Abstract
Schizosaccharomyces pombe Rho GTPases regulate actin cytoskeleton organization and cell integrity. We studied the fission yeast gene SPBC4F6.12 based on its ability to suppress the thermosensitivity of cdc42-1625 mutant strain. This gene, named pxl1+, encodes a protein with three LIM domains that is similar to paxillin. Pxl1 does not interact with Cdc42 but it interacts with Rho1, and it negatively regulates this GTPase. Fission yeast Pxl1 forms a contractile ring in the cell division region and deletion of pxl1+ causes a delay in cell–cell separation, suggesting that it has a function in cytokinesis. Pxl1 N-terminal region is required and sufficient for its localization to the medial ring, whereas the LIM domains are necessary for its function. Pxl1 localization requires actin polymerization and the actomyosin ring, but it is independent of the septation initiation network (SIN) function. Moreover, Pxl1 colocalizes and interacts with Myo2, and Cdc15, suggesting that it is part of the actomyosin ring. Here, we show that in cells lacking Pxl1, the myosin ring is not correctly assembled and that actomyosin ring contraction is delayed. Together, these data suggest that Pxl1 modulates Rho1 GTPase signaling and plays a role in the formation and contraction of the actomyosin ring during cytokinesis.
INTRODUCTION
The Rho family of GTPases regulates a variety of morphologic events leading to actin cytoskeleton remodeling in all eukaryotic cells (Ridley, 2006). Additionally, Rho GTPases regulate cell wall biosynthesis and cell integrity in fungal cells (Levin, 2005; García et al., 2006; Park and Bi, 2007). Schizosaccharomyces pombe is a highly polarized yeast growing by elongation of its ends and dividing by medial fission. Its genome contains six genes coding for Rho GTPases cdc42+ and rho1+-5+ (García et al., 2006). cdc42+ is an essential gene involved in the establishment of cell polarity (Miller and Johnson, 1994). Cdc42 is positively regulated by two GDP-GTP exchange factors (GEFs): Scd1, which is required to maintain apical growth (Chang et al., 1994); and Gef1, which plays a role in cytokinesis and in the switch to bipolar growth (Coll et al., 2003; 2007; Hirota et al., 2003). No negative regulator for Cdc42 has been described yet. rho1+ is also an essential gene that is required for maintenance of cell integrity and polarization of the actin cytoskeleton (Arellano et al., 1997). Rho1 functions are mediated by at least three targets, the (1,3)-β-d-glucan synthase (Arellano et al., 1996) and the kinases Pck1 and Pck2 (Arellano et al., 1999b). Rho1 GTPase is positively regulated by at least three GEFs: Rgf1 and Rgf2, involved in the formation of the cell wall; and Rgf3, involved in cytokinesis in fission yeast (Tajadura et al., 2004; Morrell-Falvey et al., 2005; Mutoh et al., 2005). Rho1 is negatively regulated by at least three GAPs (GTPase activating proteins): Rga1, Rga5, and Rga8 (Nakano et al., 2001; Calonge et al., 2003; Yang et al., 2003).
S. pombe cytokinesis involves the assembly of a contractile actomyosin medial ring attached to the membrane (Feierbach and Chang, 2001). The initial phase of cytokinesis is the establishment of the division site, which is defined by the position of the interphase nucleus, usually in the center of the cell, and requires the participation of the kinase Pom1, the Polo kinase Plo1, and Mid1, a protein with a PH domain (Burgess and Chang, 2005; Wolfe and Gould, 2005). Mid1, which is in the nucleus during interphase, moves to the cytoplasm at the beginning of mitosis, before the segregation of chromosomes, due to the action of Plo1 and establishes a broad band of small dots in the cell cortex that surrounds the nucleus. There Mid1 recruits the proteins that will form the medial actomyosin ring (Burgess and Chang, 2005; Wolfe and Gould, 2005). Conventional myosin II recruitment is one of the earliest events after spindle pole body separation. This myosin II is formed by hexamers with a pair of Myo2 heavy chains, a pair of Cdc4 essential light chains (Cdc4), and a pair of Rlc1 regulatory light chains (Motegi et al., 2000; Naqvi et al., 2000). S. pombe has another unconventional myosin II heavy chain, Myo3/Myp2, which is not required for the formation of the ring (Bezanilla and Pollard, 2000). The C-terminal region of Myo2 is sufficient for the myosin II accumulation to the broad band that is dependent on Mid1 and independent of F-actin (Motegi et al., 2004). Over a period of 10 min after myosin II accumulates, the IQGAP protein Rng2, the PCH protein Cdc15, and the formin Cdc12 accumulate in the dots of the broad band (Wu et al., 2003). This is followed quickly by condensation of the dots into a contractile ring in an F-actin–dependent manner that also requires the motor activity of Myo2 (Naqvi et al., 1999; Le Goff et al., 2000). It remains poorly understood how the myosin filaments assemble into a ring in association with actin and other proteins during late mitosis. Once the ring is formed, a set of proteins named septation initiation network (SIN), triggers the contraction of the actomyosin ring coordinated with the synthesis of the primary and the secondary septa that will form the new cell wall (Krapp et al., 2004; Wolfe and Gould, 2005). Mutants in components of the SIN form the ring correctly, but they do not contract the ring and deposit the septum material. The SIN controls the formation of the septum, probably by means of the regulation of the glucan synthase Bgs1/Cps1, but the mechanism is not known (Le Goff et al., 1999). SIN proteins such as Cdc11 and Cdc14 are necessary for Bgs1 location (Cortes et al., 2002). Nevertheless, any direct interaction of Bgs1 with the SIN complex or with the contractile ring has not been described. So far, Rho1 GTPase is the only known direct activator of the glucan synthase (Arellano et al., 1996).
In this article, we report the characterization of a S. pombe protein that is similar to animal cell paxillin and to Saccharomyces cerevisiae Pxl1. Paxillin is a LIM domain-containing adaptor protein localized to focal adhesions of adherent cells, in which it modulates RhoA activity, and it has been implicated in the regulation of cytoskeletal organization and cell motility (Turner, 2000; Brown and Turner, 2004; Carragher and Frame, 2004). S. cerevisiae Pxl1 is a LIM domain-containing protein that modulates Rho1 activity, and it is required for selection and/or maintenance of polarized growth sites (Gao et al., 2004; Mackin et al., 2004). LIM motifs are cysteine- and histidine-rich, zinc-coordinating domains composed of two zinc fingers. They are protein–protein interaction motifs critically involved in processes such as gene expression, cytoskeleton organization, cell adhesion, cell motility, and signal transduction (Kadrmas and Beckerle, 2004). We show here that S. pombe paxillin homologue, Pxl1, modulates Rho1 activity in the same way that S. cerevisiae Pxl1 does, but it is not required for polarized growth at the cell poles. Instead, it participates in the actomyosin ring formation and constriction during cytokinesis.
MATERIALS AND METHODS
Fission Yeast Strains, Media, and Techniques
Standard S. pombe media and genetic manipulations were used (Moreno et al., 1991). All the strains used were isogenic to wild-type strains 972 h− and 975 h+, and they are described in Supplemental Table 1. The strains were constructed by either tetrad dissection or random spore germination method. Cells were usually grown in rich medium (YES) or minimal medium (EMM) supplemented with the necessary requirements. Escherichia coli DH5α was used as host for propagation of plasmids. Cells were grown in LB medium supplemented with 50 μg/ml ampicillin when appropriate. Solid media contained 2% agar.
Plasmids and Strains Construction
The nmt1+ promoter-containing vectors pREP41X, pREP81X, pREP41-GFP, and pREP1-GST (Forsburg and Sherman, 1997) were used for the overexpression of pxl1+ that was induced by growing the cells transformed with these plasmids in the absence of thiamine for 12 h. To delete pxl1+ from the S. pombe genome, the whole open reading frame was replaced with the KanMX6 gene or the ura4+ gene by PCR-based gene targeting as described previously (Bähler et al., 1998). Stable transformants were selected and sporulated. Dissected tetrads were screened by PCR or Southern blot for the appropriate gene replacement. Genomic versions of pxl1+ with green fluorescent protein (GFP), glutathione transferase (GST), cherry red fluorescent protein (RFP), or the hemagglutinin (HA) epitope coding sequences fused at the 5′ end of the open reading frame (ORF) were generated by cloning into a BlueScript plasmid 745 base pairs of the 5′ pxl1+ flanking sequence, the corresponding tagging sequence, the pxl1+ ORF, and 460 base pairs of the 3′ pxl1+ flanking sequence. The resulting constructs were cloned into the integrative vector pJK148 that was then cut with NruI and integrated at the leu1+ locus of the leu1-32 ura4-D18 pxl1Δ strain. Transformant clones were selected in EMM without leucine and screened by PCR for the appropriate gene integration. Genomic versions of truncated GFP-pxl1 were integrated at the leu1+ locus of the leu1-32 ura4-D18 pxl1Δ strain following the same strategy.
