Abstract
Background
Campylobacter jejuni is a common cause of acute gastroenteritis and is also associated with the post-infectious neuropathies, Guillain-Barré and Miller Fisher syndromes. In the Cape Town area of South Africa, C. jejuni strains with Penner heat-stable (HS) serotype HS∶41 have been observed to be overrepresented among cases of Guillain-Barré syndrome. The present study examined the genetic content of a collection of 32 South African C. jejuni strains with different serotypes, including 13 HS∶41 strains, that were recovered from patients with enteritis, Guillain-Barré or Miller Fisher syndromes. The sequence-based typing methods, multilocus sequence typing and DNA microarrays, were employed to potentially identify distinguishing features within the genomes of these C. jejuni strains with various disease outcomes.
Methodology/Principal Findings
Comparative genomic analyses demonstrated that the HS∶41 South African strains were clearly distinct from the other South African strains. Further DNA microarray analysis demonstrated that the HS∶41 strains from South African patients with the Guillain-Barré syndrome or enteritis were highly similar in gene content. Interestingly, the South African HS∶41 strains were distinct in gene content when compared to HS∶41 strains from other geographical locations due to the presence of genomic islands, referred to as Campylobacter jejuni integrated elements (CJIEs). Only the integrated element CJIE1, a Campylobacter Mu-like prophage, was present in the South African HS∶41 strains whereas this element was absent in two closely-related HS∶41 strains from Mexico. A more distantly-related HS∶41 strain from Canada possessed both integrated elements CJIE1 and CJIE2.
Conclusion/Significance
These findings demonstrate that CJIEs may contribute to the differentiation of closely-related C. jejuni strains. In addition, the presence of bacteriophage-related genes in CJIE1 may contribute to the genomic diversity of C. jejuni strains. This comparative genomic analysis of C. jejuni provides fundamental information that potentially could lead to improved methods for analyzing the epidemiology of disease outbreaks.
Introduction
Illnesses caused by bacterial foodborne pathogens continue to be a serious health issue worldwide. In laboratory-confirmed cases of infection, Campylobacter jejuni is considered to be one of the most significant bacterial causes of human gastroenteritis [1]–[3]. The majority of C. jejuni infections result in an acute, self-limited gastrointestinal illness. However, in a small number of patients, C. jejuni infection is followed by complications, including septicaemia [4] and the development of the autoimmune neuropathies, Guillain-Barré (GBS) and Miller-Fisher (MFS) syndromes [5]–[7]. The development of these autoimmune neuropathies after C. jejuni infection is thought to be related to sialylated lipooligosaccharides (LOS) on the cell surface of C. jejuni that exhibit molecular mimicry with gangliosides on peripheral nerves [8]–[11]. The range of C. jejuni disease outcomes, extending from acute inflammatory diarrhea to the induction of the autoimmune neuropathies, could be associated with the differential expression of virulence factors in C. jejuni strains. Additionally, the broad variability in the severity and spectrum of clinical symptoms in GBS patients suggests that host susceptibility may play a substantial role in disease development [12], [13].
Recent studies have characterized collections of strains from patients with GBS in search of a specific C. jejuni genotype responsible for the development of this neuropathy. C. jejuni strains from GBS cases, with Penner heat-stable (HS) serotypes HS∶19 and HS∶41, have been shown to be overrepresented in Japan and South Africa, respectively [14]–[17]. Further genetic characterization of GBS-associated strains with HS∶41 and HS∶19 demonstrated that these serotypes represent clonal populations [18], [19], suggesting that specific virulence traits relevant to the onset of GBS may be present in strains with these serotypes. Although strains with other common serotypes have been isolated from patients with GBS, the clustering of GBS-associated strains into specific serotypes has not been observed to occur in all worldwide geographical locations [20], [21].
A major contributor to the development of GBS that is distinct from capsular polysaccharide, the Penner serotyping determinant [22], is thought to be C. jejuni LOS. In most patients who develop GBS after C. jejuni enteritis, an induction of their immune response occurs possibly due to anti-C. jejuni LOS antibodies that cross-react with ganglioside epitopes on neural tissues [7]. In particular, LOS locus classes A, B, and C contain genes involved in the biosynthesis and transfer of sialic acid, an essential component of gangliosides [23], [24], and certain genes in the biosynthesis of these specific LOS classes are proposed to be associated with the induction of the immune response [8], [10], [11], [25]. Further analyses demonstrated that a high frequency of GBS-associated strains possessed LOS locus class A [8], [9], [26], [27] while MFS-associated strains had LOS locus class B [8], [26]. Potentially, a comparative genomic study of a highly-similar collection of C. jejuni strains associated with various disease outcomes could lead to the discovery of those determinants that may contribute to the development of GBS.
Various sequence-based typing methods, including multilocus sequence typing (MLST) [28]–[34], DNA sequencing of certain virulence loci [24], [27], [35] and comparative gene indexing by DNA microarrays [36]–[42], have been exploited to both identify differences between C. jejuni strains and potential relationships between molecular markers and disease outcomes. In particular, gene indexing studies, by using whole-genome microarrays of C. jejuni strain NCTC 11168, have demonstrated several regions of intraspecies genome hypervariability between strains of C. jejuni, including the LOS, capsular polysaccharide, flagellar biosynthetic and restriction-modification loci [36]–[42].
The genome sequence data of C. jejuni strain RM1221 have provided additional information of intraspecies genome diversity in C. jejuni [43]. The genome of strain RM1221 is syntenic with the genome of C. jejuni NCTC 11168 except for four genomic islands, referred to as Campylobacter jejuni integrated elements (CJIEs), and small gene clusters. Recently, a comparative genomic analysis by using DNA microarrays demonstrated that these CJIEs were present in C. jejuni strains from various geographical locations and from both clinical and veterinary sources [40], and the coding sequences within the CJIEs from these C. jejuni strains were highly divergent when compared to strain RM1221 [40], [44]. Based on these findings, the CJIEs are postulated to be additional hypervariable genomic regions that may contribute to increase the diversity of C. jejuni strains [40].
In the present study, a comparative analysis was performed to examine the genetic content of a collection of C. jejuni strains recovered from patients admitted to the Red Cross War Memorial Children's Hospital in Cape Town, South Africa. In the Cape Town area, GBS has been estimated to occur at a high incidence in children under 15 years of age [14], [16]. Based on these findings, it has been postulated that a severe form of GBS may be prevalent in this area. Among C. jejuni strains from South African patients with GBS, serotype HS∶41 has been found to be overrepresented [14], [16]. In this comparative genomic analysis, sequence-based typing methods, MLST and whole-genome DNA microarrays, were employed to identify distinguishing features within the genomes of South African C. jejuni strains with different serotypes that were recovered from patients with GBS, MFS or enteritis.
Materials and Methods
Bacterial strains, growth conditions and chemicals
The C. jejuni reference and clinical strains used in this study are shown in Table 1. The source and characteristics of the C. jejuni strains, isolated from patients admitted to the Red Cross War Memorial Children's Hospital in Cape Town, South Africa, have been described previously [14], [16]. C. jejuni strains were grown at 42°C under microaerobic conditions (8% CO2, 4% O2, 80% N2, 8% H2) on Oxoid anaerobe basal agar (Remel Inc., Lenexa, KS) amended with 5% laked horse blood (Hema Resource and Supply Inc., Aurora, OR). PCR enzymes and reagents were purchased from New England Biolabs (Beverly, MA) or Epicentre Biotechnologies (Madison, WI). DNA sequencing chemicals and capillaries were purchased from Applied Biosystems (Foster City, CA). All other chemicals were purchased from Sigma-Aldrich Chemicals (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA).
