Abstract
A circadian (24-hour) clock regulates the light responses of fish cone horizontal cells, second order neurones in the retina that receive synaptic contact from cones and not from rods. Due to the action of the clock, cone horizontal cells are driven by cones in the day, but primarily driven by rods at night. We show here that dopamine, a retinal neurotransmitter, acts as a clock signal for the day by increasing cone input and decreasing rod input to cone horizontal cells. The amount of endogenous dopamine released from in vitro retinae was greater during the subjective day than the subjective night. Application of dopamine or quinpirole, a dopamine D2-like agonist, during the subjective night increased cone input and eliminated rod input to the cells, a state usually observed during the subjective day. In contrast, application of spiperone, a D2-like antagonist, or forskolin, an activator of adenylyl cyclase, during the subjective day reduced cone input and increased rod input. SCH23390, a D1 antagonist, had no effect. Application of Rp-cAMPS, an inhibitor of cAMP-dependent protein kinase, or octanol, an alcohol that uncouples gap junctions, during the night increased cone input and decreased rod input. Because D2-like receptors are on photoreceptor cells, but not horizontal cells, the results suggest that the clock-induced increase in dopamine release during the day activates D2-like receptors on photoreceptor cells. The resultant decrease in intracellular cyclic AMP and protein kinase A activation then mediates the increase in cone input and decrease in rod input.
Dopamine is the principal catecholamine neurotransmitter in the CNS, where it modulates a variety of functions, including locomotion, cognition, emotion, neuroendocrine regulation, and positive reinforcement. Dopamine receptors are G-protein-coupled receptors that have been classified into two general groups, D1 and D2, on the basis of their effects on cyclic AMP (cAMP) production (Missale et al. 1998). Activation of D1 receptors increases cAMP production, whereas activation of D2 receptors decreases it.
Both D1 and D2 receptors are present in the vertebrate retina (Schorderet & Nowak, 1990). In the retinae of teleost fish and New World monkeys, the dopaminergic cells are interplexiform cells, a cell type that receives synaptic input from amacrine and bipolar cells in the inner plexiform layer and makes synaptic contacts onto horizontal and bipolar cells in the outer plexiform layer (Dowling & Ehinger, 1978; Yazulla & Zucker, 1988). Dopamine, by activating D1 and D2 receptors in the retina, affects a variety of phenomena, including gap junctional permeability (Harsanyi & Mangel, 1992; Hampson et al. 1992), retinomotor movements (Pierce & Besharse, 1985; Dearry & Burnside, 1986), glutamate-gated conductances (Knapp & Dowling, 1987), and light adaptation (Witkovsky et al. 1988a). The effects of dopamine on retinal neurones are mediated by second messengers, such as cAMP, and not by directly opening and closing ion channels. The widespread distribution of dopamine receptors throughout the retina, at synapses and also at a distance from the terminals of dopaminergic neurons, as well as direct measurements of dopamine efflux from isolated retinae, suggest that dopamine acts both synaptically as a neurotransmitter and by volume transmission as a paracrine neuromodulator (Besharse et al. 1988; Witkovsky et al. 1993; Puopolo et al. 2001).
Dopamine content and release in the vertebrate retina (Wirz-Justice et al. 1984; Adachi et al. 1998) and other brain regions (Khaldy et al. 2002) are affected by a circadian clock, a type of biological oscillator that has persistent rhythmicity with a period of approximately 24 h in the absence of external timing cues (e.g. in constant darkness). In addition, a circadian clock can be entrained by cyclic light stimulation. In vertebrates, circadian clocks are located in the retina (Besharse & Iuvone, 1983; Cahill & Besharse, 1993; Tosini & Menaker, 1996; Dmitriev & Mangel, 2001), pineal gland (Robertson & Takahashi, 1988), and suprachiasmatic nucleus (Welsh et al. 1995). In the vertebrate retina, a clock regulates a variety of cellular phenomena in addition to dopamine levels, including melatonin release (Cahill & Besharse, 1993), retinomotor movements (Burnside & Nagle, 1983), horizontal cell spinule formation (Douglas & Wagner, 1983), extracellular pH (Dmitriev & Mangel, 2000, 2001), and neuronal light responses (Wang & Mangel, 1996; Manglapus et al. 1998).
Because a circadian clock regulates the light responses of horizontal cells (Wang & Mangel, 1996) and dopamine modulates rod and cone input to horizontal cells (Witkovsky et al. 1988a), we investigated whether the clock regulates cone horizontal cell light responses through dopamine pathways. We show in this study that dopamine acts as a circadian clock signal for the day by increasing cone input and decreasing rod input to fish retinal cone horizontal cells. Specifically, the findings suggest that a clock increases endogenous dopamine release during the day so that dopamine D2-like receptors on photoreceptor cells are activated. The resultant decrease in intracellular cAMP and protein kinase A activation then mediates the increase in cone input and decrease in rod input.
Methods
Preparation
Goldfish (Carassius auratus), 10-15 cm in length, were maintained at 19 °C on a 12 h-12 h light-dark cycle for at least 14 days before an experiment. During this period, the light-dark cycle was phase-advanced by 3 h so that lights ‘on’ was at 3 am and lights ‘off’ was at 3 pm. The care and use of the fish followed all guidelines of the National Institutes of Health and the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Anaesthetised (methanesulfonate, MS-222, 100 mg l−1, Sigma Chemical Co., St Louis, MO, USA) fish were decapitated and pithed. Their eyes were then enucleated and their retinae were isolated from the pigment epithelium. Prior to surgery, fish were kept in darkness for 24-48 h. All surgical procedures were performed under dim (0.2 μW cm−2) red or infrared illumination with no differential effect on the results.
Intact, isolated retinae were placed in a chamber that had a volume of 0.5 ml and superfused at 0.5 ml min−1 with a Ringer solution that contained (mm): 130 NaCl, 2.5 KCl, 20 NaHCO3, 0.7 CaCl2, 1.0 MgCl2, and 20 glucose, as described previously (Wang & Mangel, 1996; Wang et al. 1997). Oxygenation of the superfusate with a mixture of 95 % O2-5 % CO2 maintained the superfusate at pH 7.6 in the retinal chamber. After excision, the retinae were superfused in darkness for 60 min, following which horizontal cells were impaled without the aid of any light flashes. Horizontal cell responsivity and sensitivity were then assessed with dim white or monochromatic full-field test stimuli immediately following this period of darkness. Test drugs such as dopamine, quinpirole, spiperone and forskolin, which were diluted in the Ringer solution, were then superfused onto the fish retina in the subjective day or night to determine whether and how they affected the responses of horizontal cells to these light stimuli. Drugs were purchased from RBI (Natick, MA, USA).
Because circadian clocks can be affected (entrained) by light stimuli, these circadian experiments were performed under conditions of constant darkness to assess whether the clock uses dopamine to alter the light responses of L-type cone horizontal cells in the day and night. As a result, all experiments were performed during the subjective day or subjective night, that is the fish and their isolated retinae were maintained under conditions of constant darkness. Subjective day refers to the time of the circadian cycle during which illumination was previously present; subjective night refers to the time of the circadian cycle during which illumination was not previously present.
Some of the fish (n = 8) received a 5 μl injection of 6-hydroxydopamine (6-OHDA) (10 g l−1) and ascorbic acid (1 g l−1) dissolved in a NaCl solution (9 g l−1) into one eye on two consecutive days 7-14 days before the recording experiments (Harsanyi & Mangel, 1992; Wang et al. 1997). This procedure selectively destroys dopaminergic cells. The same volume of the NaCl solution containing ascorbic acid was injected into the other eye as a control. Analysis of the 6-OHDA-treated and the control retinae by high-pressure liquid chromatography with electrochemical detection has shown that the 6-OHDA treatment depletes the retinae of dopamine by an average of 98 %, compared with saline-injected controls (Harsanyi & Mangel, 1992; Wang et al. 1997).
Electrical recording
Standard intracellular recording procedures were employed as described previously (Wang & Mangel, 1996; Wang et al. 1997). Cone horizontal cells were identified by intracellular tracer injection (see Fig. 2), with spectral and intensity response curves, and by response waveform (Mangel & Dowling, 1987; Wang et al. 1997). The maximum, unattenuated intensity (Io) of full-field white light stimuli from a 100 W tungsten-halogen lamp was 2.0 × 103 μW cm−2. Intensity values indicated in the text are relative to Io. Calibrated neutral density filters and narrow-band interference filters were used to control light intensity and stimulus wavelength, respectively.
Figure 2. Horizontal cells identified morphologically as H1 cone horizontal cells are driven primarily by rods at night.