Pull-Down and Immunoprecipitation
Extracts from 5 × 108 cells expressing the different tagged proteins were obtained as described previously (Arellano et al., 1997), using 200 μl of lysis buffer (20 mM Tris-HCl, pH 8.0, 2 mM EDTA, 100 mM NaCl, and 0.5% NP-40, containing 100 μM p-aminophenyl methanesulfonyl fluoride, 2 μg/ml leupeptin, and 2 μg/ml aprotinin). Cell extracts (1 mg of total protein) were incubated with glutathione-Sepharose (GS) beads or with the corresponding antibody and protein A-Sepharose beads for 2–4 h at 4°C. The beads were washed four times with lysis buffer, and then they were resuspended in sample buffer. Proteins were separated by SDS-polyacrylamide gel electrophoresis, transferred to Immobilon-P membranes (Millipore, Billerica, MA), and blotted to detect GST-, HA-, or GFP-fused epitopes with the corresponding antibodies and the enhanced chemiluminescence (ECL) detection kit (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Total amount of protein was monitored in cell extracts aliquots and 30 μg of total protein was used directly for Western blot.
In Vivo Analysis of Rho1 Activity
The expression vector pGEX-RBD (Rho binding domain of rhotekin) (Reid et al., 1996) was used to transform E. coli. The fusion protein was produced according to the manufacturer's instructions and immobilized on glutathione-Sepharose 4B beads (GE Healthcare).
The amount of GTP-bound Rho1 was determined as described previously (Calonge et al., 2003) using a pull-down assay modified from (Ren et al., 1999). Briefly, extracts from wild-type, rga5Δ, and pxl1Δ cells containing HA-rho1+ expressed from its promoter were obtained as described previously (Arellano et al., 1997), by using 500 μl of lysis buffer (50 mM Tris, pH 7.5, 20 mM NaCl, 0.5% NP-40, 10% glycerol; 0,1 mM dithiothreitol, 1 mM NaF, 2 mM MgCl2, containing 100 μM p-aminophenyl methanesulfonyl fluoride, leupeptin, and aprotinin). Ten μg of GST-RBD fusion protein coupled to glutathione-Sepharose beads were used to immunoprecipitate the GTP-bound HA-Rho1 from 2 mg of the cell lysates. The extracts were incubated with GST-RBD beads for 2 h at 4°C, washed four times, and blotted against anti-HA (12CA5) mAb as primary antibody to detect HA-Rho1 and the ECL detection kit. Total HA-Rho1 levels were monitored in whole cell extracts (30 μg of total protein) that were used directly for Western blot and developed with anti-HA mAb.
Microscopy Techniques
For calcofluor staining, exponentially growing S. pombe cells were harvested, washed, and resuspended in a calcofluor solution (0.1 mg/ml) for 5 min at room temperature. After washing with water, cells were observed. Actin staining was performed with phalloidin-Alexa Fluor 488. To disintegrate F-actin, latrunculin A (Lat A) dissolved in dimethyl sulfoxide (DMSO) at 50 mM was added to S. pombe cultures to a final concentration of 50 μM.
Cell samples were observed using a DMXRA microscope (Leica, Wetzlar, Germany) equipped for Nomarski optics and epifluorescence, and photographed with a Sensys camera (Photometrics, Tucson, AZ). Confocal microscopy was performed on a Leica TCS SL microscope, and the images were analyzed with the Leica confocal software.
RESULTS
Pxl1 Suppresses the Thermosensitive Growth of cdc42-1625
We have constructed some thermosensitive S. pombe strains carrying different cdc42 mutant alleles with the purpose of identifying allele-specific multicopy suppressors that might participate in the Cdc42 signaling pathway. A thermosensitive mutant, cdc42-1625, was previously found to be defective in actin cable formation and secretion (Martin et al., 2007; our unpublished data). cdc42-1625-thermosensitive growth was suppressed by mild overexpression of cdc42+, scd1+, and gef1+ but not of shk1+ or shk2+, the two known Cdc42 effectors (Martin et al., 2007; Figure 1A). It was described previously that S. cerevisiae PXL1, which codes for a paxillin homologue, was able to specifically suppress some loss-of-function cdc42 alleles defective in the localization of the exocyst component Sec3 (Gao et al., 2004). We therefore cloned SPBC4F6.12, an ORF coding for a protein with similarity to S. cerevisiae Pxl1, and we tested whether overexpression of this ORF, named pxl1+, was able to suppress cdc42-1625 thermosensitive growth. As shown in Figure 1A, pxl1+ was able to rescue cdc42-1625 growth at 36°C, at the same level as scd1+, and more efficiently than gef1+, because pxl1+ rescued the growth even when the promoter was repressed. However, overexpression of pxl1+ did not suppress the morphologic defects of cdc42-1625 (Figure 1B). pxl1+ codes for a 438 amino acids protein with three LIM domains at the C-terminal region (see Supplemental Figure 1). S. pombe Pxl1 shares 21% similarity with chicken paxillin, which contains four LIM domains, and 18% similarity with S. cerevisiae Pxl1, which contains two LIM domains and is 18% similar to chicken paxillin.
Figure 1.
S. pombe pxl1+ overexpression suppresses the thermosensitive growth of cdc42-1625. (A) cdc42-1625 cells transformed with pREP81X plasmid containing different genes: cdc42+; gef1+; scd1+; shk1+; shk2+; and pxl1+. Cells were grown at 25°C and at 36°C in media with or without thiamine to repress or induce, respectively, the nmt1+ promoter. (B) Differential interphase contrast images of cdc42-1625 cells transformed with empty pREP81X or pREP81X-pxl1+ and grown 16 h without thiamine at 25 or 36°C.
S. pombe Pxl1 Interacts with Rho1 and Has a Function in Cytokinesis
LIM domains can interact specifically with other LIM domains and with many other protein domains mediating protein–protein interactions. Interestingly, the three other S. pombe proteins containing LIM domains, Rga1, Rga3, and Rga4, are Rho-GAPs (Nakano et al., 2001). We could not detect a direct interaction between Pxl1 and Cdc42 by coimmunoprecipitation, and it has been described that S. cerevisiae Pxl1 directly binds to Rho1 in vitro (Gao et al., 2004). To examine whether there was interaction between Pxl1 and Rho1, we performed coprecipitation experiments using extracts from cells carrying an HA epitope-tagged rho1+ (Arellano et al., 1997) and GFP-pxl1+ expressed from their own promoters. Cell extracts were immunoprecipitated with anti-GFP and protein A-Sepharose. As shown in Figure 2A, a band corresponding to HA-Rho1 was specifically detected. These results indicate that Pxl1 binds to Rho1, as does S. cerevisiae Pxl1 (Gao et al., 2004).
Figure 2.
Pxl1 is a negative regulator of Rho1. (A) Pxl1 interacts with Rho1. Cells expressing GFP-pxl1+ and HA-rho1+ from endogenous promoters were lysated, and immunoprecipitation was performed with anti-GFP antibody and protein A-Sepharose beads. Immunoprecipitates were analyzed by Western blot by using anti-HA (bottom) antibody. The lysates were analyzed by Western blot with anti-GFP (top) or anti-HA (middle) antibodies. (B) Differential interphase contrast and fluorescence micrographs of calcofluor-stained wild-type cells (top) and pxl1Δ cells (bottom) grown at 25°C. The arrow points to a misplaced septum. The bar corresponds to 5 μm. (C) Pxl1 modulates the amount of GTP-bound Rho1. Wild-type, rga5Δ, and pxl1Δ cells expressing HA-rho1+ from its promoter were precipitated with GST-RBD and blotted with anti-HA antibodies (top). Total HA-Rho1 in cell lysates was visualized by Western blot (middle). Data were quantified and presented as percentage relative to the wild-type extracts run in the same experiment (bottom). (D) Percentages of septating and multiseptated cells in cultures of wild-type and pxl1Δ cells transformed with pREP41 plasmids carrying the indicated genes. Cells were grown 16 h without thiamine at 28°C to allow overexpression. Percentages of septating wild-type and pxl1Δ cells carrying simultaneously the rga5Δ mutation are also included. (E) The lack of Pxl1 rescues the growth at 37°C of cells carrying the rgf3+ thermosensitive allele ehs2-1. Cells were grown in rich medium for 10 h. Initial OD600 was 0.1. (F) Same cells as in E were grown in rich medium at 32°C supplemented with 50 mM NaF for 2 d. Initial OD600 was 2 and 1:4 dilutions were successively made.
To further investigate the function of pxl1+, the ORF of this gene was replaced with KanMX6 or ura4+ genes in a diploid strain. Sporulation of the heterozygotic diploid strain containing one disrupted allele of pxl1+ yielded tetrads with four viable spores, indicating that pxl1+ is not essential for vegetative growth. The cells did not show any growth defect. However, there was a high percentage of septating cells in the pxl1Δ culture (49%; n = 200) compared with wild-type (16%; n = 200), and some multiseptated cells were observed (8%; n = 200) (Figure 2D). We could also see some misplaced septa (Figure 2B, see arrow), and some cells slightly swollen in the middle region. The cell separation defect was more noticeable at 25°C than at 36°C (data not shown).