Table 1. MLST analysis of the Campylobacter jejuni strains used in this study.
| Strainsa | Year | Countryc | Diseased | LOS class | Penner type(s)e | ST | Allele number | ||||||
| aspA | atpA | glnA | gltA | glyA | pgm | tkt | |||||||
| RM1221 (ATCC BAA-1062) | 1996 | USA | Unk | F | HS∶53 | 354 | 8 | 6 | 10 | 2 | 2 | 11 | 12 |
| RM1862 (NCTC 11168) | Unkb | UK | Enteritis | C | HS∶02 | 43 | 2 | 5 | 1 | 5 | 3 | 4 | 1 |
| RM3148 (ATCC BAA-530) | Unk | MEX | GBS | A | HS∶41 | 1672 | 1 | 8 | 2 | 42 | 4 | 256 | 9 |
| RM3149 (ATCC BAA-529) | Unk | MEX | GBS | A | HS∶41 | 1672 | 1 | 8 | 2 | 42 | 4 | 256 | 9 |
| RM3193 (260.94) | 1994 | SA | GBS | A | HS∶41 | 362 | 1 | 8 | 2 | 49 | 4 | 11 | 66 |
| RM3194 (285.94) | 1994 | SA | Enteritis | B | Unk | 1471 | 24 | 6 | 171 | 2 | 2 | 89 | 59 |
| RM3196 (233.94) | 1994 | SA | GBS | A | HS∶41 | 362 | 1 | 8 | 2 | 49 | 4 | 11 | 66 |
| RM3197 (308.95) | 1995 | SA | GBS | A | HS∶41 | 362 | 1 | 8 | 2 | 49 | 4 | 11 | 66 |
| RM3198 (367.95) | 1995 | SA | GBS | A | HS∶41 | 362 | 1 | 8 | 2 | 49 | 4 | 11 | 66 |
| RM3201 (378.96) | 1996 | SA | Enteritis | A | HS∶41 | 362 | 1 | 8 | 2 | 49 | 4 | 11 | 66 |
| RM3203 (16.97) | 1997 | SA | Enteritis | H | HS∶12 | 137 | 4 | 1 | 7 | 10 | 4 | 42 | 7 |
| RM3204 (20.97) | 1997 | SA | Enteritis | H | HS∶12 | 137 | 4 | 1 | 7 | 10 | 4 | 42 | 7 |
| RM3205 (199.97) | 1997 | SA | Enteritis | A | HS∶41 | 362 | 1 | 8 | 2 | 49 | 4 | 11 | 66 |
| RM3206 (242.98) | 1998 | SA | MFS | A | HS∶41 | 362 | 1 | 8 | 2 | 49 | 4 | 11 | 66 |
| RM3207 (250.97) | 1997 | SA | Enteritis | A | HS∶41 | 362 | 1 | 8 | 2 | 49 | 4 | 11 | 66 |
| RM3208 (1.98) | 1998 | SA | Enteritis | H | HS∶21 | 137 | 4 | 1 | 7 | 10 | 4 | 42 | 7 |
| RM3209 (24.98) | 1998 | SA | Enteritis | H | HS∶12 | 137 | 4 | 1 | 7 | 10 | 4 | 42 | 7 |
| RM3211 (96.00) | 2000 | SA | GBS | A | HS∶33 | 1472 | 2 | 127 | 2 | 42 | 4 | 90 | 25 |
| RM3430 | Unk | CAN | Unk | A | HS∶41 | 41 | 16 | 8 | 2 | 16 | 62 | 3 | 9 |
| RM4186 (390.96) | 1996 | SA | Enteritis | Unk | Unk | 354 | 8 | 6 | 10 | 2 | 2 | 11 | 12 |
| RM4187 (172.03) | 2003 | SA | Enteritis | F | Unk | 1473 | 28 | 129 | 34 | 27 | 33 | 45 | 36 |
| RM4191 (160.03) | 2003 | SA | Enteritis | G | Unk | 587 | 1 | 8 | 2 | 42 | 4 | 90 | 25 |
| RM4192 (124.03) | 2003 | SA | Enteritis | F | Unk | 1474 | 14 | 6 | 17 | 5 | 2 | 2 | 3 |
| RM4193 (MF 321317.03) | 2003 | SA | MFS | B | Unk | 730 | 2 | 5 | 4 | 5 | 2 | 2 | 1 |
| RM4194 (180.03) | 2003 | SA | Enteritis | H | Unk | 436 | 7 | 44 | 21 | 5 | 62 | 4 | 61 |
| RM4196 (126.01) | 2001 | SA | Enteritis | B | Unk | 429 | 7 | 5 | 4 | 1 | 2 | 11 | 1 |
| RM4197 (MF 996.00) | 2000 | SA | MFS | F | Unk | 257 | 9 | 6 | 2 | 4 | 62 | 4 | 5 |
| RM4269 (367.95) | 1995 | SA | GBS | A | HS∶41 | 362 | 1 | 8 | 2 | 49 | 4 | 11 | 66 |
| RM4270 (287.96) | 1996 | SA | Enteritis | A | HS∶41 | 362 | 1 | 8 | 2 | 49 | 4 | 11 | 66 |
| RM4271 (302.96) | 1996 | SA | Enteritis | B | HS∶33 | 1043 | 10 | 7 | 27 | 33 | 44 | 10 | 5 |
| RM4273 (375.96) | 1996 | SA | Enteritis | B | HS∶02 | 824 | 9 | 6 | 2 | 2 | 2 | 11 | 5 |
| RM4274 (379.96) | 1996 | SA | Enteritis | Unk | HS∶42 | 1475 | 2 | 6 | 4 | 5 | 93 | 11 | 201 |
| RM4275 (398.96) | 1996 | SA | Enteritis | H | HS∶37 | 51 | 7 | 12 | 17 | 2 | 15 | 23 | 3 |
| RM4277 (422.96) | 1996 | SA | Enteritis | F | HS∶04,16, 43,50 | 52 | 9 | 6 | 25 | 2 | 10 | 22 | 3 |
| RM4278 (423.96) | 1996 | SA | Enteritis | F | HS∶05 | 52 | 9 | 6 | 25 | 2 | 10 | 22 | 3 |
| RM4279 (424.96) | 1996 | SA | Enteritis | H | HS∶37 | 51 | 7 | 12 | 17 | 2 | 15 | 23 | 3 |
| RM4281 (1.97) | 1996 | SA | Enteritis | C | Unk | 19 | 2 | 5 | 1 | 5 | 3 | 2 | 1 |
| RM4282 (18.97) | 1996 | SA | Enteritis | H | HS∶50, 62 | 51 | 7 | 12 | 17 | 2 | 15 | 23 | 3 |
Original strain number is designated in parenthesis.
Unk, unknown.
UK, United Kingdom; USA, United States of America; SA, South Africa; MEX, Mexico; CAN, Canada.
GBS, Guillain-Barré syndrome; MFS, Miller Fisher syndrome.
Penner heat-stable (HS) serotypes.
Multilocus sequence typing of C. jejuni strains
The C. jejuni strains in Table 1 were typed by using the C. jejuni MLST primer sets as described previously [34], [45]. The Perl program MLSTparser [45] was used to extract allele sequences and assign allele numbers and sequence types. All allelic sequences were queried against the Campylobacter jejuni/coli MLST database (http://pubmlst.org/campylobacter/) to assign numbers to alleles already present in the database. Novel alleles and sequence types (STs) were submitted to the MLST database to obtain new numbers. Each novel C. jejuni ST was compared to existing C. jejuni STs by concatenating the allelic sequences at all loci for that ST and performing either pairwise BLASTN comparisons against similarly concatenated C. jejuni ST sequences or performing CLUSTALX alignments [45], [46]. A dendrogram was constructed by using the aligned concatenated sequences and the neighbor-joining method with the Kimura two-parameter distance estimation method. Subsequent phylogenetic analyses were performed by using MEGA version 2.1, as in previous studies [46].
