Abstract
Cell-attached and inside-out patch-clamp methods were employed to identify and characterize mechanosensitive (MS) ionic channels in the plasma membrane of human myeloid leukaemia K562 cells. A reversible activation of gadolinium-blockable mechanogated currents in response to negative pressure application was found in 58 % of stable patches (n = 317). I-V relationships measured with a sodium-containing pipette solution showed slight inward rectification. Data analysis revealed the presence of two different populations of channels that were distinguishable by their conductance properties (17.2 ± 0.3 pS and 24.5 ± 0.5 pS), but were indistinguishable with regard to their selective and pharmacological properties. Ion-substitution experiments indicated that MS channels in leukaemia cells were permeable to cations but not to anions and do not discriminate between Na+ and K+. The channels were fully impermeable to large organic cations such as Tris+ and N-methyl-d-glucamine ions (NMDG+). Ca2+ permeation and blockade of MS channels were examined using pipettes containing different concentrations of Ca2+. In the presence of 2 mm CaCl2, when other cations were impermeant, both outward and inward single-channel currents were observed; the I-V relationship showed a unitary conductance of 7.7 ± 1.0 pS. The relative permeability value, PCa/PK, was equal to 0.75, as estimated at physiological Ca2+ concentrations. Partial or full inhibition of inward Ca2+ currents through MS channels was observed at higher concentrations of external Ca2+ (10 or 20 mm). No MS channels were activated when using a pipette containing 90 mm CaCl2. Monovalent mechanogated currents were not significantly affected by extracellular Ca2+ at concentrations within the physiological range (0-2 mm), and at some higher Ca2+ concentrations.
It is generally accepted that temporal changes in the concentration of free ionized cytosolic Ca2+ constitute an important intracellular signal. Whereas Ca2+ channels in electrically excitable cells are well described, there is a dearth of information about Ca2+ transporters in non-excitable cells. In cells of blood origin, Ca2+ regulation and cation-transporting membrane systems may play a pivotal role in the control of cell growth and differentiation (Scharff & Foder, 1993). In particular, it has been suggested that cytosolic Ca2+ is involved in the mechanism of action of DIF-1, a potent anti-leukaemic agent that induces erythroid differentiation in K562 cells (Kubohara & Hosaka, 1999). The mechanisms responsible for the fast Ca2+ entry through the leukaemic cell membrane remain poorly understood. In a variety of blood cells, plasma membrane channels operated by receptors, store depletion or some other mechanisms are assumed to be involved (Scharff & Foder, 1993).
The data available to date indicate that in a variety of non-excitable cells, cation-transporting mechanosensitive (MS) channels may provide an effective pathway for Ca2+ influx from the extracellular medium to the cytosol. It has been shown that mechanically gated channels are present ubiquitously in various types of mammalian cell (Sachs & Morris, 1998). Using the patch-clamp technique, it has been demonstrated that non-selective cation channels that are activated by stretching of the plasma membrane exist in a number of tissues including skeletal muscle, smooth muscle cells, neurones, various epithelial and endothelial cells, and osteoblasts. In recent years, the existence of physiologically active stretch-activated channels has been demonstrated, using both single-channel and whole-cell current measurements (e.g. Vanoye & Reuss, 1999). Very little is known about the expression of calcium-permeable MS channels in cells of blood origin. Volume-regulated Cl− currents have been described in lymphocytes and myeloma cells (Levitan & Garber, 1995; Levitan et al. 1995), and it has been reported that macrophages contain stretch-sensitive K+ channels (Martin et al. 1995). The presence of non-selective cation channels was demonstrated in a patch-clamp investigation of the permeability properties of trout red blood cells (Egee et al. 1998). In previous studies, we found sodium-selective channels activated by F-actin disruption in human leukaemia K562 cells (Negulyaev et al. 1996, 2000). These channels were shown to be independent of membrane stretch. In addition, we have observed in cell-attached patches on leukaemia cells, a reversible activation of single currents that differ in their conductive properties in response to suction (Negulyaev et al. 1996; Starushchenko et al. 2000). In the study presented here, we have examined the selective characteristics and Ca2+ permeation of MS channels in K562 cells that have the properties of multipotent precursors of blood cells. The data obtained show that cation-selective stretch-activated channels permeable to Ca2+ in the physiological concentration range are expressed in the plasma membrane of leukaemia cells. The effects of extracellular Ca2+ that are of great importance for channel functioning and cellular responses have been also studied.
METHODS
Cells
Human myeloid leukaemia K562 cells obtained from a cell culture collection (Institute of Cytology, St Petersburg, Russia) were maintained in glass flasks in RPMI-1640 containing 10 % fetal bovine serum and (in some experiments) antibiotics (100 μg ml−1 streptomycin and 100 units ml−1 penicillin) at 37 °C. Cells were plated on coverslips (0.4 × 0.4 cm) 2-4 days before an experiment.