We next analyzed the level of GTP-bound Rho1 in wild-type and pxl1Δ cells. Cells lacking Pxl1 always showed higher level of GTP-bound Rho1 than wild-type cells as measured by pull-down from the extracts using GST-RBD that contains the rhotekin binding domain (Figure 2C). The increase in GTP-bound Rho1 observed in cells lacking Pxl1 was slightly lower than that observed in cells lacking Rga5, a Rho1-specific GAP whose deletion also causes increase in septating cells (Calonge et al., 2003). By contrast, no change in the GTP-bound level of Cdc42 was observed using GST-CRIB (data not shown). These results indicate that Pxl1 is a negative regulator of Rho1 in vivo. Moreover, taking into consideration that Pxl1 overexpression suppressed cdc42-1625 thermosensitivity and inhibited Rho1, it is tempting to propose that Cdc42 and Rho1 signaling pathways might be antagonistic to each other, as has been suggested previously for both S. cerevisiae (Gao et al., 2004) and S. pombe (Yang et al., 2003).
Rho1 signaling is required to maintain S. pombe cell integrity, regulating the cell wall biosynthesis, mainly β-(1-3)-glucan biosynthesis, and the actin organization (Arellano et al., 1999a). To further study the Pxl1 effect on Rho1 signaling pathway, we analyzed pxl1Δ rga5Δ cells. This double mutant strain had a higher percentage of septating cells (57%; n = 200) than the single mutants (49 and 25% in pxl1Δ and rga5Δ cultures, respectively) (Figure 2D), indicating additive defects. We also mildly overexpressed Rho1 or the two Rho1-GEFs, rgf3+ and rgf1+ that are involved in cytokinesis and general cell wall synthesis, respectively (García et al., 2006). rho1+ or rgf3+ overexpression in wild-type cells caused a slight increase in septating cells (from 16 to 18 and 19%, respectively; n = 200), and it also increased even more the percentage of septating cells in pxl1Δ cultures (from 49 to 62 and 63%, respectively; n = 200). Remarkably, rgf3+ overexpression also caused a dramatic increased in pxl1Δ multiseptated cells (from 8% to 28%; n = 200), suggesting that it has an additive effect on the pxl1Δ phenotype. By contrast, overexpression of rgf1+ caused no effect in either wild-type or pxl1Δ cultures (Figure 2D).
We also analyzed the effect of pxl1 deletion on the phenotypes of cells carrying mutant alleles of rgf3+ and rgf1+. ehs2-1 cells, carrying an rgf3+ thermosensitive allele, do not grow at 37°C and are hypersensitive to NaF (Sánchez, unpublished data), similar to lad1-1 cells that contain another rgf3+ mutant allele (Morrell-Falvey et al., 2005). Elimination of Pxl1 was able to suppress both ehs2-1 phenotypes (Figures 2, E and F), likely due to the increase in Rho1 activity. By contrast, the lack of Pxl1 did not suppress the hypersensitivivity of rgf1Δ cells to Caspofungin, a specific β-(1-3)-glucan synthase inhibitor (data not shown). Because Rgf3 activates Rho1 during cytokinesis and Rgf1 during apical growth (García et al., 2006), together our results suggest that Pxl1 is negatively modulating Rho1 activity specifically during cytokinesis.
Pxl1 Forms a Contractile Ring at the Medial Region and Its Concentration Is Higher during Cytokinesis
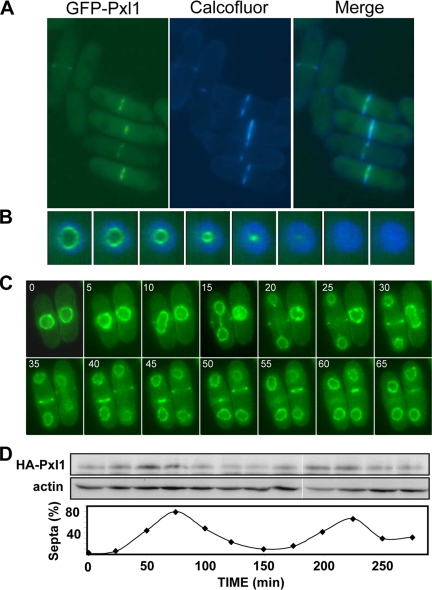
To gain further insight into the Pxl1 function, we examined the localization of Pxl1 by tagging the genomic locus with the GFP gene fused in frame to the 5′ end of pxl1+. The fusion to the 3′ end was nonfunctional. GFP-Pxl1 localized as a ring that contracts during septation and disappeared when the septum was formed (Figure 3, A and B). The Pxl1 ring was always in the inner edge of the primary septum that was specifically stained with calcofluor.
Figure 3.
Pxl1 localization and levels during cell cycle. (A) Fluorescence microscopy of log phase GFP-pxl1+ cells stained with calcofluor. (B) Three-dimensional projection of a time-lapse of GFP-pxl1+ cells stained with calcofluor. Pictures were taken at 5-min intervals. (C) Time-lapse fluorescence microscopy of cells expressing GFP-pxl1+ and cut11+-GFP grown to early log phase at 25°C. Pictures were taken at 5-min intervals. (D) cdc25-22 cells carrying endogenously expressed HA-pxl1+ were synchronized at 36°C for 4 h, and then they were transferred to 25°C, and samples for protein analysis were taken every 25 min. Proteins from cell extracts were analyzed by Western blot with monoclonal anti-HA and anti-actin antibodies. The percentage of septating cells in each sample is presented as an indicator of cell cycle progression.
To further characterize the Pxl1 ring assembly and contraction, we performed time-lapse videomicroscopy by using a strain carrying both GFP-Pxl1 and Cut11-GFP; the latter localizes to the nuclear envelope and spindle pole body (SPB) in living cells (West et al., 1998). GFP-Pxl1 seemed very faint in the medial region of the cell cortex after SPB duplication, and it formed a brighter ring in late anaphase, before septation. When the nuclei separated, Pxl1 ring contracted as the septum was formed and disappeared at the end of septation (Figure 3C).
pxl1+ is part of a gene cluster whose expression is under control of the transcription factors Sep1 and Ace2, which activate the cytokinesis program (Rustici et al., 2004; Alonso-Nunez et al., 2005). The expression of these genes, including pxl1+, peaks during late mitosis (Rustici et al., 2004). Additionally, Pxl1 can be visualized only in cells during cytokinesis; therefore, we considered the possibility that Pxl1 protein levels might be regulated in a cell cycle-dependent manner. HA-tagged Pxl1 was analyzed by Western blot in a synchronous population of cdc25-22 thermosensitive mutant cells, which arrest in late G2 at restrictive temperature (36°C). Cells were synchronized at 36°C during 4 h and transferred to permissive temperature (25°C). Septum formation was monitored to assess the synchrony. The maximum percentage of septa was reached at 75 min. HA-Pxl1 levels rose to a peak before maximum septation (50 min), and decreased when most of the cells had a septum. However, the protein did not disappear during interphase, and it was present at low levels throughout the cell cycle (Figure 3D).
Pxl1 N-Terminal Region Is Sufficient for the Localization to the Division Area and the Three LIM Domains Are Required for Pxl1 Function
To determine which portion of Pxl1 is responsible for targeting Pxl1 to the site of cell division, we generated different N-terminal GFP fusion constructs containing fragments of pxl1+ that were expressed in pxl1Δ cells under the endogenous pxl1+ promoter (Figure 4A, bottom). Interestingly, the concentration of Pxl1ΔN in the cell was much higher than that of the other Pxl1 truncations, whose protein level was very low, despite the fact that they were all expressed from the pxl1+ promoter. These results suggest that the N-terminal region regulates Pxl1 level or that the level of Pxl1 is regulated when this protein is correctly localized.
Figure 4.
Pxl1 localization depends on the N-terminal region but Pxl1 function requires the LIM domains. (A) Scheme and protein levels of GFP-Pxl1 truncations expressed under the control of pxl1+ promoter in pxl1Δ cells. The proteins were detected by Western-Blot using anti-GFP antibody and 7.5% SDS-PAGE. Molecular weight standards (right), and molecular weight of the different GFP-Pxl1 truncations (right) are indicated (B) Percentages of cells containing a single septum and multiple septa calculated from 500 pxl1Δ cells expressing different GFP-Pxl1 fragments. (C) Calcofluor and GFP fluorescence micrographs of pxl1Δ cells carrying different pxl1 fragments.
Only the full-length GFP-Pxl1 completely suppressed the cytokinesis defect of pxl1Δ cells (Figure 4B). None of the truncated derivatives were fully functional, but there was a partial complementation by the construct lacking only the C-terminal LIM domain (Pxl1Δ3). The Pxl1 truncation containing the three LIM domains but lacking the N-terminal part of the protein (Pxl1ΔN) did not localize, and it seemed dispersed throughout the cytoplasm. On the contrary, the N-terminal portion of Pxl1 lacking the three LIM domains (Pxl1Δ1,2,3) localized to the division area, although it did not rescue the phenotype of pxl1Δ cells (Figure 4C). We conclude that the N-terminal region of Pxl1 is necessary and sufficient for its localization into the contractile ring, and the LIM domains are necessary for its function. In contrast, the LIM domains of S. cerevisiae Pxl1 are the primary determinants for targeting the protein to the cortical sites of the budding yeast cells (Gao et al., 2004; Mackin et al., 2004). Indeed, we cloned S. cerevisiae Pxl1 into the S. pombe pREP41-GFP expression plasmid and used it to transform pxl1Δ cells, but S. cerevisiae GFP-Pxl1 was not localized to the division area and it was unable to suppress the cytokinesis defect of pxl1Δ cells (data not shown).