Construction of the C. jejuni DNA microarray
DNA fragments of individual open reading frames (ORFs) were amplified by using the Sigma-Genosys (The Woodlands, TX) C. jejuni ORFmer primer set specific for strain NTCT11168 coding sequences and by using primers from Operon Technologies (Alameda, CA) specific for unique sequences in strain RM1221, as previously described [40]. Additional DNA fragments were amplified from strain RM3193 (Table 1) based on the published sequence for the serotype HS∶41 capsular locus ORFs [35] and for LOS genes from locus classes A, B, C, E and F, as in previous studies [27]. A total of 1530, 227, 40 and 28 PCR products were amplified from strain NCTC 11168, strain RM1221, LOS genes and serotype HS∶41 capsule genes, respectively. The PCR products were purified on a Qiagen 8000 robot by using a Qiaquick 96-well Biorobot kit (Qiagen, Valencia, CA) and spotted in duplicate onto Ultra-GAPS glass slides (Corning Inc., Corning, N. Y.) by using an OmniGrid Accent (GeneMachines, Ann Arbor, MI), as described previously [40]. Immediately after printing, the microarrays were UV crosslinked at 300 mJoules by using a Stratalinker UV Crosslinker 1800 (Stratagene, La Jolla, CA) and stored in a desiccator. Before use, microarrays were blocked with Pronto! Pre-Hybridization Solution (Corning Inc.), according to the manufacturer's specifications.
Preparation and fluorescent labeling of genomic DNA and microarray hybridization
Genomic DNA from C. jejuni was prepared as described previously [27] or purified by using the Wizard Genomic DNA kit (Promega, Madison, WI), according to the manufacturer's specifications. Each microarray hybridization reaction consisted of genomic DNAs from the reference strains (an equal amount of DNA from C. jejuni strain NCTC11168 and strain RM1221) and from a test strain that were fluorescently labeled with indodicarbocyanine (Cy5) and indocarbocyanine (Cy3), respectively. When examining the Penner serotype HS∶41 strains, genomic DNA was purified from the reference strain RM3193. Approximately 2 µg of DNA was mixed with 5 µl 10× NEBlot labeling buffer containing random octadeoxyribonucleotides (New England Biolabs, Beverly, MA) and water to a final volume of 41 µl. This mixture was heated to 95°C for 5 min, cooled for 5 min at 4°C and added to the remainder of the labeling reaction consisting of 5 µl of 10× dNTP labeling mix (1.2 mM each dATP, dGTP, dCTP; 0.5 mM dTTP in 10 mM Tris pH 8.0; 1 mM EDTA) (Promega Corporation, Madison, WI), 3 µl of 25 nmole Amersham CyDye fluorescent nucleotides, Cy3-dUTP or Cy5-dUTP (GE Healthcare Life Sciences Corp., Piscataway, NJ), and 5 U of Klenow fragment (New England Biolabs). The labeling reactions were incubated overnight at 37°C, as previously described [40]. Labeled DNA was purified from unincorporated CyDye fluorescent label by using Qiaquick PCR Cleanup kit (Qiagen, Valencia, CA) and dried by vacuum.
DNA microarray hybridization and data analysis
Labeled reference and test DNAs were combined in a 45 µl Pronto! cDNA hybridization solution (Corning Inc., Corning, NY), heated to 95°C for 5 min and immediately centrifuged at 14,500× g for 2 min at room temperature. Fifteen µl of this hybridization mixture was added to each microarray and sealed with a coverslip. The microarray slide was placed in a hybridization chamber (Corning Inc.) and incubated at 42°C for 18 h. Following hybridization, the slides were washed and dried by centrifugation at 300× g for 10 min before scanning, as previously described [40]. At least two hybridization reactions were performed for each test strain and were quantified further as described below.
DNA microarrays were scanned by using an Axon GenePix 4000B microarray laser scanner (Molecular Devices Corporation, Sunnyvale, CA) at 532 nm (Cy3) and 635 nm (Cy5) excitation wavelengths with a 10 µm resolution, as described previously [40]. Briefly, after scanning of the DNA microarrays, features and local background intensities were detected and quantified with GenePix 4.0 software (Molecular Devices). Spots were excluded from further analysis if they contained an anomalous spot morphology or were within regions of non-specific fluorescence. The data were filtered by discarding spots with a reference signal lower than the background plus 3 standard deviations of the background. Signal intensities were corrected by subtracting the local background, and calculating the Cy5/Cy3 ratios. To compensate for unequal dye incorporation, data normalization was performed as described previously [40], [47]. Only half of the reference DNA (Cy5-labeled mixture of NCTC 11168 and RM1221 DNA) hybridized to the NCTC 11168 and RM1221 strain-specific spots, increasing the Cy3/Cy5 ratio by twofold. Therefore, the ratios for these NCTC 11168 and RM1221 strain-specific spots were divided by 2 before determining the status of the gene. The ratios for spots of each individual gene were then averaged. As previously described [40], the comparative genomic indexing analysis defined the status of a gene as present when the Cy3/Cy5 (test/reference) intensity ratio was >0.6, as divergent when the Cy3/Cy5 intensity ratio was between 0.6 and 0.3, and as highly divergent or absent when the Cy3/Cy5 intensity ratio was <0.3. For LOS class A, B, and E specific genes that are not present in the reference strains (NCTC 11168 and RM1221), the status of these genes was still determined by the Cy3/Cy5 intensity ratio. Given that the Cy5 intensity represented only nonspecific hybridization, the status of these LOS class-specific genes was defined as present when the Cy3/Cy5 (test/reference) intensity ratio was >3, and as absent when the Cy3/Cy5 intensity ratio was <3. The LOS gene status was confirmed by PCR described previously (Parker, et al., 2005). The status for all genes was converted into trinary scores (present = 2; divergent = 1; highly divergent or absent = 0). The trinary scores for all genes for each strain were further analyzed with GeneSpring microarray analysis software version 7.3 (Agilent Technologies, Santa Clara, CA) and subjected to average-linkage hierarchical clustering with the standard correlation and bootstrapping. The comparative genomic indexing analysis to assign present or absent genes of the GBS-associated strains with HS∶41 serotype was performed by using strain RM3193 as a reference strain and the GENCOM software [41], [48]. For each of these hybridizations, the Cy5 and Cy3 signal intensities were corrected by subtracting the local background before submission into the GENCOM program for determining the present or absent assignments for each gene. The microarray data has been deposited in the NCBI Gene Expression Omnibus (GEO) repository (http://www.ncbi.nlm.nih.gov/geo/) with the accession number GSE9862.
