Abstract
The origin recognition complex (ORC), first identified in Saccharomyces cerevisiae (sc), is a six-subunit protein complex that binds to DNA origins. Here, we report the identification and cloning of cDNAs encoding the six subunits of the ORC of Schizosaccharomyces pombe (sp). Sequence analyses revealed that spOrc1, 2, and 5 subunits are highly conserved compared with their counterparts from S. cerevisiae, Xenopus, Drosophila, and human. In contrast, both spOrc3 and spOrc6 subunits are poorly conserved. As reported by Chuang and Kelly [(1999) Proc. Natl. Acad. Sci. USA 96, 2656–2661], the C-terminal region of spOrc4 is also conserved whereas the N terminus uniquely contains repeats of a sequence that binds strongly to AT-rich DNA regions. Consistent with this, extraction of S. pombe chromatin with 1 M NaCl, or after DNase I treatment, yielded the six-subunit ORC, whereas extraction with 0.3 M resulted in five-subunit ORC lacking spOrc4p. The spORC can be reconstituted in vitro with all six recombinant subunits expressed in the rabbit reticulocyte system. The association of spOrc4p with the other subunits required the removal of DNA from reaction mixture by DNase I. This suggests that a strong interaction between spOrc4p and DNA can prevent the isolation of the six-subunit ORC. The unique DNA-binding properties of the spORC may contribute to our understanding of the sequence-specific recognition required for the initiation of DNA replication in S. pombe.
Keywords: replication, AT-rich sequences
Initiation of eukaryotic DNA replication occurs at multiple sites distributed throughout the entire genome. The cis-acting elements that contribute to the firing of replication origins have been studied intensively in Saccharomyces cerevisiae. The conserved sequences that are required for DNA replication, called autonomous replication sequences (ARS elements), consist of an essential A domain that contains an 11-bp ARS consensus sequence (ACS) and B domains that include sequences that enhance replication (1–3). The identification of ACS in S. cerevisiae played an important role in the isolation of the six-subunit (Orc1p–Orc6p) origin recognition complex (ORC), which specifically binds to the ACS in vivo and in vitro (4–6). Biochemical and genetic studies have shown that the genes encoding the Orc subunits are essential and required for DNA replication (7, 8). It has been shown that the ORC contains weak ATPase activity and that ATP is essential for its binding to ARS elements (9). Homologues of the ORC subunits have been found in a number of eukaryotes and in Drosophila melanogaster (dm) and Xenopus laevis (xl), These proteins have been shown to form a six-protein complex similar to that observed in S. cerevisiae (10–12). Furthermore, isolated xlORC preparations can restore the DNA replication activity of egg extracts of Xenopus depleted of the ORC proteins (11, 13). Similar experiments, carried out with recombinant dmORC proteins, can restore the DNA replication activity of egg extracts of both Xenopus and Drosophila (14). These results, and others carried out in vivo, suggest that ORC is essential for DNA replication in all eukaryotes. In the case of metazoan cells, however, the DNA sequences essential for origin function presently are unknown.
Schizosaccharomyces pombe (sp) contains some well characterized ARS elements that function as origins of replication in vivo (15–18). Those that have been analyzed in detail differ strikingly from those of S. cerevisiae. The ARS elements of S. pombe are larger than those of S. cerevisiae (between 500 and 1,000 vs. ≈100 bp). Deletion analyses indicate the S. pombe ARSs contain one or more highly AT-rich, redundant regions of 20–50 bp that appear critical for ARS function but lack a sequence analogous to the ACS of S. cerevisiae. In keeping with the marked differences in the organization of their ARSs, the origins of replication of S. pombe and S. cerevisiae function only in the homologous yeast. Based on the available protein sequences of the different ORC subunits, spORC resembles those of metazoan cells to a greater extent than those of S. cerevisiae, suggesting that the initiation of DNA replication in S. pombe may be more closely related to that of metazoan cells than of S. cerevisiae. For this reason, the purification and characterization of the ORC from S. pombe may provide information that can help to identify origin sequences of higher eukaryotes.
Here we report the identification of novel spOrc subunits corresponding to Orc3p and Orc6p. The availability of these gene products, together with previously identified spOrc subunits, have permitted us to reconstitute the subunits of the ORC into the six-subunit complex with in vitro transcription/translation products of each subunit. Furthermore, we demonstrate that the extraction of the complete six-subunit complex from chromatin requires high-salt or DNase I treatment, presumably reflecting the unique DNA-binding properties of the spOrc4 subunit.
Materials and Methods
Cloning of the S. pombe orc4.
