Abstract
In the adult brain, gamma-amino butyric acid (GABA) is the major inhibitory neurotransmitter. Understanding of the behavioral and pharmacological functions of GABA has been advanced by recent studies of mouse lines that possess mutations in various GABA receptor subtypes and associated proteins. Genetically altered mice have become important tools for discerning GABAergic function. Thus detailed knowledge of the anatomical distribution of different GABAA subtype receptors in mice is a prerequisite for understanding the neural circuitry underlying changes in normal and drug-induced behaviors seen in mutated mice. In the current study, we used in situ hybridization histochemistry with [35S]UTP-labeled riboprobes to examine the regional expression pattern of mRNA transcripts for 7 major GABAA receptor subunits in adjacent coronal brain sections (alpha 1, alpha 2, alpha 3, alpha 5, beta 2, beta 3, and gamma 2). Our results indicate that many of these GABAergic genes are co-expressed in much of the adult brain including the neocortex, hippocampus, amygdala, thalamus and striatum. However, each gene also shows a unique region-specific distribution pattern, indicative of distinct neuronal circuits that may serve specific physiological and pharmacological functions.
Keywords: mice, gamma-amino butyric acid, inhibition
Introduction
The neurotransmitter gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter involved in controlling the neural excitability in the mammalian brain. GABA exerts fast inhibitory actions by activating GABAA receptors that are formed by a co-assembly of subunits selected from α1-6, β1-3, γ1-3, ρ1-3, ε, π, and δ subunits 6, 11, 40, which form the chloride ion channel 22, 55, 59, 60. The various combinations of subunits results in a highly heterogeneous population of GABAA receptors; however, most native receptor complexes are believed to contain five subunits containing at least one member of the α, β, and γ subunit classes in a proposed stoichiometry of two α-,two β- and one γ-subunits 5, 50.
Benzodiazepine (BZs) agonists and other GABAgeric modulators (e.g. barbiturates, neurosteroids) have distinct binding sites on GABAA receptors which are allosterically linked with the GABA binding site on the GABA receptor, which forms a channel through the membrane and opens and closes to control chloride flow into the cell 59. BZ binding receptors represent around 75% of the total GABAA receptors present in the brain 39, 59. The binding properties and pharmacological action of BZs largely depend on the alpha-subunit profile of the hetero-pentameric receptors. Receptors containing the α1, α2, α3, or α5 subunits in combination with any of the β-subunits and the γ2-subunit are sensitive to BZ modulation; whereas α4- and α6-subunit-containing GABAA receptors have greatly reduced affinities for classical BZ agonists such as diazepam 25, 57, 61. Most classically defined BZ ligands display relatively similar affinities and efficacies at most BZ-sensitive GABAA receptors subtypes.
Our understanding of the behavioral and pharmacological function of GABAA receptor subtypes has been greatly advanced by the study of mouse lines that possess deleted and mutated receptor subtypes. Such mutant mouse lines lack the normal physiological and behavioral responses to BZs mediated by the mutated GABAA receptor subtype. For example, mice lacking a fully functional α1 receptor subtype fail to show the normal sedative and anticonvulsant behaviors to BZ but display typical anxiolytic responses 15, 38. In contrast, mice with mutated α2-GABAA subtypes show no anxiolytic responses to BZ but normal sedative and anticonvulsant behaviors. To date, numerous lines of mice have been generated with genetic ablation or mutations of individual GABAA receptor subunits including α1, α2, α3, α5, α6, γ2, g3, β2, and β3 47, 56. The development and use of mice with altered expression of GABAA receptor subunits have greatly advanced our understanding of the role specific GABAA subtype receptors play in response to BZs and other GABAergic modulators. They also provide a powerful tool for the development of new strategies for the treatment of disorders characterized by GABAergic dysfunction and/or treated by BZs.
Because genetically altered mice have become important tools for understanding GABAergic function, detailed knowledge of the anatomical distribution of different GABAA subtype receptors in mice is a prerequisite for understanding the neural circuitry underlying changes in normal and drug-induced behaviors seen in mutated mice. In the current study, we used in situ hybridization histochemistry with [35S]UTP-labeled riboprobes to examine the regional expression pattern of mRNAs transcripts for 7 major GABAA receptor subunits in adjacent coronal brain sections. Because differences in α subunit composition largely define specific pharmacological responses to BZs 22, 50, this study focused on the regional distribution of four α subunits: α1, α2, α3, and α5 along with β2, β3, and γ2. Various combinations of these subunits represent over 80% of BZ-sensitive GABAA receptors in the adult brain. Immunoprecipitation experiments suggest that the remaining subunits not included in this study (α4, α6, γ1, γ3, β1) account for less than 10% of GABAA receptors.
Experimental Procedures
Animals
Adult (8-12 weeks) male C57BL/6J mice weighing 22–28 g (Jackson Laboratories, Maine) were selected for this study, because this strain is the most widely used inbred strain in behavioral research and is commonly used as a background strain for the development of genetic knockout mice 1, 7, 9.
Tissue preparation
Mice were decapitated and the brains were rapidly dissected and frozen on crushed dry ice. Coronal sections (15 μm) of brains were cut on a cryostat (Leica; Nussloch, Germany) at − 20°C mounted onto SuperFrost slides (Fisher Scientific, Pittsburgh, PA), air-dried, and stored at −80°C until processed for in situ hybridization histochemistry. For each brain, sections were sequentially placed on 15 sets of slides such that each subsequent set contained adjacent sections of the brain. One slide set from each brain was used for in situ hybridization analyses of each mRNA. One slide set of from each mouse was used for cresyl violet Nissl stain.
For the hybridization, sections were thawed, and fixed in a 4% paraformaldehyde solution for 30 min. Sections were then washed twice in PBS for 5 min and treated for 7.5 min with Proteinase K (20 μg/ml) dissolved in 10mM Tris, 5mM EDTA. Slides were rinsed for 5 min PBS and fixed with 4% Paraformaldehyde for 30 min. Next, slides were rinsed were immersed for 10 min in 0.1 M triethanolamine containing 0.25% acetic anhydride then rinsed in PBS and .85% saline for 5 min each. Sections were dehydrated for 2 min in 30%, 50%, 70%, 85%, 95%, and 100% ethanol, air-dried, and stored in a desiccator prior to hybridization.
Hybridization
Hybridization riboprobes were prepared from linearized clones using T7, T3, or SP6 polymerase at high specific activity by using radioactive 35S-UTP (1250Ci/mmol, 12.5mCi/ml, NEN, Boston, MA) with no cold UTP in the polymerase reaction. Plasmid clones (GeneBank accession no.) for α1 (BI7333455) and γ2 (BC031762) were supplied by American Type Culture Collection (ATCC, Manassas, VA). Clones for α3 (NM008067), α5 (BC062112), β2 (CB526370), and β3 (BG294151) were purchased from Open Biosystems (Huntsville, AL). The α2 subunit plasmid was constructed by TOPO subcloning (Invitrogen Life technologies) of a polymerase chain reaction (PCR) product using mouse cDNA and the following customized primers: α2 sense, AGCCACTGGAGGAAAACATCT; α2 antisense, TCATGGACTGACCCCTAATACAG (Sigma Chemical, St. Louis, MO). Plasmid DNA sequencing (ISU, Ames, IA) confirmed subclone sequence and orientation. Control sense probes were made from respective cDNA vectors. The percentage of sequence alignment between clones used for riboprobe production can be found in Supplemental Data.
Following preparation of full-length sense and antisense RNA strands, the RNA was base hydrolyzed to average lengths of 50-100bp. The radiolabeled probes were isolated using Sephadex spin columns (Roche: 1273973) and diluted to a concentration of 100,000 cpm/μl in the hybridization buffer which consisted of 50% deionized formamide, 10mM dithiothreitol, 20mM Tris (pH 7.5), 300mM sodium chloride, 5 mM EDTA (pH 8.0), 10% dextran sulfate, 1 × Denhardt's solution, yeast RNA (0.5 mg/ml), and 10mM NaH2PO4. Sections were incubated overnight in humid chambers at 52°C with 75 ul/slide of hybridization buffer covered with a parafilm coverslip.
Following hybridization, slides were incubated horizontally in pre-warmed 5x SSC until coverslips floated off. Sections were washed in 5 × SSC/10 mM DTT at 50°C 30 min and treated with 50% Formamide/2× SSC/10 mM DTT at 55°C for 30 min. Next, slides were washed twice in wash buffer (10mM Tris, 5mM EDTA, 100mM NaCl) for 10 minutes at 37°C and treated with RNase A (20μg/ml) in wash buffer for 30min at 37°C. Sections were dehydrated for 2 min in 30%, 50%, 70%, 85% and 95% ethanol containing 300 mM ammonium acetate, followed by 100% ethanol. Slides were air dried and apposed to BioMax MR autoradiography film (Eastman Kodak Co., Rochester, NY) for 1-5 days. For each riboprobe, a set of slides from each brain was processed in parallel, handled identically, and all slides hybridized to the same probe were exposed to the same piece of film for the same amount of time.
Image analysis
An Epson 3700 scanner (3000 dpi) was used to scan film images and files were saved in JPEG format with compression quality 10, at a size of 32000 × 18000 pixels (∼580 MB) for intensity analyses. Signal specificities and intensities for each probe were assessed simultaneously by comparing brain sections treated with antisense or sense radiolabeled probes to brain-paste calibration standards as detailed in Supplementary Data. Hybridization signals from brain regions of interest typically fell within the 20–240 grayscale range and the near linear range of the calibration curve. For each brain region of interest (ROI), the mRNA hybridization density represents the average intensity obtained from at least five animals. Signal intensities were judged as not detectable, −; detectable, +; weak, ++; moderate, +++; or strong, ++++, +++++; corresponding to grayscale ranges 0-46, 46-84, 86-126, 126-166, 166-206, 206-255, respectively. For each probe, two independent investigators assessed the hybridization density of coronal sections and the common estimates are included in Table 1. An intensity symbol in parentheses [i.e., (+)] reflected cases where the ROI grayscale value was within 5 units of the higher range or when the ROI intensity differed at anterior-posterior regions. For visual summary of data, intensity ratings in Table 1 were converted to a numeral system ranging from 0 (not detectable) to 5 (strong) for the purpose of computing the percentage of signal strength in each brain region or functional collection of nuclei examined in this study (Figure 5). Nomenclature and boundaries of nuclei were determined with the aid of Nissl stained tissue sections and the stereotaxic atlas Paxinos and Franklin 43.
Table 1.
