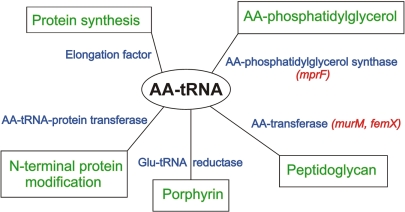
As adaptor molecules linking the codons in a mRNA to the amino acids that they specify, aminoacyl-tRNAs (AA-tRNAs) play a central role in protein biosynthesis. In addition to this critical role, AA-tRNAs are also involved in several other less well known but still important biochemical reactions (Fig. 1). For example, AA-tRNAs are used as substrates for transfer of a single amino acid to the N termini of proteins in a reaction catalyzed by the AA-tRNA-protein transferases (1, 2). The newly attached N-terminal amino acid then acts as a signal for degradation of the protein (3). In another example, the amino acid attached to the tRNA is reduced; glutamyl-tRNA reductase (4) converts the glutamyl residue of glutamyl-tRNA to glutamate 1-semialdehyde, the first precursor in the C5-pathway of porphyrin biosynthesis (5). Two other important uses of AA-tRNA that affect the properties of the cell envelope are (i) the aminoacylation of phospholipids in the cell membrane and (ii) the crosslinking of the peptidoglycan in the cell walls of Gram-positive pathogens. Two recent papers (6, 7), including the one by Roy and Ibba in a recent issue of PNAS (6), focus on these last two reactions by highlighting the role of AA-tRNA in the biosynthesis of the bacterial cell envelope that affects how the cell interacts with antibiotics and antimicrobial peptides.
Fig. 1.
Cellular processes that use AA-tRNA. The individual processes are shown in green, the enzymes/carrier proteins are shown in blue, and some relevant genes are shown in red.
The presence of a variety of aminoacyl-phosphatidylglycerol (AA-PG) compounds in bacteria was first described over four decades ago (8). The amino acids identified included lysine, alanine, arginine, and ornithine. Cell-free studies on the enzymes involved in the synthesis of these various AA-PG compounds led to the discovery that lysyl-tRNA is the donor of the amino acid (9) that is esterified to one of the 3′-hydroxyl groups of the glycerol moiety in lysyl-phosphatidylglycerol (lysyl-PG) (10). A biochemical survey showed that lysyl-PG was formed by cell extracts of Staphylococcus aureus, Bacillus megaterium, Bacillus cereus, and Clostridium welchii, an organism that also synthesizes alanyl-PG from alanyl-tRNA. Furthermore, arginyl-PG was shown to be synthesized by extracts of Enterococcus faecalis (previously called Streptococcus faecalis) (11, 12), and ornithyl-PG was found in B. cereus (13). These studies suggested the existence of different enzymes for synthesis of alanyl-PG, lysyl-PG, and possibly other AA-PGs. The enzymes displayed some specificity for tRNA recognition, because Ala-tRNACys (alanine attached to cysteine tRNA) was reported not to be a substrate for alanyl-PG formation (12). In addition, aminoethylcysteinyl-tRNALys, an analogue of lysyl-tRNALys, supported aminoethylcysteinyl-PG synthesis, whereas aminoethylcysteinyl-tRNACys did not (14). The enzymes were not further characterized.
The next advance came 30 years later during studies of bacterial immune escape mechanisms, which are directed against antimicrobial peptides of the innate immune system such as defensins and which are conserved in several pathogens. Many compounds that affect bacteria (e.g., bacteriolytic enzymes or antimicrobial peptides) are cationic and bind to the bacterial cell membrane, which is mostly anionic. Bacteria can, however, modulate the net charge of their anionic cell membrane polymers (e.g., phospholipids) by introducing positively charged groups, which would lead to reduced binding and permeability of the cationic peptides. Examination of S. aureus resistance to defensins uncovered a new gene, mprF, of unknown function conserved in many pathogens (15). A staphylococcal mprF mutant strain was much more sensitive to defensins than was the wild-type strain. The gene product was named “multiple peptide resistance factor” (MprF) and was suggested to be a new virulence factor. Also, membrane lipid analysis revealed that the mprF mutant strain did not synthesize lysyl-PG. These findings led to the notion that lysyl-PG is important for pathogenicity of S. aureus, because its presence leads to reduced binding and cellular permeability of cationic antimicrobial peptides, leading to increased resistance to defensins. Another S. aureus mprF mutation sensitized the cells to vancomycin and other antibiotics, suggesting a role for lysyl-PG in the multidrug resistance of methicillin-resistant S. aureus (16), a growing problem in staphylococcal infections, and highlighting the important role of MprF.