Electrophysiology
Single channel currents were recorded using standard cell-attached and inside-out configurations of the patch-clamp technique (Hamill et al. 1981). The membrane voltage was calculated as the potential on the intracellular membrane side minus the potential on the extracellular membrane side. Pipettes were pulled from soft glass capillaries to a resistance of 7-15 MΩ when filled with normal external solution. Membrane currents were recorded essentially as described by Negulyaev et al. (2000). Unless otherwise stated, data were filtered at 200 Hz and sampled at a rate of 1 kHz by a 12-bit A-D converter for analysis and display. The recordings were performed at room temperature (22-23 °C) on the stage of an inverted microscope that possessed Nomarsky optics (magnification 256 ×). Pieces of coverslips with adhered cells were transferred into a recording chamber filled with a normal Na+ external solution, and a giga-seal was formed between the pipette and one of the cells. For cell-attached measurements, this bath solution was replaced with the potassium-containing solution to nullify the resting membrane potential. The pipette interior was connected to a manometer with a valve to allow either application of positive and negative pressure or equilibration to atmospheric pressure. The channel open probability (Po) was determined using the following equation: Po = I/i × N, where I is the mean current determined from the amplitude histograms, i is the unitary current amplitude and N is the number of functional channels in the patch. The Goldman-Hodgkin-Katz modified constant-field equation was used to estimate the relative permeability of channels from current reversal potential (Erev) values (see Hille, 1992). Averaged data are given as the mean ± s.e.m. (number of experiments).
Solutions
The normal external solution in the bath and in the pipette typically contained (mm) 145 NaCl, 2 CaCl2, 1 MgCl2, 10 Hepes/ TrisOH. In some of the experiments, the solution contained no divalent cations. The bath solution for cell-attached measurements contained (mm) 145 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes/KOH. The cytosol-like bathing solution for inside-out measurements contained (mm) 145 KCl, 1 MgCl2, 20 Hepes/KOH, 2 EGTA and an appropriate quantity of CaCl2 (0.175 mm) to establish the final free ionized Ca2+ concentration at the desired level of 0.01 μM. In experiments with anion substitutions, KCl was replaced with 145 mm potassium glutamate, 145 mm potassium aspartate or 70 mm K2SO4 145 mm. Tris-Cl or NMDGCl were used to substitute monovalent alkali cations. To decrease the concentration of permeable electrolytes in the course of inside-out recordings, 75 mm KCl was replaced by 150 mm sucrose. To measure Ca2+ currents we used pipette solutions with different concentrations of Ca2+. The solution of high Ca2+ concentration contained (mm) 90 CaCl2, 10 Hepes/TrisOH. Solutions of lower Ca2+ concentrations contained (mm) 20 CaCl2 + 110 NMDGCl, 10 CaCl2 + 125 NMDGCl, or 2 CaCl2 + 137 NMDGCl. Experiments involving Gd3+ were carried out using the highly dissolvable salt GdCl3. The pH of all solutions was set at 7.3. All substances were purchased from Sigma.
RESULTS
Activation of MS channels in cell-attached patches on K562 cells
The first series of experiments was carried out to study the effect of mechanical stimulation on channel activity in the plasma membrane of cultured leukaemia cells. the cell-attached patch configuration is the most suitable for single-current measurements under quasi-physiological conditions. Without stimulation, the major part of cell-attached patches on K562 cells displayed no channel events. In other patches, control cell-attached recordings revealed outward currents through potassium-selective channels. Rare inward currents representing the background activity of sodium-selective channels (Negulyaev et al. 1996, 1997) were also observed in a few experiments. To examine the effect of membrane stretch on single-channel activity in human leukaemia K562 cells, negative or positive pressure was applied to the patch via the suction pipette. Positive pressure application (10-20 mmHg) induced no change in membrane current while the patches remained stable. We found that mechanically gated ion channels were activated in response to application of negative pressure(10-30 mmHg). The results of a typical experiment are presented in Fig. 1, showing activation of inward currents induced by suction at a membrane potential of −40 mV. Removal of the stimulus caused a rapid abolishment of the single currents induced by stretch (Fig. 1A). An increase in the pressure level resulted in a concomitant increase in Po. It should be emphasized that the background activity of sodium-selective and potassium-selective channels in K562 cells was not affected by suction. In cell-attached recordings, mechanically gated currents did not inactivate during prolonged (up to 300 s) stimulation. Moreover, after removal of suction the activity could be evoked repeatedly. After excising the membrane patch from the cell, the activity of MS channels was not so stable as in cell-attached recording, exhibiting a rundown in a number of experiments.
Figure 1. Activation of mechanosensitive (MS) channels in a typical cell-attached experiment on a K562 leukaemia cell.
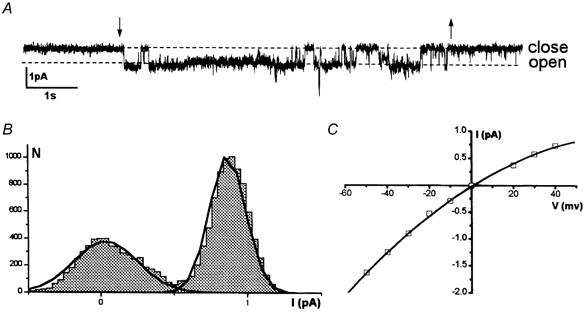
The cell-attached mode was achieved using a pipette containing a solution that included Na+. A, representative single-current records showing the effect of negative pressure application at a membrane potential of 40 mV; the application and removal of suction are indicated by the arrows. B, corresponding amplitude histogram. C, the I-V relationship shows current reversal at about zero and a slight inward rectification. Unitary conductance, which was estimated in the range of negative potentials, was 26 pS.