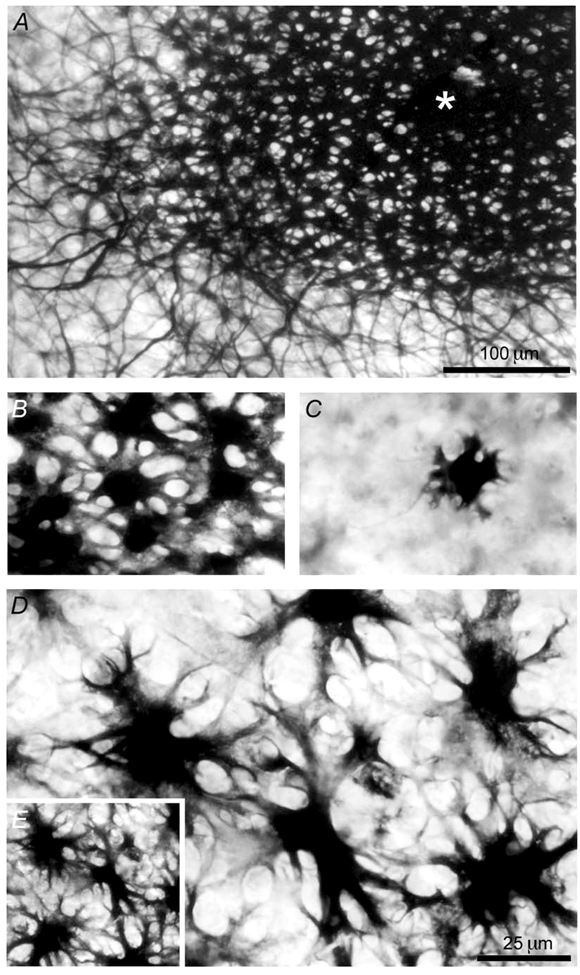
A, the horizontal cell (indicated by *) was stained with an intracellular injection of biocytin. This L-type (H1) cone horizontal cell, which is extensively coupled by gap junctions to many other L-type (H1) cone horizontal cells, produced the rod-dominated light responses in the subjective night shown in Fig. 1A. B, magnification of part of the coupled network of cells shown in A illustrates that the cells are H1 cone horizontal cells, as evidenced by the size of their somata, and the number, length, and shape of their processes. C, an uncoupled H1 cone horizontal cell, which was recorded at night in a different retina following light sensitisation and injected with biocytin, exhibits morphological features similar to those of the coupled cells shown in B. D, in contrast, a rod horizontal cell, which was recorded from a different dark-adapted retina at night and injected with biocytin, is coupled to a number of its neighbouring rod horizontal cells, all of which have different morphological features than those of the cells shown in B and C. The rod horizontal cells have somata that are twice as large in diameter as those of the H1 cone horizontal cells and their processes are more numerous, irregular and longer than those of the cone horizontal cells. E, at a comparable eccentricity and magnification, the density of coupled rod horizontal cells is lower than that of H1 cone horizontal cells, as is evident from a comparison of A and E. The scale bar in A applies to E as well and the scale bar in D also applies to B and C. The cells shown in A and E are at the same magnification, as are those in B, C and D.
Tracer injections
Micropipettes were filled at their tips with 4 % biocytin (Sigma) in 5 mm Hepes, pH 7.6, containing 1.0 m potassium chloride, and then backfilled with 2 m potassium chloride. Final DC resistances ranged between 150 and 250 MΩ. After light response measurements were obtained, biocytin was iontophoresed into the cells for 15 min using positive sinusoidal current (3 Hz, 5 nA peak-peak).
Histochemistry
After the last injection, the retina was kept in the superfusion chamber in total darkness for 30 min, then rapidly immersed in a solution of 4 % paraformaldehyde in 10 mm phosphate-buffered saline (PBS, 0.8 % NaCl, pH 7.3). The retina was then left overnight in the fixative solution at room temperature. The following morning, the retina was washed for 4 h in PBS at 4 °C and then treated under agitation at room temperature with the following concentrations of alcohol in PBS: 50 % (10 min), 70 % (15 min) and 50 % (10 min). Thereafter the retina was washed twice for 30 min in 25 mm Tris-0.5 mNaCl, pH 9.25 at room temperature and then reacted overnight with the Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) in 25 mm Tris-0.5 mNaCl, pH 9.25 containing 0.5 % Tween 20. Thereafter the retina was washed three times for 10 min each in Tris and processed for peroxidase histochemistry with 3,3′ diaminobenzidine (DAB) and cobalt intensification using the Vector Laboratories kit SK-4100. Replacing the buffer solution provided with this kit with 50 mm Tris, pH 8.6 greatly reduced the non-specific background. After a 30 min incubation in Tris containing DAB and nickel, the retinal tissue was incubated for a further 45 min in a fresh solution of DAB, nickel and hydrogen peroxide. The reaction was stopped by immersing the retina in distilled water for 10 min. The retina was then placed on a gelatinised coverslip and allowed to air dry for a few minutes. Additional drying was performed by incubating the retina in solutions of increasing alcohol concentration: 50 %, 70 %, and then 100 % for 15 min each. Finally, the retina was cleared in xylene for 1 h and flat-mounted in Histomount (National Diagnostics, Highland Park, NJ, USA). Labelled horizontal cells could then be localised under the microscope and photographed.
High Performance Liquid Chromatography (HPLC)
Reverse-phase HPLC with electrochemical detection was used to determine the total content of dopamine and its metabolite dihydroxyphenylacetic acid (DOPAC) from retinal homogenates, and the amount of endogenous dopamine released into the culture medium from explanted retinae. The HPLC system consisted of an HPLC pump (Model 515, Waters, Milford, MA, USA), an automatic, refrigerated injector (1100 series, Agilent Technologies), an electrochemical detector (Model LC-4C, BioAnalytical System, West Lafayette, IN, USA), and an adsorbsphere HS (C18) column (250 × 4.6 mm, ID 5 μm; Alltech, Deerfield, IL, USA). The potential of the glassy carbon electrode was set to +750 mV. Sensitivity was 2 nA V−1. An isocratic mobile phase composed of 0.1 m KH2 PO4, 0.0235 % octyl sodium sulfate (ACROS Organics, NJ, USA), 0.1 mm Na2-EDTA, and methanol (15 %) was adjusted to a pH of 3.10 with a solution of o-phosphoric acid (85 %). This mobile phase was delivered at 0.8 ml min−1 at 30 °C.
To determine dopamine and DOPAC content, freshly excised retinae were rapidly sonicated in 400 μl of standard solution (50 mm Na2-EDTA-1 mm Na2S2O5), containing 3333 pg 3,4-dihydroxybenzylamine (DHBA) as an internal standard. An aliquot (50 μl) of the homogenate was kept for protein determination (Lowry et al. 1951). The remainder of the homogenate was then centrifuged for 30 min (14 000 g at 4 °C) and filtered on a 0.2 μm pore-size membrane filter. A volume of 60 μl (thus containing 500 pg DHBA) of each sample was then directly injected into the HPLC system.
To determine the amount of endogenous dopamine released into the culture medium from explanted retinae, retinae were incubated in fish Ringer solution that was identical to that used for the electrophysiological experiments, but with 0.1 mm ascorbic acid added. The retinae were placed in total darkness for 1 h in a CO2 incubator that maintained conditions of 95% O2,-5% CO2 at 20 °C, water-saturated. Following this, 1 ml of supernatant was collected under infrared conditions and processed for alumina extraction, as described previously (Weiler et al. 1997).
An estimation of the concentration of dopamine in the retinal extracellular space can be calculated from the total quantity of dopamine that accumulated in the culture wells over the course of 1 h. The volume of the extracellular space has been shown to be approximately 10 % of the retinal volume (Karwoski et al. 1985). Because the average volume of the goldfish retinae we studied was 39.85 ± 2.24 μl (n = 20), the approximate extracellular volume of the retinae was 4 μl. Based on the assumption that all the dopamine measured at the end of the in vitro experiment would have diffused into 4 μl of extracellular space under in vivo conditions, as has been suggested (Witkovsky et al. 1993), the approximate extracellular concentration of dopamine in the goldfish retina can be calculated as follows:
where the concentration of dopamine is given in mol l−1, the amount of dopamine in Ringer solution is given in g, the extracellular volume of the retina is given in l, and the molar mass of dopamine is given in g mol−1.
Data analysis
Relative quantum sensitivity was determined as described previously (Naka & Rushton, 1966; Nussdorf & Powers, 1988; Wang & Mangel, 1996). Data were normalised at the wavelength of peak sensitivity (550 or 600 nm). A 1 mV criterion response was used to minimise light sensitisation of the dark-adapted state. ‘Light sensitisation’ refers to the phenomenon in which bright light (photopic range) stimulation of dark-adapted retinae increases the size of cone horizontal cell light responses in the day and night (Baldridge et al. 1995) and eliminates rod input to the cells during the night (Wang & Mangel, 1996). Red (625 nm) cone spectral sensitivity data were obtained from Harosi & MacNichol (1967) and rod spectral sensitivity data were obtained from Schwanzara (1967). The maximum, unattenuated light intensity of the stimulus at 550 nm was 7.2 × 1013 photons cm−2 sec−1.
Dose-response data were fitted to the Hill equation using a non-linear least-squares method:
where V (mV) is the peak increase in light response at a given drug concentration [C] (nm), Vmax (mV) is the maximum increase in light response to the drug, n is the Hill coefficient, and EC50 (nm) is the drug concentration that gives rise to a half-maximal response.
The Student's unpaired t test was used for all statistical comparisons. Average values in the text are given as means ± s.e.m.