Results
Multilocus sequence typing analysis of the South African strains
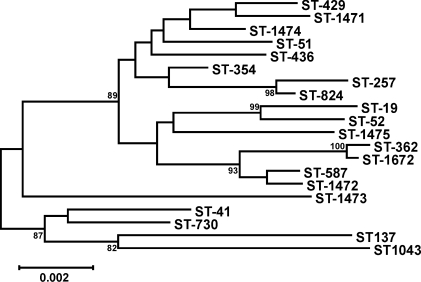
To examine the genetic variation among 32 C. jejuni clinical strains from South Africa, multilocus sequence typing (MLST) was employed to analyze the nucleic acid sequences of seven C. jejuni housekeeping loci (aspA, atpA (uncA), glnA, gltA, glyA, pgm, and tkt). As shown in Table 1, a total of 18 different sequence types (ST) were identified among these C. jejuni clinical strains. ST-51, ST-52, ST-137 and ST-362 were represented by more than one strain, and several strains with ST-51, ST-52 and ST-137 had different serotypes (Table 1). In contrast, the analysis demonstrated that C. jejuni strains from South Africa with serotype HS∶41 were all assigned ST-362. However, different sequence types were observed for three serotype HS∶41 strains from other geographical locations, Mexico and Canada. Of particular interest, the strains from Mexico shared 4 alleles (aspA, atpA, glnA and glyA) with the South African HS∶41 strains. To further examine the relatedness of the serotype HS∶41 strains from these two geographical locations, a dendrogram was constructed by using the neighbor joining method for pairwise comparisons of the concatenated seven allele sequences (Figure 1). The phylogenetic analysis demonstrated that the South African HS∶41 strains, members of ST-362, belong to the same clade as the two ST-1672 strains from Mexico with a bootstrap value of 100% (Figure 1), indicating that these strains from different geographical locations are highly similar by MLST.
Figure 1. Dendrogram of Campylobacter jejuni sequence types, including clinical strains from South Africa, Mexico and Canada.
The dendrogram was constructed by using the neighbor-joining algorithm and the Kimura two-parameter distance estimation method. Bootstrap values of >75%, generated from 500 replicates, are shown at the nodes. The scale bar represents substitutions per site.
Comparative genomic indexing of the South African strains
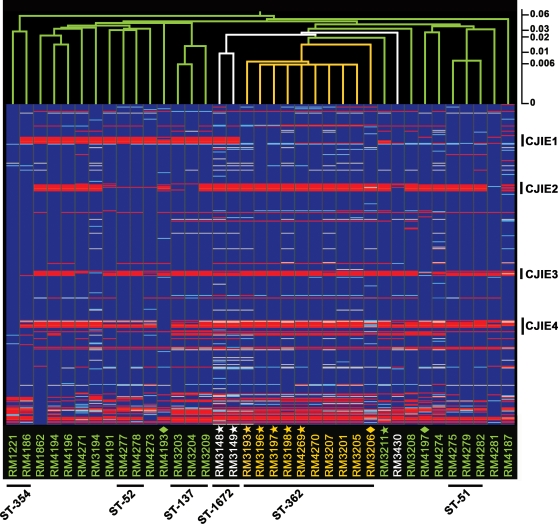
The intraspecies genetic diversity found in the South African clinical strains was examined at the whole-genome level by DNA microarrays. A comparative analysis was performed with a multi-strain C. jejuni DNA microarray based on sequence data from the genome strains NCTC 11168 and RM1221 (see Materials and Methods). In this microarray analysis, a hierarchical clustering was performed to further examine the relationship among the clinical strains by using a standard correlation function where the linkage distance between strains is represented by branch lengths in the resulting cluster. Analysis of the comparative microarray data demonstrated that strains with the same sequence type clustered together (Figure 2), demonstrating a strong correlation with our MLST analysis. A distinct subcluster with a distance score of 0.016 was observed for all serotype HS∶41 strains from South African patients with GBS, MFS or enteritis in ST-362 (Figure 2). Clustering next to this group with a distance score of 0.026 were the two GBS-associated HS∶41 strains from Mexico both with ST-1672. In contrast, strains from enteritis cases with distinct serotypes HS∶12 and HS∶37 in ST-137 and ST-51, respectively, formed two separate clusters that showed divergence from the main cluster composed of serotype HS∶41 strains (Figure 2).
Figure 2. Genome comparison of Campylobacter jejuni clinical strains by DNA microarrays analysis.
An average linkage hierarchical clustering of the C. jejuni strains with a distance score scale bar was compiled in GeneSpring version 7.3 with the standard correlation and bootstrapping (see Materials and Methods). The gene status based on cutoff values of absence and presence predictions is shown color-coded: blue, present; light blue, divergent; red, highly divergent or absent; white, no data. C. jejuni strains from South Africa with HS∶41 serotype (yellow), with other serotypes (green), or strains with HS∶41 serotype from Mexico and Canada (white) are designated vertically across the bottom. GBS-associated strains are annotated with stars; MFS-associated strains are annotated with diamonds. The four C. jejuni-integrated elements (CJIEs) and the assigned MLST sequence type (ST) for each strain cluster is indicated.
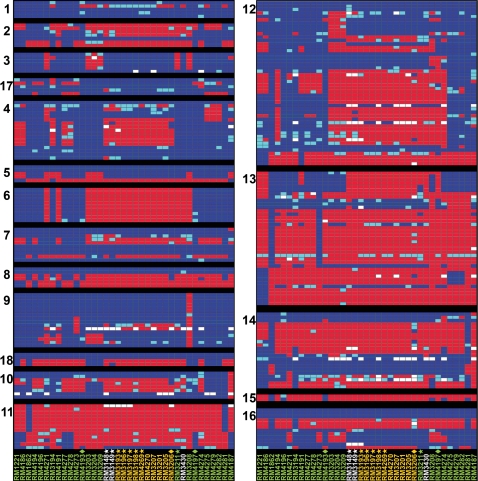
Using the trinary score for present, absent or divergent genes, 17.8% (314 of 1757) of the genes were absent or highly divergent in at least one South African strain when compared to the genome strains represented on the microarray (Figure 3). Most of the variable genes in the South African strains were found in the hypervariable genomic regions (Table 2), regions previously described to be involved in capsule (region 13) and LOS (region 11) biosynthesis, flagellar modification and O-linked glycosylation (region 12) and type I restriction-modification (region 14) [42]. Further analysis of a subset of the South African strains with the HS∶41 serotype demonstrated that genes in four hypervariable genomic regions (regions 4, 9, 12 and 13) were different from both strains NCTC 11168 and RM1221 (Figure 3). Interestingly, region 4 (CJE0340-CJE0355), encoding proteins involved in pantothenate and biotin biosynthesis and for a molybdenum ABC transporter (Table 2), was the only region that was highly divergent in all HS∶41 strains from South Africa and Mexico but present in the HS∶41 strain from Canada (Figure 3).
Figure 3. Patterns of presence, absence or divergence in the 18 hypervariable regions in Campylobacter jejuni strains.
A detailed genomic analysis of the 18 hypervariable regions (Table 2) was compiled in GeneSpring version 7.3 with the standard correlation and bootstrapping (see Materials and Methods). Each panel represents a hypervariable region, and each column corresponds to a C. jejuni strain designated vertically across the bottom, as described in the legend to Figure 2. The gene status based on cutoff values of absence and presence predictions is shown color-coded: blue, present; light blue, divergent; red, highly divergent or absent; white, no data.
