Abstract
Activated blood platelets and macrophages metabolize prostaglandin H2 into thromboxane A2 and 12(S)-hydroxyheptadeca-5Z, 8E, 10E–trienoic acid (12-HHT) in an equimolar ratio through the action of thromboxane synthase. Although it has been shown that 12-HHT is abundant in tissues and bodily fluids, this compound has long been viewed as a by-product lacking any specific function. We show that 12-HHT is a natural ligand for leukotriene B4 (LTB4) receptor-2 (BLT2), a G protein–coupled receptor that was originally identified as a low-affinity receptor for LTB4. BLT2 agonistic activity in lipid fractions from rat small intestine was identified as 12-HHT using high-performance liquid chromatography and mass spectrometry. Exogenously expressed BLT2 in mammalian cells was activated by synthetic 12-HHT, as assessed by guanosine 5′-O-(3-thio) triphosphate binding, the activation of intracellular signaling pathways, and chemotaxis assay. Displacement analysis using [3H]LTB4 showed that 12-HHT binds to BLT2 with a higher affinity than LTB4. Lipid extracts from cyclooxygenase 1–deficient mice failed to activate BLT2. Bone marrow–derived mast cells (BMMCs) isolated from wild-type mice migrated toward a low concentration of 12-HHT, whereas BMMCs from BLT2-deficient mice did not. We conclude that 12-HHT is a natural lipid agonist of BLT2 in vivo and induces chemotaxis of mast cells.
12(S)-hydroxyheptadeca-5Z, 8E, 10E–trienoic acid (12-HHT) was first identified as an enzymatic product of arachidonic acid metabolism by cyclooxygenase (Cox) derived from the vesicular gland of sheep and human platelets (1). Thromboxane A2 (TXA2) synthase catalyzes the conversion of prostaglandin H2 (PGH2) to TXA2, 12-HHT, and malondialdehyde (MDA) in an equimolar ratio. TXA2 is a potent stimulator of platelet aggregation and vascular constriction through activating the TX receptor, a member of the family of G protein–coupled receptors. TXA2 has also been shown to play a major role in thrombosis, vasoconstriction, the proliferation of vascular smooth muscle cells, and immune regulation (2). However, the functional role of 12-HHT remains elusive.
Arachidonic acid is also converted by 5-lipoxygenase (5-LO) to yield another class of metabolites termed leukotrienes and lipoxins (3, 4). 5(S), 12(R)-dihydroxy-6, 14-cis-8, 10-trans-eicosatetraenoic acid (leukotriene B4 [LTB4]) is an extremely potent lipid inflammatory mediator. Biosynthesis of LTB4 from membrane phospholipids occurs via the coordinated and sequential actions of cytosolic phospholipase A2α, 5-LO, and LTA4 hydrolase (5–8). Previously, we identified two distinct G protein–coupled receptors that bind to LTB4, LTB4 receptor-1 (BLT1), and BLT2 (9, 10). BLT1 is a high-affinity LTB4 receptor expressed in myeloid and lymphoid cells (11), whereas BLT2 is a low-affinity LTB4 receptor that is expressed rather ubiquitously (12). A recent study has shown that an LTB4–BLT1 signaling cascade regulates early phase immunological reactions by recruiting T lymphocytes (13). The LTB4–BLT1 axis is also important in atherogenesis (14), inflammatory bowel disease, and rheumatoid arthritis. In contrast, the biological role of BLT2 has not been clearly defined. Recently, we showed that when BLT2 is overexpressed in cells, it can be activated by 1–10-μM concentrations of hydroxyeicosatetraenoic acids (HETEs) (15). We have also identified a synthetic BLT2-specific agonist that activates human and mouse BLT2 at a lower concentration than LTB4 (16). These results suggest that there are other, as yet unidentified intrinsic BLT2 agonists. To identify intrinsic agonists of BLT2, we examined the activation of BLT2 by lipid extracts from various rat tissues. Lipid fractions from the rat small intestine contained BLT2 agonistic activity, which was distinct from LTB4, as assessed by HPLC. We determined the structure of the bioactive lipid using reverse-phase HPLC (RP-HPLC) and mass spectrometry, and identified 12-HHT as a natural endogenous ligand for BLT2. This result was somewhat surprising, as 12-HHT has long been considered merely a by-product of the thromboxane synthase reaction. Synthetic 12-HHT activated human and mouse BLT2, based on intracellular signalings and binding assays. In addition, 12-HHT induced chemotaxis in bone marrow–derived mast cells (BMMCs) derived from WT mice but not from BLT2-deficient mice.
