Abstract
Different subtypes of voltage-dependent Ca2+ currents in native neurones are essential in coupling action potential firing to Ca2+ influx. For most of these currents, the underlying Ca2+ channel subunits have been identified on the basis of pharmacological and biophysical similarities. In contrast, the molecular basis of R-type Ca2+ currents remains controversial. We have therefore examined the contribution of the CaV2.3 (α1E) subunits to R-type currents in different types of central neurones using wild-type mice and mice in which the CaV2.3 subunit gene was deleted. In hippocampal CA1 pyramidal cells and dentate granule neurones, as well as neocortical neurones of wild-type mice, Ca2+ current components resistant to the combined application of ω-conotoxin GVIA and MVIIC, ω-agatoxin IVa and nifedipine (ICa,R) were detected that were composed of a large R-type and a smaller T-type component. In CaV2.3-deficient mice, ICa,R was considerably reduced in CA1 neurones (79 %) and cortical neurones (87 %), with less reduction occurring in dentate granule neurones (47 %). Analysis of tail currents revealed that the reduction of ICa,R is due to a selective reduction of the rapidly deactivating R-type current component in CA1 and cortical neurones. In all cell types, ICa,R was highly sensitive to Ni2+ (100 μM: 71–86 % block). A selective antagonist of cloned CaV2.3 channels, the spider toxin SNX-482, partially inhibited ICa,R at concentrations up to 300 nm in dentate granule cells and cortical neurones (50 and 57 % block, EC50 30 and 47 nm, respectively). ICa,R in CA1 neurones was significantly less sensitive to SNX-482 (27 % block, 300 nm SNX-482). Taken together, our results show clearly that CaV2.3 subunits underlie a significant fraction of ICa,R in different types of central neurones. They also indicate that CaV2.3 subunits may give rise to Ca2+ currents with differing pharmacological properties in native neurones.
Voltage-dependent Ca2+ channels are one of the main Ca2+ entry pathways into neuronal cells and are of central importance in coupling action potential firing to Ca2+ influx. Therefore, considerable interest has focused on the molecular structures underlying different types of Ca2+ currents in neurones. Most native neurones display several types of high-threshold Ca2+ currents, which can be pharmacologically classified as L-, N-, P/Q- and R-type (Ertel et al. 2000). In addition, most neurones express T-type Ca2+ channels with a low threshold of activation, a pronounced time-dependent inactivation and a slow deactivation (Cribbs et al. 1998; Lambert et al. 1998; Lee et al. 1999a). It is generally accepted that all of these Ca2+ channels are formed by one of a number of pore-forming α1 subunits α1A-I and α1S (Birnbaumer et al. 1994; Ertel et al. 2000), in addition to ancillary subunits (Hofmann et al. 1999). On the basis of pharmacological and biophysical similarities, α1A, α1B and α1C/D subunits are thought to underlie P/Q-, N- and L-type Ca2+ currents in native neurones, respectively (Hofmann et al. 1999), while the α1G-I subunits give rise to T-type Ca2+ currents (Perez-Reyes, 1999).
In contrast, the molecular basis of R-type currents in different types of native neurones is less clear. The R-type current was first described as a component that is resistant to the combined application of organic Ca2+ channel antagonists (ICa,R; Zhang et al. 1993; Randall & Tsien, 1995). Subsequently, R-type currents with diverse biophysical properties were described in different types of CNS neurones (Eliot & Johnston, 1994; Foehring et al. 2000; Magistretti et al. 2000; Scamps et al. 2000; Tottene et al. 2000). The prime candidate as a pore-forming subunit of R-type currents is the CaV2.3 (α1E) subunit. However, the identification of current components carried by the CaV2.3 subunit in different subtypes of native neurones has been hampered by the limited number of specific blockers targeting this subunit (Randall & Tsien, 1995). Indeed, the only selective blocker of CaV2.3 in expression systems, the spider toxin SNX-482, fails to block R-type currents in many native cells (Newcomb et al. 1998). Therefore, transgenic and antisense inhibition experiments have been employed to address the contribution of CaV2.3 to R-type currents in native neurones, with controversial results. The antisense inhibition of CaV2.3 subunit expression suggests that this subunit underlies the major portion of R-type currents in cultured cerebellar granule neurones (Tottene et al. 2000). However, functional analysis of cerebellar granule cell and dorsal root ganglion cell cultures prepared from mice in which the CaV2.3 gene has been deleted showed that only a minor portion of the R-type current results from expression of CaV2.3 (Wilson et al. 2000), whereas CaV2.3 subunits give rise to a large fraction of the R-type current in amygdala neurones (Lee et al. 2002). Similar divergent results have been obtained for the pharmacological properties of the putative CaV2.3-mediated current in these preparations (Tottene et al. 2000; Wilson et al. 2000).
In view of the controversy regarding the molecular identity of the R-type current, we have addressed this question in different CNS cell types using mice in which the CaV2.3 subunit has been deleted. We demonstrate that CaV2.3 subunits underlie a significant portion of the R-type current in hippocampal CA1 and dentate granule neurones, as well as in cortical neurones.
Methods
Gene targeting of CaV2.3 (α1E) by homologous recombination
All animal experiments were carried out according to the guidelines provided by the University of Cologne and the University of Bonn animal welfare committee. For the generation of the targeting construct, genomic clones containing exons 2 and 3 of the cacna1E gene of 129/Sv mice were obtained from a genomic library (lambda fix-II, Stratagene). A 14 kb genomic fragment was subcloned into Sal I digested pBluescript-SK vector and the HindIII-Sal I fragment containing exons 2 and 3 was completely sequenced. The loxP-flanked neomycin cassette was inserted into the Nsi I site between exons 2 and 3. The third loxP site was introduced downstream of the HindIII site by a PCR-based strategy amplifying a 421-bp-long DNA fragment between the HindIII site and the PflmI site. The proper amplification was confirmed by sequencing of the DNA fragment. The recombinant HindIII-PflmI fragment containing the third loxP site was then introduced into the targeting vector.
The gene locus of CaV2.3 was targeted by homologous recombination in E14.1 embryonic stem (ES) cells. Correctly targeted clones, as shown by Southern blot analysis, were transiently transfected with the pCre-pac vector. The efficiency of the expression of the Cre-recombinase was improved by imposing a 2 day treatment with puromycin as a selection marker (Taniguchi et al. 1998). Surviving clones were analysed for the loss of the Neo cassette and the presence of a loxP-flanked exon 2 (/ fl). ES cell subclone Tα1E1E8PuNr6 had such a type-II deletion and was injected into C57Bl/6 blastocysts. Resulting male chimeras were bred to C57Bl/6 females and the CaV2.3(fl/+) genotype of agouti-coloured offspring was determined by Southern blot analysis.
Disruption of cacna1E in vivo using Cre-deleter mice
The cacna1E gene was disrupted by deleting a region containing exon 2 in vivo on mating CaV2.3(fl/+) mice with deleter mice (Schwenk et al. 1995), which express Cre-recombinase constitutively under the control of a cytomegalovirus (CMV) promoter. After mating of CaV2.3(fl/+) and deleter mice, the offspring were genotyped by Southern blot analysis. In 20 out of 83 pups (24 %) exon 2 was deleted by Cre-mediated recombination. Heterozygous CaV2.3(+/-) mice were intercrossed to derive CaV2.3-deficient mice. Genotyping the offspring of such matings showed a Mendelian inheritance with 27 of 106 (29 %) of newborn pups being CaV2.3(-/-) mice, which leads to the conclusion that a general ablation of CaV2.3 is not embryonically lethal. The CaV2.3(-/-) mice displayed normal sexual activity and reproduction, and they exhibited no obvious anatomical abnormalities. Age-matched C57Bl/6 mice were used as control animals throughout the following experiments. Mice were housed under conditions of constant temperature (22-23 °C), with light from 7.00 a.m. to 7.00 p.m., and access to food and water ad libitum.
