Abstract
There is currently no noninvasive, reliable method of assessing brain tumor malignancy or of monitoring tumor treatment response. Monitoring changes to tumor vasculature might provide an effective means of assessing both tumor aggressiveness and treatment efficacy. To date, most such research has concentrated upon tumor “microvascular” imaging, with permeability and/or perfusion imaging used to assess vessel changes at the subvoxel level. An alternative approach assesses tumor vasculature at the “macroscopic” level, calculating the numbers and shapes of the larger vessels discriminable by magnetic resonance angiography. This paper provides an overview of magnetic resonance (MR) vascular imaging at both the microscopic (dynamic MR perfusion and permeability) and macroscopic (MR angiographic) levels. The two approaches provide different, complementary information and together could provide important insights into cancer growth as well as new methods of assessing malignancy and tumor treatment response.
1 Introduction
There is clinical need for a reliable, noninvasive method of assessing brain tumor malignancy and of evaluating treatment response. The American Cancer Society estimates that in 2006 approximately 19,000 new brain tumors will be diagnosed in the US (ACS 2006). Many thousands of additional patients exhibit abnormalities of unknown significance on screening magnetic resonance (MR) studies. Malignant disease should usually be treated aggressively, whereas benign disease is often treated by watchful waiting. There is currently no effective, clinically accepted, non-invasive method of separating benign from malignant disease.
An even more difficult problem is posed by treatment monitoring. Effective therapy should usually be continued as long as it remains effective, but once it begins to fail it should be changed rapidly. The current standard of practice is to estimate tumor volumes from sequential sets of gadolinium-enhanced, T1 images (Hadley 2005). Such assessments depend upon comparison of current to past tumor volumes, do not directly evaluate what the tumor will do next, and are readily confounded by the necrosis that may appear with successful treatment, since necrosis can induce enhancement and edema indistinguishable from that of tumor growth.
A reliable method of assessing tumor metabolic or physiologic activity noninvasively would be of high value. MR-spectroscopy and Positron Emission Tomography are two imaging methods under investigation by several groups. Another promising approach is to assess tumor-associated vasculature. The large majority of such research has concentrated upon the microvasculature. Recently, several papers have proposed analyzing vessel morphology at the “macroscopic” level, using vessels segmented from magnetic resonance angiograms (MRA). The purpose of this report is to review MR imaging of tumor-associated vasculature at both the microscopic and the macroscopic levels.
2 Background Information
Tumor growth beyond minimal size is dependent upon angiogenesis, the process of new blood vessel formation from existing vessels (Folkman 1971). Vessels are recruited via the expression of agents such as Vascular Endothelial Growth Factor (VEGF) and other growth factors (Ferrara 1996, McDonald 2002). Although malignant tumors tend to be more vascular than benign lesions, some benign tumors may be more vascular than many cancers. Moreover, different regions of the same tumor may exhibit different levels of vascularization.
Cancer-associated changes to vascular morphology occur very early during tumor growth. Within 24 hours of injection of only a few 10s of cancer cells, initially healthy vessels in the tumor vicinity develop tortuosity abnormalities, with such changes emerging prior to angiogenic sprouting and affecting vessels well beyond the tumor margins (Li 2000). Tortuosity abnormalities appear across cancer types, and have been aptly described by Baish as “many smaller bends upon each larger bend” (Baish 2000) [Figure 1]. With time, effects can spread to even major named vessels, with MR-apparent alterations occurring a centimeter or more outside of any T1-gadolinium enhancing lesion [Bullitt 2007].
Figure 1.

Healthy (left) and cancer-associated (right) posterior cerebral artery segmented from time-of-flight, unenhanced brain MRA (MOTSA technique, 5 slabs, TR/TE 35msec/3msec, 0.5 × 0.5 × 0.8 mm3, 352 × 448 × 192 voxels). Note the “many smaller bends upon each larger bend” that characterize cancer-associated vessel morphology. The closest lesion detectable on gadolinium-enhanced MR was over a centimeter away.
