Abstract
Inward-rectifying potassium (Kir) channels are essential for maintaining the resting membrane potential near the K+ equilibrium and they are responsible for hyperpolarisation-induced K+ influx. We characterised the Kir current in primary cultured ovine somatotropes and examined the effect of growth hormone-releasing peptide-2 (GHRP-2) on this current and its related intracellular signalling pathways. The Kir current was, in most cases, isolated using nystatin-perforated patch-clamp techniques. In bath solution containing 5 mm K+, the Kir current was composed of both transient (fast activated) and delayed (slowly activated) components. An increase in the external K+ concentration from 5 to 25 mm induced an augmentation of ≈4-fold in the delayed part of the Kir current and both BaCl2 and CsCl dose-dependently inhibited this current, confirming the presence of the Kir current in ovine somatotropes. Moreover, this specific effect of high K+ on the Kir current was only observed in the cells that showed positive staining with anti-growth hormone (GH) antibodies, or in GC cells that belong to a rat somatotrope cell line. Application of GHRP-2 (100 nm) reversibly and significantly reduced the Kir current in bath solutions with 5 or 25 mm K+ in ovine somatotropes. In addition, we found that the reduction in the Kir current mediated by GHRP-2 was totally abolished by the pretreatments with H89 (1 μm) or Rp-cAMP (100 μm) or by intracellular dialysis of a specific protein kinase A (PKA) inhibitory peptide PKI (10 μm). The specific PKC blocker chelerythrine (1 μm) or inhibitory peptide PKC19-36 (10 μm) did not show any effects on the GHRP-2-induced decrease in the Kir current. These results suggest that the inhibition of Kir current through PKA-cAMP pathways may play an integral role in GHRP-2-induced depolarisation and GH release in ovine somatotropes.
The release of growth hormone (GH) from the anterior pituitary is predominantly controlled by two integrated actions: a stimulation from hypothalamic neuropeptides, growth hormone-releasing hormone (GHRH) and/or ghrelin, and an inhibition from somatostatin (Muller et al. 1999). Since the successful synthesis of GHRP-6 in the late 1970s, and despite its weak efficacy on GH secretion, great efforts have been made to define the pathways employed by the GHRP series of polypeptides in their regulation of GH secretion (Bowers, 1998; R. G. Smith et al. 2001). GHRP-2 is one such GH secretagogue with a strong potency for the stimulation of GH release in both humans and animals. The recent successful isolation of ghrelin, the possible endogenous ligand for the growth hormone secretagogue (GHS) receptor, has further strengthened the notion that GHRPs may play a more important role in the regulation of GH secretion and other physiological functions (Kojima et al. 1999).
Inward rectifying K+ (Kir) channels are distributed in many cell types (Isomoto et al. 1997) and the Kir currents in somatotropes of different species have been characterised (Bauer et al. 1990; Sims et al. 1991; Wulfsen et al. 2000; Gregerson et al. 2001). In primary cultured pituitary cells and GH3 cells, it has been shown that the inhibition of Kir currents may generate a higher firing rate of action potentials and subsequently enhance the secretion of prolactin (PRL) and GH (Bauer et al. 1994, 1999). Given the critical role of the Kir channels in maintaining the resting membrane potential near the K+ equilibrium and inducing K+ influx during hyperpolarisation, we hypothesise that the Kir currents may be crucial in the regulation of GH secretion by GHRP-2 in ovine somatotropes.
It has been demonstrated that GHRPs regulate the resting membrane potential through the modification of ion channels in somatotropes in different species. In primary cultured ovine and human somatotropes, voltage-gated L-and T-type Ca2+ currents are modified by GHRH and somatostatin as well as by GHRP-2 (Takei et al. 1996; Chen, 2000). GHRP-2-induced GH release in vitro was significantly inhibited by nifedipine, a specific L-type voltage-gated Ca2+ channel blocker, indicating that the Ca2+ currents are critical to the control of GH release (Roh et al. 1997; Chen, 2000). Other ion channels, e.g. the voltage-independent Ca2+-activated K+ and Na+ channels, may also be involved in the GHRP-stimulated GH secretion (McGurk et al. 1993; Kato & Sakuma, 1999). However, these channels are activated at more positive voltage levels than the Kir channels, which are normally elicited at the resting membrane potential.
The intracellular signal transduction systems by which GHRPs stimulate GH secretion in vitro are still controversial. Studies have shown that growth hormone secretagogue (GHS) receptors are coupled to phospholipase C (PLC) and intracellular Ca2+, which are mainly controlled by the transmembrane Ca2+ channels (Howard et al. 1996). Protein kinase C (PKC) has been demonstrated to be the other major signalling pathway employed by GHRPs in GH regulation (Chen, 2000). For example, pretreatment of human acromegalic tumour cells with a specific PKC inhibitor, calphostin C, completely suppressed the GHRP-6-induced GH increase (Adams et al. 1996). Meanwhile, the PKA-cAMP pathway may also be involved in GHRP-2-enhanced GH release in ovine or bovine somatotropes (Roh et al. 1997; Chen, 2000). It is possible that GHRPs may stimulate GH release through various signalling systems in different species.
In the present study, the Kir current in primary cultured ovine somatotropes was characterised and the effect of GHRP-2 on the current was examined. The involvement of second messenger systems including PKA-cAMP and PKC was also studied. Our findings indicate that the inhibition of Kir current through the PKA-cAMP signalling pathway may contribute to the GHRP-2-induced depolarisation and GH secretion in ovine somatotropes.
Methods
Chemicals
GHRP-2 was generously supplied by Professor C. Y. Bowers (Tulane University, New Orleans, LA, USA). Dulbecco's modified Eagle's medium (DMEM), N-2-hydroxyethylpiperazine-N‘-2-ethanesulphonic acid (Hepes) and carbohydrate solutions were purchased from Trace Biosciences Pty Ltd (Clayton, Victoria, Australia). Medium 199 (M199) and collagenase type-I were obtained from Worthington Biochemical Corporation (Freehold, NJ, USA) and penicillin-streptomycin fungizone solution was from CSL Ltd (Parkville, Victoria, Australia). Tissue culture reagents (DNase, hyaluronidase, trypsin inhibitor, pancreatin), sera, adenosine-5′-triphosphate (ATP), creatine phosphokinase, phosphocreatine, guanosine-5′-triphosphate (GTP), barium chloride, caesium chloride, propidium iodide (PI) and all salts for recording solutions were purchased from Sigma (St Louis, MO, USA). Tetrodotoxin (TTX) was from Alomone Labs, Jerusalem, Israel. Protein kinase A (PKA) and PKC inhibitory peptides (PKI and PKC19-36) were obtained from Research Chemicals International (Natick, MA, USA). Chelerythrine chloride, adenosine-3′,5′-cyclic phosphorothiolate-RP (Rp-cAMP) and N-(2-(p-bromocinnamyl-amino)ethyl)-5-iso-quinolinesulphonamide (H89) were purchased from Calbiochem (Alexandria, NSW, Australia). Anti-GH guinea-pig polyclonal antiserum and the goat anti-guinea-pig IgG were from Vector Laboratories (Burlingame, CA, USA).