Degenerative primers were designed according to the highly conserved amino acid sequences (YNLFD and EKRVK) of scOrc4p and hOrc4p (19, 20). A 102-bp fragment was amplified from a S. pombe cDNA library, cloned into pBluescript (Stratagene), and sequenced. To obtain both the N- and C-terminal sequences of the S. pombe orc4, PCRs were carried out with a T7 primer and specific primers against the 102-bp region, using the S. pombe library constructed in λ ZAPII as the template. The 2.3-kbp (N-terminal) and the 1.1-kbp (C-terminal) PCR products formed were cloned into pBluescript. The N- and C-terminal sequences of S. pombe orc4 were obtained after sequencing. These sequences were used as primers to amplify the full-length orc4 (3 kbp) from a S. pombe cDNA library.
S. pombe Strains.
Strain KM1, expressing spOrc1p with six histidines and two copies of hemagglutinin (HA) epitope tags at the C terminus, was constructed as follows. An NdeI restriction site was introduced immediately before the stop codon of the Orc1 ORF and was used to insert a linker encoding six histidines and two copies of the HA epitope. A genomic fragment containing Orc1-HA-His6 was transformed into an orp1–4 temperature-sensitive (ts) mutant [h−ura4-D18 orp1–4 (21)], and transformants were isolated after incubation at 36°C. Chromosomal integration of the genomic fragment was established by PCR, and expression of the spOrc1p containing the HA epitope was confirmed by immunoblotting.
Strain KM2 expressing the spOrc4p with the FLAG epitope at the C terminus was constructed as follows. The 1.1-kbp C-terminal fragment of orc4+ was subcloned into the BamHI site of plasmid pCG1 that contained the ura4+. Two copies of the FLAG epitope linker were inserted into the AflII site immediately before the stop codon of the orc4+; the DNA was linearized with BclI and introduced into strain KM1 (h− ura4-D18 orp1+∷2HA6his). This transformation yielded strain KM2 (h− ura4-D18 orp1+∷2HA6his orp4+∷2FLAG[ura4+]), harboring a single integration of the plasmid at the orc4+ locus that was obtained by selection for ura+ cells. Plasmid integration and expression of the FLAG epitope were confirmed by PCR and immunoblotting, respectively.
Purification of spORC.
S. pombe cells (strain KM1) were grown to an OD600 of 1.5 in 150 liters of YE medium (3% glucose plus 0.5% yeast extract) supplemented with uracil. The cells were harvested and washed with 1.2 M sorbitol. To prepare spheroplasts, the cells were suspended in 600 ml of buffer S (10 mM Tris⋅HCl, pH 7.4/1.2 M sorbitol/100 mM NaCl/5 mM EDTA/14 mM 2-mercaptoethanol) containing lysing enzyme (0.3 mg/ml; Sigma) and zymolase 20T (0.3 mg/ml; ICN) until the OD600 (in 1% SDS) was reduced to less than 10% of the starting value. The spheroplasts were collected by centrifugation at 5,000 × g and washed two times with buffer S. The spheroplasts were lysed with three pellet volumes of buffer N (20 mM Hepes⋅NaOH, pH 7.6/50 mM NaCl/10 mM magnesium acetate/1 mM ATP/0.02% NP-40/1 mM PMSF/2 μg/ml pepstatin A/2 μg/ml leupeptin/5 μg/ml aprotinin) containing 1% Triton X-100 on ice for 30 min. The lysate was centrifuged (15,000 × g for 20 min at 4°C) to isolate chromatin. The chromatin pellet was extracted twice with 600 ml of 0.3 M NaCl in buffer N on ice for 30 min followed by centrifugation at 15,000 × g for 20 min. The combined chromatin extracts (3 mg/ml, 1.2 liters) were loaded onto a 50-ml Ni column (Invitrogen) equilibrated with buffer N containing 10 mM imidazole. The column was washed with 10 column volumes of buffer N containing 15 mM imidazole and 0.3 M NaCl. Bound protein was eluted with three column volumes of 200 mM imidazole in buffer N containing 0.3 M NaCl. The elution of spOrc1, 2, and 5 proteins was monitored by immunoblotting with antibodies against HA, Orc2p, and Orc5p. The peak fractions containing these proteins were pooled (2 mg/ml, 80 ml), concentrated to 15 ml by centricon centrifugation (Amicon), and loaded onto a 300-ml Sephacryl S-300 gel-filtration column equilibrated with buffer N containing 0.3 M NaCl. The fraction containing Orc1, 2, and 5 proteins was pooled and loaded onto an 1-ml column of protein A-agarose (Upstate Biotechnology, Lake Placid, NY), which was cross-linked with the mAb C12A5 (2 mg IgG/ml of anti-HA beads; Boehringer Mannheim). After washing the column several times with buffer N containing 0.3 M NaCl, the beads were incubated with 1 ml of HA peptide (5 mg/ml; Boehringer Mannheim) in buffer N containing 0.3 M NaCl at 4°C for 4 hr, and the eluate was collected. This step was repeated with a fresh solution of HA peptide. The combined eluates (20 μg/ml, 2 ml) were concentrated by centricon centrifugation to 0.2 ml, which then was centrifuged through a 5-ml, 15–35% glycerol gradient containing 0.3 M NaCl in buffer H (20 mM Hepes⋅NaOH, pH 7.6/10 mM magnesium acetate/1 mM DTT/1 mM ATP/1 mM EDTA/1 mM EGTA/0.02% NP-40). Fractions collected from the bottom of the gradient were subjected to 10% SDS/PAGE and analyzed for proteins by immunoblotting and by silver staining. From 3.6 g of protein (chromatin extract), 18 μg of spORC was recovered in the pooled peak of glycerol gradient fractions.