Isolation of microsomal membranes and immunoblotting
Brain microsomes were isolated according to standard procedures (Flockerzi et al. 1986), as described in detail by Pereverzev et al. (1998). Half of a brain was used to isolate microsomes without freezing the tissue. Aliquots of microsomal membranes were stored at −80 °C.
Preparation of acutely isolated cells
Isolated hippocampal or neocortical neurones were prepared as described previously (Beck et al. 1998). Experiments on CaV2.3(-/-) and (+/+) mice were interleaved. Mice were aged 2–15 months. For comparisons of CaV2.3(-/-) and (+/+) animals, mice were always age-matched. Animals were decapitated under deep ether anaesthesia and the brain rapidly removed and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (mm): NaCl 125, KCl 3, CaCl2 2, MgCl2 1, NaH2PO4 1.25, glucose 20 and NaHCO3 25 (pH 7.4, osmolarity 305 mosmol l−1, 95 % CO2-5 % O2). Hippocampal or neocortical slices (400 μm) were prepared with a vibratome and transferred to a storage chamber with ACSF where they were maintained at room temparature. After an equilibration period of at least 60 min, enzymatic digestion was carried out for 10 min at 37 °C and 5 min at room temperature in 5 ml of incubation medium containing (mm): sodium methanesulfonate 145, KCl 3, CaCl2 0.5, MgCl2 1, Hepes 10, glucose 15 and pronase (protease type XIV, Sigma) 2 mg ml−1 (pH 7.4, osmolarity 310 mosmol l−1, 100 % O2). After washing with enzyme-free incubation medium, the area of interest (CA1 region, dentate gyrus or neocortex) was dissected and triturated with fire-polished glass pipettes, and the cell suspension placed in a Petri dish. The isolated cells were superfused with an extracellular solution containing (mm): sodium methanesulfonate 125, TEA-Cl 20, 4-aminopyridine (4-AP) 4, BaCl2 5, MgCl2 1, KCl 3, glucose 10, Hepes 10, TTX 0.5 μM (pH 7.4, osmolarity 315 mosmol l−1). In the CA1 and the cortical preparation, dissociated neurones that displayed a pyramidal shape, a single well-defined apical dendrite and smaller basal dendrites were selected for recording. In the dentate gyrus, smaller neurones with an ovoid soma and a single apical dendrite were selected. The CaV2.3-selective antagonist SNX-482 was obtained from the Peptide Institute (Osaka, Japan). Other Ca2+ channel toxins were obtained from Bachem Biochemica (Heidelberg, Germany). All other chemicals were obtained from Sigma. Stock solutions of these toxins (0.2-1 mm) were prepared in deoxygenated solutions containing 0.1 % bovine serum albumin (BSA), 100 mm NaCl, 10 mm Trizma, 1 mm EDTA, pH 7.5 (HCl). Toxins were applied in extracellular solution containing 0.1 mg ml−1 cytochrome c to prevent unspecific binding to tubing. Nifedipine stock solutions were prepared in DMSO (10 mm). The different extracellular solutions were applied with a superfusion pipette placed at a distance of 30–50 μm from the cell body. The superfusion rate was adjusted by hydrostatic pressure.
Patch-clamp whole-cell recording
Patch pipettes with a resistance of 3–4 MΩ were fabricated from borosilicate glass capillaries and filled with an intracellular solution containing (mm): caesium methanesulfonate 87.5, TEA-Cl 20, CaCl2 0.5, MgCl2 5, BAPTA 5, Hepes 10, glucose 10, adenosine-5′-triphosphate (Na2-ATP) 10 and guanosine-5′-triphosphate (GTP) 0.5 (pH 7.2 NaOH, osmolarity adjusted to 300 mosmol l−1 with sucrose). Tight-seal whole-cell recordings were obtained at room temperature (21-24 °C) according to Hamill et al. (1981). Membrane currents were recorded using a patch-clamp amplifier (EPC9, HEKA Elektronik, Lambrecht/Pfalz, Germany) and collected online with the ‘TIDA for Windows’ acquisition and analysis program (HEKA Elektronik). Series resistance compensation was employed to improve the voltage-clamp control (70 %) so that the maximal residual voltage error did not exceed 1 mV (Sigworth et al. 1995). A liquid junction potential of 10 mV was measured between the intra- and extracellular solutions and corrected online so that without correction the voltages given in this paper would be 10 mV more positive. The holding potential for most recordings was −80 mV.
Data analysis
The voltage dependence of activation and inactivation was characterised using standard protocols. The conductance (G) was calculated according to:
| (1) |
The reversal potential VCa was estimated from the current-voltage (I-V) relationship, which was quite linear close to the reversal potential (values for wild-type: 27.4 ± 3.3 mV, 22.0 ± 2.9 mV and 29.1 ± 3.0 mV for CA1 neurones, dentate granule cells and neocortical neurones, respectively). The data points for the conductance G were fitted with a Boltzmann equation:
| (2) |
where Gmax is the maximum Ca2+ current, V1/2 is the voltage at which G is half of Gmax, and k indicates the slope of the relationship between channel activation/inactivation and membrane voltage. This procedure was used rather than utilising the Goldman-Hodgkin-Katz equation because of the linear behaviour of I-V curves close to the reversal potential, possibly due to some contamination by activation of an outward conductance at positive voltages. Thus, data points were best fitted by the procedure described herein. It should be noted that conductances obtained using eqn (1) do not reflect the open probability of the channel population, because this would assume a linear I-V relationship of the open channel. Nevertheless, they are suitable for comparing voltage-dependent activation between groups. Data points were normalised to the maximal conductance, averaged and plotted.
The time course of deactivation of Ca2+ currents resistant to organic Ca2+ channel antagonists (ICa,R) was determined following brief (5 ms) test pulses to 0 mV (Cota, 1986; Randall & Tsien, 1997). From these recordings, traces obtained following complete blockade of Ca2+ currents by application of 1 mm Ni2+ were subtracted. Time constants of deactivation (τ) were extracted from the resulting traces by fitting with a double exponential function:
| (3) |
where I(t) is the current amplitude at the time point t, A1 and A2 are the amplitudes of the first and second components, respectively, and A0 is a constant offset. The peak of the tail currents was taken as a starting point for fitting and the relative proportions of A0-A2 were determined at this time point.
Concentration-response curves were plotted at the logarithmic scale and fitted with a logarithmically transformed Hill equation of the form:
| (4) |
where c is the concentration of the used substance, Emax the maximal possible effect, EC50 the concentration at which a half-maximal effect was obtained and E the effect evoked by the concentration c. γ is the Hill coefficient.
The Levenberg-Marquardt least-squares algorithm was used for all fits. Statistical analysis for significance of differences between measured variables was carried out using Student's t test with the level of significance set to < 0.05. In the case of multiple data sets, one-way ANOVA was used. Data are presented as means ± s.e.m.