Cell preparation
The adult sheep pituitary glands were obtained at the time of slaughter from a local abattoir and then subjected to collagenase/ pancreatin treatments to dissociate the cells as described previously (Chen et al. 1994a). Briefly, whole pituitary glands were divested of encapsulating tissue and the neurohypophysis and pituitary stalk tissue. The anterior pituitary glands were then minced and placed into calcium-free phosphate-buffered solution (PBS) with added bovine serum albumin (BSA, 0.5 %) for approximately 5 min. The tissue fragments were gently washed and incubated in DNase (20 μg ml−1), hyaluronidase (1 mg ml−1), trypsin inhibitor (0.5 μg ml−1), pancreatin (0.5 μg ml−1) and collagenase (3 mg ml−1) for 30-40 min (at 37 °C in a shaking water bath). After centrifugation at 650 g, the dissociated cells were suspended in the M199 and counted. Cell yield was usually (2-4) × 107 per pituitary gland, with more than 95 % viability (trypan blue exclusion test). The cell suspension (3-5 ml) was then placed, under sterile conditions, above a column of layers of an increasing density of Percoll solutions in a centrifuge tube. In our experimental condition, seven Percoll dilutions were prepared for the discontinuous density gradient as follows: 1.10, 1.074, 1.071, 1.068, 1.063, 1.058, 1.040 and 1.029 g ml−1 (calibrated by density mark beads from Pharmacia Biotech, Uppsala, Sweden). The tube was spun at ≈1800 g for 30 min at 4 °C in a centrifuge with the brake set to zero. About 80 % of cells in fractions 2 and 3 with densities ranging from 1.063-1.071 were identified as somatotropes, which was confirmed by immunohistochemical staining (Chen et al. 1994a). Cells were then removed from each layer and the fractions with higher percentages of somatotropes were collected and subsequently seeded into 35 mm culture dishes containing DMEM supplemented with 10 % fetal calf serum (FCS), 1 % (v/v) l-glutamine (200 mm) and 1 % (v/v) penicillin-streptomycin fungizone solution in a humidified incubator (37 °C, 5 % CO2). The culture medium was replenished every 2-3 days and electrophysiological recordings were performed between days 4 and 14 in culture.
Electrophysiological recording
On the day of recording, culture medium was replaced by patch-clamp bath solution through a gravity pressure perfusion system at least 10 min before recording. All the recordings were made using nystatin-perforated patch-clamp techniques except for the studies on the blocking effect of PKI and the specific inhibitory PKC peptide. Electrodes were pulled by a Sutter P-87 microelectrode puller from borosilicate micropipettes with an inner filament (Clark Electromedical Instruments, Pangbourne, Reading, UK) and had an initial input resistance of 2-5 MΩ. All recordings were made using the Axopatch 200A amplifier (Axon Instruments). The bath solution was composed of the following (mm): 140 NaCl, 5 KCl, 0.5 MgCl2, 0.5 CaCl2, 1 CdCl2, 10 glucose and 10 Hepes, adjusted to pH 7.4 with NaOH; osmolarity 300 mosmol l−1 with sucrose. To prevent contamination by Na+ current, TTX was added to the bath solution at a final concentration of 1 μm and this bath solution was used for up to 3 days only, due to the degradation of TTX. The pipette solution was composed of the following (mm): 55 KCl, 75 K2SO4, 8 MgSO4 and 10 Hepes. Just before recording, nystatin (150 μg ml−1) was added to the pipette solution. In experiments requiring the use of classic whole-cell recording (WCR), the pipette solution was composed of the following (mm): 140 KCl, 5 NaCl, 0.5 MgCl2, 10 EGTA and 10 Hepes. An ATP regenerating system (2 mm ATP, 5 mm Na2-phosphocreatine, 20 U ml−1 creatine phosphokinase) plus 0.1 mm GTP were added to the pipette solution (pH adjusted to 7.4 and osmolarity to 300 mosmol l−1) in the studies with PKI or PKC19-36 (see Results section for details).
After obtaining a high-resistance seal, the voltage in the pipette was held at −80 mV with pulses (10 mV, 200 ms duration) delivered periodically to monitor the access resistance. The access to the cell interior was judged by the appearance of a membrane capacitance transient current 2-5 min after forming a seal. Whole-cell capacitance (mean ± s.e.m. 7.4 ± 0.5 pF, n = 81 recorded cells) and series resistance were compensated (≈80 %) before experimentation and leak current was routinely subtracted using the options offered by the Clampex-7 program (Axon Instruments). We also monitored the change in series resistance over the course of each experiment, and the recordings with significant change in series resistance or with a series resistance over 30 MΩ were excluded from the final data analysis. The electrical signal was filtered at 2 kHz with a low-pass filter built into the amplifier, and the sweeps were sampled at 1 ms intervals between data points in our recording protocols.
Cell culture dishes were fixed on the stage of an Olympus inverted microscope, and a gravity pressure system was used to perfuse the cells at a rate of approximately 1 ml min−1. PKA-cAMP and PKC blockers, including H89, Rp-cAMP and chelerythrine, were added by hand to the culture dishes containing the cells to be recorded when the perfusion system was temporarily on pause. Cells were incubated with blockers for at least 5 min in the bath solution in order to achieve an even distribution (the concentrations cited in the Results section are the final concentrations after blockers were diluted in the bath solution). Clamp control studies showed that this procedure did not cause appreciable changes in the currents. GHRP-2 diluted in bath solution or vehicle was applied through the perfusion system. Application of vehicle from the same system did not change the Kir currents in our recording conditions. All experiments were performed at room temperature (20-22 °C).
Immunocytochemistry
Cells to be stained were marked soon after the recordings were completed. Dishes were gently rinsed with 0.05 m phosphate-buffered saline and then washed in 0.1 m phosphate buffer (PB) for 40 min. Cells were fixed for 30 min using 4 % paraformaldehyde at room temperature. After washing, cells were incubated in the blocking solution (10 % normal goat serum, 0.3 % Triton X-100 and 0.24 % streptavidin in PB) for 30 min. The dishes were again washed and cells were incubated for 48 h at 4 °C with guinea-pig anti-oGH polyclonal antiserum (1:5) diluted in a solution containing: 10 % normal goat serum, 0.24 % biotin, 1 mg ml−1 NaNH3 and 0.3 % Triton X-100. Soon after washing, dishes were incubated with biotinylated goat anti-guinea-pig IgG (1:500, Vector Laboratories) in PB for 1 h, further washed and reacted with avidin-FITC (1:500, Vector) for 1 h. Cells were then washed and counterstained with PI at 1:100 for 30 min. The reaction was stopped after the dishes were rinsed for 5 min in PB. Finally, the dishes were cover-slipped with Dako antefix mounting medium (Dako, Carpinteria, CA, USA). The GH staining was examined and photographed using an Olympus BX-50 fluorescent microscope fitted with a Fujix HC-2000 high-resolution digital camera.
Data analysis
pCLAMP 7.0 software (Axon Instruments) was used to acquire data during the recording and to analyse the data afterwards. Student's paired t test was used as appropriate to evaluate the statistical significance of differences between two group means and the effect was considered to be significant at the level of less than 5 %. Group data are expressed as means ± s.e.m. in the Results section. The concentrations cited in the Results were the final concentrations after an even distribution. The traces in figures were representatives of at least four recordings under the same experimental conditions unless indicated otherwise in the text.
Results
The Kir current in ovine somatotropes
Membrane ion channels including voltage-dependent outward K+, Ca2+ and TTX-sensitive Na+ currents have been characterised in somatotropes from different species (Chen et al. 1994b; Kato & Sakuma, 1997; Xu et al. 1999). To isolate the Kir current, cells were bathed in the solution containing 5 mm K+ supplemented with 1 μm TTX and 1 mm CdCl2 to prevent possible contamination by Ca2+ and Na+ currents. The Kir current was recorded by a series of voltage commands from −160 to −40 mV at the holding potential of −50 mV using nystatin-perforated patch-clamp techniques (Fig. 1A). At least two distinctive components of the Kir current were identified: an initial rapidly activated and inactivating (transient) component in the first 20-30 ms, followed by a slowly activated (delayed) component in the remaining 200 ms of the voltage step (Fig. 1A). Due to the significant overlapping between the residual capacitance and the transient Kir current, an expanded time scale is used to separate them (inset in Fig. 1). The current versus voltage (I-V) curves of the two Kir components exhibited inwardly rectifying kinetics, especially for the delayed part of the Kir current (Fig. 1B). It should be noted that there were a small number of cells recorded with predominantly the transient current. Cells that possessed different Kir kinetics or were unresponsive to high external K+ were subsequently excluded from the study because they were not stained by anti-GH antibodies (see sections below).