Construction of DNA Templates Used for in Vitro Transcription and Translation.
cDNAs encoding the six subunits of spORC were cloned into pET-derived vectors (Novagen) for expression under the control of the T7 RNA polymerase promoter. A HA coding sequence was inserted at the N terminus of the cDNA coding for spOrc5p. The DNA sequence coding for the T7 epitope tag was fused to the N terminus of the cDNA of spOrc4p. The C-terminal half of spOrc4p (amino acids 516–972) was amplified by PCR and cloned into pET28a and then fused with the T7 epitope tag at the N terminus. The resulting expression vectors were: pET24, Orc1; pET24, Orc2; pET15b, Orc3; pET28a, Orc4; pET15b, Orc5; pET19b, Orc6; and pET28a, Orc4ΔN.
In Vitro Transcription/Translation of spORC Subunits.
The in vitro transcription/translation reactions were carried out with the TNT-coupled Reticulocyte Lysate System (Promega) according to the manufacturer’s protocol. Reaction mixtures (50 μl) containing 1.8 μg of plasmid DNA, reaction buffer, phage T7 RNA polymerase, 20 μM of each amino acid except methionine, 20 μCi of l-[35S]methionine (1,000 Ci/mmol; Amersham), 50 units of RNasin, and 25 μl of rabbit reticulocyte lysate were incubated at 30°C for 90 min. The concentrations of the different cDNA templates used were adjusted to obtain near-stoichiometric expression of each protein.
Immunoprecipitation of in Vitro Transcription and Translation Products.
Translation products (10 μl) were incubated with either 0.1 μl of polyclonal antisera (Orc2 or 6), 1 μg of HA (Orc1), or T7 mAb (Orc4; Sigma). When products were treated with DNase I, mixtures were incubated with DNase I (1,000 unit/ml; Boehringer Mannheim) on ice for 30 min before antibody addition. After incubation on ice for 1 hr, mixtures were diluted to 130 μl with buffer H and immunocomplexes were adsorbed to 15 μl of protein A-agarose beads equilibrated with buffer H containing 0.1 M NaCl. After incubation at 4°C for 1 hr with shaking, the beads were collected by centrifugation and washed five times with 1 ml of buffer H containing 0.1 M NaCl and 0.2% NP-40. Bound complexes were boiled for 5 min in SDS sample buffer (62.5 mM Tris⋅HC1, pH 6.8/1% SDS/2.5% 2-mercaptoethanol/5% glycerol) and subjected to SDS/PAGE; gels were then fixed in 10% methanol/7.5% acetic acid, dried, and then exposed with an intensifying screen (Kodak) at −80°C for autoradiography.
Immunoprecipitation of the ORC and Orc Subunits from S. pombe Chromatin Preparations.
Cells (strain KM2), grown to an OD600 of 1 in 20 ml of YE medium, were harvested and chromatin was isolated as described above. The isolated chromatin was incubated with 0.3 ml of 0.1 M NaCl in buffer H containing DNase I (1,000 units/ml) at 4°C for 30 min to extract the ORC proteins and then centrifuged for 15 min at 15,000 × g at 4°C. HA or FLAG cross-linked agarose beads (15 μl; Sigma) were added to the supernatant, and the mixture was incubated for 2 hr at 4°C with shaking. Beads containing the immune complexes then were washed four times with 1 ml of buffer H containing 0.2% Nonidet P-40 and 0.1 M NaCl, boiled for 5 min in SDS sample buffer, and subjected to SDS/PAGE. The presence of spOrc subunits then was determined by Western blotting.
Results
Identification and Cloning of the Six spOrc Subunits.