Results
Pharmacological isolation of Ca2+ currents resistant to organic Ca2+ channel blockers
R-type Ca2+ currents (ICa,R) are known to be present in different types of native CNS neurones, and are characterised by their resistance to organic Ca2+ channel antagonists (Randall & Tsien, 1995; Kavalali et al. 1997; Magnelli et al. 1998; Foehring et al. 2000). We analysed ICa,R in dissociated CA1 pyramidal cells, dentate granule cells as well as in neocortical neurones. After establishing the whole-cell configuration of the patch-clamp technique, ICa,R was isolated by pharmacologically blocking L-, P/Q- and N-type Ca2+ currents with saturating concentrations (Burley & Dolphin, 2000) of a combination of Ca2+ channel antagonists (ω-conotoxin (CgTx) GVIA (2 μM), ω-CgTx MVIIC (3 μM), ω-agatoxin (AgaTx) IVA (200 nm), nifedipine (10 μM); Fig. 1A and B). A full block of these Ca2+ channel components was allowed to develop for around 5 min (Fig. 1B). ICa,R constituted 38.2 ± 17.1 % (n = 8), 15.0 ± 5.0 % (n = 5) and 21.7 ± 7.4 % (n = 6) of the total Ba2+ current in hippocampal CA1 neurones, dentate granule cells and neocortical neurones, respectively (test pulses to 0 mV). ICa,R invariably showed a strong inactivation during the test pulse, consistent with the properties of R-type currents described previously (Foehring et al. 2000).
Figure 1. Pharmacological isolation of Ca2+ currents resistant to organic Ca2+ channel antagonists (ICa,R) by combined application of ω-conotoxin (CgTx) GVIA (2 μM), ω-CgTx MVIIC (3 μM), ω-agatoxin (AgaTx) IVa (200 nm) and nifedipine (10 μM) in wild-type mice.

A, Ba2+ currents were elicited with voltage jumps to 0 mV (200 ms) following a conditioning prepulse to −100 mV (2 s, see inset). The holding potential in this recording was −50 mV. The current trace following the saturation of the block is marked with an asterisk. B, time course of block of the peak Ba2+ current by combined application of the Ca2+ channel antagonists (horizontal bar). Care was always taken to ensure that the block was saturated before analysis of ICa,R current properties.
Contribution of CaV2.3 subunits to ICa,R in CA1 pyramidal neurones, dentate granule cells and neocortical neurones
To determine which portion of ICa,R is mediated by CaV2.3 subunits, the properties of Ba2+ currents in mice lacking expression of the CaV2.3 subunit (Fig. 2A) were examined. As shown in Fig. 2B, the amplitude of ICa,R elicited with voltage steps to −10 mV was markedly decreased in CaV2.3-deficient mice. In CA1 neurones, the current amplitude normalised to cell capacitance was 20.4 ± 3.9 pA pF−1 (n = 9) in wild-type and 4.3 ± 1.3 pA pF−1 (n = 10) in CaV2.3-deficient animals, corresponding to a 79 % decrease (Fig. 2B, left-most panels, Fig. 2Ca). In neocortical neurones, a more pronounced decrease of 87 % was noted from 25.1 ± 5.3 (n = 9) to 3.4 ± 0.9 pA pF−1 (n = 9; Fig. 2B, right-most panels, Fig. 2Ca). In contrast, ICa,R in dentate granule cells was less affected in CaV2.3-deficient mice (12.2 ± 2.3 pA pF−1, n = 12 in wild-type and 6.5 ± 1.6 pA pF−1 in CaV2.3-deficient mice, n = 7, 47 % decrease, Fig. 2B and Ca). Thus, ICa,R in CA1 and neocortical neurones was mediated mostly by CaV2.3 subunits, with a smaller contribution in dentate granule cells. We also assessed the amplitude of the compound current consisting of L-, N- and P/Q-type currents by quantifying the current component blocked by coapplication of ω-CgTx GVIA and MVIIC, ω-AgaTx IVA and nifedipine. In contrast to ICa,R, this current component was unaltered in CaV2.3-deficient mice (Fig. 2Cb).
Figure 2. ICa,R is considerably reduced in CaV2.3(-/-) mice.
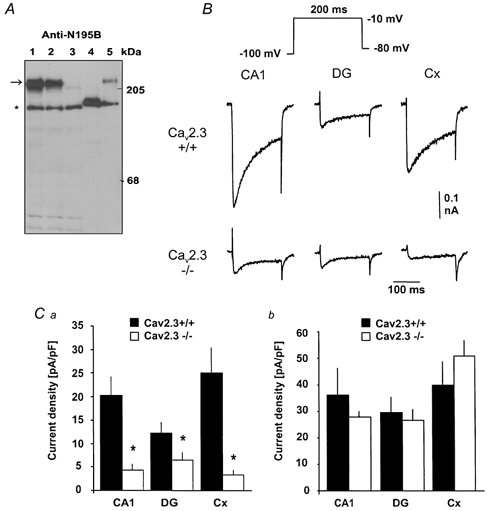
A, analysis of CaV2.3 protein levels using Western blot analysis of microsomal membrane proteins. Microsomes (24 μg) were isolated from the brains of wild-type mice (lane 1), from heterozygous litter mates (lane 2) and from CaV2.3 null mutants (lane 3). Microsomal membranes from untransfected (lane 4; 5 μg) and stably transfected with CaV2.3 human embryonic kidney (HEK)-293 cells (lane 5; 2 μg) were used as negative and positive controls, respectively. The primary antibody (Anti-N195B) is directed against an epitope of CaV2.3 common to all known CaV2.3 splice variants. A polypeptide of 246 kDa (CaV2.3) was detected in wild-type and heterozygous mice as well as in stably transfected HEK-293 cells (arrow). No staining was observed at this position in CaV2.3 null mutant mice or untransfected HEK-293 cells (lanes 3 and 4). Unspecific staining was detected as a faint band at 205 kDa and a major band (*), neither of which is related to CaV2.3 because they were also detected after preabsorption of the serum by the antigenic peptide (not shown). B, ICa,R in CA1 pyramidal neurones (CA1), dentate granule cells (DG) and neocortical neurones (Cx) in both wild-type (upper traces) and CaV2.3-deficient mice (lower traces). ICa,R was elicited with the voltage-step protocol shown in the inset. Calibration bars apply to all traces. Ca, the amplitude of ICa,R elicited with the voltage step to −10 mV was significantly (*P < 0.05) decreased in CaV2.3-deficient mice (open bars) compared to wild-type mice (filled bars) in all cell types studied. The amplitude of ICa,R elicited with command pulses to −10 mV was normalised to cell capacitance. Cb, normalised amplitude of the current component blocked by the combination of Ca2+ channel antagonists used to isolate ICa,R (see Fig. 1A). No difference between wild-type mice and mice lacking CaV2.3 subunits was detected.