Figure 1. The presence of two types of inward rectifying K+ currents in ovine somatotropes with standard K+ (5 mm) bath solution.
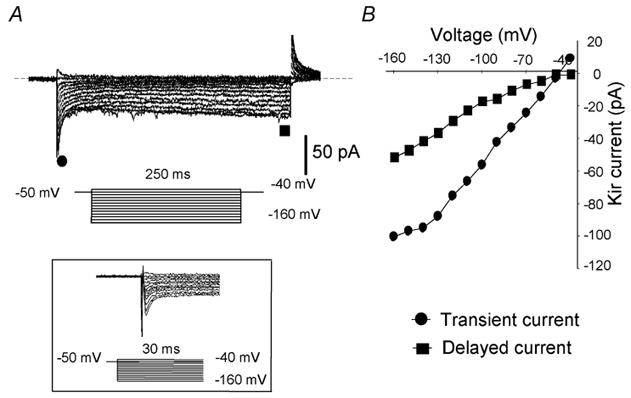
A, the Kir currents were recorded with a 10 mV interval by hyperpolarising test pulses from −160 to −40 mV with the holding potential at −50 mV. The dashed line indicates the zero current level. Inset: the capacitance and the transient Kir component are distinguishable on an expanded time scale. B, current-voltage relationships of two subtypes of Kir currents measured at the highest points for the rapid initial current (•) and at the end of the 250 ms voltage step for the delayed current (▪).
Responses to high external K+ in the Kir current
Since one of the characteristics of the Kir current is that its conductance increases proportionately with the extracellular K+ concentration (Hagiwara & Takahashi, 1974; Kubo et al. 1993), we examined this specific current property in somatotropes by bathing the cells in a normal K+ (5 mm) solution followed by a challenge with higher K+ (25 mm). The cells were subsequently classified into two subgroups based on their responses to higher K+. In the first group of cells (Fig. 2A), an increase in the external K+ from 5 to 25 mm caused a 3- to 4-fold reversible enlargement in the delayed Kir current, while the transient part of the Kir current exhibited only a marginal increase (compare Fig. 2Aa and b). This response pattern was observed in the majority of cells recorded. In contrast, no such changes occurred in the other group of cells (compare Fig. 2Ba and b). The I-V curves (produced from the maximal values in delayed currents recorded at the testing pulses) indicate that the responses to high K+ in these two subgroups of cells were apparently different (Fig. 2Ad and Bd). After calculation, the ratio of the Kir conductances in the responsive cells with 5 mm and 25 mm external K+ was 0.43, a value very close to the ratio of the square roots of the external K+ concentrations: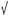 5/
5/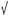 25 + 0.44, as previously described (Kuryshev et al. 1997). We also examined the effect of high K+ on the Kir current in a known GH cell line, GC cells. A similar phenomenon was observed although the increase in the Kir current as a result of high external K+ was smaller (compare Fig. 2Ab and Cb). The summarised data for a group of GC cells is shown in Fig. 2Cd. Since only the delayed Kir current had typical inward rectifying kinetics and was sensitive to changes in extracellular K+ and GHRP-2 (see sections below), we only targeted this part of the Kir current in the following experiments.
25 + 0.44, as previously described (Kuryshev et al. 1997). We also examined the effect of high K+ on the Kir current in a known GH cell line, GC cells. A similar phenomenon was observed although the increase in the Kir current as a result of high external K+ was smaller (compare Fig. 2Ab and Cb). The summarised data for a group of GC cells is shown in Fig. 2Cd. Since only the delayed Kir current had typical inward rectifying kinetics and was sensitive to changes in extracellular K+ and GHRP-2 (see sections below), we only targeted this part of the Kir current in the following experiments.
Figure 2. Responses of the Kir current to high external K+ (25 mm) in ovine somatotropes and GC cells.
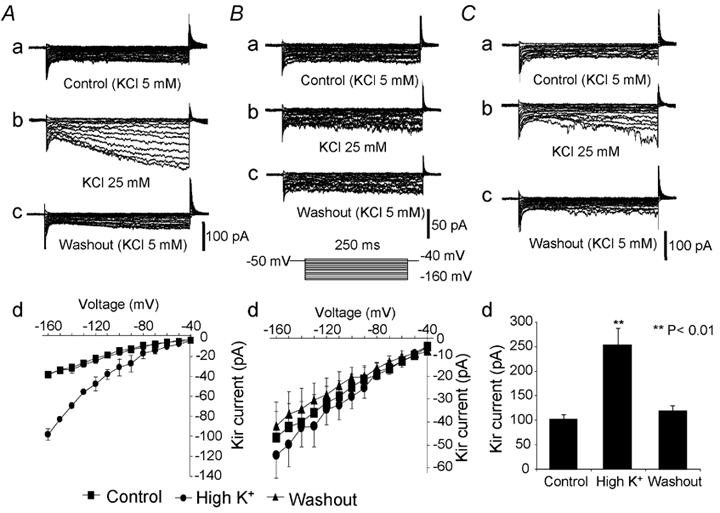
Current traces are shown from representative cells in response to high external K+. The Kir currents were recorded with a 10 mV interval by hyperpolarising test pulses from −160 to −40 mV with the holding potential at −50 mV. The I-V curves (Ad and Bd) were constructed from the peak values of the delayed Kir currents. A, data from a representative cell responsive to high external K+. Traces show the Kir current in control (Aa), after application of 25 mm K+ (Ab), and recovery in Kir after 25 mm K+ was replaced with 5 mm K+ (Ac). Ad, I-V curves showing the effect of high K+ (25 mm) on the Kir current (mean ± s.e.m., n = 8). B, data from a representative cell unresponsive to high external K+. Traces show the Kir current in control (Ba), after application of 25 mm K+ (Bb), and recovery after 25 mm K+ was replaced with 5 mm K+ (Bc). Bd, I-V curves showing the effect of high K+ (25 mm) on the Kir current (mean ± s.e.m., n = 6). C, data from a representative GC cell in response to high external K+. Traces show the Kir current in control (Ca), after application of 25 mm (K+) (Cb), and recovery after 25 mm K+ was replaced with 5 mm K+ (Cc). Cd, the effect of high K+ (25 mm) on the Kir current in GC cells (mean ± s.e.m., n = 4). The data were generated from the peak values of the Kir current evoked by a hyperpolarising pulse to −160 mV from a holding potential of −50 mV.
GH staining
In order to differentiate between the cells responding differently to high K+, we performed GH immunocytochemical staining on these cells. To mark the recorded cell, a rectangle (lines or dashed lines) was drawn on the bottom of the dish using a second pipette located near the cell soon after the recordings were completed (Fig. 3Aa and Ba). In a group of six cells with characteristic responses to higher external K+, positive GH staining was observed in all the cells, confirming that these were somatotropes (Fig. 3Aa and b). However, no GH staining was detected on cells without such a response to high K+ (Fig. 3Ba and b). It was also noted that GH staining was not evenly distributed in some cells, especially after the WCR (Fig. 3Ab). We concluded that, at least in our culture conditions, cells that did not show a characteristic response to high K+ were not somatotropes. Based on this correlation, all cells were challenged with 25 mm K+ after the experimental recordings were completed. Cells lacking the specific response to high K+ in their Kir current were excluded from the final data analysis.
Figure 3. GH immunostaining of cultured pituitary cells after electrophysiological recording.
