Abstract
We report four adult patients who presented with septic pulmonary emboli and community-acquired methicillin-resistant Staphylococcus aureus bacteremia associated with deep tissue infections, such as pyomyositis, osteomyelitis, and prostatic abscess. The patients lacked evidence of right-sided endocarditis or thrombophlebitis. This association, previously described in children, may also be important in adults.
CASE REPORT
Four adult patients from the community presented to a large urban hospital (John H. Stroger Jr. Hospital of Cook County, Chicago, IL) with septic pulmonary emboli and community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) bacteremia from December 2005 to February 2007. Each patient underwent a transesophageal echocardiogram that was negative for endocarditis. In addition, clinical and radiographic evaluations for deep-vein thrombosis were negative. No history or stigmata of intravenous drug use, thrombophlebitis, or prior intravascular-catheter use were found. Instead, each patient was diagnosed with a deep tissue infection or abscess as a possible primary focus of infection.
Patient 1.
A 36-year-old obese man with type 2 diabetes mellitus presented with 1 week of fever, cough, chest pain, and difficulty walking. Computed tomography (CT) of the chest demonstrated bilateral pulmonary nodules, some cavitary, consistent with septic pulmonary emboli (Fig. 1a). Blood cultures grew MRSA on admission and remained positive for 12 days. Despite receipt of appropriate antimicrobials, the patient remained intermittently febrile for 3 weeks. CT of the abdomen/pelvis, as well as magnetic resonance imaging (MRI) of the spine did not reveal thrombophlebitis or abscess. On hospital day 25, a gallium scan demonstrated increased uptake in the left thigh. CT of the lower extremities revealed an enhancing fluid collection extending the full length of the left quadriceps muscle, consistent with pyomyositis, without evidence of deep-vein thrombosis (Fig. 1c). Surgical drainage revealed gram-positive cocci in clusters on Gram stain; the culture (obtained during vancomycin therapy) was negative. The patient defervesced after drainage and recovered with 6 weeks of vancomycin therapy.
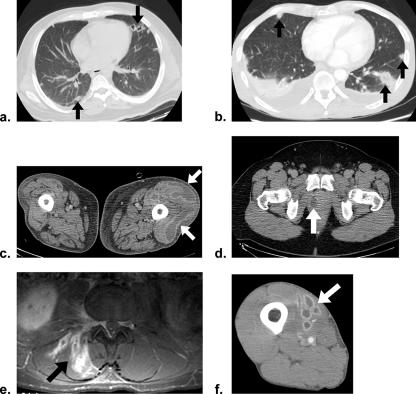
FIG. 1.
Clinical images from case patients (the arrows indicate areas of interest). (a and b) CT images of the chest showing peripheral pulmonary nodules consistent with septic pulmonary emboli for patients 1 and 4, respectively. (c) Patient 1, left thigh pyomyositis (CT). (d) Patient 2, prostatic abscess (CT). (e) Patient 3, right erector spinae pyomyositis with adjacent osteomyelitis at L2 vertebra (magnetic resonance imaging, T1 weighted). (f) Patient 4, right thigh pyomyositis (CT).
Patient 2.
A 55-year-old man with a history of hypertension, benign prostatic hypertrophy, and chronic rectal hemorrhoids was admitted with urinary hesitancy and rectal pain for 4 days. He had chills, but no pulmonary complaints. On examination, the patient was febrile and had large nonbleeding external rectal hemorrhoids and an enlarged nontender prostate. Blood cultures from the first 6 days of hospitalization were positive for MRSA. Pelvic CT revealed an enhancing cystic lesion in the inferior aspect of the prostate, consistent with a prostatic abscess, without evidence of pelvic thrombophlebitis (Fig. 1d). Pulmonary nodules consistent with septic pulmonary emboli were noted on CT. The patient was treated with 4 weeks of vancomycin and improved without surgical drainage.
Patient 3.
