Abstract
To enhance therapeutic efficacy and reduce adverse effects, practitioners of traditional Chinese medicine (TCM) prescribe a combination of plant species/minerals, called formulae, based on clinical experience. Nearly 100,000 formulae have been recorded, but the working mechanisms of most remain unknown. In trying to address the possible beneficial effects of formulae with current biomedical approaches, we use Realgar-Indigo naturalis formula (RIF), which has been proven to be very effective in treating human acute promyelocytic leukemia (APL) as a model. The main components of RIF are realgar, Indigo naturalis, and Salvia miltiorrhiza, with tetraarsenic tetrasulfide (A), indirubin (I), and tanshinone IIA (T) as major active ingredients, respectively. Here, we report that the ATI combination yields synergy in the treatment of a murine APL model in vivo and in the induction of APL cell differentiation in vitro. ATI causes intensified ubiquitination/degradation of promyelocytic leukemia (PML)-retinoic acid receptor α (RARα) oncoprotein, stronger reprogramming of myeloid differentiation regulators, and enhanced G1/G0 arrest in APL cells through hitting multiple targets compared with the effects of mono- or biagents. Furthermore, ATI intensifies the expression of Aquaglyceroporin 9 and facilitates the transportation of A into APL cells, which in turn enhances A-mediated PML-RARα degradation and therapeutic efficacy. Our data also indicate A as the principal component of the formula, whereas T and I serve as adjuvant ingredients. We therefore suggest that dissecting the mode of action of clinically effective formulae at the molecular, cellular, and organism levels may be a good strategy in exploring the value of traditional medicine.
Keywords: traditional chinese medicine, active ingredient, tetraarsenic tetrasulfide, synergism, systems biology
The complexity of medicine suggests that treatment protocols should be carefully designed, and the construction of a prescription is an art in fighting disease. Increasing evidence demonstrates that, in treating illnesses, including cancer (1) and HIV/AIDS (2), treatment regimens containing multiple drugs with distinct but related mechanisms can usually amplify the therapeutic efficacies of each agent, leading to maximal therapeutic efficacy with minimal adverse effects. Interestingly, combination therapy has been advocated for >2,500 years by prescriptions called formulae (3, 4) in traditional Chinese medicine (TCM), a unique medical system assisting the ancient Chinese in dealing with disease. Typically, formulae consist of several types of medicinal herbs or minerals, in which one represents the principal component, and others serve as adjuvant ones to assist the effects or facilitate the delivery of the principal component (3, 4). It is believed that, at least in some formulae, multiple components could hit multiple targets and exert synergistic therapeutic efficacies (3, 4). However, essential compounds have not been identified in most formulae, whereas precise mechanisms of formulae remain to be addressed by using molecular approaches, thus hampering the modernization of TCM.
Acute promyelocytic leukemia (APL), the M3 type of acute myeloid leukemia (AML), is characterized by the accumulation of immature promyelocytes in bone marrow (BM) and the presence of a specific chromosome translocation t(15;17) (q22;q21), which generates the leukemogenic PML-RARα fusion gene in a vast majority of patients. APL is uniquely sensitive to the differentiation inducer all-trans retinoic acid (ATRA), which causes the modulation and catabolism of PML-RARα oncoprotein (5, 6). Interestingly, arsenic trioxide (ATO) (7, 8), an ancient remedy in both TCM and Western medicine, has been proven effective in treating APL, and the key molecular mechanism has been revealed to be related to the degradation of promyelocytic leukemia (PML)-retinoic acid receptor α (RARα) (9). A recent report on the ATRA/ATO combination yields the 4-year disease-free survival of >90% of APL patients (10). Of note, in parallel to this advancement in the understanding and treatment of APL, accomplished mainly by the hematology/oncology community in China and other countries, a group of TCM doctors in China designed a Realgar-Indigo naturalis formula (RIF) in the 1980s (11) entirely based on TCM theories, in which mined ore, realgar, is the principal element, whereas Indigo naturalis, Salvia miltiorrhiza, and Radix psudostellariae are adjuvant components to assist the effects of realgar. Intriguingly, recent multicenter clinical trials showed that a complete remission rate of 96.7% (12) to 98% (11) and a 5-year overall survival rate of 86.88% (13) were achieved in APL patients receiving RIF, with moderate adverse effects such as gastrointestinal discomfort and rash. Realgar, in combination with Indigo naturalis, also showed anti-APL activity (14). The literature documents the antitumor activity of S. miltiorrhiza (15), whereas R. psudostellariae is believed to be able to strengthen immune activity and seems not to be essential for RIF. Studies showed that tetraarsenic tetrasulfide (As4S4, A) (8), indirubin (I) (16), and tanshinone IIA (T) (17) are the major active ingredients of realgar, Indigo naturalis, and S. miltiorrhiza, respectively (18). These results not only demonstrate the clinical efficacy of, but also suggest the possible synergistic effects among, the three components of RIF, warranting the mechanistic exploration of this formula.