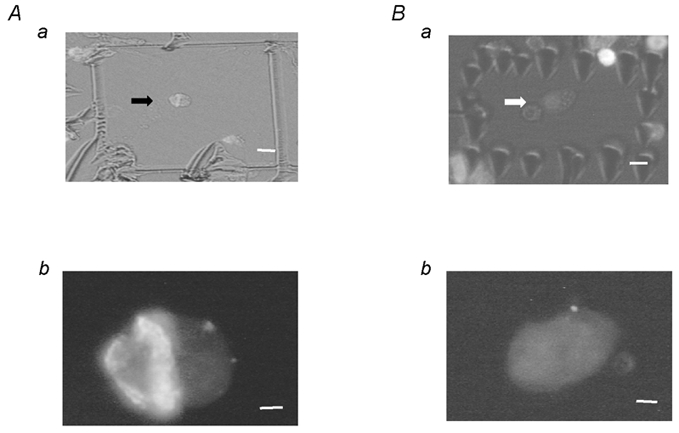
A, positive GH staining in a representative cell showing response to high external K+ as in Fig. 2A. Aa shows the recorded cell as a rectangle drawn by a second pipette located close to the recording pipette after the recording was completed. Ab shows the positive staining of GH under a fluorescence microscope. A representative cell with negative GH staining is shown in B. The recording conditions correspond to those in Fig. 2B. Scale bars in upper and lower panels represent 4 and 1 μm, respectively.
Effects of BaCl2 and CsCl on the Kir current in somatotropes
The Kir channels display a particularly high affinity for monovalent and divalent cations (Kubo et al. 1993). We thus tested the inhibitory effects of CsCl and BaCl2 on the Kir current in ovine somatotropes. To systematically evaluate the effects of these blockers effectively, a large control Kir current was recorded in the bath with 25 mm K+. Increasing concentrations of BaCl2 at 10, 50 and 150 μm dose-dependently blocked the Kir current, as shown by the I-V curves in Fig. 4A. A very similar blocking effect was also evident with a series of concentrations of CsCl at 10, 50 and 250 μm (Fig. 4B).
Figure 4. Dose-dependent curves of the Kir current in response to BaCl2 and CsCl.
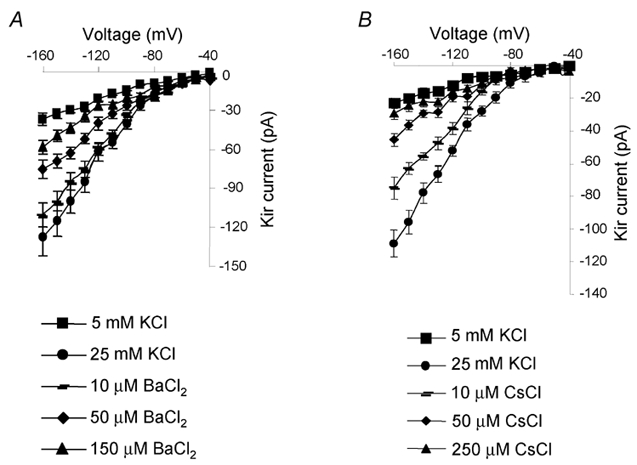
A, high K+ (25 mm) induces a significant increase in the Kir current (indicated by ▪ at 5 mm K+ and • at 25 mm K+). BaCl2 dose-dependently reduces the Kir current (mean ± s.e.m., n = 4). B, high K+ (25 mm) induces a significant increase in the Kir current (indicated by ▪ at 5 mm K+ and • at 25 mm K+). CsCl dose-dependently reduces the Kir current (mean ± s.e.m., n = 4).
In summary, the high selectivity to K+, the approximately square root dependence of conductance on the external K+ concentration and the special sensitivity to Ba2+ and Cs+ suggested that the current we obtained in ovine somatotropes is indeed the Kir current.
Effect of GHRP-2 on the Kir current in ovine somatotropes
The response of the Kir current to GHRP-2 is shown in Fig. 5A, in which the traces were taken from recordings in a representative cell. GHRP-2 (100 nm) significantly and reversibly decreased the Kir current when cells were bathed in 5 mm external K+. When further challenged by 25 mm external K+, this current showed a typical increase as previously described, suggesting that the recorded cells were somatotropes. The response pattern in the Kir current to GHRP-2 and high K+ is also depicted in the I-V curves (Fig. 5B). In order to verify these findings, we further examined the effect of GHRP-2 on the Kir current using a bath solution with higher external K+ (25 mm). A similar responsiveness in the Kir current was recorded. In comparison, the reduction in the Kir current by GHRP-2 in the bath solution with 25 mm K+ was, nevertheless, more prominent than that in 5 mm K+ solution (compare Fig. 6A and B).
Figure 5. Effect of GHRP-2 on the Kir current in ovine somatotropes.
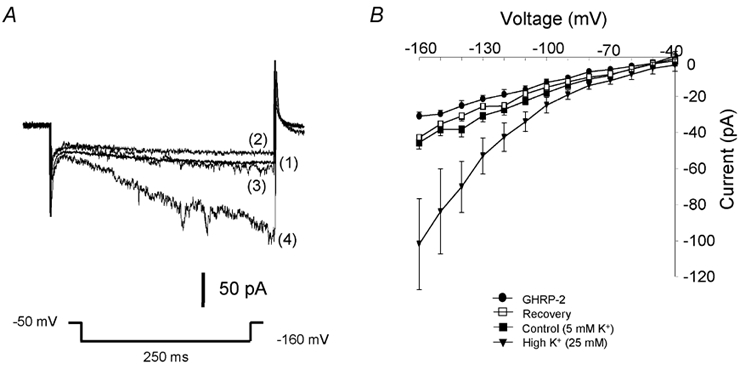
A, traces are shown from a representative cell. The Kir current was measured at the voltage step to −160 mV with the holding potential of −50 mV. Traces show the Kir currents in control (1), 3 min after the application of 100 nm GHRP-2 (2), 3 min after removal of GHRP-2 (3) and finally challenged by 25 mm external K+ (4). B, I-V curves of the Kir current show that GHRP-2 significantly and reversibly decreases the amplitude of the Kir current (mean ± s.e.m., n = 6). All cells responsive to GHRP-2 also respond well to high external K+.
Figure 6. Effects of GHRP-2 on the Kir current at different K+ concentrations.
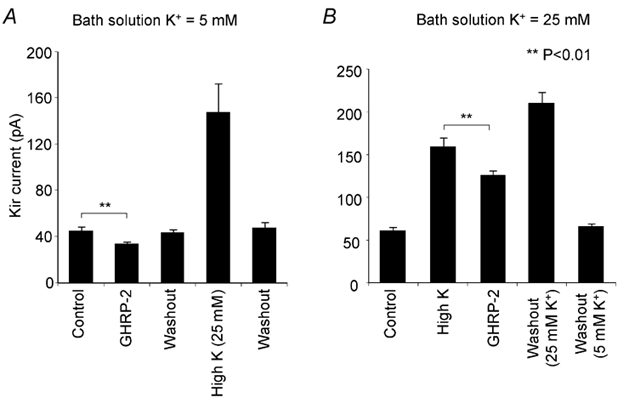
Graphs were generated from the peak values of the Kir current evoked by a hyperpolarising pulse to −160 mV from a holding potential of −50 mV. GHRP-2 significantly and reversibly decreases the amplitude of the Kir current in both standard (5 mm external K+ in A) and high K+ (25 mm external K+ in B) conditions (mean ± s.e.m., n = 4). All cells respond well to GHRP-2 and high external K+.
We found that, at 5 mm extracellular K+ (close to physiological conditions), the Kir currents obtained were much more stable and the recordings lasted longer than that at 25 mm K+. It was decided, therefore, that the effect of GHRP-2 on the Kir current should be examined in the bath solution with 5 mm K+ in the following experiments.