Results
A circadian clock regulates rod and cone input to cone horizontal cells
The light responses of goldfish retinal L-type cone horizontal cells exhibit a circadian rhythm when the fish are maintained in constant darkness (Fig. 1A), as shown previously (Wang & Mangel, 1996). Cone horizontal cells are a type of second order cell in the fish retina that receives synaptic contact from cones, but not from rods (Stell & Lightfoot, 1975; Downing & Djamgoz, 1989). During the subjective day, the responses are cone-mediated and similar to classic responses previously reported for these cells (Naka & Rushton, 1966; Mangel & Dowling, 1987). In contrast, during the subjective night, the responses are rod-dominated. Compared to the day, the responses at night are slower, smaller in size and longer in duration, and the response threshold is considerably lower, as shown previously (Wang & Mangel, 1996). Moreover, the spectral sensitivity of the cells resembles that of red (625 nm) cones (Harosi & MacNichol, 1974) during the day (see Fig. 7) and rods (Schwanzara, 1967) during the night (Wang & Mangel, 1996).
Figure 1. A circadian clock regulates the light responses of goldfish cone horizontal cells.
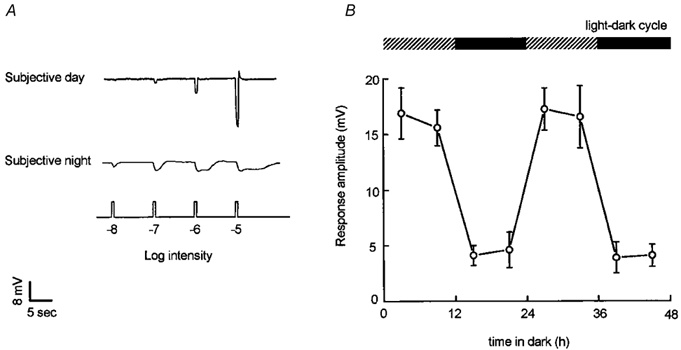
A, cone input to L-type cone horizontal cells predominates during the subjective day and rod input predominates during the subjective night. Compared to the day, the responses at night are slower, smaller in size, longer in duration, and response threshold is approximately a hundred times lower. Retinae were dark-adapted for at least 1 hr after excision, following which L-type cone horizontal cells were impaled without the aid of any light flashes. Responses of the cells to dim full-field white light flashes (ranging from -8 log Io to -5 log Io) were then recorded.The responses of two different cells are shown in the subjective day and night. Similar results were obtained from 43 other cells. B, average responses to a bright light stimulus (-3 log Io) as a function of time in the dark are greater during the subjective day than during the subjective night, indicating that a circadian clock regulates cone input to cone horizontal cells. The presence of a recording from a cone horizontal cell was confirmed following cell impalement by flashing a series of dim (≤ -6 log Io) lights. Following this, a single bright (-3 log Io) light was flashed. Data were averaged only from responses to this single bright light stimulus (one bright light stimulus per retina). In each case, a response to a single bright light stimulus (-3 log Io) was obtained at the time indicated. Surgical isolation of the retina occurred approximately 2 h before this bright light response was recorded. Each data point represents averages obtained from 5-8 cells (1 cell per retina). Intensity values indicated in Figs. 1, 4, 6,and 8 are relative to the maximum, unattenuated intensity (Io) of full field white light stimuli generated by the photostimulator (see Methods).
Figure 7. Endogenous activation of D2-like receptors mediates circadian clock regulation of rod and cone input to cone horizontal cells.
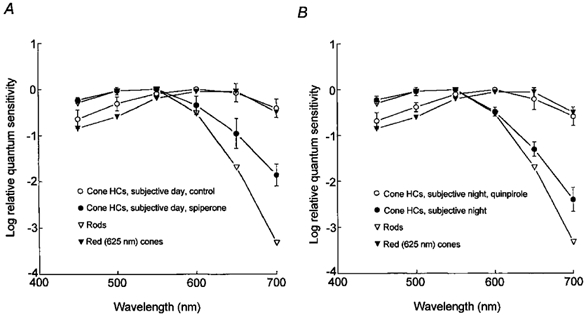
A, application of spiperone during the subjective day (ZT 03, second cycle) altered the average spectral sensitivity of L-type cone horizontal cells to resemble that of goldfish rod horizontal cells (and rods) (Schwanzara, 1967), rather than red (625 nm) cones (Harosi & MacNichol, 1974), for wavelengths ≤ 600 nm. B, in contrast, application of quinpirole during the subjective night (ZT 15, second cycle) altered the average spectral sensitivity of L-type cone horizontal cells to resemble that of goldfish red (625 nm) cones and cone horizontal cells in the day (Harosi & MacNichol, 1974), rather than rod horizontal cells and rods (Schwanzara, 1967). Each data point represents the average of 5 to 8 cells (1 cell per retina).
A circadian rhythm in cone input to cone horizontal cells is illustrated in Fig. 1B, which depicts average responses to a bright light stimulus (-3 log Io) as a function of time in the dark. Following constant darkness, average responses to a bright stimulus are greater during the subjective day (Zeitgeber time (ZT) 03 and 09, first and second cycles) than during the subjective night (ZT 15 and 21, first and second cycles), as shown previously (Wang & Mangel, 1996). The results shown in Fig. 1 thus reveal the presence of a circadian rhythm in cone horizontal cell responsiveness to bright light stimulation, and indicate that cone input to cone horizontal cells is greatly reduced during the subjective night and that rod input is present at night, but not in the day.
Although the light responses of cone horizontal cells were dominated by rod input at night and resembled those of rod horizontal cells, the two types of cells could be distinguished by the size of their light responses (i.e. average response to -5 log Io stimulus: cone horizontal cells, -5 mV; rod horizontal cells, -20 mV), by the fact that light sensitisation, after dark-adapted measurements were obtained, produced a rod-cone shift in cone horizontal cells, but not in rod horizontal cells (see Wang & Mangel, 1996), and by intracellular tracer injection. The cone horizontal cell, whose night-time light responses are depicted in Fig. 1A, was injected intracellularly with biocytin following recording of its light responses. As shown in Fig. 2A, this L-type (H1) cone horizontal cell, which was coupled by gap junctions to many other cells of like type, as has been reported previously (Tornqvist et al. 1988; Baldridge & Ball, 1991), produced rod-dominated light responses during the subjective night. Moreover, in every case (n = 9) in which biocytin was injected into cells that exhibited light responses at night similar to those shown in Fig. 1A, the cell was identified morphologically as an H1 cone horizontal cell (Stell & Lightfoot, 1975; Downing & Djamgoz, 1989). As shown in Fig. 2B, magnification of part of the coupled network of cells shown in Fig. 2A illustrates that the cells are H1 cone horizontal cells, as evidenced by the size of their somata and the number, length, and shape of their processes (Stell & Lightfoot, 1975; Downing & Djamgoz, 1989).
The identification of the coupled cells illustrated in Fig. 2A and 2B as H1 cone horizontal cells can also be confirmed by comparing their morphological features to uncoupled H1 cone horizontal cells (Fig. 2C) and to rod horizontal cells (Fig. 2D). The cell shown in Fig. 2C was injected with biocytin at night following light sensitisation of the retina, a procedure that uncouples horizontal cells (Tornqvist et al. 1988; Baldridge & Ball, 1991). Its morphological features, which distinguish it as an H1 cone horizontal cell, are similar to those of the coupled cells shown in Fig. 2B. In contrast, a rod horizontal cell, which was recorded from a dark-adapted retina at night and injected with biocytin, is shown in Fig. 2D coupled to a number of its neighbouring rod horizontal cells, as described previously (Tsukamoto et al. 1987). Its light responses were rod-driven and relatively large in amplitude (20 mV) to bright stimuli (-3 log Io), as described previously (Wang & Mangel, 1996), but its morphological features are clearly different from those of the H1 cone horizontal cells shown in Fig. 2A-C. That is, the rod horizontal cells have cell bodies that are twice as large in diameter as those of the cone horizontal cells shown in Fig. 2A-C, and their processes are more numerous, irregular, and longer than those of cone horizontal cells (Stell & Lightfoot, 1975; Tsukamoto et al. 1987). The micrographs shown in Fig. 2B-D are all at the same magnification. The mean soma size of H1 cone horizontal cells (12.7 ± 0.1 μm, n = 11; five cells from each of 11 retinae were averaged) injected with biocytin in our experiments was significantly different (P < 0.001) from that of the biocytin-injected rod horizontal cells (23.0 ± 0.2 μm, n = 10; five cells from each of 10 retinae were averaged). Finally, at a comparable eccentricity and magnification, the density of coupled rod horizontal cells was lower than that of H1 cone horizontal cells, as is evident from a comparison of Fig. 2A and 2E, which illustrate H1 cone horizontal cells and rod horizontal cells, respectively, at similar magnifications.