RESULTS AND DISCUSSION
Acetone-soluble lipids from rat small intestine activate BLT2
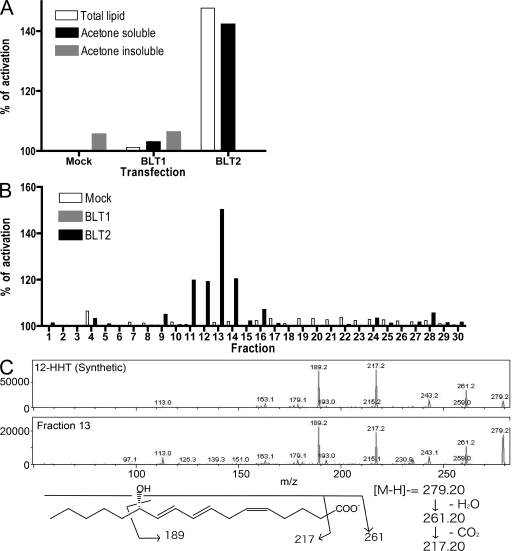
We extracted lipids from several rat organs and analyzed their ability to activate BLT2 that was heterologously expressed in Chinese hamster ovary (CHO) cells using a cytosensor microphysiometor assay. Cytosensor measures proton flux that is not dependent on or biased by intracellular signals (17). CHO cells that stably expressed human BLT2 (CHO-BLT2) were activated by total lipid and acetone-soluble lipid fractions from the rat small intestine, whereas cells that expressed human BLT1 (CHO-BLT1) and mock transformants were not (Fig. 1 A). These results were consistent with the high expression of mouse BLT2 in the small intestine (16). The acetone-soluble lipid fraction from the rat small intestine was fractionated by RP-HPLC, and the activities of the collected fractions were analyzed using a cytosensor. We also analyzed authentic LTB4, 12-HETE, and 15-HETE (12- and 15-HETE are weak agonists for BLT2) by RP-HPLC using the same conditions. LTB4 eluted in fraction 6, 15-HETE eluted in fraction 13, and 12-HETE eluted in fractions 14 and 15 (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20072329/DC1), and both CHO-BLT1 and CHO-BLT2 cells were activated after exposure to fraction 6 (Fig. S1 A). When we examined the activity of the fractionated acetone-soluble lipid from rat intestine, we found that fractions 7 and 8 activated CHO-BLT2 cells but not CHO-BLT1 cells (Fig. S1 B). These results indicated that there is a BLT2-specific agonist in the lipid fraction of the rat intestine, and that this activity is distinct from LTB4 or HETEs. Previously, we reported that several HETEs also weakly activate BLT2 (15). However, in the current analysis, it appeared that the concentration of HETEs in fractions 13, 14, and 15 was too low to activate BLT2 (Fig. S1 A). Therefore, we focused on identifying the BLT2 agonist in fractions 7 and 8.
Figure 1.
Structural determination of a BLT2 agonist isolated from the rat intestine. Total lipid was extracted from the rat small intestine by the Bligh and Dyer method and separated into acetone-soluble and insoluble fractions. (A) CHO cells stably expressing BLT1 or BLT2 were stimulated with lipid fractions, and activity was monitored by cytosensor assay. (B) Acetone-soluble lipids from the rat small intestine were analyzed by RP-HPLC (MeOH/H2O/AcOH = 70:30:0.01), and fractions were collected every 1 min. The agonistic activity was examined by cytosensor assay. (C) Tandem mass spectra of 12-HHT (synthetic) and the m/z 279.2 peak of fraction 13. The proposed fragmentation is indicated.
Determination of lipid structure by HPLC and mass spectrometry
To determine the structure of the BLT2-active lipid from the rat small intestine, we analyzed the acetone-soluble lipid fraction by RP-HPLC and mass spectrometry. To improve the separation on RP-HPLC, we used a solvent of methanol/H2O/AcOH at a ratio of 70:30:0.01 (vol/vol/vol), rather than 75:25:0.01, to extend the retention time. Under these conditions, fraction 13 showed the highest BLT2 agonist activity, and mock transformants or CHO-BLT1 cells were not activated by this fraction (Fig. 1 B). Fractions 11–15 were then subjected to electrospray ionization–mass spectrometry by flow injection. The main peak in fraction 13 was located at m/z 279.2 and represented the likely candidate for the BLT2-active molecule. This peak was low in fractions 12 and 14 (Fig. S1 C). Using the enhanced resolution mode, we determined that the detailed numerical value of the active molecule was 279.18 (Fig. S1 D). LTB4 is a C-20 unsaturated fatty acid with two hydroxyl groups and one carboxyl group, and 12- and 15-HETE are C-20 unsaturated fatty acids with one hydroxyl group and one carboxyl group (Fig. S1 E). In our analysis, the BLT2-active molecule eluted between the fractions that contained LTB4 and HETEs. Based on calculated formulas and retention time, which reflects the polarity and solubility of the molecule, we speculated that the target agonist molecule was C17H27O3 (m/z 279.196), with one hydroxyl group and one carboxyl group. These results strongly implicated 12-HHT as the lipid agonist because the formula of 12-HHT is C17H27O3, and 12-HHT is a C-17 unsaturated fatty acid with one hydroxyl group and one carboxyl group. Furthermore, in the enhanced product scan mode, the fragmentation patterns of chemically synthesized 12-HHT and the target agonist molecule in fraction 13 were similar (Fig. 1 C). Collectively, these results indicated that the BLT2-active lipid in fraction 13 was 12-HHT. To examine the retention times of 12-HHT and the bioactive lipid, we analyzed a mixture of LTB4, 12-HHT, and 12- and 15-HETE by RP-HPLC. 12-HHT (Fig. S1 A) and the bioactive lipid (Fig. S1 B) eluted in the same fraction, between LTB4 and HETEs, and both were able to activate BLT2 in the cytosensor assay.