The MS channels were identified in 58 % of stable patches (n = 317) on K562 cells; the activity of two to four, or more channels was recorded with increasing pressure level (see Fig. 2). According to their gating properties, MS channels in K562 cells could be identified as typical stretch-activated channels. This was confirmed by the fact that addition of 20 mm Gd to the pipette fully prevented the activation of all types of MS channels (not shown). We also found in cell-attached experiments that a high concentration of the diuretic amiloride (1 mm) caused a full inhibition of stretch-activated MS channels in K562 cells.
Figure 2. The activity of MS channels of different conductances.
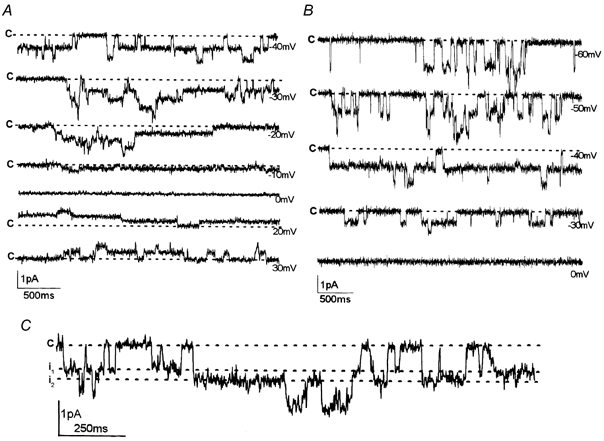
Representative single-current recordings from three cell-attached patches. The holding membrane potentials are indicated near the traces; c indicates the closed state. A, single currents characterized by a unitary conductance of 18 pS; at least three to four channels of the same conductance were activated in this patch. B, single currents characterized by a unitary conductance of 25 pS; at least two channels of the same conductance were active in this patch. C, current record demonstrating the coexistence of channels with different conductance levels (17 pS and 25 pS) in the same patch. The holding potential was 40 mV. The amplitude of single-channel events i1 and i2 was 0.68 pA and 0.98 pA, respectively. Single currents are likely to represent the activity of two different channels functioning independently in the patch.
I-V data analysis
Figure 2 shows single currents induced by suction in cell-attached patches at different holding potentials. The data revealed that MS channels were active throughout the range of holding potentials tested and Po was not affected by membrane voltage. The unitary I-V curves measured for MS channels with Na+ in the pipette showed slight inward rectification; the Erev of currents was close to zero (Figs 1C, 2A, B). In the range of negative membrane potentials, the I-V curve could be approximated as a linear regression to estimate single-channel conductance. I-V relationships measured with Na+ in the pipette solution showed unitary conductance values in the range 10-30 pS (see Figs 1-3). Importantly, MS channels characterized by different conductance values displayed very similar gating properties and selective characteristics. Two conductance levels of about 17 and 25 pS were predominant; representative current recordings shown in Fig. 2A, B demonstrate the channel activity induced by suction in two different patches. Different conductances only rarely coexisted in the same patch. Figure 2C shows one of these examples: channel events corresponding to conductance levels of 17 and 25 pS were observed. At a holding potential of −40 mV, the amplitudes of these single-channel openings were i1 = 0.68 pA and i2 = 0.98 pA. There were no direct transitions between levels i1 and i2, representing possible conductance states. On the contrary, successive openings of two independent channels of different conductance levels occurred. At the same time, there was no direct transition from the closed state (zero current) to the level equal to the sum of the current amplitude (i1 + i2). Thus, according to the classical scheme (Colquhoun & Sigworth, 1995), examination of single currents provided no evidence that the different conductances represent different sub-states of the same MS channel. Consequently, we had to consider that there were different populations of MS channel in the K562 cell plasma membrane. Data analysis revealed two major channel populations having the following conductance values: 17.2 ± 0.3 pS (n = 37) and 24.5 ± 0.5 pS (n = 34). In a few experiments, MS channels characterized by lower unitary conductance values (10.9 ± 0.4 pS; n = 20) were observed.
Figure 3. Selective properties of MS channels in K562 cells.
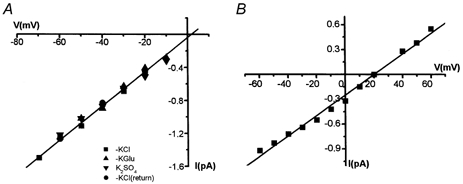
A, I-V data obtained from an inside-out patch with different anions in the cytosol-like bathing solution: KCl, potassium glutamate (KGlu), K2SO4. The unitary conductance was 19 pS. B, I-V curve obtained from an inside-out patch when 50 % of the electrolyte in the bath solution was replaced with sucrose. The reversal potential was about +20 mV, indicating that MS channels have a high cation specificity.
It should be emphasized that activity of several channels of the same conductance was observed frequently, whereas coexistence of difference channel types was only rarely encountered. This imbalance compelled us to assume that the populations of MS channels are characterized by cluster organization. Alternatively, it seems reasonable to suggest that the MS channel properties are controlled by some intracellular mechanisms that provide rather stable conductance levels. As a result, different conductance states may be realized in the same channel protein, although we did not record any direct transitions in the course of our single-current measurements.
