Abstract
Varietal data from 27 crop species from five continents were drawn together to determine overall trends in crop varietal diversity on farm. Measurements of richness, evenness, and divergence showed that considerable crop genetic diversity continues to be maintained on farm, in the form of traditional crop varieties. Major staples had higher richness and evenness than nonstaples. Variety richness for clonal species was much higher than that of other breeding systems. A close linear relationship between traditional variety richness and evenness (both transformed), empirically derived from data spanning a wide range of crops and countries, was found both at household and community levels. Fitting a neutral “function” to traditional variety diversity relationships, comparable to a species abundance distribution of “neutral ecology,” provided a benchmark to assess the standing diversity on farm. In some cases, high dominance occurred, with much of the variety richness held at low frequencies. This suggested that diversity may be maintained as an insurance to meet future environmental changes or social and economic needs. In other cases, a more even frequency distribution of varieties was found, possibly implying that farmers are selecting varieties to service a diversity of current needs and purposes. Divergence estimates, measured as the proportion of community evenness displayed among farmers, underscore the importance of a large number of small farms adopting distinctly diverse varietal strategies as a major force that maintains crop genetic diversity on farm.
Keywords: conservation on farm, diversity estimates, traditional varieties
Crop genetic resources have long been crucial to agricultural production, and the second half of the last century saw considerable effort in collecting, characterizing, and conserving this diversity in seed banks (ex situ conservation). Although these efforts have led to a worldwide network of ex situ gene banks and botanical gardens, these facilities cannot accommodate the full range of useful diversity in economically useful plant species, nor can they conserve the dynamic processes of crop evolution and farmers' knowledge of crop selection and management inherent in the development and evolution of local cultivars (1, 2).
Over the last two decades, the conservation of genetic resources on farm has received increasing attention (3, 4). In many parts of the world, traditional crop varieties [“landraces” in the sense of Harlan (5)] are still grown in traditional farming systems (6, 7) and constitute important elements of the production systems and of the farmers' livelihood strategies. Although an accurate gauge of the diversity present on farms at a global scale is still lacking, there is a consensus of the erosion of the genetic diversity that supports world food production (8). The evidence for this comes largely from individual short-term, single-country, or single-species studies (4), and often diversity information is captured only as a list of variety names. Furthermore, indices that could scale up or monitor these studies over time and space are unformulated.
Richness and evenness are two key notions of biological diversity (9, 10). Richness refers to the number of different kinds of individuals regardless of their frequencies. Evenness, however, measures how similar the frequencies of the different variants are, with low evenness indicating dominance by one or a few types. Applying these concepts at the scale of traditional varieties requires prior determination of the identity of the varieties. These simple but powerful concepts of diversity are often forgotten when small-scale or single-crop studies are conducted.
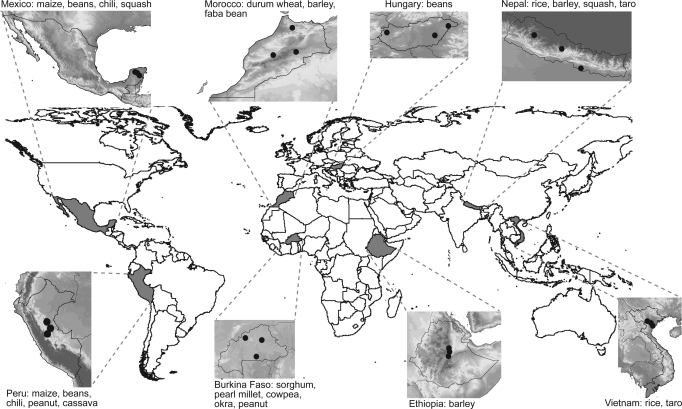
Over the last 10 years, a coordinated global partnership of researchers in eight countries and on five continents has measured the amount and distribution of genetic diversity present in farmers' fields of 27 crop species (11) (Fig. 1). Through this partnership, countries worked together to collate biologically and culturally diverse datasets into a small number of globally applicable diversity indices to compare across farmer households and communities. This paper: (i) synthesizes the total body of diversity data gathered in the study, (ii) demonstrates that considerable crop genetic diversity is maintained on farm, (iii) provides evidence of broadly based relationships between different measures of crop diversity, and (iv) demonstrates that these measures provide a useful framework for the conservation and management of diversity in farmers' fields (on farm) and an appropriate basis for developing indicators of on-farm diversity.
