Abstract
p62 has been proposed to mark ubiquitinated protein bodies for autophagic degradation. We report that the Drosophila melanogaster p62 orthologue, Ref(2)P, is a regulator of protein aggregation in the adult brain. We demonstrate that Ref(2)P localizes to age-induced protein aggregates as well as to aggregates caused by reduced autophagic or proteasomal activity. A similar localization to protein aggregates is also observed in D. melanogaster models of human neurodegenerative diseases. Although atg8a autophagy mutant flies show accumulation of ubiquitin- and Ref(2)P-positive protein aggregates, this is abrogated in atg8a/ref(2)P double mutants. Both the multimerization and ubiquitin binding domains of Ref(2)P are required for aggregate formation in vivo. Our findings reveal a major role for Ref(2)P in the formation of ubiquitin-positive protein aggregates both under physiological conditions and when normal protein turnover is inhibited.
Introduction
The mammalian polyubiquitin binding protein p62 is a multifunctional scaffold protein that serves a large variety of cellular functions (Wooten et al., 2006; for review see Moscat et al., 2007). The single Drosophila melanogaster p62 homologue, refractory to Sigma P (ref(2)P/CG10360), was originally characterized in a screen for modifiers of sigma virus multiplication (Carré-Mlouka et al., 2007) and shares similar functional motifs (Avila et al., 2002; Carré-Mlouka et al., 2007). Ref(2)P has been shown to interact genetically with Drosophila atypical PKC and to participate in the Toll signaling pathway (Avila et al., 2002; Goto et al., 2003).
Ubiquitin-containing protein aggregates are among the most characteristic features of human neurodegenerative diseases, and mouse models have indicated that autophagy is crucial to prevent their accumulation (for review see Rubinsztein, 2006). The mammalian p62 protein is known to closely associate with neural aggregates and inclusion bodies found in the most common neural degenerative disorders (Zatloukal et al., 2002) and has been shown to bind the autophagic protein Atg8/LC3, but its physiological role in aggregate formation and/or clearance has not been elucidated (Bjorkoy et al., 2005; Pankiv et al., 2007).
In this paper, we present that the D. melanogaster p62 homologue Ref(2)P is a major component of protein aggregates formed during normal aging in D. melanogaster adult brain. Ref(2)P is also a major component of protein aggregates in flies that are defective in autophagy, flies that have impaired proteasomal function, and D. melanogaster models of human neurodegenerative diseases. Importantly, both the abilities of Ref(2)P to multimerize (through its Phox and Bem1p [PB1] domain) and to bind ubiquitinated proteins (through its ubiquitin-associated [UBA] domain) are necessary functions required during the in vivo formation of protein aggregates in the adult brain.
Results and discussion
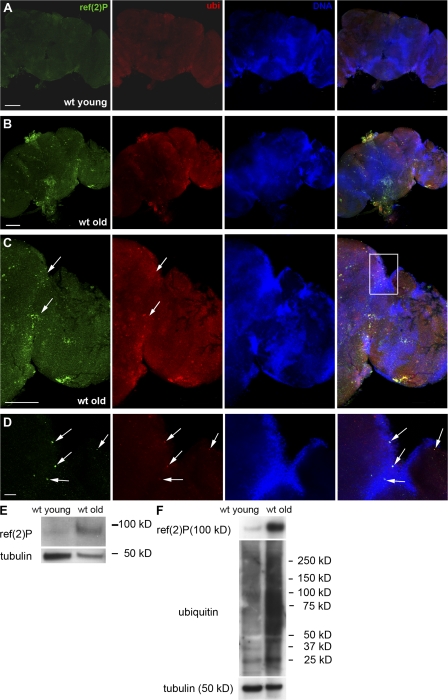
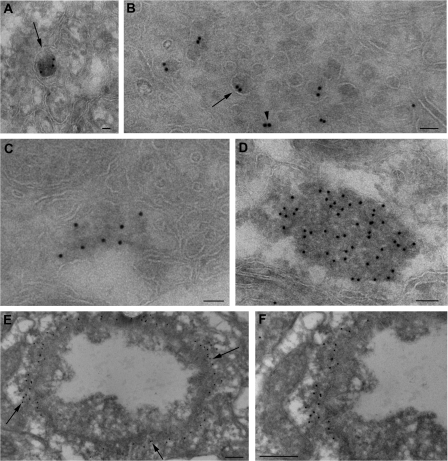