Dopamine levels are higher during the subjective day than in the subjective night
Retinal dopamine and dopamine precursor levels are higher in the subjective day than in the night in the rat (Wirz-Justice et al. 1984) and quail (Manglapus et al. 1999). To determine whether a similar day-night difference occurs in fish, we used reversed-phase HPLC to measure dopamine levels in the subjective day and night. As shown in Fig. 3A, dopamine content of retinal homogenates was similar (P > 0.5) in the subjective day and night. In contrast, DOPAC, the primary metabolite of dopamine in the retina, was significantly (P < 0.001) greater in the subjective day than in the night (Fig. 3B). Because these latter results suggest that dopamine release is greater in the subjective day than in the subjective night, we directly measured endogenous dopamine release from intact neural retinae in the subjective day and night. As shown in Fig. 3C, endogenous dopamine release from the goldfish retina into the culture medium was significantly (P < 0.001) greater in the subjective day than in the night. Based on the values shown in Fig. 3C and the assumptions described in the Methods, the approximate concentration of dopamine in the extracellular space of the goldfish retina, as measured by HPLC, was 181 ± 11 nm during the subjective day and 98 ± 8 nm during the subjective night.
Figure 3. Release of endogenous dopamine in the goldfish retina is higher in the subjective day than in the subjective night.
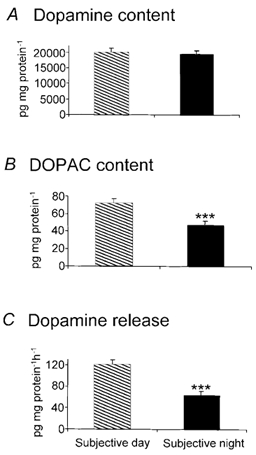
Although dopamine content of retinal homogenates (A) was similar in the subjective day and night, DOPAC content (B), which reflects dopamine utilisation, and endogenous dopamine release from in vitro retinae into the Ringer solution (C) were significantly greater in the subjective day, compared to the subjective night. In C, retinae were maintained in fish Ringer solution in total darkness for 1 h. Subjective day data were obtained during the second cycle at Zeitgeber time (ZT) 6 and subjective night data during the second cycle at ZT 18 (ZT 0 corresponds to dawn). ***, significant difference between subjective day and night values (P < 0.001).
Effects of dopamine on retinal horizontal cells depend on time of subjective day
Superfusion of dopamine (1-5000 nm) during the subjective night increased cone input and eliminated rod input to the cells (Fig. 4A), a state typically observed during the subjective day. In contrast, dopamine application during the subjective day had no effect (1-10 nm) or increased (100-5000 nm) responses slightly (Fig. 4B). Destruction of dopaminergic cells following 6-hydroxydopamine (6-OHDA) pre-treatment increased rod input and decreased cone input during the subjective day and had no effect during the subjective night (Fig. 4C). Prior intraocular injection of saline as a control had no effect on the light responses of horizontal cells in the subjective day or night (data not shown).
Figure 4. Exogenous dopamine application increases cone input and decreases rod input to goldfish retinal L-type cone horizontal cells.
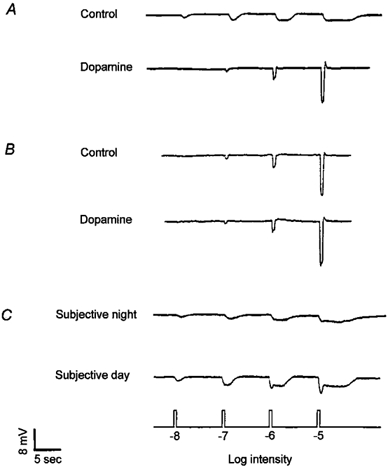
A, superfusion of dopamine (100 nm) during the subjective night increased cone input and eliminated rod input to the cells, so that light responses closely resembled those typically observed during the day. B, superfusion of dopamine (100 nm) during the subjective day produced a slight increase in the size of the light responses. C, destruction of dopaminergic cells following pre-treatment with 6-hydroxydopamine (see Methods) increased rod input and decreased cone input during the subjective day. Light responses during the subjective night were not affected. Similar results were obtained from 9 (A), 8 (B), and 13 (C) cells. Subjective day data in this and all subsequent figures were obtained under conditions of constant darkness during the second cycle at ZT 3 and 9. No differences were observed between ZT 3 and 9. Subjective night data in this and all subsequent figures were obtained at ZT 15 and 21; no differences were observed between ZT 15 and 21.
These results suggest that dopamine acts as a circadian signal for the day, that is the clock increases dopamine release in the subjective day and decreases it in the subjective night, in agreement with our HPLC measurements (Fig. 3C). The increase in dopamine concentration in the day then increases cone input and decreases rod input to cone horizontal cells. Figure 5A, which depicts the average increase in response size to a light stimulus (-3 log Io) as a function of dopamine concentration, illustrates clearly that exogenous dopamine increases cone horizontal cell light responses at night, but has little effect in the subjective day. The dose-response data at night are fitted by a sigmoid curve with a Hill coefficient (n) of 1.14 and a half-saturation value (EC50) of 6.5 nm, values in good agreement with those obtained in a study of dopamine-mediated inhibition of fish cone elongation (Hillman et al. 1995). In contrast, in the subjective day, the dose-response data are fitted by a sigmoid curve with a Hill coefficient of 1.09 and an apparent EC50 of 83.0 nm. When the data in Fig. 5A were replotted as average light response amplitudes (Fig. 5B), rather than as average increases in light response amplitude (Fig. 5A), the dose-response curve during the subjective day closely overlapped that during the night for concentrations above approximately 20 nm, indicating that exogenous dopamine had a small effect when applied to dark-adapted retinae at doses greater than 20 nm.
Figure 5. Effect of dopamine on horizontal cell light response amplitude in the day and night.
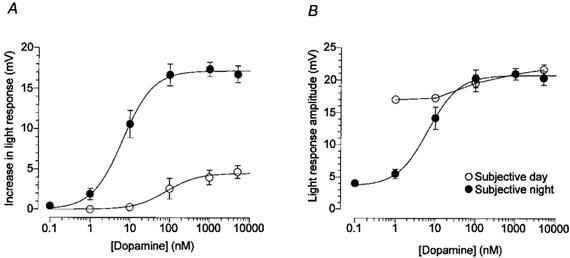
A, average increase in light response amplitude as a function of dopamine concentration in the subjective day and night. Exogenous dopamine increases horizontal cell light responses at night, but has little or no effect in the day. Data were fitted to the Hill equation (see Methods). B, average light response amplitude as a function of dopamine concentration in the subjective day and night. Dopamine has little (100-5000 nm) or no (1-10 nm) effect on the amplitude of light responses during the subjective day, possibly because endogenous dopamine is already bound to its receptors. Night-time data were fitted to the Hill equation. Average response increase (A) and average response amplitude (B) to a -3 log Io light stimulus are shown. Each data point represents the average of 6-17 cells (1 cell per retina). Bars represent the s.e.m.
The difference between the daytime and night-time dose- response curves in Fig. 5B may be due to activation of dopamine receptors by endogenous dopamine in the subjective day, but not in the subjective night. Inspection of the curves indicated that the amplitude of light responses saturated at a dopamine concentration of about 20 nm. At lower concentrations (e.g. 1-10 nm), exogenous dopamine application had no effect on the amplitude of light responses during the subjective day (Fig. 5A), presumably because endogenous dopamine had already activated dopamine receptors. At night, however, exogenous dopamine application greatly increased response amplitude because the level of endogenous dopamine was below the threshold of dopamine receptor activation (approximately 0.5 nm), as evidenced by the lack of effect of the D2-like antagonist spiperone at night (see Fig. 6 legend). Assuming that the efficacy of dopamine in modulating intracellular cAMP and protein kinase A is similar in the day and night, these electrophysiological data suggest that the circadian-induced dopamine concentration in the outer retina is approximately 20 nm during the subjective day and less than 0.5 nm during the night. In addition, the fact that exogenous dopamine at very low concentrations (1-5 nm) can affect cone horizontal cells during the subjective night, but not in the subjective day, also suggests that the day-night difference cannot be explained by a hyperactive dopamine transporter at night.
Figure 6. The effects of the clock on cone horizontal cells are mediated by activation of D2-like, and not D1-like, receptors.
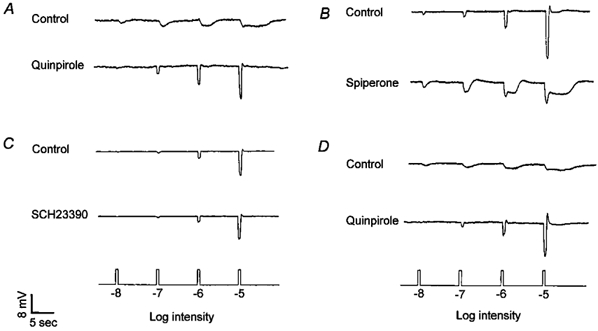
A, application of quinpirole (1 μm), a D2 receptor agonist, during the subjective night increased cone input and eliminated rod input to cone horizontal cells, an effect similar to that of dopamine. B, application of spiperone (1 μm), a general D2-like receptor antagonist, during the subjective day reduced cone input and introduced rod input to the cone horizontal cells. C, SCH23390 (10 μm), a D1 antagonist, had no effect during the subjective day. D, application of quinpirole (1 μm) at night to retinae pre-treated with 6-OHDA increased cone input and decreased rod input to the cells. The recordings shown are representative of results obtained on 12 (A), 16(B), 9 (C), and 6(D) cells.