Fig. 1.
Map showing location of study areas for the crops included (site details are presented in SI).
Results
Basic Richness Units.
Twenty-seven major food subsistence crop species were surveyed. For each crop, Table 1 lists the relationship between farmer-named varieties and the “basic diversity units” used to measure richness as one of three kinds: (A) the farmer-named varieties coincided with the basic richness units, (B) the variety names underestimated the units farmers were using to manage diversity, or (C) the variety names overestimated (different names for the same variety) for at least some varieties but underestimated (same name for different varieties) for other varieties. The basic diversity units for categories B and C resulted from discussions with farmers to define subclasses or morphotypes that were recognizable [see supporting information (SI) for supporting literature for the establishment of basic diversity units by country and project crop]. In cases where farmers' classification of traditional varieties of a single crop included more than one species, the species were grouped by crop to calculate richness and evenness (Table 1).
Table 1.
Rational and procedure defining units of diversity in 27 crops species by eight countries
| Crop | Country | Variety name – units of diversity relationship (A, B, C)* | Breeding system Cl, In, Po, Oc† | Major starch source |
|---|---|---|---|---|
| Barley (Hordeum vulgare) | Nepal | A | In | Yes |
| Rice (Oryza sativa) | Nepal, Vietnam | A | In | Yes |
| Finger millet (Eleusine coracana) | Nepal | A | In | |
| Bean‡ (Phaseolus vulgaris, Phaseolus lunatis, Vigna unguiculata) | Burkina Faso, Mexico, Peru | A | In | |
| Peanut (Arachis hypogaea) | Burkina Faso, Peru | A | In | |
| Okra (Abelmoschus esculentus) | Burkina Faso | A | Oc | |
| Squash (Cucurbita maxima, Cucurbita mixta, Cucurbita moschata, Cucurbita pepo, Luffa cylindrica) | Mexico, Nepal§ | A | Oc | |
| Chili (Capsicum annuum, Cucurbita chinense, Cucurbita baccatum, Cucurbita pubescens, Cucurbita frutescens) | Peru*, Mexico | A | Po | |
| Taro (Colocasia esculenta, Xanthosoma spp.) | Nepal, Vietnam | A | Cl | |
| Barley (Hordeum vulgare) | Ethiopia, Morocco | B | In | Yes |
| Durum wheat (Triticum durum) | Morocco | B | In | Yes |
| Pearl millet (Pennisetum glaucum) | Burkina Faso | B | Oc | Yes |
| Bean (Phaseolus vulgaris) | Hungary§ | B | In | |
| Maize (Zea mays) | Mexico, Peru | C | Oc | Yes |
| Sorghum (Sorgum bicolor) | Burkina Faso | C | Po | Yes |
| Faba bean (Vicia faba) | Morocco | C | Po | |
| Cassava (Manihot esculenta) | Peru | C | Cl | Yes |
*A, variety names (names used direction as the units farmers manage); B, variety names underestimate the units farmers are using to manage diversity; C, variety names both overestimated (different names for the same variety) for some varieties and underestimated (same name for different varieties) for other varieties. See Sadiki et al. (14) and SI for further details of variety names and units of diversity relationships.
†Cl, clonal; In, inbreeding; Po, partially outcrossing; Oc, outcrossing.
‡Beans (common bean, lima bean, and cowpea), chili, squash. and taro are managed as crop complexes. Each of these crop complexes occupy the same ecological and cultural niche, respectively, and are considered by farmers as a single crop types.
§Average household area planted to traditional varieties of the crop is <50 m2 (i.e., crop managed as few plants in home gardens).
Overall Diversity Estimates.
Table 2 lists, for each crop, the total land areas and averages of the diversity statistics at both the farm and community levels. Overall, the study encompassed an area of 63,600 ha planted with the target crops. Traditional varieties dominated the planting at most of the sites (from 80% to 100% of the total crop area). The exception was rice, where the range was from 7% to 100% across the six sites (see SI). Across all crops, farmers on average grew more than one traditional variety, because the overall average richness per farm was 1.82. The on-farm richness of traditional varieties ranged from 1.38 to 4.25 per household. At the community level, the richness indices indicated that communities harbored a large number of varieties. The mean number of varieties per community ranged from 4 (durum wheat) to 60 (cassava). The number of varieties differed significantly within and among countries (estimates for individual countries and crops are given in SI). For example, rice richness in Vietnam varied from 9 to 74 varieties per community.