In trying to approach the rationale of formula design in TCM, here we use the treatment of APL with RIF as a working model. A, I, and T were used as active compounds of realgar, Indigo naturalis, and S. miltiorrhiza, respectively, and the efficacies and mechanisms of ATI combination on APL were tested in vivo and in vitro.
Results
In Vivo Therapeutic Efficacies of ATI on a Murine APL Model.
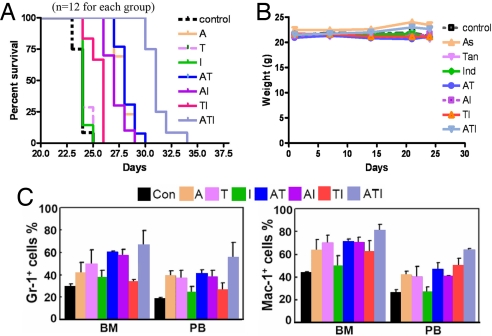
In FVB/NJ mice injected with 1 × 105 cells expressing PML-RARα (19, 20), a statistically significant prolongation of median overall survival was observed in mice treated with ATI combination compared with animals treated with vehicle control or mono- or bitherapy of A, T, and I (Fig. 1A). In the monotherapy groups, A showed the most potent therapeutic efficacy. The ATI protocol decreased the weight of spleen and liver [supporting information (SI) Fig. 6] but did not cause weight loss in animals (Fig. 1B). Terminally differentiated granulocytes dominated the bone marrow (BM) and spleen of ATI-treated mice but were rare or occurred much less frequently in other treatment groups (SI Fig. 7). Consistent with this, cells expressing granulocytic differentiation antigens Gr-1 and Mac-1 in BM and spleens of mice treated with ATI were higher than in other treatment groups (Fig. 1C). A or T alone also induced some APL cell differentiation. Unlike vehicle control, the ATI regimen significantly reduced disseminated disease and prevented destruction of tissue architectures (SI Fig. 8). These results suggest the ATI combination intensifies therapeutic efficacies, but not adverse effects, on murine APL model.
Fig. 1.
In vivo therapeutic efficacies of ATI on an APL murine model. (A) ATI significantly prolongs the life span of mice bearing PML-RARα-positive leukemic cells compared with those treated with mono- or bitreatment of A, T, and I (P < 0.001). (B) ATI does not cause loss of body weight, suggesting that ATI might probably not cause severe toxicity. (C) Treatment with ATI results in cell maturation revealed by an accumulation of Gr-1 and Mac-1-positive cells in BM and peripheral blood (PB). (Dosage of agents used: A, 10 mg/kg; T, 50 mg/kg; I, 50 mg/kg. A is administrated by i.v. injection, whereas T and I are given orally.)
ATI Causes Synergistic Effects on Human APL Cell Differentiation in Vitro.
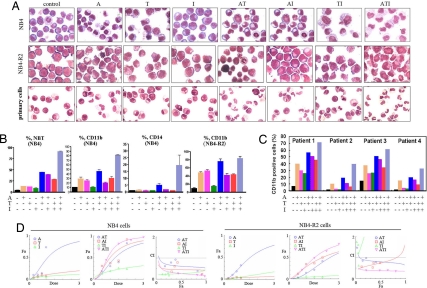
We found that A or T but not I alone induced a certain degree of morphological features of cell differentiation in a human APL cell line NB4 (21) and primary culture of fresh APL cells, whereas the ATI combination induced a much higher level of maturation not only in these cells but also in the ATRA-resistant NB4-R2 cells (22) (Fig. 2A). Cells, upon ATI, showed higher activity to produce oxidative bursts detected by the NBT reduction assay and showed up-regulated CD11b/CD14 expression compared with those treated with vehicle or mono- or bitreatment of A, T, and I (Fig. 2 B and C). To test whether A and/or T and/or I exert synergic or additive effects on APL cells, the dose–effect curves of single or combined drug treatment were analyzed by the median-effect method (23), where the combination indexes (CI) of less than, equal to, and more than 1 indicate synergistic, additive, and antagonistic effects, respectively. We found that A, T, and I at concentrations ranging from 0.25 to 1.0 μM had CI values <1 (Fig. 2D), indicating synergic effects among the agents.