Table 2. Intraspecies hypervariable regions in Campylobacter jejuni.
| Region | Genesa | Proposed function |
| 1 | CJE0031-CJE0035 (Cj0032-Cj0036) | Type IIS restriction/modification |
| 2 | CJE0051-CJE0055 (Cj0055c-Cj0059c) | Unknown |
| 3 | CJE0170-CJE0175 (Cj0177-Cj0182) | Putative iron transport, biopolymer transport, putative TonB transport protein |
| 17 | CJE0308-CJE0313 (Cj0258-Cj0263) | Putative zinc transport; dihydroorotase; homodimeric type |
| 4 | CJE0340–CJE0355 (Cj0294-Cj0310c) | Pantothenate and biotin biosynthesis pathway; molybdenum ABC transporter |
| 5 | CJE0470-CJE0472 (Cj0421c-Cj0425) | Unknown |
| 6 | CJE0530-CJE0538 (Cj0480c-Cj0490) | Unknown; UxaA family hydrolase |
| 7 | CJE0666-CJE0673 (Cj0561c-Cj0571) | Unknown |
| 8 | CJE0728-CJE0732 (Cj0625-Cj0629) | Hydrogenase formation proteins; type III restriction/modification |
| 9 | CJE0828-CJE0844 (Cj0727-Cj0755) | Phosphate regulated genes; iron uptake; TonB transport protein; ferric enterobactin uptake receptor |
| 18 | CJE0944-CJE0947 (Cj0857c-Cj0860) | Unknown |
| 10 | CJE1047-CJE1056 (Cj0967-Cj0975) | Unknown |
| 11 | CJE1278-CJE1281 (Cj1135-Cj1145c) | Lipooligosaccharide biosynthesis |
| 12 | CJE1485-CJE1532 (Cj1293-Cj1343) | Flagellar modification; O-linked glycosylation |
| 13 | CJE1601-CJE1622 (Cj1414c-Cj1449c) | Capsule biosynthesis |
| 14 | CJE1714-CJE1733 (Cj1543c-Cj1563c) | Type I restriction/modification; unknown |
| 15 | (Cj1677-Cj1679) | Unknown |
| 16 | CJE1888-CJE1896 (Cj1717c-Cj1729c) | 2-isopropylmalate synthase; isopropylmalate dehydrogenase; 3-isopropylmalate dehydratase; unknown |
Analysis of the comparative microarray data of the LOS region showed that all six strains from South African patients with GBS possessed class A (Table 1). This finding correlated with previous reports that demonstrated that the class A locus, a class that has been associated with the GM1 ganglioside-like structure [8], [9], [49], was overrepresented in GBS-associated strains [8], [26], [27]. In the present study, this LOS locus class was identified also in the four enteritis-associated strains with the HS∶41 serotype (Table 1). Similar to previous findings [50], most GBS-associated strains examined in this study bound cholera toxin (data not shown), which binds highly selectively to GM1 gangliosides [9], [51], demonstrating that these strains may have a similar LOS outer core structure. In contrast, the GBS-associated strains RM3196 and RM3198 failed to bind cholera toxin. These observations suggested that strains RM3196 and RM3198 with a class A LOS locus may prevalently synthesize ganglioside mimics with an outer core structure that is different from a GM1-like ganglioside, as recently demonstrated [9]. Interestingly, the MFS-associated strains RM3206, RM4193, and RM4197 with distinct LOS locus classes A, B, and F, respectively, also failed to bind cholera toxin (data not shown), as demonstrated previously in a recent study showing a lack of cholera toxin binding by all MFS-associated strains [50].
To identify potential differences within the genome of GBS-associated strains, the genetic relatedness of the HS∶41 South African strains from GBS and enteritis patients was examined further by using the GENCOM software [41], [48] and the GBS-associated strain RM3193 as a reference strain (see Materials and Methods). In this particular analysis, the cutoff values between divergent and similar genes were established to represent a 95% nucleotide sequence identity for each gene examined between the reference strain RM3193 and the remaining South African strains with HS∶41 serotype (Table 1). The regression slope had a linear relationship between the logarithms of the red Cy5 (reference RM3193 strain) and the green Cy3 (tested HS∶41 strain) fluorescence (data not shown). These results indicated that the genome content of these GBS- and enteritis-associated strains with HS∶41 serotype, a serotype considered to be clonal and highly stable [19], was very similar. Based on this DNA microarray analysis, differences in gene content could not be identified in the HS∶41 South African strains from GBS and enteritis patients.
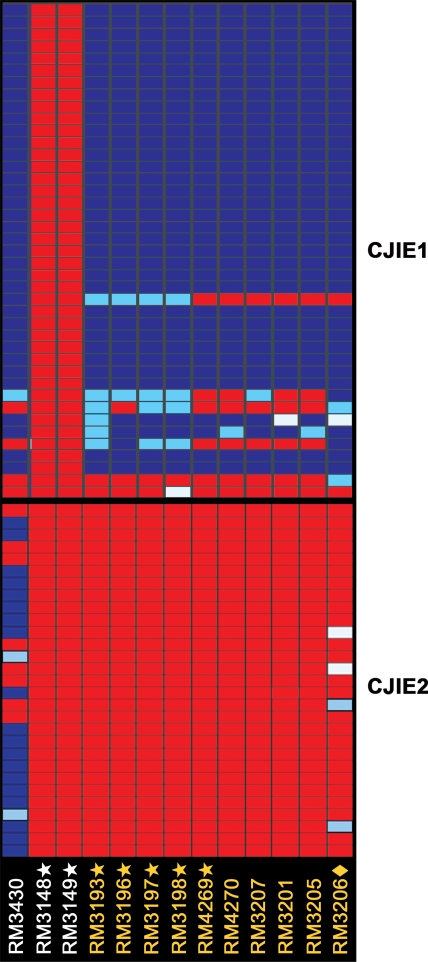
The comparative genomic analysis further assessed for the presence of CJIEs, genomic integrated elements initially identified in strain RM1221 [40], [43], in the C. jejuni strains from South Africa. From the hierarchical cluster analysis that examined the relationships among the four CJIEs for each strain, the presence of genes in at least one of the four CJIEs was identified in each South African strain (Figure 2). Interestingly, the clustering analysis demonstrated that all serotype HS∶41 strains from South Africa contained most genes in the CJIE1 genomic element, as in strain RM1221 (Figure 4), whereas genes in the other three genomic elements, CJIE2, CJIE3, and CJIE4, were absent in all of these strains (Figures 2 and 4). By using our trinary score for genes present, absent or divergent in sequence (see Materials and Methods), no distinct features in the CJIE1 element were observed for any of the HS∶41 South African strains from GBS patients when compared to strains from enteritis patients (Figure 4). Furthermore, the hybridization patterns for the genes in the CJIEs were compared with serotype HS∶41 strains from other geographical locations. The hierarchical analysis demonstrated the presence of genes for both CJIE1 and CJIE2 genomic elements in strain RM3430, a serotype HS∶41 strain from Canada (Figure 4). When compared to CJIE genes represented in the DNA microarray, both CJIE1 and CJIE2 genes were absent in the Mexican HS∶41 strains RM3148 and RM3149 (Figure 4). These results indicated that the inclusion of CJIE sequences, RM1221-like genomic elements, in our microarray analysis distinguished the gene content of the clonal HS∶41 South African strains from three HS∶41 strains from Canada and Mexico.
Figure 4. Patterns of presence, absence or divergence in the Campylobacter jejuni-integrated elements.
A detailed genomic analysis of the genomic integrated elements CJIE1 (top) and CJIE2 (bottom) was compiled in GeneSpring version 7.3 with the standard correlation and bootstrapping (see Materials and Methods). Each column corresponds to a C. jejuni strain designated vertically across the bottom, as described in the legend to Figure 2. The gene status based on cutoff values of absence and presence predictions is shown color-coded: blue, present; light blue, divergent; red, highly divergent or absent; white, no data.
Discussion
The present study performed a comparative genomic analysis, by using the sequence-based typing methods MLST and DNA microarrays, to examine the genetic polymorphism of C. jejuni strains that were isolated from South African patients with various disease outcomes, enteritis, GBS or MFS. The hierarchical cluster analysis by DNA microarrays showed that C. jejuni strains with the same sequence type clustered together. Our MLST and microarray analysis also demonstrated that all of the South African strains with HS∶41 serotype, a serotype considered to be clonal and highly stable [19], are clearly distinct from the other South African strains and that they are also distinct from other HS∶41 strains from other three strains from Canada and Mexico. Although several reports demonstrated regions of high variability between other strains of C. jejuni and strain NCTC 11168 [36]–[39], [41], [42], the gene indexing analysis performed in this study, by using a microarray containing genes from both genome strains NCTC11168 and RM1221 [40], identified a total of 18 intraspecies hypervariable regions in the genome from the South African strains, as previously reported for other C. jejuni strains from various geographical locations and sources [40]. Intriguingly, only hypervariable region 4 (CJE0340-CJE0355), encoding proteins involved in pantothenate and biotin biosynthesis and for a molybdenum ABC transporter, was highly divergent in all HS∶41 strains from South Africa and Mexico but present in the HS∶41 strain from Canada. A previous study documented that this region was distinct between strain NCTC 11168 and RM1221, and similar results were obtained when the hybridization pattern for additional C. jejuni isolates from various locations and sources was examined [40]. These findings have led to the proposal that an additional level of diversity may be found in the hypervariable region 4 [40]. Still to be determined is the role of genomic diversity within this region and whether the divergence of these genes is characteristic of certain serotype HS∶41 strains.