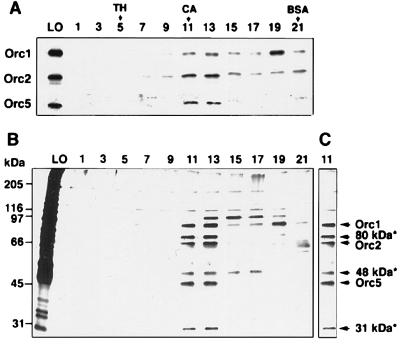
To facilitate the isolation of the spORC, we isolated chromatin from S. pombe cells that expressed the Orc1p containing two HA epitopes and six histidines at its C terminus. Early studies showed that elution of Orc1p and Orc2p during a number of ion-exchange chromatographic steps resulted in the separation of these proteins, suggesting that high-salt concentrations dissociated the complex. Because of this difficulty, we used affinity and sizing purification steps to avoid the use of high-salt concentrations. The purification procedure developed for the isolation of the spORC included four steps that involved selective binding and elution from a Ni chelate column, S-300 gel filtration, adsorption to HA antibody–protein A-agarose beads, followed by HA peptide elution and glycerol gradient centrifugation. The presence of Orc subunits in each fraction was monitored by Western blot analysis. The results showed that the Orc1, 2, and 5 proteins comigrated after the final glycerol gradient step, though some dissociation, particularly of Orc1 and Orc2, was noted (Fig. 1A). In the peak fraction (fraction 11), these three proteins appeared to sediment coincidentally, close to the position in the gradient at which catalase peaked, similar to that observed for scORC (4) and dmORC preparations (22). Silver-stained gels of the glycerol gradient fractions and of the peak fraction are shown in Fig. 1 B and C, respectively. A number of protein bands of approximately 90, 80, 68, 45, and 31 kDa were noted that cosedimented with the Orc 1, 2, and 5 proteins. The 90-, 68-, and 45-kDa protein bands corresponded to Orc1, Orc2, and Orc5 proteins, respectively. Three additional polypeptides of 80, 48, and 31 kDa (noted by asterisks in Fig. 1C) were characterized further by peptide sequencing. Three tryptic peptides were isolated from the 80-kDa band. The amino acid sequences of two of these peptides matched those present in the fission yeast database (Sanger center) under accession number AF188642. The cDNA sequence deduced from this cosmid lacked a termination codon. Based on the sequence of this cosmid, the N-terminal region of this clone was amplified by PCR and the product formed was used as a probe to screen a S. pombe cDNA library. A full-length cDNA clone was isolated that encoded a polypeptide of 690 aa, with a calculated molecular mass of 78 kDa. The sequence of the third tryptic peptide derived from the 80-kDa band also was present within this deduced protein sequence. The amino acid sequence of this protein was found to contain 16% identity (30% homology) to the scOrc3p, 17% identity (34% homology) to the dmOrc3p, and 18% identity (35% homology) to hOrc3p (Fig. 2). Based on this information, we identified the 80-kDa protein as the spOrc3p.
Figure 1.
Glycerol gradient sedimentation of spORC. The concentrated spORC fraction (0.2 ml) eluted from HA antibody cross-linked protein A-agarose beads (as described in Materials and Methods) was loaded onto a 5-ml, 15–35% glycerol gradient containing 0.3 M NaCl in buffer H and centrifuged at 460,000 × g for 14 hr at 4°C; protein standards were centrifuged in parallel gradients. Gradient fractions (20 μl) were subjected to 10% SDS/PAGE separation and immunoblotted with antibodies against spOrc1, 2, and 5 proteins (A) or silver-stained (B). The position of the Orc subunits and the protein bands present in the peak (fraction 11) that were analyzed by peptide sequencing are indicated by asterisks (C). Arrows indicate the position of molecular mass standards: TH, thyroglobulin (669 kDa); CA, catalase (232 kDa); BSA (66 kDa). Lanes: M, molecular mass markers; LO, load on; 1–21, glycerol gradient fraction numbers.
Figure 2.
Sequence alignment of the spOrc3 subunit with D. melanogaster, X. laevis, human, and S. cerevisiae Orc3 proteins by using the GCG pileup program. Identical residues are indicated by shaded boxes, and similar residues are indicated by clear boxes. The sequences corresponding to the three tryptic fragments isolated from the purified spOrc3 subunit are underlined.
Trypsin digestion of the 31-kDa protein band (Fig. 1C) yielded four distinct peptides whose sequences matched that of a 27-kDa hypothetical protein from S. pombe (GenBank accession no. AL031788). A blast search failed to reveal any protein homologous to this 265-aa polypeptide. However, we identified this protein as spOrc6p based on its similar molecular mass to Orc6p isolated from other organisms and its ability to interact with other spOrc subunits as described below (see Figs. 4 and 5). Overall, the spOrc6p showed 9% identity (21% homology) to the scOrc6p and 17% identity (34% homology) to the dmOrc6p (Fig. 3). Orc proteins purified from Xenopus egg extracts also included a protein of similar size (27 kDa) (12).
Figure 4.
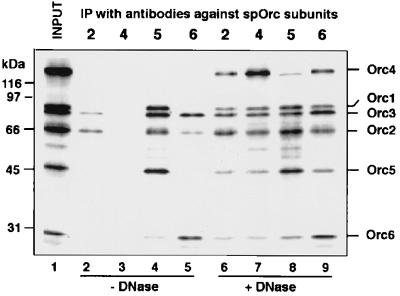
Immunoprecipitation complexes formed after the simultaneous in vitro transcription/translation of the six spORC subunits. In vitro transcription/translation products derived from plasmids encoding all six spORC subunits (lane 1) were incubated on ice either without (lanes 2–5) or with DNase I (1,000 units/ml) (lanes 6–9). Complex formation was checked by immunoprecipitation (indicated as IP) with antibodies against Orc2p (lanes 2 and 6), Orc4p (anti-T7, lanes 3 and 7), Orc5p (HA, lanes 4 and 8), and Orc6p (lanes 5 and 9). After washing, the immunoprecipitated materials were subjected to SDS/PAGE and then autoradiographed.