Table 2.
Community and household area statistics and estimates of diversity for traditional varieties in crops
| Crop | Total community area devoted to the crop,* ha | Mean community percent farm area devoted to traditional varieties, % | Number of households (or farms) (total = 4,074) | Average area for traditional varieties,† ha | Range of community means of farm areas for traditional varieties, ha | Avg. farm richness | Avg. farm evenness‡ | Community richness | Community evenness‡ | Average divergence |
|---|---|---|---|---|---|---|---|---|---|---|
| Rice | 22,182 | 38 | 777 | 0.20 | 0.035–0.35 | 2.18 | 0.25 | 34.83 | 0.77 | 0.67 |
| Barley | 10,790 | 98 | 308 | 0.79 | 0.12–1.86 | 1.39 | 0.16 | 6.33 | 0.60 | 0.72 |
| Maize | 8,588 | 97 | 449 | 1.83 | 0.72–3.68 | 1.57 | 0.18 | 8.50 | 0.60 | 0.68 |
| Cassava | 4,183 | 100 | 159 | 0.48 | 0.26–0.63 | 2.05 | 0.33 | 60.33 | 0.96 | 0.66 |
| Faba bean | 3,825 | 100 | 87 | 1.29 | 0.76–1.76 | 1.77 | 0.28 | 6.50 | 0.68 | 0.60 |
| Durum wheat | 3,064 | 82 | 354 | 0.34 | 0.35–1.67 | 1.49 | 0.21 | 3.50 | 0.57 | 0.64 |
| Beans | 2,642 | 98 | 524 | 0.74 | 0.0015–0.79 | 1.80 | 0.27 | 8.92 | 0.63 | 0.58 |
| Pearl millet | 2,365 | 100 | 49 | 0.76 | 0.56–0.99 | 2.42 | 0.47 | 12.67 | 0.86 | 0.46 |
| Peanut | 2,176 | 100 | 96 | 0.51 | 0.22–1.09 | 1.69 | 0.24 | 7.50 | 0.70 | 0.64 |
| Sorghum | 1,811 | 100 | 52 | 1.25 | 0.95–1.72 | 4.25 | 0.69 | 23.33 | 0.91 | 0.25 |
| Squash | 1,417 | 100 | 562 | 1.00 | 0.0004–3.05 | 1.51 | 0.22 | 8.01 | 0.66 | 0.63 |
| Okra | 265 | 100 | 51 | 0.36 | 0.309–0.397 | 2.22 | 0.48 | 10.00 | 0.80 | 0.40 |
| Finger millet | 248 | 100 | 337 | 0.09 | 0.036–0.20 | 1.38 | 0.15 | 14.00 | 0.67 | 0.76 |
| Chili | 30 | 100 | 175 | 0.10 | 0.0001–0.19 | 1.42 | 0.16 | 6.17 | 0.70 | 0.76 |
| Taro | 24 | 100 | 361 | 0.03 | 0.0069–0.053 | 1.44 | 0.12 | 17.20 | 0.65 | 0.81 |
| Weighted average | 1.82 | 0.26 | 14 | 0.70 | 0.63 |
*Includes total area of traditional and modern varieties.
†These averages exclude the farms with <50 m2 (i.e., home gardens).
‡These averages are the complement of the relevant Simpson indices (SI).
There was appreciable evenness at the farm level and particularly at the community level. In general, farm evenness statistics indicated that farm diversity is not made up of one dominant and other very rare varieties. Instead, any two samples drawn at random at the farm level differed in varietal source in 25% of the cases. Evenness at the community level was impressively high, with a mean of 0.70. The last column in Table 2 lists the estimates of divergence. This measure reflects the potential of any two randomly chosen households within the same community to grow different varieties and ranged from 0.25 to 0.81, depending on the crop.
Patterns Among Categories of Crop Species.
The 27 crops species were grouped into four broad categories of breeding system and two of crop use (Table 1). Table 3 gives the averages of the diversity measures for these categories. At the farm level, the categories did not differ significantly for diversity. However, at the community level, significant differences in community richness were found, with variety richness for clonal species much higher than the other systems (Table 3). The major staples had higher richness and evenness than the nonstaples, a difference significant at the community level. No significant differences were found in divergence among either breeding systems or use types. Clonally propagated crops had high richness for a given evenness, whereas outcrossing and partially outcrossing crops tended to have more even frequencies across communities.