Evaluation of the Hybridization Distribution of GABAA mRNA Receptor Subunits
| CEREBRAL CORTEX | α1 | α2 | α3 | α5 | β2 | β3 | γ2 |
|---|---|---|---|---|---|---|---|
| Layer 2 | ++ | ++++ | +++ | + | +++ | +++ | +++ |
| Layers 3 | ++(+) | ++++ | +++ | + | +++ | +++ | +++ |
| Layers 4 | +++(+) | ++(+) | ++ | + | +++(+) | +++ | +++(+) |
| Layer 5 | ++(+) | ++(+) | +++(+) | ++(+) | ++(+) | ++(+) | +++ |
| Layer 6 | +++ | ++(+) | ++++ | ++(+) | +++ | ++(+) | +++(+) |
| Agranular insular cortex | ++ | ++(+) | +++ | +++ | +++ | ++++ | +++ |
| Piriform cortex | +++ | +++ | ++++ | +(+) | ++(+) | ++++ | +++ |
| Perirhinal cortex | +++ | ++ | +++(+) | ++ | ++ | ++ | +++ |
| Endopiriform nucleus | +++ | ++ | ++++ | ++++ | +++ | +++++ | +++ |
| BASAL GANGLIA | |||||||
| N accumbens: core | − | +++++ | − | + | + | ++++ | +++ |
| N accumbens: shell | − (+) | ++++ | + | + | + | ++++ | +++ |
| Caudate putamen | − (+) | ++++ | − (+) | −(+) | + | +++ | +++ |
| Ventral pallidum | +++(+) | ++ | ++(+) | +(+) | +++(+) | + | +++ |
| Globus pallidus | ++++ | ++(+) | ++ | − | +++ | + | +++ |
| Subthalamic nucleus | +++(+) | +++(+) | +++(+) | + | +++(+) | +++ | +++(+) |
| Substantia nigra, compact | ++ | + | +++(+) | +(+) | +++(+) | + | ++ |
| Substantia nigra, reticular | ++++(+) | + | ++ | − | +++(+) | − | ++ |
| SEPTAL AND BASAL FOREBRAIN | |||||||
| Lateral septum | ++ | +++(+) | +++ | + | ++ | +++(+) | +++ |
| Medial septum | ++++ | ++ | +++ | − | ++++ | ++ | +++(+) |
| Bed n. stria terminalis | ++ | +++(+) | ++++ | ++ | ++ | ++++ | +++ |
| Olfactory tubercle | ++ | ++++ | +++ | − | +++ | +++++ | ++(+) |
| HIPPOCAMPUS | |||||||
| CA1 | +++(+) | +++++ | ++ | +++++ | +++(+) | +++++ | +++(+) |
| CA2 | ++(+) | +++++ | − | +++++ | ++ | +++++ | +++++ |
| CA3 | ++(+) | +++++ | − | +++++ | ++ | +++++ | +++(+) |
| Dentate gyrus | +++ | +++++ | + | +++++ | +++(+) | +++++ | +++++ |
| Subiculum | ++++ | +++++ | ++++ | ++(+) | +++ | ++++ | +++ |
| Parasubiculum | ++++ | +++++ | +++ | ++++ | +++(+) | ++++ | +++ |
| Presubiculum | ++++ | +++++ | +++ | +++ | +++ | +++++ | +++ |
| Entorhinal cortex (L/M) | ++ | ++++ | +++ | ++++ | ++(+) | ++++ | +++ |
| AMYGDALA | |||||||
| Anterior amygdala area | ++++ | ++++ | +++ | − | +++ | +++(+) | +++ |
| Lateral nucleus | +++(+) | ++++(+) | ++++(+) | ++ | ++(+) | ++++ | +++ |
| Basolateral nucleus | +++ | +++++ | ++++(+) | ++ | ++(+) | ++++ | +++(+) |
| Central nucleus | − | ++++ | ++(+) | ++(+) | + | ++++ | ++(+) |
| Basomedial nucleus | ++(+) | ++++ | +++ | ++(+) | ++ | +++ | +++ |
| Amygdalohippocampal area | ++ | +++++ | +++++ | ++ | ++ | ++++ | +++ |
| Medial nucleus | ++ | +++(+) | +++ | +(+) | ++ | +++(+) | +++ |
| THALAMUS AND EPITHALAMUS | |||||||
| Paraventricular nucleus | ++ | ++++ | ++++ | +++ | +++(+) | +++(+) | ++ |
| Anterodorsal thalamus | +++(+) | ++++ | − | ++(+) | ++++(+) | +++ | +++(+) |
| Anteromedial thalamus | ++(+) | + | − | −(+) | +++(+) | +++ | ++ |
| Anteroventral thalamus | ++(+) | + | − | −(+) | +++ | +++ | ++ |
| Reticular nucleus | − | − | +++(+) | − | − | ++(+) | ++(+) |
| Ventral lateral thalamus | ++(+) | − | − | − | ++++ | ++ | ++ |
| Ventral posterior thalamus | +++(+) | − | − | − | +++(+) | ++ | +++ |
| Mediodorsal thalamic nuclei | ++(+) | +(+) | ++ | − | ++(+) | ++ | +++ |
| Laterodorsal thalamic | ++(+) | + | +++ | − | +++(+) | ++ | ++++(+) |
| Medial Habenula | − | ++++ | + | − | − | ++++ | +++++ |
| Lateral Habenula | + | ++ | +++ | + | − | ++ | +++ |
| Zona incerta dorsal/ventral | +++(+) | +++(+) | +++ | ++ | +++(+) | +++ | +++ |
| PO area | ++ | −(+) | − | − | ++(+) | ++ | ++ |
| parafascicular area | ++ | +++(+) | ++(+) | − | ++++ | +++ | +++ |
| APT | ++++ | + | +++ | +(+) | +++ | ++ | +++ |
| Dorsal lateral geniculate | +++ | + | − | − | ++++ | ++ | ++(+) |
| Ventral lateral geniculate | +++ | +++ | +++ | ++ | +++ | ++ | ++++ |
| SG/POT | ++++ | +++(+) | +++(+) | ++ | +++(+) | ++ | +++ |
| Medial geniculate | +++ | + | −(+) | − | +++++ | +++ | +++ |
| PP/PIL | ++(+) | +++(+) | +++ | ++ | +++ | ++ | +++ |
| HYPOTHALAMUS | |||||||
| Preoptic area: medial/lateral | ++ | +++ | ++++ | ++++ | + | ++++ | +++ |
| Lateral hypothalamic area | ++(+) | ++(+) | ++(+) | + | +++ | ++ | ++(+) |
| Reuniens nucleus | +++ | ++++ | +++(+) | ++ | +++ | +++ | +++ |
| Anterior hypothalamic area | ++ | ++++(+) | ++++ | + | ++ | +++ | +++(+) |
| Paraventricular nuclei | ++ | ++++(+) | ++(+) | −(+) | + | ++(+) | ++++(+) |
| Dorsomedial nucleus | ++ | ++++ | ++++ | ++ | ++ | +++ | +++(+) |
| Ventromedial hypothalamic area | +++ | ++++ | ++++ | +++++ | ++ | +++++ | ++++(+) |
| Posterior hypothalamic area | ++(+) | +++ | +++ | ++ | ++(+) | +++ | +++ |
| Supramammillary nuclei | ++ | ++(+) | +++(+) | ++ | ++ | ++++ | ++(+) |
| Mammillary nuclei | +++ | ++++ | +++ | +++(+) | +++ | ++++ | +++(+) |
| MIDBRAIN | |||||||
| Superior colliculus: superficial | ++ | ++++ | ++++ | ++ | +(+) | ++++ | +++ |
| Superior colliculus: intermediate | +++(+) | ++(+) | +++ | ++ | ++ | +++ | +++ |
| Superior colliculus: deep layers | +++(+) | ++ | +++ | + | ++ | +++ | ++(+) |
| Red nucleus | ++++(+) | −(+) | +++ | − | ++++ | + | +++ |
| Ventral tegmental area | +(+) | + | +++ | − | + | ++ | +++ |
| Periaqueductal gray | ++(+) | +++ | +++ | + | +++(+) | +++ | +++ |
| Dorsal raphe nucleus | +++ | +++ | ++(+) | + | ++ | +++ | ++++ |
| Median raphe nucleus | +++ | +++ | ++(+) | + | ++ | +++ | +++ |
| Pontine nuclei | ++++ | +++ | ++ | ++ | ++ | +++ | ++++ |
| Pontine reticular nucleus, oral part | +++ | ++ | +(+) | + | ++(+) | +++ | ++ |
| Lateral lemniscus | − | ++(+) | ++ | +++ | −(+) | +++(+) | +++ |
| Inferior colliculus: central | +++++ | ++ | ++(+) | + | +++(+) | ++ | +++(+) |
| Inferior colliculus: external | +++(+) | +(+) | ++ | + | +++ | ++ | +++ |
(−) No detectable signal; (+) Detectable signal; (++) Weak signal; (+++) Moderate signal; (++++), (+++++) Strong signal
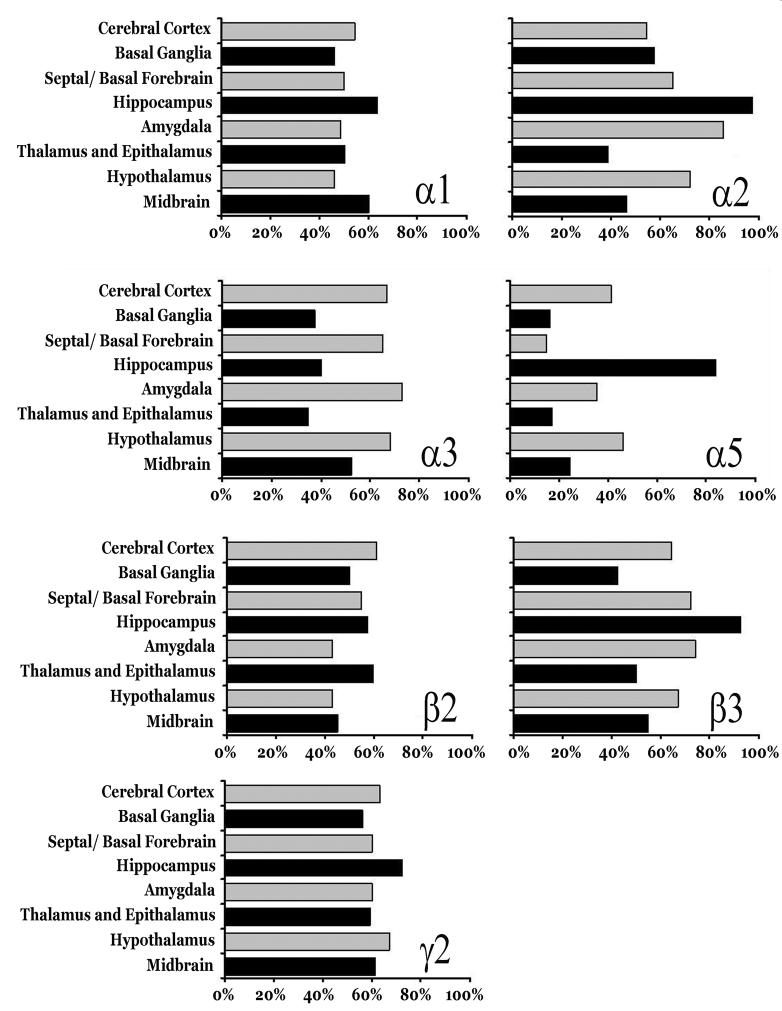
Figure 5.