Pxl1 Localization to the Division Area Is Dependent on the Actomyosin Ring but Not on the SIN Pathway
We examined whether Pxl1 localization to the medial ring was dependent on actin polymerization by promoting the disassembly of actin by using 50 μM Lat A in G2 synchronized cdc25-22 cells expressing GFP-pxl1+. Cells were arrested at 36°C for 4 h, and then they were returned to permissive temperature in the presence of Lat A or DMSO. After 20 min Pxl1 could already be observed forming a medial ring in DMSO-treated cells. By contrast, in Lat A-treated cells GFP-Pxl1 was visualized as punctuated membrane structures accumulating at the tips (Figure 5A). To see whether actin polymerization was also required to maintain Pxl1 in a medial ring, Lat A was added 40 min after switching the cdc25-22 cells to permissive temperature. At that time GFP-Pxl1 was already localized to a ring, and cells were initiating septation, as assessed by calcofluor staining. GFP-Pxl1 disappeared from the division site in Lat A-treated cells in which the ring had already been formed, and it was observed again as punctuated structures at the poles (Figure 5B). Therefore, Pxl1 requires F-actin to localize and to maintain its localization in the medial ring.
Figure 5.
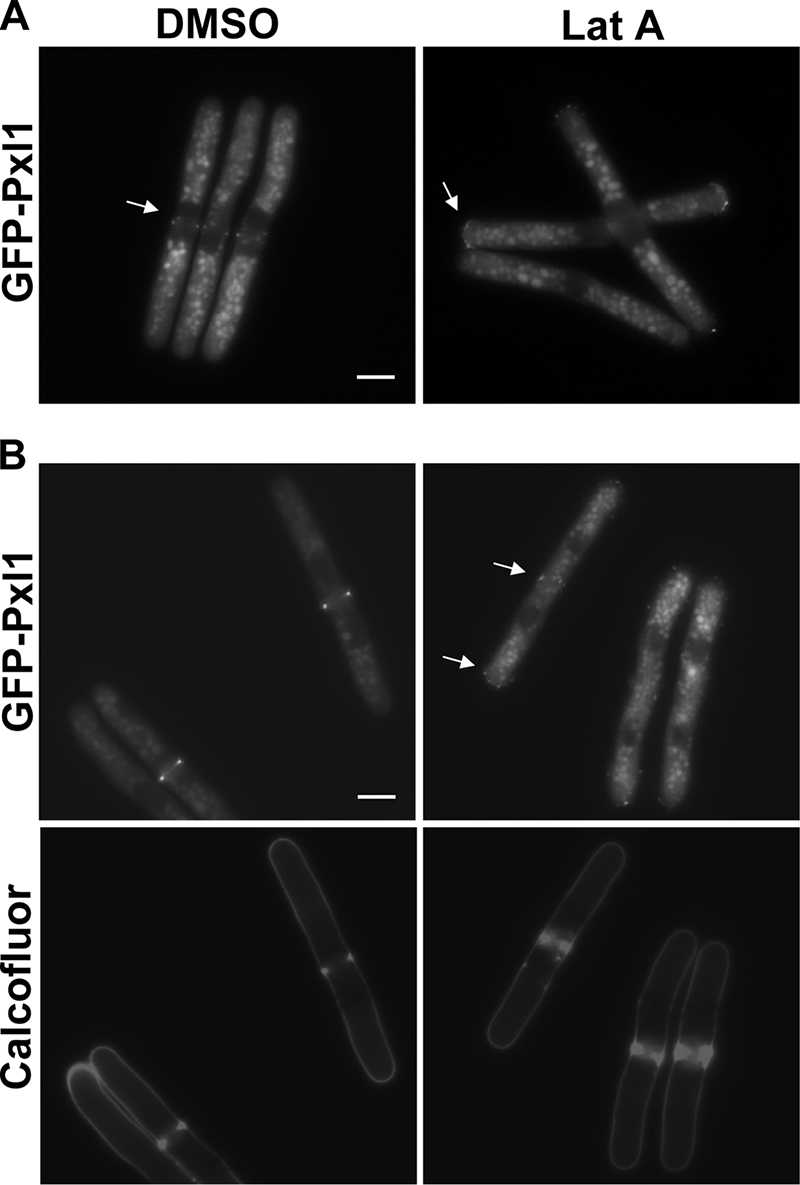
Pxl1 localization depends on actin polymerization. cdc25-22 GFP-pxl1+ cells grown at 25°C to mid-log phase, and then they were shifted to 36°C for 4 h and shifted back to 25°C. At time 0 (A) and 40 min (B), 1% DMSO or 50 μM Lat A was added. Cells were stained with calcofluor, and then they were analyzed by fluorescence microscopy 20 min after the addition of Lat A. The arrows point the GFP-Pxl1. Bar, 5 μm.
To further investigate how Pxl1 localization is regulated, we analyzed GFP-Pxl1 in different septation mutants (Supplemental Figure 2). Cdc15 is an FCH domain-containing protein necessary for the actomyosin ring formation. cdc15-140 cells are impaired in the assembly of the actomyosin ring when grown at 36°C (Carnahan and Gould, 2003). GFP-Pxl1 was not recruited to the medial region in cdc15-140 cells grown at 36°C, and it was diffused in the cytoplasm (Supplemental Figure 2). cdc12-112 is a thermosensitive allele of the gene coding for the essential Cdc12 formin. Cells carrying cdc12-112 do not form actomyosin rings at the restrictive temperature (Chang et al., 1997). GFP-Pxl1 did not form a ring in cdc12-112 cells grown at 36°C, but in some cells GFP spots were observed in the membrane at sites in which division should have occurred (Supplemental Figure 2, see arrows). Interestingly, the same localization was observed for Cdc15-GFP in cdc12-112 mutant cells. cdc4-8 is a thermosensitive allele of the gene coding for the essential myosin light chain, and it does not form actomyosin rings at the restrictive temperature (McCollum et al., 1995). GFP-Pxl1 showed up as a bright cytoplasmic spot in the medial region of cdc4-8 cells grown at the restrictive temperature (Supplemental Figure 2). We conclude from these results that the actomyosin ring is necessary for the localization of Pxl1 to a ring at the division site.
To test whether Pxl1 localization required the SIN proteins, essential for the formation of the division septum after assembly of the actomyosin ring, we analyzed GFP-Pxl1 in cdc11-119 mutant cells. Cdc11 is a protein required for the localization of the known SIN components, except Sid4, to the spindle pole body. At the restrictive temperature, cdc11-119 mutant cells assemble the actomyosin ring but do not form a septum (Krapp et al., 2004). GFP-Pxl1 localized properly in cdc11-119 cells grown at the restrictive temperature (Supplemental Figure 2). These observations indicate that Pxl1 localization is not SIN pathway dependent.
Cdc16 is a negative regulator of the GTPase Spg1 that activates the SIN. At the restrictive temperature, cdc16-116 mutants form multiple well oriented septa in one cell (Furge et al., 1998). GFP-Pxl1 only localized to one of the multiple septa formed by cdc16-116 cells grown at the restrictive temperature (Supplemental Figure 2). Therefore, Pxl1 does not require the SIN inactivation to disappear from the division area.
Pxl1 Colocalizes with the Actomyosin Ring Proteins
To see whether Pxl1 is part of the actomyosin ring, we constructed a strain carrying Pxl1 tagged with cherry RFP at the N terminus, and we analyzed the simultaneous localization of cherry RFP-Pxl1 and other proteins belonging to the contractile ring tagged with GFP or YFP. We observed colocalization of GFP-Myo2 and cherry RFP-Pxl1 during ring constriction (Figure 6A). However, at the early steps of cytokinesis, when Myo2 was present as a broad band of dots, Pxl1 was not there, suggesting that Pxl1 arrives at the cell division area later. Cdc12 and Cdc15 also concentrated in small nodes around the equator over a period of 10 min after myosin II occurred, followed quickly by lateral condensation of the nodes into a contractile ring. We observed colocalization of cherryRFP-Pxl1 with either Cdc12-3YFP or GFP-Cdc15 during cytokinesis (Figure 6, B and C). However, we could observe some cells in early cytokinesis with Cdc12-3YFP that did not have cherryRFP-Pxl1 (Figure 6B, inset). We also observed that cherryRFP-Pxl1 formed a lateral dot, whereas GFP-Cdc15 was forming small nodes around the equator at early stages of cytokinesis (Figure 6C, inset). These results suggest that Pxl1 forms part of the actomyosin ring and is recruited to the medial region after myosin II and Cdc12, perhaps together with Cdc15.
Figure 6.
Pxl1 colocalizes with actomyosin ring proteins and physically interacts with Rlc1 and Cdc15. Cells containing cherry RFP-Pxl1 and GFP-Myo2 Sad1-GFP (A), Cdc12-3YFP (B), or GFP-Cdc15 (C) were grown to log phase, and then they were examined by fluorescence microscopy. A separated cell (inset) is shown to see the localization at early stages of cytokinesis. (D) Interaction of Rlc1-GFP and GST-Pxl1. Extracts of cells expressing rlc1+-GFP and GST-pxl1+ at physiological levels were immunoprecipitated with GS-beads and probed with anti-GFP antibodies. Extracts were assayed for the level of GST-Pxl1 and Rlc1-GFP by Western blot. (E) Interaction of GFP-cdc15 and HA-Pxl1. Extracts of cells carrying GFP-cdc15+ and HA-pxl1+ expressed at endogenous levels were immunoprecipitated with anti-HA antibodies and probed with anti-GFP antibodies. Extracts were assayed for levels of HA-pxl1 and GFP-Cdc15 by Western blot.