Dopamine activates D2-like receptors during the subjective day
The clock-induced increased level of endogenous dopamine during the subjective day activates D2-like, and not D1-like, receptors. Application of quinpirole (1 μm), a D2-like receptor agonist, during the subjective night increased cone input and eliminated rod input to the cells (Fig. 6A), an effect similar to that of dopamine. Application of spiperone (0.5-10 μm), a general D2-like receptor antagonist, during the subjective day reduced cone input and introduced rod input to the horizontal cells (Fig. 6B). In contrast, SCH23390 (10 μm), a D1 antagonist, had no effect during the subjective day (Fig. 6C). Application of spiperone alone or in conjunction with SCH23390 during the subjective night had no effect on the light responses (data not shown), suggesting that the level of endogenous dopamine at night was below the threshold of dopamine receptor activation. Finally, application of quinpirole or dopamine to retinae pre-treated with 6-OHDA increased cone input and decreased rod input to the cells when applied at night (Fig. 6D), indicating that the D2-like receptors, which are activated during the subjective day, are downstream from the dopaminergic interplexiform cells.
The effects of dopamine and dopamine ligands on the dark resting membrane potential of L-type cone horizontal cells depended on the time of day. As reported previously (Wang & Mangel, 1996), the dark resting potential of cone horizontal cells during the subjective night (-37.5 ± 1.4 mV; n = 24) was not significantly different (P > 0.5) from that during the subjective day (-36.9 ± 1.2 mV; n = 20). As shown in Table 1, dopamine and quinpirole hyperpolarised the cells by about 6 mV at night, but had no effect during the day. In contrast, spiperone depolarised the cells in the day, but not at night. Finally, SCH23390 had no effect.
Table 1.
Effects of dopamine and dopamine ligands on horizontal cell membrane potential in the day and night
| Test drug | Membrane potential change (mV) | |
|---|---|---|
| Day | Night | |
| Dopamine (100 nm) | −1.7 ± 2.0 (8) | −5.8 ± 3.1 (9) |
| Quinpirole (1 μm) | −2.1 ± 1.8 (6) | −6.4 ± 2.7 (12) |
| Spiperone (1 μm) | 4.1 ± 1.6 (16) | 0.7 ± 0.6 (7) |
| SCH23390 (10 μm) | 0.6 ± 0.5 (9) | 0.4 ± 0.7 (6) |
Values are means ± s.e.m. Numbers in parentheses in the column on the right indicate numbers of cells tested.
Spectral sensitivity measurements demonstrated that rod and cone input to cone horizontal cells is modulated by endogenous activation of D2-like receptors. L-type (H1) cone horizontal cells receive synaptic contact primarily from red (625 nm) cones (Stell & Lightfoot, 1975; Downing & Djamgoz, 1989) and spectral sensitivity measurements (Fig. 7A) during the subjective day support this. Figure 6A also shows that application of spiperone during the subjective day (ZT 03, second cycle) altered the average spectral sensitivity of L-type cone horizontal cells to resemble that of goldfish rod horizontal cells (and rods; Schwanzara, 1967), rather than red cones (Harosi & MacNichol, 1974). In contrast, application of quinpirole during the subjective night (ZT 15, second cycle) altered the average spectral sensitivity of L-type cone horizontal cells (Fig. 7B) to resemble that of goldfish red cones (Harosi & MacNichol, 1974) and cone horizontal cells during the day, rather than rod horizontal cells and rods (Schwanzara, 1967). Interestingly, the greater relative spectral sensitivity of cone horizontal cells in the far red region of the spectrum during the subjective night, compared to rod horizontal cells, may indicate that L-type cone horizontal cells still receive some input from red cones at night, even though they are primarily driven by rod input. A greater than expected sensitivity at night in the far red region of the spectrum was also observed in electroretinogram studies of goldfish (Nussdorf & Powers, 1988) and Japanese quail (Manglapus et al. 1998) retinae. The relative spectral sensitivity of rod horizontal cells closely resembled that of goldfish rods (cf. Schwanzara, 1967; Wang & Mangel, 1996).
In the above spectral sensitivity measurements, a 1 mV criterion response was used to minimise light sensitisation of the dark-adapted state. This response amplitude represents approximately 25 % of the maximum dark-adapted response of a cone horizontal cell at night. In the day, the dark-adapted maximum response of a cone horizontal cell is about 17 mV. Therefore, we also compared spectral sensitivity using a criterion response of 25 % of Vmax. Comparable results to those of Fig. 7 were obtained.
Increase in cAMP and protein kinase A activation enhance rod input
As suggested by the findings shown in Fig. 8, the decreased activation of D2-like receptors during the night, which increased the level of cAMP and the activation of protein kinase A, enhanced rod input and decreased cone input to cone horizontal cells. Application of forskolin (10-20 μm), an activator of adenylyl cyclase, during the subjective day reduced cone input and increased rod input (Fig. 8A) in all cases tested (n = 13). Dibutyryl cAMP (0.5-1.0 mm) had similar effects, but application of 1,9-dideoxy-forskolin (10-20 μm), an analogue of forskolin that does not activate adenylyl cyclase, had no effect (data not shown). In about half of the cases (n = 7), forskolin initially depolarised the cells by a few millivolts and increased their light responses by about 33 %, a finding that may be attributed to an enhancement by cAMP of glutamate-gated conductances in the horizontal cells (Knapp & Dowling, 1987). Following this enhancement, responses always decreased below initial levels and rod input became evident (about 20 min later). Although forskolin application during the subjective night had no effect on the light responses of the cells in all cases tested, it blocked the effects of a subsequent dopamine (100 nm) application (Fig. 8B). Application of Rp-cAMPS (100 μm), a membrane-permeant inhibitor of cAMP-dependent protein kinase, during the subjective night (Fig. 8C), or octanol (2 mm), an alcohol that uncouples gap junctions, increased cone input and decreased rod input (Fig. 8D). Octanol also had a similar effect when it was applied during the subjective night in the presence of spiperone.
Figure 8. Increases in intracellular cAMP and protein kinase A activation increase rod input and decrease cone input to cone horizontal cells at night.
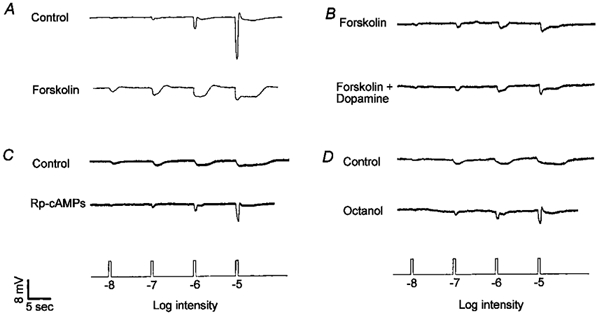
A, superfusion of forskolin (10 μm), an activator of adenylyl cyclase, during the subjective day reduced cone input and increased rod input. B, forskolin application during the subjective night blocked the effects of a subsequent dopamine application. C, application of Rp-cAMPS (100 μm), an inhibitor of cAMP-dependent protein kinase, during the subjective night increased cone input and decreased rod input. D, application of octanol (2 mm), an alcohol that uncouples gap junctions, during the subjective night increased cone input and decreased rod input. Similar results were obtained from 13 (A), 6 (B), 9 (C), and 10 (D) cells.
Discussion
Dopamine activates D2-like receptors on rods and cones during the subjective day
These findings suggest that dopamine acts as a circadian clock signal for the day. During the day, the clock increases endogenous dopamine release in the retina. The dopamine activates D2-like receptors that decrease rod input and increase cone input to cone horizontal cells via decreases in intracellular cAMP and protein kinase A activation. During the night, the clock decreases dopamine levels below the threshold for activation of D2-like receptors and cone horizontal cell responses become rod-dominated. In other words, even in the absence of light stimulation, the clock activates D2-like receptors to a greater extent in the day, compared to the night. It is likely that the D2 receptors that are involved with this circadian phenomenon are located on photoreceptor cells, because: (1) D2-like receptors are found on photoreceptors (Dearry & Burnside, 1986; Yazulla & Lin, 1995), and not on horizontal cells (Knapp & Dowling, 1987; Lasater, 1987; Harsanyi & Mangel, 1992); and (2) the effects of exogenous dopamine and quinpirole persist following destruction of dopaminergic cells with 6-OHDA (Fig. 6D). The clock thus regulates rod and cone input to cone horizontal cells by activating extrasynaptic D2 receptors on photoreceptor cells and not by activating D2 autoreceptors on dopaminergic cells (Harsanyi & Mangel, 1992; Rashid et al. 1993; Yazulla & Lin, 1995; Wang et al. 1997). These findings are consistent with previous observations that a circadian clock regulates rod-cone dominance of the electroretinogram (Manglapus et al. 1999) and retinomotor movements (Dearry & Burnside, 1986; Besharse et al. 1988) by activation of D2-like extrasynaptic receptors.