The expression pattern and localization of Ref(2)P in D. melanogaster tissues are not known. To explore the subcellular localization of the Ref(2)P protein and its participation in the formation of protein aggregates, we used immunofluorescence confocal microscopy to determine its expression pattern in adult D. melanogaster neurons. In young wild-type adult brains (2 d old) stained with anti-ref(2)P and anti-ubiquitin antibodies, Ref(2)P- or ubiquitin-positive structures were not detected in any region of the brain (n = 45; Fig. 1 A). In contrast, 8-wk-old flies showed a significant number of Ref(2)P- and ubiquitin-positive structures in both neuropil and cortical regions of the adult brain (n = 30; Fig. 1, B and C), with double-positive structures primarily detected in cortical regions of the central brain and optic lobes (Fig. 1, B–D). This staining pattern is distinct from the presence of occasional autofluorescent structures that are reported to accumulate in old brains (Fig. S1, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200711108/DC1). Western blot analysis showed that old flies have higher levels of Ref(2)P protein than young flies (Fig. 1 E). In addition, Western analysis of detergent-fractionated proteins demonstrated a significant accumulation of Ref(2)P and insoluble ubiquitinated proteins in old flies (Fig. 1 F). To further analyze the nature of Ref(2)P-positive structures, we used electron microscopy and immunogold labeling of ultrathin cryosections of old wild-type D. melanogaster adult brains (n = 5). Ref(2)P was localized in electron-dense masses ranging from 50 nm to 1 μm in diameter that were or were not surrounded by a limiting membrane (Fig. 2, A–D). Occasionally, Ref(2)P appeared to participate in shell-like structures that surrounded aggregated filamentous material (Fig. 2, E and F). Collectively, these data show that the levels of Ref(2)P protein in the adult brain increase with age and that Ref(2)P is a component of protein aggregates accumulating during the normal aging process in the D. melanogaster brain.
Figure 1.
Ref(2)P localization and expression in the adult brain of wild-type flies. (A) Confocal micrographs of adult brain of a young (2 d old) wild-type fly. Positive staining for Ref(2)P and ubiquitin is not evident. (B–D) Confocal micrographs of adult brain of an old (8 wk old) wild-type fly. Ref(2)P accumulates in protein aggregates that often colocalize with ubiquitin (arrows). The white rectangle in C indicates the area shown in D. (E and F) Western blot analysis of total cell lysates (E) and insoluble protein fraction (F) of wild-type young and old adult heads probed with anti-ref(2)P and anti-ubiquitin antibodies, demonstrating a significant increase in Ref(2)P and insoluble ubiquitinated protein levels in old flies. wt, wild type. Bars: (A–C) 100 μm; (D) 10 μm.
Figure 2.
Ref(2)P localizes in protein aggregates in old flies during normal aging. (A–D) Electron micrographs of adult brain of old wild-type flies. Ref(2)P localizes in electron-dense structures ranging from 50 nm to 1 μm in diameter that are surrounded (arrows) or not (arrowhead) by a limiting membrane. (E and F) Electron micrographs of wild-type brain of old flies demonstrating that Ref(2)P forms shell-like structures that surround aggregated filamentous material (arrows). Bars: (A–C) 50 nm; (D) 100 nm; (E and F) 200 nm.