To further characterize the interaction of Pxl1 with the ring proteins, we performed immunoprecipitation of extracts from cells expressing GST-pxl1+ and rlc1+-GFP at endogenous levels. We observed coprecipitation of Rlc1-GFP with GST-Pxl1 by using GS beads (Figure 6D). Additionally, we performed immunoprecipitation of extracts from cells carrying HA-pxl1+ and GFP-cdc15+ expressed at endogenous levels, and we also observed coimmunoprecipitation of GFP-Cdc15 with HA-Pxl1 by using anti-HA antibodies (Figure 6E). These results suggest that these proteins might form a complex during cytokinesis.
Genetic Interactions between pxl1+ and Genes Regulating Cytokinesis
To better understand the molecular function of Pxl1, we looked for possible genetic interaction of pxl1+ with other genes encoding proteins forming the actomyosin ring or participating in cytokinesis. Several double mutants of these genes and pxl1Δ were generated, and their growth at different temperatures was examined (Table 1 and Supplemental Figure 3). Mid1 interacts with Myo2 and anchors the myosin at the medial cortex (Motegi et al., 2004). There were synthetic fitness (slow growth) defects between pxl1Δ and mid1Δ. Thus, pxl1Δ mid1Δ double mutant was not viable at 25°C (Supplemental Figure 3A), and the cells grew very slowly at 32°C. There was also a strong genetic interaction among pxl1+, and the genes coding the components of myosin II. Thus, pxl1Δ rlc1Δ double mutant was not viable at 25°C, and the cells grew very slowly at 28 or 32°C (Supplemental Figure 3B). rlc1+, coding for the regulatory light chain of myosin II, is not essential, and the phenotype of rlc1Δ cells is a cytokinesis defect similar to that of cells lacking Pxl1 (Le Goff et al., 2000). In the same way, myo2-E1 mutant cells are thermosensitive, and they have a cytokinesis defect (Balasubramanian et al., 1998). myo2-E1 defects were drastically aggravated in cells lacking Pxl1. Thus, myo2-E1 pxl1Δ mutant cells grew slowly at 25°C, and they could not grow at temperatures of 28°C or higher (Table 1, Supplemental Figure 3B). It was not possible to obtain double mutant strains carrying pxl1Δ and cdc4-8, a thermosensitive allele of the gene coding for the myosin essential light chain. The pxl1Δ cdc4-8 spores germinated and generated branched multiseptated cells that could not form colonies (Supplemental Figure 3D). These germinating spores were similar to myo2Δ spores, forming short filaments with septa that failed to cleave (Kitayama et al., 1997).
Table 1.
Genetic interactions between pxl1+ and genes regulating cytokinesis
| Strain | Gene product | Phenotype of double mutant with pxl1Δ |
|
|---|---|---|---|
| Growth | Septation | ||
| mid1Δ | Anillin | SG | A |
| cdc4-8 | Essential myosin light chain | SL | |
| rlc1Δ | Regulatory light chainA | SG | A |
| myo2-E1 | Myosin II heavy chain | SG | A |
| rng2-D5 | IQGAP | SL | |
| cdc15-140 | FCH protein | SG | A |
| cdc12-112 | Formin | NE | A |
| cps1-12 | Bgs1 glucan synthase subunit | SL | |
| cps1-N12 | Bgs1 glucan synthase subunit | SL | |
| cps1-191 | Bgs1 glucan synthase subunit | SL | |
| cwg1-1 | Bgs4 glucan synthase subunit | NE | NE |
| myo3Δ | Myosin II heavy chain | NE | A |
| spn3Δ | Septin | NE | A |
| mid2Δ | Anillin | NE | A |
Growth of each double mutant strain was compared with those of the corresponding parental strains. SL, synthetic lethal; SG, slow growth; A aggravated; and NE no effect.
The IQGAP-related protein Rng2p is a component of the actomyosin ring, and it is required for ring formation after assembly of F-actin at the division site (Eng et al., 1998). It was not possible to obtain double mutant strains carrying rng2-D5 and pxl1Δ mutations. The germinated spores formed short filaments as pxl1Δ cdc4-8 and myo2Δ spores (Supplemental Figure 3D). There was also genetic interaction between pxl1+ and cdc15+. Double mutant cells carrying pxl1Δ and the thermosensitive cdc15-140 allele did not grow at temperatures of 28°C or higher (Supplemental Figure 3B). Surprisingly, the absence of Pxl1 did not aggravate the growth phenotype of cdc12-112 cells; thus, the cdc12-112 pxl1Δ double mutant grew as well as cdc12-112 cells at 32°C (Supplemental Figure 3C). However, the percentage of septating and multiseptated cells was higher in cdc12-112 pxl1Δ than in pxl1Δ cells (data not shown). These results suggest that Pxl1 plays a role in actomyosin ring formation.
Interestingly, we also observed lethality in double mutant strains carrying pxl1Δ and any of the bgs1+-thermosensitive alleles, cps1-N12, cps1-12, and cps1-191. By contrast, the lack of Pxl1 did not aggravate the growth defect of cwg1-1, a bgs4-thermosensitive mutant (Table 1). Bgs1 and Bgs4 are the (1,3)β-d-glucan synthase catalytic subunits responsible for biosynthesis of the primary septum and cell wall, respectively (Cortes et al., 2005, 2007). These results suggest that Pxl1 and Bgs1 are contributing to some essential process in cytokinesis that might be related to primary septum synthesis, because that is the role of Bgs1 (Cortes et al., 2007), and septum synthesis is coordinated with the actomyosin ring contraction.
We did not observe synthetic lethality with myo3Δ; although the multiseptation phenotype of pxl1Δ myo3Δ was more severe than the parental phenotypes (Table 1). Similarly, the lack of Pxl1 had no effect on the growth of either mid2Δ or spn3Δ, but the double mutants showed additive septation defects (Table 1). Together, these results point toward Pxl1 collaborating with proteins that participate in the formation of the actomyosin ring and the primary septum but not with the proteins that act later in cytokinesis such as Mid2 or the septins.
Pxl1 Is Required for Proper Myosin II Organization into the Actomyosin Ring
We have described that Pxl1 localization is actomyosin ring-dependent and that there is colocalization of myosin II and Pxl1 during ring contraction. Additionally there is strong genetic interaction between pxl1+ and other genes related to the actomyosin ring formation. So we next compared the behavior of myosin II and other ring components in pxl1+ and pxl1Δ strains. Fluorescence microscopy of cells endogenously expressing any of the myosin II chains GFP-Myo2 (and the SPB marker Sad1-GFP), Rlc1-GFP, or Cdc4-GFP showed that myosin II localized to the division area in cells lacking Pxl1, but the formation of the myosin contractile ring was not always correct in these cells (Figure 7). Cells carrying tagged myosin chains in a wild-type background do not have a visible phenotype. However, pxl1Δ cells carrying tagged myosin chains showed a stronger septation defect than pxl1Δ cells, suggesting that the tagged myosin chains are not fully functional. With all of them, we observed irregular myosin rings in pxl1Δ cells (47, 50, and 51% with GFP-Myo2, Rlc1-GFP, and Cdc4-GFP, respectively; n = 125) that were not present in wild-type cells. Most rings had disorganized filaments connected to them or floating nearby (Figure 7B). We could also see more than one ring (Figure 7 and Supplemental Movies S2, S4, and S6) in some cells (19, 18, and 22% with GFP-Myo2, Rlc1-GFP, and Cdc4-GFP, respectively; n = 125). In the cells in which there was more than one ring, only one of them contracted and formed a septum, whereas the other ring or rings remained noncontracted (Figure 7C and Supplemental Movies S2, S4, and S6). The absence of Pxl1 did not cause an increase in total amount of myosin II, as detected by Western blot of the light chains Cdc4 and Rlc1 (data not shown). Therefore, the phenotypes observed are probably due to a defect in myosin II organization and interaction with other ring proteins.
Figure 7.
Pxl1 is required for proper myosin II localization to the actomyosin ring. (A) Fluorescence microscopy of wild-type and pxl1Δ cells carrying GFP-myo2+ sad1+-GFP, rlc1+-GFP, and cdc4+-GFP endogenously expressed. (B) Confocal fluorescence images of GFP-Myo2 and Sad1-GFP in pxl1Δ cells. (C) Fluorescence microscopy of a calcofluor-stained pxl1Δ cell expressing rlc1-GFP to see the septum formation while one of the myosin rings was not contracted. (D) Localization of Myo3-GFP in wild-type and pxl1Δ cells. Arrows point to irregular rings.
We observed an increased septation defect of pxl1Δ myo3Δ double mutant compared with the parental strains. Therefore, considering that Myo3/Myp2 is the other heavy chain of myosin II and also participates in cytokinesis, we analyzed Myo3-GFP localization in cells lacking Pxl1. Fluorescence microscopy of these cells showed a significant number of irregular rings (41%; n = 90) (Figure 7D), but we never saw more than one ring formed. There was also a considerably higher percentage of cells that accumulated Myo3-GFP at the end of cytokinesis (Figure 7D). This last effect could be caused, at least partially, by a delay in cell separation.