Selective reduction of a rapidly deactivating, putative R-type Ca2+ current in CaV2.3-deficient mice
The somatodendritic ICa,R is composed of an R-type, as well as a smaller T-type component (Fisher et al. 1990). We have therefore addressed the question of whether only R-type or both of these components are reduced in CaV2.3-deficient mice. In order to discriminate R- and T-type components, we have made use of the fact that the deactivation time course of R-type current components is markedly faster than that of T-type currents (Randall & Tsien, 1997). We have therefore fitted the deactivation time course of ICa,R following brief (5 ms) depolarisations that activate both R- and T-type currents (Fig. 3A, inset) with a sum of two exponential functions. This protocol allowed the discrimination of a rapidly deactivating, putative R-type component from a slowly deactivating, putative T-type component (Cota, 1986; Randall & Tsien, 1997). At a potential of −60 mV, a faster time constant in the range of 300–400 μs and a slower time constant in the range of 6–10 ms could be observed (see Table 1 for values). The faster component (filled bars, Fig. 3B) comprised the main portion of tail current decay in wild-type mice (90.2 ± 2.3, 62.4 ± 9.7 and 85.8 ± 3.7 % for CA1 neurones, dentate granule cells and neocortical neurones, respectively, at −60 mV; compare Fig. 3Ba and Bb, n = 5 for all measurements; Thompson & Schwindt, 1991; Foehring et al. 2000). In mice lacking CaV2.3 subunits, the time constants of both components were not significantly changed. However, the amplitude contribution of the rapidly decaying, putative R-type component was markedly reduced (by 90.4 %, 76.6 % and 90.9 % for CA1 neurones, dentate granule cells and neocortical neurones, respectively; n = 5 for all measurements, P < 0.05, Fig. 3Ba). In contrast, the more slowly decaying, putative T-type component remained unaffected in CA1 and cortical neurones (Fig. 3Bb). Some reduction in this component was observed in dentate granule cells from CaV2.3 knockout mice (P < 0.05), but to a significantly lower degree than the reduction in the rapidly decaying component (P < 0.05, compare Fig. 3Ba and Bb). Taken together, these results suggest that genetic deletion of CaV2.3 subunits selectively affects R-type Ca2+ currents in cortical and CA1 neurones.
Figure 3. Selective reduction of a rapidly deactivating, putative R-type current in CaV2.3(-/-) mice.

A, deactivation kinetics were determined following a brief (5 ms) test pulse following a conditioning prepulse to −100 mV (see inset). The holding potential for these recordings was −80 mV. Recordings shown on a semilogarithmic scale were generated by subtracting traces obtained after complete blockade of inward currents with 1 mm Ni2+. The resulting Ba2+ current tails were fitted with a biexponential function shown as a dashed line superimposed on representative example traces. Traces are shown beginning from the peak amplitude. Averaged time constants are given in Table 1. B, the amplitude contributions of the fast- (Ba) and the slowly deactivating components (Bb) were derived from the fits, normalised to the cell capacitance and averaged in wild-type (filled bars) and CaV2.3-deficient mice (open bars). The rapidly decaying, putative R-type component was considerably reduced in CaV2.3-deficient mice (*P < 0.05).
Table 1.
Functional properties of Ca2+ Currents reslstant to organic Ca2+ channel antagonists (ICa,R) in hippocampal and neocortical cells in wild-type mice and CaV2.3(−/−)mice
| Parameter | CA1 | Dentate gyrus | Neocortex |
|---|---|---|---|
| CaV2.3(+/+)mice | |||
| V1/2,act (mV) | -13.3 ± 2.7(9) | -14.0 ± 1.8(11) | -13.7 ± 2.1(9) |
| k(mV) | 8.8 ± 0.5 | 8.4 ± 0.7 | 7.2 ± 0.2 |
| V1/2,inact(mV) | -70.3 ± 3.3(8) | -73.1 ± 1.7(11) | -67.8 ± 2.4(11) |
| k(mv) | 12.9 ± 0.6 | 14.8 ± 0.6 | 13.4 ± 0.4 |
| Deactivation(-60 mV) | |||
| Tfast(ms) | 0.297 ± 0.046(5) | 0.386 ± 0.073(5) | 0.422 ± 0.057(5) |
| Tslow(ms) | 6.34 ± 1.08 | 6.19 ± 0.72 | 13.64 ± 3.13 |
| CaV2.3(-/-)mice | |||
| V1/2,act(mv) | -25.6 ± 4.3(7) | -23.9 ± 1.5(7) | -20.4 ± 2.4(8) |
| k(mV) | 10.8 ± 1.5 | 9.6 ± 0.6 | 12.8 ± 1.3 |
| V1/2,inact(mV) | -63.0 ± 3.9(7) | -68.4 ± 2.6(6) | -57.6 ± 6.5(5) |
| k(mV) | 13.1 ± 2.0 | 15.5 ± 1.7 | 18.9 ± 2.9 |
V1/2,act voltage of half-maximal activation; V1/2,inact voltage of half-maximal inactivation; k, slope factor. Numbers in parentheses indicate number of neurones. Values are given as means ± s.e.m.
Voltage dependence of ICa,R in wild-type and CaV2.3(-/-) mice
Next, we analysed the voltage-dependent activation and inactivation behaviour of ICa,R in CA1 pyramidal cells, dentate granule cells, and neocortical cells in wild-type mice (see example traces in Fig. 4Aa and Ab) and mice lacking CaV2.3 subunits (Fig. 4Ba and Bb) using the voltage protocols shown in the insets. In wild-type mice, this analysis yielded similar voltages of half-maximal activation and inactivation for CA1 pyramidal cells (n = 8, Fig. 4C, filled squares), dentate granule neurones (n = 11, Fig. 4D, filled circles) and neocortical neurones (n = 11, Fig. 4E, filled triangles, see also Table 1). These values were in good agreement with the properties of cloned CaV2.3 channels, and R-type currents in native neurones (Ellinor et al. 1993; Randall & Tsien, 1997; Nakashima et al. 1998; Pereverzev et al. 1998; Foehring et al. 2000; Magistretti et al. 2000).
Figure 4. Voltage dependence of ICa,R in wild-type and CaV2.3-deficient mice.
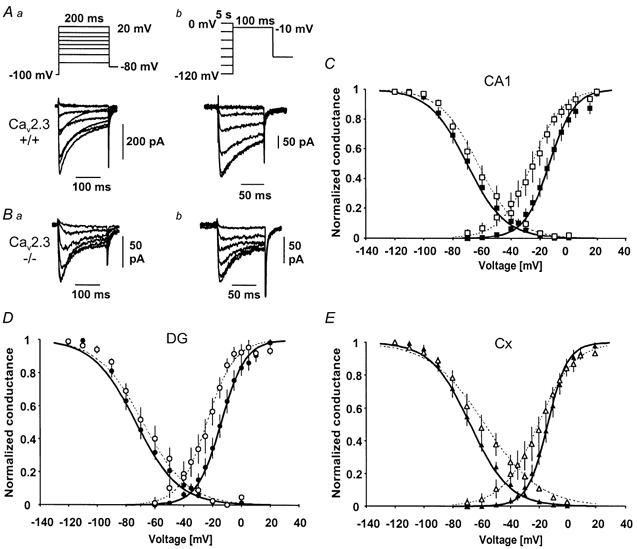
A, voltage dependence of activation (Aa) and inactivation (Ab) in a CA1 neurone from a wild-type mouse. The activation behaviour was analysed with voltage jumps to various command voltages (200 ms) following a conditioning prepulse to −100 mV (2 s, see inset). Inactivation was induced with a 5 s conditioning pulse to various voltages followed by a test pulse to −10 mV (50 ms, see inset). B, voltage dependence of activation (Ba) and inactivation (Bb) in a CA1 neurone from a CaV2.3-deficient mouse. C, D, E, voltage-dependent activation and inactivation behaviour for wild-type mice (filled symbols) and CaV2.3-deficient mice (open symbols) in CA1 neurones (C), dentate granule neurones (DG, D) and cortical neurones (Cx, E). Data points for the steady-state activation curve were obtained by calculating the conductance from peak Ba2+ current values (see Aa, Ba), and plotting normalised and averaged values vs. the command voltage. The inactivation curve was constructed from normalised and averaged peak conductances and plotted vs. the voltage of the conditioning pulse. Boltzmann functions were fitted to the data points using the Levenberg-Marquardt least-squares algorithm and are shown superimposed on the data points. The half-maximal activation and inactivation voltages averaged across individual experiments are given in Table 1.
