Abstract
Formins are regulated actin-nucleating proteins that are widespread among eukaryotes. Overexpression of unregulated formins in budding yeast is lethal and causes a massive accumulation of disorganized cable-like filaments. To explore the basis of this lethality, a cDNA library was screened to identify proteins whose overexpression could rescue the lethality conferred by unregulated Bnr1p expression. Three classes of suppressors encoding actin-binding proteins were isolated. One class encodes proteins that promote the assembly of actin cables (TPM1, TPM2, and ABP140), suggesting that the lethality was rescued by turning disorganized filaments into functional cables. The second class encodes proteins that bind G-actin (COF1, SRV2, and PFY1), indicating that reduction of the pool of actin available for cable formation may also rescue lethality. Consistent with this, pharmacological or genetic reduction of available actin also protected the cell from overproduction of unregulated Bnr1p. The third class consists of Las17p, an activator of the formin-independent Arp2/3p-dependent actin nucleation pathway. These results indicate that proper assembly of actin cables is sensitive to the appropriate balance of their constituents and that input into one pathway for actin filament assembly can affect another. Thus, cells must have a way of ensuring a proper balance between actin assembly pathways.
INTRODUCTION
The ability to polarize is an essential feature of all cells (Nelson, 2003). It is most clearly seen in the distinct functional domains characteristic of epithelial cells, migrating cells, and neurons. Another manifestation of cell polarity is the ability of cells to select an axis for cell division and then segregate their organelles along that axis. In budding yeast, the axis of cell division is determined early in the cell cycle by bud site selection. The bud grows by polarized growth and organelles are then segregated along this axis (Pruyne et al., 2004b).
The actin cytoskeleton is responsible for this polarized growth (Pruyne et al., 2004b). The yeast actin cytoskeleton consists primarily of cortical patches, which are sites of endocytosis (Engqvist-Goldstein and Drubin, 2003), and actin cables (Adams and Pringle, 1984; Kilmartin and Adams, 1984), which are bundles of filaments arising from the bud cortex and neck and extending into the mother cell (Pruyne et al., 2004a). The cables play several roles in promoting proper bud growth, serving as tracks for the transport of secretory vesicles to support cell expansion (Govindan et al., 1995; Pruyne et al., 1998; Schott et al., 1999), for the segregation of organelles, including the vacuole, mitochondria, endoplasmic reticulum, and peroxisomes (Simon et al., 1995; Du et al., 2001; Hoepfner et al., 2001; Fehrenbacher et al., 2002; Weisman, 2003; Fagarasanu et al., 2006) and for the transport of mRNAs (Takizawa et al., 1997) and the tips of cytoplasmic microtubules for nuclear orientation (Beach et al., 2000; Yin et al., 2000; Hwang et al., 2003). Most of these transport activities, including secretory vesicle transport, are dependent on the yeast MYO2 gene that encodes the heavy chain of the essential myosin-V; the nonessential heavy chain encoded by MYO4 transports specific mRNAs and cortical endoplasmic reticulum into the bud along actin cables (Takizawa and Vale, 2000; Estrada et al., 2003; Schmid et al., 2006).
The spontaneous nucleation of filaments from G-actin occurs very slowly, so the assembly of actin containing structures is driven by the activity of nucleation factors. The regulated localization and activation of these factors controls the distribution of different actin-based structures. Yeast has two classes of actin nucleators, the Arp2/3 complex that nucleates assembly of the actin filaments of patches (Winter et al., 1999b), and the two formin homologues, Bni1p and Bnr1p, that are the nucleators for cables (Evangelista et al., 2002; Pruyne et al., 2002; Sagot et al., 2002a,b; Pruyne et al., 2004a).
Regulation of the Arp2/3 complex is through the action of activators that greatly increase its very weak intrinsic actin nucleation activity (Moseley and Goode, 2006). Budding yeast has five known activators: Las17p (a member of the Wiskott-Aldrich syndrome protein family), Myo3p and Myo5p (two myosin-I family members), and Abp1p and Pan1p (related to Eps15; Duncan et al., 2001; Goode et al., 2001). Of these, Abp1p and Pan1p have relatively weak activity, Myo3p and Myo5p require the cofactor Vrp1p (a WIP family member) for their full activity, and Las17p exhibits strong Arp2/3-stimulatory activity on its own (Rodal et al., 2003; Sun et al., 2006). Like other WASp family members, the C-terminus of Las17p includes a conserved WH2 (WASp homology 2) domain that binds G-actin, and an A (acidic) region, which interacts with the Arp2/3 complex and is necessary to stimulate nucleation (Winter et al., 1999a).
Regulation of the formins has been proposed to involve activation through a conformational change induced by Rho-GTPases (Alberts, 2001; Dong et al., 2003; Pruyne et al., 2004a). The formins Bni1p and Bnr1p share homologous N-terminal RBD (Rho-binding domain) and adjacent C-terminal proline-rich FH1 (formin homology 1) and FH2 (formin homology 2) domains. The FH2 domain contains the actin nucleating activity and binds the filament barbed end, protecting it from the inhibitory effects of capping protein (Zigmond et al., 2003; Moseley et al., 2004). The FH1 domain recruits complexes of actin monomers bound to the protein profilin, enhancing transfer of actin to the barbed end (Evangelista et al., 1997; Vavylonis et al., 2006). Mammalian formin homologues with similar domain structures are autoinhibited by an interaction between a sequence C-terminal to the FH2 domain called the DAD (Dia-autoregulatory domain; Alberts, 2001) and a sequence overlapping the RBD called the DID (diaphanous inhibitory domain; Otomo et al., 2005). Association of a GTP-bound Rho protein with the RBD disrupts the DID/DAD interaction, relieving the inhibition (Watanabe et al., 1999; Rose et al., 2005). The N-terminal regions of Bni1p and Bnr1p have been implicated in proper localization of the proteins to the cell cortex (Fujiwara et al., 1998; Kikyo et al., 1999; Ozaki-Kuroda et al., 2001).
In support of this model, overexpression in yeast of formin constructs lacking the predicted N-terminal regulatory motif is lethal and leads to an aberrant accumulation of cable-like actin filaments (Evangelista et al., 2002; Sagot et al., 2002a). Here we report the identification of genes whose overexpression can suppress the lethality conferred by such a constitutively active Bnr1p-derived construct. Analysis of specific suppressors suggests that they function by shifting the balance of actin between patches and cables, thereby revealing the need to have a normal mechanism to achieve such a balance.
MATERIALS AND METHODS
Construction of Yeast Strains and Plasmids
Yeast strains used in this study are described in Table 1 and plasmids in Table 2.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| ABY204 | MATα ade2-101 ade3 leu2,112 his3Δ200 ura3-52 tpm1Δ::LEU2 | Lab collection |
| Y1239 | MATa his3Δ1 leu2Δ0 metΔ15 ura3Δ0 | Evangelista et al. (2002) |
| Y1240 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Evangelista et al. (2002) |
| ABY1802 | MATa his3Δ1 lys2Δ leu2Δ0 ura3Δ0 bni1Δ::KanR | Evangelista et al. (2002) |
| ABY1807 | MATa his3Δ1 met15Δ0 leu2Δ0 ura3Δ0 tpm1-2::LEU2 tpm2Δ::KanR | Evangelista et al. (2002) |
| ABY2000 | MATα his3Δ1 leu2Δ1 lys2Δ1 ura3Δ0 bnr1Δ::KanR bni1-11::ura3Δ::HIS3 | Derived from Y4133 Evangelista et al. (2002) |
| ABY2001 | MATα his3Δ1 leu2Δ0 metΔ15 ura3Δ0 ade2Δ::GAL1-10 promoter-Bnr1ΔRBD::KanR | This study |
| ABY2007 | MATa his3Δ1 leu2Δ0 metΔ15 ura3Δ0 bni1Δ::KanR bnr1-6::HIS3 | This study |
| ABY2015 | MAT a/α his3Δ1/his3Δ1 met15Δ0/MET15 leu2Δ0/leu2Δ0lys2Δ0/LYS2 ura3Δ0/ura3Δ0 ACT1/act1Δ::KanR | This study |
| ABY2061 | MATα his3Δ1 leu2Δ0 metΔ15 ura3Δ0 ade2Δ::GAL1-10 promoter-Bnr1ΔRBD::KanR aip1Δ:: KanR | This study |
| aip1Δ | MATa his3Δ1 leu2Δ0 metΔ15 ura3Δ0 aip1Δ:: KanR | ATCC (Manassas VA) |
Table 2.