The work of Roy and Ibba (6) presents a thorough analysis of two different Clostridium perfringens proteins, MprF1 and MprF2, as AA-PG synthases. C. perfringens MprF2 is an 851-aa protein with a membrane-inserted hydrophobic N-terminal domain and a hydrophilic C-terminal domain. MprF homologues are present in a large number of bacteria and even in some archaea. Using a special Escherichia coli strain that allows high expression of membrane proteins, Roy and Ibba characterized the C. perfringens mprF1 and mprF2 gene products in vivo and in vitro. Each enzyme was shown to have distinct amino acid specificity; MprF1 catalyzes alanyl-PG formation, whereas MprF2 catalyzes lysyl-PG formation. A careful analysis showed that, under physiological conditions, the affinity of MprF2 protein for Lys-tRNALys was comparable to that of the elongation factor EF-Tu, the carrier of AA-tRNAs to the ribosome during protein synthesis. Studies with different tRNAs and a tRNA minihelix indicated that the primary determinant for AA-tRNA recognition by MprF1 and MprF2 was the amino acid moiety attached to the tRNA. In view of early studies suggesting some tRNA specificity in recognition of AA-tRNA by the AA-PG synthases (12, 14) and by enzymes involved in peptidoglycan synthesis (17), further work—particularly on the role of the tRNA acceptor stem nucleotides and the discriminator base—may, however, be desirable.
The paper by Lloyd et al. (7) focuses on the biosynthesis and properties of peptidoglycan. This essential cell wall component, located outside the cytoplasmic membrane, gives the bacterial cell wall strength and shape. The peptidoglycan layer is a linear carbohydrate polymer of alternating N-acetylmuramic acid and N-acetylglucosamine residues with an appended stem peptide of 4–5 aa linked to each of the N-acetylmuramic acid residues. The stem peptides are cross-linked either directly or through interpeptide bridges between the lysine of one chain and alanine of the other chain. The structure of the interpeptide bridges is to some degree genus-specific, and amino acids in the interpeptide bridges are inserted by AA-transferases using AA-tRNA as substrates (e.g., see refs. 18 and 19).
In S. aureus, including the methicillin-resistant strains, a pentaglycine bridge cross-links the peptidoglycan stem peptides (18, 20). Inhibition of pentaglycine bridge formation reduces methicillin resistance, leading to β-lactam hypersusceptibility (21). The enzyme catalyzing the first step in the synthesis of the pentaglycine bridge in S. aureus peptidoglycan was shown to be encoded by fmhB (also called femX), an essential gene (20). Similar work carried out in Streptococcus pneumoniae (7) has also shown that high-level penicillin resistance is associated with modifications in the structure of the peptidoglycan (22). Penicillin-resistant pneumococcal strains contained mostly abnormal branched stem peptides with Ala-Ala or Ser-Ala dipeptides linking the ε-amino group of the lysine residue in one stem peptide to alanine in the other stem peptide (22). In contrast, the penicillin-sensitive Pneumococcus strains had primarily linear stem peptides. Based on work done in S. aureus, the pneumo-coccal murM gene was identified from its sequence similarity with S. aureus FemX (23). A murM gene disruption in penicillin-resistant S. pneumoniae generated a penicillin-sensitive strain that contained mainly linear stem peptides. Thus, the presence of branched stem peptides in S. pneumoniae is critical for penicillin resistance. The recent work by Lloyd et al. (7) characterizes the S. pneumoniae MurM protein (406 aa) from penicillin-resistant and -sensitive clinical isolates. This enzyme catalyzes the first step in the synthesis of the branched stem peptide by attaching either alanine or serine to the ε-amino group of the stem peptide's lysine residue. The MurM enzyme from a penicillin-resistant strain was shown to have a much higher alanylation activity compared with one from the sensitive strain.