In CaV2.3-deficient mice, a more hyperpolarised threshold of activation was observed in all cell types (Fig. 4C–E). Correspondingly, the voltage of half-maximal activation was significantly (P < 0.05) shifted in a hyperpolarising direction in CA1 neurones (n = 7, Fig. 4C, open squares) and dentate granule neurones (n = 7, Fig. 4D, open circles). In cortical neurones, the voltage of half-maximal activation (n = 8, Fig. 4E, open triangles, see also Table 1) was not significantly different compared to wild-type mice (P = 0.052). The inactivation curves did not differ in mice lacking CaV2.3 subunits and wild-type mice (Fig. 4C–E). The more hyperpolarised threshold of activation and voltage of half-maximal activation is consistent with a higher contribution of T-type Ca2+ currents to the compound current ICa,R in CaV2.3-deficient mice. Such a higher contribution would be expected as a consequence of a selective reduction of R-type Ca2+ currents (see Fig. 3). The lack of significant changes in the inactivation behaviour is most probably explained by rather similar properties of voltage-dependent inactivation of R-type/CaV2.3 and T-type currents (Randall & Tsien, 1997; Klöckner et al. 1999), especially if CaV2.3 subunits are coexpressed with accessory subunits (Stephens et al. 1997).
Pharmacological properties of ICa,R
Recombinant CaV2.3 channels show distinctive pharmacological properties. For instance, CaV2.3-mediated Ca2+ currents in expression systems were shown to be sensitive to nanomolar concentrations of the spider toxin SNX-482 (Tottene et al. 2000; Vajna et al. 2001). Application of various concentrations of SNX-482 in wild-type mice (10, 30, 100 and 300 nm, Fig. 5A) led to a pronounced concentration-dependent reduction of ICa,R in cortical neurones and dentate granule neurones (block of 56.9 ± 9.4 %, n = 4 and 50.0 ± 7.0 %, n = 4, respectively, 300 nm SNX-482). The data points were fitted with the logarithmically transformed Hill equation (eqn (4), see Methods, EC50 30 and 47 nm, maximal block Emax 65.5 and 53.1 %, Hill coefficient 1.09 and 0.93 for cortical neurones and dentate granule neurones, respectively). The ICa,R in CA1 neurones proved to be significantly less sensitive to SNX-482 (block of 27.9 ± 1.9 %, n = 7, 300 nm SNX-482), as suggested previously (Newcomb et al. 1998). Pronounced block of ICa,R was only observed at very high concentrations of SNX-482 (1 μM), which are thought to exert unspecific effects (Neelands et al. 2000; Bourinet et al. 2001) and did not saturate in this concentration range. For this reason, the values obtained for CA1 neurones with fitting as above were considered unreliable.
Figure 5. Pharmacological properties of ICa,R.

A, time course of a representative experiment during application of 10–300 nm SNX-482 (Aa, see bars for duration of application). The amplitude of ICa,R in a cortical neurone from a wild-type mouse is depicted over time. Current traces elicited at the time points indicated by the lower-case letters are shown in panel Ab. The voltage step is shown in the inset and was applied following a 2 s hyperpolarising prepulse to −100 mV. The holding potential in all pharmacological experiments was −50 mV. B, concentration dependence of ICa,R block by SNX-482 in CA1 neurones (squares), dentate granule cells (circles) and cortical neurones (triangles) in wild-type mice. Data points were fitted with the logarithmically transformed Hill equation (eqn (4), see Methods) and the fitted curves are superimposed on the data points (dashed curve: cortex). The fitted curve for CA1 neurones is given for illustrative purposes only. Note that block by SNX-482 does not seem to saturate completely during some applications, either due to a slow onset of the block, or to some degree of unavoidable rundown. C, fraction of Ba2+ current blocked by application of 100 μM Ni2+ in the different neurone types from wild-type mice.
In mice lacking CaV2.3 channels, it proved difficult to examine the pharmacology of ICa,R due to the small amplitude of this residual current. We did succeed in obtaining a limited number of recordings in which the effects of SNX-482 on ICa,R could be assessed in dentate granule neurones (1 μM SNX-482, n = 2) and CA1 neurones (1 μM, n = 2; 300 nm SNX-482, n = 5). The portion of ICa,R blocked by SNX-482 was markedly reduced in CA1 neurones from CaV2.3(-/-) mice (300 nm: from 2.1 ± 0.2 to 0.7 ± 0.1 pA pF−1, P < 0.05; 1 μM: 4.7 ± 0.8 to 0.4 pA pF−1). Likewise, in dentate granule cells, the SNX-482-sensitive component amounted to 3.3 ± 0.8 pA pF−1 (300 nm), while only 1.2 pA pF−1 was blocked in CaV2.3(-/-) mice, even at a blocker concentration of 1 μM. Even though these results show that the SNX-482-sensitive portion of ICa,R is reduced in mice lacking CaV2.3, they also suggest effects of SNX-482 on channels other than CaV2.3. Indeed, we found that application of 300 nm SNX-482 onto CA1 neurones from mice lacking CaV2.3 subunits results in a modest but significant block of the total whole-cell Ca2+ current (16.1 ± 4.9 % block, n = 4). These effects were not further analysed, but demonstrate that SNX-482 does not exclusively block CaV2.3 currents at these concentrations, as suggested previously by other studies (Neelands et al. 2000; Bourinet et al. 2001).
CaV2.3 Ca2+ channels are also highly sensitive to the divalent cation Ni2+ (Ellinor et al. 1993), even though Ni2+ is not specific for these channels (Zhang et al. 1993; Soong et al. 1993; Pereverzev et al. 1998). In wild-type mice, Ni2+ (100 μM) potently inhibited ICa,R in all cell types under investigation (CA1 neurones: 73.0 ± 1.1 %, n = 5; neocortical cells: 86.0 ± 3.2 %, n = 6; and dentate granule cells: 71.4 ± 7.4 %, n = 5, Fig. 5B). In CaV2.3-deficient mice, ICa,R also proved to be sensitive to 100 μM Ni2+ in all cell types under investigation (CA1 neurones: 62.3 ± 4.9 % block, n = 5, neocortical cells: 74.0 ± 0.8 % block, n = 4; and dentate granule cells: 89.0 ± 11.1 % block, n = 4), possibly due to the Ni2+ sensitivity of some T-type channel subunits (Lee et al. 1999b).