Table 3.
Overall trends for categories of crops classified by breeding systems and use
| Classification | No. of crops | N† | Farm richness | Farm evenness | Community richness | Community evenness | Divergence |
|---|---|---|---|---|---|---|---|
| Breeding system | |||||||
| Outcrossing | 4 | 17 | 1.73 | 0.28 | 9.4 | 0.70 | 0.60 |
| Partially outcrossing | 3 | 11 | 2.26 | 0.33 | 10.9 | 0.75 | 0.59 |
| Inbreeding | 6 | 35 | 1.75 | 0.23 | 12.8 | 0.66 | 0.64 |
| Clonal | 2 | 8 | 1.70 | 0.20 | 33.4 | 0.77 | 0.76 |
| Mann–Whitney test | NS | NS | ** | NS | NS | ||
| Use | |||||||
| Main staple | 7 | 29 | 2.1 | 0.29 | 20.5 | 0.73 | 0.62 |
| Others | 8 | 42 | 1.65 | 0.23 | 9.5 | 0.67 | 0.65 |
| Mann–Whitney test | NS | NS | * | * | NS |
*, P < 0.05; **, P < 0.01; ***, P < 0.001.
†Number of communities.
Relationships Among Diversity Measures.
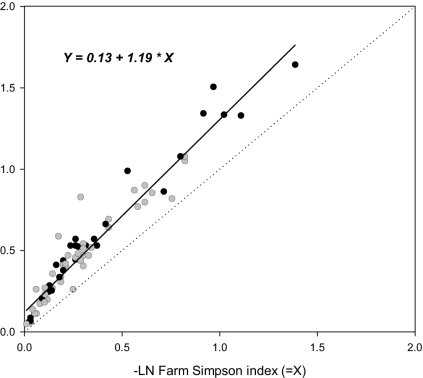
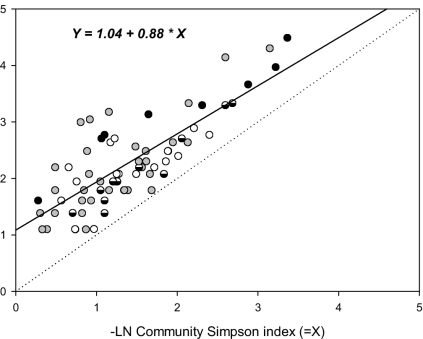
Table 4 summarizes the relationships among the three measures of diversity (richness, evenness, and divergence) inter se and their relationship with crop area. Figs. 2–4 display three of these relationships. Richness and evenness were highly correlated at both the farm and community levels (Table 4; Figs. 2 and 3). Over the whole study, the correlation between farm richness and evenness accounted for ≈94% of the variance, although it was closer at the farm than at the community level. The richness–evenness relationship was also mirrored in the Spearman rank correlation coefficients (12), computed for the overall data, for among and within the 26 combinations of individual crops in each country. Figs. 2 and 3 plot the regression of richness on evenness at the farm and community levels, both variables transformed to a logarithm scale. The fit is linear (P < 0.0001 and P < 0.1000, respectively). The implications of this major result are discussed below.
Table 4.
Pearson and Spearman rank correlation coefficients for diversity and area variables
| Relationships |
Pearson correlation, r | Spearman Rank correlation |
|||
|---|---|---|---|---|---|
| X | Y | Overall | Among CCC† | Within CCC‡ | |
| Farm evenness | Farm richness | 0.95*** | 0.97*** | 0.94*** | 0.82*** |
| Community evenness | Community richness | 0.81*** | 0.73*** | 0.67*** | 0.46*** |
| Divergence | Community richness | −0.05 | −0.07 | 0.06 | −0.14 |
| Farm field area | Farm richness | 0.34* | 0.44*** | 0.41 | 0.26 |
| Farm field area | Farm evenness | 0.36* | 0.49*** | 0.52** | 0.26* |
| Farm field area | Divergence | −0.37** | −0.51*** | −0.53*** | −0.28 |
| Community area | Community richness | 0.04 | −0.01 | −0.05 | 0.42* |
| Community area | Community evenness | 0.09 | 0.04 | 0.02 | 0.22 |
| Community area | Divergence | −0.24 | −0.21 | −0.30 | −0.14 |
All variables are transformed. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
†Among CCC (specific combination of country, community, and crop) was derived as follows: the estimates for each of the communities (usually three) within a specific combination of crop and country (e.g., barley in Ethiopia) were averaged. These 26 averages were then ranked over the whole study.