Fig. 2.
ATI induces terminal differentiation of NB4, NB4-R2, and primary leukemic cells harvested from APL patients. (A) Morphological examination of APL cells treated with ATI. (B) Detection of NBT reduction activity and CD11b/CD14 expression of cells treated with ATI. (C) Expression of CD11b in primary leukemic cells treated with ATI in vitro. Concentration of agents used: A, 0.375 μM; T, 0.75 μM; I, 0.75 μM. (D) Combination of A, T, and I exerts synergic effects on NB4 and NB4-R2 cells, as reflected by the median-effect method of Chou and Talalay (23).
ATI Causes Enhanced Ubiquitination and Degradation of PML-RARα Oncoprotein.
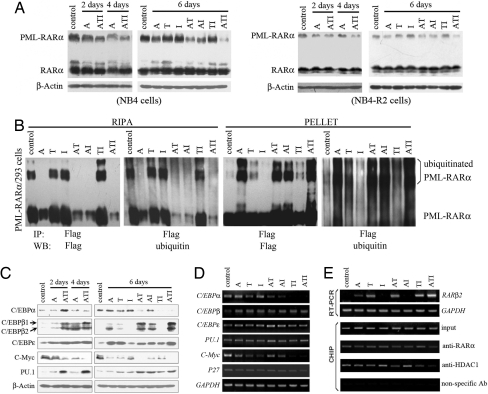
Like ATO, As4S4 is able to trigger the degradation of the PML-RARα oncoprotein (Fig. 3A). Interestingly, T or I augmented A-triggered catabolism of PML-RARα, whereas the ATI combination further enhanced this effect (Fig. 3A). Previous studies showed that ATO shifts PML/PML-RARα from the nucleoplasm onto the nuclear matrix and induces their degradation via the proteasome pathway (24). By Western blot analysis of the PML-RARα contents of RIPA (the cytoplasm and most of the nucleoplasm) and pellet (the nuclear matrix and some chromatin components) fractions (24) in PML-RARα-expressing 293 cells pretreated with proteasome inhibitor MG-132, we found that A, but not T and/or I, induced a clear shift from the nucleoplasm to the nuclear matrix and an apparent ubiquitination of PML-RARα (Fig. 3B), whereas TI increased A-triggered relocalization and ubiquitination of the oncoprotein (Fig. 3 B). Immunofluorescence staining using an anti-PML antibody showed that untreated NB4 and NB4-R2 cells exhibited hundreds of micropunctates in the nuclei and cytoplasm, whereas A down-regulated PML-RARα and restored the normal PML speckles (SI Fig. 9). Although T, I, or a TI combination did not affect the staining pattern, these two compounds enhanced the effects of A (SI Fig. 9), suggesting that degradation of PML-RARα by A can be synergized by T and I. That MG-132 could induce a significant accumulation of PML-RARα (Fig. 3B) provided strong evidence that the synergistic effect of A, T, and I occurred at the level of protein ubiquitination. These data, together with the in vivo and cell biology data, suggest that A is the principal ingredient in this formula, whereas T and I could serve as adjuvant components.
Fig. 3.
ATI triggers degradation of PML-RARα oncoprotein and reprogramming of differentiation regulators. (A) Enhanced degradation of PML-RARα is seen in NB4 (Left) and NB4-R2 (Right) cells treated with the ATI combination compared with those treated with mono- or bitreatment of A, T, and I. (B) TI intensifies matrix transfer from the nucleoplasm and ubiquitination of PML-RARα caused by A. Cells are pretreated with MG-132 for 1 h and then with the protocols indicated for 6 h. Western blot is performed as described (24). (C) Effects of ATI on differentiation regulators of NB4 cells at the protein level. (D) Effects of the ATI combination on differentiation regulators at the mRNA level, assessed by semiquantitative RT-PCR. (E) ATI treatment up-regulates the expression of RARβ2 at the mRNA level; this might be due to the dissociation of RARβ2 from HDAC1 upon ATI treatment.
ATI Triggers Relief of Transcription Suppression.