Time course of the effect of GHRP-2 on the Kir current
The time course of the response to GHRP-2 in Kir current is shown in Fig. 7. The inhibitory effect of GHRP-2 on the Kir current occurred soon after its administration and then it gradually reached the maximum in about 3-4 min. A full and fast recovery of the Kir current was observed 2-3 min after the removal of GHRP-2. It was also apparent that there was an instant and remarkable increase in the Kir current in response to high K+ (25 mm).
Figure 7. Time-dependent curve of the Kir current in response to GHRP-2.
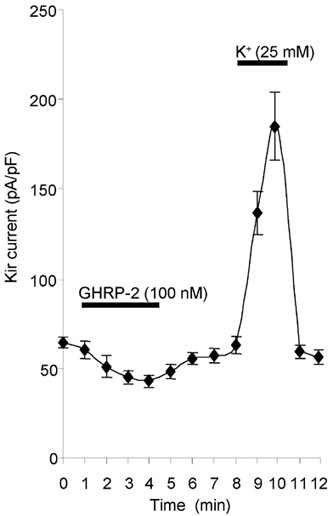
GHRP-2 (100 nm) significantly decreases and high external K+ increases the Kir current (mean ± s.e.m., n = 5).
Involvement of the PKA-cAMP system in the response of Kir current to GHRP-2
We finally examined the possible involvement of the second messenger signalling pathways in the inhibitory effect of GHRP-2 on Kir current. A series of blockers has been successfully employed previously in the electrophysiological assessment of the involvement of PKA-cAMP in GHRH-induced change in Ca2+ and K+ currents in somatotropes (Chen, 2000; Xu et al. 2000). In this study, the cell-permeable blocker H89 (1 μm) or Rp-cAMP (100 μm) was added directly to the bath solution and an even distribution was achieved in 5 min. The basal Kir current was unchanged when the cells were individually incubated with H89 or Rp-cAMP. However, the GHRP-2-induced decrease in the Kir current was completely abolished in the presence of these reagents (Fig. 8A and B). PKI is a synthetic PKA inhibitory peptide that is not able to permeate through the cell membrane and it was subsequently included in the pipette solution to be dialysed into the cells as previously described (Chen, 1997). After the administration of PKI (10 μm), the reduction in Kir current mediated by GHRP-2 was also totally inhibited (Fig. 8C). These results indicate that an intact PKA-cAMP system is required in the GHRP-2-induced reduction in the Kir current.
Figure 8. Involvement of cAMP-PKA in the response of Kir current to GHRP-2.
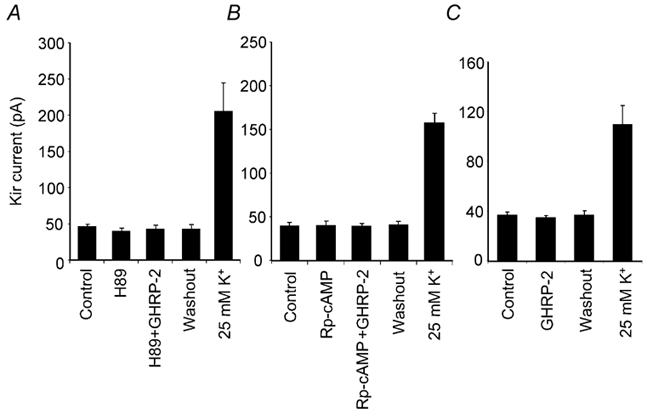
The effects of H89, Rp-cAMP and the PKA inhibitory peptide (PKI) on GHRP-2-induced reduction in the Kir current in ovine somatotropes. Statistical data were derived from the peak values of the Kir current evoked by a hyperpolarising pulse to −160 mV from a holding potential of −50 mV. A, the data (mean ± s.e.m., n = 4) show that the application of H89 (1 μm, 5 min) did not modify the basal Kir current, but prevented GHRP-2 from reducing the Kir current. B, the data (mean ± s.e.m., n = 5) show that the application of Rp-cAMP (100 μm, 5 min) did not modify the basal Kir current, but prevented GHRP-2 from reducing the Kir current. C, because PKI is not membrane permeable, internal dialysis was required to introduce this peptide into the recorded cell. The conventional whole-cell recording configuration was employed in this set of experiments as detailed in the Methods. The data (mean ± s.e.m., n = 4) show that inclusion of PKI (10 μm) in the internal solution for 10 min abolished GHRP-2-induced reduction in the Kir current. The Kir current in all cells included in these groups responded well to 25 mm K+.
Involvement of the PKC system in the response of the Kir current to GHRP-2
To assess the involvement of the PKC system, we evaluated the possible inhibitory effects of chelerythrine and PKC19-36 on the GHRP-2-induced effect on the Kir current. Both chemicals were shown to effectively and selectively inhibit PKC activities in previous studies under similar culture conditions (Xu et al. 1999). Incubation of the cells with chelerythrine at a concentration of 1 μm for 5 min did not change the basal current amplitude, and the decrease in Kir current by GHRP-2 was sustained in the presence of chelerythrine (Fig. 9A). Furthermore, we micro-dialysed PKC19-36, a specific PKC inhibitory peptide (10 μm), into the cells. The GHRP-2-induced reduction in Kir current was not affected (Fig. 9B). These results strongly suggest that the PKC signalling pathway is not involved in the GHRP-2-mediated decrease in Kir current in ovine somatotropes.
Figure 9. Involvement of the PKC system in the Kir current response to GHRP-2.
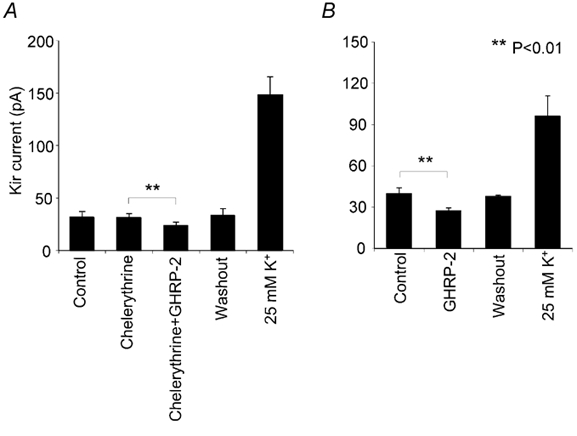
Using the same experimental procedure as in Fig. 8, the effects of chelerythrine and a specific PKC inhibitory peptide PKC19-36 were tested. Statistical data were derived from the peak values of the Kir current evoked by a hyperpolarising pulse to −160 mV from a holding potential of −50 mV. A, the data (mean ± s.e.m., n = 5) show that the application of chelerythrine (1 μm, 5 min) did not modify the Kir current. GHRP-2 caused a significant and reversible reduction in the amplitude of the Kir current. B, the PKC inhibitory peptide PKC19-36 is not membrane permeable. Internal dialysis was therefore used to introduce this peptide into the recorded cell. The conventional whole-cell recording configuration was employed in this set of experiments as detailed in the Methods. The results (mean ± s.e.m., n = 4) show that the inclusion of PKC19-36 (10 μm) in the internal solution for 10 min did not influence the GHRP-2-induced reduction in the Kir current. Again, Kir in all the cells included in these groups responded well to 25 mm K+.
Discussion
Recent studies on the expression of different Kir currents in GH3/B6 (a rat GH cell line) and rat anterior pituitary gland cells have provided some evidence that the Kir current may play an important role in the regulation of pituitary functions (Wulfsen et al. 2000; Gregerson et al. 2001). Using nystatin-perforated patch-clamp and immunohistochemistry techniques, the present study demonstrates the presence of the Kir current in primary cultured ovine somatotropes, and shows that the current possesses similar kinetic characteristics to those identified in primary cultured pituitary cells in other species (Kuryshev et al. 1997; Takano et al. 1997a). More importantly, the Kir current was significantly and reversibly reduced by GHRP-2, suggesting that such a modification in the Kir current may contribute to GHRP-2-induced depolarisation and GH secretion in ovine somatotropes. In addition, the PKA- cAMP system was demonstrated to be involved in the reduction of the Kir current mediated by GHRP-2.