Plasmids used in this study
| Plasmid | Backbone | Selectable marker | Features | Source |
|---|---|---|---|---|
| p3100 | pRS315 | LEU2 | MYO3 under GAL1-10 promoter | C. Boone |
| pLG011 | pRS315 | LEU2 | BNR1 1141-7294 | This study |
| p015 | pRS316 | URA3 | Bnr1-ΔRBD under GAL1-10 promoter | Pruyne et al. (2004a) |
| pLG024 | pRS316 | URA3 | C-terminally 3HA-tagged Bnr1-ΔRBD under GAL1-10 promoter | This study |
| pLG028 | pRS316 | URA3 | Bnr1-ΔRBD followed by KanR marker, with flanking ADE2 5′ and 3′ UTR | This study |
| pLG034 | pRS303 | HIS3 | Integrates 3′ to BNR1 | This study |
| pLG041 | pRS316 | URA3 | TPM1 under GAL1-10 promoter | This study |
| pLG044 | pRS316 | URA3 | COF1 under GAL1-10 promoter | This study |
| pLG051 | pRS316 | URA3 | LAS17 (612-1990) under GAL1-10 promoter | This study |
| pLG060 | pRS303 | HIS3 | Integrates 3′ to BNR1 to introduce bnr1-6 | This study |
| pLG068 | pRS316 | URA3 | TPM2 under GAL1-10 promoter | This study |
| pLG072 | pRS316 | URA3 | SRV2 under GAL1-10 promoter | This study |
| pLG081 | pRS316 | URA3 | ABP140 (1-654) under GAL1-10 promoter | This study |
| pLG089 | pRS316 | URA3 | LAS17 under GAL1-10 promoter | This study |
| pLG096 | pRS316 | URA3 | PFY1 under GAL1-10 promoter | This study |
| pLG210 | pRS316 | URA3 | pfy1-4 under GAL1-10 promoter | This study |
| pLG244 | pRS316 | URA3 | GAL1-10 promoter followed by BNR1 3′ UTR | This study |
| pLG245 | pRS316 | URA3 | pfy1-14 under GAL1-10 promoter | This study |
| pLG282 | pRS316 | URA3 | BNR1 under GAL1-10 promoter | This study |
| pCM36 | pRS316 | URA3 | las17ΔA under GAL1-10 promoter | B. Goode |
| pCM37 | pRS316 | URA3 | las17ΔWH2-A under GAL1-10 promoter | B. Goode |
| PUG6 | N/A | N/A | KanR cassette | Guldener et al. (1996) |
| pYGS108 | pRS316 | URA3 | HA-PAN1 under GAL1-10 promoter | M. Cai |
The strategy used to construct myo2 temperature-sensitive mutations (Schott et al., 1999) was used here to construct bnr1 temperature-sensitive mutations. This strategy involves generating a library of PCR-mutagenized bnr1 FH2 products that are then used to replace the genomic sequence and transformants screened for temperature sensitivity. pLG011, which contains BNR1 1141-7294, was cut with MfeI to release BNR1 2810-4420, which encodes the FH2 region and 3′ untranslated region (UTR). This fragment was circularized by ligation and used directly as a template for 10 PCR cycles using primers CCATCGATCGGGCGGYGGTCGTGTTGACAA and GCTCTAGACCACCGCCCGAAAAGGG. This generated a linear product with the distal region of the 3′UTR fused at the MfeI site to the FH2 coding sequence followed by the proximal 3′UTR sequence. The PCR product was then cut with ClaI and XbaI, sites that had been introduced by the PRC primers, and the product was ligated into ClaI/XbaI-cut pRS303. The resulting plasmid, pLG034, was cut with PvuII to release a 2-kb fragment that was used as a template for mutagenic PCR (Schott et al., 1999), with primers spanning the BlpII site upstream of the FH2 region and SstII in the vector The mutagenized PCR product was cut with BlpI and SstII and ligated into pLG034 and then transformed into bacteria. Transformants were pooled and plasmid DNA was isolated from them. This library of PCR-mutagenized bnr1 FH2 sequence was cut with MfeI and transformed into ABY1802 (bni1Δ) with selection for HIS3 to replace the endogenous sequence. About 500 transformants were isolated and screened for growth at 14, 30, and 37°C. Six strains viable at 14 and 30°C but not at 37°C were isolated. Genomic DNA of ABY2007 (bni1Δbnr1-6) was cut with MfeI and ligated to recover plasmid pLG060 bearing the mutagenized bnr1 FH2 sequence.
To allow for galactose-inducible expression of Bnr1p lacking the RBD, a KanR cassette was generated by PCR from PUG6 (Guldener et al., 1996) and cloned into the NotI site downstream of BNR1 sequence in p015 (Pruyne et al., 2004a), followed by 497-bp ADE2 3′ sequence (1501–1998) cloned between NotI and SstII. A 365-bp sequence of ADE2 5′ UTR(−23 to −390) was cloned into KpnI site upstream of the GAL1-10 promoter with a SstII site added upstream of ADE2 5′UTR. The resulting plasmid, pLG028, was cut with SstII and transformed into Y1240 (wild type). This strain was called ABY2001.
To allow for overexpression of Bnr1p ΔRBD in aip1Δ background, ABY2001 was crossed to aip1Δ purchased from ATCC (Manassas VA), followed by sporulation. A spore containing both Bnr1ΔRBD and aip1Δ was selected and named ABY2061.
To generate a BNR1 gene tagged with hemagglutinin (HA), a fragment of BNR1 from the endogenous BamHI site to the C terminus was amplified by PCR to eliminate the stop codon and append three HA sequences and a 3′ NotI site. The fragment was ligated into BamHI/NotI-cut p015 (Pruyne et al., 2004a), resulting in pLG024.
To generate the Bnr1p overexpression plasmid, pLG244 was first made by insertion of a NheI site between the MluI and NotI sites and insertion of BNR1 3′UTR (4129–4628) between the NotI and SstII sites of the pRS316-GAL1-10 plasmid (Liu et al., 1992). The BNR1 open reading frame (ORF) was then cloned by PCR from a plasmid containing full-length BNR1 sequence into the NheI and NotI sites of pLG209, resulting in pLG282.
The coding sequences of pfy1-4 and pfy1-14 were cloned by PCR from pBG832 and pBG833 (unpublished results provided by Dr. B. Goode, Brandeis University), respectively, and inserted between the NheI and NotI sites of the pRS316-GAL1-10 plasmid (Liu et al., 1992).
pLG089 was constructed by retrieving the LAS17 sequence from p3186 (Tong et al., 2002) by cutting with BamHI and NotI, followed by ligation into the pRS316-GAL1-10 plasmid (Liu et al., 1992).
Overexpression Suppression Screen
A GAL1-10 promoter-driven cDNA library (Liu et al., 1992) was transformed into ABY2001 and plated on SGal. About 200,000 transformants were screened for viability. Plasmids were recovered from surviving colonies and transformed back into ABY2001 to confirm their ability to suppress. Plasmids that continued to rescue the galactose-induced lethality were then sequenced.
Immunoblot with Bnr1p Antibodies
Rabbit polyclonal antiserum was raised against recombinant GST-Bnr1p FH1-FH2-COOH (residues 757-1375) purified from bacteria as described (Pruyne et al., 2004a). Antibodies used in this study were affinity-purified first on excess glutathione S-transferase (GST) and then on GST- Bnr1p FH1-FH2-COOH. Standard Western blotting was performed using Bnr1p FH1-FH2-COOH antibody at 1:50.