Transcription factors, e.g., CCAAT/enhancer-binding proteins (C/EBPs) and PU.1, are critical for normal myelopoiesis and granulocytic maturation and are suppressed in APL. We found that in NB4 cells treated with the ATI combination, C/EBPα at protein level was up-regulated on day 2 and subsequently decreased, whereas C/EBPβ1/2 and C/EBPε were up-regulated (Fig. 3 C and D). Further analysis showed that A or T alone modulated these proteins, and combined use of ATI intensified the effect (Fig. 3C). The oncogene C-Myc blocks myeloid differentiation, and its down-regulation is critical for myeloid cell differentiation (25). Interestingly, down-regulation of C-Myc at both the protein and mRNA levels was detected in cells treated with ATI compared with cells treated with mono- or biagents (Fig. 3 C and D). RARβ2 is an RAR target gene that contains the strongest natural RA response element so far (βRARE) in its promoter (26, 27). We found that both T and A up-regulated RARβ2 at the mRNA level, whereas the ATI combination significantly intensified RARβ2 up-regulation (Fig. 3E Upper). Using the ChIP assay, we identified the dissociation of RARβ2 from histone deacetylase 1 (HDAC1) (Fig. 3E Lower), which might contribute to the activation of RARβ2 transcription. Suppression of transcription factors by the dominant-negative effect of PML-RARα is involved in APL pathogenesis, and the ATI combination might relieve the repression of the normal function of transcription machinery in APL cells, suggesting the rationality of RIF in treating APL.
ATI Induces G1/G0 Arrest of APL Cells with Effects on Key Regulators of Cell Cycle Progression.
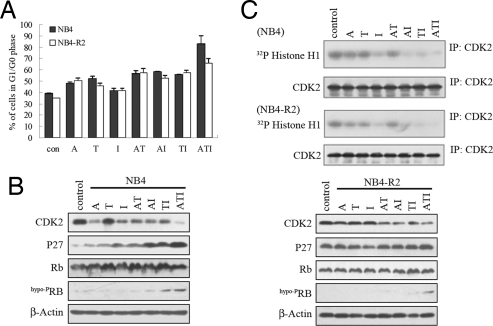
We tested whether ATI treatment could act on cell cycle progression and found that T alone increased cellular compartment in the G1/G0 phase, whereas AT, AI, and TI combinations further caused G1/G0 blockage (Fig. 4A). Intriguingly, 83.7% ± 6.7% of NB4 cells treated with ATI were at the G1/G0 phase, demonstrating an enhanced effect of the compounds in inhibiting the G1/S transition (Fig. 4A). Similar results were observed in NB4-R2 cells treated with ATI (Fig. 4A). It has been shown that I is a potent inhibitor of cyclin-dependent kinase 2 (CDK2), which is critical for cell cycle progression from the G1 to S phase (16). We found that A and I down-regulated CDK2 in NB4 and NB4-R2 cells, whereas the ATI combination further enhanced this effect (Fig. 4B). CDK2 has been shown to be able to phosphorylate histone H1 (28), whereas phosphorylated H1 is increased during the transition from the G1 to S phase (29). Regimens containing I markedly decreased phosphorylated H1 (Fig. 4C). P27 is an important CDK inhibitor, which negatively regulates the progression from G1 into S phase. We showed that the protein but not the mRNA level of P27 was dramatically increased during A, T, I, and the combinatory treatment on NB4 cells (Figs. 3D and 4B). The ATI combination also up-regulated P27 in NB4-R2 cells (Fig. 4B Right). Normally, Rb governs cells' entry into the S phase (30). We found that the expression of the Rb protein was slightly increased in treatment groups containing T (T, AT, TI, and ATI) in NB4 cells, whereas hypophosphorylated Rb (hypoPRB, the functional form of Rb as a tumor suppressor in blocking the cell cycle) was significantly up-regulated in the ATI treatment group (Fig. 4B). These observations indicated that A, T, and I had a synergistic/additive effect on inducing G0/G1 arrest of APL cells.
Fig. 4.
Effects of ATI on cell cycle progression and cell cycle modulators. (A) ATI treatment leads to the G1/G0 arrest of NB4 and NB4-R2 cells. (B) Effects of the ATI combination on important cell cycle regulators in NB4 (Left) and NB4-R2 (Right) cells. (C) Down-regulation of CDK2 by ATI treatment on NB4 (Upper) and NB4-R2 (Lower) cells results in decrease of phosphorylated H1. Cells are treated with A, T, I, and the combined drugs for 24 h. CDK2 is immunoprecipitated (IP) from cell lysates and assayed for kinase activity using Histone H1 as a substrate. The efficiency of IP is confirmed and quantitated by Western blot analysis, and CDK2 at each treatment group is adjusted to the same level.