A 45-year-old man was admitted with acute low back pain radiating to the right thigh, with fever, nausea, and vomiting; he had no pulmonary complaints. Physical examination revealed only fever and pain over the lumbar spine. Blood cultures were positive for MRSA through the first 5 days of hospitalization. The patient underwent magnetic resonance imaging of the abdomen and pelvis, which revealed enhancement of the L2 vertebra with an overlying phlegmon of the erector spinae muscle, consistent with vertebral osteomyelitis with contiguous pyomyositis (Fig. 1e). The lung images by CT scan revealed bilateral pleural-based infiltrates consistent with septic pulmonary emboli. The patient was initially treated with vancomycin for 5 days; however, because of suspected drug fever, he was switched to 6 weeks of intravenous trimethoprim-sulfamethoxazole for a full recovery.
Patient 4.
A 64-year-old alcoholic man was admitted with a history of being assaulted, resulting in prolonged exposure to the cold and 10-finger frostbite. Upon admission, the patient was noted to have fever and pulmonary rales. A CT of the chest showed bilateral infiltrates consistent with septic pulmonary emboli (Fig. 1b). Initial blood cultures were positive for MRSA and remained positive through 8 days. We noted mild enlargement of the right thigh, without warmth or pain; there were no other stigmata of thrombosis or phlebitis. A CT of the right lower extremity showed pyomyositis of the quadriceps muscle, without adjacent deep venous thrombosis (Fig. 1f). The patient was treated with 6 weeks of vancomycin and cleared his bacteremia without surgical drainage.
Microbiologic analysis.
We tested a clinical bloodstream isolate of S. aureus from each case patient for oxacillin resistance, genotype by pulsed-field gel electrophoresis, and the presence of the genes lukS-PV and lukF-PV encoding the Panton-Valentine leukocidin toxin. All four isolates were methicillin resistant by disk diffusion susceptibility testing in accordance with the Clinical Laboratory Standards Institute guidelines (1). The isolates were further typed by pulsed-field gel electrophoresis (SmaI restriction endonuclease digestion) and found to be indistinguishable from each other and from a USA300-0114 reference strain (6, 9). All four isolates carried genes for Panton-Valentine leukocidin toxin, confirmed using a PCR assay as previously described by Johnsson et al. (5).
The four adult patients in this series presented with pyomyositis (three patients, one with contiguous lumbar osteomyelitis) and prostatic abscess (one patient) as the likeliest sources of MRSA bacteremia and septic pulmonary emboli. The four patients were not epidemiologically linked by time or location and were infected by the most common endemic clone of community-acquired MRSA in the United States, USA300-0114, which typically carries Panton-Valentine leukocidin toxin, along with other virulence factors (9). In patients 1 and 4, the deep tissue foci were occult, requiring careful and prolonged clinical investigation for diagnosis (Table 1). All four patients survived with prolonged antimicrobial therapy; patient 1 also required surgical drainage.
TABLE 1.
Summary of patient characteristics
| Patient | Age (yr)/sexa | Days of hospitalization | Days of confirmed bacteremia | Days to primary focus diagnosis | Prior medical history | Site of infection | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 36/M | 60 | 12 | 26 | Diabetes mellitus, obesity | Thigh pyomyositis | Surgical debridement, vancomycin | Recovered |
| 2 | 55/M | 13 | 6 | 0 | Hypertension | Prostatic abscess | Vancomycin | Recovered |
| 3 | 46/M | 11 | 5 | 5 | None | Lumbar osteomyelitis and pyomyositis | Vancomycin; trimethoprim-sulfamethoxazole | Recovered |
| 4 | 65/M | 13 | 8 | 9 | Prior assault, homelessness | Thigh pyomyositis | Vancomycin | Recovered |
M, male.
Septic pulmonary emboli are usually associated with right-sided endocarditis or other intravascular disease (2). However, septic pulmonary emboli arising from primary deep tissue infections, such as osteomyelitis, septic arthritis, cellulitis, and, rarely, pyomyositis, have been increasingly described in pediatric patients with CA-MRSA infections (3, 7, 10). To our knowledge, these associations have not been previously described among adult patients.