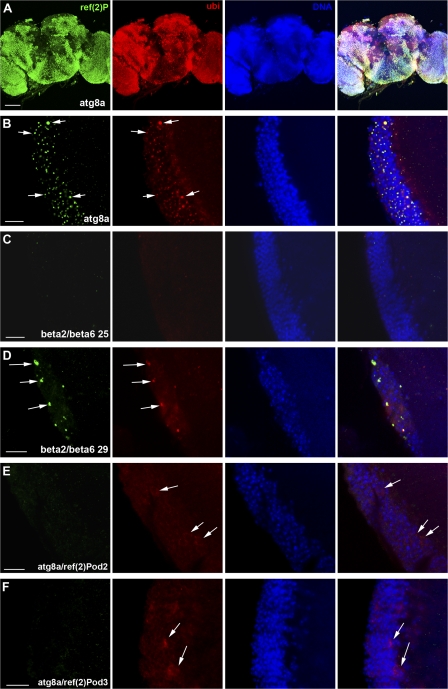
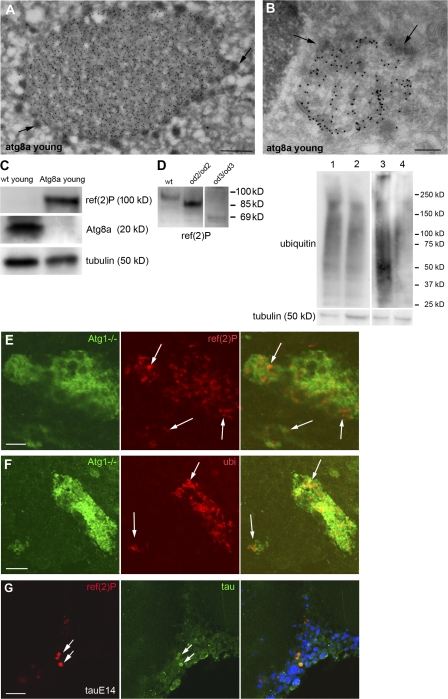
Cytosolic proteins are turned over either by the autophagic pathway or the more selective ubiquitin–proteasome pathway (for review see Yorimitsu and Klionsky 2005; Wooten et al., 2006). In mice whose central nervous system was made defective for autophagy by removing either atg5 or 7, ubiquitin-containing aggregates were observed (Hara et al., 2006; Komatsu et al., 2006). To test whether Ref(2)P is participating in protein aggregates formed upon autophagic or proteasomal dysfunction, we impaired these two pathways genetically. We therefore investigated flies containing a P-element insertion in the atg8a gene, P{SuPor-P }atg8aKG07569. Immunofluorescence preparations of adult brains of young atg8aKG07569 mutant flies (n = 50) demonstrated a striking positive staining for Ref(2)P in all regions of the adult brain. Ref(2)P was localized in aggregates with a diameter of 0.5–2 μm that also stained positively for ubiquitin (Fig. 3, A and B). Lysates from young adult heads were examined by Western blotting, which showed that atg8aKG07569 mutants accumulate Ref(2)P protein compared with young wild-type flies (Fig. 4 C). Ultrastructural analysis of these aggregates revealed that they were not surrounded by a limiting membrane and they contained amorphous granular material resembling the sequestosome morphology. Interestingly, they were surrounded by small electron-dense vesicles (Fig. 4, A and B). Moreover, we investigated brains of D. melanogaster young adults where clones of neurons were made mutant for the atg1 gene that is necessary for autophagy (Scott et al., 2004; 2007). Neurons of young adults mutant for atg1 specifically accumulated Ref(2)P- and ubiquitin-positive structures in a cell-autonomous manner (Fig. 4, E and F).
Figure 3.
Ref(2)P localizes to protein aggregates in flies that have impaired autophagy or proteasome function. The formation of these aggregates is inhibited in atg8a/ref(2)P double mutant flies. (A and B) atg8a mutants exhibiting a striking positive staining for Ref(2)P and ubiquitin staining in all the regions of the adult brain. As it is shown in high magnification of the optic lobe (medulla region; B), Ref(2)P- and ubiquitin-positive aggregates are observed (arrows). (C) Normal phenotype is observed in β2 and 6 dominant-negative temperature-sensitive mutants at 25°C. (D) Accumulation of Ref(2)P- and ubiquitin-positive aggregates are observed in β2 and 6 mutants at 29°C (arrows). (E and F) double mutants for atg8a and ref(2)P loss-of-function alleles lacking the PB1 (E) or UBA (F) domain do not form ubiquitinated protein aggregates. Ubiquitin exhibits a cytoplasmic staining pattern (arrows). Genotypes: (A and B) atg8aKG07569/Y, (C and D) elavGal4/+; UAS-β6/+; UAS-β2/+, (E) atg8aKG07569/Y; ref(2)Pod2/ref(2)Pod2, and (F) atg8aKG07569/Y; ref(2)Pod3/ref(2)Pod3. Bars: (A) 100 μm; (B–F) 10 μm.
Figure 4.