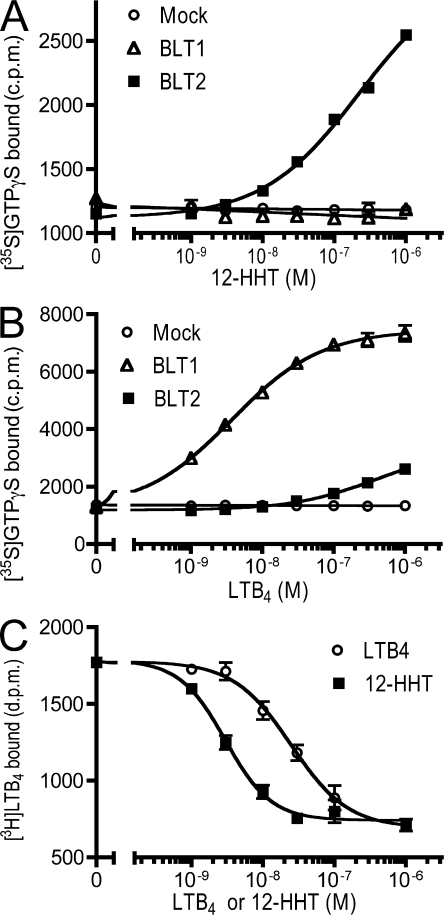
12-HHT activates BLT2 that is heterologously expressed in mammalian cells
Although cytosensor analysis is a useful screening tool for the identification of compounds based on their ability to activate cell metabolism, it does not identify specific intracellular signaling pathways that are activated after receptor engagement. To determine whether 12-HHT–mediated activation of BLT2 induced the GDP-GTP exchange activity of G proteins, we performed a guanosine 5′-O-(3-thio) triphosphate (GTPγS) binding assay using membrane preparations from CHO-BLT1 and CHO-BLT2 cells. 12-HHT increased GTPγS binding in membrane preparations from CHO-BLT2 cells in a dose-dependent manner, but not in membranes from CHO-BLT1 cells or mock transformants (Fig. 2 A). LTB4 induced a much lower level of GTPγS binding in membrane preparations from CHO-BLT2 cells as compared with CHO-BLT1 cells (Fig. 2 B). Although ligand-binding experiments are the most definitive method for demonstrating ligand–receptor interactions, radiolabeled [3H]12-HHT is not readily available. Therefore, we performed a displacement assay using [3H]LTB4 and either 12-HHT or LTB4. In membrane preparations of CHO-BLT2 cells, the IC50 values of 12-HHT and LTB4 in the presence of 5 nM [3H]LTB4 were 2.8 and 25 nM, respectively (Fig. 2 C). These results suggested that BLT2 is a high-affinity receptor for 12-HHT, and that 12-HHT and LTB4 occupy the same binding site on BLT2.
Figure 2.
12-HHT binds BLT and activates G proteins. (A and B) The dose–response curves of the [35S]GTPγS binding assay using membrane preparations of CHO cells stably expressing BLT1 or BLT2. Data represent the mean ± SEM (n = 3). (C) Membrane preparations of CHO-BLT2 cells were incubated with 5 nM [3H]LTB4, and specific binding was measured in the presence of the indicated concentrations of LTB4 or 12-HHT, as depicted. Data represent the mean ± SEM (n = 3).