Permeation properties
In some of the experiments, channel activity could also be evoked by negative pressure application after patch excision. Inside-out recordings showed similar kinetic properties of mechanogated currents. I-V curves measured with Na+ as the major cation in the pipette and K+ in the cytosol-like bath solution were similar to those obtained in cell-attached recordings (Figs 1, 3). To identify reliably MS channels in the plasma membrane of K562 cells we examined their selective properties via ion substitution. Figure 3A demonstrates that substitution of anions in the cytosol-like bathing solution caused no changes in the amplitude of single-channel openings; in different experiments where Cl−, SO42-, glutamate and aspartate anions were tested. This suggests that the channels are likely to be impermeable to anions, or else they do not discriminate between anions at all. The latter possibility seems to be very unlikely. However, to confirm directly the cation specificity of MS channels, single currents induced by suction in inside-out patches were measured after a non-electrolyte solution replaced half of the saline solution (Fig. 3B). Thus, both the anion and cation concentration were diminished, whereas the tonicity of the cytosol-like bathing solution was not altered. This is a simple and unequivocal test that allows the determination of channel cation or anion specificity. In our experiments, this substitution led to a shift in the I-V curve to more positive potentials (i.e. the value of Erev shifted from zero to 20.1 ± 0.9 mV). This indicates that MS channels are highly cation/anion selective.
In the next series of experiments, I-V relationships of MS channels were measured with the substitution of cations in the extra- and intracellular solutions (Fig. 4). When the pipette solution contained large organic cations (Tris+ or NMDG+) instead of Na+, only outward currents through MS channels were recorded; no currents of the opposite (inward) direction were observed in the range of potentials up to −150 mV. Figure 4 also demonstrates the analogous results obtained with inside-out patches using the control Na+ solution in the pipette and 145 mm NMDGCl in the cytosol-like bathing solution. In this case, we observed only inward mechanogated currents; no reversal of currents took place at potentials up to +60 mV. Thus, current recordings made over a wide range of membrane potentials revealed unequivocally that the MS channels in these K562 cells were not permeable to organic cations such as Tris+ and NMDG+. These results also confirm that MS channels in leukaemic cells are permeable to cations but not to anions, and do not discriminate between Na+ and K+. Moreover, the data obtained allowed us to examine the cation permeability of MS channels in further experiments using these impermeant cations for partial cation substitution.
Figure 4. MS channels are not permeable to organic cations.
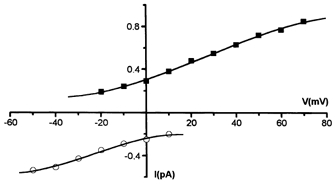
I-V relationships of MS channels with 145 mm NMDGCl in the pipette (cell-attached recording, filled squares), and in the cytosol-like bathing solution (inside-out recording, open circles). Single-current measurements were performed over a wide range of membrane potentials: at potentials up to −150 mV with NMDG in the pipette, no inward currents were observed; at potentials up to +60 mV with NMDG in the cytosol-like bathing solution, no outward currents were observed.
Ca2+ permeation and blocking effects
It was found in our experiments on K562 cells that physiological concentrations (1-2 mm) of Ca2+ and Mg2+ did not prevent the activation of MS channels. In some of the experiments, mechanogated currents were recorded with no bivalent cations present (Ca2+ and Mg2+) in the sodium-containing pipette solution. The data obtained revealed channel populations with two major conductance levels (17 and 25 pS) similar to the values measured in the normal solution.
To determine the Ca+ permeability of MS channels, cell-attached measurements with different concentrations of Ca2+ in the pipette solution were performed; other cations in the pipette were impermeant NMDG and Tris (Fig. 5). In the presence of 2 mm CaCl2, outward and inward single-channel currents could be observed; the I-V relationship showed a unitary conductance of 7.7 ± 1.0 pS (n = 4) and Erev = −39 ± 2.2 mV (Fig. 5A). The relative permeability value (PCa/PK) estimated with 2 mm Ca2+ in the pipette was equal to 0.75.
Figure 5. Ca2+ permeation and blockade of MS channels.
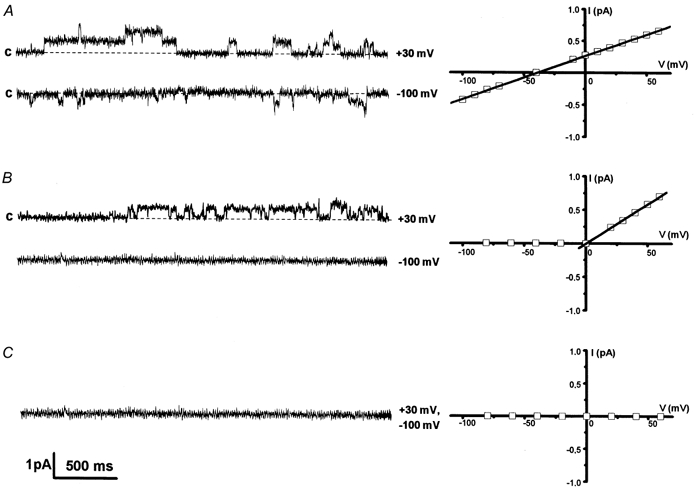
Mechanogated currents (on the left) and corresponding I-V relationships (on the right) measured in cell-attached experiments with various Ca2+ concentrations (mm) in the pipette; NMDG was added to maintain the ionic strength; c indicates the closed state. A, in the presence of 2 mm CaCl2, single-channel activity in the inward (at −100 mV) and outward (at +30 mV) direction was recorded. The I-V curve shows unitary conductance of 8 pS and a reversal potential of −39 mV. B, in the presence of 20 mm CaCl2, no inward currents were observed, whereas outward channel activity was recorded. C, at 90 mm CaCl2, MS currents were inhibited over the entire voltage range studied.