Ref(2)P is a component of protein aggregates in flies defective in autophagy and D. melanogaster models of human neurodegenerative diseases. (A and B) Protein aggregates in atg8aKG07569 mutants exhibit sequestosome morphology and are positively labeled for Ref(2)P (A) and ubiquitin (B). Protein aggregates are surrounded by small electron-dense vesicles (arrows). (C) Western blot analysis of cell lysates of wild type and atg8aKG07569 young adult heads demonstrating a significant increase of Ref(2)P protein levels in atg8aKG07569 flies. (D, left) Ref(2)P protein migration profile in ref(2)P loss-of-function alleles. Ref(2)P mutant protein for the PB1 domain has an apparent molecular mass of 85 kD (ref(2)Pod2/ref(2)Pod2) and the Ref(2)P mutant protein for the UBA domain has an apparent molecular mass of 69 kD (ref(2)Pod3/ref(2)Pod3). (D, right) Western blot analysis of insoluble protein fractions of adult heads of double mutants for atg8a and ref(2)P probed for ubiquitin. Genotypes: (1) atg8aKG07569/Y; ref(2)Pod2/Cyo, (2) atg8aKG07569/Y; ref(2)Pod2/ref(2)Pod2, (3) atg8aKG07569/Y; ref(2)Pod3/CyO, and (4) atg8aKG07569/Y; ref(2)Pod3/ref(2)Pod3. The insoluble ubiquitinated protein profile of the double mutants is diminished compared with the one of the respective control heterozygous for ref(2)P. (E and F) Neuronal cells mutant for atg1 (green) accumulate Ref(2)P- (E) and ubiquitin-positive (F) structures (arrows), whereas the normal surrounding cells do not exhibit positive staining. (G) Confocal micrographs of the optic lobe (medulla region) of young flies expressing a mutated form of human tau protein exhibiting Ref(2)P- and tau-positive aggregates (arrows). Genotypes: (E and F) yw/UAS-CD8-GFP, hs-flp; tubGal4/+; atg1Δ3D, FRT80/tubGal80, FRT80, and (G) elav/+;UAS-E14/+. Bars: (A) 500 nm; (B) 200 nm; (E) 2 μm; (F) 5 μm; (G) 10 μm.
Pros26 (DTS5) and Prosbeta2 (DTS7) are temperature-sensitive dominant-negative mutations of the D. melanogaster β6 and 2 subunits of the proteasome (Neuburger et al., 2006). Using the upstream activator sequence (UAS)/Gal4 system (Brand and Perrimon, 1993), we targeted Pros26 and Prosbeta2 expression in the adult brain to cause proteasome dysfunction. At 25°C (permissive temperature), proteasome function was intact and the brain morphology was normal (Fig. 3 C). However, at 29°C (restrictive temperature), a massive accumulation of aggregates that stained positively for Ref(2)P and ubiquitin was observed mainly in the cortex (n = 20; Fig. 3 D). Additionally, Western blot analysis of detergent fractionated proteins demonstrated a significant accumulation of Ref(2)P protein in proteasome mutant flies at 29°C (Fig. S1 C). Collectively, these data demonstrate that Ref(2)P is localized to protein aggregates in the adult brain of young flies defective in autophagy and flies whose proteasomal function is genetically inactivated.
Ref(2)P protein contains three domains involved in protein–protein interactions: PB1, ZZ, and UBA. The N-terminal end consists of a PB1 domain (aa 6–88) involved in multimerization. The ZZ zinc finger and the UBA domains are located between aa 121 and 165 and aa 554 and 594, respectively (Carré-Mlouka et al., 2007). To address the role of Ref(2)P in aggregate formation, we used two loss-of-function alleles of ref(2)P (Wyers et al., 1995). The ref(2)Pod2 loss-of-function allele results in a protein lacking the PB1 domain and has a molecular mass of 85 kD, compared with the 100 kD mass of the wild-type protein (Fig. 4 D). In the ref(2)Pod3 loss-of-function mutant, the protein lacks the UBA domain and has a molecular mass of 69 kD (Fig. 4 D). These two ref(2)P mutations appear unfunctional for sigma virus ability to multiply and for male fertility (homozygous males are sterile; Wyers et al., 1995). To test whether these two loss-of-function mutations affect the formation of protein aggregates in the adult brain in the absence of autophagy, we decided to test the role of the loss-of-function ref(2)P mutants in an atg8a mutant genetic background. Importantly, young double mutant flies for atg8aKG07569 and ref(2)Pod2 or atg8aKG07569 and ref(2)Pod3 did not exhibit protein aggregates in the cell cortex of the adult brain compared with the single atg8aKG07569 mutant flies (Fig. 3, E–F). Moreover, ubiquitin exhibited cytoplasmic staining (Fig. 3, E–F), and fractionation and Western blot analysis of double mutant brains showed that their insoluble ubiquitinated protein profile was diminished compared with that of control flies (atg8aKG07569/Y; ref(2)Pod2orod3/CyO; Fig. 4 D). This is consistent with a paper published when the present manuscript was under review, reporting that loss of p62 suppresses the formation of protein aggregates in autophagy-deficient neurons and hepatocytes in mice (Komatsu et al., 2007). Collectively, these data show that a functional Ref(2)P protein is necessary for the formation of aggregates of ubiquitinated proteins in vivo in atg8a mutant genetic background. Furthermore, it suggests that both the PB1 and the UBA domain of D. melanogaster Ref(2)P are required for the formation of protein aggregates in vivo.