Inclusion in this microarray of the coding sequences within the four CJIEs, identified initially in strain RM1221 [40], [43], differentiated the South African HS∶41 strains from the two closely-related HS∶41 strains from Mexico. Only CJIE1, a Campylobacter Mu-like prophage [40], [43], was present in the South African HS∶41 strains whereas all four CJIEs were absent in the HS∶41 strains from Mexico. A more distantly-related HS∶41 strain from Canada possessed both CJIE1 and CJIE2. In addition, the presence of bacteriophage-related genes in CJIE1 may probably contribute to increasing the genomic diversity of these C. jejuni strains. These findings demonstrated that these CJIEs are additional regions of high variability between strains of C. jejuni and that these genomic regions could be utilized in the differentiation of closely-related C. jejuni strains.
The comparative analysis further investigated any potential association between specific classes of LOS biosynthesis loci and GBS. Our DNA microarray analysis demonstrated that all of GBS-associated strains possessed the class A LOS whereas only 17% (4 of 24) of the enteritis-control strains also possessed this class of LOS. Indeed, it has been speculated that the C. jejuni-induced neuropathies are probably related to the molecular mimicry between gangliosides in nerve tissue and LOS on the C. jejuni cell surface [7]. Biochemical and serological studies have revealed that GBS-associated strains exhibit ganglioside-like structures in their LOS [50], [52]. More specifically, strains possessing the class A locus have been shown to express an LOS outer core that mimics gangliosides [23]–[25], and the occurrence of the class A locus has been correlated strongly with GBS-associated strains [8], [9], [26], [27]. Finally, the only substantial differences between GBS-associated and control C. jejuni strains appears to date to reside in the LOS-biosynthetic gene locus [8], [25], [26], [53]. Recent comparative genomic analysis have proposed that GBS markers may be located only in the LOS biosynthesis region [25], [53], [54].
Previous studies that employed MLST and DNA microarrays could not identify GBS- specific genetic markers by comparing the genomes of C. jejuni strains from various geographical locations, serotypes, and disease outcomes [39], [55]. Furthermore, no molecular markers specific to the GBS syndrome were detected after analyzing a highly clonal HS∶41 population from South African patients by using a different typing technique, high-throughput amplified fragment length polymorphism [53]. Similar results were obtained in the present study by using DNA microarrays to compare the genome content of South African C. jejuni GBS-associated and enteritis strains with the HS∶41 serotype. There are several potential reasons why no GBS-specific molecular markers in C. jejuni were identified in this study. First, our DNA microarray was based on the genome sequence of strains NCTC 11168 and RM1221, and genes that are not present in these strains used to construct the microarray will not be detected. Furthermore, single nucleotide changes that may result in point mutations leading to biological differences, but not detectable by DNA microarrays, may be an important factor in the development of GBS. However, these genetic differences could be disclosed potentially by a more detailed DNA analysis identifying single-nucleotide polymorphisms (SNPs). Just recently, SNPs have been used successfully for comparing entire genomes in clinically-relevant strains of Mycobacterium tuberculosis [56], Helicobacter pylori [57], E. coli [58] and S. enterica [59]. Furthermore, specific C. jejuni GBS-features may rather involve differential expression of Campylobacter virulence factors and could be revealed by expression analysis of GBS-associated strains. Indeed, this approach might identify regulatory or virulence-related genes or gene loci that represent mechanisms important in the development of molecular mimicry associated with GBS. Given that there is broad variability in the severity and spectrum of clinical symptoms in GBS patients [12], [13], host-susceptibility may play a substantial role in the development of GBS [60], [61]. Thus, further characterization of the interaction between the host and C. jejuni is needed to better understand the pathogenesis of Campylobacter-induced GBS.
Acknowledgments
The authors wish to thank Sharon T. Horn, Anna H. Bates and Guilin Wang for technical assistance and Irving Nachamkin and David Woodward for providing bacterial strains used in this study. This publication made use of the Campylobacter MultiLocus Sequence Typing website (http://pubmlst.org/campylobacter/) developed by Keith Jolley [62] and sited at the University of Oxford, United Kingdom. This work was part of a United States collaboration in the European Commission Fifth Framework Project QLK1-CT-2002-0220, “CAMPYCHECK.”
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the United States Department of Agriculture, Agricultural Research Service CRIS project number 5325-42000-045 and the South African Medical Research Council.
References
- 1.Allos BM. Campylobacter jejuni infections: update on emerging issues and trends. Clin Infect Dis. 2001;32:1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- 2.Snelling WJ, Matsuda M, Moore JE, Dooley JS. Under the microscope: Campylobacter jejuni. Lett Appl Microbiol. 2005;41:297–302. doi: 10.1111/j.1472-765X.2005.01788.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller WG, Mandrell RE. Prevalence of Campylobacter in the food and water supply: incidence, outbreaks, isolation and detection. In: Ketley J, Konkel ME, editors. Campylobacter: Molecular and Cellular Biology. Norfolk, UK: Horizon Bioscience; 2005. pp. 101–163. [Google Scholar]
- 4.Blaser MJ, Berkowitz ID, LaForce FM, Cravens J, Reller LB, et al. Campylobacter enteritis: clinical and epidemiologic features. Ann Intern Med. 1979;91:179–185. doi: 10.7326/0003-4819-91-2-179. [DOI] [PubMed] [Google Scholar]
- 5.Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 6.Overell JR, Willison HJ. Recent developments in Miller Fisher syndrome and related disorders. Curr Opin Neurol. 2005;18:562–566. doi: 10.1097/01.wco.0000173284.25581.2f. [DOI] [PubMed] [Google Scholar]
- 7.Yuki N, Koga M. Bacterial infections in Guillain-Barré and Fisher syndromes. Curr Opin Neurol. 2006;19:451–457. doi: 10.1097/01.wco.0000245367.36576.e9. [DOI] [PubMed] [Google Scholar]
- 8.Godschalk PC, Heikema AP, Gilbert M, Komagamine T, Ang CW, et al. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barré syndrome. J Clin Invest. 2004;114:1659–1665. doi: 10.1172/JCI15707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godschalk PC, Kuijf ML, Li J, St Michael F, Ang CW, et al. Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barré and Miller Fisher syndromes. Infect Immun. 2007;75:1245–1254. doi: 10.1128/IAI.00872-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Belkum A, van den Braak N, Godschalk P, Ang W, Jacobs B, et al. A Campylobacter jejuni gene associated with immune-mediated neuropathy. Nat Med. 2001;7:752–753. doi: 10.1038/89831. [DOI] [PubMed] [Google Scholar]