Light Microscopy
Immunofluorescence microscopy with rabbit antibodies to yeast actin, Tpm1p, Myo2p, and mouse antibodies to the HA epitope was performed as described (Pruyne et al., 1998; Evangelista et al., 2002). Pictures were acquired with a Nikon Eclipse TE-2000U microscope (Melville, NY) on a confocal imaging system (UltraView LCI, PerkinElmer, Norwalk, CT) using a Nikon 100× 1.4 NA lens and digital camera (C4742–95-12ERG; Hamamatsu, Bridgewater, NJ).
To count the number of actin patches and measure the relative intensity of individual patches, pictures of Z-series were imported into ImageJ (NIH; http://rsb.info.nih.gov/ij/) and projected onto one plane. Relative intensity was calculated by selecting the area of a patch and then dividing the average intensity by the area.
Latrunculin A Halo Assay
Y1240 and ABY2001 carrying pRS316 were grown to midlog phase (OD600 = 0.5). 2-ml cell cultures were mixed with 2 ml 50°C 1% agar and poured onto SGal-Ura plates. Filter paper soaked with 10 μl 10 μM latrunculin A (LatA) in DMSO or 10 μl DMSO were placed onto the cell-agar surface. Plates were then incubated at 26°C.
RESULTS
Bnr1p Nucleates Actin Cable Assembly In Vivo
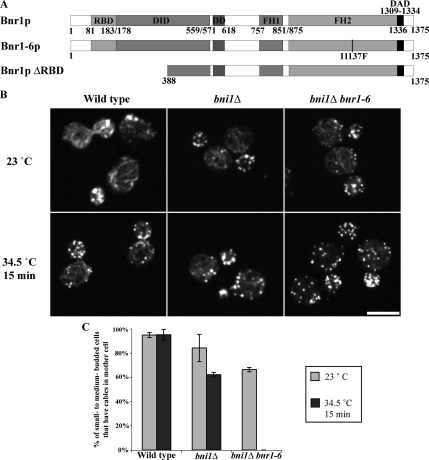
Yeast has two formins, Bni1p and Bnr1p. Bni1p is localized to sites of polarized growth, that is, the presumptive budding site in unbudded cells, the bud tip in small- to medium-budded cells, the bud cortex in large-budded cells, and the bud neck in dividing cells (Fujiwara et al., 1998; Ozaki-Kuroda et al., 2001; Pruyne et al., 2004a; Buttery et al., 2007). Consistent with its known nucleation activity, overexpression of Bni1p induces the accumulation of cable-like filaments in the bud (Evangelista et al., 2002; Sagot et al., 2002a). Bnr1p is localized to the bud neck after bud emergence and remains there during the rest of the cell cycle (Kikyo et al., 1999; Pruyne et al., 2004a; Buttery et al., 2007). We have previously shown that purified recombinant Bnr1p FH1-FH2 domains nucleate actin filaments in vitro, and overexpression of Bnr1p lacking the RBD results in the accumulation of excessive actin filaments in vivo (Pruyne et al., 2004a). To further confirm its biological function, we generated bnr1 temperature-sensitive alleles in a bni1Δ background. Six mutants were recovered, and all had mutations in the Bnr1p FH2 domain that result in loss of actin cables at the restrictive temperature. One of these alleles, bnr1-6, is caused by an I1137F mutation in the FH2 domain (Figure 1A). Actin antibody staining showed that bnr1-6 bni1Δ cells have wild-type actin cables coming from the bud neck when grown at room temperature. On temperature shift, these cells lost actin cables in 15 min (Figure 1, B and C), confirming Bnr1p's role as a nucleator for actin cables assembled from the bud neck.
Figure 1.
Loss of Bnr1p function in bni1Δ cells causes loss of actin cables. (A) Bnr1p constructs used in this study: RBD, DID, dimerization domain (DD), FH1 and FH2, and DAD. (B) Localization of actin at room temperature (23°C) and after shifting to 34.5°C for 15 min in wild-type (Y1239), bni1Δ(ABY1802), and bni1Δbnr1-6 (ABY2007) cells. Bar, 5 μm. (C) Percentage of small- to medium-budded cells with actin cables in the mother cell at 23 and 34.5°C.
Overexpression of Unregulated Bnr1p Is Lethal
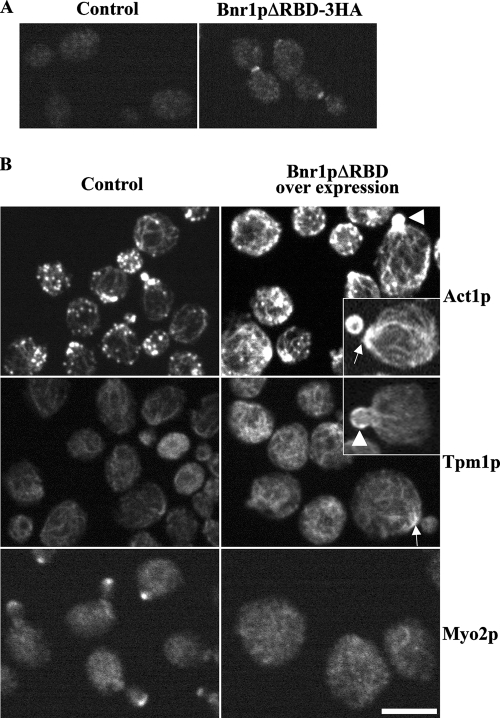
It has been reported that overexpression of the C-terminal half of Bnr1p causes growth inhibition, abnormal cell morphology, and actin patch delocalization (Kikyo et al., 1999). We explored in greater detail how overexpression of unregulated Bnr1p affects cytoskeletal organization, cell morphology, and growth. Like its mammalian homologues, Bnr1p is believed to be autoregulated through interaction between the DID and DAD, and we have shown previously that induction of Bnr1p lacking the RBD (Bnr1pΔRBD, Figure 1A) from the GAL1-10 promoter results in overproduction of actin filaments in the cell (Pruyne et al., 2004a). On further examination, we found that yeast bearing the Gal-inducible Bnr1pΔRBD grew on glucose-containing media (repressive conditions) but failed to grow on galactose-containing media, confirming that overexpression of unregulated Bnr1p is lethal (Figure S1A). This is likely due to the constitutive activity of Bnr1pΔRBD, because overexpression of full-length Bnr1p only caused a slight growth defect (Figure S1A).
To investigate the cause of lethality conferred by overexpression of Bnr1pΔRBD, cells were grown overnight in galactose and examined for cell morphology and the organization of the actin cytoskeleton. Overexpressed HA-tagged Bnr1pΔRBD localized to the bud neck (Figure 2A), just like endogenous full-length Bnr1p (Kikyo et al., 1999; Pruyne et al., 2004a). Most cells were unbudded, consistent with the lethality of Bnr1pΔRBD upon overexpression. In a few budded cells, the mother cell was enlarged and round (Figure 2B), indicating that the normal polarized secretion to the bud was compromised. The cells accumulated actin filaments in the mother cell around the neck (indicated by white arrow) and in the bud (indicated by white arrowhead) that also contained the cable-specific protein, tropomyosin (Figure 2B). In general, cells overexpressing Bnr1pΔRBD tend to have fewer patches (see Figure 4C) and more cables than control cells. However, these cables are not long and polarized as in wild-type cells, but are usually short and randomly oriented, as most clearly seen by tropomyosin staining (Figure 2B). Again, overexpression of full-length Bnr1p does not induce this phenotype (Figure S1, B and C). This suggests that unregulated Bnr1p induces excessive nonfunctional filaments at the bud and bud neck, which may block secretion to the bud. Myo2p is normally enriched at sites of polarized growth after transporting post-Golgi secretory vesicles along polarized cables, so its localization provides a convenient marker for assessing the sites of polarized growth (Pruyne et al., 1998; Schott et al., 1999). In cells overexpressing Bnr1pΔRBD, Myo2p was not polarized in the majority of cells (Figures 2B and 4B), consistent with the suggestion that growth is not polarized and explaining why the cells become large and round. Thus, the overexpression of unregulated Bnr1p appears to be toxic due to the excessive accumulation of cable-like filaments that somehow disrupt polarized secretion to the bud.
Figure 2.