p62 is known to associate with protein aggregates found in the most common neurodegenerative disorders (Zatloukal et al., 2002). To test the functional conservation of Ref(2)P during the formation of neural aggregates, we examined known D. melanogaster models of human neurodegenerative diseases (Marsh and Thompson, 2006). The mutant human tau protein (Fulga et al., 2007) was expressed throughout the D. melanogaster central nervous system using the pan-neural elav-Gal4 driver (Fig. S1 D). Immunofluorescence microscopy analysis of whole mount brains revealed the presence of Ref(2)P-positive structures in the adult brain that colocalized with tau (Fig. 4 G). These data demonstrate that Ref(2)P can also be a component of protein aggregates in D. melanogaster models of human neurodegenerative diseases.
The formation of protein aggregates is a major feature in many neurodegenerative diseases. The mechanisms of their formation are currently under extensive research. In this paper, we used D. melanogaster adult brain as a model tissue to study the formation of protein aggregates and show that Ref(2)P, the D. melanogaster homologue of mammalian p62, is a major component of protein aggregates in the D. melanogaster adult brain. These aggregates are formed during normal aging, when it is known that proteasomal and autophagic activity diminishes (Vernace et al., 2007; Simonsen et al., 2008). This decrease likely contributes to the reduced mobility and subsequent death of old flies. Ref(2)P is also a major component of protein aggregates in flies that are defective in autophagy, flies that have impaired proteasomal function, and D. melanogaster models of human neurodegenerative diseases. Importantly, both the PB1 and the UBA domains of Ref(2)P are necessary for the formation of protein aggregates, suggesting that both the abilities of Ref(2)P to multimerize and bind ubiquitinated proteins are necessary functions required during the in vivo formation of protein aggregates.
Materials and methods
Fly strains and clonal analysis
Fly crosses and experiments were performed at 25°C unless noted otherwise. The w1118 strain was used as a wild-type control. The P-element insertions y1P{SuPor-P }Atg8a[KG07569]/FM7c, w1118; P{UAS-Pros261.B }2B; and P{UAS-Prosβ21.B }1B and the pan-neural driver elav–GAL4C155 were obtained from the Bloomington Drosophila Stock Center. The UAS line for the mutated human tau (UAS-E14) was a gift from M. Feany (Harvard Medical School, Boston, MA). The atg1Δ3D FRT80 line was a gift from T.P. Neufeld (University of Minnesota, Minneapolis, MN). The ref(2)Pod2 and ref(2)Pod3 flies are described in Wyers et al., (1995). For the induction of clones in the adult brain, larvae were heat shocked on days 4 and 5 (L2 and L3 of larvae development) for 1 h and 30 min at 37°C in a circulating water bath. The UAS CD8 GFP, hsflp; tubGal4/CyO; tubGal80 FRT80 line used for clonal analysis was a gift from T. Schupbach (Princeton University, Princeton, NJ).