Presence of Kir in ovine somatotropes
The Kir currents in both GH3 cells and primary cultured rat anterior pituitary cells have been characterised (Bauer et al. 1990; Barros et al. 1994; Schafer et al. 1999), and the expression of the Kir channels and their subunits in these cells has been demonstrated more recently (Wulfsen et al. 2000; Gregerson et al. 2001). We characterised the Kir current in primary cultured ovine somatotropes in a bath solution containing a standard concentration of external K+ (5 mm) using nystatin-perforated patch-clamp recordings. At the negative voltage steps up to −160 mV, the Kir current was elicited and comprised transient (fast activated) and delayed (slowly activated) components, both of which were time and voltage dependent. After a 5-fold increase in the external K+, the majority of cells responded with an increase in both transient and delayed Kir components; however, the delayed current was increased much more prominently (≈4-fold). The other cells did not exhibit such a specific response to high external K+ in the Kir current. These two characteristic effects necessitated the use of GH immunostaining to differentiate between these cells, either morphologically or functionally. We confirmed that cells without the typical response to 25 mm K+ were indeed not somatotropes due to the fact that no GH staining was detected. It was also found that most cells with positive GH staining were relatively small in size (6-8 μm). This distinctive correlation eventually not only allowed us to differentiate somatotropes from other cells, but also assisted in confirming that the Kir current was a property of somatotropes.
We have been aware that the Kir current in GH3 cells has been particularly well characterised and that this cell line has served as a useful model in the investigation of the role of Kir currents in the regulation of hormone secretion (Bauer et al. 1990; Schafer et al. 1999). In the present study, the Kir current in ovine somatotropes showed similar characteristics to those reported previously (Bauer et al. 1990; Kuryshev et al. 1997). The characteristic effect of high K+ on the delayed component of Kir current has only been previously described in primary cultured rat corticotropes and human somatotropes (Sims et al. 1991; Takano et al. 1997b). However, these responses differed from those of the GH3 cell line, in which the Kir current was predominantly the fast-activated transient component (Bauer et al. 1990; Schafer et al. 1999; Wu et al. 2001). The reason for the discrepancy is still unclear. It is possible that Kir channels (a large family) in different cell types may comprise different Kir subunits possessing rather distinctive biophysical properties. Some of these Kir subunits may finally determine the cell functions. Therefore, further studies on the expression of different Kir subunits in ovine somatotropes would be of great value in providing a better understanding of their biophysical properties.
Physiological role of the Kir current in ovine somatotropes
The essential functions of Kir channels have been well recognised in lactotropes, neuroblastoma cells, blood vessel cells and cardiac myocytes (Arcangeli et al. 1995; Sanguinetti et al. 1995; Schafer et al. 1999; Jiang et al. 2001; Wu et al. 2001). Since Kir channels can be activated at approximately the resting membrane potential, these channels may be particularly important in the control of cell excitability. Studies have shown that these channels are involved in the regulation of [Ca2+]i levels, which are critical to exocytosis in pituitary endocrine cells. For instance, the Kir channels may influence the onset and frequency of Ca2+ action potential firing in rat anterior pituitary cells (Bauer, 1998; Bauer et al. 1999). In GH3 cells, the modification of ether-à-go-go (erg) Kir currents is responsible for, at least partially, the second phase of Ca2+ increase induced by thyrotropin-releasing hormone (TRH; Bauer et al. 1990). Moreover, the inhibition of the Kir current may result in direct membrane depolarisation in GH3 cells (Kuryshev et al. 1997). In the present study, the Kir current in ovine somatotropes was obtained using a similar protocol to that of other investigators, and GHRP-2, as well as Ba2+ and Cs+, reversibly decreased the Kir current. Since Cs+ and/or Ba2+ are capable of depolarising cell membranes (Ransom & Sontheimer, 1995; Kuryshev et al. 1997), our results suggest that the Kir current may be important in the depolarisation of ovine somatotropes.
Modulation of the Kir current by GHRP-2
It has been demonstrated that somatostatin and GHRH may modify a number of physiological functions via different K+ channels, including the Kir channels (Sims et al. 1991; Chen et al. 1994b). Somatostatin hyperpolarises cells and therefore inhibits their excitability through Kir channels in pancreatic α- and β-cells, neurons and pituitary cells (Mihara et al. 1987; P. A. Smith et al. 2001). In somatotropes, both voltage-gated K+ and the Kir channels contribute to the regulation of GH release mediated by GHRH or somatostatin (Yamashita et al. 1986; Sims et al. 1991; Chen et al. 1994b; Xu et al. 1999). These observations led us to hypothesise that, apart from the other cation channels, the Kir current in somatotropes may be important in their functional regulation. In this study, the Kir current in ovine somatotropes is reduced in a dose-dependent fashion by Ba2+ or Cs+, and reversibly decreased by GHRP-2. These findings are consistent with the observations that increased GH secretion results from the reduction in the Kir current by TRH secretagogues and Ba2+ or Cs+ in GH3 cells (Barros et al. 1994; Charles et al. 1999). Meanwhile, the Kir current may be crucial in determining the frequency of [Ca2+]i oscillations (Charles et al. 1999; Jiang et al. 2001). Therefore, it is possible that the reduction in the Kir current in ovine somatotropes, together with the modification of other channels, influences the [Ca2+]i oscillations that lead to the increase in GH secretion by GHRP-2 (Chen & Clarke, 1995).
The response to GHRP-2 in the Kir current was quick, but apparently slower than that to high external K+ (25 mm), suggesting that GHRP-2 and high external K+ modified the Kir current via different mechanisms. Most cells showed an instant but progressive reduction in the Kir current within 3-4 min and a complete recovery was achieved soon after the removal of GHRP-2. As expected, all these cells also responded well to 25 mm external K+. Therefore, the somatotropes with a characteristic Kir current represent the major subgroup of cells in our experimental conditions.
Signalling pathways involved in the Kir current response to GHRP-2
The biophysical properties of ion channels can be affected by protein kinases, in particular PKA and PKC, either in native tissues or when these channels are heterogeneously expressed in Xenopus oocytes (Walsh & Kass, 1988; Henry et al. 1996). However, controversy still exists with regard to the possible intracellular signalling systems involved in the regulation of the Kir current in specific cells. The involvement of PKA has been revealed in the modification of delayed rectifier K+ current in primary cultured cardiomyocytes (Karle et al. 2002) and in some Kir channel subtypes expressed in kidney cells or oocytes (Wischmeyer & Karschin, 1996; Xu et al. 1996; Liou et al. 1999). It has also been shown that the activation of Kir channels may require the activation of PKC in other cells. For example, the phosphorylation of PKC modulates the responsiveness of the Kir channel in epithelial cells or a kidney (tsA201) cell line (Light et al. 2000). In oocytes co-expressed with the HERG K+ channel and the TRH receptor, a PKC-dependent pathway may link the TRH receptor to the modulation of HERG (Barros et al. 1998). The Kir channel-related signalling systems become even more complicated following the reports that TRH may induce PKC activation via the generation of IP3 and diacylglycerol (Martin et al. 1990), whereas others showed that neither PKA nor PKC was necessary for the TRH-induced modulation of erg currents (Barros et al. 1992; Schledermann et al. 2001).