Figure 5.
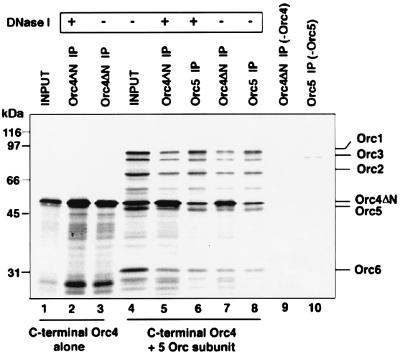
Influence of spOrc4pΔN on complex formation. In vitro transcription/translation product of the plasmid encoding the C-terminal region of spOrc4p (Orc4pΔN, lane 1), which was incubated with (lane 2) or without DNase 1 (lane 3), was immunoprecipitated (indicated by IP) with anti-T7 antibody. The spOrc4pΔN was coexpressed in the presence of the five other subunits in an in vitro transcription/translation reaction (lane 4), and the reaction mixture was incubated with (lanes 5 and 6) or without DNase I (1,000 units/ml) (lanes 7 and 8). The products were immunoprecipitated with antibodies against T7 (lanes 5 and 7) or HA (Orc5p, lanes 6 and 8). As controls, transcription/translation reactions that coexpressed five of the subunits but lacked either Orc4 or Orc5 were subjected to immunoprecipitation with anti-T7 antibody (lane 9) or anti-HA antibody (lane 10), respectively. Proteins that bound to the protein A-agarose beads were subjected to SDS/PAGE, after which the gels were dried and autoradiographed.
Figure 3.
Sequence alignment of the D. melanogaster, S. cerevisiae, and S. pombe Orc6 proteins using the GCG pileup program. Identical residues are indicated by shaded boxes, and similar residues are indicated by clear boxes. Underlined regions indicate amino acid sequences corresponding to the four tryptic fragments that were derived from the purified spOrc6 protein.
Protein sequencing of the 48-kDa protein (Fig. 1C) identified this protein as the S. pombe homologue of elongation factor 1α, an abundant cellular protein that most likely copurified with the spORC by nonspecific adsorption to the various resins used. No further studies with this protein were carried out.
The above data suggest that we have isolated a spORC devoid of Orc4p. The sequence of Orc4p is highly conserved from S. cerevisiae to human (4, 12, 20). To search for spORC4, PCR was carried out with degenerative primers complementary to regions of scOrc4p and hOrc4p that are highly homologous. A full-length cDNA clone encoding a predicted protein of 972 aa was obtained as described in Materials and Methods. The sequence of this cDNA was identical to that of the spOrc4p reported by Chuang and Kelly (23). Overall, the C-terminal half of spOrc4p (Orc4pΔN) is approximately 35% identical and 63% homologous to h- and xlOrc4 proteins and 28% identical and 58% similar to the scOrc4p. Thus, as reported (23), spOrc4pΔN is highly homologous to the Orc4 protein of other species, whereas the N-terminal half is unique to fission yeast.
Reconstitution of a Six-Subunit spORC in Vitro.
To prove that the newly identified spOrc3, 4, and 6 proteins could form a complex with known spOrc subunits, immunoprecipitation experiments were carried out with in vitro transcription/translation products generated from DNAs encoding all six spOrc subunits. As shown in Fig. 4, full-length products of all six subunits were formed (with minor breakdown products) in the in vitro transcription/translation reaction (lane 1). However, immunoprecipitation with antibodies specific for either spOrc2, 4, 5, or 6 proteins failed to precipitate the six-subunit complex (lanes 2–5). However, a number of immunoprecipitation reactions revealed the formation of various subcomplexes of Orc polypeptides including Orc2p-Orc3p (lane 2), Orc1p-Orc2p-Orc3p-Orc5p-Orc6p (lane 4), and Orc2p-Orc3p-Orc6p (lane 5). Orc4p, tagged with a T7 epitope at its N terminus, was not immunoprecipitated by mAb against the T7 epitope (lane 3). Because spOrc4p binds strongly to DNA (23), we suspected that this protein bound to DNA during the in vitro expression reaction, preventing it from complexing with the other Orc subunits. To explore this possibility, reaction mixtures were treated with DNase I after the in vitro transcription/translation reaction but before the immunoprecipitation step. As shown in Fig. 4 (lanes 6–9), after DNase I treatment, antibodies against either Orc2, 4, 5, or 6 proteins precipitated all of the subunits, indicating that a six-subunit complex had formed.