It is worth noting that peptidoglycan is covalently linked to wall teichoic acid, another class of polyanionic molecules in the cell walls of Gram-positive bacteria (24). Interestingly, d-alanine, covalently attached through ester linkages to teichoic acids (25), is also thought to modulate the net anionic charge of the teichoic acids. Furthermore, there is a good correlation between the d-alanyl ester content of teichoic acids and resistance of the bacteria to peptides of the innate immune system such as defensins and antibiotics such as vancomycin (26). Because it is d-alanine and not l-alanine that is linked to the teichoic acids, transfer of d-alanine does not involve AA-tRNA but involves a d-alanine carrier protein in which d-alanine is covalently linked to the 4′-phosphopantetheine prosthetic group of the carrier protein through a thioester bond (24).
In summary, recent studies on AA-PG synthases and the peptidoglycan related AA-transferases and the genes encoding them have highlighted the versatility of AA-tRNA in donating activated amino acids to very different acceptors in the cell. In addition, knowledge of the properties and important role of these enzymes and the genes encoding them has led to suggestions that inhibitors of these enzymes would increase the sensitivity of many bacterial pathogens to proteins of the innate immunity system and extend the action range of currently used antibiotics (27).
Footnotes
The authors declare no conflict of interest.
See companion article on page 4667 in issue 12 of volume 105.
References
- 1.Leibowitz MJ, Soffer RL. Enzymatic modification of proteins. 3. Purification and properties of a leucyl, phenylalanyl transfer ribonucleic acid protein transferase from Escherichia coli. J Biol Chem. 1970;245:2066–2073. [PubMed] [Google Scholar]
- 2.Watanabe K, et al. Protein-based peptide-bond formation by aminoacyl-tRNA protein transferase. Nature. 2007;449:867–871. doi: 10.1038/nature06167. [DOI] [PubMed] [Google Scholar]
- 3.Varshavsky A. The N-end rule: Functions, mysteries, uses. Proc Natl Acad Sci USA. 1996;93:12142–12149. doi: 10.1073/pnas.93.22.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moser J, et al. V-shaped structure of glutamyl-tRNA reductase, the first enzyme of tRNA-dependent tetrapyrrole biosynthesis. EMBO J. 2001;20:6583–6590. doi: 10.1093/emboj/20.23.6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jahn D, Verkamp E, Söll D. Glutamyl-transfer RNA: A precursor of heme and chlorophyll biosynthesis. Trends Biochem Sci. 1992;17:215–218. doi: 10.1016/0968-0004(92)90380-r. [DOI] [PubMed] [Google Scholar]
- 6.Roy H, Ibba M. RNA-dependent lipid remodeling by bacterial multiple peptide resistance factors. Proc Natl Acad Sci USA. 2008;105:4667–4672. doi: 10.1073/pnas.0800006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd AJ, et al. Characterization of tRNA-dependent peptide bond formation by MurM in the synthesis of Streptococcus pneumoniae peptidoglycan. J Biol Chem. 2008;283:6402–6417. doi: 10.1074/jbc.M708105200. [DOI] [PubMed] [Google Scholar]
- 8.Macfarlane MG. Phosphatidylglycerols and lipoamino acids. Adv Lipid Res. 1964;2:91–125. doi: 10.1016/b978-1-4831-9938-2.50009-1. [DOI] [PubMed] [Google Scholar]
- 9.Lennarz WJ, Nesbitt JA, III, Reiss J. The participation of sRNA in the enzymatic synthesis of O-l-lysyl phosphatidylgylcerol in Staphylococcus aureus. Proc Natl Acad Sci USA. 1966;55:934–941. doi: 10.1073/pnas.55.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lennarz WJ, Bonsen PP, van Deenen LL. Substrate specificity of O-l-lysylphosphatidylglycerol synthetase. Enzymatic studies on the structure of O-l-lysylphosphatidylglycerol. Biochemistry. 1967;6:2307–2312. doi: 10.1021/bi00860a005. [DOI] [PubMed] [Google Scholar]
- 11.Gould RM, Lennarz WJ. Biosynthesis of aminoacyl derivatives of phosphatidylglycerol. Biochem Biophys Res Commun. 1967;26:512–515. doi: 10.1016/0006-291x(67)90578-5. [DOI] [PubMed] [Google Scholar]