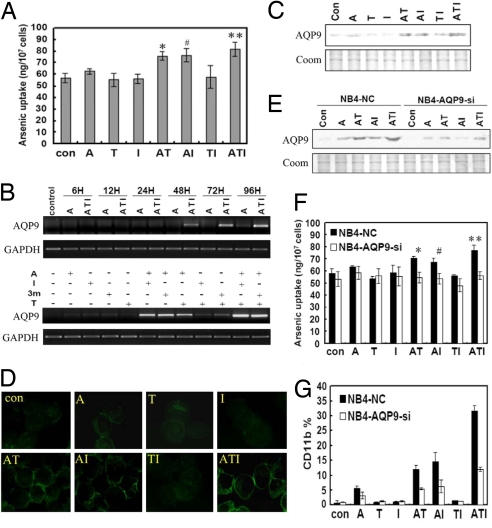
T and I Increase Cellular Uptake of Arsenic Through Cooperating with A in Inducing Up-Regulation of the Transmembrane Protein Aquaglyceroporin 9 (AQP9).
It has been shown that AQP9 serves as the transmembrane transporter of arsenic compounds (31). A question can thus be raised: could T and/or I exert any effect on the transportation of A through AQP9? To address this, NB4 cells were pretreated with different protocols for 4 days, washed, and then incubated with 2.5 μM A for 4 h. The intracellular arsenic concentration ([As]i) was carefully evaluated by an atomic absorption spectrometer system. Intriguingly, cells treated with ATI had the highest [As]i, whereas [As]i in cells treated with AT and AI but not TI was higher than in cells treated with A (Fig. 5A), demonstrating that T and/or I assist arsenic uptake. It has been reported that MRP-1 is involved in arsenic efflux (32), and recently, AQP9 has been shown to mediate arsenic uptake by leukemic cells and determine arsenic sensitivity (31). We found that expression of MRP-1 was not changed in NB4 cells treated with these compounds (data not shown). Interestingly, ATI treatment induced strong expression of AQP9 after 24–96 h (Fig. 5 B and C). Further analysis revealed T, I, or Indirubin-3′-monoxime (3m), an analog of I, cooperated with A in up-regulating AQP9 at both the mRNA (Fig. 5B) and/or protein (Fig. 5C) levels, whereas TI or T3m moderately caused the elevation of AQP9. The immunofluorescence assay confirmed the cooperation of T and I with A in up-regulating AQP9 expression, whereas the strongest effect was seen in the ATI combination (Fig. 5D).
Fig. 5.
T and I facilitate arsenic uptake by malignant promyelocytes via up-regulation of AQP9. (A) Combinatory use of T and/or I increases intracellular arsenic concentration ([As]i) in NB4 cells (*, P = 0.002; #, P = 0.004; **, P = 0.002). (B and C) T and/or I in combination with A up-regulate AQP9 expression at both the mRNA (B) and protein (C) levels (Coom, stained with Coomassie blue). (D) Immunofluorescence analysis of AQP9 expression in NB4 cells treated with the ATI combination. (E) AQP9-specific siRNA down-regulates AQP9 expression by approximately one-half in NB4 cells (NB4-AQP9-Si) compared with cells treated with nonsilencing siRNA control (NC). (F) Treatment with AQP9-specific siRNA reduces [As]i in NB4-AQP9-Si cells upon ATI compared with NB4-NC cells (*, P = 0.018; #, P = 0.037; **, P = 0.009). (G) Treatment with AQP9-specific siRNA inhibits differentiation of NB4 cells on ATI, revealed by analysis of CD11b expression.
To validate the importance of AQP9 in arsenic uptake, RNA interference (RNAi) was used to suppress AQP9 gene expression. Transfection of the AQP9 siRNA expression vector into NB4 cells resulted in a decrease of AQP9 by approximately one-half (Fig. 5E). Consequently, AQP9 silencing led to a decrease of [As]i of cells treated with A, AT, AI, or the ATI combination (Fig. 5F). Moreover, AQP9 knockdown inhibited cell maturation induced by ATI, as revealed by CD11b expression (Fig. 5G). These results suggest that AQP9 is critical to arsenic transportation, and T and I facilitate arsenic uptake through up-regulation of AQP9.
Discussion
In the past few years, the pharmaceutical industry has seen a shift from the search for “magic bullets” that specifically target a single disease-causing molecule to the pursuit of combination therapies that comprise more than one active ingredient (33). Interestingly, TCM has advocated combinatory therapeutic strategies for >2,500 years. Based on the symptoms and characteristics of patients and guided by the theories of TCM, formulae are designed to contain a combination of different kinds of plants or minerals to improve clinical efficacy. One example is RIF, whose efficacies in treating APL have been well established recently (12, 13).