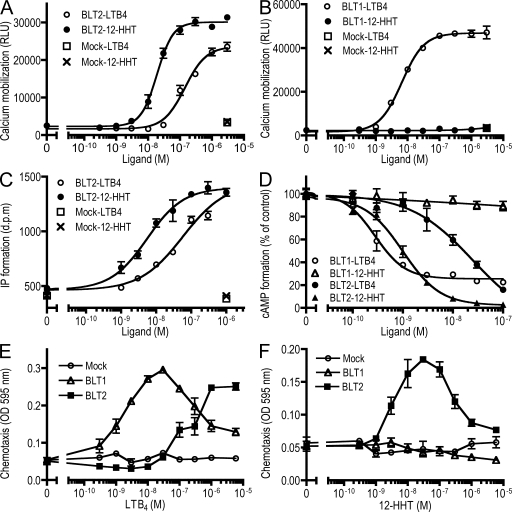
Previously, we showed that the activation of BLT1 and BLT2 is coupled to the Gi and Gq families of G proteins (12, 18). To determine whether 12-HHT could induce the activation of Gq, we examined 12-HHT–dependent calcium mobilization in CHO-BLT1 and CHO-BLT2 cells. The EC50 values of 12-HHT and LTB4 in CHO-BLT2 cells were 19 and 142 nM, respectively (Fig. 3 A). The EC50 value of LTB4 in CHO-BLT1 cells was 6.6 nM, whereas 12-HHT failed to induce calcium mobilization in CHO-BLT1 cells, even at concentrations as high as 3.3 μM (Fig. 3 B). The activation of receptors that are coupled to Gq family proteins induces phospholipase C activation, followed by the production of inositol triphosphate (IP3). To identify the Gq family members that are involved 12-HHT–mediated activation of BLT2, we performed an IP accumulation assay using COS-7 cells that were transiently cotransfected with expression vectors for BLT2 and various Gq family proteins. There was a significantly higher level of IP accumulation in cells that coexpressed BLT2 and G14 (Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20072329/DC1), whereas coexpression of Gq and G11 with BLT2 resulted in a relatively weak increase and no effect of G16 in IP accumulation. These findings are in contrast to previous data in BLT1-mediated signaling (18). In cells that coexpressed BLT2 and G14, the EC50 of 12-HHT and LTB4 for the accumulation of IP was 5.4 and 43 nM, respectively (Fig. 3 C). 12-HHT also activated mouse BLT2, as shown by IP accumulation assay using cells that were cotransfected with expression vectors for mouse BLT2 and G14 (Fig. S2 B).
Figure 3.
12-HHT–induced intracellular signaling is mediated by BLT2. (A and B) Intracellular calcium mobilization in (A) CHO-BLT2 and (B) CHO-BLT1 cells after exposure to 12-HHT or LTB4 was analyzed by FlexStation (MDS Analytical Technologies). Data represent the mean ± SEM (n = 3). (C) The dose–response curves of the IP accumulation assay in coexpressing COS-7 cells transfected with BLT2 and G14. Data represent the mean ± SEM (n = 3). (D) CHO cells stably expressing BLT1 or BLT2 were stimulated with 50 μM of forskolin and the indicated concentrations of LTB4 and 12-HHT, and the levels of cAMP in the cells were determined. Data represent the mean ± SEM (n = 4). (E and F) Chemotaxis assay of CHO-BLT1 and CHO-BLT2 cells was examined using a Boyden chamber assay. Data represent the mean ± SEM (n = 3).
The activity that resulted from the combined expression of specific receptors and G proteins may reflect the expression pattern or function of these proteins in vivo. Although all Gq proteins share a capacity to activate phospholipase Cβ, they differ markedly in their biochemical properties and tissue distribution. Gq and G11 are the most similar among this class of G proteins and are fairly widely expressed. G14 exhibits a more diverse expression pattern (kidney, liver, lung, and testis), whereas G16 expression appears to be limited to hematopoietic cells. Human and mouse BLT1 are expressed exclusively in leukocytes, which also express G16. Human BLT2 expression correlates with the distribution of Gq, G11, and G14, which suggests that BLT2 is coupled to these G proteins in vivo.
To determine whether 12-HHT could induce the activation of Gi proteins, we performed a series of cAMP and chemotaxis assays in CHO-BLT1 and CHO-BLT2 cells. The IC50 of 12-HHT and LTB4 for adenylyl cyclase inhibition in CHO-BLT2 cells was 1 and 14 nM, respectively (Fig. 3 D). 12-HHT–dependent adenylyl cyclase inhibition in CHO-BLT2 cells was completely blocked by pretreatment with pertussis toxin (Fig. S2 C). The IC50 of LTB4 in CHO-BLT1 cells was 0.3 nM, whereas 12-HHT had no effect (Fig. 3 D). Previously, we showed that LTB4 induces chemotaxis through the activation of BLT2 (12, 15, 16). To determine whether 12-HHT also stimulated chemotaxis through BLT2, we performed a Boyden chamber chemotaxis assay of CHO-BLT2 cells exposed to 12-HHT or LTB4. The concentration of 12-HHT required for maximal chemotaxis was 30 nM, whereas that of LTB4 was >1,000 nM (Fig. 3, E and F). 12-HHT failed to induce chemotaxis in CHO-BLT1 cells and mock cells (Fig. 3 F). Pretreatment of CHO-BLT2 cells with pertussis toxin completely abolished 12-HHT–induced chemotaxis (Fig. S2 D). These results suggested that 12-HHT is a high-affinity ligand for human and mouse BLT2 and activates Gq and Gi family G proteins.
