal of Neurophysiology. 1986;55:527–539. doi: 10.1152/jn.1986.55.3.527. [DOI] [PubMed] [Google Scholar]
- Jafri MS, Moore KA, Taylor GE, Weinreich D. Histamine H1 receptor activation blocks two classes of potassium current, IK(rest) and IAHP, to excite ferret vagal afferents. Journal of Physiology. 1997;503:533–546. doi: 10.1111/j.1469-7793.1997.533bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis JD, Devor M. Altered excitability in large diameter cutaneous afferents following nerve injury: consequences for chronic pain. In: Devor M, Rowbotham MC, Wiesenfeld-Hallin Z, editors. Proceedings of the 9th World Congress on Pain. Seattle: IASP Press; 2000. pp. 119–135. [Google Scholar]
- Lancaster E, Oh EJ, Weinreich D. Vagotomy decreases excitability in primary vagal afferent somata. Journal of Neurophysiology. 2001;85:247–251. doi: 10.1152/jn.2001.85.1.247. [DOI] [PubMed] [Google Scholar]
- Lancaster E, Weinreich D. Sodium currents in vagotomized primary afferent neurons of the rat. Journal of Physiology. 2001;536:445–458. doi: 10.1111/j.1469-7793.2001.0445c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL. Upregulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. Journal of Neuroscience. 2001;21:1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Pinna J, McLachlan EM, Gallego R. Distinct mechanisms for activation of Cl− and K+ currents by Ca2+ from different sources in mouse sympathetic neurones. Journal of Physiology. 2000;527:249–264. doi: 10.1111/j.1469-7793.2000.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. Journal of Physiology. 1985;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelowitz D, Kunze DL. Characterization of calcium currents in aortic barroreceptor neurons. Journal of Neurophysiology. 1992;68:509–517. doi: 10.1152/jn.1992.68.2.509. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Strittmatter P, Strittmatter SM. GAP-43 augmentation of G protein-mediated signal transduction is mediated by both phosphorylation and palmitoylation. Journal of Neurochemistry. 1998;70:983–992. doi: 10.1046/j.1471-4159.1998.70030983.x. [DOI] [PubMed] [Google Scholar]
- Petersen OH, Cancela JM. Attraction or repulsion by local Ca2+ signals. Current Biology. 2000;10:R311–314. doi: 10.1016/s0960-9822(00)00438-3. [DOI] [PubMed] [Google Scholar]
- Sánchez-Vives MV, Gallego R. Calcium-dependent chloride current induced by axotomy in rat sympathetic neurons. Journal of Physiology. 1994;475:391–400. doi: 10.1113/jphysiol.1994.sp020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RH, McGuirk SM, Dolphin AC. Modulation of divalent cation-activated chloride ion currents. British Journal of Pharmacology. 1988;94:653–662. doi: 10.1111/j.1476-5381.1988.tb11572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RH, Sutton KG, Griffin A, Stapleton SR, Currie KP. Aspects of calcium-activated chloride currents: a neuronal perspective. Pharmacological Therapeutics. 1995;66:535–565. doi: 10.1016/0163-7258(95)00018-c. [DOI] [PubMed] [Google Scholar]
- Strittmatter SM, Connon SC, Ross EM, Higashijima T, Fishman MC. GAP-43 augments G protein-coupled receptor transduction in Xenopus laevis oocytes. Proceedings of the National Academy of Sciences of the USA. 1993;90:5327–5331. doi: 10.1073/pnas.90.11.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Cummins TR, Dib-Hajj S, Fjell J, Black JA. Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain. Muscle and Nerve. 1999;22:1177–1187. doi: 10.1002/(sici)1097-4598(199909)22:9<1177::aid-mus3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Weinreich D, Moore KA, Taylor GE. Allergic inflammation in isolated vagal sensory ganglia unmasks silent NK-2 tachykinin receptors. Journal of Neuroscience. 1997;17:7683–7693. doi: 10.1523/JNEUROSCI.17-20-07683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Tsien RY. Calcium current activated by depletion of calcium stores in Xenopus oocytes. Journal of General Physiology. 1997;109:703–715. doi: 10.1085/jgp.109.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]