Summary of hybridization distribution of each defined as the percentage of signal strength in each defined brain region or functional collection of nuclei examined in this study.
Results
The mRNA distributions of 7 different GABAA receptor subunits are depicted at 4 different coronal sections in Figures 1-4. The antisense [35S] riboprobe signal was specific to neuronal areas and was very low to absent over white matter regions. In Table 1, a qualitative and semi-quantitative evaluation of the mRNA distribution levels of the GABAA receptor subunits is summarized. Figure 5 illustrates the percentage of signal strength in each brain region. Descriptions of various mRNA coexpression patterns can be found in Supplemental Data.
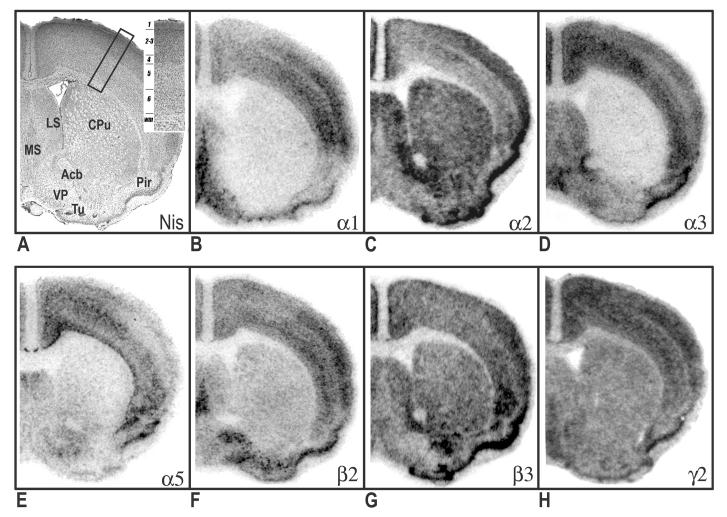
Figure 1.
Distribution of hybridization signals for 7 different GABAA receptor subunit mRNAs in the mouse brain. Nissil (A) and bright-field (B-H) photomicrographs correspond roughly to coronal sections 1mm anterior to Bregma as referenced by Paxinos and Franklin (2002). Inset in Nissil photomicrograph shows cortical layers. Acb, nucleus accumbens; CPu, caudate putamen; LS, lateral septal nucleus; MS, medial septal nucleus; Pir, piriform cortex; Tu, olfactory tubercle; VP, ventral pallidum.
Figure 4.
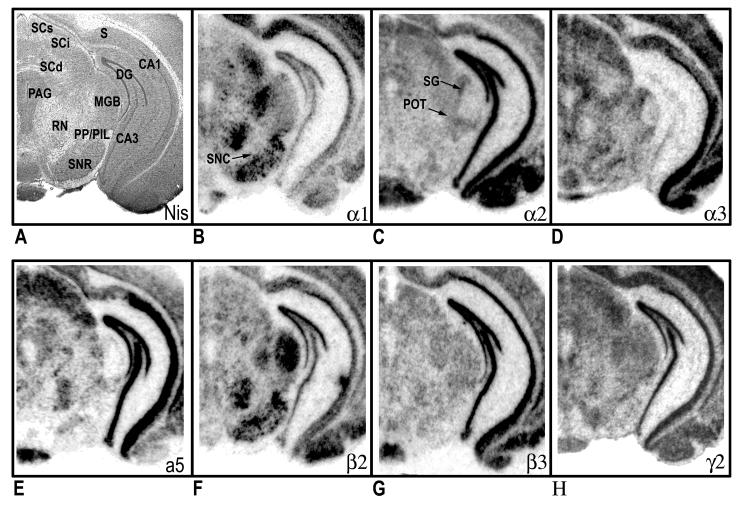
Distribution of hybridization signals for 7 different GABAA receptor subunit mRNAs in the mouse brain. Nissil (A) and bright-field (B-H) photomicrographs correspond roughly to coronal sections 3.5mm posterior to Bregma as referenced by Paxinos and Franklin (2002). CA1 field of the hippocampus; CA3 field of the hippocampus; MGB, medial geniculate body; PAG, periaqueductal gray; PIL, posterior intralaminar thalamic nucleus; POT, posterior thalamic nucleus, triangular; PP, peripeducular nucleus; RN, red nucleus; S, subicular complex; SCd, superior colliculus, deep layers; SCi, superior colliculus, intermediate layers; SCs, superior colliculus, superficial layers; SG, suprageniculate thalamic nucleus; SNC, substantia nigra, compact part; SNR, substantia nigra, reticular part.
Distribution of mRNA for the alpha subunits
Alpha1
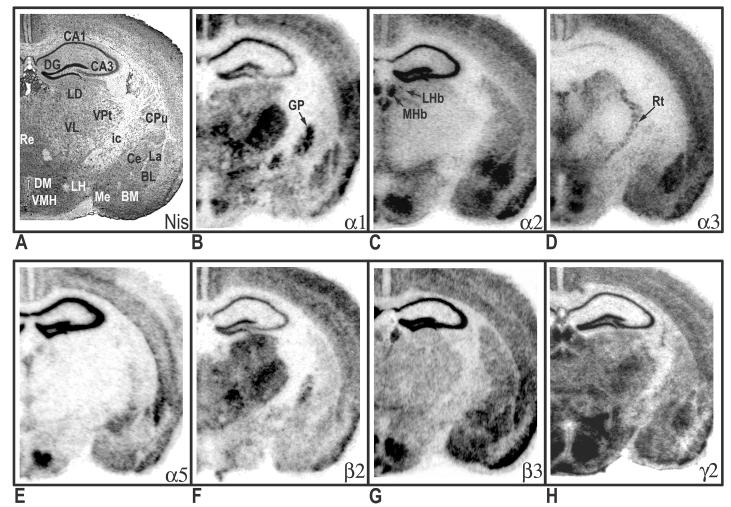
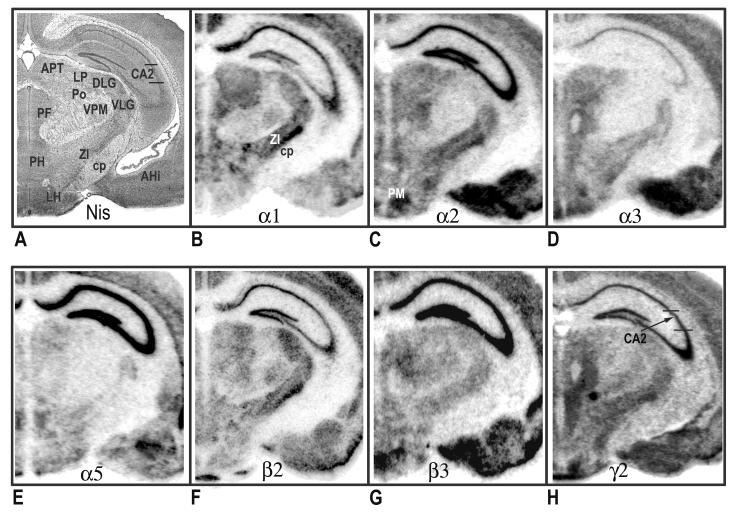
In rostral sections (Fig. 1B), particularly high mRNA levels of α1 were evident in the medial septum, globus pallidus, ventral pallidum and cortex. In contrast, the caudate and nucleus accumbens revealed weak or no hybridization. The relative levels of α1-subunit mRNA in cortical layers of the motor cortex were as follows: 4>3=6>5=2. Clear regional distribution patterns were seen the hippocampus, amygdala, and thalamus (Fig. 2B). In the amygdala, moderate to strong labeling was seen in the lateral (La) and basolateral (BL) nuclei; whereas no detectable signal was noted in the central (Ce) nuclei. The relative levels of α1-subunit mRNA at the level of the hippocampus were as follows: CA1>DG>CA2=CA3. Overall, α1 mRNA was highly expressed in the thalamus. Most notable was the ventral posterior nucleus, which exhibited the highest levels of thalamic expression. Also evident at this level was prominent α1expression in the globus pallidus. Most nuclei of the hypothalamus showed relatively weak α1 signals (Figs. 3B & 4B), with the exception of the ventromedial hypothalamic area, which revealed moderate transcript levels. The level of expression in the mammillary complex was notably heterogeneous: moderate expression was evident in the mammillary nuclei and no detectable signal was evident in the supramammillary nuclei. At caudal levels, strong α1 expression was detected in the reticular part of the substantia nigra and red nucleus (Fig. 4B). In the midbrain, a high level of α1 mRNA expression was observed in the central nucleus of the inferior colliculus (IC). Slightly lower, but still substantial α1expression was evident in the deep layers of the superior colliculus (SC). Other midbrain structures containing relatively moderate α1 signals included the pontine and raphe nuclei.
Figure 2.
Distribution of hybridization signals for 7 different GABAA receptor subunit mRNAs in the mouse brain Nissil (A) and bright-field (B-H) photomicrographs correspond roughly to coronal sections 1mm posterior to Bregma as referenced by Paxinos and Freanklin (2002). BL, basolateral amygdaloid nucleus; BM, basomedial amygdaloid nucleus CA1, CA2, CA3 fields of the hippocampus proper; Ce, central amygdaloid nucleus; CPu, caudate putamen; DG, dentate gyrus; DM, dorsomedial hypothalamic nucleus; ic, internal capsule; GP, globus pallidus; La, lateral amygdaloid nucleus; LD, laterodorsal thalamic nucleus; LH, lateral hypothalamic area; LHb, lateral habenular nucleus; Me, medial amygdaloid nucleus; MHb, medial habenular nucleus; Re, reuniens thalamic nucleus; Rt, reticular thalamic nucleus; VMH, ventromedial hypothalamic area; VL, ventrolateral thalamic nucleus; VPt, ventral posterior thalamic nuclei.