Using a series of specific PKA and PKC blockers added to either the pipette solution (if impermeable through cell membrane) or the bath solution, we have demonstrated previously that the reduction of voltage-gated K+ channels by GHRH was mediated by the PKC system (Xu et al. 1999). In this study, we used the same blocking reagents and found that H89, Rp-cAMP and the inhibitory peptide (PKI) completely abolished the GHRP-2-induced reduction in Kir current in ovine somatotropes. The PKC specific blocker chelerythrine and PKC inhibitory peptide (PKC19-36), however, had no influence on the effect of GHRP-2 on the Kir current. These data collectively suggest that only the PKA-cAMP system, rather than the PKC pathway, is responsible for mediating the GHRP-2-induced reduction in Kir current, although both signalling pathways have been implicated in GHRP-2-induced GH secretion in ovine somatotropes in vitro (Chen et al. 1996). Further studies on the specific Kir subunits involved in this GHRP-2-mediated effect in primary cultured somatotropes would be helpful in clarifying this issue.
In conclusion, our results demonstrate the presence of the Kir current in ovine somatotropes, and show that GHRP-2 reversibly and significantly reduces this current through the PKA-cAMP pathway. In physiological conditions, this Kir current contributes to the maintenance of resting membrane potential, and reduction in the Kir current by GHRP-2 may cause depolarisation of the somatotropes, consequently activating the voltage-gated Ca2+ channels. The reduction in Kir current, together with the direct opening of voltage-gated Ca2+ channels, elevates the level of [Ca2+]i, which subsequently triggers the exocytosis of GH granules.
Acknowledgments
This work was supported by the Australian NH & MRC. R. Xu is a recipient of a Monash Graduate Scholarship awarded by Monash University, Australia. We are grateful to Professor Evan Simpson and Dr Chris Reid for their critical reading of the manuscript. The authors also thank Maria Hernandez and Neveen Tawadros for the technical assistance in tissue culture and immunostaining of GH, and Sue Panckridge for making the photographs.
References
- Adams EF, Lei T, Buchfelder M, Bowers CY, Fahlbusch R. Protein kinase C-dependent growth hormone releasing peptides stimulate cyclic adenosine 3′,5′-monophosphate production by human pituitary somatotropinomas expressing gsp oncogenes: evidence for cross talk between transduction pathways. Molecular Endocrinology. 1996;10:432–438. doi: 10.1210/mend.10.4.8721987. [DOI] [PubMed] [Google Scholar]
- Arcangeli A, Bianchi L, Becchetti A, Faravelli L, Coronnello M, Mini E, Olivotto M, Wanke E. A novel inward-rectifying K+ current with a cell-cycle dependence governs the resting potential of mammalian neuroblastoma cells. Journal of Physiology. 1995;489:455–471. doi: 10.1113/jphysiol.1995.sp021065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros F, Delgado LM, del Camino D, de la Pena P. Characteristics and modulation by thyrotropin-releasing hormone of an inwardly rectifying K+ current in patch-perforated GH3 anterior pituitary cells. Pflügers Archiv. 1992;422:31–39. doi: 10.1007/BF00381510. [DOI] [PubMed] [Google Scholar]
- Barros F, Gomez-Varela D, Viloria CG, Palomero T, Giraldez T, de la Pena P. Modulation of human erg K+ channel gating by activation of a G protein-coupled receptor and protein kinase C. Journal of Physiology. 1998;511:333–346. doi: 10.1111/j.1469-7793.1998.333bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros F, Villalobos C, Garcia-Sancho J, Del Camino D, de la Pena P. The role of the inwardly rectifying K+ current in resting potential and thyrotropin-releasing-hormone-induced changes in cell excitability of GH3 rat anterior pituitary cells. Pflügers Archiv. 1994;426:221–230. doi: 10.1007/BF00374775. [DOI] [PubMed] [Google Scholar]
- Bauer CK. The erg inwardly rectifying K+ current and its modulation by thyrotrophin-releasing hormone in giant clonal rat anterior pituitary cells. Journal of Physiology. 1998;510:63–70. doi: 10.1111/j.1469-7793.1998.063bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CK, Davison I, Kubasov I, Schwarz JR, Mason WT. Different G proteins are involved in the biphasic response of clonal rat pituitary cells to thyrotropin-releasing hormone. Pflügers Archiv. 1994;428:17–25. doi: 10.1007/BF00374747. [DOI] [PubMed] [Google Scholar]
- Bauer CK, Meyerhof W, Schwarz JR. An inward-rectifying K+ current in clonal rat pituitary cells and its modulation by thyrotropin-releasing hormone. Journal of Physiology. 1990;429:169–189. doi: 10.1113/jphysiol.1990.sp018250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CK, Schafer R, Schiemann D, Reid G, Hanganu I, Schwarz JR. A functional role of the erg-like inward-rectifying K+ current in prolactin secretion from rat lactotrophs. Molecular and Cellular Endocrinology. 1999;148:37–45. doi: 10.1016/s0303-7207(98)00241-x. [DOI] [PubMed] [Google Scholar]
- Bowers CY. Growth hormone-releasing peptide (GHRP) Cellular and Molecular Life Sciences. 1998;54:1316–1329. doi: 10.1007/s000180050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AC, Piros ET, Evans CJ, Hales TG. L-type Ca2+ channels and K+ channels specifically modulate the frequency and amplitude of spontaneous Ca2+ oscillations and have distinct roles in prolactin release in GH3 cells. Journal of Biological Chemistry. 1999;274:7508–7515. doi: 10.1074/jbc.274.11.7508. [DOI] [PubMed] [Google Scholar]
- Chen C. G(o)2 and Gi3 proteins mediate the action of somatostatin on membrane Ca2+ and K+ currents in ovine pituitary somatotrophs. Clinical and Experimental Pharmacology and Physiology. 1997;24:639–645. doi: 10.1111/j.1440-1681.1997.tb02105.x. [DOI] [PubMed] [Google Scholar]
- Chen C. Growth hormone secretagogue actions on the pituitary gland: multiple receptors for multiple ligands. Clinical and Experimental Pharmacology and Physiology. 2000;27:323–329. doi: 10.1046/j.1440-1681.2000.03258.x. [DOI] [PubMed] [Google Scholar]
- Chen C, Heyward P, Zhang J, Wu D, Clarke IJ. Voltage-dependent potassium currents in ovine somatotrophs and their function in growth hormone secretion. Neuroendocrinology. 1994a;59:1–9. doi: 10.1159/000126631. [DOI] [PubMed] [Google Scholar]
- Chen C, Vincent JD, Clarke IJ. Ion channels and signal transduction pathways in the regulation of growth hormone secretion. Trends in Endocrinology and Metabolism. 1994b;5:227–233. doi: 10.1016/1043-2760(94)p3080-q. [DOI] [PubMed] [Google Scholar]
- Chen C, Wu D, Clarke IJ. Signal transduction systems employed by synthetic GH-releasing peptides in somatotrophs. Journal of Endocrinology. 1996;148:381–386. doi: 10.1677/joe.0.1480381. [DOI] [PubMed] [Google Scholar]
- Gregerson KA, Flagg TP, O'Neill TJ, Anderson M, Lauring O, Horel JS, Welling PA. Identification of G protein-coupled, inward rectifier potassium channel gene products from the rat anterior pituitary gland. Endocrinology. 2001;142:2820–2832. doi: 10.1210/endo.142.7.8236. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. Journal of Membrane Biology. 1974;18:61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Henry P, Pearson WL, Nichols CG. Protein kinase C inhibition of cloned inward rectifier (HRK1/KIR2. 3) K+ channels expressed in Xenopus oocytes. Journal of Physiology. 1996;495:681–688. doi: 10.1113/jphysiol.1996.sp021625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Kurachi Y. Inwardly rectifying potassium channels: their molecular heterogeneity and function. Japanese Journal of Physiology. 1997;47:11–39. doi: 10.2170/jjphysiol.47.11. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Si JQ, Lasarev MR, Nuttall AL. Two resting potential levels regulated by the inward-rectifier potassium channel in the guinea-pig spiral modiolar artery. Journal of Physiology. 2001;537:829–842. doi: 10.1111/j.1469-7793.2001.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle CA, Zitron E, Zhang W, Kathofer S, Schoels W, Kiehn J. Rapid component I(Kr) of the guinea-pig cardiac delayed rectifier K+ current is inhibited by beta(1)-adrenoreceptor activation, via cAMP/protein kinase A-dependent pathways. Cardiovascular Research. 2002;53:355–362. doi: 10.1016/s0008-6363(01)00509-0. [DOI] [PubMed] [Google Scholar]