‡Within CCC: the values for each community were ranked (1, 2, or 3) within a specific combination of crop and country. The resulting 25 possible estimates of rank correlation were averaged. (Only one community was surveyed for barley in Nepal).
Fig. 2.
Relationship between farm evenness and farm richness, both on a logarithm scale. Black, main staple; gray, nonmain staple; 2 × 2 contingency χ (P = 0.03).
Fig. 3.
Relationship between community evenness and community richness, both on a logarithm scale. White, outcrossing; semifilled, partial outcrossing; gray, inbreeding; black, clonal.
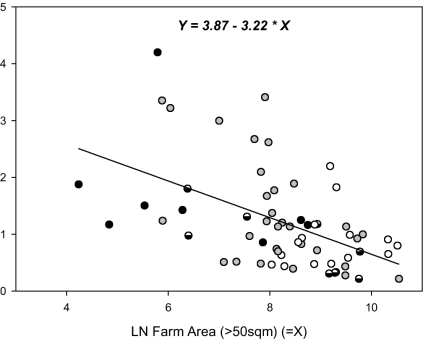
Fig. 4.
Relationship between farm area and divergence, both on a logarithm scale. White, outcrossing; semifilled, partial outcrossing; gray, inbreeding; black, clonal. The graph excludes farms with <50 m2 per household (i.e., home gardens).
Fig. 2 graphs the data points coded whether the crop is typed as a staple or nonstaple species (Table 1). A contingency χ2 test revealed that staples were significantly (P = 0.0012) more often located above the line (i.e., had excess richness for a given Simpson value) compared with nonstaple crops. There was no such pattern among breeding systems at the farm level. In contrast, Fig. 3 displays the data points according to breeding system. Crops with an open breeding system (partial to complete outcrossing) were significantly (P = 0.028) more often located below the line than inbreeders and clonals, implying the latter crops had higher richness for a given Simpson index. At the community level, there was no significant effect on use type.
Field-Size Effects.
In biodiversity assessment in general, area variables have been proposed as surrogate indicators of diversity (13). Table 4 gives the overall correlations between the area growing a particular crop on a farm and the diversity measures. Although larger fields tended on average to have higher varietal diversity, the relationship between area and divergence was negative (Fig. 4). Communities having smaller farm-field areas showed more differentiation in varietal composition than those with larger areas. Fig. 4 codes the data points for breeding systems and shows the trend for field size to be larger for crops with more open breeding. As well, staples were significantly (P = 0.024) more likely to lie above the fitted regression of richness on area.
Discussion
Landrace Diversity on Farm.
This study is based on a wide range of crops species growing in traditional agroecosystems. The farms were sampled to represent a broad picture of the varietal diversity on ≈63,600 ha. Perhaps the most remarkable finding is that farmers who chose to grow traditional varieties more often than not are growing more than one variety of crop, presumably a deliberate choice for diversity. Moreover, the average Simpson index diversity at the farm level is 0.26, equivalent in a two-varietal system to a frequency for the dominant type of <0.85. In addition, community richness (omitting cassava) is 8-fold that of farm richness, a result that underscores the importance of the divergence between farms within the local community.
There has been serious debate about the variety names as a basis for arriving at estimates at variety numbers and richness values (4, 14). In part, the issues resemble the problems associated with using species occurrences and density in conservation decisions for natural communities (15). Two issues are that the reliability of names as indicators of population ancestry is likely to decrease as the geographic scale of sampling increases, and that pairs of populations of a crop differing in name are not equally divergent genetically. The hierarchical approach adopted here (i.e., surveying farms within communities) gives a framework for testing the correspondence between named varieties and actual genetic divergence for specific crop–country situations (16). Yet, apart from being convenient variables to tabulate on a broad scale, variety names have additional advantages for use in summary measures of diversity. First, they focus attention on farmers themselves as key factors in maintaining crop diversity. Second, farmer management of the diversity is itself in part a self-adapting system (17). For example, the belief that a named recognizable population is adapted to a particular soil or disease regime leads to particular actions by farmers based on that belief. This is likely to set up a powerful selection routine that will work to improve that population for the farmers' preferred trait and lead to a self-sustaining system.
Richness–Evenness Relationship.