Figure 3.
Distribution of hybridization signals for 7 different GABAA receptor subunit mRNAs in the mouse brain. Nissil (A) and bright-field (B-H) photomicrographs correspond roughly to coronal sections 2.25 mm posterior to Bregma as referenced by Paxinos and Freanklin (2002). AHi, amygdalohippocampus area; APT, anterior pretectal nucleus; cp, cerebral peducle; CA2 field of the hippocampus; DLG, dorsal lateral geniculate nucleus; LH, lateral hypothalamic area; LP, lateral posterior thalamic nucleus; PF, parafascicular thalamic nucleus; PH, posterior hypothalamic area; PM, premammillary nucleus; Po, posterior thalamic nuclear group; VLG, ventral lateral geniculate nucleus; VPM, ventral posteromedial thalamic nucleus; ZI, zona incerta.
Alpha2
In the telencephalon, the hybridization signal for the α2-subunit mRNA was relatively strong in the lateral septum, nucleus accumbens and caudate putamen (Fig. 1C). In the nucleus accumbens, strong labeling was present in both the shell and core divisions. Prominent α2 expression was also present in the bed nucleus of the stria terminalis and olfactory tubercle. The distribution of α2 mRNA in the cortex was heterogeneous, with higher expression in outer layers: 2=3>4=6=5 (Fig. 1C). Strong expression of α2-subunit mRNA was found throughout the hippocampus and amygdala (Fig. 2C). In the amygdala, strong α2-mRNA signals clearly defined the boundaries of the La, BL, Ce and basomedial nuclei. The α2-subunit was also prominent in all fields of the hippocampus and related areas, including the subicular complex (subiculum, presubiculum and parasubiculum) and the entorhinal cortex. Compared with other brain regions, the density α2 mRNA was weakest in the thalamus, with the exception of the paraventricular nucleus and anterodorsal thalamus, which showed strong α2 expression. The zona incerta, parafascicular (PF) area, and ventral lateral geniculate showed more moderate signal levels (Fig. 3C). In addition, intense α2 mRNA was evident in the medial habenula. Labeling was more prominent in the hypothalamus, with the strong mRNA levels observed in most evaluated nuclei including, the preoptic nuclei, anterior, lateral, and ventromedial hypothalamic areas. In the midbrain region, the periaqueductal gray, raphe nuclei, and pontine nuclei showed intermediate levels of α2 mRNA. Labeling for α2 in the substantia nigra was barely detectable. The distribution mRNA in the SC was moderately strong in the superficial layers and weak labeling in the deep layers (Fig. 4C). The IC and ventral tegmental area (VTA) revealed relatively weak hybridization signals.
Alpha3
Relative levels of α3-subunit mRNA in rostral brain regions were strongest in cortex and septal region (Fig. 1D). In the cortex, α3 mRNA was expressed at highest levels in the inner layers, with slightly lower but still substantial expression evident in outer layers: 6>5>2=3>4. No appreciable expression α3 mRNA was identified in the nucleus accumbens. Compared with other subunits, α3 labeling the hippocampus proper was sparse, limited mainly to moderate labeling in the CA1 (Fig. 2D). In contrast, intense expression was evident in the subiculum proper. In the amygdala, the La and BL nuclei revealed the most intense α3-mRNA hybridization signals; however, moderate hybridization signals were evident in the Ce, basomedial, and medial nuclei. Several nuclei of the thalamus revealed relatively moderate α3-mRNA signals including, the mediodorsal, laterodorsal, and anterior pretectal nuclei. Also notable at this level was the presence of moderate to strong α3 mRNA labeling in the reticular nucleus. The latter observation was in contrast to other α subunits, which revealed weak or undetectable signals in the reticular nucleus. In the hypothalamus, hybridization signals in the ventral medial nucleus, anterior hypothalamic area and over the preoptic areas were most intense. Within the midbrain, expression in the superficial layers of the SC was strong and diminished slightly in deeper layers (Fig. 4D). At this level, labeling for the α3 subunit in the substantia nigra was moderate to strong in the compact part and weak in the reticular part. Other regions of the midbrain containing moderate α3 signals included the red nucleus, VTA, and periaqueductal gray.
Alpha5
The mRNA distribution for the α5-subunit was considerably less prominent when compared to other subunits. Within the rostral sections, α5 hybridization signals were largely resticted to cortical structures. The hybridization signal in the cerebral cortex was mostly limited to the inner layers 5 and 6 (Fig. 1E). Weak α5 mRNA expression was evident in the bed nucleus of the stria terminalis. Compared with other brain areas, α5-subunit mRNA was particularly prominent in the hippocampus proper, with all subfields exhibiting strong expression (Fig. 2E). Moderate expression was detected in the associated subicular complex (Fig. 4E). Likewise, moderate α5-mRNA signals were also evident in restricted nuclei of the amygdala, including the La, Ce and basomedial nuclei, with weak but still evident expression in the BL. Overall, nuclei in the thalamus had among the lowest α5-mRNA expression levels (Fig. 3E). A notable exception was the paraventricular nucleus, which exhibited strong expression. In contrast to the thalamus, stronger α5-mRNA levels were noted in various nuclei of the hypothalamus including, preoptic areas, ventromedial hypothalamic area, and mammillary nuclei. In the midbrain, moderate α5 mRNA expression was evident in the outer layers of the SC and in the ventral nucleus of the lateral lemniscus. At this level, no detectable signals were identified in the substantia nigra (Fig. 4E). Other regions of the midbrain containing detectable α5 hybridization included the SC and pontine nuclei.
Distribution of mRNA for the beta and gamma2 subunits
Beta2
With some exceptions, the overall pattern of β2-mRNA labeling closely resembled the distribution of the α1 subunit. As seen with α1, prominent β2 labeling was identified in the medial septum, ventral pallidum and globus pallidus (Fig. 1F). In contrast, hybridization signals in the caudate and nucleus accumbens were barely detectable. In the cortex, hybridization signals for the β2-subunit were relatively homogeneous, with moderate to strong β2 hybridization identified in all layers. The relative levels of β2-subunit mRNA in the hippocampus were as follows: DG=CA1>CA2=CA3. The amygdala expressed weak to moderate amounts of β2-subunit mRNA, with some heterogeneity of expression among nuclei. The intensity of labeling was higher in the BL and La amygdala than in the medial and Ce amygdala nuclei (Fig. 2F). In the thalamus, the regional distribution of β2 mRNA was similar to α1 subunit pattern, with moderate to high transcript level in most nuclei examined. However, the reticular nucleus and habenular complex contained no β2 hybridization signals. In contrast to α1, nuclei of the medial geniculate expressed high levels of β2 mRNA (Fig. 4F). Compared with other brain areas, β2 labeling was somewhat less prominent in the hypothalamus (Fig. 3F). Within this region, the lateral hypothalamic area, reuniens nucleus, substantia nigra, and mammillary nuclei displayed only moderate β2 levels. In the tectum, expression in the IC and SC were moderate and weak, respectively. Most prominent in the midbrain, was the strong labeling for subunit β2 in the red nucleus. With the exception of moderate to strong labeling in the periaqueductal gray, all other midbrain structures displayed weak β2 hybridization signals.
Beta3
In rostral sections, distribution of the β3 mRNA was particularly high in the nucleus accumbens, with slightly lower but still substantial expression in the caudate and lateral septum (Fig. 1G). Prominent β3 expression was also evident in the bed nucleus of the stria terminalis and olfactory tubercle. In contrast, the ventral pallidum, globus pallidus and medial septum revealed weak hybridization. At the level of the motor cortex, β3 expression was relatively homogeneous, with no prominent distinction between cortical layers. The hippocampus formation revealed the most intense hybridization signals for β3, with homogeneous expression across all CA regions and the dentate gyrus (Fig. 2G). Likewise, the pattern of hybridization appeared continuously strong throughout hippocampal cortical areas. In the amygdala, the regional distribution of β3 mRNA was similar to the α2-subunit pattern, with high-level signals in the La, BL and Ce nuclei. Overall, transcript expression of β3 was weak in the thalamus; however, moderate β3 signals were common in some anterior thalamic nuclei including, the paraventricular nucleus and anterior nuclei of thalamus (Fig. 3G). In the posterior portion, β3 expression was moderate in the PF area and medial geniculate. Also noted was strong β3 expression in the medial habenula. At the level of the hypothalamus, prominent β3 mRNA levels were evident in the preoptic nuclei, ventromedial hypothalamic areas, and mammillary nuclei. Within this region, signals in the substantia nigra were barely detectable. The β3 distribution in the SC was strong in the superficial layers and diminished slightly in deeper layers (Fig. 4G). In contrast, expression in the IC was weak. Also in the midbrain, moderate α5 hybridization was detected in the pontine nuclei, periaqueductal gray, raphe nuclei and lateral lemniscus.
Gamma 2
In contrast to the discrete topographic pattern observed for most subunit mRNAs, the distribution of the γ2-subunit mRNA was more evenly distributed across all brain regions, possible due to its required presence in virtually all BZ- GABAA receptors. Expression in the cortex was moderate and homogeneous, with little apparent distinction among layers (Fig. 1H). Most nuclei of the basal ganglia expressed moderate hybridization signals for γ2 mRNA, with the exception of the substantia nigra, which showed weak expression. Likewise, in the basal forebrain, moderate labeling identified the septal nuclei and bed nucleus of the stria terminalis. Intense γ2 mRNA expression was apparent in all fields of the hippocampus proper (Fig. 2H), whereas in the entorhinal cortex and associated subicular complex, transcript expression was intermediate. Hybridization signals for the γ2-subunit mRNA was evident throughout the thalamus, with the anterodorsal nucleus, ventral lateral geniculate, and laterodorsal nucleus exhibited the most intense labeling. Labeling for the γ2 was weak to moderate in the remaining thalamic nuclei. The distribution of γ2 mRNA in the hypothalamus was heterogeneous (Fig. 3H). In this region, high levels of expression of receptor mRNA was evident in the paraventricular nuclei (PVN) and ventromedial hypothalamic area. Levels were less intense in the anterior hypothalamic area, dorsomedial nucleus, and mammillary nuclei. The supramammillary nuclei and lateral hypothalamic area revealed weak γ2 mRNA signals. Overall, midbrain and brainstem nuclei levels of γ2 mRNA were intermediate and uniform (Fig. 4H). Notable exceptions were the pontine nuclei, and dorsal raphe nucleus, which exhibited more intense expression.