We could not see the localization of Cdc15 in pxl1Δ cells because tagged Cdc15 (either GFP-Cdc15 or Cdc15-GFP) was lethal in combination with the lack of Pxl1, suggesting that tagged Cdc15 is not totally functional and Pxl1 is essential in that situation. We then compared Cdc12-3YFP localization in pxl1+ and pxl1Δ cells, and we did not observe any difference (Supplemental Figure 4A). Because the formin Cdc12 seemed properly organized in the actin ring during cytokinesis in cells lacking paxillin, we performed actin staining to see whether we could detect actin ring disorganization. Most actin rings in cells lacking Pxl1 were very similar to those in wild-type cells, although more disorganized patches were observed around the ring, and in very few cells (<1%) a double ring was detected (Supplemental Figure 4B, see inset, bottom right corner).
Because the main defect in pxl1Δ cells is an increase in the percentage of septating cells (45–50%), we also analyzed the localization of different proteins required for proper septum formation and for cell separation. In particular, we analyzed Bgs1p and Bgs4p, the (1,3)β-d-glucan synthase catalytic subunits, and both were correctly localized (Supplemental Figure 5). However, we observed that many cells have incomplete Bgs1-GFP (60 vs. 34% in wild-type cells) or Bgs4-GFP (40 vs. 18% in wild-type cells) fluorescent signal in the division area, suggesting that these cells are delayed in the process of septum formation. We also analyzed Spn1-GFP, one of the fission yeast septins that is essential for septin ring formation (An et al., 2004); Sec8-GFP from the exocist complex, involved in the targeting of enzymes responsible for septum cleavage (Wang et al., 2002); and the glucanases Eng1-GFP and Agn1-GFP, which are responsible for the degradation of the primary septum and the lateral cell wall, respectively (Martin-Cuadrado et al., 2003; Garcia et al., 2005). These four proteins all localized correctly to the septum area in pxl1Δ cells (Supplemental Figure 6). Therefore, our results suggest that the main defect of cells lacking Pxl1 is myosin II misorganization into the actomyosin ring.
Pxl1 Collaborates in the Constriction of the Actomyosin Ring
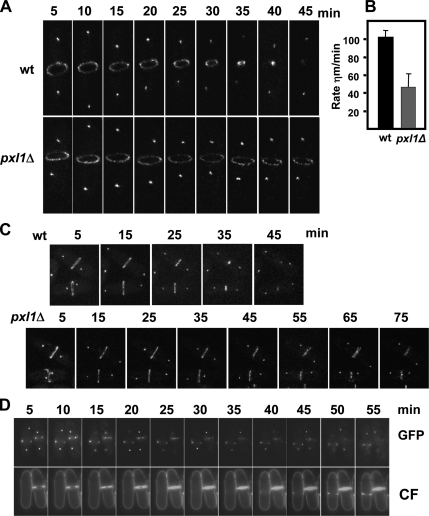
Myosin II is responsible for actomyosin ring contraction during animal cell cytokinesis (Matsumura, 2005), and it is essential for cytokinesis in fission yeast (Kitayama et al., 1997). Our results indicate that Pxl1 function could be related to myosin II organization; therefore, we tried to determine whether the delay observed in pxl1Δ cell separation was due to a delay in ring assembly or in ring constriction. Time-lapse confocal microscopy at 25°C of cells expressing both GFP-Myo2 and Sad1p-GFP was used to analyze the SPB localization and the ring constriction. As shown in Figure 8, A and B, the rate of ring closure was much slower in pxl1Δ cells than in a wild-type strain. Although the time for ring constriction was variable among different pxl1Δ cells, we calculated the average ring constriction rate at 25°C using cells from several independent experiments. Constriction was always slower in pxl1Δ (45 ± 15 nm/min; n = 20) than in wild-type cells (92 ± 10 nm/min n = 10). Additionally, the rings remained for a much longer time during the late stages of cytokinesis in pxl1Δ cells (Figure 8C). We also observed simultaneously the ring constriction and septum formation by calcofluor staining of cells carrying both GFP-Myo2 and Sad1-GFP, and in some cases, as with the left cell shown in Figure 8D, the myosin ring was formed but the septum formation was deferred at least 40 min. We can conclude that a delay in ring contraction could be the cause of the increased percentage of septating cells observed in pxl1Δ cell cultures. A similar septation phenotype was observed in rlc1Δ cells (Le Goff et al., 2000), in myo2-E1 mutant cells grown at the restrictive temperature (Balasubramanian et al., 1998), and in cells in which myo2+ was switched off (Kitayama et al., 1997).
Figure 8.
Ring constriction during cytokinesis is delayed in pxl1Δ cells. Analysis of Myo2 ring contraction in wild-type and pxl1Δ cells grown at 25°C. (A) Time-lapse confocal fluorescence microscopy of GFP-Myo2 Sad1-GFP in wild-type and pxl1Δ cells. (B) Rate of ring constriction at 25°C of wild type (n = 10) and pxl1Δ cells (n = 20) calculated from the time-lapse confocal images in three independent experiments. (C and D) Time-lapse fluorescence images of different GFP-Myo2 Sad1-GFP in wild-type and pxl1Δ cells during cytokinesis. In D, cells were stained with calcofluor.
DISCUSSION
The genome of S. pombe contains an ORF coding a protein that shares similarity with S. cerevisiae Pxl1 (Gao et al., 2004; Mackin et al., 2004) and paxillin from animal cells; therefore, we named it pxl1+. Mild overexpression of this gene was able to suppress the thermosensitive growth lethality of cdc42-1625 mutant with defects in actin cable formation and secretion (Martin et al., 2007). Coimmunoprecipitation of Pxl1 and Rho1, and the biochemical data suggest that Pxl1 acts as a negative regulator of Rho1, modulating the level of GTP-Rho1 in vivo. Thus, the lack of Pxl1 causes an increase in GTP-bound Rho1 slightly lower than that produced by the absence of Rga5, a specific Rho1 GAP. The genetic interaction with rgf3+ shown in Figure 2, D–F also strongly supports the relevance of these results. In contrast, we could not see interaction between Pxl1 and Cdc42. Therefore, pxl1+ suppression of cdc42-1625 thermosensitivity might not be due to a direct activation of the Cdc42 signaling pathway altered in cdc42-1625, but rather to an indirect effect caused by a decrease in Rho1 signaling. Antagonistic cross-talk of these two GTPases was first reported in fibroblasts, in which Cdc42 and RhoA mutually inhibit each other (Nobes and Hall, 1995), and it has been described in various processes of animal cells (Sander et al., 1999).
Pxl1 does not have a clear RhoGAP domain; therefore, we explored the possibility that the interaction between Rho1 and Pxl1 was mediated through a Rho1GAP but two-hybrid assays performed using Pxl1 as bait and the three Rho1 GAPs, Rga1, Rga5 and Rga8 did not show any interaction. Additionally, there was coimmunoprecipitation of Rho1 and Pxl1 in rga5Δ and rga8Δ strains (data not shown), suggesting that the interaction was not mediated by those Rho1 GAPs. S. pombe Pxl1 seems to behave similarly to budding yeast Pxl1 with respect to its interaction with Rho1 GTPase (Gao et al., 2004). However, S. cerevisiae Pxl1 was not able to suppress pxl1Δ phenotype when expressed in S. pombe (data not shown). There is a major difference in the localization of these two paxillin-like proteins: S. cerevisiae Pxl1 localizes to sites of polarized growth and LIM domains are required to target the protein to these sites; by contrast, S. pombe Pxl1 localizes to the area of cellular division and it is the N-terminal region of the molecule, not the LIM domains, that is necessary and sufficient to target the molecule to the division site. It is possible that the mechanism of Rho1 modulation is similar in both paxillin-like proteins, but the spatial regulation allows a different function of these molecules in budding and fission yeasts.
Pxl1 localization experiments indicate that it is a nonessential member of the contractile ring that is recruited to the division area after myosin II and Cdc12, immediately before the ring is formed. Additionally, Pxl1 interacts with Cdc15 and Rlc1. These results, supported by the genetic interactions with other septation genes, suggest a role of Pxl1 in actomyosin ring organization that is corroborated by fluorescence microscopy of the GFP-tagged myosin chains in pxl1Δ cells. It has been proposed that the interaction between the Myo2 fibers formed from the nodes in the broad medial band and the F-actin cables formed in the same area might pull nodes together and laterally condense the networks of Myo2 fibers and F-actin cables into a contractile ring. Myo2 is stabilized when the ring forms and in turn it contributes to actin polymerization. Pxl1, together with Cdc15, might aid in the coalescence of the myosin II and F-actin cables into a ring. Myo2 fibers connected to the ring can be observed in pxl1Δ cells, indicating that the coalescence has not been properly achieved. Moreover, the additional myosin ring formed in some pxl1Δ cells is noncontractile, suggesting that myosin is not stably associated to the actin ring in the absence of Pxl1.