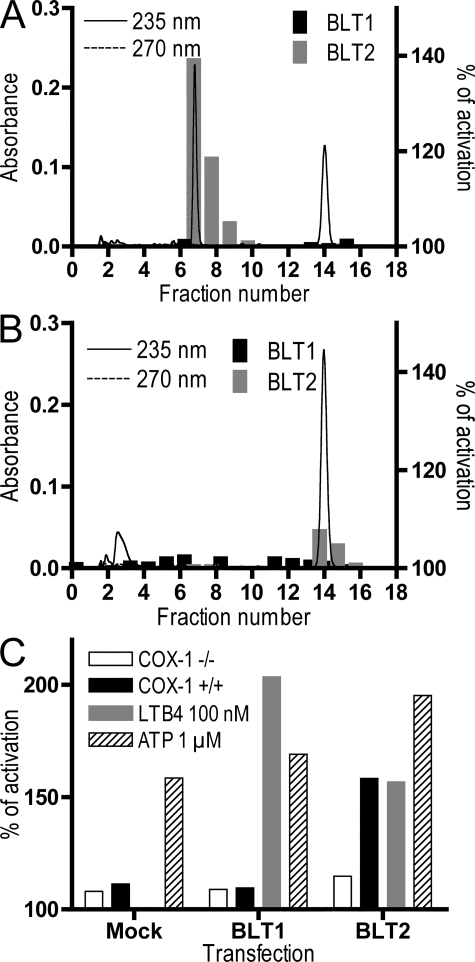
Platelet-derived 12-HHT activates BLT2
Activated platelets produce TXA2, 12-HHT, and MDA from PGH2. We stimulated human platelets with arachidonic acid and extracted the lipid fraction from these cells. The extracted lipids were fractionated by RP-HPLC, and the fractions were analyzed by cytosensor assay. Fig. 4, A and B show the HPLC chromatograph and the results of the cytosensor assays for each fraction. The BLT2-active fractions from human platelets were fractions 7 and 8 (Fig. 4 A). Pretreatment of platelets with 10 μM aspirin completely abolished BLT2 agonistic activity, and there was a concomitant loss of UV absorbance at 235 nm in fractions 7 and 8 (Fig. 4 B). Aspirin treatment resulted in the appearance of weak BLT2 agonistic activity in fractions 14 and 15, and a high level of absorbance at 235 nm in these fractions. Based on the UV spectrum and the HPLC profile, we speculate that this weak agonistic activity is 12-HETE (Fig. S1 A). None of the fractions exhibited UV absorbance at 270 nm or BLT1-specific activity, which suggested that platelets do not produce detectable amounts of LTB4. These results indicated that human platelets produce 12-HHT, a natural agonist of BLT2, and that Cox activity is required for the biosynthesis of 12-HHT. It is likely that the inhibition of the Cox pathway in platelets by pretreatment with aspirin increased the production of 12-HETE (Fig. 4 B) by shunting arachidonic acid from the Cox to the 12-LO pathway (1). Shao et al. reported that BLT2 was expressed in mouse platelets (19). 12-HHT did not display agonistic activity in platelet aggregation (unpublished data); thus, the role of 12-HHT and 12-HETE in platelet function remains to be determined.
Figure 4.
Activation of BLT2 by platelet-derived 12-HHT requires Cox-1. (A and B) Human platelets were pretreated with vehicle (A) or 10 μM aspirin (B) and then incubated with 10 μM of arachidonic acid. Lipids were extracted and analyzed by RP-HPLC. Fractions were collected every 1 min, and agonistic activity toward CHO-BLT1 and CHO-BLT2 cells was analyzed by cytosensor assay. The continuous and dashed lines show absorbance at 235 and 270 nm, respectively. (C) Lipids were extracted from the small intestines of Cox-1–deficient and WT mice, and agonistic activity toward CHO-mock, CHO-BLT1, and CHO-BLT2 cells was examined by cytosensor assay. ATP was used as a control for the activation of endogenous purinergic receptors in CHO cells.
Lipids extracted from Cox-1–deficient mice fail to activate BLT2
It has been shown that Cox activity is important for 12-HHT production. To determine the effect of Cox-1 deficiency on the production of 12-HHT, we extracted lipids from the small intestines of Cox-1–deficient mice. As seen in Fig. 4 C, lipids from Cox-1–deficient mice contained much lower BLT2 agonistic activity than those from WT mice. These results indicated that Cox-1 is required for the biosynthesis of 12-HHT in vivo, and that 12-HHT is the major ligand of BLT2 in the intestine.