Overexpression of unregulated Bnr1p induces accumulation of disorganized cable-like filaments and delocalized Myo2p. (A) Localization of HA-Bnr1pΔRBD, as visualized by immunofluorescence with antibody to the HA-tag, after 2-h galactose induction. (B) Immunolocalization of actin (Act1p), tropomyosin (Tpm1p), and Myo2p in wild-type cells containing a control plasmid or overexpressing Bnr1pΔRBD after 10-h galactose induction. White arrows, excess filaments at the bud neck; arrowheads, excess filaments in the bud. Bar, 5 μm.
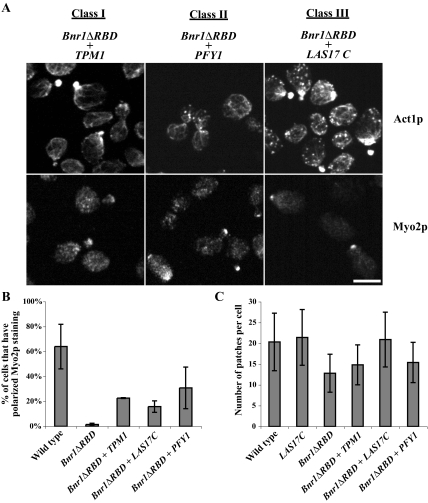
Figure 4.
Suppression of Bnr1pΔRBD overexpression phenotype by three classes of suppressors. (A) Immunolocalization of actin (Act1p) and Myo2p in cells overexpressing Bnr1pΔRBD and one representative of each class of suppressors. Exponentially growing cells were fixed and processed for immunofluorescence microscopy as indicated. Bar, 5 μm. (B) Percentage of cells that had polarized Myo2p staining (bud tip in small- to medium-budded cells and bud neck in large-budded cells) in wild-type cells and strains overexpressing the denoted genes. (C) The number of actin patches in wild-type cells and strains overexpressing the denoted genes.
Identification of Overexpression Suppressors of Bnr1pΔRBD Overexpression Lethality
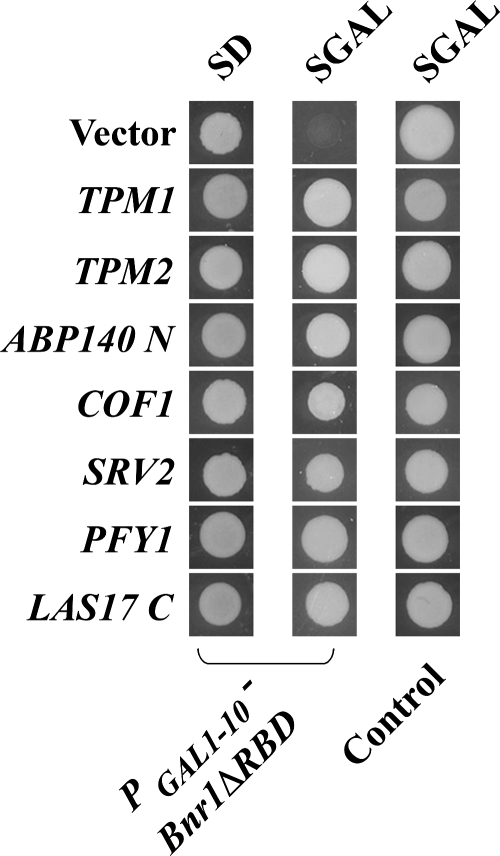
In a previous study, Evangelista et al. (1997) used a GAL1-10 cDNA library to identify genes whose overexpression could suppress the lethality conferred by overexpression of the FH1FH2 domains of Bni1p. The three strongest suppressors identified encoded profilin (PFY1) and tropomyosins (TPM1, TPM2). To identify factors that might modulate Bnr1p-dependent filament assembly, the GAL1-10-cDNA library was similarly screened for genes whose overexpression could suppress the lethality caused by Bnr1pΔRBD overexpression. The screen yielded 49 suppressors, and the suppressing cDNAs were found to be derived from 20 different genes (Table 3). A larger spectrum of suppressors, including PFY1, TPM1, and TPM2, were recovered and could be placed into four classes based on the functional properties of their protein products: 1) proteins that bind F-actin and normally organize the structure of actin cables, 2) proteins that bind monomeric actin and regulate F-actin turnover, 3) a major activator of the Arp2/3 complex, 4) proteins unrelated to the actin cytoskeleton, including chaperones, α -tubulin, proteins involved in galactose metabolism, and proteins involved in protein synthesis and modification machinery. In this study, we focus on the first three classes encoding genes whose products participate in actin organization and regulation. The ability of these genes to suppress the lethality conferred by Bnr1pΔRBD is shown in Figure 3.
Table 3.
Identity of genes isolated as overexpression suppressors of Bnr1pΔRBD overexpression lethality
| Gene name | Times isolated | |
|---|---|---|
| Class I | TPM1 | 8 |
| TPM2 | 2 | |
| ABP140 N | 1 | |
| Class II | PFY1 | 2 |
| COF1 | 1 | |
| SRV2 | 3 | |
| Class III | LAS17 C | 1 |
| Class IV | SSE1 | 2 |
| TUB1 | 1 | |
| PGM1 | 1 | |
| PGM2 | 8 | |
| GAL11 | 1 | |
| SEC53 | 9 | |
| GBP2, PAB1 | 2 each | |
| RPS2, MSN2, SNF5, RPG1, STP4 | 1 each |
Figure 3.
Suppression of Bnr1pΔRBD overexpression lethality by the identified genes. Bnr1pΔRBD overexpression cells (ABY2001) or wild-type cells (Y1240) containing a control vector (pRS316) or the identified cDNAs after growing for 2 d on glucose-containing media (SD) or 4 d on galactose media (SGAL).
Class I Suppressors: Actin Cable Components
The first class of suppressors included TPM1, TPM2, and the N-terminal-coding region (residues 1–218) of ABP140 (ABP140N). TPM1 and TPM2 encode tropomyosin isoforms, which bind selectively to the F-actin of cables to stabilize them (Liu and Bretscher, 1989a,b; Drees et al., 1995; Pruyne et al., 1998). Abp140p is an actin-bundling protein found associated with both actin patches and cables (Asakura et al., 1998). Localization of actin in the suppressed strains overexpressing both Bnr1pΔRBD and Tpm1p showed they have a rather normal actin cytoskeleton, but with fewer actin patches (Figure 4, A and C). When compared with cells just overproducing Bnr1pΔRBD alone, they have less short and randomly oriented cables. Moreover, localization of Myo2p was partially restored to sites of cell growth (Figure 4, A and B), consistent with the ability of the suppressed strains to grow. As Tpm1p, Tpm2p, and Abp140p all function to organize actin cables, their capacity to suppress the lethality of Bnr1pΔRBD overexpression is presumably due to their ability to turn disorganized actin filaments into functional cables. Another possibility is that excessive short filaments nucleated by overexpressed Bnr1p ΔRBD makes tropomyosin the limiting factor, which is suppressed by overexpression of tropomyosin.
Class II Suppressors: G-Actin–binding Proteins
The second group that can suppress Bnr1pΔRBD-induced lethality included COF1, SRV2, and PFY1. The protein products of these three genes have been well characterized for their role in regulating the actin cytoskeleton (Moseley and Goode, 2006). Cells overexpressing class II suppressors and Bnr1pΔRBD had relatively normal actin cables and a nearly normal number of actin patches (Figure 4, A and C). Class II suppressors seem to limit the actin used by the activated formin, resulting in a restoration of the number of patches and a normal distribution of cables. As expected, Myo2p localization was restored to the bud tip (Figure 4, A and B), thereby permitting polarized growth.
Cof1p (cofilin) binds to ADP-containing actin filaments and severs them (Lappalainen and Drubin, 1997; Okreglak and Drubin, 2007). It seems unlikely that this function of Cof1p suppresses Bnr1p ΔRBD overexpression lethality as more nonfunctional short filaments should be produced. Because the ability of Cof1p to disassemble actin filaments is tightly coupled to its cofactor Aip1p (Balcer et al., 2003; Okada et al., 2006), we tested if overexpression of Cof1p could still suppress in aip1Δ background. We found that it suppressed even better when Aip1p is absent (Figure 5). An additional property of cofilin is its ability to bind monomeric actin (Hayden et al., 1993). Overexpression of cofilin may therefore increase the level of cofilin–actin and thereby reduce the level of actin available to Bnr1pΔRBD to assemble filaments.