The interactions between myosin II and actin in the ring are believed to generate the force that constricts animal cells to divide in two daughter cells (Matsumura, 2005). A lower concentration of myosin II properly organized in the actomyosin ring may cause a reduction in the contraction force, which in turn causes the delay or even the halt in the ring contraction that we have seen in some pxl1Δ cells during the time-lapse experiments. Other mutations affecting myosin II, such as rlc1Δ, myo2-E1, and ring2-D5, cause a similar phenotype (Balasubramanian et al., 1998; Le Goff et al., 2000; Mulvihill and Hyams, 2003), suggesting that myosin II activity is required for proper cytokinesis. Disassembly of the myosin rings is also slowed down in pxl1Δ cells, and a strong accumulation of Myo3 is observed. These effects might also cause additional delay in septation and cell separation. Zebra fish embryos with reduced myosin activity also exhibit at late stages of cytokinesis a stabilized contractile ring apparatus that suggests a role for myosin function in the disassembly of the contractile ring (Urven et al., 2006).
The actomyosin ring is not essential for cytokinesis in S. cerevisiae, which can still complete cytokinesis in its absence, possibly by localized cell wall synthesis (Bi et al., 1998). In fission yeast it is not known whether ring constriction is the force that triggers septum formation or if septum formation forces ring constriction and cytokinesis as has recently been proposed (Johnson et al., 2005). Indeed, bgs1+ shut-off and deletion also causes a multiseptation phenotype (Cortes et al., 2007). The lethal interaction observed between pxl1Δ cells and several bgs1 mutant strains suggests that both ring constriction and primary septum formation might collaborate in the completion of S. pombe cytokinesis.
Whether Pxl1 modulation of Rho1 activity is related to Pxl1 function in actomyosin ring formation and contraction remains unknown. Further studies will be required to clarify the possible involvement of Rho1 in S. pombe contractile ring formation and cytokinesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. C. Ribas, B. Santos, and H. Valdivieso for useful comments. We thank to D. Posner for language revision. We thank M. Balasubramanian (University of Singapore), F. Chang (Columbia University, New York, NY), S. Erdman (Syracuse University, Syracuse, NY), K. Gould (Vanderbilt University, Nashville, TN), T. Pollard (Yale University, New Haven, CT), J. C. Ribas (CSIC, Spain), Y. Sánchez (University of Salamanra, Spain), V. Simanis (ISREC, Switzerland), and R. Y. Tsien (University of California, San Diego) for generous gifts of strains, and plasmids. Thanks to D. Riveline and C. Castro for technical help with the microscopy. M. Pinar was supported by a fellowship from the Spanish Ministerio de Educación. This work was supported by grant BIO2004-0834 from the Comisión Interministerial de Ciencia y Tecnología, Spain.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-07-0718) on February 6, 2008.
REFERENCES
- Alonso-Nunez M. L., An H., Martin-Cuadrado A. B., Mehta S., Petit C., Sipiczki M., del Rey F., Gould K. L., de Aldana C. R. Ace2p controls the expression of genes required for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell. 2005;16:2003–2017. doi: 10.1091/mbc.E04-06-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An H., Morrell J. L., Jennings J. L., Link A. J., Gould K. L. Requirements of fission yeast septins for complex formation, localization and function. Mol. Biol. Cell. 2004;15:5551–5564. doi: 10.1091/mbc.E04-07-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano M., Coll P. M., Pérez P. Rho GTPases in the control of cell morphology, cell polarity, and actin localization in fission yeast. Microsc. Res. Tech. 1999a;47:51–60. doi: 10.1002/(SICI)1097-0029(19991001)47:1<51::AID-JEMT5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Arellano M., Duran A., Perez P. Rho 1 GTPase activates the (1–3)beta-D-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. EMBO J. 1996;15:4584–4591. [PMC free article] [PubMed] [Google Scholar]
- Arellano M., Duran A., Perez P. Localization of the Schizosaccharomyces pombe Rho1 GTPase and its involvement in the organization of the actin cytoskeleton. J. Cell Sci. 1997;110:2547–2555. doi: 10.1242/jcs.110.20.2547. [DOI] [PubMed] [Google Scholar]
- Arellano M., Valdivieso M. H., Calonge T. M., Coll P. M., Durán A., Pérez P. Schizosaccharomyces pombe protein kinase C homologues, pck1p and pck2p, are targets of rho1p and rho2p and differentially regulate cell integrity. J. Cell Sci. 1999b;112:3569–3578. doi: 10.1242/jcs.112.20.3569. [DOI] [PubMed] [Google Scholar]
- Bähler J., Wu J.-Q., Longtine M. S., Shah N. G., McKenzie A., 3rd, Steever A. B., Wach A., Philippsen P., Pringle J. R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., McCollum D., Chang L., Wong K. C., Naqvi N. I., He X., Sazer S., Gould K. L. Isolation and characterization of new fission yeast cytokinesis mutants. Genetics. 1998;149:1265–1275. doi: 10.1093/genetics/149.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla M., Pollard T. D. Myosin-II tails confer unique functions in Schizosaccharomyces pombe: characterization of a novel myosin-II tail. Mol. Biol. Cell. 2000;11:79–91. doi: 10.1091/mbc.11.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E., Maddox P., Lew D. J., Salmon E. D., McMillan J. N., Yeh E., Pringle J. R. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. The J. Cell Biol. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Turner C. E. Paxillin: adapting to change. Physiol. Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- Burgess D. R., Chang F. Site selection for the cleavage furrow at cytokinesis. Trends Cell Biol. 2005;15:156–162. doi: 10.1016/j.tcb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Calonge T. M., Arellano M., Coll P. M., Perez P. Rga5p is a specific Rho1p GTPase-activating protein that regulates cell integrity in Schizosaccharomyces pombe. Mol. Microbiol. 2003;47:507–518. doi: 10.1046/j.1365-2958.2003.03312.x. [DOI] [PubMed] [Google Scholar]
- Carnahan R. H., Gould K. L. The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J. Cell Biol. 2003;162:851–862. doi: 10.1083/jcb.200305012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragher N. O., Frame M. C. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14:241–249. doi: 10.1016/j.tcb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Coll P. M., Rincon S. A., Izquierdo R. A., Perez P. Hob3p, the fission yeast ortholog of human BIN3, localizes Cdc42p to the division site and regulates cytokinesis. EMBO J. 2007;26:1865–1877. doi: 10.1038/sj.emboj.7601641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll P. M., Trillo Y., Ametzazurra A., Perez P. Gef1p, a new guanine nucleotide exchange factor for Cdc42p, regulates polarity in Schizosaccharomyces pombe. Mol. Biol. Cell. 2003;14:313–323. doi: 10.1091/mbc.E02-07-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J. C., Carnero E., Ishiguro J., Sanchez Y., Duran A., Ribas J. C. The novel fission yeast (1,3)beta-D-glucan synthase catalytic subunit Bgs4p is essential during both cytokinesis and polarized growth. J. Cell Sci. 2005;118:157–174. doi: 10.1242/jcs.01585. [DOI] [PubMed] [Google Scholar]
- Cortes J. C., Ishiguro J., Duran A., Ribas J. C. Localization of the (1,3)beta-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J. Cell Sci. 2002;115:4081–4096. doi: 10.1242/jcs.00085. [DOI] [PubMed] [Google Scholar]
- Cortes J. C., Konomi M., Martins I. M., Munoz J., Moreno M. B., Osumi M., Duran A., Ribas J. C. The (1,3)beta-D-glucan synthase subunit Bgs1p is responsible for the fission yeast primary septum formation. Mol. Microbiol. 2007;65:201–217. doi: 10.1111/j.1365-2958.2007.05784.x. [DOI] [PubMed] [Google Scholar]
- Chang E. C., Barr M., Wang Y., Jung V., Xu H. P., Wigler M. H. Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell. 1994;79:131–141. doi: 10.1016/0092-8674(94)90406-5. [DOI] [PubMed] [Google Scholar]
- Chang F., Drubin D., Nurse P. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J. Cell Biol. 1997;137:169–182. doi: 10.1083/jcb.137.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng K., Naqvi N. I. Rng2p, a protein required for cytokinesis in fission yeast, is a component of the actomyosin ring and the spindle pole body. Curr. Biol. 1998;8:611–621. doi: 10.1016/s0960-9822(98)70248-9. [DOI] [PubMed] [Google Scholar]
- Feierbach B., Chang F. Cytokinesis and the contractile ring in fission yeast. Curr. Opin. Microbiol. 2001;4:713–719. doi: 10.1016/s1369-5274(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Forsburg S. L., Sherman D. A. General purpose tagging vectors for fission yeast. Gene. 1997;191:191–195. doi: 10.1016/s0378-1119(97)00058-9. [DOI] [PubMed] [Google Scholar]