12-HHT induces the migration of BMMCs through BLT2
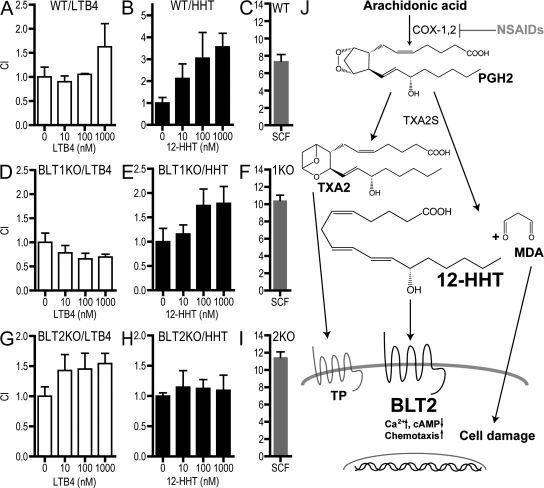
Lundeen et al. (20) previously reported that mouse BMMCs (mBMMCs) express BLT2 and migrate in response to LTB4 and 12-HETE. We performed a similar migration assay using mBMMCs derived from WT, BLT1-KO (21), and BLT2-KO (unpublished data) mice. The maturation of mast cells was confirmed by flow cytometry (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20072329/DC1). BMMCs from WT and BLT2-KO mice migrated in response to LTB4, whereas cells from BLT1-KO mice did not (Fig. 5, A, D, and G). WT and BLT1-KO cells migrated in response to 12-HHT, whereas BLT2-KO cells did not (Fig. 5, B, E, and H). As a positive control, we observed that stem cell factor (SCF) induced robust chemotaxis through the c-kit receptor in WT, BLT1-KO, and BLT2-KO BMMCs to a similar extent (Fig. 5, C, F, and I). LTB4 and 12-HHT induced weaker chemotaxis in mBMMCs than SCF, as reported previously (20), and we speculate that this is caused by the lower expression of BLT1 and BLT2 in these cells. These results indicate that functional BLT2 is expressed in BMMCs and mediates 12-HHT–dependent cell migration.
Figure 5.
12-HHT–induced mBMMC chemotaxis is mediated by BLT2. Chemotaxis of WT BMMCs in response to (A) LTB4, (B) 12-HHT, and (C) 25 ng/ml SCF. Chemotaxis of BLT1-KO BMMCs in response to (D) LTB4, (E) 12-HHT, and (F) 25 ng/ml SCF. Chemotaxis of BLT2-KO BMMCs in response to (G) LTB4, (H) 12-HHT, and (I) 25 ng/ml SCF. Data represent the mean ± SEM (n = 3). (J) Schematic representation of the production and action of 12-HHT.
Putative biological roles of the 12-HHT–BLT2 signaling axis
Although the biological role of the BLT1–LTB4 signaling axis has been investigated in detail, the physiological and pathological roles of BLT2 remain largely unknown. Our findings demonstrate that platelets produce 12-HHT in a Cox-dependent manner, and that 12-HHT functions as a BLT2 ligand. We also demonstrated that 12-HHT induces chemotaxis in mast cells through the activation of endogenous BLT2. In addition to their role in the allergic response, mast cells are multipotent effector cells in the formation of atherothrombosis (22). Atherogenic plaques consist of mast cells, macrophages, and platelets, all of which produce TXA2 and 12-HHT (23, 24). We previously reported that mouse BLT2 is expressed in mouse keratinocytes, and that LTB4 and BLT2-specific agonists induced extracellular signal-regulated kinase activation and cell migration (16). Thus, 12-HHT also may activate keratinocytes in vivo.
MDA is produced along with 12-HHT from PGH2, and forms adducts with the proteins or phospholipids. Such adducts have been detected in atherosclerotic lesions of the human aorta, suggesting that they may play a role in atherogenesis (25). MDA has also been shown to form endogenous DNA adducts that may contribute to human genetic diseases and cancer (26). Thus, the synergistic activity of 12-HHT and MDA in certain pathological conditions remains an intriguing possibility.
In summary, we have partially purified a natural ligand for BLT2 from the rat small intestine and identified the ligand as 12-HHT by liquid chromatography–mass spectrometry. 12-HHT is a very abundant metabolite of the arachidonic acid cascade and has been considered to be merely a by-product, without any biological activity, for almost 30 yr. Our present findings provide evidence for a novel functional role of 12-HHT as a BLT2-ligand and represent a critical milestone in the understanding of the function of 12-HHT. Intrinsically expressed BLT2 in mast cells responds to 12-HHT (Fig. 5), suggesting a novel important role of 12-HHT as an intercellular messenger between platelets, macrophages, and mast cells in vivo.
MATERIALS AND METHODS
Materials.
LTB4, 12-HHT, and 12- and 15-HETE were purchased from Cayman Chemical. cDNAs encoding the various G proteins were obtained from Guthrie cDNA Resource Center. pcDNA3-HA-BLT1 and BLT2 were previously described (12, 16, 27).
Cell culture, transfection, and cell sorting.
All cells were established and maintained as previously described (18).
Extraction of lipid fractions, RP-HPLC, and mass spectrometry analysis.