- 11.Perera VN, Nachamkin I, Ung H, Patterson JH, McConville MJ, et al. Molecular mimicry in Campylobacter jejuni: role of the lipo-oligosaccharide core oligosaccharide in inducing anti-ganglioside antibodies. FEMS Immunol Med Microbiol. 2007;50:27–36. doi: 10.1111/j.1574-695X.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- 12.Tatsumoto M, Koga M, Gilbert M, Odaka M, Hirata K, et al. Spectrum of neurological diseases associated with antibodies to minor gangliosides GM1b and GalNAc-GD1a. J Neuroimmunol. 2006;177:201–208. doi: 10.1016/j.jneuroim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 13.van der Meché FG, Visser LH, Jacobs BC, Endtz HP, Meulstee J, et al. Guillain-Barré syndrome: multifactorial mechanisms versus defined subgroups. J Infect Dis. 1997;176(Suppl 2):S99–102. doi: 10.1086/513779. [DOI] [PubMed] [Google Scholar]
- 14.Goddard EA, Lastovica AJ, Argent AC. Campylobacter 0∶41 isolation in Guillain-Barré syndrome. Arch Dis Child. 1997;76:526–528. doi: 10.1136/adc.76.6.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroki S, Saida T, Nukina M, Haruta T, Yoshioka M, et al. Campylobacter jejuni strains from patients with Guillain-Barré syndrome belong mostly to Penner serogroup 19 and contain β-N-acetylglucosamine residues. Ann Neurol. 1993;33:243–247. doi: 10.1002/ana.410330304. [DOI] [PubMed] [Google Scholar]
- 16.Lastovica AJ, Goddard EA, Argent AC. Guillain-Barré syndrome in South Africa associated with Campylobacter jejuni O∶41 strains. J Infect Dis. 1997;176(Suppl 2):S139–143. doi: 10.1086/513796. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi M, Koga M, Yokoyama K, Yuki N. Epidemiology of Campylobacter jejuni isolated from patients with Guillain-Barré and Fisher syndromes in Japan. J Clin Microbiol. 2005;43:335–339. doi: 10.1128/JCM.43.1.335-339.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nachamkin I, Engberg J, Gutacker M, Meinersman RJ, Li CY, et al. Molecular population genetic analysis of Campylobacter jejuni HS∶19 associated with Guillain-Barré syndrome and gastroenteritis. J Infect Dis. 2001;184:221–226. doi: 10.1086/322008. [DOI] [PubMed] [Google Scholar]
- 19.Wassenaar TM, Fry BN, Lastovica AJ, Wagenaar JA, Coloe PJ, et al. Genetic characterization of Campylobacter jejuni O∶41 isolates in relation with Guillain-Barré syndrome. J Clin Microbiol. 2000;38:874–876. doi: 10.1128/jcm.38.2.874-876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endtz HP, Ang CW, van Den Braak N, Duim B, Rigter A, et al. Molecular characterization of Campylobacter jejuni from patients with Guillain-Barré and Miller Fisher syndromes. J Clin Microbiol. 2000;38:2297–2301. doi: 10.1128/jcm.38.6.2297-2301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engberg J, Nachamkin I, Fussing V, McKhann GM, Griffin JW, et al. Absence of clonality of Campylobacter jejuni in serotypes other than HS∶19 associated with Guillain-Barré syndrome and gastroenteritis. J Infect Dis. 2001;184:215–220. doi: 10.1086/322010. [DOI] [PubMed] [Google Scholar]
- 22.Karlyshev AV, Linton D, Gregson NA, Lastovica AJ, Wren BW. Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Mol Microbiol. 2000;35:529–541. doi: 10.1046/j.1365-2958.2000.01717.x. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert M, Brisson JR, Karwaski MF, Michniewicz J, Cunningham AM, et al. Biosynthesis of ganglioside mimics in Campylobacter jejuni OH4384. Identification of the glycosyltransferase genes, enzymatic synthesis of model compounds, and characterization of nanomole amounts by 600-MHz 1H and 13C NMR analysis. J Biol Chem. 2000;275:3896–3906. doi: 10.1074/jbc.275.6.3896. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert M, Karwaski MF, Bernatchez S, Young NM, Taboada E, et al. The genetic basis for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J Biol Chem. 2002;277:327–337. doi: 10.1074/jbc.M108452200. [DOI] [PubMed] [Google Scholar]
- 25.Godschalk PC, van Belkum A, van den Braak N, van Netten D, Ang CW, et al. PCR-restriction fragment length polymorphism analysis of Campylobacter jejuni genes involved in lipooligosaccharide biosynthesis identifies putative molecular markers for Guillain-Barré syndrome. J Clin Microbiol. 2007;45:2316–2320. doi: 10.1128/JCM.00203-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koga M, Gilbert M, Takahashi M, Li J, Koike S, et al. Comprehensive analysis of bacterial risk factors for the development of Guillain-Barré syndrome after Campylobacter jejuni enteritis. J Infect Dis. 2006;193:547–555. doi: 10.1086/499969. [DOI] [PubMed] [Google Scholar]
- 27.Parker CT, Horn ST, Gilbert M, Miller WG, Woodward DL, et al. Comparison of Campylobacter jejuni lipooligosaccharide biosynthesis loci from a variety of sources. J Clin Microbiol. 2005;43:2771–2781. doi: 10.1128/JCM.43.6.2771-2781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colles FM, Jones K, Harding RM, Maiden MC. Genetic diversity of Campylobacter jejuni isolates from farm animals and the farm environment. Appl Environ Microbiol. 2003;69:7409–7413. doi: 10.1128/AEM.69.12.7409-7413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dingle KE, Colles FM, Ure R, Wagenaar JA, Duim B, et al. Molecular characterization of Campylobacter jejuni clones: a basis for epidemiologic investigation. Emerg Infect Dis. 2002;8:949–955. doi: 10.3201/eid0809.02-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dingle KE, Colles FM, Wareing DR, Ure R, Fox AJ, et al. Multilocus sequence typing system for Campylobacter jejuni. J Clin Microbiol. 2001;39:14–23. doi: 10.1128/JCM.39.1.14-23.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duim B, Godschalk PC, van den Braak N, Dingle KE, Dijkstra JR, et al. Molecular evidence for dissemination of unique Campylobacter jejuni clones in Curacao, Netherlands Antilles. J Clin Microbiol. 2003;41:5593–5597. doi: 10.1128/JCM.41.12.5593-5597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning G, Dowson CG, Bagnall MC, Ahmed IH, West M, et al. Multilocus sequence typing for comparison of veterinary and human isolates of Campylobacter jejuni. Appl Environ Microbiol. 2003;69:6370–6379. doi: 10.1128/AEM.69.11.6370-6379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sails AD, Swaminathan B, Fields PI. Utility of multilocus sequence typing as an epidemiological tool for investigation of outbreaks of gastroenteritis caused by Campylobacter jejuni. J Clin Microbiol. 2003;41:4733–4739. doi: 10.1128/JCM.41.10.4733-4739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker CT, Miller WG, Horn ST, Lastovica AJ. Common genomic features of Campylobacter jejuni subsp. doylei strains distinguish them from C. jejuni subsp. jejuni. BMC Microbiol. 2007;7:50. doi: 10.1186/1471-2180-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlyshev AV, Champion OL, Churcher C, Brisson JR, Jarrell HC, et al. Analysis of Campylobacter jejuni capsular loci reveals multiple mechanisms for the generation of structural diversity and the ability to form complex heptoses. Mol Microbiol. 2005;55:90–103. doi: 10.1111/j.1365-2958.2004.04374.x. [DOI] [PubMed] [Google Scholar]