The close linear relationship between traditional variety richness and evenness, empirically derived here from data spanning a wide range of crops and countries, is important from two perspectives. First, it implies that estimates of richness can be approximated by those of evenness. Richness is the diversity statistic of most importance in conservation. However, it has the drawback that it inherently depends on sample size, but evenness can be estimated from small samples. The finding of a close overall relationship between the two measures means that one statistic, appropriately transformed, can be used for an approximation of the other. Although richness is known to contribute to Simpson measures (9, 10), the standardized variance of frequencies (i.e., evenness) is a substantial component. Hence, the Simpson index receives ecologists' support as a measure of evenness diversity.
Second, the deviation of any bivariate point from the line itself carries potentially important information for conservation management. Departures above the line indicate above-average richness for a given evenness and suggest the dominance of one variety with much of the richness held in low frequencies. Departures below the line indicate a comparatively more even distribution of types. Such departures may provide a starting point for the development of testable hypotheses on the different social, economic, or environmental factors affecting the relationship. For example, staple crops had high richness or high dominance (Fig. 2) and high insurance diversity (diversity for future use), as might be supposed for the main crop of the farm, whereas the trend for nonstaples was to higher evenness and higher diversity for immediate use.
The comparison of richness and evenness values across a wide range of crops and continents can lead to a global average relationship of richness and evenness at household and community levels, particularly for areas where local crop genetic diversity dominates the landscape. It is of interest to ask what the relationship might be for a specific model, analogous to the comparison of the richness–evenness relationship for DNA diversity (e.g., the Tajima test) or in species abundance distributions of “neutral ecology” (18, 19). Fitting a neutral “function” to the traditional-variety diversity relationships would provide an additional benchmark from which to assess standing diversity on farm. High dominance, with much of the richness held at low frequencies, indicates a management strategy for diversity maintained as an insurance to meet future environmental changes or social and economic needs. However, an even frequency distribution of varieties implies that farmers are selecting varieties to service a diversity of specific current needs and purposes. The difference between the two situations has implications for the use and conservation of traditional-variety diversity.
Area as a Predictor of Diversity.
In considering variables that might be useful as indicators of genetic diversity, Brown and Brubaker (17) suggested that the area planted to a specific crop, an approximation of population size, could serve as an indicator of genetic diversity for temporal and spatial comparisons for any crop within a particular agricultural production system. That suggestion parallels the species diversity–area relationships that are well known in community ecology (12). In this present study, both richness and evenness diversity are significantly correlated with farmer area. Although only a small amount of the variance in genetic diversity is explained by these two predictors, the relationship arose from the broad sweep of data in the study and suggests that the use of area of a crop grown by farmers may be useful as an indicator of diversity. This suggests it may be important to monitor changes of farm size over time.
Divergence.
Measured as the proportion of community evenness displayed between farms or households, is an indicator of the extent to which two farms have diverged by adopting different varietal strategies. High divergence implies the community is maintaining genetic diversity among farms. A functional interpretation of our results is that local habitats or smaller field areas are heterogeneous, requiring neighboring farmers to grow different varieties, particularly for the communities' staple crops (Fig. 4). However, the apportionment measure of divergence has at least one serious disadvantage. It refers only to the diversity actually present, and it can take misleadingly high values when there is only a small amount of diversity. For example, if all farms except one are growing one variety, and the exceptional farm has just a few plants of another variety, then the divergence estimate is maximal at 100%. Nevertheless, our estimates underscore the importance of a large number of small farms adopting distinctly different varietal strategies as a major force in retaining crop genetic diversity on farm. If this inference is well founded, it means that well intentioned intervention that unifies landscapes genetically may threaten such diversity in the long term.
Materials and Methods
Study Sites.
Research was carried out from 1998 to 2005 in Burkina Faso, Ethiopia, Hungary, Mexico, Morocco, Nepal, Peru, and Vietnam, through the collaboration of national research and education institutes with local nongovernment agencies and farmer communities. Twenty-five communities were involved in the work across eight countries. The communities consisted of groups of villages that shared (to varying degrees) agroecological regions, common markets, and planting materials.w These communities were selected to encompass environmental, cultural, technological, and economic differences. Fig. 1 shows the location of sites and target crop species (site details are presented in SI). Site elevations ranged from sea level in the Yucatan Peninsula, Mexico, to >3,000 m in the Nepal highlands. The environments included arid and semiarid climates in Burkina Faso and Morocco, temperate areas in Hungary and Nepal; tropical highlands in Ethiopia; and tropical and subtropical lowlands in Mexico, Amazonian Peru, and Vietnam. The farming systems were either rain-fed, irrigated, or shifting cultivation. Economic development ranged from less-developed economies, such as Ethiopia and Nepal, to more-developed economies, such as Hungary (20).