The cause of this vessel morphological change is probably cancer-induced alteration of the vessel wall. Such alterations include modification of the basement membrane, endothelial cell fenestrae, reduction of pericytes and smooth muscle, and the formation of blind saccular extensions (McDonald 2002). This vessel wall disruption also often produces a “leaky vessel”, in which intravascular contents leak into interstitial tissues. Successful tumor treatment leads to regression of tumor-associated vascular morphological and permeability alterations (Yuan 1996, Baish 2000, Li 2000, Bullitt 2004 2007, Willett 2004, Jain 2005). The time course is rapid (hours to days) when anti-angiogenic treatment is administered.
3 Microscopic Imaging: Dynamic Perfusion and Permeability
The goals of dynamic MR perfusion and permeability imaging are to assess regional cerebral blood flow, volume, and permeability via sequential image acquisitions during contrast injection. Changes in intensity values are compared over time within a single voxel or cluster of voxels following injection of contrast. The choice of contrast agent may be gadolinium or a larger molecule that leaks into the extravascular space under conditions of high permeability but not otherwise. Figure 2 illustrates a cerebral blood flow map in a patient with a malignant tumor.
Figure 2.
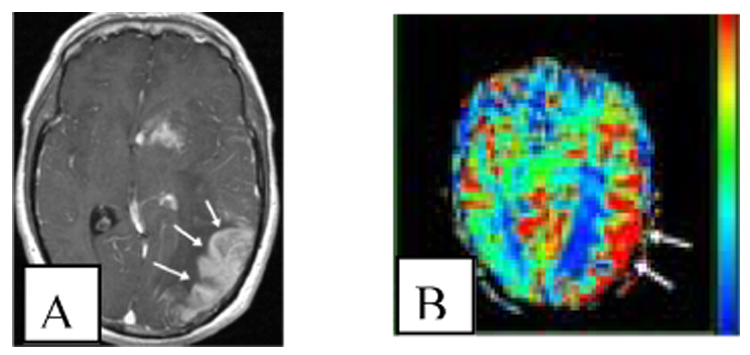
Blood flow increase in high grade glioma. A: The patient has a mass in the left parietal lobe which shows enhancement (arrows) on post contrast T1 weighted image (axial, gadolinium-enhanced, TR/TE 1700/4.38, voxel size 1 × 1 × 1.5 mm3, voxels 256 × 256 × 130). B: The corresponding cerebral blood flow map (T2*-weighted EPI sequence, TR/TE 54/2000) shows a corresponding area of high blood flow (arrows). Red indicates higher blood flow, blue lower. Interpretation may be complicated by normally high blood flow in grey matter and surface vessels.
A generic advantage of perfusion and permeability imaging is that they both incorporate microvascular information. However, a disadvantage is that voxels are large and the data are noisy. Values may vary across healthy subjects and from time to time in an individual patient. Furthermore, seizures alter both vascular permeability and blood flow (Leonhardt 2005). Corticosteroid administration also alters values (Ostergaard 1999). Such effects complicate tumor evaluation.
The assumption when evaluating tumors by MR perfusion/permeability is that at least some regions within higher grade tumors will exhibit higher blood flow and greater permeability than do lower grade tumors. Results obtained from correlating glioma grade with regional blood volume/flow do indeed suggest that higher grade tumors tend to display higher blood flow/volume, but both specificity and sensitivity remain a concern (Aronen 2002, Jackson 2002, Covarrubias 2004). Permeability measurements also suggest that higher grade tumors tend to exhibit higher permeability. However, similar to the results for perfusion, there is overlap of values (Roberts 2000, Provenzale 2002, Covarrubias 2004).
When making the distinction between radiation necrosis and tumor growth, one would expect that an actively growing tumor should possess higher blood flow/volume than a purely necrotic lesion. Indeed, Sugahara analyzed regional cerebral blood volume in 20 patients, concluding that a normalized rCBV ratio higher than 2.6 was indicative of malignancy and a value less than 0.6 of necrosis (Sugahara 2000). Unfortunately, 12 of 20 tumors fell into the indeterminate range. Better results were reported by Hazle, who used T1 weighted permeability and noted that necrotic and tumorous regions enhanced at different rates (Hazle 1997).