In this study, we find that, in a murine APL model, combined use of the active components of RIF, namely A, T, and I, significantly prolongs the life span of mice bearing PML-RARα-positive leukemic cells. At the cellular level, the ATI induced the terminal differentiation of APL cells in vivo and in vitro and overcame ATRA resistance. Assessment of the CI value by the median-effect method directly demonstrates the synergic effect of A, T, and I on NB4 and NB4-R2 cells. These results clearly show that components of RIF exert effects of mutual reinforcement. However, ATI does not show an intensified adverse effect in the APL murine model, as revealed by change in body weight, consistent with the safety of RIF observed in the clinic. These observations are in agreement with the rationality of the formula: mutual reinforcement of the compounds and reduction of adverse effects.
A large body of evidence indicates that PML-RARα is the causative oncoprotein of APL. Interestingly, A, which exhibits the strongest therapeutic effects in the murine APL model, has also been shown to be the principal agent of RIF in targeting PML-RARα. Furthermore, the TI combination enhances A-induced ubiquitination and degradation of PML-RARα, augmenting the effects of A. Thus, the RIF formula, although designed by TCM doctors in the premolecular era of APL, proves its rationale by modern biochemical analysis. In addition, PML-RARα acts as a dominant-negative mutant over wild-type RARα and prevents the activation of key target genes essential for myeloid differentiation (6). In this study, the ATI combination is found to be able to act on some key transcription factors in myelopoiesis and results in the up-regulation of C/EBPα, C/EBPβ1/2, C/EBPε, PU.1, and RARβ2 and the down-regulation of C-Myc. Using the ChIP assay, we show that dissociation of RARβ2 from HDAC1 might contribute to the activation of RARβ2 transcription. These results indicate that ATI not only targets PML-RARα but also at least partly restores the normal function of the myeloid cell transcriptional machinery, leading to the terminal differentiation of APL cells.
Maintenance of normal cell cycle progression requires a balance of positive and negative regulators. The effects of ATI on cell cycle distribution are quite interesting, in that a high percentage of cells arrested at the G1/G0 phase can be reached when the three compounds are used simultaneously in both ATRA-sensitive NB4 and ATRA-resistant NB4-R2 cells. In the ATI treatment group, CDK2, a critical regulator of S phase entry (34), is greatly down-regulated, and consequently phosphorylated H1 is eliminated. In contrast, P27, a well established inhibitor of the cyclin/CDK complex at the transition of the cell cycle, can be significantly induced by A, T, and I, particularly when the three compounds are used in combination. In addition, the functional status of the Rb protein, a pivotal factor in the negative control of the cell cycle through repressing the transcription of the gene required for the transition from G1 to S phase, can also be regulated by ATI. Of note, in untreated NB4-R2 and NB4 cells, hypophosphorylated Rb is undetectable, whereas the ATI combination, but not any single agent of the three, significantly induces its expression. This phenomenon not only suggests that ATI might trigger protein modification but also indicates that formulae might exert therapeutic effects through mechanisms beyond each component.
Finally, it is worth pointing out that, in the principle of designing a formula in TCM, some adjuvant components should be considered to facilitate the delivery of the principal element to the disease site in the body. It may be an interesting observation in this work that ATI treatment increases [As]i of NB4 cells compared with arsenic treatment alone, whereas A combined with T and/or I significantly up-regulates AQP9, a transmembrane protein playing an essential role in arsenic uptake and determining cellular arsenic sensitivity (31). Because AQP9 was up-regulated 24 h after ATI treatment, some other events might also be involved in the enhancement of arsenic action by T and I at the earlier stage. Interestingly, ATI treatment could not up-regulate AQP9 in HL60 or K562 cells (date not shown), indicating a cell context-based selectivity of AQP9 modulation by ATI. Furthermore, the silencing of AQP9 by specific siRNA inhibited arsenic uptake, decreased [As]i, and inhibited the differentiation of NB4 cells induced by ATI. These results suggest that AQP9 mediates, to a significant extent, the therapeutic effects of RIF, and that T and I might assist A in the formula through modulating this key transporter.
In summary, we show that the dissection of the mode of action of clinically well established TCM formulae such as RIF should be possible by combined application of both analytical and synthetic research approaches at the molecular, cellular, and organism levels. This study may be considered a useful pilot trial in exploring the value of traditional formulae on a larger scale and in helping to bridge Western and the Eastern medicines in the era of systems biology.
Materials and Methods
Reagents.
Details on A, I, T, and other materials used can be found in SI Materials and Methods.
Animals.
Mice were bred and maintained in a specific pathogen-free environment. Six- to eight-week-old FVB/NJ mice were i.v. injected with 1 × 105 cells expressing PML-RARα (20) via the tail vein. When they became moribund, the mice were killed, and splenic cells were isolated and inoculated into secondary recipients for passage (19). Five days after leukemic cell transplantation, the mice were treated with A and/or T and/or I at the doses indicated in the legend for Fig. 1.