- Furge K. A., Wong K., Armstrong J., Balasubramanian M., Albright C. F. Byr4 and Cdc16 form a two-component GTPase-activating protein for the Spg1 GTPase that controls septation in fission yeast. Curr. Biol. 1998;8:947–954. doi: 10.1016/s0960-9822(98)70394-x. [DOI] [PubMed] [Google Scholar]
- Gao X. D., Caviston J. P., Tcheperegine S. E., Bi E. Pxl1p, a paxillin-like protein in Saccharomyces cerevisiae, may coordinate Cdc42p and Rho1p functions during polarized growth. Mol. Biol. Cell. 2004;15:3977–3985. doi: 10.1091/mbc.E04-01-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I., Jimenez D., Martin V., Duran A., Sanchez Y. The alpha-glucanase Agn1p is required for cell separation in Schizosaccharomyces pombe. Biol. Cell. 2005;97:569–576. doi: 10.1042/BC20040096. [DOI] [PubMed] [Google Scholar]
- García P., Tajadura V., Garcia I., Sanchez Y. Role of Rho GTPases and Rho-GEFs in the regulation of cell shape and integrity in fission yeast. Yeast. 2006;23:1031–1043. doi: 10.1002/yea.1409. [DOI] [PubMed] [Google Scholar]
- Hirota K., Tanaka K., Ohta K., Yamamoto M. Gef1p and Scd1p, the two GDP-GTP exchange factors for Cdc42p, form a ring structure that shrinks during cytokinesis in. Mol. Biol. Cell. 2003;14:3617–3627. doi: 10.1091/mbc.E02-10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. F., Yoo B. Y., Calleja G. B., Kozela C. P. Second thoughts on septation by the fission yeast, Schizosaccharomyces pombe: pull vs. push mechanisms with an appendix–dimensional modelling of the flat and variable septa. 2005;88:1–12. doi: 10.1007/s10482-004-7074-2. [DOI] [PubMed] [Google Scholar]
- Kadrmas J. L., Beckerle M. C. The LIM domain: from the cytoskeleton to the nucleus. Nat. Rev. Mol. Cell Biol. 2004;5:920–931. doi: 10.1038/nrm1499. [DOI] [PubMed] [Google Scholar]
- Kitayama C., Sugimoto A., Yamamoto M. Type II myosin heavy chain encoded by the myo2 gene composes the contractile ring during cytokinesis in Schizosaccharomyces pombe. J. Cell Biol. 1997;137:1309–1319. doi: 10.1083/jcb.137.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A., Gulli M. P., Simanis V. SIN and the art of splitting the fission yeast cell. Curr. Biol. 2004;14:R722–R730. doi: 10.1016/j.cub.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Le Goff X., Motegi F., Salimova E., Mabuchi I., Simanis V. The S. pombe rlc1 gene encodes a putative myosin regulatory light chain that binds the type II myosins myo3p and myo2p. J. Cell Sci. 2000;113:4157–4163. doi: 10.1242/jcs.113.23.4157. [DOI] [PubMed] [Google Scholar]
- Le Goff X., Woollard A., Simanis V. Analysis of the cps1 gene provides evidence for a septation checkpoint in Schizosaccharomyces pombe. Mol. Gen. Genet. 1999;262:163–172. doi: 10.1007/s004380051071. [DOI] [PubMed] [Google Scholar]
- Levin D. E. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackin N. A., Sousou T. J., Erdman S. E. The PXL1 gene of Saccharomyces cerevisiae encodes a paxillin-like protein functioning in polarized cell growth. Mol. Biol. Cell. 2004;15:1904–1917. doi: 10.1091/mbc.E04-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cuadrado A. B., Duenas E., Sipiczki M., Vazquez de Aldana C. R., del Rey F. The endo-beta-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J. Cell Sci. 2003;116:1689–1698. doi: 10.1242/jcs.00377. [DOI] [PubMed] [Google Scholar]
- Martin S. G., Rincon S. A., Basu R., Perez P., Chang F. Regulation of the formin for3p by cdc42p and bud6p. Mol. Biol. Cell. 2007;18:4155–4167. doi: 10.1091/mbc.E07-02-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15:371–377. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- McCollum D., Balasubramanian M. K., Pelcher L. E., Hemmingsen S. M., Gould K. L. Schizosaccharomyces pombe cdc4+ gene encodes a novel EF-hand protein essential for cytokinesis. J. Cell Biol. 1995;130:651–660. doi: 10.1083/jcb.130.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. J., Johnson D. I. Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. Mol. Cell Biol. 1994;14:1075–1083. doi: 10.1128/mcb.14.2.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Morrell-Falvey J. L., Ren L., Feoktistova A., Haese G. D., Gould K. L. Cell wall remodeling at the fission yeast cell division site requires the Rho-GEF Rgf3p. J. Cell Sci. 2005;118:5563–5573. doi: 10.1242/jcs.02664. [DOI] [PubMed] [Google Scholar]
- Motegi F., Mishra M., Balasubramanian M. K., Mabuchi I. Myosin-II reorganization during mitosis is controlled temporally by its dephosphorylation and spatially by Mid1 in fission yeast. J. Cell Biol. 2004;165:685–695. doi: 10.1083/jcb.200402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motegi F., Nakano K., Mabuchi I. Molecular mechanism of myosin-II assembly at the division site in Schizosaccharomyces pombe. J. Cell Sci. 2000;113:1813–1825. doi: 10.1242/jcs.113.10.1813. [DOI] [PubMed] [Google Scholar]
- Mulvihill D. P., Hyams J. S. Role of the two type II myosins, Myo2 and Myp2, in cytokinetic actomyosin ring formation and function in fission yeast. Cell Motil. Cytoskeleton. 2003;54:208–216. doi: 10.1002/cm.10093. [DOI] [PubMed] [Google Scholar]
- Mutoh T., Nakano K., Mabuchi I. Rho1-GEFs Rgf1 and Rgf2 are involved in formation of cell wall and septum, while Rgf3 is involved in cytokinesis in fission yeast. Genes Cells. 2005;10:1189–1202. doi: 10.1111/j.1365-2443.2005.00908.x. [DOI] [PubMed] [Google Scholar]
- Nakano K., Mutoh T., Mabuchi I. Characterization of GTPase-activating proteins for the function of the Rho-family small GTPases in the fission yeast Schizosaccharomyces pombe. Genes Cells. 2001;6:1031–1042. doi: 10.1046/j.1365-2443.2001.00485.x. [DOI] [PubMed] [Google Scholar]
- Naqvi N. I., Eng K., Gould K. L., Balasubramanian M. K. Evidence for F-actin-dependent and -independent mechanisms involved in assembly and stability of the medial actomyosin ring in fission yeast. EMBO J. 1999;18:854–862. doi: 10.1093/emboj/18.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N. I., Wong K. C., Tang X., Balasubramanian M. K. Type II myosin regulatory light chain relieves auto-inhibition of myosin-heavy-chain function. Nat. Cell Biol. 2000;2:855–858. doi: 10.1038/35041107. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Park H. O., Bi E. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol. Mol. Biol. Rev. 2007;71:48–96. doi: 10.1128/MMBR.00028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid T., Furuyashiki T., Ishizaki T., Watanabe G., Watanabe N., Fujisawa K., Morii N., Madaule P., Narumiya S. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the rho-binding domain. J. Biol. Chem. 1996;271:13556–13560. doi: 10.1074/jbc.271.23.13556. [DOI] [PubMed] [Google Scholar]
- Ren X. D., Kiosses W. B., Schwartz M. A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Rustici G., Mata J., Kivinen K., Lio P., Penkett C. J., Burns G., Hayles J., Brazma A., Nurse P., Bahler J. Periodic gene expression program of the fission yeast cell cycle. Nat. Genet. 2004;36:809–817. doi: 10.1038/ng1377. [DOI] [PubMed] [Google Scholar]
- Sander E. E., ten Klooster J. P., van Delft S., van der Kammen R. A., Collard J. G. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajadura V., Garcia B., Garcia I., Garcia P., Sanchez Y. Schizosaccharomyces pombe Rgf3p is a specific Rho1 GEF that regulates cell wall beta-glucan biosynthesis through the GTPase Rho1p. J. Cell Sci. 2004;117:6163–6174. doi: 10.1242/jcs.01530. [DOI] [PubMed] [Google Scholar]
- Turner C. E. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2000;2:E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- Urven L. E., Yabe T., Pelegri F. A role for non-muscle myosin II function in furrow maturation in the early zebrafish embryo. J. Cell Sci. 2006;119:4342–4352. doi: 10.1242/jcs.03197. [DOI] [PubMed] [Google Scholar]
- Wang H., Tang X., Liu J., Trautmann S., Balasundaram D., McCollum D., Balasubramanian M. K. The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell. 2002;13:515–529. doi: 10.1091/mbc.01-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R. R., Vaisberg E. V., Ding R., Nurse P., McIntosh J. R. cut11(+): A gene required for cell cycle-dependent spindle pole body anchoring in the nuclear envelope and bipolar spindle formation in Schizosaccharomyces pombe. Mol. Biol. Cell. 1998;9:2839–2855. doi: 10.1091/mbc.9.10.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe B. A., Gould K. L. Split decisions: coordinating cytokinesis in yeast. Trends Cell Biol. 2005;15:10–18. doi: 10.1016/j.tcb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Wu J. Q., Kuhn J. R., Kovar D. R., Pollard T. D. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Developmental cell. 2003;5:723–734. doi: 10.1016/s1534-5807(03)00324-1. [DOI] [PubMed] [Google Scholar]
- Yang P., Qyang Y., Bartholomeusz G., Zhou X., Marcus S. The novel Rho GTPase-activating protein family protein, Rga8, provides a potential link between Cdc42/p21-activated kinase and Rho signaling pathways in the fission yeast, Schizosaccharomyces pombe. J. Biol. Chem. 2003;278:48821–48830. doi: 10.1074/jbc.M306819200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.