Discussion
Voltage-dependent Ca2+ channels constitute a major link between neuronal excitability and intracellular Ca2+. Therefore, the question of which Ca2+ channel subunits underlie these currents in native neurones has been a subject of intensive research. For some Ca2+ channel α1 subunits, specific antagonists have permitted the dissection of their contribution to Ca2+ currents in native cells. In the case of R- and T-type Ca2+ currents, the lack of specific antagonists has necessitated the use of antisense (Piedras-Renteria et al. 1997; Lambert et al. 1998; Piedras-Renteria & Tsien, 1998; Tottene et al. 2000) or transgenic strategies (Wilson et al. 2000; Lee et al. 2002) to ablate the expression of specific α1 subunits. R-type currents resistant to organic Ca2+ channel antagonists (ICa,R) can be found in most neurones, such as neocortical and striatal neurones (Foehring et al. 2000), CA1 neurones (Ishibashi et al. 1995), dentate granule cells (Eliot & Johnston, 1994; Beck et al. 1998) and cerebellar granule neurones (Forti et al. 1994; Tottene et al. 1996, 2000; Schramm et al. 1999). They have also been recorded from the insulinoma cell line INS-1 and show a moderate sensitivity towards SNX-482 (Vajna et al. 2001). Nevertheless, the molecular basis of these currents has remained unknown. In the present study, knock-out mice lacking the CaV2.3 Ca2+ channel subunit were used to investigate whether this subunit underlies R-type Ca2+ currents in different types of central neurones known to express CaV2.3 mRNA (Soong et al. 1993; Hendriksen et al. 1997; Foehring et al. 2000). Neocortical and CA1 pyramidal neurones, as well as hippocampal dentate granule cells were analysed, all of which expressed Ca2+ currents resistant to organic Ca2+ channel antagonists, ICa,R, with properties reminiscent of cloned CaV2.3 subunits (see also Foehring et al. 2000). Experiments in mice lacking CaV2.3 subunits show unequivocally that this subunit underlies the major portion of ICa,R in CA1 and neocortical neurones (≈79 and 87 %), and a smaller fraction of this current in dentate granule neurones (≈47 %). Furthermore, our analyses of tail currents show a specific reduction of a rapidly deactivating, putative R-type current in mice lacking CaV2.3 subunits, with no (in CA1, cortical cells) or significantly less (in dentate granule cells) effects on a small, slowly deactivating putative T-type current component (Klöckner et al. 1999). The selective reduction of R-type currents in CaV2.3 mice would be expected to cause an increased contribution of T-type currents to ICa,R in these animals, consistent with the more hyperpolarised voltage-dependent activation curve we observed in CaV2.3(-/-) mice.
Molecular basis of ICa,R in other cell types
Hitherto, two studies have addressed the question of the molecular basis of R-type currents using either antisense knockdown (Tottene et al. 2000) or transgenic techniques (Wilson et al. 2000). The former study demonstrated, similar to our study, that CaV2.3 subunits underlie a major portion of R-type currents in cerebellar granule cell cultures (Tottene et al. 2000). This is unlike findings in cerebellar granule cell and dorsal root ganglion cell cultures from mice lacking the CaV2.3 subunit, in which only a minor portion of the R-type current seems to be due to the expression of CaV2.3 (Wilson et al. 2000). There may be several reasons for these discrepancies. For instance, in the pharmacological isolation used by Wilson et al. the concentration of ω-agatoxin IVA used (200 nm) may have been insufficient for a complete block of Q-type components (half-blocking concentration ≈90 nm in cerebellar granule cells, see Randall & Tsien, 1995), resulting in a contribution of such currents to the putative R-type current. In our study, as well as in the study by Tottene et al. addition of ω-conotoxin MVIIC most probably allowed us to avoid contamination of R-type currents by Q-type components. Alternatively, it may be possible that either T-type channel subunits (especially in dorsal root ganglion neurones, see Lovinger & White, 1989; Hilaire et al. 1997), or as yet uncloned subunits, giving rise to blocker-resistant currents may underlie part of the putative R-type current under the specific culture conditions chosen by Wilson et al. (2000). Nevertheless, these contrasting observations may also reflect a molecular diversity of R-type currents in the central nervous system.
Pharmacological properties of ICa,R in central neurones
The specific pharmacological properties common to most of the isoforms of CaV2.3 studied so far include a high sensitivity to Ni2+ blockade (Soong et al. 1993), but this substance may also block other Ca2+ channels at relatively low concentrations (Zhang et al. 1993; Schneider et al. 1994; Williams et al. 1994). To date, the peptide neurotoxin SNX-482 is thought to be the only selective, high-affinity antagonist targeting CaV2.3 channels (Newcomb et al. 1998; Tottene et al. 2000). On the basis of their sensitivity to the spider toxin SNX-482, three components of the R-type current were distinguished in cultured cerebellar granule cells (Tottene et al. 2000). Two of these components are completely blocked by 100 nm SNX-482. A third component was only blocked at very high concentrations of SNX-482, which also affect other current components (Newcomb et al. 1998; Tottene et al. 2000).
Our results also suggest that CaV2.3 subunits give rise to both SNX-482-sensitive and SNX-482-resistant components of ICa,R. In CA1 neurones, CaV2.3 subunits underlie 80–90 % of ICa,R, but only around 50 % of this current is blocked by high (300 nm) concentrations of SNX-482, suggesting that the remainder of the CaV2.3-mediated current corresponds to the relatively SNX-482 resistant current component observed in cerebellar granule neurones (Tottene et al. 2000). In dentate granule neurones, CaV2.3 subunits underlie only part (≈50 %) of ICa,R. A large part of this component appeared to be blocked by 300 nm SNX-482. Thus, our results suggest both that CaV2.3 subunits may give rise to Ca2+ currents with differing SNX-482 sensitivity, and that the relative contribution of these components may vary from cell type to cell type.
Possible molecular basis for the diversity of CaV2.3-mediated Ca2+ currents in native neurones
Taken together, the results of the present study and other published data suggest that the biophysical and pharmacological properties of R-type currents in native neurones are diverse, even in those cases where antisense or transgenic approaches have confirmed the molecular identity of the channel protein. One explanation for this variation could be the expression of different CaV2.3 splice variants in different neurone types. At least six different splice forms, varying in the II-III loop and the C-terminus, can be deduced from RT-PCR studies (Marubio et al. 1996; Vajna et al. 1998; Schramm et al. 1999). Some of these show a differential expression pattern during development and in the mature brain (Pereverzev et al. 1998; Schramm et al. 1999). However, the biophysical and pharmacological properties of these C-terminal splice variants are quite similar (Pereverzev et al. 1998; Schramm et al. 1999), and in particular both variants are sensitive to SNX-482 when expressed in human embryonic kidney (HEK)-293 cells. It has been suggested that the binding site for the peptide toxin could be occluded by an auxiliary subunit (Newcomb et al. 1998), but coexpression of the human α1Ed subunit with two auxiliary subunits, human β3 or α2δ2, did not influence inward currents in HEK-293 cells (Vajna et al. 2001).
In summary, our study shows unequivocally that a large part of the R-type current in the central neurone types we have investigated is carried by CaV2.3 subunits. Taken together with published data on CaV2.3-mediated currents in native cells (Tottene et al. 2000), our results indicate that CaV2.3 subunits may underlie R-type currents with different functional properties. In view of the importance of the CaV2.3 subunit in pain responses and spatial memory (Saegusa et al. 2000; Kubota et al. 2001), future studies should address the molecular basis for this intriguing functional diversity.
Acknowledgments
This research was supported by a University of Bonn Medical Center grant ‘BONFOR’ (H.B.), the German-Israel collaborative research program of the MOS and the BMBF (H.B.), a grant from the Deutsche Forschungsgemeinschaft (DFG EL 122/7) and the Center of Molecular Medicine Cologne/Zentrum für Molekularbiologische Medizin Köln (BMBF 01 KS 9502 to T.S.). We are grateful to Professor Klaus Rajewsky and Drs Werner Müller and Ralph Kühn for their helpful gifts of reagents. We thank Professor M. Taniguchi for the pCre-pac vector. We thank, Dr Xio-hua Chen and Dr George Miljanich for their support, Dr Wolfgang Nastainczyk (Universität des Saarlandes, Homburg) for the synthesis of peptides and antibodies, and Professor Y. Yaari for a critical revision of the manuscript.