Cell Culture, Cell Viability, Cell Differentiation, and Cell Cycle.
NB4 and NB4-R2 cell lines were kindly provided by M. Lanotte from Hôpital Saint-Louis, Paris, France. Fresh leukemia cells were obtained from four APL patients with informed consent. Cells were cultured, and their major biological features were assayed as described (19). The dose–effect curves of single or combined drug treatment were analyzed by the median-effect method (23) by using Calcusyn Software (Biosoft).
Semiquantitative RT-PCR.
Semiquantitative RT-PCR was performed by using the primers listed in SI Table 1, according to a method described in ref. 19.
Immunofluorescence, Western Blot Assays, and ChIP.
Immunofluorescence and Western blot assays were performed as described (9) by using the antibodies indicated. ChIP was conducted as described (19). Semiquantitative RT-PCR was performed by using the primers listed in SI Table 1.
Immunoprecipitation (IP) and CDK2 Kinase Assay.
Cells were lysed and proteins purified for IP and kinase assay of CDK2 according to protocols provided by the reagent manufacturer (Santa Cruz Biotechnology).
Measurement of Arsenic Concentration.
Cells were pretreated with A, T, and I alone or in combination, as indicated, for 4 days. After being washed with PBS, cells were resuspended in RPMI medium 1640 supplemented with 10% FBS and 2.5 μM As4S4 for 4 h. After being washed with PBS, cell samples were broken by ultrasonic wave, and total inorganic trivalent As(III) were determined as described (9).
Knockdown of AQP9 by RNAi.
RNAi candidate target sequences to human AQP9 were designed (SI Table 2). Details for the RNAi experiment can be found in SI Materials and Methods.
Statistical Analysis.
Assays were set up in triplicate, and the results were expressed as the mean ± SD. See SI Materials and Methods for details of the statistical analysis.
Supplementary Material
Acknowledgments.
This work was supported in part by the National Natural Science Foundation of China; the Chinese National High Tech Program (863) the Chinese National Key Program for Basic Research (973); the Shanghai Municipal Commission for Science and Technology; the Shanghai Commission for Education; the Shanghai Municipal Bureau for Public Health; and the Shanghai Leading Academic Discipline Program (Y0201); and by grants from the Administration of Traditional Chinese Medicine of Guangdong Province.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712365105/DC1.
References
- 1.Chabner BA, Roberts TG., Jr Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 2.Perelson AS, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. The Inner Canon of Emperor Huang. Beijing: Chinese Medical Ancient Books Publishing House; 2003. [Google Scholar]
- 4.Anonymous. Formularies for 52 Kinds of Disorders. Beijing: Culture Relics Press; 1979. [Google Scholar]
- 5.Huang ME, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572. [PubMed] [Google Scholar]
- 6.Zhou GB, Zhao WL, Wang ZY, Chen SJ, Chen Z. Retinoic acid and arsenic for treating acute promyelocytic leukemia. PLoS Med. 2005;2:33–38. doi: 10.1371/journal.pmed.0020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang TD, et al. Arsenic trioxide, a therapeutic agent for APL. Oncogene. 2001;20:7146–7153. doi: 10.1038/sj.onc.1204762. [DOI] [PubMed] [Google Scholar]
- 8.Zhu J, Chen Z, Lallemand-Breitenbach V, de Thé H. How acute promyelocytic leukaemia revived arsenic. Nat Rev Cancer. 2002;2:705–713. doi: 10.1038/nrc887. [DOI] [PubMed] [Google Scholar]
- 9.Chen GQ, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with down-regulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 10.Liu YF, et al. Long-term follow-up confirms the benefit of all-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) as front line therapy for newly diagnosed acute promyelocytic leukemia. Blood. 2006;108:170a. [Google Scholar]
- 11.Huang SL, et al. Clinical study on the treatment of acute promyelocytic leukemia with Composite Indigo Naturalis tablets. Chin J Hematol. 1995;16:26–28. [Google Scholar]
- 12.The Cooperation Group of Phase II Clinical Trial of Compound Huangdai Tablet. Phase II clinical trial of compound Huangdai tablet in newly diagnosed acute promyelocytic leukemia. Chin J Hematol. 2006;27:801–804. [Google Scholar]