Figure 5.
Suppression of Bnr1pΔRBD overexpression lethality by COF1 is not dependent on Aip1p. Top two rows, a control plasmid or PGAL1-10 -COF1 expressed in PGAL1-10 -Bnr1ΔRBD cells (ABY2001). Bottom row, expression of PGAL1-10 -COF1 in PGAL1-10 -Bnr1ΔRBD, aip1Δ cells (ABY2061). Cells were grown for 2 d on glucose-containing media (SD) or 4 d on galactose (SGAL).
Srv2p is a multifunction protein. Its N terminal domain functions as a CAP (adenylyl cyclase associated protein), and its C-terminal domain as a regulator of the actin cytoskeleton. It has been shown Srv2p performs its second function by binding monomeric actin (Freeman et al., 1995). Thus, Srv2p may also suppress by reducing available G-actin. In addition, Srv2p regulates actin filament turnover. During actin filament disassembly, cofilin complexes with ADP-actin, and this inhibits ADP–ATP exchange. Srv2p enhances ADP–ATP exchange on actin by handing off ADP-actin from cofilin to profilin (discussed below; Balcer et al., 2003). Therefore, suppression of Bnr1pΔRBD overexpression lethality by Srv2p overexpression might not only be due to Srv2p's G-actin–binding activity, but also its ability to promote rapid actin turnover.
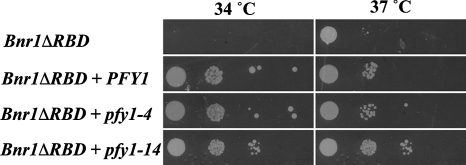
Pfy1p (profilin) binds monomeric actin and stimulates ADP–ATP exchange. In this sense, it would be expected to promote filament assembly because only ATP-actin is used for assembly in vivo. Profilin also has the ability to bind to the proline-rich sequences in the FH1 domains of formins, including Bnr1p (Wasserman, 1998). By recruiting profilin–actin complexes to the FH1 domain it accelerates the elongation of actin filaments nucleated by formins (Kovar et al., 2006). How might profilin overexpression suppress Bnr1pΔRBD overexpression lethality? There are two plausible hypotheses. First, although profilin–actin is the favored substrate over free actin for filament elongation, it might be a poorer substrate for nucleation than actin alone. Thus, when profilin is over produced, elongation is favored over nucleation, resulting in cells with long functional filaments, rather than less functional short ones. A second possibility is that when profilin is over produced, an excess of profilin over G-actin is present, so that free profilin might compete with profilin–actin for the FH1 on the formins and thereby reduce F-actin assembly. To distinguish between these two possibilities, we used two temperature-sensitive PFY1 alleles, pfy1-4 that is conditionally defective in actin binding and pfy1-14 that is conditionally defective in poly-proline binding (Wolven et al., 2000). Overexpression of both pfy1-4 and pfy1-14 were able to suppress the reduced growth conferred by Bnr1pΔRBD overexpression at 37°C (restrictive temperature; Figure 6). Because both alleles are expected to compromise the ability of FH1 to feed profilin–actin complexes to the FH2 domain (Pfy1–4p by binding FH1 without actin and Pfy1–14p by competing with endogeneous profilin for limiting actin and its recruitment to the FH1 domain), this supports the idea that PFY1 overexpression compromises the ability of Bnr1ΔRBD to nucleate filaments.
Figure 6.
Suppression of the Bnr1p ΔRBD overexpression lethality by pfy1-4 and pfy1-14. Dilution series of the Bnr1pΔRBD-overexpressing yeast with PGAL1-10-driven PFY1, pfy1-4, or pfy1-14 plasmid on SGAL plates at permissive (34°C) and restrictive (37°C) temperatures.
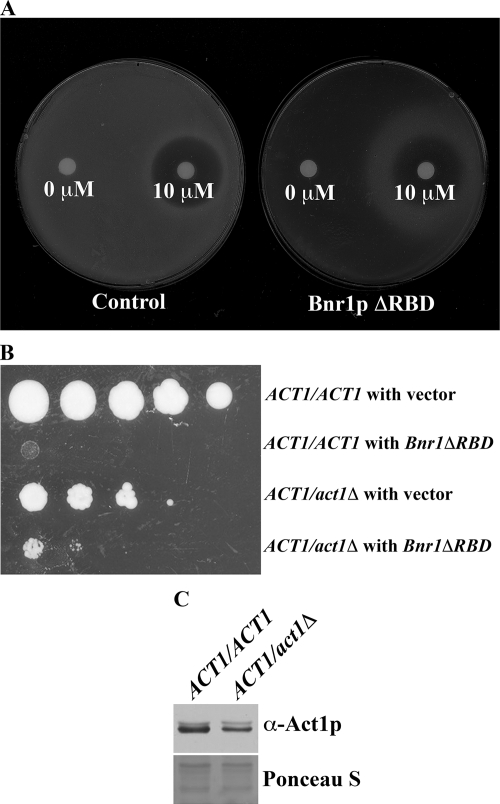
Analysis of the preceding suppressors suggests that they diminish the ability of the overexpressed Bnr1pΔRBD to assemble actin filaments by reducing accessibility of the formin to actin for filament nucleation or elongation. Another possible explanation, which is not necessarily incompatible with the former, is that they suppress the lethality by promoting rapid actin turnover. Ideally this could be tested with mutants that are defective in G-actin binding, but with normal actin turnover–promoting activity. However, such mutants do not exist, because a mutant that is defective in G-actin binding will have weakened ability in actin turnover. To test the former possibility that reducing available G-actin is sufficient to suppress Bnr1pΔRBD overexpression lethality, we examined the effect of reducing the availability of G-actin pharmacologically and genetically. LatA is an actin monomer sequestering drug, which at saturating levels can cause the loss of all filamentous actin structures from the cell (Ayscough et al., 1997; Karpova et al., 1998). We tested whether intermediate levels of LatA could suppress the overexpression lethality of Bnr1pΔRBD. A gradient of LatA was formed by placing a LatA-soaked filter on a lawn of GAL-driven Bnr1pΔRBD cells plated on galactose media. The highest levels of LatA killed both wild-type and Bnr1pΔRBD-expressing cells, and the Bnr1pΔRBD-expressing cells died in the absence of LatA, but intermediate levels of the drug rescued growth of the Bnr1pΔRBD cells (Figure 7A).
Figure 7.
Reducing the available G-actin suppresses the overexpression lethality of Bnr1pΔRBD. (A) LatA suppressed Bnr1pΔRBD-induced lethality. Wild-type cells (left) and cells overexpressing Bnr1pΔRBD were plated on galactose-containing media. Filters soaked with 10 μl DMSO (0 μM) or 10 μM LatA in DMSO (10 μM) were placed onto the plates and then incubated for 6 d at 26°C. (B) Deletion of one copy of ACT1 in diploid cells partially suppressed Bnr1pΔRBD-induced lethality. An equal density of the indicated strains were diluted and spotted on SGal plates. (C) Level of Act1p in ACT1/ACT1 and ACT1/act1Δ as detected by immunoblot.
To reduce the level of actin genetically, we assessed whether diploid yeast heterozygous for the loss of the actin gene (ACT1/act1Δ) could tolerate Bnr1pΔRBD overexpression. Compared with ACT1/ACT1 cells, the heterozygous ACT1/act1Δ cells grew poorly, presumably because of the lowered level of Act1p (Figure 7C), but with the expression of Bnr1pΔRBD, the ACT1/ACT1 cells died, whereas the heterozygotes were able to grow weakly (Figure 7B). Thus, genetic and pharmacological manipulations confirm that the overall reduction of actin available for assembly is able to remediate the lethal effects of overexpression of deregulated Bnr1p.