Because the spOrc4p C-terminal region is highly homologous to Orc4 proteins of other eukaryotes, it is likely that this region is responsible for its ability to complex with the other proteins of the ORC. To examine this possibility, a cDNA containing spOrc4pΔN, tagged with a T7 epitope at its N terminus, was constructed. The spOrc4pΔN derivative was coexpressed in vitro with the other spOrc subunits (Fig. 5, lane 4). As shown in Fig. 5, immunoprecipitation with Orc5 antibodies indicated that the spOrc4pΔN was complexed with other Orc subunits (lanes 6 and 8). Immunoprecipitation with T7 antibody also demonstrated that the spOrc4pΔN formed a complex with other Orc subunits (lanes 5 and 7) and formation of the complex, in contrast to reactions containing the complete spOrc4p (Fig. 4), was unaffected by DNase I digestion before immunoprecipitation (compare lanes 5 and 6 with lanes 7 and 8). These results suggest that the spOrc4pΔN can form a stable complex with other subunits of the ORC in vitro.
Identification of the Six-Subunit ORC from S. pombe Chromatin Extracts.
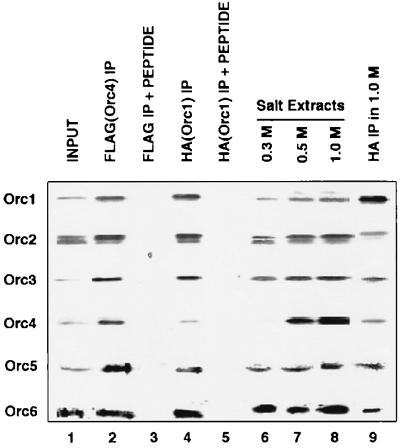
Based on the above results, it seemed likely that the conditions used to isolate the ORC from chromatin (0.3 M NaCl, described in Fig. 1) did not extract Orc4p. To investigate this possibility further, we altered the S. pombe chromosomal Orc4p by placing a FLAG peptide tag at its C terminus so that the distribution of this protein could be followed during the isolation procedure. Chromatin preparations isolated from S. pombe cells (as described in Materials and Methods) were subjected to DNase I treatment as well as extraction at various salt concentrations (Fig. 6). SDS/PAGE analysis followed by Western blot analysis showed that the Orc4p was extracted with DNase I (lane 1) or by 0.5 M and 1 M NaCl (lanes 7 and 8) but poorly with 0.3 M NaCl (lane 6, the conditions previously used in Fig. 1). Furthermore, immunoprecipitation with antibodies against the HA (lanes 4 and 9) or FLAG (lane 2) epitope showed that spOrc4p existed as a complex with the other subunits of the ORC under these conditions. These results support the conclusion that the spORC consists of a six-subunit complex, similar to the ORC preparations isolated from other eukaryotic species.
Figure 6.
Association of spOrc4p with other Orc subunits in vivo. Sp chromatin was extracted with DNase I (1,000 units/ml, lane 1) or with a solution containing the indicated concentrations of NaCl (lanes 6–8). Extracted chromatin fractions were analyzed by immunoblotting with antibodies against either Orc1p (HA), Orc2p, Orc3p, Orc4p (FLAG), Orc5p, or Orc6p. The chromatin fractions that were incubated with DNase I or extracted with 1 M NaCl were immunoprecipitated with anti-HA antibody (Orc1p, lanes 4 and 9) or anti-FLAG antibody (Orc4p, lane 2). As controls for the immunoprecipitation experiments, the HA peptide (1 mg/ml, lane 3) or the FLAG peptide (100 μg/ml, lane 5) was added during the immunoprecipitation step. Proteins adsorbed to beads were first separated by SDS/PAGE, transferred to nitrocellulose paper, and then immunoblotted with antibodies as described above.
Discussion
To identify the genes encoding unknown subunits of the spORC, the complex was purified from cells expressing an Orc1p tagged with HA and six histidine residues. Proteins corresponding to spOrc3p (80 kDa) and spOrc6p (31 kDa), which comigrated with Orc1, 2, and 5 proteins, were isolated, cloned, and sequenced. Alignment of spOrc3p with Orc3p of Xenopus, human, and Drosophila showed only limited regions of identity and homology. These findings suggest that Orc3p is poorly conserved during evolution, whereas the other Orc subunits (Orc1, 2, 4ΔN, and 5 proteins) are highly conserved. It is interesting to note that the molecular mass of the Orc3 proteins of S. pombe, Xenopus, and human are larger than their Orc2 proteins (11, 20), whereas the Orc3 proteins of Drosophila and S. cerevisiae are smaller than their Orc2 proteins (4, 10).
Weak evolutionary conservation also was noted for spOrc6p. At present, only the scOrc6p and dmOrc6p sequences are available in the database. Based on this information, spOrc6p does not show regions significantly identical or homologous to the known Orc6 proteins. The molecular mass of spOrc6p (31 kDa) is closer to that of Drosophila (30 kDa) than scOrc6p (50 kDa). An immunopurified xlORC fraction also included a 29-kDa protein, likely to be the xlOrc6p (12).