- 12.Gould RM, Thornton MP, Liepkalns V, Lennarz WJ. Participation of aminoacyl transfer ribonucleic acid in aminoacyl phosphatidylglycerol synthesis. II. Specificity of alanyl phosphatidylglycerol synthetase. J Biol Chem. 1968;243:3096–3104. [PubMed] [Google Scholar]
- 13.Houtsmuller UM, van Deenen LL. Identification of a bacterial phospholipid as an O-ornithine ester of phosphatidyl glycerol. Biochim Biophys Acta. 1963;70:211–213. doi: 10.1016/0006-3002(63)90743-1. [DOI] [PubMed] [Google Scholar]
- 14.Nesbitt JA, III, Lennarz WJ. Participation of aminoacyl transfer ribonucleic acid in aminoacyl phosphatidylglycerol synthesis. I. Specificity of lysyl phosphatidylglycerol synthetase. J Biol Chem. 1968;243:3088–3095. [PubMed] [Google Scholar]
- 15.Peschel A, et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med. 2001;193:1067–1076. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruzin A, et al. Inactivation of mprF affects vancomycin susceptibility in Staphylococcus aureus. Biochim Biophys Acta. 2003;1621:117–1121. doi: 10.1016/s0304-4165(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 17.Villet R, et al. Idiosyncratic features in tRNAs participating in bacterial cell wall synthesis. Nucleic Acids Res. 2007;35:6870–6883. doi: 10.1093/nar/gkm778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuhashi M, Dietrich CP, Strominger JL. Biosynthesis of the peptidoglycan of bacterial cell walls. III. The role of soluble ribonucleic acid and of lipid intermediates in glycine incorporation in Staphylococcus aureus. J Biol Chem. 1967;242:3191–3206. [PubMed] [Google Scholar]
- 19.Bumsted RM, Dahl JL, Söll D, Strominger JL. Biosynthesis of the peptidoglycan of bacterial cell walls. X. Further study of the glycyl transfer ribonucleic acids active in peptidoglycan synthesis in Staphylococcus aureus. J Biol Chem. 1968;243:779–782. [PubMed] [Google Scholar]
- 20.Rohrer S, Ehlert K, Tschierske M, Labischinski H, Berger-Bächi B. The essential Staphylococcus aureus gene fmhB is involved in the first step of peptidoglycan pentaglycine interpeptide formation. Proc Natl Acad Sci USA. 1999;96:9351–9356. doi: 10.1073/pnas.96.16.9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strandén AM, Ehlert K, Labischinski H, Berger-Bächi B. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J Bacteriol. 1997;179:9–16. doi: 10.1128/jb.179.1.9-16.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Bustos J, Tomasz A. A biological price of antibiotic resistance: Major changes in the peptidoglycan structure of penicillin-resistant pneumococci. Proc Natl Acad Sci USA. 1990;87:5415–5419. doi: 10.1073/pnas.87.14.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filipe SR, Tomasz A. Inhibition of the expression of penicillin resistance in Streptococcus pneumoniae by inactivation of cell wall muropeptide branching genes. Proc Natl Acad Sci USA. 2000;97:4891–4896. doi: 10.1073/pnas.080067697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuhaus FC, Baddiley J. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in Gram-positive bacteria. Microbiol Mol Biol Rev. 2003;67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baddiley J, Buchanan JG, Martin RO, RajBhandary UL. Teichoic acid from the walls of Staphylococcus aureus H. 2. Location of phosphate and alanine residues. Biochem J. 1962;85:49–56. doi: 10.1042/bj0850049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peschel A, Vuong C, Otto M, Götz F. The d-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob Agents Chemother. 2000;44:2845–2847. doi: 10.1128/aac.44.10.2845-2847.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staubitz P, Neumann H, Schneider T, Wiedemann I, Peschel A. MprF-mediated biosynthesis of lysylphosphatidylglycerol, an important determinant in staphylococcal defensin resistance. FEMS Microbiol Lett. 2004;231:67–71. doi: 10.1016/S0378-1097(03)00921-2. [DOI] [PubMed] [Google Scholar]