We found that when the pipette solution contained 90 mm CaCl2, no activation of MS channels occurred throughout the voltage range tested (Fig. 5C). At a pipette CaCl2 concentration of 20 mm, the outward mechanogated currents activated in response to pressure application were observed at positive potentials, whereas the inward currents were fully inhibited (Fig. 5B). When the pipette solution contained 10 mm CaCl2, rare inward openings of MS channels could be measured at potentials more negative than −20 mV. Presumably, the inhibition of mechanogated Ca2+ currents at increasing extracellular concentrations of Ca2+ may be due, at least partially, to the modification of channel kinetics, resulting in an apparent decrease in Po. I-V curves obtained at 10 and 20 mm CaCl2 displayed typical outward rectification; for outward currents, the single-channel conductance measured at positive potentials was about 12-13 pS. Inward channel openings recorded with 10 mm Ca2+ in the pipette solution and in the voltage range −100 to −30 mV corresponded to a conductance value that did not exceed 10 pS. These data imply a blocking effect or saturation of open channel currents at higher concentration of Ca2+. Thus, an increased level of extracellular Ca2+ resulted in a full or partial inhibition of Ca2+ currents through MS channels in K562 cells.
A question arises as to whether monovalent currents through MS channels could be blocked at higher Ca2+ concentrations. Figure 6 shows inward and outward currents recorded in a representative cell-attached patch after the addition of 20 mm CaCl2 to the sodium-containing (115 mm NaCl) pipette solution. MS channel activity was observed as at both negative and positive membrane potentials. Single-channel conductance was about 13 pS in these patches. Taking into account that the Na+ concentration was diminished to maintain ionic strength in these experiments, it is reasonable to consider that this value may correspond to one of the main conductance levels found for MS channels under normal conditions (i.e. 16 pS at 145 mm NaCl). However, we have not made a detailed, quantitative estimation of the possible effect of external Ca2+ on mechanogated Na+ currents for two reasons: (1) we have no data on the possible saturation of Na+ currents and conductance for these channels and (2) at least two conductance levels were measured in normal Na+ solution. If we assume that currents and conductances are linearly dependent upon the Na+ concentration (activity), we can conclude no significant effect of increasing Ca2+ in the range 0-20 mm on monovalent currents through MS channels.
Figure 6. Monovalent mechanogated currents at a high concentration of external Ca2+.
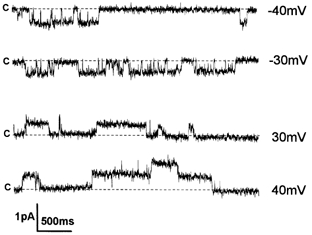
Cell-attached current records showing the activity of MS channels at positive and negative membrane potentials. The pipette solution contained 20 mm CaCl2 and 115 mm NaCl; c indicates the closed state. Unitary conductance was equal to 13.3 pS.
DISCUSSION
Very little is known about MS calcium-permeable channels in cells of blood origin. In the present study, MS cationic channels in the plasma membrane of cultured human myeloid leukaemia K562 cells were first identified and then examined. The data obtained confirmed our preliminary results on K562 cells showing stretch-sensitive activation of single-channel currents in cell-attached patches. Moreover, MS channels identified in leukaemia cells displayed some similar features with non-selective cation channels that could be activated by suction in fish erythrocytes (Egee et al. 1998). Previous studies on blood cells revealed other channel types involved in cell mechanosensitivity and volume regulation: K+ and Cl− channels activated during osmotic swelling in frog erythrocytes (Hamill, 1983), stretch-sensitive K+ channels in human macrophages (Martin et al. 1995) and volume-regulated anion channels in lymphocytes and myeloma cells (Levitan & Garber, 1995; Levitan et al. 1995). In our experiments on K562 leukaemia cells, the background activity of potassium- and sodium-selective channels was not directly affected by pressure application. We found that MS channels were permeable to Ca2+ in the physiological range of concentration. Therefore, the indirect effects of mechanical stimulation on the non-voltage-gated Na+ channels may be assumed due to the calcium-dependent channel regulation via actin cytoskeleton rearrangement previously shown in K562 cells (Maximov et al. 1997). These data suggest that the actin-severing protein gelsolin, which is activated by even a local increase of cytosolic Ca2+, provides an important physiological mechanism controlling ion-channel activity and signal transduction in a variety of non-excitable tissues including blood cells.
It is known that mechanogated channels function as physiological transducers in mechanosensory cells; more general and basic roles such as cell volume and growth regulation in animal cells have been also considered (see Hamill & McBride, 1997; Sachs & Morris, 1998). These functions of MS channels may be realized in blood cells and their precursors. Numerous haemopoietic growth factors were reported to evoke Ca2+ mobilization; thus, an essential role of Ca2+ signalling in cell proliferation and differentiation processes was suggested (Scharff & Foder, 1993). In particular, putative anti-tumour agents were shown to suppress cell growth and to induce erythroid differentiation in human leukaemia K562 cells that had properties of multipotent precursors of blood cells (Asahi et al. 1995; Kubohara & Hosaka, 1999). Investigations of the signalling pathway have indicated that an increase in cytosolic Ca2+ is involved in the mechanisms of action of DIF-1 and its analogues, and is required for their anti-leukaemic activity. Importantly, the authors suggested that similar mechanisms could exist in different mammalian cells, emphasizing their therapeutic potential in the treatment of cancer (Kubohara, 1999; Kubohara & Hosaka, 1999). The MS channels described here may provide one of the physiological pathways for Ca2+ influx in different blood cells that are stimulated mechanically during their normal and pathological functioning.