Anti-tumor agents specifically targeted to combat the effects of VEGF are now becoming available. The effects of therapy upon the vasculature may be complex, however. After one week of anti-VEGF therapy in mice with colon cancer, Wieldiers noted both a decrease in vascular density and an increase in overall perfusion (Wildiers 2006). This increase in perfusion, despite a diminution in vascular density, may be related to normalization of tortuosity in the larger feeding vessels (Jain 2005). By contrast, Willett describes both a decrease in vessel density and a decrease in tumor perfusion and blood volume following anti-VEGF treatment (Willett 2004), as might be expected following tumor regression.
4 Macroscopic Imaging: Vessel shape analysis from MRA
A different approach to the analysis of tumor associated vasculature is via a quantitative, statistical measure of vessel shape provided by the vessels segmented from time-of-flight, non-enhanced MRA. Much less is known about the relative disadvantages and advantages of this method both because it is relatively new and because work has been done predominantly by only one group. A disadvantage of this approach is that it inherently cannot analyze vessels of diameter less than the voxel size used during image acquisition. A second disadvantage is that image processing time may require an hour. An advantage, however, is that individual vessels can be followed over time. The fundamental difference between the two approaches is that microscopic imaging provides information that includes clusters of vessels too small to be individually discerned by MR (thus including information that macroscopic imaging cannot) but that macroscopic imaging provides multiple measures of vessel morphology.
Bullitt describes a blinded study in which MRA images of 30 tumor subjects were analyzed prior to total gross resection of each lesion, with all but one lesion (a false negative) correctly classified as malignant or benign on the basis of vessel tortuosity (Bullitt 2005). Cases included those difficult to classify by any current method and included hypervascular benign tumors, irradiated cancers, radiation necrosis, hemorrhagic lesions, and “pinpoint” lesions. Tiny cancers occupying only a few voxels on gadolinium enhanced MR could correctly be defined as malignant on the basis of vessel shape because vessel tortuosity abnormalities affect the vessels not only within the cancer but in the surrounding neighborhood as well.
An important question is how large a cancer must be before vessel shape abnormalities can be perceived by MRA. In a study that evaluated mice genetically engineered to develop carcinoma, cancers larger than 1 mm3 could be correctly classified as malignant on the basis of vessel tortuosity (Bullitt 2006).
Normalization of vessel tortuosity as visualized by MRA appears to occur during successful treatment of human cancer patients (Bullitt 2004, 2007). Figure 3 illustrates vessel shape normalization in a patient who underwent MRA imaging prior to initiation of treatment and 2 months after beginning a successful course of chemotherapy. Note that the later image demonstrates not only disappearance of tiny abnormal vessels but also resolution of tortuosity abnormalities in large vessels. Vessels at the second time point also exhibit normalization of vessel radii, with enlargement of the initially very narrow, tortuous right frontopolar artery and reduction in radius of the initially engorged left frontopolar artery. This enlargement of some vessels with concomitant decrease in radius of others may explain the previous contradictory reports on the increase/decrease of perfusion levels following tumor treatment (Wildiers 2006, Willett 2004). Insufficient data is available, however, to know if the macroscopic imaging approach will ultimately prove clinically useful when analyzing tumors of a variety of types and under various treatment regimens.
Figure 3.
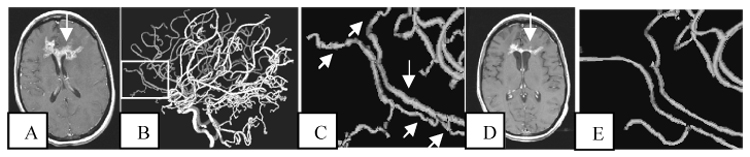
Improvement of vessel tortuosity abnormalities during successful treatment. A: Axial slice of a T1-GAD baseline scan showing a large tumor (arrow). Acquisition parameters are the same as outlined in Figure 2. B: Lateral view of the vessels segmented from the baseline MRA, with MRA acquisition parameters the same as described in Figure 1. Rectangle outlines the region magnified in C. C: Tortuosity abnormalities involve both smaller branches and the large frontopolar arteries (arrows). D: The tumor has regressed at month 2 of treatment (arrow). E: At month 2 the small, abnormal vessel branches have largely disappeared and there is normalization of the larger vessels’ shapes. The tumor continued to regress during the following 8 months.