Only the spOrc4p was missing from the purified ORC preparations isolated from chromatin. Based on the size of Orc4 proteins identified in Drosophila, Xenopus, and human, we anticipated that the spOrc4p would be approximately 45 kDa. All proteins present in the glycerol gradient peak fraction that migrated in this region after SDS/PAGE analysis were sequenced; they were shown to be either degradation products of the other Orc subunits or possibly spurious contaminants. Because Orc4 proteins are highly conserved from S. cerevisiae to human, PCR experiments were performed with degenerative primers that resulted in the identification of a protein of 972 aa whose sequence was identical to that reported by Chuang and Kelly (23). We believe that the absence of the spOrc4p from our purified ORC complex can be explained by the finding that spOrc4p was not extracted from chromatin with 0.3 M NaCl whereas the other five subunits were extracted at this salt concentration. This salt concentration readily extracts the six-subunit ORC from S. cerevisiae (24) and human cells (unpublished observations). The strong DNA-binding properties of the spOrc4p may be due to its unique 500-aa N-terminal extension that includes nine AT-hook motifs, possessing the sequence RGRP (23). This N-terminal region contains no homology to any other eukaryotic Orc4p. The strong DNA-binding property of the RGRP motif appears to be involved in the recognition of the unique structure of the narrow minor groove of AT-rich regions rather than a specific nucleotide sequence (25–28). Fission yeast ARS elements have multiple AT-rich regions that are required for ARS function but no specific essential sequence equivalent to the ACS of S. cerevisiae (17, 18). Therefore, it is possible that the spORC strongly binds to origin sequences through the multiple AT-hook motifs of spOrc4p and that extraction with 0.3 M NaCl is not ample to dissociate the spOrc4p–DNA complex but can release the other subunits as a five-subunit complex. This possibility is supported by our findings that extraction of chromatin with 0.5 M or 1 M NaCl, as well as after DNase I treatment, yielded the spORC containing spOrc4p. In addition, immunoprecipitation of in vitro transcription/translation products treated with DNase I resulted in the isolation of the six-subunit ORC, whereas immunoprecipitation of products that were not DNase I-treated resulted in the isolation of subassemblies of Orc subunits devoid of spOrc4p. Furthermore, spOrc4pΔN, which shows high homology to the full-length Orc4p of other eukaryotes, formed a stable complex with other Orc subunits. These results are consistent with the fact that the full-length and N-terminal half of spOrc4p have strong DNA-binding properties, whereas the C-terminal fragment does not (23). It is interesting to note that the purified five-subunit complex, devoid of spOrc4p, was unable to bind to ars1 or ars3002 DNA sequences (unpublished data). In addition, our finding that the five-subunit complex isolated from chromatin was relatively salt-sensitive and tended to disassemble at high ionic strength may be a unique property of this incomplete complex. Further studies comparing the properties of the five- and six-subunit complexes will be necessary to address this question.
The availability of each of the S. pombe Orc subunits and the ability to clone, express, and isolate the six-subunit ORC complex from baculovirus-infected cells (unpublished data) should permit a detailed evaluation of the DNA-binding properties of the spORC and its interaction with multiple, redundant, AT-rich sequences of S. pombe ARSs.
Acknowledgments
We thank Dr. P. Nurse of Imperial Cancer Research Fund for kindly providing the orc1–4 ts (temperature-sensitive) mutant cell and Dr. D. Young of Calgary University for providing the ZAPII S. pombe cDNA library. We also thank Drs. Z.-Q. Pan and Z. Kelman for critical reading of the manuscript. We are indebted to Drs. Hediye Erdjument-Bromage and Paul Tempst of the Protein Center at Sloan-Kettering Institute for their help in determining the peptide sequences reported here and to David Valentin for the preparation of yeast cells used in these studies. This work was supported by Grant GM38559 (to J.H.). J.H. is a Professor of the American Cancer Society.
Abbreviations
- ARS
autonomous replication sequence
- ACS
ARS consensus sequence
- ORC
origin recognition complex
- sc
Saccharomyces cerevisiae
- sp
Schizosaccharomyces pombe
- dm
Drosophila melanogaster
- HA
hemagglutinin
- xl
Xenopus laevis
- h
human
- Orc4pΔN
C-terminal half of spOrc4p
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF188642).