Rats were purchased from Japan Clea, and Cox-1–deficient mice were purchased from Taconic. All animal experiments were conducted in accordance with the Guidelines of Animal Research at the University of Tokyo and Kyushu University and were approved by the Animal Ethics Committees of both universities. Small intestines of adult female Wistar rats or mice were homogenized in chloroform/methanol (1:2; C/M), and lipids were extracted by the Bligh and Dyer method. To separate acetone-soluble and insoluble fractions, dried lipids were reconstituted with acetone and incubated for 1 h at 4°C. After centrifugation at 1,000 g for 5 min, the pellet was washed twice with ice-cold acetone. Acetone-soluble fractions were combined, dried, and dissolved in C/M. The HPLC column (4.6 × 150 mm; COSMOSIL 5C18-AR; Nacalaitesque) was equipped with a diode array detector (System Gold module 168; Beckman Coulter) and eluted with MeOH/H2O/AcOH at a flow rate of 1 ml/min. Absorbance was monitored at 235 and 270 nm. Mass spectrometry analyses were performed using a 4000 Q-TRAP system (Applied Biosystems). Spectra were recorded in negative ion mode, the composition of the mobile phase was acetonitrile/methanol/water 6:7:2 (plus 0.1% ammonium formate), and the flow rate was 4 μl/min−1.
Binding and signaling assays.
Cytosensor, calcium mobilization (27), cAMP, IP accumulation, GTPγS, LTB4 binding, and chemotaxis assays (18) were performed as described previously.
Preparation of human platelets and arachidonic acid stimulation.
50 ml of human blood were collected from healthy volunteers under written informed consent, added to 1 ml of 0.25 M EDTA, and subjected to centrifugation at 1,400 g for 3 min to obtain platelet-rich plasma. The supernatants were centrifuged twice at 100 g for 25 min to remove leukocytes, and then the supernatant was centrifuged at 1,000 g for 17 min. The resulting pellets were resuspended in 2 ml of wash buffer (0.113 M NaCl, 4.3 mM K2HPO4, 4.3 mM K2HPO4, 4.3 mM NaHPO4, and 5.5 mM sucrose [pH 6.5]), washed twice, and resuspended in 4 ml of resuspension buffer (0.14 M NaCl, 5.5 mM glucose, and 15 mM Tris-HCl [pH 7.5]). 3 × 108 platelets were incubated with or without 1 mM aspirin at 37°C for 5 min and stimulated with 50 μM of arachidonic acid at 37°C for 15 min. The lipid fraction was recovered using C/M.
Chemotaxis of BMMCs.
Mice that were deficient in BLT1 (21) and BLT2 (unpublished data) were backcrossed to BALB/c mice for 10 generations. BMMCs were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 0.1 mM of nonessential amino acids, and 50% WEHI-3 cell-conditioned medium (WEHI-3 cells were provided by M. Murakami, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan). The BMMC migration assay was performed as previously described (20).
Online supplemental material.
Fig. S1 shows the behavior on HPLC and the structural determination of BLT2 ligand from rat intestine. Fig. S2 shows 12-HHT–induced intracellular signaling through BLT2. Fig. S3 shows the expression of c-kit and FcεRI on mBMMCs used in this experiment. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20072329/DC1.
Supplemental Material
Acknowledgments
We thank Drs. T. Houjyo, M. Ishida, and H. Nakanishi for technical advice on lipid extraction and mass spectrometric analyses. We thank Drs. M. Nakamura, S. Ishii, N. Uozumi, Y. Kita, H. Shindou, and all affiliates of the University of Tokyo for valuable advice and discussions.
This work was supported in part by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology, and grants from the Center for NanoBio Integration Program at the University of Tokyo, the Takeda Science Foundation, the Mitsubishi Foundation, the Naito Foundation, the Uehara Memorial Foundation, the Astellas Foundation for Research on Metabolic Disorders, and the Japan Society for the Promotion of Science (Global Centers of Excellence Program).
The authors have no conflicting financial interests.
References
- 1.Hamberg, M., J. Svensson, and B. Samuelsson. 1974. Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc. Natl. Acad. Sci. USA. 71:3824–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narumiya, S. 2003. Prostanoids in immunity: roles revealed by mice deficient in their receptors. Life Sci. 74:391–395. [DOI] [PubMed] [Google Scholar]
- 3.Serhan, C.N., Y. Lu, S. Hong, and R. Yang. 2007. Mediator lipidomics: search algorithms for eicosanoids, resolvins, and protectins. Methods Enzymol. 432:275–317. [DOI] [PubMed] [Google Scholar]
- 4.Samuelsson, B., S.E. Dahlen, J.A. Lindgren, C.A. Rouzer, and C.N. Serhan. 1987. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 237:1171–1176. [DOI] [PubMed] [Google Scholar]