Class III Suppressors: Las17p, Promoting Activation of Arp2/3-mediated Actin Assembly
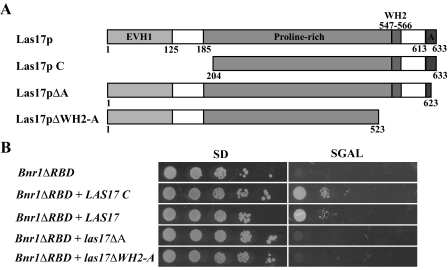
A clone of the C-terminal region (encoding residues 204–633) of LAS17 (LAS17C) comprises the third group of suppressors of Bnr1pΔRBD-induced lethality (Figure 8). Similarly, overexpression of full-length LAS17 also suppresses Bnr1pΔRBD overexpression lethality (Figure 8). As a homolog of the WASp family proteins, Las17p is a modular protein with an actin monomer–binding WH2 domain, followed by an acidic domain (A domain), which together function as a major activator of the Arp2/3 complex (Winter et al., 1999a; Rodal et al., 2003; Sun et al., 2006). Two possible explanations for suppression by Las17p are that it functions either by reducing available G-actin or by stimulating the Arp2/3 to be more active in patch formation and thereby indirectly depleting the G-actin available to the formins. To distinguish between these possibilities, we overexpressed Las17p lacking the A domain, yet retaining the WH2 domain (Las17pΔA), or Las17p lacking both the WH2 domain and the A domain (Las17pΔWH2-A). Neither could suppress the lethality (Figure 8), suggesting that increased activation of the Arp2/3 complex is necessary to suppress the lethality conferred by Bnr1pΔRBD overexpression. Consistent with this, Bnr1pΔRBD-overexpressing cells rescued by Las17pC have more actin patches compared with the first two classes of suppressors (Figure 4C).
Figure 8.
Suppression of Bnr1pΔRBD overexpression lethality by Las17p requires the A domain. (A) Schematic representation of the LAS17 constructs used. EVH1, Ena/VASP homology 1; WH2, WASp homology 2; A, acidic domain. (B) Dilution series of Bnr1pΔRBD overexpressing yeast with PGAL1-10 -LAS17, LAS17 C, las17ΔA, or las17ΔWH2-A plasmid on glucose- (SC) and galactose-containing (SGAL) plates.
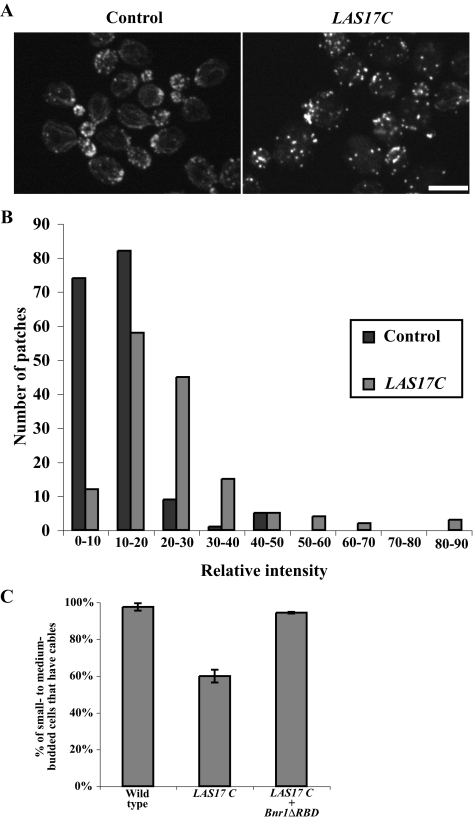
These results suggest that increased Arp2/3-dependent filament assembly can shift actin assembly away from formin-dependent assembly. In yeast, the products of Arp2/3 stimulated filaments form the cortical patches, whereas the formin-stimulated filaments assemble into actin cables (Evangelista et al., 2002; Pruyne et al., 2002, 2004a; Sagot et al., 2002a,b). We assayed if the overactivation of the Arp2/3-complex by Las17p in wild-type yeast affects actin organization. We found that overexpression of Las17p C did not increase the number of actin patches in a cell (Figure 4C), but resulted in increased intensity of actin patches (Figure 9, A and B). We also found that the overexpression of Las17p C depleted actin cables in ∼40% of small- to medium-budded cells (Figure 9, A and C). Further, overexpression of Las17p C also lowers the restrictive temperature of yeast with defects in actin cable stability (tpm1Δ and tpm1-2 tpm2Δ) or actin cable assembly (bni1-11 bnr1Δ and bnr1-6 bni1Δ; Table 4). Thus, the overproduction of actin patches occurs to the detriment of cable assembly.
Figure 9.
Overexpression of LAS17C enhances Arp2/3-dependent F-actin in wild-type cells. (A) Localization of actin in cells after 10-h galactose induction containing either a control plasmid or overexpressing LAS17C. Bar, 5 μm. (B) Comparison of actin patch intensity in cells containing either a control plasmid or overexpressing LAS17C. (C) Percentage of small- to medium-budded cells that have actin cables in control cells or cells overexpressing LAS17C or LAS17C and Bnr1ΔRBD.
Table 4.
Overexpression of LAS17C lowers the restrictive temperature of mutants with conditional actin cable defects
| ABY204 (tpm1Δ) |
ABY1807 (tpm1-2 tpm2Δ) |
ABY2000 (bni1-11 bnr1Δ) |
ABY2007 (bni1Δbnr1-6) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 23°C | 32°C | 34°C | 23°C | 32°C | 34°C | 23°C | 32°C | 23°C | 36.5°C | |
| Vector | ++ | ++ | ++ | ++ | + | − | ++ | −/+ | ++ | ++ |
| LAS17C | + | −/+ | − | ++ | − | − | ++ | − | + | −/+ |
The LAS17C overexpression plasmid was introduced into the indicated strains which were then streaked on galactose-containing media and incubated at the indicated temperatures. ++, wild-type growth, −, no observable growth, +, moderate growth, −/+, poor growth.
More interestingly, the reduction in cables in Las17 C–overexpressing cells could be rescued by overexpression of Bnr1pΔRBD (Figure 9C). Taken together, our data suggest that yeast needs to balance actin between actin patches and actin cables, which is likely achieved at the nucleation level of both pathways.
DISCUSSION
Formins are critical cytoskeletal-regulatory proteins, promoting the proper assembly and organization of subsets of actin filaments (Evangelista et al., 2002; Sagot et al., 2002a). In this article, we show that similar to the Bni1p formin, overexpression of the Bnr1p formin lacking its regulatory Rho-binding domain is lethal. Yeast overexpressing this construct showed a massive accumulation of cable-like filaments around the neck and in the bud and a defect in the myosin-dependent polarization of secretion that normally occurs along cables. Identification of overexpression suppressors of this lethality confirmed the idea that it is this aberrant actin assembly that is the cause of the lethality.
Suppressors isolated in this screen encode proteins involved in several aspects of actin filament dynamics and architecture. Interestingly, all the recognizable cytoskeletal factors seemed to function by modulating the levels of constituent components of the actin cables. Overexpression of additional cable components, such as tropomyosin or Abp140p, rescued lethality caused by excess formin activity, as could suppressors that act to reduce the actin pool available to the formin. Reducing the G-actin pool by treatment with LatA or deletion of one copy of ACT1 in diploid cells similarly suppressed the unregulated Bnr1p overexpression lethality. Identification of cofilin, Srv2p, and profilin as suppressors also suggests the possibility that more rapid actin turnover plays a role in moderating the lethal effects of Bnr1pΔRBD overexpression.