Ca2+ influx through MS channels is considered to play an important role in Ca2+ signalling in a variety of non-excitable cells (Christensen, 1987; Lumpkin & Hudspeth, 1995; Hoyer et al. 1994, 1996). Our results represent the first identification of calcium-permeable MS channels in leukaemia cells. These channels were activated in both cell-attached and inside-out patches by negative pressure application. Without stimulation, the Po value for MS channels was close to zero. In terms of gating, the channels described here could be assigned to the stretch-activated type. We have demonstrated that MS channels in K562 cells were highly selective for cations over anions and they were non-selective with regard to Na+ and K+. The conductance values of MS channels in leukaemia cells for monovalent cations resemble those reported for stretch-activated cationic channels in endocardial endothelium (Hoyer et al. 1994) and in Reissner's membrane (Yeh et al. 1998), and for channels reconstituted by expression of the α-subunit of the epithelial Na+ channel cloned from osteoblasts (Kizer et al. 1997). Thus, it may be concluded that leukaemia MS channels are rather similar to the stretch-activated cation channels described in cells of various types (Guharay & Sachs, 1984; Sachs & Morris, 1998). Typically for this type, the channels were shown to be blocked by Gd and to be permeable to Ca2+. However, in numerous studies, conductive characteristics for bivalent cations were obtained at rather high extracellular concentrations of Ca2+ or Ba2+ (Hoyer et al. 1994, 1996; Kizer et al. 1997). The advantage of our study is the measurement of mechanogated Ca2+ currents and Ca2+ permeability in the physiological concentration range.
Channel conductance calculated from the I-V relationship with 2 mm CaCl2 in the pipette was about 8 pS, which is comparable with the values measured for much higher Ca2+ concentrations. This may be due to the inhibition of channel currents at the higher bivalent ion concentrations used. The blocking action of Ca2+ on stretch-activated channels in Xenopus oocytes has been described previously (Taglietti & Toselli, 1988; Yang & Sachs, 1989). An inhibitory effect of Ca2+ and Ba2+ on mechanogated monovalent currents was analysed when the concentration of bivalent cations varied within 0-2 mm (Taglietti & Toselli, 1988). Analogous variations of external Ca2+ did not alter the amplitude or kinetics of MS monovalent currents in leukaemia cells. Yang & Sachs (1989) noted a reduction in channel open time and in the amplitude of the current caused by addition of Ca2+ to a sodium-containing bath solution. Unlike our data on K562 cells, an increase in external Ca2+ (Ba2+) up to 70 mm (Taglietti & Toselli, 1988) or even 160 mm (Yang & Sachs, 1989) did not result in a full inhibition of inward mechanogated Ca2+ currents in frog oocytes. Marchenko & Sage (1997) also reported that pressure-activated currents in the endothelium were reduced by extracellular Ca2+. The slope conductance for inward currents was diminished from 33 pS in calcium-free solution to 6 pS in isotonic CaCl2 solution. Recently, Zhang & Hamill (2000) examined the activation of mechanogated channels initiated by the removal of external Ca2+ and other known stimuli. The blocking effects of extracellular Ca2+ that are of great interest for MS channels were examined in our experiments on leukaemia cells. Importantly, we found that an increase of external Ca2+ resulted in a partial or full inhibition of mechanogated Ca2+ currents; at a high concentration (90 mm CaCl2 in the pipette solution), Ca2+ completely inhibited channel MS activity. It should be emphasized that monovalent mechanogated currents were not significantly affected by the variations of external Ca2+ in the physiological concentration range and at some higher levels. The data allow us to suppose that the pore-forming region of the channel includes an anion site that is characterized by an apparent binding constant for Ca2+ that is higher than 2 mm and possibly equal to 10 mm; a site position near the extracellular membrane surface is more probable. Binding of Ca2+ could result in an inhibition of Ca2+ currents and did not alter monovalent ion fluxes. The complete inhibition of channel activity at high Ca2+ concentrations may be due to the existence of one more binding site.
Over the last decade, patch-clamp studies together with fluorescence imaging data have revealed an apparent ubiquity of MS (or mechanogated) channels (Hamill & McBride, 1996; Sachs & Morris, 1998). These data challenged two different views with regard to the functional significance of these channels in living cells. On the one hand, MS channels, including stretch-activated cation channels, are thought to mediate a variety of functions in both excitable and non-excitable tissues. On the other hand, specific reports have questioned the reality of mechanogated channels as biological transducers and proposed that such channels are artefacts of patch-clamp recording (for review see Hamill & McBride, 1996). It has been suggested that the effect varies with ion channel and cell type and presumably arises because of the disruption of membrane-cytoskeleton interactions. It is worth noting that functional coupling between Na+ channels and the actin cytoskeleton has been clarified in our previous studies on leukaemia K562 cells (Negulyaev et al. 1996, 2000; Maximov et al. 1997). Patch-clamp data obtained in different configurations and using cytochalasin D, gelsolin and different forms of G-actin support the idea that microfilament organization remains essentially undamaged during the course of single-current recordings on K562 cells. Since the cortical cytoskeleton is predominant in erythroleukaemia cells and erythrocytes, it may be that K562 cells present a suitable model with which to examine stretch-activated cation channels and mechanotransduction.