References
- Beck H, Steffens R, Heinemann U, Elger CE. Properties of voltage-activated calcium currents in acutely dissociated human hippocampal granule cells. Journal of Neurophysiology. 1998;77:1526–1537. doi: 10.1152/jn.1997.77.3.1526. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Campbell KP, Catterall WA, Harpold MM, Hofmann F, Horne WA, Mori Y, SchwartZ A, Snutch TP, Tanabe T, et al. The naming of voltage-gated calcium channels. Neuron. 1994;13:505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Bourinet E, StotZ SC, Spaetgens RL, Dayanithi G, Lemos J, Nargeot J, Zamponi GW. Interaction of SNX482 with domains III and IV inhibits activation gating of alpha1E (CaV2. 3). calcium channels. Biophysical Journal. 2001;81:79–88. doi: 10.1016/S0006-3495(01)75681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley JR, Dolphin AC. Overlapping selectivity of neurotoxin and dihydropyridine calcium channel blockers in cerebellar granule neurones. Neuropharmacology. 2000;39:1740–1755. doi: 10.1016/s0028-3908(99)00266-x. [DOI] [PubMed] [Google Scholar]
- Cota G. Calcium channel currents in pars intermedia cells of the rat pituitary gland. Kinetic properties and washout during intracellular dialysis. Journal of General Physiology. 1986;88:83–105. doi: 10.1085/jgp.88.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, PereZ-Reyes E. Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circulation Research. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- Eliot LS, Johnston D. Multiple components of calcium current in acutely dissociated dentate gyrus granule neurones. Journal of Neurophysiology. 1994;72:762–777. doi: 10.1152/jn.1994.72.2.762. [DOI] [PubMed] [Google Scholar]
- Ellinor PT, Zhang J-F, Randall AD, Zhou M, SchwarZ TL, Tsien RW, Horne WA. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993;363:455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, PereZ-Reyes E, SchwartZ A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- Fisher RE, Gray R, Johnston D. Properties and distribution of single voltage-gated calcium channels in adult hippocampal neurons. Journal of Neurophysiology. 1990;64:91–104. doi: 10.1152/jn.1990.64.1.91. [DOI] [PubMed] [Google Scholar]
- Flockerzi V, Oeken HJ, Hofmann F. Purification of a functional receptor for calcium-channel blockers from rabbit skeletal-muscle microsomes. European Journal of Biochemistry. 1986;161:217–224. doi: 10.1111/j.1432-1033.1986.tb10145.x. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Mermelstein PG, Song WJ, Ulrich S, Surmeier DJ. Unique properties of R-type calcium currents in neocortical and neostriatal neurons. Journal of Neurophysiology. 2000;84:2225–2236. doi: 10.1152/jn.2000.84.5.2225. [DOI] [PubMed] [Google Scholar]
- Forti L, Tottene A, Moretti A, Pietrobon D. Three novel types of voltage-dependent calcium channels in rat cerebellar neurons. Journal of Neuroscience. 1994;14:5243–5256. doi: 10.1523/JNEUROSCI.14-09-05243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hendriksen H, Kamphuis W, Lopes Da Silva FH. Changes in voltage-dependent calcium channel alpha1-subunit mRNA levels in the kindling model of epileptogenesis. Brain Research. Molecular Brain Research. 1997;50:257–266. doi: 10.1016/s0169-328x(97)00196-4. [DOI] [PubMed] [Google Scholar]
- Hilaire C, Diochot S, Desmadryl G, Richard S, Valmier J. Toxin-resistant calcium currents in embryonic mouse sensory neurons. Neuroscience. 1997;80:267–276. doi: 10.1016/s0306-4522(97)00101-2. [DOI] [PubMed] [Google Scholar]
- Hofmann F, Lacinova L, Klugbauer N. Voltage-dependent calcium channels: from structure to function. Reviews of Physiology, Biochemistry and Pharmacology. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Rhee JS, Akaike A. Regional difference of high voltage-activated Ca2+ channels in rat CNS neurones. NeuroReport. 1995;6:1621–1624. doi: 10.1097/00001756-199508000-00008. [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Zhuo M, Bito H, Tsien RW. Dendritic Ca2+ channels characterized by recordings from isolated hippocampal dendritic segments. Neuron. 1997;18:651–663. doi: 10.1016/s0896-6273(00)80305-0. [DOI] [PubMed] [Google Scholar]
- Klöckner U, Lee JH, Cribbs LL, Daud A, Hescheler J, Pereverzev A, PereZ-Reyes E, Schneider T. Comparison of the Ca2+ currents induced by expression of three cloned alpha1 subunits, alpha1G, alpha1H and alpha1I, of low-voltage-activated T-type Ca2+ channels. European Journal of Neuroscience. 1999;11:4171–4178. doi: 10.1046/j.1460-9568.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- Kubota M, Murakoshi T, Saegusa H, Kazuno A, Zong S, Hu Q, Noda T, Tanabe T. Intact LTP and fear memory but impaired spatial memory in mice lacking CaV2. 3 (alpha1E). channel. Biochemical and Biophysical Research Communications. 2001;282:242–248. doi: 10.1006/bbrc.2001.4572. [DOI] [PubMed] [Google Scholar]
- Lambert RC, McKenna F, Maulet Y, Talley EM, Bayliss DA, Cribbs LL, Lee JH, PereZ-Reyes E, FeltZ A. Low-voltage-activated Ca2+ currents are generated by members of the CaVT subunit family (α1G/H). in rat primary sensory neurons. Journal of Neuroscience. 1998;18:8605–8613. doi: 10.1523/JNEUROSCI.18-21-08605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klöckner U, Schneider T, PereZ-Reyes E. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. Journal of Neuroscience. 1999a;19:1912–1921. doi: 10.1523/JNEUROSCI.19-06-01912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Gomora JC, Cribbs LL, PereZ-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block alpha1H. Biophysical Journal. 1999b;77:3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Choi S, Lee T, Kim HL, Chin H, Shin HS. Molecular basis of R-type calcium channels in central amygdala neurons of the mouse. Proceedings of the National Academy of Sciences of the USA. 2002;99:3276–3281. doi: 10.1073/pnas.052697799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G. Post-natal development of burst firing behavior and the low-threshold transient calcium current examined using freshly isolated neurons from rat dorsal root ganglia. Neuroscience Letters. 1989;102:50–57. doi: 10.1016/0304-3940(89)90306-6. [DOI] [PubMed] [Google Scholar]
- Magistretti J, Brevi S, De Curtis M. A blocker-resistant, fast-decaying, intermediate-threshold calcium current in palaeocortical pyramidal neurons. European Journal of Neuroscience. 2000;12:2376–2386. doi: 10.1046/j.1460-9568.2000.00125.x. [DOI] [PubMed] [Google Scholar]
- Magnelli V, Baldelli P, Carbone E. Antagonist-resistant calcium currents in rat embryo motoneurons. European Journal of Neuroscience. 1998;10:1810–1825. doi: 10.1046/j.1460-9568.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- Marubio LM, Roenfeld M, Dasgupta S, Miller RJ, Philipson LH. Isoform expression of the voltage-dependent calcium channel alpha1E. Receptors and Channels. 1996;4:243–251. [PubMed] [Google Scholar]