A particularly interesting finding was that mis-incorporation of actin filaments into cables could be suppressed by the enhanced assembly of actin into cortical patches by overproduction of the Arp2/3 activator Las17p. Analysis of Las17p truncations showed this activity depended on the A domain required for Arp2/3 activation (Winter et al., 1999a). Las17p represents only one of five patch-associated Arp2/3 activators of budding yeast, making the absence of the other activators curious. However, among the Arp2/3 activators of yeast, Abp1p and Pan1p exhibit relatively weak activity and so might have been insufficient to significantly shift the actin pool toward patch assembly (Duncan et al., 2001; Goode et al., 2001; Moseley and Goode, 2006). The myosin-I homologues Myo3p and Myo5p exhibit strong activity, but only in combination with the WIP homolog, Vrp1p, and so expression of either of these alone would have been unlikely to increase patch-associated filament assembly (Evangelista et al., 2000). However, Las17p is a potent Arp2/3 activator that requires no cofactor (Rodal et al., 2003; Moseley and Goode, 2006; Sun et al., 2006), making its recovery by this screen feasible. In fact, we confirmed that overexpression of Pan1p and Myo3p did not suppress Bnr1pΔRBD overexpression lethality (unpublished data).
Together, these results suggests that the detrimental effects of formin overactivity do not result simply in excess filament assembly, but from assembly of filaments that are of improper composition, having an excess of actin versus other components such as tropomyosin. Reduction of actin by various means or increase of other cable components was able to relieve the lethality. Absent from the list were factors such as kinases or phosphatases, which might be expected to regulate incorporation of available proteins into the cables, suggesting that regulation of the assembly of cable components is regulated largely by the level of formin activity and the size of the pool of cable constituents present in the cell, as well as the level of competing Arp2/3-driven actin assembly into cortical patches.
It has long been known that actin patches and actin cables are the two major actin containing organizations in yeast. Here we demonstrated for the first time that cells need to balance actin between these two structures. Because it is not easy to measure how actin is distributed, we can only assay if changes in one organization affects the other. Here we showed that overexpression of Bnr1pΔRBD stimulates cable assembly and reduces the number of actin patches in a cell. On the other hand, overexpression of Las17p C increase the intensity of actin patches and reduces actin cables. More interestingly, when Bnr1pΔRBD and Las17p C are co-overexpressed, a balance is reconstituted. Our data also strongly suggested that such a balance is achieved through the nucleation of both pathways. It will be interesting to discover how the activity levels of the formins and patch-associated components are normally regulated to achieve the optimal balance in actin assembly between the various essential actin cytoskeletal structures that coexist in the yeast cell.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. David Pruyne for many fruitful discussions and help with the manuscript. We are indebted to Dr. Bruce Goode, who suggested the experiments involving LAS17 and kindly provided several of the reagents used in this study. This work was supported by Public Health Service, National Institute of General Medical Sciences Grant GM39066.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0520) on January 30, 2008.
REFERENCES
- Adams A. E., Pringle J. R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts A. S. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J. Biol. Chem. 2001;276:2824–2830. doi: 10.1074/jbc.M006205200. [DOI] [PubMed] [Google Scholar]
- Asakura T., Sasaki T., Nagano F., Satoh A., Obaishi H., Nishioka H., Imamura H., Hotta K., Tanaka K., Nakanishi H., Takai Y. Isolation and characterization of a novel actin filament-binding protein from Saccharomyces cerevisiae. Oncogene. 1998;16:121–130. doi: 10.1038/sj.onc.1201487. [DOI] [PubMed] [Google Scholar]
- Ayscough K. R., Stryker J., Pokala N., Sanders M., Crews P., Drubin D. G. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcer H. I., Goodman A. L., Rodal A. A., Smith E., Kugler J., Heuser J. E., Goode B. L. Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1. Curr. Biol. 2003;13:2159–2169. doi: 10.1016/j.cub.2003.11.051. [DOI] [PubMed] [Google Scholar]
- Beach D. L., Thibodeaux J., Maddox P., Yeh E., Bloom K. The role of the proteins Kar9 and Myo2 in orienting the mitotic spindle of budding yeast. Curr. Biol. 2000;10:1497–1506. doi: 10.1016/s0960-9822(00)00837-x. [DOI] [PubMed] [Google Scholar]
- Buttery S. M., Yoshida S., Pellman D. Yeast formins Bni1 and Bnr1 utilize different modes of cortical interaction during the assembly of actin cables. Mol. Biol. Cell. 2007;18:1826–1838. doi: 10.1091/mbc.E06-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Pruyne D., Bretscher A. Formin-dependent actin assembly is regulated by distinct modes of Rho signaling in yeast. J. Cell Biol. 2003;161:1081–1092. doi: 10.1083/jcb.200212040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees B., Brown C., Barrell B. G., Bretscher A. Tropomyosin is essential in yeast, yet the TPM1 and TPM2 products perform distinct functions. J. Cell Biol. 1995;128:383–392. doi: 10.1083/jcb.128.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Pypaert M., Novick P., Ferro-Novick S. Aux1p/Swa2p is required for cortical endoplasmic reticulum inheritance in Saccharomyces cerevisiae. Mol. Biol. Cell. 2001;12:2614–2628. doi: 10.1091/mbc.12.9.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. C., Cope M. J., Goode B. L., Wendland B., Drubin D. G. Yeast Eps15-like endocytic protein, Pan1p, activates the Arp2/3 complex. Nat. Cell Biol. 2001;3:687–690. doi: 10.1038/35083087. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein A. E., Drubin D. G. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- Estrada P., Kim J., Coleman J., Walker L., Dunn B., Takizawa P., Novick P., Ferro-Novick S. Myo4p and She3p are required for cortical ER inheritance in Saccharomyces cerevisiae. J. Cell Biol. 2003;163:1255–1266. doi: 10.1083/jcb.200304030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista M., Blundell K., Longtine M. S., Chow C. J., Adames N., Pringle J. R., Peter M., Boone C. Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- Evangelista M., Klebl B. M., Tong A. H., Webb B. A., Leeuw T., Leberer E., Whiteway M., Thomas D. Y., Boone C. A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex. J. Cell Biol. 2000;148:353–362. doi: 10.1083/jcb.148.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista M., Pruyne D., Amberg D. C., Boone C., Bretscher A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 2002;4:32–41. doi: 10.1038/ncb718. [DOI] [PubMed] [Google Scholar]
- Fagarasanu M., Fagarasanu A., Rachubinski R. A. Sharing the wealth: peroxisome inheritance in budding yeast. Biochim. Biophys. Acta. 2006;1763:1669–1677. doi: 10.1016/j.bbamcr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher K. L., Davis D., Wu M., Boldogh I., Pon L. A. Endoplasmic reticulum dynamics, inheritance, and cytoskeletal interactions in budding yeast. Mol. Biol. Cell. 2002;13:854–865. doi: 10.1091/mbc.01-04-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman N. L., Chen Z., Horenstein J., Weber A., Field J. An actin monomer binding activity localizes to the carboxyl-terminal half of the Saccharomyces cerevisiae cyclase-associated protein. J. Biol. Chem. 1995;270:5680–5685. doi: 10.1074/jbc.270.10.5680. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Tanaka K., Mino A., Kikyo M., Takahashi K., Shimizu K., Takai Y. Rho1p-Bni1p-Spa2p interactions: implication in localization of Bni1p at the bud site and regulation of the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell. 1998;9:1221–1233. doi: 10.1091/mbc.9.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode B. L., Rodal A. A., Barnes G., Drubin D. G. Activation of the arp2/3 complex by the actin filament binding protein abp1p. J. Cell Biol. 2001;153:627–634. doi: 10.1083/jcb.153.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan B., Bowser R., Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J. Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldener U., Heck S., Fielder T., Beinhauer J., Hegemann J. H. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden S. M., Miller P. S., Brauweiler A., Bamburg J. R. Analysis of the interactions of actin depolymerizing factor with G- and F-actin. Biochemistry. 1993;32:9994–10004. doi: 10.1021/bi00089a015. [DOI] [PubMed] [Google Scholar]