Cation-permeable MS channels are thought to be closely related to the epithelial Na+ channel (ENaC)/degenerin family of channel proteins that are characterized by the presence of two hydrophobic domains (Corey & Garcia-Anoveros, 1996; Kizer et al. 1997). Studying cation-transporting pathways in non-excitable cells, we found non-voltage-gated sodium-selective channels in macrophages (Negulyaev & Vedernikova, 1994), carcinoma cells (Negulyaev et al. 1994) and leukaemia cells (Negulyaev et al. 1996, 1997). Amiloride-sensitive Na+ channels in other cells of blood origin have also been reported (Bubien & Warnock, 1993; Bradford et al. 1995; Achard et al. 1996). Several lines of evidence show that the characteristics of these channels are similar to those of the well-known ENaC (Benos et al. 1995). It is reasonable to assume that the MS channels identified in the present study and the Na+ channels described previously in leukaemia cells (Negulyaev et al. 1996, 1997) belong to the same superfamily of channel proteins that mediate cation membrane permeability, and are characterized principally by the similarity of their molecular organization and functional properties. The blocking effect of amiloride found in our experiments confirm the similarity of stretch-activated cationic channels and sodium-selective channels in leukaemia cells. Sodium-selective channels were reported to be impermeable to bivalent cations (Ca2+ and Ba2+ at a concentration of 100 mm). The data obtained here show that high extracellular concentrations of Ca2+ may block cationic channels, whereas at lower concentrations the channels may be permeable to this cation.
Acknowledgments
This work was supported by the Russian Basic Research Foundation, grant no. 02-04-48251, and by the Royal Swedish Academy of Sciences.
REFERENCES
- Achard JM, Bubien JK, Benos DJ, Warnock DG. Stretch modulates amiloride sensitivity and cation selectivity of sodium channels in human B lymphocytes. American Journal of Physiology. 1996;270:C224–C234. doi: 10.1152/ajpcell.1996.270.1.C224. [DOI] [PubMed] [Google Scholar]
- Asahi K, Sakurai A, Takahashi N, Kubohara Y, Okamoto K, Tanaka Y. DIF-1, morphogen of Dictyostelium discoideum, induces the erythroid differentiation in murine and human leukemia cells. Biochemical and Biophysical Research Communications. 1995;208:1036–1039. doi: 10.1006/bbrc.1995.1438. [DOI] [PubMed] [Google Scholar]
- Benos DJ, Awayda MS, Ismailov II, Johnson JP. Structure and function of amiloride-sensitive Na+ channels. Journal of Membrane Biology. 1995;143:1–18. doi: 10.1007/BF00232519. [DOI] [PubMed] [Google Scholar]
- Bradford AL, Ismailov II, Achard JM, Warnock DG, Bubien JK, Benos DJ. Immunopurification and functional reconstitution of a Na+ channel complex from rat lymphocytes. American Journal of Physiology. 1995;269:C601–C611. doi: 10.1152/ajpcell.1995.269.3.C601. [DOI] [PubMed] [Google Scholar]
- Bubien JK, Warnock DG. Amiloride-sensitive sodium conductance in human B lymphoid cells. American Journal of Physiology. 1993;265:C1175–C1183. doi: 10.1152/ajpcell.1993.265.4.C1175. [DOI] [PubMed] [Google Scholar]
- Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature. 1987;330:66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single-channel records. In: Sakmann B, Neher E, editors. Single Channel Recording. 2. New York: Plenum; 1995. pp. 483–587. [Google Scholar]
- Corey DP, Garcia-Anoveros J. Mechanosensation and the DEG/ENaC ion channels. Science. 1996;273:323–324. doi: 10.1126/science.273.5273.323. [DOI] [PubMed] [Google Scholar]
- Egee S, Mignen O, Harvey BJ, Thomas S. Chloride and non-selective cation channels in unstimulated trout red blood cells. Journal of Physiology. 1998;511:213–224. doi: 10.1111/j.1469-7793.1998.213bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. Journal of Physiology. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP. Potassium and chloride channels in red blood cells. In: Sakmann B, Neher E, editors. Single Channel Recording. 2. New York: Plenum; 1983. pp. 451–471. [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacological Reviews. 1996;48:231–252. [PubMed] [Google Scholar]
- Hamill OP, McBride DW., Jr Induced membrane hypo/hyper-mechanosensitivity: a limitation of patch-clamp recording. Annual Review of Physiology. 1997;59:621–631. doi: 10.1146/annurev.physiol.59.1.621. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels in Excitable Membranes. Sunderland, MA, USA: Sinauer Associates; 1992. [Google Scholar]
- Hoyer J, Distler A, Haase W, Gogelein H. Ca2+ influx through stretch-activated cation channels activates maxi K+ channels in porcine endocardial endothelium. Proceedings of the National Academy of Sciences of the USA. 1994;91:2367–2371. doi: 10.1073/pnas.91.6.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer J, Kohler R, Haase W, Distler A. Up-regulation of pressure-activated Ca2+-permeable cation channel in intact vascular endothelium of hypertensive rats. Proceedings of the National Academy of Sciences of the USA. 1996;93:11253–11258. doi: 10.1073/pnas.93.20.11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizer N, Guo XL, Hruska K. Reconstitution of stretch-activated cation channels by expression of the alpha-subunit of the epithelial sodium channel cloned from osteoblasts. Proceedings of the National Academy of Sciences of the USA. 1997;94:1013–1018. doi: 10.1073/pnas.94.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubohara Y. Effects of differentiation-inducing factors of Dictyostelium discoideum on human leukemia K562 cells: DIF-3 is the most potent anti-leukemic agent. European Journal of Pharmacology. 1999;381:57–62. doi: 10.1016/s0014-2999(99)00548-8. [DOI] [PubMed] [Google Scholar]