Immunofluorescence labeling and confocal microscopy
D. melanogaster adult brains were carefully dissected in PBS and immediately fixed in PBS containing 4% formaldehyde for 1 h. After fixation, the samples were incubated in blocking solution (PBS containing 0.3% Triton X-100 and 0.3% BSA) for 30 min. The brains were then incubated overnight at 4°C in blocking solution containing the primary antibodies. The primary antibodies used in the present study were the following: mouse anti–mono- and polyubiquitinated proteins (Clone FK2; BIOMOL International, L.P.) used at a concentration of 1:500; rabbit polyclonal antibody against D. melanogaster Ref(2)P protein (Wyers et al., 1995) used at a dilution of 1:1,000; and mouse anti-tau1 (Millipore) used at a dilution of 1:1,000. After incubation with the respective primary antibody, brains were washed four times with blocking solution. The secondary antibodies, conjugated with either Cy2 or 3, were purchased from Jackson Immunoresearch Laboratories. Draq5 (Biostatus Limited) was used to stain DNA at a dilution of 1:1,000. Finally, the brains were mounted in antifading mounting medium (Prolong Antifade; Invitrogen) and observed under a confocal laser scanning microscope (LSM 510 META; Carl Zeiss, Inc.) equipped with NeoFluar 16×/0.50 NA, 63×/1.4 NA, and 100×/1.45 NA oil immersion objectives at 20°C. Image processing and analysis were done with LSM 510 software (version 3.2; Carl Zeiss, Inc.), ImageJ (National Institutes of Health), and Photoshop CS2 (Adobe).
Western blot analysis
Biochemical analysis of D. melanogaster adult heads was performed as previously described (Finley et al., 2003, Fulga et al., 2007; Simonsen et al., 2008). The anti-GABARAP antibody used for detecting Atg8a was a gift from T. Ueno and E. Kominami (Juntendo University, Tokyo, Japan).
Electron microscopy
For cryoimmunocytochemistry, the brains were fixed in 4% formaldehyde and 0.1% glutaraldehyde in 0.1 M Sørensen phosphate buffer, pH 7.4. The samples were washed in PBS, infiltrated with 2.3 M sucrose, mounted on sample stubs, and frozen in liquid nitrogen. Sectioning was done on a cryomicrotome (FCS; Leica). Sections were collected with a 1:1 mixture of 2% methylcellulose and 2.3 M sucrose, transferred to formvar/carbon-coated grids, and labeled with the primary antibodies described in Immunofluorescence labeling and confocal microscopy, followed by a secondary rabbit anti–mouse bridging antibody (Dako) and protein A–gold conjugate. Specimens were observed in a JEM-1230 (JEOL) operating at 80 KV. Micrographs were recorded with a digital camera (Morada; Olympus) using ITEM software (Olympus).
Online supplemental material
Fig. S1 shows accumulation of protein deposits in the brains of old flies, proteasome mutant flies, and tau-expressing flies. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200711108/DC1.
Supplementary Material
Acknowledgments
We are very grateful to Thomas P. Neufeld, Mel Feany, Trudi Schupbach, Takashi Ueno, Eiki Kominami and Terje Johansen for fly lines, antibodies and helpful suggestions.
This work was supported by grants from the Hartmann Family foundation (H. Stenmark), the Functional Genomics Program of the Norwegian Research Council (I.P. Nezis, A. Simonsen, T.E. Rusten, H. Stenmark and A. Brech), and the Norwegian Cancer Society (A. Simonsen).
Abbreviations used in this paper: PB1, Phox and Bem1p; UAS, upstream activator sequence; UBA, ubiquitin-associated.