- Hoepfner D., van den Berg M., Philippsen P., Tabak H. F., Hettema E. H. A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J. Cell Biol. 2001;155:979–990. doi: 10.1083/jcb.200107028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang E., Kusch J., Barral Y., Huffaker T. C. Spindle orientation in Saccharomyces cerevisiae depends on the transport of microtubule ends along polarized actin cables. J. Cell Biol. 2003;161:483–488. doi: 10.1083/jcb.200302030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova T. S., McNally J. G., Moltz S. L., Cooper J. A. Assembly and function of the actin cytoskeleton of yeast: relationships between cables and patches. J. Cell Biol. 1998;142:1501–1517. doi: 10.1083/jcb.142.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikyo M., Tanaka K., Kamei T., Ozaki K., Fujiwara T., Inoue E., Takita Y., Ohya Y., Takai Y. An FH domain-containing Bnr1p is a multifunctional protein interacting with a variety of cytoskeletal proteins in Saccharomyces cerevisiae. Oncogene. 1999;18:7046–7054. doi: 10.1038/sj.onc.1203184. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Adams A. E. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J. Cell Biol. 1984;98:922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar D. R., Harris E. S., Mahaffy R., Higgs H. N., Pollard T. D. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124:423–435. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Lappalainen P., Drubin D. G. Cofilin promotes rapid actin filament turnover in vivo. Nature. 1997;388:78–82. doi: 10.1038/40418. [DOI] [PubMed] [Google Scholar]
- Liu H., Krizek J., Bretscher A. Construction of a GAL1–regulated yeast cDNA expression library and its application to the identification of genes whose overexpression causes lethality in yeast. Genetics. 1992;132:665–673. doi: 10.1093/genetics/132.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. P., Bretscher A. Disruption of the single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell. 1989a;57:233–242. doi: 10.1016/0092-8674(89)90961-6. [DOI] [PubMed] [Google Scholar]
- Liu H. P., Bretscher A. Purification of tropomyosin from Saccharomyces cerevisiae and identification of related proteins in Schizosaccharomyces and Physarum. Proc. Natl. Acad. Sci. USA. 1989b;86:90–93. doi: 10.1073/pnas.86.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley J. B., Goode B. L. The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol. Mol. Biol. Rev. 2006;70:605–645. doi: 10.1128/MMBR.00013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley J. B., Sagot I., Manning A. L., Xu Y., Eck M. J., Pellman D., Goode B. L. A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol. Biol. Cell. 2004;15:896–907. doi: 10.1091/mbc.E03-08-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., Ravi H., Smith E. M., Goode B. L. Aip1 and cofilin promote rapid turnover of yeast actin patches and cables: a coordinated mechanism for severing and capping filaments. Mol. Biol. Cell. 2006;17:2855–2868. doi: 10.1091/mbc.E06-02-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okreglak V., Drubin D. G. Cofilin recruitment and function during actin-mediated endocytosis dictated by actin nucleotide state. J. Cell Biol. 2007;178:1251–1264. doi: 10.1083/jcb.200703092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otomo T., Otomo C., Tomchick D. R., Machius M., Rosen M. K. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol. Cell. 2005;18:273–281. doi: 10.1016/j.molcel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Ozaki-Kuroda K., Yamamoto Y., Nohara H., Kinoshita M., Fujiwara T., Irie K., Takai Y. Dynamic localization and function of Bni1p at the sites of directed growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001;21:827–839. doi: 10.1128/MCB.21.3.827-839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D., Evangelista M., Yang C., Bi E., Zigmond S., Bretscher A., Boone C. Role of formins in actin assembly: nucleation and barbed end association. Science. 2002;297:612–615. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- Pruyne D., Gao L., Bi E., Bretscher A. Stable and dynamic axes of polarity use distinct formin isoforms in budding yeast. Mol. Biol. Cell. 2004a;15:4971–4989. doi: 10.1091/mbc.E04-04-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D., Legesse-Miller A., Gao L., Dong Y., Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 2004b;20:559–591. doi: 10.1146/annurev.cellbio.20.010403.103108. [DOI] [PubMed] [Google Scholar]
- Pruyne D. W., Schott D. H., Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 1998;143:1931–1945. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- Rodal A. A., Manning A. L., Goode B. L., Drubin D. G. Negative regulation of yeast WASp by two SH3 domain-containing proteins. Curr. Biol. 2003;13:1000–1008. doi: 10.1016/s0960-9822(03)00383-x. [DOI] [PubMed] [Google Scholar]
- Rose R., Weyand M., Lammers M., Ishizaki T., Ahmadian M. R., Wittinghofer A. Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature. 2005;435:513–518. doi: 10.1038/nature03604. [DOI] [PubMed] [Google Scholar]
- Sagot I., Klee S. K., Pellman D. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 2002a;4:42–50. doi: 10.1038/ncb719. [DOI] [PubMed] [Google Scholar]
- Sagot I., Rodal A. A., Moseley J., Goode B. L., Pellman D. An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 2002b;4:626–631. doi: 10.1038/ncb834. [DOI] [PubMed] [Google Scholar]
- Schmid M., Jaedicke A., Du T. G., Jansen R. P. Coordination of endoplasmic reticulum and mRNA localization to the yeast bud. Curr. Biol. 2006;16:1538–1543. doi: 10.1016/j.cub.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Schott D., Ho J., Pruyne D., Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 1999;147:791–808. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon V. R., Swayne T. C., Pon L. A. Actin-dependent mitochondrial motility in mitotic yeast and cell-free systems: identification of a motor activity on the mitochondrial surface. J. Cell Biol. 1995;130:345–354. doi: 10.1083/jcb.130.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Martin A. C., Drubin D. G. Endocytic internalization in budding yeast requires coordinated actin nucleation and myosin motor activity. Dev. Cell. 2006;11:33–46. doi: 10.1016/j.devcel.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Takizawa P. A., Sil A., Swedlow J. R., Herskowitz I., Vale R. D. Actin-dependent localization of an RNA encoding a cell-fate determinant in yeast. Nature. 1997;389:90–93. doi: 10.1038/38015. [DOI] [PubMed] [Google Scholar]
- Takizawa P. A., Vale R. D. The myosin motor, Myo4p, binds Ash1 mRNA via the adapter protein, She3p. Proc. Natl. Acad. Sci. USA. 2000;97:5273–5278. doi: 10.1073/pnas.080585897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong A. H., et al. A combined experimental and computational strategy to define protein interaction networks for peptide recognition modules. Science. 2002;295:321–324. doi: 10.1126/science.1064987. [DOI] [PubMed] [Google Scholar]
- Vavylonis D., Kovar D. R., O'Shaughnessy B., Pollard T. D. Model of formin-associated actin filament elongation. Mol. Cell. 2006;21:455–466. doi: 10.1016/j.molcel.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S. FH proteins as cytoskeletal organizers. Trends Cell Biol. 1998;8:111–115. doi: 10.1016/s0962-8924(97)01217-8. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Kato T., Fujita A., Ishizaki T., Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1999;1:136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- Weisman L. S. Yeast vacuole inheritance and dynamics. Annu. Rev. Genet. 2003;37:435–460. doi: 10.1146/annurev.genet.37.050203.103207. [DOI] [PubMed] [Google Scholar]
- Winter D., Lechler T., Li R. Activation of the yeast Arp2/3 complex by Bee1p, a WASP-family protein. Curr. Biol. 1999a;9:501–504. doi: 10.1016/s0960-9822(99)80218-8. [DOI] [PubMed] [Google Scholar]
- Winter D. C., Choe E. Y., Li R. Genetic dissection of the budding yeast Arp2/3 complex: a comparison of the in vivo and structural roles of individual subunits. Proc. Natl. Acad. Sci. USA. 1999b;96:7288–7293. doi: 10.1073/pnas.96.13.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolven A. K., Belmont L. D., Mahoney N. M., Almo S. C., Drubin D. G. In vivo importance of actin nucleotide exchange catalyzed by profilin. J. Cell Biol. 2000;150:895–904. doi: 10.1083/jcb.150.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Pruyne D., Huffaker T. C., Bretscher A. Myosin V orientates the mitotic spindle in yeast. Nature. 2000;406:1013–1015. doi: 10.1038/35023024. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Evangelista M., Boone C., Yang C., Dar A. C., Sicheri F., Forkey J., Pring M. Formin leaky cap allows elongation in the presence of tight capping proteins. Curr. Biol. 2003;13:1820–1823. doi: 10.1016/j.cub.2003.09.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.