5 Discussion
The microscopic and macroscopic imaging methods outlined above have different advantages and disadvantages. Microscopic vessel analysis has the advantage of including tiny vessels at subvoxel resolution. However, large and small vessels are considered together, the approach may be sensitive to acute changes in the environment, and individual vessels cannot be followed over time. Conversely, macroscopic vessel analysis can follow individual vessels over time and is likely to be less susceptible to acute environmental change, but vessels smaller in diameter than the voxel acquisition size cannot be assessed. On theoretical grounds, one might expect the two approaches to provide complementary information. Indeed, a recent comparative study concluded that there was no correlation between vessel tortuosity measurements and regional cerebral blood flow/volume across tumors of varying histological type (Parikh 2004).
Much remains unknown about cancer-associated vascular abnormalities. In a thoughtful review, McDonald notes that it is not only the mechanisms by which the abnormalities are produced that are poorly understood, but also that it is not yet clear what the implications are for the rate of cancer growth, predisposition to metastasis, and the delivery of macromolecular therapeutics to tumor cells (McDonald 2002). As illustrated by the radius changes shown in Figure 3, the same therapeutic agent may exert differing effects upon different parts of the vasculature within the same patient. Which parameters (blood flow, blood volume, permeability, vessel number, vessel tortuosity) are most important when assessing tumor activity is unknown. Similarly, the time course over which “clinically meaningful vascular normalization” occurs during treatment is also unknown, and the critical phrase “clinically meaningful vascular normalization” has yet to be defined.
The fact that the “macro” and “micro” imaging techniques appear to offer complementary information provides the opportunity to examine a number of important questions. As one example, Jain has suggested that the abnormal tortuosity of vessels feeding a cancer may reduce blood flow to a tumor, thus undesirably reducing the delivery of therapeutic agents to the tumor bed. This hypothesis has led to the potential recommendation of treating cancer by first delivering an anti-angiogenic agent to normalize the vasculature, thus intentionally increasing blood flow to the tumor, and then providing direct anti-tumor treatment [Jain 2005]. This hypothesis could be examined directly by using “macro” imaging techniques to quantify the radius and tortuosity of individual feeding vessels prior to and after anti-angiogenic treatment while simultaneously examining tumor blood flow and volume using “micro” imaging techniques. As a second example, abnormal, cancer-associated vessel permeability is believed to be associated with growth-factor induced changes to the vessel wall [McDonald 2002]. Many different growth factors may be involved, however, and it is unknown whether vessel tortuosity changes are induced by the same growth factor(s) that modulate vessel permeability. If the micro and macro imaging techniques provide different information, it is possible that the two may provide information about different growth factors and that the combination of the two imaging modalities could provide more information about tumor therapeutic response than either could alone. Thirdly, different parts of the same tumor may respond differently to the same therapy, as may different metastatic lesions within the same patient. The combination of micro and macro vascular imaging could help explain some of the current discrepancies in the literature in which perfusion is sometimes reported as increased and sometimes as decreased following anti-angiogenic therapy [Wildiers 2006, Willett 2004]. More specifically, the combination of micro/macro imaging might also help elucidate which lesions or portions of a lesion are “responding” or “not responding” to therapy (with the ultimate definition of what “responding to therapy” really means when correlated with clinical outcome), and what the importance and potential correlations of the different measures of vessel shape, vessel perfusion, and vessel permeability are likely to be.
The development of new pharmacological agents that target molecular pathways offers new hope to cancer patients. An ongoing challenge is the delineation of a method that can determine both noninvasively and early during a treatment course how effective a therapy will prove to be. We believe that the study of tumor-associated vascular changes should offer important new insight into how tumors grow and respond (or fail to respond) to treatment. These same imaging methods may also provide clinicians an accurate means of non-invasively assessing a tumor’s malignancy and its response to treatment.
Acknowledgments
This work was supported by R01 EB000219 NIH-NIBIB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth Bullitt, Email: bullitt@med.unc.edu.
David A. Reardon, Email: reard003@mc.duke.edu.