References
- Avila, A., N. Silverman, M.T. Diaz-Meco, and J. Moscat. 2002. The Drosophila atypical protein kinase C-ref(2)p complex constitutes a conserved module for signaling in the toll pathway. Mol. Cell. Biol. 22:8787–8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkoy, G., T. Lamark, A. Brech, H. Outzen, M. Perander, A. Overvatn, H. Stenmark, and T. Johansen. 2005. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171:603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, A.H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118:401–415. [DOI] [PubMed] [Google Scholar]
- Carré-Mlouka, A., S. Gaumer, P. Gay, A.M. Petitjean, C. Coulondre, P. Dru, F. Bras, S. Dezélée, and D. Contamine. 2007. Control of sigma virus multiplication by the ref(2)P gene of Drosophila melanogaster: an in vivo study of the PB1 domain of Ref(2)P. Genetics. 176:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley, K.D., P.T. Edeen, R.C. Cumming, M.D. Mardahl-Dumesnil, B.J. Taylor, M.H. Rodriguez, C.E. Hwang, M. Benedetti, and M. McKeown. 2003. blue cheese mutations define a novel, conserved gene involved in progressive neural degeneration. J. Neurosci. 23:1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulga, T.A., I. Elson-Schwab, V. Khurana, M.L. Steinhilb, T.L. Spires, B.T. Hyman, and M.B. Feany. 2007. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat. Cell Biol. 9:139–148. [DOI] [PubMed] [Google Scholar]
- Goto, A., S. Blandin, J. Royet, J.M. Reichhart, and E.A. Levashina. 2003. Silencing of Toll pathway components by direct injection of double-stranded RNA into Drosophila adult flies. Nucleic Acids Res. 31:6619–6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, T., K. Nakamura, M. Matsui, A. Yamamoto, Y. Nakahara, R. Suzuki-Migishima, M. Yokoyama, K. Mishima, I. Saito, H. Okano, and N. Mizushima. 2006. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 441:885–889. [DOI] [PubMed] [Google Scholar]
- Komatsu, M., S. Waguri, T. Chiba, S. Murata, J. Iwata, I. Tanida, T. Ueno, M. Koike, Y. Uchiyama, E. Kominami, and K. Tanaka. 2006. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 441:880–884. [DOI] [PubMed] [Google Scholar]
- Komatsu, M., S. Waguri, M. Koike, Y.S. Sou, T. Ueno, T. Hara, N. Mizushima, J. Iwata, J. Ezaki, S. Murata, et al. 2007. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 131:1149–1163. [DOI] [PubMed] [Google Scholar]
- Marsh, J.L., and L.M. Thompson. 2006. Drosophila in the study of neurodegenerative disease. Neuron. 52:169–178. [DOI] [PubMed] [Google Scholar]
- Moscat, J., M.T. Diaz-Meco, and M.W. Wooten. 2007. Signal integration and diversification through the p62 scaffold protein. Trends Biochem. Sci. 32:95–100. [DOI] [PubMed] [Google Scholar]
- Neuburger, P.J., K.J. Saville, J. Zeng, K.A. Smyth, and J.M. Belote. 2006. A genetic suppressor of two dominant temperature-sensitive lethal proteasome mutants of Drosophila melanogaster is itself a mutated proteasome subunit gene. Genetics. 173:1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv, S., T.H. Clausen, T. Lamark, A. Brech, J.A. Bruun, H. Outzen, A. Øvervatn, G. Bjørkøy, and T. Johansen. 2007. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282:24131–24145. [DOI] [PubMed] [Google Scholar]
- Rubinsztein, D.C. 2006. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 443:780–786. [DOI] [PubMed] [Google Scholar]
- Scott, R.C., O. Schuldiner, and T.P. Neufeld. 2004. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell. 7:167–178. [DOI] [PubMed] [Google Scholar]
- Scott, R.C., G. Juhasz, and T.P. Neufeld. 2007. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr. Biol. 17:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen, A., R.C. Cumming, A. Brech, P. Isakson, D.R. Schubert, and K.D. Finley. 2008. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila.Autophagy. 4:176–184. [DOI] [PubMed] [Google Scholar]
- Vernace, V.A., L. Arnaud, T. Schmidt-Glenewinkel, and M.E. Figueiredo-Pereira. 2007. Aging perturbs 26S proteasome assembly in Drosophila melanogaster.FASEB J. 21:2672–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten, M.W., X. Hu, J.R. Babu, M.L. Seibenhener, T. Geetha, M.G. Paine, and M.C. Wooten. 2006. Signaling, polyubiquitination, trafficking, and inclusions: sequestosome 1/p62's role in neurodegenerative disease. J. Biomed. Biotechnol. 2006:62079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyers, F., A.M. Petitjean, P. Dru, P. Gay, and D. Contamine. 1995. Localization of domains within the Drosophila Ref(2)P protein involved in the intracellular control of sigma rhabdovirus multiplication. J. Virol. 69:4463–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu, T., and D.J. Klionsky. 2005. Autophagy: molecular machinery for self-eating. Cell Death Differ. 12:1542–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatloukal, K., C. Stumptner, A. Fuchsbichler, H. Heid, M. Schnoelzer, L. Kenner, R. Kleinert, M. Prinz, A. Aguzzi, and H. Denk. 2002. p62 is a common component of cytoplasmic inclusions in protein aggregation diseases. Am. J. Pathol. 160:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.