Sampling on Farms and in Communities.
Information was collected from a total number of 2,041 households in 26 communities, for an average 2.2 crops per household, or 4,074 records in all. Household size ranged from nuclear families of two to five people to extended family households in Burkina Faso averaging 18 persons. For each target crop, only those households that grew at a least one traditional variety of the crop under investigation were included in the analysis. The number of modern varieties of a specific crop available to the community was recorded to reflect the extent of exotic genetic resources available to households from any source but not necessarily bought and planted by the farmers surveyed in the study. The proportion of the farm growing traditional varieties was measured to indicate the percentage of the crop that consisted of local varieties.
Nomenclatural Procedures.
Consistency of variety names was an issue given attention early in the study, because it was essential to the proper analysis of diversity data. Thus, we recorded the name each farmer gave to each variety, together with the descriptors the farmer used to recognize the variety in question and distinguish it from others (21). These sets of traits, together with the names and traits used by different farmers, photos of varieties, and, in some cases, common garden plots of all local varieties, were used to arrive at the “basic diversity units” for each crop. The diversity units were based on agreement among farmers that the units at hand were different. The process of arriving at distinguishable units included the removal of duplicates (i.e., two varieties with different names that the farmers agreed were actually the same items) and separating larger units into discrete units (i.e., two varieties referred to with the same name by two or more different farmers but recognized as different units). Sadiki et al. (16) give specific details, and Jarvis and Campilan (22) provide guidelines on how this was done for different crops (see SI for supporting literature for the establishment of basic diversity units by country and project crop). These “basic diversity units” were used in this study to calculate richness.
Area Planted.
The area planted with each variety was estimated by using local area measurements, converted to square meters and hectares. The area growing both modern and traditional varieties was noted to calculate the total area planted with the crop and the proportion of the farm growing traditional varieties (data are presented in SI). Farms with no traditional varieties were excluded from averages. Although this biases some measures of diversity, the areas chosen for the studies were already those where such traditional varieties were prevalent. However, farms that have no traditional varieties of a crop do not contribute information about levels and patterns of traditional variety diversity. The time period of the sample was the standing crop over 1 year. For cases where there were two cropping seasons, the land area was doubled. The area of home garden crops, such as chili and squash in Nepal, for which farmers grew <10 plants, was estimated by using leaf area covered (canopy) and was multiplied by the number of plants grown on the farm. Following Magurran (10), we measured evenness as the complement of d (= 1-D), where D is the Simpson measure of dominance.x
Diversity Estimates.
Average farm richness was calculated as the average number of traditional varieties per household, excluding households that grew no traditional varieties. The Simpson index itself is a measure of dominance, and it is more convenient to tabulate its complement (1-SI) as the estimate of evenness diversity, including only farms that grew at least one traditional variety. The index is relatively insensitive to the correct identification of rare varieties but assumes the commoner varieties are reliably identified. Total community richness was calculated by summing the number of distinct traditional varieties found across villages in the community. Percentage divergence (i.e., the partition of diversity between and within farms) was calculated as the difference between community and farm index values divided by the community Simpson index [analogous to the genetic divergence measures used by Hamrick and Godt (23)].
Supplementary Material
Acknowledgments.
This work was made possible through the input of numerous scientists worldwide. We thank Dr. Stephen Brush for assistance during the formulation stage of the work and Drs. Paul Hattersley, Brad Murray, and anonymous reviewers for comments. This work was supported by grants from the Swiss Agency for Development and Cooperation (SDC), the Netherlands Directorate-General for International Cooperation (DGIS), the Bundesministerium für Wirtschaftliche Zusammenarbeit/Deutsche Gesellschaft für Technische Zusammenarbeit (BMZ/GTZ), the Japanese International Cooperation Agency (JICA), the International Development and Research Center (IDRC) Canada, and the governments of Spain and Peru.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800607105/DC1.
The word “community” is interpreted differently depending on national administrative regions, local cultures, and language. To standardize the community unit of analysis, collaborating partners agreed that a “community” would consist of one or more villages linked by a common agroecological system, local markets, and/or seed exchange system. Equivalent local terms for community units are found in SI.