References
- 1.Van Houten J V, Newlon C S. Mol Cell Biol. 1990;10:3917–3925. doi: 10.1128/mcb.10.8.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marahrens Y, Stillman B. Science. 1992;255:817–823. doi: 10.1126/science.1536007. [DOI] [PubMed] [Google Scholar]
- 3.Newlon C S, Theis J F. Curr Opin Genet Dev. 1993;3:752–758. doi: 10.1016/s0959-437x(05)80094-2. [DOI] [PubMed] [Google Scholar]
- 4.Bell S P, Stillman B. Nature (London) 1992;357:128–134. doi: 10.1038/357128a0. [DOI] [PubMed] [Google Scholar]
- 5.Bell S P, Kobayashi R, Stillman B. Science. 1993;262:1844–1849. doi: 10.1126/science.8266072. [DOI] [PubMed] [Google Scholar]
- 6.Fox C A, Loo S, Dillin A, Rine J. Genes Dev. 1995;9:911–924. doi: 10.1101/gad.9.8.911. [DOI] [PubMed] [Google Scholar]
- 7.Liang C, Weinreich M, Stillman B. Cell. 1995;81:667–676. doi: 10.1016/0092-8674(95)90528-6. [DOI] [PubMed] [Google Scholar]
- 8.Aparicio O M, Weinstein D M, Bell S P. Cell. 1997;91:59–69. doi: 10.1016/s0092-8674(01)80009-x. [DOI] [PubMed] [Google Scholar]
- 9.Klemm R D, Austin R J, Bell S P. Cell. 1997;88:493–502. doi: 10.1016/s0092-8674(00)81889-9. [DOI] [PubMed] [Google Scholar]
- 10.Gossen M, Pak D T, Hansen S K, Acharya J K, Botchan M R. Science. 1995;270:1674–1677. doi: 10.1126/science.270.5242.1674. [DOI] [PubMed] [Google Scholar]
- 11.Rowles A, Chong J P, Brown L, Howell M, Evan G I, Blow J J. Cell. 1996;87:287–296. doi: 10.1016/s0092-8674(00)81346-x. [DOI] [PubMed] [Google Scholar]
- 12.Tugal T, Zou-Yang X H, Gavin K, Pappin D, Canas B, Kobayashi R, Hunt T, Stillman B. J Biol Chem. 1998;273:32421–32429. doi: 10.1074/jbc.273.49.32421. [DOI] [PubMed] [Google Scholar]
- 13.Romanowski P, Madine M A, Rowles A, Blow J J, Laskey R A. Curr Biol. 1996;6:1416–1425. doi: 10.1016/s0960-9822(96)00746-4. [DOI] [PubMed] [Google Scholar]
- 14.Chesnokov I, Gossen M, Remus D, Botchan M. Genes Dev. 1999;13:1289–1296. doi: 10.1101/gad.13.10.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maundrell K, Wright A P, Piper M, Shall S. Nucleic Acids Res. 1985;13:3711–3722. doi: 10.1093/nar/13.10.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubey D D, Zhu J, Carlson D L, Sharma K, Huberman J A. EMBO J. 1994;13:3638–3647. doi: 10.1002/j.1460-2075.1994.tb06671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clyne R K, Kelly T J. EMBO J. 1995;14:6348–6357. doi: 10.1002/j.1460-2075.1995.tb00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubey D D, Kim S M, Todorov I T, Huberman J A. Curr Biol. 1996;6:467–473. doi: 10.1016/s0960-9822(02)00514-6. [DOI] [PubMed] [Google Scholar]
- 19.Bell S P, Mitchell J, Leber J, Kobayashi R, Stillman B. Cell. 1995;83:563–568. doi: 10.1016/0092-8674(95)90096-9. [DOI] [PubMed] [Google Scholar]
- 20.Quintana D G, Hou Z, Thome K C, Hendricks M, Saha P, Dutta A. J Biol Chem. 1997;272:28247–28251. doi: 10.1074/jbc.272.45.28247. [DOI] [PubMed] [Google Scholar]
- 21.Grallert B, Nurse P. Genes Dev. 1996;10:2644–2654. doi: 10.1101/gad.10.20.2644. [DOI] [PubMed] [Google Scholar]
- 22.Gossen M, Pak D T, Hansen S K, Acharya J K, Botchan M R. Science. 1995;270:1674–1677. doi: 10.1126/science.270.5242.1674. [DOI] [PubMed] [Google Scholar]
- 23.Chuang R Y, Kelly T J. Proc Natl Acad Sci USA. 1999;96:2656–2661. doi: 10.1073/pnas.96.6.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donovan S, Harwood J, Drury L S, Diffley J F. Proc Natl Acad Sci USA. 1997;94:5611–5616. doi: 10.1073/pnas.94.11.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon M J, Strauss F, Varshavsky A. Proc Natl Acad Sci USA. 1986;83:1276–1280. doi: 10.1073/pnas.83.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeves R, Nissen M S. J Biol Chem. 1990;265:8573–8582. [PubMed] [Google Scholar]
- 27.Bustin M, Reeves R. Prog Nucleic Acid Res Mol Biol. 1996;54:35–100. doi: 10.1016/s0079-6603(08)60360-8. [DOI] [PubMed] [Google Scholar]
- 28.Huth J R, Bewley C A, Nissen M S, Evans J N, Reeves R, Gronenborn A M, Clore G M. Nat Struct Biol. 1997;4:657–665. doi: 10.1038/nsb0897-657. [DOI] [PubMed] [Google Scholar]