Discussion
The present study documents the comparative neuroanatomical topography of 7 GABAA receptor subunit mRNAs in the mouse brain for the purpose of providing normative data need to direct future research using mice. Subunit mRNAs levels were assessed in the present in situ hybridization investigation with riboprobes and the results of our observations in individual nuclei are presented in Table 1. As summarized in Figure 5, the strongest expression for α1 was observed in the hippocampus and midbrain, followed by the cerebral cortex; whereas the lowest α1 expression was observed in the basal ganglia. Throughout the hippocampus, α2 hybridization signal was extremely strong. In addition, relative strong labeling was seen in amygdala and hypothalamus. Overall, the weakest expression of α2 was observed in the thalamus. Compared to other regions, the relative abundance of α3 was highest in the amygdala, hypothalamus, and cerebral cortex; while low signal strengths were noted in the thalamus, hippocampus, and basal ganglia. By far, the most intense hybridization signals for α5 mRNA were observed in the hippocampus and weak signals were observed in basal ganglia thalamus and septum. For the β2 subunit, on average the strongest mRNA signals were seen in the cerebral cortex, thalamus, and hippocampus compared to weaker expression within the amygdala and hypothalamus. The strongest expression the β3 was observed in the hippocampus, followed by the amygdala and septal/basal forbrain region. Finally, the signal strength for subunit γ2 was rather uniformly distributed across all regions; however, labeling for γ2 was most prominent in the hypothalamus and hippocampus.
When using the same probe, it is reasonable to make comparisons about relative abundance of each subunit across brain regions; however, precautions should be taken when comparing across subunits. Due to potential differences in the sensitivities of the subunit-specific riboprobes used, the relative abundance of one subunit compared with another cannot be judged without some level of uncertainty. With this qualification in mind, it is still of importance to consider how our findings compare to past studies examining the regional distribution of various GABAA subunits.
Comparison with previous mRNA and immunocytochemical reports of subunit distributions
Cerebral cortex
A subtle laminar segregation among various subunits characterized the cerebral cortex. In layers 2 and 3, strong hybridization was observed for the α2 subunit with more weak to moderate expression for other subunit with the exception α5,which was all but absent. mRNA expression in layer 4 was strongest for α1, β2, and γ2 mRNA transcripts. In layers 5 and 6, mRNA expression for subunits γ2 and α3 were moderate to strong, whereas other mRNA transcripts were less intense. The current findings are in line with in situ hybridization studies of these subunits in rat cortex 17, 62, 63, with some distinguishing exceptions. Most notable was the stronger α2 signal observed in upper cortical layers seen in the current study. Furthermore, our results indicate that the expression of α1 mRNA in layer 2 was markedly less than layer 3, which is at odds with some rat hybridization data showing both layers express high levels of α1 mRNA 62, 63. The paucity of α1 expression in layer 2 is also at odds with immunocytochemical reports from the rats 22, 29, 44, which are otherwise largely in accord with the distribution of other subunits in this study. On the bases of these past studies, differences in cortical α1 and α2 mRNA levels indicate differences between rats and mice. However, in the current study, caudal cortical regions display stronger α1 hybridization in layer 2, as is evident in other studies 17.
In other defined cortical areas, including the agranular insular, piriform, perirhinal, endopiriform, entorhinal, both the α1 and γ2 subunits generally showed moderate expression, with the exception of the agranular insular cortex which showed weak expression of the α1 subunit. The α2 subunit expression was generally weak in these cortical areas. In contrast, both the α3 and β3 subunits displayed relatively strong expression. These findings are in excellent agreement with some mRNA studies of subunit distribution in the rat 3, 62. However, in a study examining α1, β2, and γ2 transcript distribution, Duncan et al. 17 reported intense α1 and β2 expression in the piriform cortex which is at odds with our findings.
Basal ganglia
The basal ganglia were characterized by a salient regional segregation among various subunits. Subunit expression in the core and shell of the nucleus accumbens was restricted to relatively intense α2 and β3 hybridization, along with a more moderate γ2 signal. Similarly, in the caudate putamen, prominent hybridization was evident for α2, β3, and γ2 subunits. Relatively weak or undetectable levels of α1, α3, and α5 subunits mRNA transcripts were seen in the nucleus accumbens and caudate. In contrast, α1, β2, and γ2 subunits were expressed prominently in the globus pallidus and ventral pallidum, whereas α2, α3, β3, and α5 displayed relatively weak expression. In the substatia nigra, we observed a strong α1 hybridization signal in the pars reticularis along with a slightly less intense β2 signal. Hybridization in the pars compacta appeared as moderate to strong for the α3 and β2 subunits and weak for other subunits; however the boundaries of this division of the substatia nigra could not be defined without some degree of ambiguity. Lastly, in the subthalamic nucleus, moderate to strong hybridization was seen for all analyzed subunits with the exception of α5, which was absent.
Our subunit distribution data from basal ganglia and substantia nigra are in general agreement with previous rat GABAA subunit in situ hybridization data 3, 17, 19, 27, 35, 42, 62 and immunocytochemical data 12, 26, 48. However, our mouse study did demonstrate a stronger relative γ2 levels in the caudate when compared to several studies 3, 62. In addition, in comparison to past studies, we observed stronger expression of most subunits in the subthalamic nucleus. For example, Wisden et al. 62 reported a moderate α1, β2, and γ2 expression and an absence of other subunits in this nucleus. Whether these discrepancies represent a species difference or a difference in defining the boundaries of the subthalamic nucleus is unclear.
Septal and basal forebrain
The medial and lateral subdivisions of the septum were outlined by specific distributions of subunits. The lateral nucleus showed very prominent α2 and β3 hybridization, along with a more moderate γ2 subunit signal and weak α1, β2 hybridization. In contrast, α1, β2 and γ2 subunits were most prominent in the medial nucleus; whereas, α2 and β3 were weak. Both septum subdivisions showed moderate α3 mRNA signal and an absence of α5 subunit transcript. In the bed nucleus of the stria terminalis, the signal intensity for α2, β3, and α3 subunits were strong, whereas γ2 was moderate and α1, α5, and β2 exhibited the weakest mRNA signals. Hybridization labeling of all subunit except α5 was evident in the dense cell layer of olfactory tubericle and cell clusters of the Island of Calleja, although the labeling was strong for the α2 and β3 subunits only. A comparison of our finding with previous rat GABAA subunit in situ hybridization data reveals broad agreement 3, 17, 19, 33, 62. However, in contrast to our data, a number of studies have shown only a minor presence of γ2 in the bed nucleus of the stria terminalis 2, 19, 62. We also recognized a moderate presence of α3 mRNA in the medial septum which was not identified by some studies 3, 62. Interestingly, the septal and basal forebrain subunit distribution seen in this study is more aligned with immunocytochemical rat data 22, 24, 44.
Hippocampus
The expression of mRNA within CA regions of the hippocampus proper was confined to pyramidal layers. All CA regions expressed very strong mRNA hybridization levels for α2, α5, and β3 subunits and slightly less γ2 hybridization. Likewise strong expression of these subunits was evident in the granular layer of the dentate gyrus. The expression of both α1 and β2 subunit transcripts were moderate in CA1 and the dentate gyrus with slightly weaker levels in CA2 and CA3. Localization of transcripts encoding the α3 subunit was limited to weak expression in CA1. Overall, this is in good general agreement with previous mRNA reports of the subunit distributions in the rat 3, 32, 53, 62; however, in some of these reports the levels of β2 mRNA in CA regions were characterized as weak, suggesting a difference between species.
Overall, our mRNA expression levels coincide with the subunit immunoreactivity distributions 14, 22, 44, 51, however as expected, the localization of subunit expression was predominantly identified in dendritic fields of pyramidal neurons. A noteworthy exception is the relative scarcity of α5 immunoreactivity within the dentate gyrus seen in some studies 22, 51, which is at odds with the relatively strong expression of this subunit identified in most studies.
In other regions of the hippocampal formation such as the subicular complex and entorhinal cortex, the expression of all mRNAs subunits was clearly present. In the entorhinal cortex, mRNA hybridization was prominent for α2, α5, and β3 whereas α1 and β2 subunits were weak. In the subiculum proper, the expression of α1, α2, α3, and β3 mRNA was strong, whereas β2 and γ2 was more moderate. In other regions of the subicular complex, the expression of all mRNA subunits was more uniformly distributed with moderate to strong signal intensities. Few in situ hybridization studies have examined and contrasted differences among subunit expression intensity in these latter brain regions. However, our data best match the subunit distributions described by Araki and Tohyama 3, who reported moderate to strong signals throughout the subicular complex and entorhinal cortex for α1, α3, β2, β3, and γ2 subunits see also,45. Studies using GABAA subunit antibodies vary in their descriptions of the subunit expression patterns in the above structures 22, 44. However, these studies have reliably reported a general weak presence of α2 and strong presence of α1 in the subicular complex, which are opposite to what we observed in the mouse.
Amygdala
The expression pattern of subunits in the amygdaloid complex clearly revealed the boundaries of individual nuclei. In the La and BL nuclei, particularly strong mRNA labeling was observed for the α2, α3,and β3 subunits with more moderate signals from α1, β2, and γ2 and weak α5 expression. The Ce nucleus was characterized by strong α2 and β3 labeling, and weak to moderate α3, α5, β2, and γ2 hybridization. The presence of α1 was undetected in the Ce nucleus and the expression of α3 was limited to the medial division of the Ce nucleus. The basomedial, medial, and amygdalohippocampal regions nuclei of the mouse amygdala showed similar distribution profiles with moderate to strong α2 and β3, and weak α1, α5, β2 and γ2 hybridization. The α3 subunit, however, showed strong hybridization in the amygdalohippocampal area and moderate expression in the former nuclei. Lastly, an analysis at the level of the anterior amygdala area revealed the prominent expression of α1 and α2 mRNA transcripts along with moderate α3, β2, γ3, and an absence of α5 mRNA.