In one pediatric cohort, Gonzalez et al. described seven children with septic pulmonary emboli, of whom six had osteomyelitis and one had septic arthritis (3). Soft tissue infections have also been implicated: Miyashita et al. reported a case of cellulitis caused by CA-MRSA leading to septic pulmonary emboli (7). Although CA-MRSA has been noted as an increasingly common cause of pyomyositis (8), there have been few reports linking pyomyositis with septic pulmonary emboli. Wong et al. reported pyomyositis caused by MRSA associated with septic pulmonary emboli in a 7-year-old child (10), although the genotype was not described. Yuksel et al. also described an 8-year-old girl with pyomyositis and septic pulmonary emboli due to S. aureus without providing information regarding methicillin resistance or genotype (11).
The cases described in this series extend to the adult population the clinical association of deep tissue infection with CA-MRSA bacteremia and septic pulmonary emboli. For adults presenting with septic pulmonary emboli and CA-MRSA bacteremia, a search for deep tissue infections beyond the more common intravascular sources may therefore be important.
The pathogenesis of septic pulmonary emboli in our patients remains speculative. As demonstrated in prior pediatric studies (3, 4), deep tissue infections may be associated with local venous, and presumably septic, thrombophlebitis with septic pulmonary emboli. It is therefore plausible that our patients' deep tissue infections were complicated by local septic thrombophlebitis that could not be detected using the imaging modalities available, although tricuspid valve endocarditis that eluded detection by transesophageal echocardiography or an alternative, clinically occult focus of septic thrombophlebitis cannot be definitively excluded.
Acknowledgments
We acknowledge Thomas Rice and Alla Aroutcheva for their laboratory work in characterizing the S. aureus strains.
Footnotes
Published ahead of print on 30 January 2008.
REFERENCES
- 1.CLSI. 2005. Performance standards for antimicrobial susceptibility testing. CLSI approved standard M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 2.Cook, R. J., R. W. Ashton, G. L. Aughenbaugh, and J. H. Ryu. 2005. Septic pulmonary embolism: presenting features and clinical course of 14 patients. Chest 128162-166. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez, B. E., K. G. Hulten, M. K. Dishop, L. B. Lamberth, W. A. Hammerman, E. O. Mason, Jr., and S. L. Kaplan. 2005. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus infection. Clin. Infect. Dis. 41583-590. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez, B. E., J. Teruya, D. H. Mahoney, Jr., K. G. Hulten, R. Edwards, L. B. Lamberth, W. A. Hammerman, E. O. Mason, Jr., and S. L. Kaplan. 2006. Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics 1171673-1679. [DOI] [PubMed] [Google Scholar]
- 5.Johnsson, D., P. Molling, K. Stralin, and B. Soderquist. 2004. Detection of Panton-Valentine leukocidin gene in Staphylococcus aureus by LightCycler PCR: clinical and epidemiological aspects. Clin. Microbiol. Infect. 10884-889. [DOI] [PubMed] [Google Scholar]
- 6.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 415113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyashita, T., Y. Shimamoto, H. Nishiya, Y. Koshibu, H. Sugiyama, Y. Ono, T. Satoh, H. Haraoka, J. Nakano, K. Ohta, T. Sato, N. Morinaga, and M. Noda. 2002. Destructive pulmonary embolism in a patient with community-acquired staphylococcal bacteremia. J. Infect Chemother. 899-102. [DOI] [PubMed] [Google Scholar]
- 8.Pannaraj, P. S., K. G. Hulten, B. E. Gonzalez, E. O. Mason, Jr., and S. L. Kaplan. 2006. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 43953-960. [DOI] [PubMed] [Google Scholar]
- 9.Tenover, F. C., L. K. McDougal, R. V. Goering, G. Killgore, S. J. Projan, J. B. Patel, and P. M. Dunman. 2006. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J. Clin. Microbiol. 44108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong, K. S., T. Y. Lin, Y. C. Huang, S. H. Hsia, P. H. Yang, and S. M. Chu. 2002. Clinical and radiographic spectrum of septic pulmonary embolism. Arch. Dis. Child. 87312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuksel, H., O. Yilmaz, S. Orguc, H. S. Yercan, and D. Aydogan. 2007. A pediatric case of pyomyositis presenting with septic pulmonary emboli. Joint Bone Spine 74491-494. [DOI] [PubMed] [Google Scholar]