- 13.Xiang Y, et al. The influence on long-term survey of the patients with acute promyelocytic leukemia treated with compound huangdai tablets and chemotherapy. Chin J Clin Hematol. 2007;16:204–206. [Google Scholar]
- 14.Zhou A, Qing-Huang-San (Realgar in combination with Indigo) in treatment of leukemia. Chin J Integr Tradit West Med. 1998;18:582–583. [Google Scholar]
- 15.Chen L, Huang J. Review on the research of the effect of Danshen (salvia miltiorrhiza) and its extract on tumor metastasis. J Zhejiang Univ Trad Chin Med. 2006;30:449–452. [Google Scholar]
- 16.Hoessel R, et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1:60–67. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- 17.Sung HJ, Choi SM, Yoon Y, An KS. Tanshinone IIA, an ingredient of Salvia miltiorrhiza BUNGE, induces apoptosis in human leukemia cell lines through the activation of caspase-3. Exp Mol Med. 1999;31:174–178. doi: 10.1038/emm.1999.28. [DOI] [PubMed] [Google Scholar]
- 18.Huang SL, Xiang Y. Beijing: People's Medical Publishing House; 2004. Current advances in realgar, white arsenic and related formulae in treating leukemia. [Google Scholar]
- 19.Zhou GB, et al. Oridonin, a diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion protein and shows potent antitumor activity with low adverse effects on t(8;21) leukemia in vitro and in vivo. Blood. 2007;109:3441–3450. doi: 10.1182/blood-2006-06-032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown D, et al. A PMLRARalpha transgene initiates murine acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1997;94:2551–2556. doi: 10.1073/pnas.94.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanotte M, et al. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3) Blood. 1991;77:1080–1086. [PubMed] [Google Scholar]
- 22.Duprez E, Benoit G, Flexor M, Lillehaug JR, Lanotte M. A mutated PML/RARA found in the retinoid maturation resistant NB4 subclone, NB4–R2, blocks RARA and wild-type PML/RARA transcriptional activities. Leukemia. 2000;14:255–261. doi: 10.1038/sj.leu.2401683. [DOI] [PubMed] [Google Scholar]
- 23.Chou TC, Talalay P. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur J Biochem. 1981;115:207–216. doi: 10.1111/j.1432-1033.1981.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, et al. Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1997;94:3978–3983. doi: 10.1073/pnas.94.8.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvakumaran M, Liebermann D, Hoffman B. The proto-oncogene c-myc blocks myeloid differentiation independently of its target gene ornithine decarboxylase. Blood. 1996;88:1248–1255. [PubMed] [Google Scholar]
- 26.Fazi F, et al. Heterochromatic gene repression of the retinoic acid pathway in acute myeloid leukemia. Blood. 2007;109:4432–4440. doi: 10.1182/blood-2006-09-045781. [DOI] [PubMed] [Google Scholar]
- 27.de Thé H, Vivanco-Ruiz MM, Tiollais P, Stunnenberg H, Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990;343:177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- 28.Herrera RE, Chen F, Weinberg RA. Increased histone H1 phosphorylation and relaxed chromatin structure in Rb-deficient fibroblasts. Proc Natl Acad Sci USA. 1996;93:11510–11515. doi: 10.1073/pnas.93.21.11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talasz H, Helliger W, Puschendorf B, Lindner H. In vivo phosphorylation of histone H1 variants during the cell cycle. Biochemistry. 1996;35:1761–1767. doi: 10.1021/bi951914e. [DOI] [PubMed] [Google Scholar]
- 30.Shah MA, Schwartz GK. Cell cycle-mediated drug resistance: an emerging concept in cancer therapy. Clin Cancer Res. 2001;7:2168–2181. [PubMed] [Google Scholar]
- 31.Leung J, Pang A, Yuen WH, Kwong YL, Tse EWC. Relationship of expression of aquaglyceroporin 9 with arsenic uptake and sensitivity in leukemia cells. Blood. 2007;109:740–746. doi: 10.1182/blood-2006-04-019588. [DOI] [PubMed] [Google Scholar]
- 32.Kimura A, et al. MRP-1 expression levels determine strain-specific susceptibility to sodium arsenic-induced renal injury between C57BL/6 and BALB/c mice. Toxicol Appl Pharmacol. 2005;203:53–61. doi: 10.1016/j.taap.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Qiu J. ‘Back to the future’ for Chinese herbal medicines. Nat Rev Drug Discov. 2007;6:506–507. doi: 10.1038/nrd2350. [DOI] [PubMed] [Google Scholar]
- 34.Hinds PW. Cdk2 dethroned as master of S phase entry. Cancer Cell. 2003;3:305–307. doi: 10.1016/s1535-6108(03)00084-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.