J. Keith Smith, Email: jksmith@med.unc.edu.
References
- 1.ACS (American Cancer Society) Cancer Facts and Figures 2006. Atlanta: American Cancer Society; 2006. [Google Scholar]
- 2.Aronen HJ, Perkio J. Dynamic susceptibility contrast MRI of gliomas. Neuroimag Clin N Am. 2002;12:501–523. doi: 10.1016/s1052-5149(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 3.Baish JS, Jain RK. Fractals and cancer. Cancer Research. 2000;60:3683–3688. [PubMed] [Google Scholar]
- 4.Bullitt E, Ewend MG, Aylward S, et al. Abnormal vessel tortuosity as a marker of treatment response of malignant gliomas: Preliminary report. Technology in Cancer Research and Treatment. 2004;3:577–584. doi: 10.1177/153303460400300607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullitt E, Zeng D, Gerig G, et al. Vessel tortuosity and brain tumor malignancy: A blinded study. Academic Radiology. 2005;12:1232–1240. doi: 10.1016/j.acra.2005.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullitt E, Wolthusen A, Brubaker L, et al. Malignancy-associated vessel tortuosity: A computer-assisted, MRA study of choroid plexus carcinoma in genetically engineered mice. AJNR. 2006;27:612–619. [PMC free article] [PubMed] [Google Scholar]
- 7.Bullitt E, Lin NU, Smith JK, et al. Blood Vessel Morphological Changes as Visualized by MRA During Treatment of Brain Metastases, accepted Radiology. 2007 doi: 10.1148/radiol.2453061889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covarrubias DJ, Rosen BR, Lev MH. Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist. 2004;9:528–537. doi: 10.1634/theoncologist.9-5-528. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey MF, Condon BR, Hadley DM. Measurement of tumor “size” in recurrent malignant glioma: 1D, 2D, or 3D? AJNR. 2005;26:770–776. [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J. Tumor angiogenesis: therapeutic implications. New England Journal of Medicine. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 12.Hazle JD, Jackson EF, Schomer DF, et al. Dynamic imaging of intracranial lesions using fast spin echo imaging: differentiation of brain tumors and treatment effects. J Magn Reson Imaging. 1997;7:1084–1093. doi: 10.1002/jmri.1880070622. [DOI] [PubMed] [Google Scholar]
- 13.Jackson A, Kassner A, Annesley-Williams D, et al. Abnormalities in the recirculation phase of contrast agent bolus passage in cerebral gliomas: comparison with relative blood volume and tumor grade. AJNR. 2002;23:7–14. [PMC free article] [PubMed] [Google Scholar]
- 14.Jain RK. Normalization of the tumor vasculature: An emerging concept in anti-angiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 15.Leonhardt G, de Greiff A, Weber J, et al. Brain perfusion following single seizures. Epilepsia. 2005;46:1943–1949. doi: 10.1111/j.1528-1167.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 16.Li CH, Shan S, Huang Q, et al. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J Natl Cancer Inst. 2000;92:143–147. doi: 10.1093/jnci/92.2.143. [DOI] [PubMed] [Google Scholar]
- 17.McDonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Research. 2002;62:5381–5385. [PubMed] [Google Scholar]
- 18.Ostergaard L, Hochberg FH, Rabinov JD, et al. Early changes measured by magnetic resonance imaging in cerebral blood flow, blood volume, and blood-brain barrier permeability following dexamethasone treatment in patients with brain tumors. J Neurosurg. 1999;90:300–305. doi: 10.3171/jns.1999.90.2.0300. [DOI] [PubMed] [Google Scholar]
- 19.Parikh A, Smith JK, Ewend MG, et al. Correlation of MR perfusion imaging and vessel tortuosity parameters in assessment of intracranial neoplasms. Technology in Cancer Research and Treatment. 2004;3:585–590. doi: 10.1177/153303460400300608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Provenzale JM, Wang GR, Brenner T, et al. Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. AJR. 2002;178:711–716. doi: 10.2214/ajr.178.3.1780711. [DOI] [PubMed] [Google Scholar]
- 21.Roberts HC, Roberts TPL, Brasch RC, et al. Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR. 2000;21:891–899. [PMC free article] [PubMed] [Google Scholar]
- 22.Sugahara T, Yukunori K, Tomiguchi S, et al. Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR. 2000;21:901–909. [PMC free article] [PubMed] [Google Scholar]
- 23.Wildiers H, Guetens G, De Boeck G, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. J Pancreas. 2006;7:163–173. doi: 10.1038/sj.bjc.6601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willett CG, Boucher Y, Di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan F, Chen Y, Dellian M, et al. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc. Natl. Acad. Sci. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]