Due to BZ- GABAA receptor's purported involvement in the anxiolytic response to BZs, the expression of various combinations of GABAA subunit in the amygdala has been the subject of numerous studies. Our results are in good general agreement with previous in situ hybridization studies 3, 62, although the hybridization intensity of subunits diverged to some extent in a few amygdala regions. Consistent with these studies, we found weak α1 mRNA expression in the Ce nucleus and moderate to strong homogenous expression of α1, α2 and α3 in the La and BL nucleus. Others however have reported strong α1 mRNA levels in the Ce versus La and BL nucleus 18. We did observe a higher expression of α3 mRNA in the mouse Ce nucleus compared to that observed by other groups in the rat, however as described above, its location was restricted. In contrast to the rat hybridization study by Wisden 62, our observations also clearly showed the expression of α5 in the Ce and La amygdala, albeit weak. The decided presence of α3 and α5 in the Ce nucleus has also been identified by rt-PCR in a study comparing of the distribution of GABAA subunit mRNAs in the Ce amygdala to the lateral/basolateral amygdala complex 23. In addition, this study revealed no differences in the levels of α2, β2, or β3 between the Ce amygdala and lateral/basolateral amygdala complex, whereas the α1, α3, α5, and γ2 transcripts were more prominent in the lateral/basolateral complex.
Compared to rat immunoreactivity studies, our data from amygdala regions generally matched the subunit distribution maps described by Fritschy and Mohler 22. Others, however, are at variance with the differential expression profile of α1 and α2 within the Ce and lateral/basolateral amygdala reported in this paper. For example, some data show prominent α1 immunostaining in the Ce nucleus 33, 44and a relatively weak α2 subunit immunoreactivity in the lateral/ basolateral complex 33. Given differences among studies, it is difficult to conclude the microdistribution of GABAA in this region varies among species. However, our in situ hybridization data are in excellent agreement with the distribution of α1, α2, and α3 subunit proteins in the mouse Ce, La and BL nuclei of the amygdala, including the clear lack of α1 in the Ce nucleus 37.
Thalamus and epithalamus
Globally, the al, β2, and γ2 subunits were the predominant subunits expressed in the thalamus. The presence of α5 hybridization were largely absent in the thalamus; whereas, α2, α3 and β3 subunits generally showed low expression in most thalamic nuclei. In addition to comparable expression levels, the α1, β2, and γ2 subunits also showed similar spatial distributions within the thalamus, as seen in other in situ hybridization studies of rat brains 16, 17. In the mouse, α1, β2 and γ2 mRNA subunits were expressed with similar intensities in many thalamic nuclei, including moderate to strong levels in the anteromedial, anteroventral, ventral lateral, ventral posterior, PO area, and dorsal lateral geniculate. Differences in the relative abundance of α1 and β2 were apparent in the PF area and medial geniculate, where transcripts encoding the β2 subunit were markedly stronger. More uniform expression of subunits was identified in a number of nuclei, including the zona incerta, ventral lateral geniculate and nuclei surrounding the mediate geniculate (SG/POT and PP/PIL), where the appearance of all subunits transcripts was moderate or strong with the exception α5. Restricted subunit expression distinguished the medial habenula, which contained strong expression of transcripts encoding the α2, β3, and γ2 subunits. Labeling in the lateral habenula was largely limited to moderate α3, γ2 and weak α2, β3 hybridization. In the reticular nucleus, we observed a moderate to strong α3 hybridization signal and less intense γ2 and β3 expression levels, whereas the appearance of other subunits transcripts was not evident. The spatial distribution and relative abundance of thalamic subunit mRNA differs to some degree with the mRNA and antibody subunit distributions seen in rats 2, 17, 22, 44, 62. For example, our study revealed an overall level of γ2 that was more intensely expressed in a number of nuclei when compared to some of the studies cited above. Some of these studies have also noted a complete absence of β3 in many thalamic nuclei; whereas our finding revealed clear β3 signals in the examined nuclei, albeit weak in most cases. Past subunit distribution studies are also at odds with the stronger presence of α2 and α3 subunits seen in the zona incerta and ventral lateral geniculate of the current mouse study 22, 44, 62. Furthermore, in the case of the ventral posterior nucleus, our detection of moderate/strong α1 hybridization is in agreement with past antibody, but not mRNA studies. Also of note was the distribution pattern in nuclei surrounding the mediate geniculate. To our knowledge, past mRNA or antibodies studies have not recognized prominent expression of most GABAA subunits in these sites.
Hypothalamus
Overall, the most intense hybridization signals in the hypothalamus were from α2, α3, β3 and γ2 subunits. Transcripts for α1, α5, and β2 subunits showed lower expression in the hypothalamus; however, there were exceptions in some nuclei as noted below. In the case of α1 and α2 expression, past studies report mixed results with regard to whether the overall level of α1 or α2 is more prominently expressed in the hypothalamus. Our mouse data is in keeping with most GABAA subunit antibodies and RNA probes studies which have revealed higher levels of α2 subunit throughout the hypothalamus 4, 22, 62. However, others have revealed greater α1 levels when compared to α2 mRNAs or proteins 30. Areas showing moderate to strong levels of α2, α3, and β2 mRNA transcripts included the dorsomedial nucleus, preoptic, anterior, and ventromedial hypothalamic areas. The presence of α5 was strong in the preoptic and ventromedial areas and weak in other hypothalamic nuclei. In the lateral and posterior hypothalamic areas, all subunits examined in this study revealed weak to moderate mRNA expression. These results are largely in line with hypothalamic α subunits protein levels reported by Backberg et al. 4. However, in that study, and others 22, 44, the α1 subunit exhibited strong immunoreactivity in the lateral hypothalamic area which is at variance with the weak mRNA expression seen in current mouse report. Past mRNA and antibody studies which have examined the distribution of α5 in preoptic areas have also reported lower levels of α5 subunit than we observed in this study 22, 44, 62.
Numerous studies have examined the distribution of various subunit combinations in the PVN in order to elucidate the modulatory circuits involved in hormonal, autonomic and behavioral responses to stressors. In the current mouse study, we found transcripts encoding the α2 and γ2 subunits were strong. The α1, α3 and β3 subunits generally showed lower expression levels, and the α5 and β2 subunits were virtually absent. In general, past studies have revealed a broad range of subunit expression in the PVN and are without consensus regarding the subunit expression levels. For example, some studies examining the differential expression of α subunits have reported levels of α2 mRNA and protein expression to be greater than other alpha subunits 8, 22, 62; however, others have shown no differences 4, 19 or greater α1 presence 30, 44. Likewise, past reports indicate no consensus with regard to beta levels 20, 44, 62.
Midbrain and Pons
In the midbrain, different distribution patterns of α1 and α2 subunits clearly outlined subdivisions of the SC. In the superficial layers, the expression of α2 subunit transcripts was strong, whereas transcripts encoding the α1 were weak. In contrast, the intermediate and deep layers showed very prominent α1 hybridization, while the expression of mRNA for α2 subunit was relatively weak. The red nucleus was characterized by strong α1 and γ2 mRNA levels, along with moderate α3 and β2 and weak α2 hybridization. In the periaqueductal gray, dorsal and median raphe nuclei, moderate hybridization was seen for the α1, α2, α3, and β3 subunits. These nuclei also displayed similar moderate to strong γ2 signals, an absence of α5, and weak β2 hybridization, with the exception of periaqueductal gray, which showed strong β2 levels. In the ventral tegmental area, mRNA expression was limited to moderate α3 and γ2 levels and a weak β3 signal. Comparisons of our observations in the mouse midbrain with previous rat GABAA subunit immunocytochemical data reveal some differences 26, 27. Most notable was the stronger α2 signal observer in the periaqueductal gray, dorsal and median raphe nuclei seen in the current study. Past in situ hybridization comparative reports of GABAA subunit mRNAs in the midbrain have been limited to the description of only a few discrete brain regions including the substantia nigra, red nucleus, IC, and SC. Our mRNA results diverged only slightly from that observed by Wisden et al. 62. However, in contrast to the Wisden et al. study, we observed a moderate α3 signal in the red nucleus which was absent in that study. To our knowledge, in situ hybridization studies have not been previously reported showing differential subunit mRNA expression levels in the periaqueductal gray, dorsal and median raphe nuclei, and VTA. However, as seen in this study, a weak expression of α1 mRNA in the VTA has been reported in both the mouse and rat 17, 31. In contrast, immunocytochemical studies have demonstrated obvious α1 subunit expression in the VTA 22, 44, 48.
In contrast to the above-mentioned areas, the expression and levels of GABAA mRNA subunits in the IC have been described by a number of laboratories investigating subunit alterations in the central auditory system associated with developmental and various experimental manipulations 13, 28, 36. In the mouse, we observed an intense hybridization for subunit α1 mRNA along with moderate to strong β2 and γ2 expression in the central nucleus of the IC. Signals for mRNA encoding other subunits were relatively weak. The distribution patterns were the same in the external capsule of the IC; however, the signal levels were slightly weaker. In the lateral lemniscus, α5, β3, and γ2 were moderate and to a lesser extent α2; whereas only weak α3 and β2 was detectable and no α1 mRNA was identified. The above findings are in excellent agreement with both regional distribution mRNA subunits 36, 41, 49, 62 and subunit proteins 22, 44.
Comparison summary and limitations
As noted above, the regional distribution of GABAA receptor mRNA in the mouse often correlates with the distribution of GABAA receptor mRNA and protein levels observed in the rat; however, discrepancies exist in several brain areas. These differences in distribution and expression levels may represent species-specific dissimilarities in GABAA function. Overall our study revealed stronger labeling for β3 and γ2 than that seen in other comprehensive studies comparing subunit signal strengths 2, 3, 62. Conversely, our stronger γ2 mRNA levels more closely match that reported in a study examining γ2 transcript distribution alone 2; thus, differences in hybridization sensitivity among probes may partially explain some discrepancies. Given that BZ receptors require the presence of the γ2 subunit and that those containing the γ1- or γ3-subunit represent only minor populations 46, 54, one might expect strong γ2 mRNA expression. It is worth noting that the γ2 mRNA exists in two variant forms due to alternate splicing of the precursor mRNA 58. Although, the relative abundances of each version depend on the brain region 58, our γ2 probe hybridizes to both of these mRNA variants.
Particular brain areas of variance between mice and rats included the subthalamic nucleus were we observed stronger expression of a number of subunits. We also recognized a moderate presence of α3 mRNA in the medial septum which was not identified by other mRNA rat studies 3, 62. In the amygdala, mice clearly showed the expression of α5 in the Ce and La amygdala, which is at odds with most rat studies. In the thalamus stronger presence of α2 and α3 subunits in the zona incerta and ventral lateral geniculate was seen. We found prominent expression most GABAA subunits in nuclei surrounding the mediate geniculate.