Although the HPLC (Fig. 3) and electrophysiological (Fig. 5) results both indicate that endogenous dopamine release is greater during the subjective day compared to the subjective night, the magnitude of the day-night difference apparently depends on the method used for the measurements. The electrophysiological results suggested that the day-night difference was approximately 40:1 and that the level of endogenous dopamine release at night was very low, as evidenced by the lack of effect of the D2 antagonist spiperone at night. In contrast, HPLC measurements of dopamine overflow into the medium suggested that endogenous dopamine release was 50 % greater during the subjective day than the subjective night and that dopamine release at night was relatively high. This apparent discrepancy, however, can probably be attributed to differences between the two techniques, because the HPLC results represent dopamine overflow from dopaminergic neurones into the medium, whereas the electrophysiological results depend on both dopamine receptor activity and the extracellular dopamine concentration. There are at least four characteristics of the two techniques that can help explain the different results. First, when dopamine release from freshly excised retinae was measured using HPLC, dopamine present in the extracellular space diffused into the medium over the course of 1 h (see Methods), a situation that probably generated a relatively high background level at night (cf. Witkovsky et al. 1993). Second, dopamine diffusion over a distance from the extracellular space into the medium might decrease the subjective day-subjective night difference that was present at D2 receptors in the retina, because dopamine uptake in the retina is concentration dependent (Witkovsky et al. 1993), and therefore might be more active during the subjective day than the subjective night. Third, D2-like receptors become desensitised within minutes to hours from constant exposure to dopamine (Bates et al. 1991). Because desensitisation of D2-like receptors has been suggested to occur in the vertebrate retina (Schorderet and Nowak, 1990) and because endogenous dopamine activates D2-like receptors during the subjective day, the responsiveness of D2 receptors in the retina probably decreases during the subjective day. Consequently, higher doses of exogenous dopamine may be needed to activate D2-like receptors during the subjective day because endogenous dopamine binds D2 receptors and desensitises them. Thus, desensitisation of D2-like receptors during the subjective day may have increased the magnitude of the day-night difference in extracellular dopamine concentration that was estimated from our electrophysiological data by raising the daytime values. In contrast, desensitisation of D2-like receptors during the subjective day would not alter the magnitude of the day-night difference in dopamine release that was measured with HPLC, because these data are independent of D2 receptor sensitivity. Fourth, electrophysiological measurements were obtained during superfusion of the retina, a procedure that continuously washed dopamine from the extracellular space and thus increased the apparent subjective day-subjective night difference. On the other hand, because the superfusion rate was relatively low (0.5 ml min−1) in our experiments (see Methods), the amount of dopamine washed from the extracellular space may be relatively small. To conclude, then, the exact concentration of dopamine at D2 receptor sites in the in vivo outer retina cannot be known with certainty. It is likely, however, that the in vivo subjective day-subjective night values in the outer retina are somewhere between those measured by the two techniques. Moreover, the conclusion that the clock increases dopamine release during the subjective day, compared to the subjective night, is strengthened by the fact that two independent measures, namely HPLC and electrical recording, have both provided evidence in support of it.
Circadian clock and light regulate dopamine release differently
The release of dopamine depends on distinct circadian and light adaptive processes. The circadian clock, which is likely intrinsic to the retina itself (Besharse & Iuvone, 1983; Cahill & Besharse, 1993; Tosini & Menaker, 1996; Dmitriev & Mangel, 2001), modulates the dopamine concentration in the fish outer retina in the low nanomolar range (cf. Hillman et al. 1995; Fig. 3 and Fig. 5). Flickering and sustained light stimuli in the photopic range during the day may then further increase dopamine concentration to the high nanomolar to low micromolar level (Harsanyi & Mangel, 1992; Witkovsky et al. 1993; Weiler et al. 1997) so that D1 receptors are also activated. In intact brain regions, such as in intact retina, D2 receptors are two to three orders of magnitude more sensitive to dopamine than D1 receptors (Harsanyi & Mangel, 1992; Missale et al. 1998).
The effects of dopamine that are reported here were observed in retinae kept in constant darkness, that is no light stimuli were flashed before horizontal cell impalement. It has recently been reported that dark-adapted retinae possess a high degree of sensitivity to light stimuli in the low photopic range and brighter (Morgan & Boelen, 1996; Wang & Mangel, 1996). In fact, we have found that a single flash of light at night brighter than -4.5 log Io (i.e. in the low photopic range) can significantly increase fish cone horizontal cell response size, as well as eliminate rod input to the cells. Earlier studies (Mangel & Dowling, 1985, 1987; Yang et al. 1988; Tornqvist et al. 1988), which had suggested that dopamine release was higher in the dark than in the light, were performed before it was known that dark-adapted retinae were highly sensitive to stimuli in the photopic range or that the actions and release of dopamine are dependent on the time of day. It thus seems likely that a number of the discrepancies between these earlier findings and those reported here might be due to the fact that the retinae in the earlier studies were light sensitised, because occasional flashes of light in the photopic range were used to facilitate cell impalement. On the other hand, other differences between the findings reported previously (Yang et al. 1988; Tornqvist et al. 1988) and those reported here, such as those involving elimination of dopaminergic neurones with 6-OHDA, are not easily explained.
Underlying mechanisms
Rod-cone coupling
A complete description of the processes that result from the actions of dopamine and lead to the modulation of rod and cone input to cone horizontal cells is not yet available. When dopamine release is low at night, it is unclear how rod input reaches cone horizontal cells or how the efficacy of cone input to the cells decreases. However, the following scenario can account for our findings in part (Fig. 9). Because goldfish cone horizontal cells receive synaptic contact from cones and not from rods (Stell & Lightfoot, 1975; Downing & Djamgoz, 1989) and rod-cone gap junctions are present in numerous vertebrates (Raviola & Gilula, 1973), including fish (Witkovsky et al. 1974), the most parsimonious explanation for our findings is that dopamine mediates circadian clock regulation of rod and cone input to cone horizontal cells by modulating rod-cone coupling. Specifically, the clock increases dopamine levels during the day so that D2 receptors on photoreceptor cells are activated. Activation of D2-like receptors on photoreceptor cells has been shown to decrease intracellular cAMP (Nir et al. 2002). This in turn decreases rod-cone coupling via decreases in cAMP content and protein kinase A activation. Thus, it is possible that rod input reaches cone horizontal cells at night because of a circadian clock-induced decrease in dopamine concentration that increases the conductivity of rod-cone gap junctions. The finding that octanol, an alcohol that uncouples gap junctions, acts like dopamine (Fig. 8D) supports this suggestion. In addition, the finding that octanol increases cone input to cone horizontal cells, in addition to decreasing rod input, supports the previous suggestion (Mangel et al. 1994) that cone-mediated signals are shunted at cone pedicles at night when rod-cone coupling is high, because rods outnumber cones in the goldfish retina by an average of about 12-fold (Stell & Harosi, 1976). It should be noted that there is precedence to suggest that cAMP increases the coupling between rods and cones at night. Although the gap junctions between retinal horizontal cells are uncoupled by cAMP (Lasater, 1987; DeVries & Schwartz, 1989), gap junctional coupling is enhanced by cAMP in liver, heart and other systems (Dermietzel & Spray, 1993), presumably due to differences in connexin type in horizontal cells, compared to liver and heart.
Figure 9. Proposed circadian clock pathway in the fish retina.
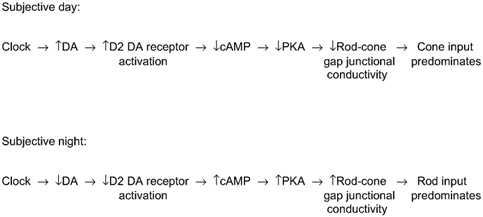
The clock increases dopamine (DA) levels in the day so that D2-like receptors on photoreceptor cells are activated. This in turn lowers intracellular cAMP and protein kinase A (PKA) levels in photoreceptors, decreasing the conductance between rod-cone gap junctions so that cone input dominates cone horizontal cells. At night, rod input dominates cone horizontal cells, because the clock has decreased dopamine levels below the threshold of activation of the D2-like receptors. As a result, the intracellular cAMP level in photoreceptor cells increases, raising the conductance between rod-cone gap junctions. See Discussion for details.
The clock-induced decrease in the amplitude of cone horizontal cell light responses at night (Fig. 1) also probably involves a decrease in the gain of the synapse between cones and cone horizontal cells. This is so, because the light responses of cone horizontal cells at night, which are primarily rod-driven, are significantly smaller in size than those of rod horizontal cells at night (Wang & Mangel, 1996). The decrease in synaptic gain at night may be due in part to a clock-induced decrease in extracellular pH (Dmitriev & Mangel, 2000, 2001), an effect which would reduce calcium influx into cone pedicles and transmitter release from cones (Barnes et al. 1993), and/or to a decrease in the glutamate-gated conductance of horizontal cells (Knapp & Dowling, 1987), an effect which might result from a decrease in the activation of horizontal cell D1 receptors at night. However, the finding that the dark resting potential of cone horizontal cells is similar in the subjective day and night suggests that the clock does not modulate the glutamate-gated conductance of horizontal cells. The relatively small effects of dopamine and D2 ligands on the dark resting potential (Table 1) support this view.