Suppose the inverse of the coefficient of variation of variety frequencies is the basic concept of evenness; then, the Simpson index of diversity includes a richness-diversity component (9).
References
- 1.Bellon M, Pham J-L, Jackson MT. In: Plant Genetic Conservation: The In Situ Approach. Maxted N, Ford-Lloyd BV, Hawkes JG, editors. London: Chapman and Hall; 1997. pp. 261–289. [Google Scholar]
- 2.Fowler C, Hodgkin T. Plant genetic resources for food and agriculture: assessing global availability. Annu Rev Env Resour. 2005;29:10.1–10.37. [Google Scholar]
- 3.Brush S. In situ conservation of landraces in centers of crop diversity. Crop Sci. 1995;35:346–354. [Google Scholar]
- 4.Bretting PK, Duvick DN. Dynamic conservation of plant genetic resources. Adv Agron. 1997;61:1–51. [Google Scholar]
- 5.Harlan JR. Crops and Man. 1st Ed. Madison, WI: American Society of Agronomy and Crop Science Society of America; 1975. [Google Scholar]
- 6.Brush S. Farmers' Bounty: Locating Crop Diversity in the Contemporary World. New Haven, CT: Yale Univ Press; 2004. [Google Scholar]
- 7.Teshome A, Brown AHD, Hodgkin T. Diversity in landraces of cereal and legume crops. Plant Breed Rev. 2001;21:221–261. [Google Scholar]
- 8.Food and Agriculture Organization of the United Nations. The State of the Worlds' Plant Genetic Resources for Food and Agriculture. Rome: Food and Agriculture Organization; 1998. [Google Scholar]
- 9.Frankel OH, Brown AHD, Burdon JJ. The Conservation of Plant Biodiversity. Cambridge, UK: Cambridge Univ Press; 1995. [Google Scholar]
- 10.Magurran AE. Measuring Biological Diversity. Oxford, UK: Blackwell; 2003. [Google Scholar]
- 11.Jarvis DI, T. Hodgkin T. In: Genes in the Field: On-Farm Conservation of Crop Diversity. Brush SB, editor. Boca Raton, FL: Lewis; 2000. pp. 261–278. [Google Scholar]
- 12.Siegel S. Nonparametric Statistics. New York: McGraw–Hill; 1956. [Google Scholar]
- 13.MacArthur RH, Wilson EO. The Theory of Island Biogeography. Princeton, NJ: Princeton Univ Press; 1967. [Google Scholar]
- 14.Perales HR, Benz BF, Brush SB. Maize diversity and ethnolinguistic diversity in Chiapas, Mexico. Proc Natl Acad Sci USA. 2005;102:949–954. doi: 10.1073/pnas.0408701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieseberg LH, Wood TE, Baack EJ. The nature of plant species. Nature. 2006;440:524–527. doi: 10.1038/nature04402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadiki M, et al. In: Managing Biodiversity in Agricultural Ecosystems. Jarvis DI, Padoch C, Cooper HD, editors. New York: Columbia Univ Press; 2007. pp. 34–76. [Google Scholar]
- 17.Brown AHD, Brubaker CL. In: Managing Plant Genetic Diversity. Engels J, Brown AHD, Jackson MT, Rao VR, editors. Wallingford, UK: CABI; 2001. pp. 249–262. [Google Scholar]
- 18.McGill BJ. A test of the unified neutral theory of biodiversity. Nature. 2003;422:881–885. doi: 10.1038/nature01583. [DOI] [PubMed] [Google Scholar]
- 19.Bell G. Neutral macroecology. Science. 2001;293:2413–2418. doi: 10.1126/science.293.5539.2413. [DOI] [PubMed] [Google Scholar]
- 20.Smale M. Valuing Crop Biodiversity: On-Farm Genetic Resources and Economic Change. Oxfordshire, UK: CABI; 2006. [Google Scholar]
- 21.Jarvis DI, et al. A Training Guide for in Situ Conservation On-Farm. Rome: International Plant Genetic Resources Institute; 2000. Ver 1. [Google Scholar]
- 22.Jarvis DI, Campilan DM. Crop Genetic Diversity to Reduce Pests and Diseases On-Farm: Participatory Diagnosis Guidelines. Rome: Bioversity International; 2006. Ver 1. [Google Scholar]
- 23.Hamrick JL, Godt MJW. Allozyme diversity in cultivated crops. Crop Sci. 1997;37:26–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.