- Kato M, Sakuma Y. Regulation by growth hormone-releasing hormone and somatostatin of a Na+ current in the primary cultured rat somatotroph. Endocrinology. 1997;138:5096–5100. doi: 10.1210/endo.138.12.5589. [DOI] [PubMed] [Google Scholar]
- Kato M, Sakuma Y. The effect of GHRP-6 on the intracellular Na+ concentration of rat pituitary cells in primary culture. Journal of Neuroendocrinology. 1999;11:795–800. doi: 10.1046/j.1365-2826.1999.00394.x. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Kuryshev YA, Haak L, Childs GV, Ritchie AK. Corticotropin releasing hormone inhibits an inwardly rectifying potassium current in rat corticotropes. Journal of Physiology. 1997;502:265–279. doi: 10.1111/j.1469-7793.1997.265bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light PE, Bladen C, Winkfein RJ, Walsh MP, French RJ. Molecular basis of protein kinase C-induced activation of ATP-sensitive potassium channels. Proceedings of the National Academy of Sciences of the USA. 2000;97:9058–9063. doi: 10.1073/pnas.160068997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou HH, Zhou SS, Huang CL. Regulation of ROMK1 channel by protein kinase A via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. Proceedings of the National Academy of Sciences of the USA. 1999;96:5820–5825. doi: 10.1073/pnas.96.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk JF, Pong SS, Chaung LY, Gall M, Butler B, Arena JP. Growth hormone secretagogues modulate potassium currents in rat somatotrophs. Society for Neuroscience Abstracts. 1993;19(part 1):1559. [Google Scholar]
- Martin TF, Hsieh KP, Porter BW. The sustained second phase of hormone-stimulated diacylglycerol accumulation does not activate protein kinase C in GH3 cells. Journal of Biological Chemistry. 1990;265:7623–7631. [PubMed] [Google Scholar]
- Mihara S, North RA, Surprenant A. Somatostatin increases an inwardly rectifying potassium conductance in guinea-pig submucous plexus neurones. Journal of Physiology. 1987;390:335–355. doi: 10.1113/jphysiol.1987.sp016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiological Reviews. 1999;79:511–607. doi: 10.1152/physrev.1999.79.2.511. [DOI] [PubMed] [Google Scholar]
- Ransom CB, Sontheimer H. Biophysical and pharmacological characterization of inwardly rectifying K+ currents in rat spinal cord astrocytes. Journal of Neurophysiology. 1995;73:333–346. doi: 10.1152/jn.1995.73.1.333. [DOI] [PubMed] [Google Scholar]
- Roh SG, He ML, Matsunaga N, Hidaka S, Hidari H. Mechanisms of action of growth hormone-releasing peptide-2 in bovine pituitary cells. Journal of Animal Science. 1997;75:2744–2748. doi: 10.2527/1997.75102744x. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Schafer R, Wulfsen I, Behrens S, Weinsberg F, Bauer CK, Schwarz JR. The erg-like potassium current in rat lactotrophs. Journal of Physiology. 1999;518:401–416. doi: 10.1111/j.1469-7793.1999.0401p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schledermann W, Wulfsen I, Schwarz JR, Bauer CK. Modulation of rat erg1, erg2, erg3 and HERG K+ currents by thyrotropin-releasing hormone in anterior pituitary cells via the native signal cascade. Journal of Physiology. 2001;532:143–163. doi: 10.1111/j.1469-7793.2001.0143g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims SM, Lussier BT, Kraicer J. Somatostatin activates an inwardly rectifying K+ conductance in freshly dispersed rat somatotrophs. Journal of Physiology. 1991;441:615–637. doi: 10.1113/jphysiol.1991.sp018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PA, Sellers LA, Humphrey PP. Somatostatin activates two types of inwardly rectifying K+ channels in MIN-6 cells. Journal of Physiology. 2001;532:127–142. doi: 10.1111/j.1469-7793.2001.0127g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RG, Leonard R, Bailey AR, Palyha O, Feighner S, Tan C, McKee KK, Pong SS, Griffin P, Howard A. Growth hormone secretagogue receptor family members and ligands. Endocrine. 2001;14:9–14. doi: 10.1385/ENDO:14:1:009. [DOI] [PubMed] [Google Scholar]
- Takano K, Yasufuku-Takano J, Kozasa T, Nakajima S, Nakajima Y. Different G proteins mediate somatostatin-induced inward rectifier K+ currents in murine brain and endocrine cells. Journal of Physiology. 1997a;502:559–567. doi: 10.1111/j.1469-7793.1997.559bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K, Yasufuku-Takano J, Teramoto A, Fujita T. Gi3 mediates somatostatin-induced activation of an inwardly rectifying K+ current in human growth hormone-secreting adenoma cells. Endocrinology. 1997b;138:2405–2409. doi: 10.1210/endo.138.6.5185. [DOI] [PubMed] [Google Scholar]
- Takei T, Takano K, Yasufuku-Takano J, Fujita T, Yamashita N. Enhancement of Ca2+ currents by GHRH and its relation to PKA and [Ca2+]i in human GH-secreting adenoma cells. American Journal of Physiology. 1996;271:E801–807. doi: 10.1152/ajpendo.1996.271.5.E801. [DOI] [PubMed] [Google Scholar]
- Walsh KB, Kass RS. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988;242:67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- Wischmeyer E, Karschin A. Receptor stimulation causes slow inhibition of IRK1 inwardly rectifying K+ channels by direct protein kinase A-mediated phosphorylation. Proceedings of the National Academy of Sciences of the USA. 1996;93:5819–5823. doi: 10.1073/pnas.93.12.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SN, Lo YK, Kuo BI, Chiang HT. Ceramide inhibits the inwardly rectifying potassium current in GH(3) lactotrophs. Endocrinology. 2001;142:4785–4794. doi: 10.1210/endo.142.11.8508. [DOI] [PubMed] [Google Scholar]
- Wulfsen I, Hauber HP, Schiemann D, Bauer CK, Schwarz JR. Expression of mRNA for voltage-dependent and inward-rectifying K channels in GH3/B6 cells and rat pituitary. Journal of Neuroendocrinology. 2000;12:263–272. doi: 10.1046/j.1365-2826.2000.00447.x. [DOI] [PubMed] [Google Scholar]
- Xu R, Clarke IJ, Chen S, Chen C. Growth hormone-releasing hormone decreases voltage-gated potassium currents in GH4C1 cells. Journal of Neuroendocrinology. 2000;12:147–157. doi: 10.1046/j.1365-2826.2000.00430.x. [DOI] [PubMed] [Google Scholar]
- Xu R, Roh SG, Loneragan K, Pullar M, Chen C. Human GHRH reduces voltage-gated K+ currents via a non-cAMP-dependent but PKC-mediated pathway in human GH adenoma cells. Journal of Physiology. 1999;520:697–707. doi: 10.1111/j.1469-7793.1999.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZC, Yang Y, Hebert SC. Phosphorylation of the ATP-sensitive, inwardly rectifying K+ channel, ROMK, by cyclic AMP-dependent protein kinase. Journal of Biological Chemistry. 1996;271:9313–9319. doi: 10.1074/jbc.271.16.9313. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Shibuya N, Ogata E. Hyperpolarization of the membrane potential caused by somatostatin in dissociated human pituitary adenoma cells that secrete growth hormone. Proceedings of the National Academy of Sciences of the USA. 1986;83:6198–6202. doi: 10.1073/pnas.83.16.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]