Although direct synaptic contacts from rods onto cone horizontal cells in the goldfish retina have not been observed, a morphological connection such as this cannot be conclusively excluded. If rods directly contact cone horizontal cells in the goldfish, then an alternative explanation for the findings reported here might hold that the clock-induced increase in dopamine release and D2 receptor activation during the day would somehow suppress rod-to-cone horizontal cell transmission (Akopian & Witkovsky, 1996) and augment cone-to-cone horizontal cell transmission. However, the means by which D2 receptor activation would augment a cone-to-cone horizontal cell pathway while simultaneously suppressing a rod-to-cone horizontal cell pathway is presently not clear. It should also be noted that the above scenario and that described in the text accompanying Fig. 9 are not mutually exclusive. That is, it is possible that both phenomena occur, especially in retinae such as amphibian retinae, in which horizontal cells receive direct synaptic contacts from both rods and cones (Witkovsky et al. 1988b). However, it is very unlikely that rod input reaches fish cone horizontal cells at night via rod horizontal cells. No evidence of a chemical or electrical signal or synaptic contact from a rod horizontal cell to a cone horizontal cell has ever been obtained in any vertebrate retina. Moreover, the data presented here indicate that such a signal would have to be sign-conserving, an unlikely possibility since horizontal cells are inhibitory.
Day-night morphological changes in the outer retina probably cannot account for the circadian changes reported here. Light-sensitisation of fish cone horizontal cell light responses (see Fig. 1 in Wang & Mangel, 1996) and the effects of dopamine on cone horizontal cell light responses (Fig. 4) can occur during the night about 10 times faster than retinomotor movements (Burnside & Nagle, 1983) or horizontal cell spinule formation (Douglas & Wagner, 1983). Thus, dopamine-mediated transmission from cones to cone horizontal cells can occur even when cones are elongated and horizontal cell spinules are relatively sparse. This suggests that retinomotor movements and horizontal cell spinule formation and dissolution do not themselves underlie the circadian clock effects of dopamine on transmission from rods and cones to cone horizontal cells.
Different mechanisms underlie circadian clock- and light-activated phenomena
It is important to note that circadian and light-adaptive phenomena are different and may utilise different mechanisms. Although it has been reported that light stimulation of previously dark-adapted retinae during the day increases the conductivity of rod-cone gap junctions (Yang & Wu, 1989) through activation of dopamine D2-like receptors (Krizaj et al. 1998), it is possible that the clock, in contrast to light stimulation of dark-adapted retinae, increases the conductivity of rod-cone gap junctions at night. In fact, as shown in Fig. 1 of Wang & Mangel (1996) and elsewhere (Witkovsky et al. 1988a; Yang & Wu, 1989), bright (photopic range) light stimulation of dark-adapted, but not light-adapted, retinae produces rod-mediated responses in horizontal cells during the day, a phenomenon that has been named the ‘rod plateau potential’ or ‘rod tail’. In contrast, the clock produces rod-mediated responses in fish horizontal cells to light stimulation at night. Thus, dopamine may reduce rod-cone coupling if it is released by the clock, but increases rod-cone coupling if it is released by bright light stimulation of dark-adapted retinae in the day. It is therefore likely that the underlying dopamine-mediated circadian and light-evoked processes are different.
A circadian clock may utilise dopamine in other brain regions
In summary, our findings demonstrate at the single cell level that a circadian clock utilises dopamine to modulate neuronal activity and connections in the vertebrate retina. It is possible that activation of D2-like receptors mediates circadian modulation of neuronal activity and connections in other regions of the CNS, such as the basal ganglia, limbic system, and suprachiasmatic nucleus, where dopamine functions as a transmitter (Viswanathan et al. 1994; Missale et al. 1998) and where dopamine levels exhibit day/night differences (Khaldy et al. 2002). Disruptions in circadian clock pathways and in the circadian regulation of dopamine action may contribute in part to features of Parkinson's disease, schizophrenia and seasonal affective disorder.
Acknowledgments
This investigation was supported in part by grants to S.C.M. from the National Institutes of Health (EY05102), the National Science Foundation (IBN-9819981), and the Plum Foundation, and by a National Eye Institute CORE Grant (EY03039) to the University of Alabama at Birmingham. C.R. and Y.W. were supported in part by Postdoctoral Fellowships from Fight for Sight/Prevent Blindness America and the Helen Keller Eye Research Foundation, respectively. We thank Drs John Dowling and Tim Kraft for critical reading of an earlier version of this manuscript and Drs Kent Keyser, Robin Lester, and David Weiss for helpful discussions.
References
- Adachi A, Nogi T. Phase-relationship and mutual effects between circadian rhythms of ocular melatonin and dopamine in the pigeon. Brain Research. 1998;792:361–369. doi: 10.1016/s0006-8993(98)00206-6. [DOI] [PubMed] [Google Scholar]
- Akopian A, Witkovsky P. D2 dopamine receptor-mediated inhibition of a hyperpolarization-activated current in rod photoreceptors. Journal of Neurophysiology. 1996;76:1828–1835. doi: 10.1152/jn.1996.76.3.1828. [DOI] [PubMed] [Google Scholar]
- Baldridge WH, Ball AK. Background illumination reduces horizontal cell receptive-field size in both normal and 6-hydroxydopamine-lesioned goldfish retinas. Visual Neuroscience. 1991;7:441–450. doi: 10.1017/s0952523800009731. [DOI] [PubMed] [Google Scholar]
- Baldridge WH, Weiler R, Dowling JE. Dark-suppression and light-sensitization of horizontal responses in the hybrid bass retina. Visual Neuroscience. 1995;12:611–620. doi: 10.1017/s0952523800008907. [DOI] [PubMed] [Google Scholar]
- Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proceedings of the National Academy of Sciences of the USA. 1993;90:10081–10085. doi: 10.1073/pnas.90.21.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates MD, Senogles SE, Bunzow JR, Liggett SB, Civelli O, Caron MG. Regulation of responsiveness at D2 receptors by receptor desensitization and adenylyl cyclase sensitization. Molecular Pharmacology. 1991;39:55–63. [PubMed] [Google Scholar]
- Besharse JC, Iuvone PM. Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature. 1983;305:133–135. doi: 10.1038/305133a0. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Iuvone PM, Pierce ME. Regulation of rhythmic photoreceptor metabolism: a role for post-receptoral neurons. Progress in Retinal Research. 1988;7:21–61. [Google Scholar]
- Burnside B, Nagle B. Retinomotor movements of photoreceptors and retinal pigment epithelium: mechanisms and regulation. Progress in Retinal Reseach. 1983;2:67–109. [Google Scholar]
- Cahill GM, Besharse JC. Circadian clock functions localised in Xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- Dearry A, Burnside B. Dopaminergic regulation of cone retinomotor movement in isolated teleost retinas. I. Induction of cone contraction is mediated by D2 receptors. Journal of Neurochemistry. 1986;46:1006–1031. doi: 10.1111/j.1471-4159.1986.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Spray DC. Gap junctions in the brain: where, what type, how many and why? Trends in Neurosciences. 1993;16:186–192. doi: 10.1016/0166-2236(93)90151-b. [DOI] [PubMed] [Google Scholar]
- Dmitriev AV, Mangel SC. A circadian clock regulates the pH of the fish retina. Journal of Physiology. 2000;522:77–82. doi: 10.1111/j.1469-7793.2000.0077m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev AV, Mangel SC. Circadian clock regulation of pH in the rabbit retina. Journal of Neuroscience. 2001;21:2897–2902. doi: 10.1523/JNEUROSCI.21-08-02897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RH, Wagner H-J. Endogenous control of spinule formation in horizontal cells of the teleost retina. Cell and Tissue Research. 1983;229:443–449. doi: 10.1007/BF00214985. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Ehinger B. The interplexiform cell system. I. Synapses of the dopaminergic neurones of the goldfish retina. Proceedings of the Royal Society B. 1978;201:7–26. doi: 10.1098/rspb.1978.0030. [DOI] [PubMed] [Google Scholar]
- Downing JEG, Djamgoz MBA. Quantitative analysis of cone photoreceptor-horizontal cell connectivity patterns in the retina of a cyprinid fish: electron microscopy of functionally identified and HRP-labelled horizontal cells. Journal of Comparative Neurology. 1989;289:537–553. doi: 10.1002/cne.902890402. [DOI] [PubMed] [Google Scholar]
- Hampson E C G M, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. Journal of Neuroscience. 1992;12:4911–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harosi FI, MacNichol EF. Visual pigments of goldfish cones. Journal of General Physiology. 1974;63:279–304. doi: 10.1085/jgp.63.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsanyi K, Mangel SC. Activation of dopamine D2 receptors increases the electrical coupling between fish horizontal cells by inhibiting dopamine release. Proceedings of the National Academy of Sciences of the USA. 1992;89:9220–9224. doi: 10.1073/pnas.89.19.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman DW, Lin D, Burnside B. Evidence for D4 receptor regulation of retinomotor in isolated teleost cone inner-outer segments. Journal of Neurochemistry. 1995;64:1326–1335. doi: 10.1046/j.1471-4159.1995.64031326.x. [DOI] [PubMed] [Google Scholar]