- 5.Samuelsson, B., and C.D. Funk. 1989. Enzymes involved in the biosynthesis of leukotriene B4. J. Biol. Chem. 264:19469–19472. [PubMed] [Google Scholar]
- 6.Shimizu, T., and L.S. Wolfe. 1990. Arachidonic acid cascade and signal transduction. J. Neurochem. 55:1–15. [DOI] [PubMed] [Google Scholar]
- 7.Zeldin, D.C. 2001. Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 276:36059–36062. [DOI] [PubMed] [Google Scholar]
- 8.Haeggstrom, J.Z. 2004. Leukotriene A4 hydrolase/aminopeptidase, the gatekeeper of chemotactic leukotriene B4 biosynthesis. J. Biol. Chem. 279:50639–50642. [DOI] [PubMed] [Google Scholar]
- 9.Brink, C., S.E. Dahlen, J. Drazen, J.F. Evans, D.W. Hay, S. Nicosia, C.N. Serhan, T. Shimizu, and T. Yokomizo. 2003. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol. Rev. 55:195–227. [DOI] [PubMed] [Google Scholar]
- 10.Tager, A.M., and A.D. Luster. 2003. BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot. Essent. Fatty Acids. 69:123–134. [DOI] [PubMed] [Google Scholar]
- 11.Yokomizo, T., T. Izumi, K. Chang, Y. Takuwa, and T. Shimizu. 1997. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 387:620–624. [DOI] [PubMed] [Google Scholar]
- 12.Yokomizo, T., K. Kato, K. Terawaki, T. Izumi, and T. Shimizu. 2000. A second leukotriene B4 receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J. Exp. Med. 192:421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luster, A.D., and A.M. Tager. 2004. T-cell trafficking in asthma: lipid mediators grease the way. Nat. Rev. Immunol. 4:711–724. [DOI] [PubMed] [Google Scholar]
- 14.Jala, V.R., and B. Haribabu. 2004. Leukotrienes and atherosclerosis: new roles for old mediators. Trends Immunol. 25:315–322. [DOI] [PubMed] [Google Scholar]
- 15.Yokomizo, T., K. Kato, H. Hagiya, T. Izumi, and T. Shimizu. 2001. Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J. Biol. Chem. 276:12454–12459. [DOI] [PubMed] [Google Scholar]
- 16.Iizuka, Y., T. Yokomizo, K. Terawaki, M. Komine, K. Takami, and T. Shimizu. 2005. Characterization of a mouse second leukotriene B4 receptor, mBLT2. J. Biol. Chem. 280:24816–24823. [DOI] [PubMed] [Google Scholar]
- 17.Gronert, K., S.P. Colgan, and C.N. Serhan. 1998. Characterization of human neutrophil and endothelial cell ligand-operated extracellular acidification rate by microphysiometry: impact of reoxygenation. J. Pharmacol. Exp. Ther. 285:252–261. [PubMed] [Google Scholar]
- 18.Kuniyeda, K., T. Okuno, K. Terawaki, M. Miyano, T. Yokomizo, and T. Shimizu. 2007. Identification of the intracellular region of the leukotriene B4 receptor type 1 that is specifically involved in Gi activation. J. Biol. Chem. 282:3998–4006. [DOI] [PubMed] [Google Scholar]
- 19.Shao, W.H., A. Del Prete, C.B. Bock, and B. Haribabu. 2006. Targeted disruption of leukotriene B4 receptors BLT1 and BLT2: a critical role for BLT1 in collagen-induced arthritis in mice. J. Immunol. 176:6254–6261. [DOI] [PubMed] [Google Scholar]
- 20.Lundeen, K.A., B. Sun, L. Karlsson, and A.M. Fourie. 2006. Leukotriene B4 receptors BLT1 and BLT2: expression and function in human and murine mast cells. J. Immunol. 177:3439–3447. [DOI] [PubMed] [Google Scholar]
- 21.Terawaki, K., T. Yokomizo, T. Nagase, A. Toda, M. Taniguchi, K. Hashizume, T. Yagi, and T. Shimizu. 2005. Absence of leukotriene B4 receptor 1 confers resistance to airway hyperresponsiveness and Th2-type immune responses. J. Immunol. 175:4217–4225. [DOI] [PubMed] [Google Scholar]
- 22.Kovanen, P.T. 2007. Mast cells: multipotent local effector cells in atherothrombosis. Immunol. Rev. 217:105–122. [DOI] [PubMed] [Google Scholar]
- 23.Sun, J., G.K. Sukhova, P.J. Wolters, M. Yang, S. Kitamoto, P. Libby, L.A. MacFarlane, J. Mallen-St Clair, and G.P. Shi. 2007. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat. Med. 13:719–724. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi, T., Y. Tahara, M. Matsumoto, M. Iguchi, H. Sano, T. Murayama, H. Arai, H. Oida, T. Yurugi-Kobayashi, J.K. Yamashita, et al. 2004. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J. Clin. Invest. 114:784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchida, K. 1999. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc. Med. 9:109–113. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhary, A.K., M. Nokubo, G.R. Reddy, S.N. Yeola, J.D. Morrow, I.A. Blair, and L.J. Marnett. 1994. Detection of endogenous malondialdehyde-deoxyguanosine adducts in human liver. Science. 265:1580–1582. [DOI] [PubMed] [Google Scholar]
- 27.Okuno, T., H. Ago, K. Terawaki, M. Miyano, T. Shimizu, and T. Yokomizo. 2003. Helix 8 of the leukotriene B4 receptor is required for the conformational change to the low affinity state after G-protein activation. J. Biol. Chem. 278:41500–41509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.