- Nakashima YM, Todorovic SM, Pereverzev A, Hescheler J, Schneider T, Lingle CJ. Properties of Ba2+ currents arising from human alpha1E and alpha1E-beta3 constructs expressed in HEK293 cells: physiology, pharmacology, and comparison to native T-type Ba2+ currents. Neuropharmacology. 1998;37:957–972. doi: 10.1016/s0028-3908(98)00097-5. [DOI] [PubMed] [Google Scholar]
- Neelands TR, King AP, Macdonald RL. Functional expression of L-, N-, P/Q-, and R-type calcium channels in the human NT2-N cell line. Journal of Neurophysiology. 2000;84:2933–2944. doi: 10.1152/jn.2000.84.6.2933. [DOI] [PubMed] [Google Scholar]
- Newcomb R, Szoke B, Palma A, Wang G, Chen X, Hopkins W, Cong R, Miller J, Urge L, Tarczy-Hornoch K, Loo JA, Dooley DJ, Nadasdi L, Tsien RW, Lemos J, Miljanich G. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- Pereverzev A, Klöckner U, Grabsch H, Vajna R, Olyschläger S, Viatchenko-Karpinski S, Schröder R, Hescheler J, Schneider T. Structural diversity of the voltage-dependent Ca2+ channel alpha1E subunit. European Journal of Neuroscience. 1998;10:916–925. doi: 10.1046/j.1460-9568.1998.00099.x. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Three for T: molecular analysis of the low voltage-activated calcium channel family. Cellular and Molecular Life Sciences. 1999;56:660–669. doi: 10.1007/s000180050460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedras-Renteria ES, Chen CC, Best PM. Antisense oligonucleotides against rat brain alpha1E DNA and its atrial homologue decrease T-type calcium current in atrial myocytes. Proceedings of the National Academy of Sciences of the USA. 1997;94:14936–14941. doi: 10.1073/pnas.94.26.14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedras-Renteria ES, Tsien RW. Antisense oligonucleotides against alpha1E reduce R-type calcium currents in cerebellar granule cells. Proceedings of the National Academy of Sciences of the USA. 1998;95:7760–7765. doi: 10.1073/pnas.95.13.7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall A, Tsien RW. Pharmacological dissection of multiple types of calcium channel currents in rat cerebellar granule neurons. Journal of Neuroscience. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall AD, Tsien RW. Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology. 1997;36:879–893. doi: 10.1016/s0028-3908(97)00086-5. [DOI] [PubMed] [Google Scholar]
- Saegusa H, Kurihara T, Zong S, Minowa O, Kazuno A, Han W, Matsuda Y, Yamanaka H, Osanai M, Noda T, Tanabe T. Altered pain responses in mice lacking alpha 1E subunit of the voltage-dependent Ca2+ channel. Proceedings of the National Academy of Sciences of the USA. 2000;97:6132–6137. doi: 10.1073/pnas.100124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scamps F, Vigues S, Restituito S, Campo B, Roig A, Charnet P, Valmier J. Sarco-endoplasmic ATPase blocker 2,5-di(tert-butyl)-1,4-benzohydroquinone inhibits N-, P-, and Q- but not T-, L-, or R-type calcium currents in central and peripheral neurons. Molecular Pharmacology. 2000;58:18–26. doi: 10.1124/mol.58.1.18. [DOI] [PubMed] [Google Scholar]
- Schneider T, Wei X, Olcese R, Costantin JL, Neely A, Palade P, PereZ-Reyes E, Qin N, Zhou J, Crawford GD, et al. Molecular analysis and functional expression of the human type E neuronal Ca2+ channel alpha 1 subunit. Receptors and Channels. 1994;2:255–270. [PubMed] [Google Scholar]
- Schramm M, Vajna R, Pereverzev A, Tottene A, Klöckner U, Pietrobon D, Hescheler J, Schneider T. Isoforms of alpha1E voltage-gated calcium channels in rat cerebellar granule cells – detection of major calcium channel alpha1 transcripts by reverse transcription-polymerase chain reaction. Neuroscience. 1999;92:565–575. doi: 10.1016/s0306-4522(99)00013-5. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Research. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth FJ, Affolter H, Neher E. Design of the EPC-9, a computer-controlled patch-clamp amplifier. 2. Software. Journal of Neuroscience Methods. 1995;56:203–215. doi: 10.1016/0165-0270(94)00129-5. [DOI] [PubMed] [Google Scholar]
- Soong TW, Stea A, Hodson CD, Dubel SJ, Vincent SR, Snutch TP. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- Stephens GJ, Page KM, Burley JR, Berrow NS, Dolphin AC. Functional expression of rat brain cloned alpha1E calcium channels in COS-7 cells. Pflügers Archiv. 1997;433:523–532. doi: 10.1007/s004240050308. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Sanbo M, Watanabe S, Naruse I, Mishina M, Yagi T. Efficient production of Cre-mediated site-directed recombinants through the utilization of the puromycin resistance gene, pac: a transient gene-integration marker for ES cells. Nucleic Acids Research. 1998;26:679–680. doi: 10.1093/nar/26.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Schwindt W. Development of calcium current subtypes in isolated rat hippocampal pyramidal cells. Journal of Physiology. 1991;439:671–689. doi: 10.1113/jphysiol.1991.sp018687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottene A, Moretti A, Pietrobon D. Functional diversity of P-type and R-type calcium channels in rat cerebellar neurons. Journal of Neuroscience. 1996;16:6353–6363. doi: 10.1523/JNEUROSCI.16-20-06353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottene A, Volsen S, Pietrobon D. Alpha(1E) subunits form the pore of three cerebellar R-type calcium channels with different pharmacological and permeation properties. Journal of Neuroscience. 2000;20:171–178. doi: 10.1523/JNEUROSCI.20-01-00171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajna R, Klöckner U, Pereverzev A, Weiergraber M, Chen X, Miljanich G, Klugbauer N, Hescheler J, PereZ-Reyes E, Schneider T. Functional coupling between ‘R-type’ Ca2+ channels and insulin secretion in the insulinoma cell line INS-1. European Journal of Biochemistry. 2001;268:1066–1075. doi: 10.1046/j.1432-1327.2001.01969.x. [DOI] [PubMed] [Google Scholar]
- Vajna R, Schramm M, Pereverzev A, Arnhold S, Grabsch H, Klöckner U, PereZ-Reyes E, Hescheler J, Schneider T. New isoform of the neuronal Ca2+ channel alpha1E subunit in islets of Langerhans and kidney – distribution of voltage-gated Ca2+ channel alpha1 subunits in cell lines and tissues. European Journal of Biochemistry. 1998;257:274–285. doi: 10.1046/j.1432-1327.1998.2570274.x. [DOI] [PubMed] [Google Scholar]
- Williams ME, Marubio LM, Deal CR, Hans M, Brust PF, Philipson LH, Miller RJ, Johnson EC, Harpold MM, Ellis SB. Structure and functional characterization of neuronal alpha 1E calcium channel subtypes. Journal of Biological Chemistry. 1994;269:22347–22357. [PubMed] [Google Scholar]
- Wilson SM, Toth PT, Oh SB, Gillard SE, Volsen S, Ren D, Philipson LH, Lee EC, Fletcher CF, Tessarollo L, Copeland NG, Jenkins NA, Miller RJ. The status of voltage-dependent calcium channels in alpha 1E knock-out mice. Journal of Neuroscience. 2000;20:8566–8571. doi: 10.1523/JNEUROSCI.20-23-08566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-F, Randall AD, Ellinor PT, Horne WA, Sather W, Tanabe T, SchwarZ TL, Tsien RW. Distinctive pharmacology and kinetics of cloned neuronal calcium channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]