- Kubohara Y, Hosaka K. The putative morphogen, DIF-1, of Dictyostelium discoideum activates Akt/PKB in human leukemia K562 cells. Biochemical and Biophysical Research Communications. 1999;263:790–796. doi: 10.1006/bbrc.1999.1468. [DOI] [PubMed] [Google Scholar]
- Levitan I, Almonte C, Mollard P, Garber SS. Modulation of a volume-regulated chloride current by F-actin. Journal of Membrane Biology. 1995;147:283–294. doi: 10.1007/BF00234526. [DOI] [PubMed] [Google Scholar]
- Levitan I, Garber SS. Voltage-dependent inactivation of volume-regulated Cl− current in human T84 colonic and B-cell myeloma cell lines. Pflügers Archiv. 1995;431:297–299. doi: 10.1007/BF00410203. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Hudspeth AJ. Detection of Ca2+ entry through mechanosensitive channels localizes the site of mechanoelectrical transduction in hair cells. Proceedings of the National Academy of Sciences of the USA. 1995;92:10297–10301. doi: 10.1073/pnas.92.22.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko SM, Sage SO. A novel mechanosensitive cationic channel from the endothelium of rat aorta. Journal of Physiology. 1997;498:419–425. doi: 10.1113/jphysiol.1997.sp021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DK, Bootcov MR, Campbell TJ, French PW, Breit SN. Human macrophages contain a stretch-sensitive potassium channel that is activated by adherence and cytokines. Journal of Membrane Biology. 1995;147:305–315. doi: 10.1007/BF00234528. [DOI] [PubMed] [Google Scholar]
- Maximov AV, Vedernikova EA, Hinssen H, Khaitlina SY, Negulyaev YA. Ca-dependent regulation of Na+-selective channels via actin cytoskeleton modification in leukemia cells. FEBS Letters. 1997;412:94–96. doi: 10.1016/s0014-5793(97)00754-0. [DOI] [PubMed] [Google Scholar]
- Negulyaev YA, Khaitlina SY, Hinssen H, Shumilina EV, Vedernikova EA. Sodium channel activity in leukemia cells is directly controlled by actin polymerization. Journal of Biological Chemistry. 2000;275:40933–40937. doi: 10.1074/jbc.M008219200. [DOI] [PubMed] [Google Scholar]
- Negulyaev YA, Maximov AV, Vedernikova EA, Katina IE. Voltage-insensitive Na channels of different selectivity in human leukemic cells. General Physiology and Biophysics. 1997;16:163–173. [PubMed] [Google Scholar]
- Negulyaev YA, Vedernikova EA. Sodium-selective channels in membranes of rat macrophages. Journal of Membrane Biology. 1994;138:37–45. doi: 10.1007/BF00211067. [DOI] [PubMed] [Google Scholar]
- Negulyaev YA, Vedernikova EA, Maximov AV. Disruption of actin filaments increases the activity of sodium-conducting channels in human myeloid leukemia cells. Molecular Biology of the Cell. 1996;12:1857–1864. doi: 10.1091/mbc.7.12.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negulyaev YA, Vedernikova EA, Mozhayeva GN. Several types of sodium-conducting channel in human carcinoma A-431 cells. Biochimica et Biophysica Acta. 1994;1194:171–175. doi: 10.1016/0005-2736(94)90217-8. [DOI] [PubMed] [Google Scholar]
- Sachs F, Morris CE. Mechanosensitive ion channels in nonspecialized cells. Reviews of Physiology Biochemistry and Pharmacology. 1998;132:1–77. doi: 10.1007/BFb0004985. [DOI] [PubMed] [Google Scholar]
- Scharff O, Foder B. Regulation of cytosolic calcium in blood cells. Physiological Reviews. 1993;73:547–582. doi: 10.1152/physrev.1993.73.3.547. [DOI] [PubMed] [Google Scholar]
- Starushchenko AV, Mamin AG, Neguliaev IA, Vedernikova EA. Activation of mechanosensitive ion channels in the plasma membrane of K562 cells. (in Russian) Tsitologiia. 2000;42:669–674. [PubMed] [Google Scholar]
- Taglietti V, Toselli M. A study of stretch-activated channels in the membrane of frog oocytes: interactions with Ca2+ ions. Journal of Physiology. 1988;407:311–328. doi: 10.1113/jphysiol.1988.sp017417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoye CG, Reuss L. Stretch-activated single K+ channels account for whole-cell currents elicited by swelling. Proceedings of the National Academy of Sciences of the USA. 1999;96:6511–6516. doi: 10.1073/pnas.96.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- Yeh TH, Herman P, Tsai MC, Tran Ba Huy P, Van Den Abbeele T. A cationic nonselective stretch-activated channel in the Reissner's membrane of the guinea pig cochlea. American Journal of Physiology. 1998;274:C566–C576. doi: 10.1152/ajpcell.1998.274.3.C566. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hamill OP. Calcium-, voltage- and osmotic stress-sensitive currents in Xenopus oocytes and their relationship to single mechanically gated channels. Journal of Physiology. 2000;523:83–99. doi: 10.1111/j.1469-7793.2000.t01-2-00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