- 36.Champion OL, Gaunt MW, Gundogdu O, Elmi A, Witney AA, et al. Comparative phylogenomics of the food-borne pathogen Campylobacter jejuni reveals genetic markers predictive of infection source. Proc Natl Acad Sci U S A. 2005;102:16043–16048. doi: 10.1073/pnas.0503252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorrell N, Mangan JA, Laing KG, Hinds J, Linton D, et al. Whole genome comparison of Campylobacter jejuni human isolates using a low-cost microarray reveals extensive genetic diversity. Genome Res. 2001;11:1706–1715. doi: 10.1101/gr.185801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leonard EE, 2nd, Takata T, Blaser MJ, Falkow S, Tompkins LS, et al. Use of an open-reading frame-specific Campylobacter jejuni DNA microarray as a new genotyping tool for studying epidemiologically related isolates. J Infect Dis. 2003;187:691–694. doi: 10.1086/368268. [DOI] [PubMed] [Google Scholar]
- 39.Leonard EE, 2nd, Tompkins LS, Falkow S, Nachamkin I. Comparison of Campylobacter jejuni isolates implicated in Guillain-Barré syndrome and strains that cause enteritis by a DNA microarray. Infect Immun. 2004;72:1199–1203. doi: 10.1128/IAI.72.2.1199-1203.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker CT, Quiñones B, Miller WG, Horn ST, Mandrell RE. Comparative genomic analysis of Campylobacter jejuni strains reveals genomic diversity due to genomic elements similar to those present in C. jejuni strain RM1221. J Clin Microbiol. 2006;44:4125–4135. doi: 10.1128/JCM.01231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearson BM, Pin C, Wright J, I'Anson K, Humphrey T, et al. Comparative genome analysis of Campylobacter jejuni using whole genome DNA microarrays. FEBS Lett. 2003;554:224–230. doi: 10.1016/s0014-5793(03)01164-5. [DOI] [PubMed] [Google Scholar]
- 42.Taboada EN, Acedillo RR, Carrillo CD, Findlay WA, Medeiros DT, et al. Large-scale comparative genomics meta-analysis of Campylobacter jejuni isolates reveals low level of genome plasticity. J Clin Microbiol. 2004;42:4566–4576. doi: 10.1128/JCM.42.10.4566-4576.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fouts DE, Mongodin EF, Mandrell RE, Miller WG, Rasko DA, et al. Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol. 2005;3:e15. doi: 10.1371/journal.pbio.0030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barton C, Ng LK, Tyler SD, Clark CG. Temperate bacteriophages affect pulsed-field gel electrophoresis patterns of Campylobacter jejuni. J Clin Microbiol. 2007;45:386–391. doi: 10.1128/JCM.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller WG, On SL, Wang G, Fontanoz S, Lastovica AJ, et al. Extended multilocus sequence typing system for Campylobacter coli, C. lari, C. upsaliensis, and C. helveticus. J Clin Microbiol. 2005;43:2315–2329. doi: 10.1128/JCM.43.5.2315-2329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoddard RA, Miller WG, Foley JE, Lawrence J, Gulland FMD, et al. Campylobacter insulaenigrae isolates from northern elephant seals (Mirounga angustirostris) in California. Appl Environ Microbiol. 2007;73:1729–1735. doi: 10.1128/AEM.01816-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anjum MF, Lucchini S, Thompson A, Hinton JC, Woodward MJ. Comparative genomic indexing reveals the phylogenomics of Escherichia coli pathogens. Infect Immun. 2003;71:4674–4683. doi: 10.1128/IAI.71.8.4674-4683.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pin C, Reuter M, Pearson B, Friis L, Overweg K, et al. Comparison of different approaches for comparative genetic analysis using microarray hybridization. Appl Microbiol Biotechnol. 2006;72:852–859. doi: 10.1007/s00253-006-0536-x. [DOI] [PubMed] [Google Scholar]
- 49.Yuki N, Taki T, Inagaki F, Kasama T, Takahashi M, et al. A bacterium lipopolysaccharide that elicits Guillain-Barré syndrome has a GM1 ganglioside-like structure. J Exp Med. 1993;178:1771–1775. doi: 10.1084/jem.178.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ang CW, Laman JD, Willison HJ, Wagner ER, Endtz HP, et al. Structure of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity and clinical features of Guillain-Barré and Miller Fisher patients. Infect Immun. 2002;70:1202–1208. doi: 10.1128/IAI.70.3.1202-1208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Angstrom J, Teneberg S, Karlsson KA. Delineation and comparison of ganglioside-binding epitopes for the toxins of Vibrio cholerae, Escherichia coli, and Clostridium tetani: evidence for overlapping epitopes. Proc Natl Acad Sci U S A. 1994;91:11859–11863. doi: 10.1073/pnas.91.25.11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nachamkin I, Liu J, Li M, Ung H, Moran AP, et al. Campylobacter jejuni from patients with Guillain-Barré syndrome preferentially expresses a GD(1a)-like epitope. Infect Immun. 2002;70:5299–5303. doi: 10.1128/IAI.70.9.5299-5303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Godschalk PC, Bergman MP, Gorkink RF, Simons G, van den Braak N, et al. Identification of DNA sequence variation in Campylobacter jejuni strains associated with the Guillain-Barré syndrome by high-throughput AFLP analysis. BMC Microbiol. 2006;6:32. doi: 10.1186/1471-2180-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taboada EN, van Belkum AF, Yuki N, Acedillo RR, Godschalk PC, et al. Comparative genomic analysis of Campylobacter jejuni associated with Guillain-Barré and Miller Fisher syndromes: neuropathogenic and enteritis-associated isolates can share high levels of genomic similarity. BMC Genomics. 2007;8:359. doi: 10.1186/1471-2164-8-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dingle KE, Van Den Braak N, Colles FM, Price LJ, Woodward DL, et al. Sequence typing confirms that Campylobacter jejuni strains associated with Guillain-Barre and Miller-Fisher syndromes are of diverse genetic lineage, serotype, and flagella type. J Clin Microbiol. 2001;39:3346–3349. doi: 10.1128/JCM.39.9.3346-3349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gutacker MM, Smoot JC, Migliaccio CA, Ricklefs SM, Hua S, et al. Genome-wide analysis of synonymous single nucleotide polymorphisms in Mycobacterium tuberculosis complex organisms: resolution of genetic relationships among closely related microbial strains. Genetics. 2002;162:1533–1543. doi: 10.1093/genetics/162.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299:1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 58.Zhang W, Qi W, Albert TJ, Motiwala AS, Alland D, et al. Probing genomic diversity and evolution of Escherichia coli O157 by single nucleotide polymorphisms. Genome Res. 2006;16:757–767. doi: 10.1101/gr.4759706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hasenstein JR, Zhang G, Lamont SJ. Analyses of five gallinacin genes and the Salmonella enterica serovar Enteritidis response in poultry. Infect Immun. 2006;74:3375–3380. doi: 10.1128/IAI.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caporale CM, Papola F, Fioroni MA, Aureli A, Giovannini A, et al. Susceptibility to Guillain-Barré syndrome is associated to polymorphisms of CD1 genes. J Neuroimmunol. 2006;177:112–118. doi: 10.1016/j.jneuroim.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 61.Magira EE, Papaioakim M, Nachamkin I, Asbury AK, Li CY, et al. Differential distribution of HLA-DQ beta/DR beta epitopes in the two forms of Guillain-Barré syndrome, acute motor axonal neuropathy and acute inflammatory demyelinating polyneuropathy (AIDP): identification of DQ beta epitopes associated with susceptibility to and protection from AIDP. J Immunol. 2003;170:3074–3080. doi: 10.4049/jimmunol.170.6.3074. [DOI] [PubMed] [Google Scholar]
- 62.Jolley KA, Chan MS, Maiden MC. mlstdbNet - distributed multi-locus sequence typing (MLST) databases. BMC Bioinformatics. 2004;5:86. doi: 10.1186/1471-2105-5-86. [DOI] [PMC free article] [PubMed] [Google Scholar]