Some dissimilarity may have resulted from variations in procedures used for subunit detection. For instance, Wisden et al. 62 and Araki et al. 3 used single 44-45-base oligonucleotide probes for their comprehensive in situ hybridization studies designed to determine multiple GABAA subunit mRNA distributions. Our detection system employed riboprobes which were prepared from linearized clones and base hydrolyzed to average lengths of 50-100bp. This procedure resulted in a mixture of synthesized probes which targeted different regions of each subunit mRNA. In addition, some studies used emulsion 17 while we used exposure on film. Nevertheless, our riboprobes provided an excellent hybridization signal-to-noise ratio. We cannot eliminate the possibility that our subunit-specific riboprobes cross-hybridized with other mRNA subunits that shared homogeneous gene domains. Some discrepancies between mRNA and protein levels may simply reflect the fact that neurons synthesize subunit receptor proteins from mRNA in their cell bodies and transport it to postsynaptic sites located in their dendrites and extrasynaptic loci 10.
Significance
Diazepam and other classical BZs are among the most widely prescribed therapeutic agents, having a variety clinical uses based on their anxiolytic, hypnotic, anticonvulsant, and antispastic effects. However, the therapeutic effects of BZs are often accompanied by frequent abuse and unwanted side effects; thus, there is great interest in understanding the behavioral and pharmacological function of GABAA receptor subtypes in hopes of developing benzodiazepine site ligands with higher therapeutic selectivity and reduced side effect profiles.
Important insights into the behavioral and pharmacological function of GABAA receptor subunits has been revealed by studies of mouse lines which possess deleted and genetically altered receptor subunits sites 21, 34, 38. However, there is an increasing awareness that anatomical and behavioral differences between mice and other well-studied rodents (rats) may limit the interpretation and usefulness of studies using gene-targeting strategies in mice. Descriptions of the distribution profiles of various GABAA subunits in mice have been generally confined to reports showing alterations in ligand recognition sites in limited areas of the brains of mice lacking individual GABAA receptor subunits 34, 38, 52. Unfortunately, at present the heterogeneity in subtypes of receptors exceed that observed by ligand-binding autoradiography due to the current lack of ligands specific for most receptor subtypes. This study provides a record of the regional distribution and the cellular localization of the main GABAA receptor mRNAs within the mouse brain. The regional distribution of subunit receptors often correlates with the distribution seen in rats; however, the microdistribution of GABAA mRNA varies between these species. Because inbred and genetically altered mice have become important tools for understanding GABAergic function, these data provide the necessary normative data need to direct future research using mice.
Supplementary Material
Acknowledgements
We would also like to thank Dr. Todd M. Preuss, Amanda Green, Daphne Pierre, and Natalie Balkema for their technical contributions to this manuscript.
Grant Sponsor: Funding support was provided by NARSAD, Rockefeller Brothers Fund, NIH (MH069884, DA019624, and F32 MH073389-01), Yerkes Research Center (RR-00165), and the Center for Behavioral Neuroscience (NSF agreement IBN-987675).
Abbreviations
- Acb
nucleus accumbens
- AHi
amygdalohippocampus area
- APT
anterior pretectal nucleus
- BL
basolateral amygdaloid nucleus
- BM
basomedial amygdaloid nucleus
- CA1
CA1 field of the hippocampus proper
- CA2
CA2 field of the hippocampus proper
- CA3
CA3 field of the hippocampus proper
- Ce
central amygdaloid nucleus
- cp
cerebral peducle
- CPu
caudate putamen
- DG
dentate gyrus
- DLG
dorsal lateral geniculate nucleus
- DM
dorsomedial hypothalamic nucleus
- GP
globus pallidus
- ic
internal capsule
- La
lateral amygdaloid nucleus
- LD
laterodorsal thalamic nucleus
- LH
lateral hypothalamic area
- LHb
lateral habenular nucleus
- LP
lateral posterior thalamic nucleus
- LS
lateral septal nucleus
- Me
medial amygdaloid nucleus
- MGB
medial geniculate body
- MHb
medial habenular nucleus
- MS
medial septal nucleus
- PAG
periaqueductal gray
- PF
parafascicular thalamic nucleus
- PH
posterior hypothalamic area
- PIL
posterior intralaminar thalamic nucleus
- Pir
piriform cortex
- PM
premammillary nucleus
- Po
posterior thalamic nuclear group
- POT
posterior thalamic nucleus, triangular
- PP
peripeducular nucleus
- Re
reuniens thalamic nucleus
- RN
red nucleus
- Rt
reticular thalamic nucleus
- S
subicular complex
- SCd
superior colliculus, deep layers
- SCi
superior colliculus, intermediate layers
- SCs
superior colliculus, superficial layers
- SG
suprageniculate thalamic nucleus
- SNC
substantia nigra, compact part
- SNR
substantia nigra, reticular part
- Tu
olfactory tubercle
- VL
ventrolateral thalamic nucleus
- VLG
ventral lateral geniculate nucleus
- VMH
ventromedial hypothalamic area
- VP
ventral pallidum
- VPt
ventral posterior thalamic nuclei
- VPM
ventral posteromedial thalamic nucleus
- ZI
zona incerta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Submitted to: Charles R. Gerfen, Section Editor: Neuroscience Lab of Systems Neuroscience, NIMH (Bethesda, MD, USA) June 4, 2007
Supplemental data associated with article can be found in online version.
References
- 1.Anagnostopoulos AV, Mobraaten LE, Sharp JJ, Davisson MT. Transgenic and knockout databases: Behavioral profiles of mouse mutants. Physiology & Behavior. 2001;73:675–689. doi: 10.1016/s0031-9384(01)00525-x. [DOI] [PubMed] [Google Scholar]
- 2.Araki T, Sato M, Kiyama H, Manabe Y, Tohyama M. Localization of GABAA-receptor [gamma]2-subunit mRNA-containing neurons in the rat central nervous system. Neuroscience. 1992;47:45–61. doi: 10.1016/0306-4522(92)90119-m. [DOI] [PubMed] [Google Scholar]
- 3.Araki T, Tohyama M. Region-specific expression of GABAA receptor [alpha]3 and [alpha]4 subunits mRNAs in the rat brain. Molecular Brain Research. 1992;12:293–307. doi: 10.1016/0169-328x(92)90132-u. [DOI] [PubMed] [Google Scholar]
- 4.Backberg M, Ultenius C, Fritschy JM, Meister B. Cellular localization of GABA receptor alpha subunit immunoreactivity in the rat hypothalamus: relationship with neurones containing orexigenic or anorexigenic peptides. J Neuroendocrinol. 2004;16:589–604. doi: 10.1111/j.1365-2826.2004.01207.x. [DOI] [PubMed] [Google Scholar]
- 5.Backus KH, Arigoni M, Drescher U, Scheurer L, Malherbe P, Mohler H, Benson JA. Stoichiometry of a recombinant GABAA receptor deduced from mutation-induced rectification. Neuroreport. 1993;5:285–288. doi: 10.1097/00001756-199312000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 7.Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behavioural Brain Research. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 8.Birzniece V, Turkmen S, Lindblad C, Zhu D, Johansson IM, Backstrom T, Wahlstrom G. GABA(A) receptor changes in acute allopregnanolone tolerance. Eur J Pharmacol. 2006;535:125–134. doi: 10.1016/j.ejphar.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 9.Bothe GWM, Bolivar VJ, Vedder MJ, Geistfeld JG. Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes, Brain and Behavior. 2004;3:149–157. doi: 10.1111/j.1601-183x.2004.00064.x. [DOI] [PubMed] [Google Scholar]
- 10.Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- 11.Burt DR, Kamatchi GL. GABAA receptor subtypes: from pharmacology to molecular biology. Faseb J. 1991;5:2916–2923. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- 12.Caruncho HJ, Liste I, Rozas G, Lopez-Martin E, Guerra MJ, Labandeira-Garcia JL. Time course of striatal, pallidal and thalamic alpha 1, alpha 2 and beta 2/3 GABAA receptor subunit changes induced by unilateral 6-OHDA lesion of the nigrostriatal pathway. Brain Res Mol Brain Res. 1997;48:243–250. doi: 10.1016/s0169-328x(97)00097-1. [DOI] [PubMed] [Google Scholar]
- 13.Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age-related changes in GABA(A) receptor subunit composition and function in rat auditory system. Neuroscience. 1999;93:307–312. doi: 10.1016/s0306-4522(99)00121-9. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Huang X, Zeng XJ, Sieghart W, Tietz EI. Benzodiazepine-mediated regulation of alpha1, alpha2, beta1-3 and gamma2 GABA(A) receptor subunit proteins in the rat brain hippocampus and cortex. Neuroscience. 1999;93:33–44. doi: 10.1016/s0306-4522(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 15.Crestani F, Martin JR, Mohler H, Rudolph U. Mechanism of action of the hypnotic zolpidem in vivo. Br J Pharmacol. 2000;131:1251–1254. doi: 10.1038/sj.bjp.0703717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cullinan WE. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. J Comp Neurol. 2000;419:344–351. doi: 10.1002/(sici)1096-9861(20000410)419:3<344::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Duncan GE, Breese GR, Criswell HE, McCown TJ, Herbert JS, Devaud LL, Morrow AL. Distribution of [3H]zolpidem binding sites in relation to messenger RNA encoding the alpha 1, beta 2 and gamma 2 subunits of GABAA receptors in rat brain. Neuroscience. 1995;64:1113–1128. doi: 10.1016/0306-4522(94)00433-6. [DOI] [PubMed] [Google Scholar]
- 18.Facciolo RM, Alo R, Canonaco M, Franzoni MF. Early phylogenetic value of the major GABA(A) receptor subunit mRNAs in the telencephalon. Exp Brain Res. 2002;142:504–511. doi: 10.1007/s00221-001-0972-x. [DOI] [PubMed] [Google Scholar]
- 19.Fenelon VS, Herbison AE. In vivo regulation of specific GABAA receptor subunit messenger rnas by increased gaba concentrations in rat brain. Neuroscience. 1996;71:661–670. doi: 10.1016/0306-4522(95)00492-0. [DOI] [PubMed] [Google Scholar]
- 20.Fenelon VS, Sieghart W, Herbison AE. Cellular localization and differential distribution of GABAA receptor subunit proteins and messenger RNAs within hypothalamic magnocellular neurons. Neuroscience. 1995;64:1129–1143. doi: 10.1016/0306-4522(94)00402-q. [DOI] [PubMed] [Google Scholar]