- Karwoski C, Frambach DA, Proenza L. Laminar profile of resistivity in frog retina. Journal of Neurophysiology. 1985;54:1607–1619. doi: 10.1152/jn.1985.54.6.1607. [DOI] [PubMed] [Google Scholar]
- Khaldy H, Leon J, Escames G, Bikjdaouene L, Garcia JJ, Acuna-Castroviego D. Circadian rhythms of dopamine and dihydroxyphenyl acetic acid in the mouse striatum: effects of pinealectomy and of melatonin treatment. Neuroendocrinology. 2002;75:201–208. doi: 10.1159/000048238. [DOI] [PubMed] [Google Scholar]
- Knapp AG, Dowling JE. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. Nature. 1987;325:437–439. doi: 10.1038/325437a0. [DOI] [PubMed] [Google Scholar]
- Krizaj D, Gabriel R, Owen WG, Witkovsky P. Dopamine D2 receptor-mediated modulation of rod-cone coupling in the Xenopus retina. Journal of Comparative Neurology. 1998;398:529–538. [PMC free article] [PubMed] [Google Scholar]
- Lasater EM. Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cyclic AMP-dependent protein kinase. Proceedings of the National Academy of Sciences of the USA. 1987;84:7319–7323. doi: 10.1073/pnas.84.20.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Mangel SC, Baldridge WH, Weiler R, Dowling JE. Threshold and chromatic sensitivity changes in fish cone horizontal cells following prolonged darkness. Brain Research. 1994;659:55–61. doi: 10.1016/0006-8993(94)90862-1. [DOI] [PubMed] [Google Scholar]
- Mangel SC, Dowling JE. Responsiveness and receptive field size of carp horizontal cells are reduced by prolonged darkness and dopamine. Science. 1985;229:1107–1109. doi: 10.1126/science.4035351. [DOI] [PubMed] [Google Scholar]
- Mangel SC, Dowling JE. The interplexiform-horizontal cell system of the fish retina: effects of dopamine, light stimulation and time in the dark. Proceedings of the Royal Society B. 1987;231:91–121. doi: 10.1098/rspb.1987.0037. [DOI] [PubMed] [Google Scholar]
- Manglapus MK, Iuvone PM, Underwood H, Pierce ME, Barlow RB. D opamine mediates circadian rhythms of rod-cone dominance in the Japanese quail retina. Journal of Neuroscience. 1999;19:4132–4141. doi: 10.1523/JNEUROSCI.19-10-04132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglapus MK, Uchiyama H, Buelow NF, Barlow RB. Circadian rhythms of rod-cone dominance in the Japanese quail retina. Journal of Neuroscience. 1998;18:4775–4784. doi: 10.1523/JNEUROSCI.18-12-04775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiological Reviews. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Boelen MK. A retinal dark-light switch: a review of the evidence. Visual Neuroscience. 1996;13:399–409. doi: 10.1017/s0952523800008087. [DOI] [PubMed] [Google Scholar]
- Naka K-I, Rushton WAH. An attempt to analyse colour reception by electrophysiology. Journal of Physiology. 1966;185:556–586. doi: 10.1113/jphysiol.1966.sp008002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir I, Harrison JM, Haque R, Low MJ, Grandy DK, Rubinstein M, Iuvone PM. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. Journal of Neuroscience. 2002;22:2063–2073. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussdorf JD, Powers MK. Spectral sensitivity of the electroretinogram b-wave in dark-adapted goldfish. Visual Neuroscience. 1988;1:159–168. doi: 10.1017/s0952523800001437. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Besharse JC. Circadian regulation of retinomotor movements. I. Interaction of melatonin and dopamine in the control of cone length. Journal of General Physiology. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puopolo M, Hochstetler SE, Gustincich S, Wightman RM, Raviola E. Extrasynaptic release of dopamine in a retinal neuron: activity dependence and transmitter modulation. Neuron. 2001;30:211–225. doi: 10.1016/s0896-6273(01)00274-4. [DOI] [PubMed] [Google Scholar]
- Rashid K, Baldridge WH, Ball AK. Evidence for D2 receptor regulation of dopamine release in the goldfish retina. Journal of Neurochemistry. 1993;61:2025–2033. doi: 10.1111/j.1471-4159.1993.tb07438.x. [DOI] [PubMed] [Google Scholar]
- Raviola E, Gilula NB. Gap junctions between photoreceptor cells in the vertebrate retina. Proceedings of the National Academy of Sciences of the USA. 1973;70:1677–1681. doi: 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LM, Takahashi JS. Circadian clock in cell culture: II. In vitro photic entrainment of melatonin oscillation from dissociated chick pineal cells. Journal of Neuroscience. 1988;8:22–30. doi: 10.1523/JNEUROSCI.08-01-00022.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorderet M, Nowak JZ. Retinal dopamine D1 and D2 receptors: characterisation by binding or pharmacological studies and physiological functions. Cellular and Molecular Neurobiology. 1990;10:303–325. doi: 10.1007/BF00711177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanzara SA. The visual pigments of fresh water fishes. Vision Research. 1967;7:121–148. doi: 10.1016/0042-6989(67)90079-x. [DOI] [PubMed] [Google Scholar]
- Stell WK, Harosi FI. Cone structure and visual pigment content in the retina of the goldfish. Vision Research. 1976;16:647–657. doi: 10.1016/0042-6989(76)90013-4. [DOI] [PubMed] [Google Scholar]
- Stell WK, Lightfoot DO. Colour-specific interconnections of cones and horizontal cells in the retina of the goldfish. Journal of Comparative Neurology. 1975;159:473–501. doi: 10.1002/cne.901590404. [DOI] [PubMed] [Google Scholar]
- Tornqvist K, Yang X-L, Dowling JE. Modulation of cone horizontal cell activity in the teleost fish retina. III. Effects of prolonged darkness and dopamine on electrical coupling between horizontal cells. Journal of Neuroscience. 1988;8:2279–2288. doi: 10.1523/JNEUROSCI.08-07-02279.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Yamada M. Morphological and physiological studies of rod-driven horizontal cells with special reference to the question of whether they have axons and axon terminals. Journal of Comparative Neurology. 1987;255:305–316. doi: 10.1002/cne.902550213. [DOI] [PubMed] [Google Scholar]
- Viswanathan N, Weaver DR, Reppert SM, Davis FC. Entrainment of the foetal circadian pacemaker by prenatal injections of the dopamine agonist SKF38393. Journal of Neuroscience. 1994;14:5393–5398. doi: 10.1523/JNEUROSCI.14-09-05393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Harsanyi K, Mangel SC. Endogenous activation of dopamine D2 receptors regulates dopamine release in the fish retina. Journal of Neurophysiology. 1997;78:439–449. doi: 10.1152/jn.1997.78.1.439. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mangel SC. A circadian clock regulates rod and cone input to fish retinal cone horizontal cells. Proceedings of the National Academy of Sciences of the USA. 1996;93:4655–4660. doi: 10.1073/pnas.93.10.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler R, Baldridge WH, Mangel SC, Dowling JE. Modulation of endogenous dopamine release in the fish retina by light and prolonged darkness. Visual Neuroscience. 1997;14:351–356. doi: 10.1017/s0952523800011470. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurones dissociated from rat suprachiasmatic nucleus express independently phased firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Da Prada M, Reme CE. Circadian rhythm in rat retinal dopamine. Neuroscience Letters. 1984;45:21–25. doi: 10.1016/0304-3940(84)90323-9. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Nicholson C, Rice ME, Bohmaker K, Meller E. Extracellular dopamine concentration in the retina of the clawed frog, Xenopus laevis. Proceedings of the National Academy of Sciences of the USA. 1993;90:5667–5671. doi: 10.1073/pnas.90.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, Shakib M. Interreceptoral junctions in the teleost retina. Investigative Ophthalmology and Visual Science. 1974;13:996–1009. [PubMed] [Google Scholar]
- Witkovsky P, Stone S, Besharse JC. Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain Research. 1988a;449:332–336. doi: 10.1016/0006-8993(88)91048-7. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Stone S, Macdonald ED. Morphology and synaptic connections of HRP-filled axon-bearing horizontal cells in the Xenopus retina. Journal of Comparative Neurology. 1988b;275:29–38. doi: 10.1002/cne.902750104. [DOI] [PubMed] [Google Scholar]
- Yang X-L, Tornqvist K, Dowling JE. Modulation of cone horizontal cell activity in the teleost fish retina. II. Role of interplexiform cells and dopamine in regulating light responsiveness. Journal of Neuroscience. 1988;8:2269–2278. doi: 10.1523/JNEUROSCI.08-07-02269.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-L, Wu SM. Modulation of rod-cone coupling by light. Science. 1989;244:352–354. doi: 10.1126/science.2711185. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Lin Z-S. Differential effects of dopamine depletion on the distribution of 3H-SCH23390 and 3H-spiperone binding sites in the goldfish retina. Vision Research. 1995;17:2409–2414. [PubMed] [Google Scholar]
- Yazulla S, Zucker CL. Synaptic organisation of dopaminergic interplexiform cells in the goldfish retina. Visual Neuroscience. 1988;1:13–29. doi: 10.1017/s0952523800000997. [DOI] [PubMed] [Google Scholar]