- 21.Fradley R, Guscott M, Bull S, Hallett DJ, Goodacre SC, Wafford KA, Garrett EM, Newman R, O'Meara GF, Whiting PJ, Roshal TW, Dawson GR, Reynolds DS, Atack J. Differential contribution of GABAA receptor subtypes to the anticonvulsant efficacy of benzodiazepine site ligands. J Psychopharmacol. 2007 doi: 10.1177/0269881106067255. 0269881106067255. [DOI] [PubMed] [Google Scholar]
- 22.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 23.Fujimura J, Nagano M, Suzuki H. Differential expression of GABA(A) receptor subunits in the distinct nuclei of the rat amygdala. Brain Res Mol Brain Res. 2005;138:17–23. doi: 10.1016/j.molbrainres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Gao B, Hornung JP, Fritschy JM. Identification of distinct GABAA-receptor subtypes in cholinergic and parvalbumin-positive neurons of the rat and marmoset medial septum-diagonal band complex. Neuroscience. 1995;65:101–117. doi: 10.1016/0306-4522(94)00480-s. [DOI] [PubMed] [Google Scholar]
- 25.Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji DJ, Whiting PJ. Cloning of cDNAs encoding the human gamma-aminobutyric acid type A receptor alpha 6 subunit and characterization of the pharmacology of alpha 6-containing receptors. Mol Pharmacol. 1996;49:253–259. [PubMed] [Google Scholar]
- 26.Hartig W, Brauer K, Fritschy J-M, Bruckner G, Bigl V. Regional and cellular expression sites of the [alpha]1 subunit of GABAA receptors in the rat basal forebrain: a cytochemical study with glutamic acid decarboxylase, choline acetyltransferase, calcium-binding proteins and nitric oxide synthase as second markers. Brain Research. 1995;692:215–226. doi: 10.1016/0006-8993(95)00631-y. [DOI] [PubMed] [Google Scholar]
- 27.Henderson Z. Expression of GABAA receptor subunit messenger RNA in non-cholinergic neurons of the rat basal forebrain. Neuroscience. 1995;65:1077–1086. doi: 10.1016/0306-4522(94)00542-d. [DOI] [PubMed] [Google Scholar]
- 28.Holt AG, Asako M, Lomax CA, MacDonald JW, Tong L, Lomax MI, Altschuler RA. Deafness-related plasticity in the inferior colliculus: gene expression profiling following removal of peripheral activity. J Neurochem. 2005;93:1069–1086. doi: 10.1111/j.1471-4159.2005.03090.x. [DOI] [PubMed] [Google Scholar]
- 29.Hutcheon B, Fritschy JM, Poulter MO. Organization of GABA receptor alpha-subunit clustering in the developing rat neocortex and hippocampus. Eur J Neurosci. 2004;19:2475–2487. doi: 10.1111/j.0953-816X.2004.03349.x. [DOI] [PubMed] [Google Scholar]
- 30.Inglefield JR, Sieghart W, Kellogg CK. Immunohistochemical and neurochemical evidence for GABAA receptor heterogeneity between the hypothalamus and cortex. J Chem Neuroanat. 1994;7:243–252. doi: 10.1016/0891-0618(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 31.Johanek LM, Cullinan WE, Vaughn LK. Increased mRNA expression for the alpha(1) subunit of the GABA(A) receptor following nitrous oxide exposure in mice. Brain Res Mol Brain Res. 2001;89:41–49. doi: 10.1016/s0169-328x(01)00060-2. [DOI] [PubMed] [Google Scholar]
- 32.Kamphuis W, De Rijk TC, Lopes da Silva FH. Expression of GABAA receptor subunit mRNAs in hippocampal pyramidal and granular neurons in the kindling model of epileptogenesis: an in situ hybridization study. Molecular Brain Research. 1995;31:33–47. doi: 10.1016/0169-328x(95)00022-k. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann WA, Humpel C, Alheid GF, Marksteiner J. Compartmentation of alpha 1 and alpha 2 GABA(A) receptor subunits within rat extended amygdala: implications for benzodiazepine action. Brain Res. 2003;964:91–99. doi: 10.1016/s0006-8993(02)04082-9. [DOI] [PubMed] [Google Scholar]
- 34.Kralic JE, Korpi ER, O'Buckley TK, Homanics GE, Morrow AL. Molecular and Pharmacological Characterization of GABAA Receptor alpha 1 Subunit Knockout Mice. J Pharmacol Exp Ther. 2002;302:1037–1045. doi: 10.1124/jpet.102.036665. [DOI] [PubMed] [Google Scholar]
- 35.Li XQ, Dong L, Liu ZH, Luo JY. Expression of gamma-aminobutyric acid A receptor subunits alpha1, beta1, gamma2 mRNA in rats with hepatic encephalopathy. World J Gastroenterol. 2005;11:3319–3322. doi: 10.3748/wjg.v11.i21.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao WH, Van Den Abbeele T, Herman P, Frachet B, Huy PT, Lecain E, Marianowski R. Expression of NMDA, AMPA and GABA(A) receptor subunit mRNAs in the rat auditory brainstem. II. Influence of intracochlear electrical stimulation. Hear Res. 2000;150:12–26. doi: 10.1016/s0378-5955(00)00167-2. [DOI] [PubMed] [Google Scholar]
- 37.Marowsky A, Fritschy JM, Vogt KE. Functional mapping of GABA A receptor subtypes in the amygdala. Eur J Neurosci. 2004;20:1281–1289. doi: 10.1111/j.1460-9568.2004.03574.x. [DOI] [PubMed] [Google Scholar]
- 38.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 39.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 40.Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- 41.Milbrandt JC, Hunter C, Caspary DM. Alterations of GABAA receptor subunit mRNA levels in the aging Fischer 344 rat inferior colliculus. J Comp Neurol. 1997;379:455–465. doi: 10.1002/(sici)1096-9861(19970317)379:3<455::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 42.Nicholson LF, Faull RL, Waldvogel HJ, Dragunow M. The regional, cellular and subcellular localization of GABAA/benzodiazepine receptors in the substantia nigra of the rat. Neuroscience. 1992;50:355–370. doi: 10.1016/0306-4522(92)90429-6. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 2001. [Google Scholar]
- 44.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 45.Poulter MO, Barker JL, O'Carroll AM, Lolait SJ, Mahan LC. Co-existent expression of GABAA receptor beta 2, beta 3 and gamma 2 subunit messenger RNAs during embryogenesis and early postnatal development of the rat central nervous system. Neuroscience. 1993;53:1019–1033. doi: 10.1016/0306-4522(93)90486-y. [DOI] [PubMed] [Google Scholar]
- 46.Quirk K, Gillard NP, Ragan CI, Whiting PJ, McKernan RM. gamma-Aminobutyric acid type A receptors in the rat brain can contain both gamma 2 and gamma 3 subunits, but gamma 1 does not exist in combination with another gamma subunit. Mol Pharmacol. 1994;45:1061–1070. [PubMed] [Google Scholar]
- 47.Rudolph U, Mohler H. Analysis of GABAA Receptor Function and Dissection of the Pharmacology of Benzodiazepines and General Anesthetics through Mouse Genetics. Annual Review of Pharmacology and Toxicology. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- 48.Schwarzer C, Berresheim U, Pirker S, Wieselthaler A, Fuchs K, Sieghart W, Sperk G. Distribution of the major gamma-aminobutyric acid(A) receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. J Comp Neurol. 2001;433:526–549. doi: 10.1002/cne.1158. [DOI] [PubMed] [Google Scholar]
- 49.Shiraishi S, Shiraishi Y, Oliver DL, Altschuler RA. Expression of GABA(A) receptor subunits in the rat central nucleus of the inferior colliculus. Brain Res Mol Brain Res. 2001;96:122–132. doi: 10.1016/s0169-328x(01)00282-0. [DOI] [PubMed] [Google Scholar]
- 50.Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, Adamiker D. Structure and subunit composition of GABAA receptors. Neurochemistry International. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 51.Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABA(A) receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 52.Sur C, Wafford KA, Reynolds DS, Hadingham KL, Bromidge F, Macaulay A, Collinson N, O'Meara G, Howell O, Newman R, Myers J, Atack JR, Dawson GR, McKernan RM, Whiting PJ, Rosahl TW. Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice. J Neurosci. 2001;21:3409–3418. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tietz EI, Huang X, Chen S, Ferencak WF., 3rd Temporal and regional regulation of alpha1, beta2 and beta3, but not alpha2, alpha4, alpha5, alpha6, beta1 or gamma2 GABA(A) receptor subunit messenger RNAs following one-week oral flurazepam administration. Neuroscience. 1999;91:327–341. doi: 10.1016/s0306-4522(98)00516-8. [DOI] [PubMed] [Google Scholar]
- 54.Togel M, Mossier B, Fuchs K, Sieghart W. gamma-Aminobutyric acidA receptors displaying association of gamma 3-subunits with beta 2/3 and different alpha-subunits exhibit unique pharmacological properties. J Biol Chem. 1994;269:12993–12998. [PubMed] [Google Scholar]
- 55.Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vicini S, Ortinski P. Genetic manipulations of GABAA receptor in mice make inhibition exciting. Pharmacology & Therapeutics. 2004;103:109–120. doi: 10.1016/j.pharmthera.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 57.Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human gamma-aminobutyric acidA receptors containing the alpha 4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- 58.Whiting P, McKernan R, Iversen L. Another Mechanism for Creating Diversity in {gamma}-Aminobutyrate Type A Receptors: RNA Splicing Directs Expression of Two Forms of {gamma}2 Subunit, One of Which Contains a Protein Kinase C Phosphorylation Site. PNAS. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whiting PJ. GABA-A receptor subtypes in the brain: a paradigm for CNS drug discovery? Drug Discov Today. 2003;8:445–450. doi: 10.1016/s1359-6446(03)02703-x. [DOI] [PubMed] [Google Scholar]
- 60.Whiting PJ, McKernan RM, Wafford KA. Structure and pharmacology of vertebrate GABAA receptor subtypes. Int Rev Neurobiol. 1995;38:95–138. doi: 10.1016/s0074-7742(08)60525-5. [DOI] [PubMed] [Google Scholar]
- 61.Wisden W, Herb A, Wieland H, Keinanen K, Luddens H, Seeburg PH. Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor alpha 4 subunit. FEBS Lett. 1991;289:227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- 62.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang JH, Araki T, Sato M, Tohyama M. Distribution of GABAA-receptor alpha 1 subunit gene expression in the rat forebrain. Brain Res Mol Brain Res. 1991;11:239–247. doi: 10.1016/0169-328x(91)90032-s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.