Abstract
CcpA globally regulates transcription in response to carbohydrate availability in many gram-positive bacteria, but its role in Streptococcus mutans remains enigmatic. Using the fructan hydrolase (fruA) gene of S. mutans as a model, we demonstrated that CcpA plays a direct role in carbon catabolite repression (CCR). Subsequently, the expression of 170 genes was shown to be differently expressed (≥2-fold) in glucose-grown wild-type (UA159) and CcpA-deficient (TW1) strains (P ≤ 0.001). However, there were differences in expression of only 96 genes between UA159 and TW1 when cells were cultivated with the poorly repressing substrate galactose. Interestingly, 90 genes were expressed differently in wild-type S. mutans when glucose- and galactose-grown cells were compared, but the expression of 515 genes was altered in the CcpA-deficient strain in a similar comparison. Overall, our results supported the hypothesis that CcpA has a major role in CCR and regulation of gene expression but revealed that in S. mutans there is a substantial CcpA-independent network that regulates gene expression in response to the carbohydrate source. Based on the genetic studies, biochemical and physiological experiments demonstrated that loss of CcpA impacts the ability of S. mutans to transport and grow on selected sugars. Also, the CcpA-deficient strain displayed an enhanced capacity to produce acid from intracellular stores of polysaccharides, could grow faster at pH 5.5, and could acidify the environment more rapidly and to a greater extent than the parental strain. Thus, CcpA directly modulates the pathogenic potential of S. mutans through global control of gene expression.
Carbon catabolite repression (CCR) in bacteria involves complex regulatory networks that activate or silence selected genes in response to carbon source and availability (10). In low-G+C-content gram-positive bacteria, CCR is controlled by HPr, a phosphoenolpyruvate:sugar phosphotransferase system (PTS) protein, and a transcriptional regulator, CcpA (23, 43). HPr is central to PTS-mediated sugar transport. Phosphorylation of HPr at histidine-15 is catalyzed by enzyme I of the PTS at the expense of phosphoenolpyruvate, and HPr-(His-P) can donate the phosphoryl group to enzyme II (EII) permeases of the PTS, which concomitantly phosphorylate and internalize a wide variety of carbohydrates (26, 34). When cells are grown under conditions that trigger CCR, an HPr kinase that is activated by elevated levels of particular glycolytic intermediates, usually fructose-1,6,-bisphosphate (F-1,6-bP) or glucose-6-phosphate, can phosphorylate HPr at serine-46 at the expense of ATP or PPi (19, 35). HPr-(Ser-46-P) forms a complex with CcpA, stimulating CcpA binding to conserved catabolite responsive elements (CRE) in the promoter regions of a variety of genes to repress or activate transcription (47). Thus, the amount of carbohydrate flowing through glycolysis is the primary trigger for CcpA binding in these bacteria. In addition to CCR (43), CcpA can regulate sporulation (24, 46), antibiotic resistance (36), and expression of virulence attributes in a number of different bacteria (9, 36). The CcpB and CcpC proteins of Bacillus subtilis and some other gram-positive bacteria (16, 25) also participate in control of CCR, albeit to a less significant degree.
Streptococcus mutans is the primary etiological agent of human dental caries (22). The ability of this organism to cause disease is dependent on adherence to the tooth surface, formation of biofilms, conversion of a wide variety of carbohydrates to organic acids through glycolysis, and the ability to tolerate low pH (11). One of the established virulence attributes of S. mutans is a fructan hydrolase encoded by the fruA gene (12, 13). S. mutans can convert sucrose extracellularly to homopolymers of glucose (glucans) or fructose (fructans); the former serve primarily as adhesive scaffolding for biofilm accumulation, and the latter serve as a source of storage polysaccharides that the organism can access when exogenous sources of carbohydrate are not present (11, 27). The FruA enzyme is a secreted β-fructosidase that releases fructose from fructan polymers, and the expression of fruA is inducible by growth in the presence of its substrates and is sensitive to CCR (13, 14). Induction requires activation of transcription by an unusual four-component signal transduction system, which consists of a classical two-component system (LevRS) and two predicted surface-associated sugar binding proteins (LevQT) that together sense fructose and activate fruA expression (50). Analysis of the cis elements in the promoter region of fruA revealed the presence of two highly conserved CRE sequences that may serve as binding sites for the apparent CcpA homologue of S. mutans (49). Deletion of the CRE sequences resulted in significant alleviation of CCR, but inactivation of the apparent ccpA gene of S. mutans did not relieve CCR of fruA in cells cultivated with combinations of glucose and the fructan homopolymer inulin (49). In other cases (21, 39), inactivation of ccpA, which has also been called regM, did not relieve repression of CCR-sensitive S. mutans genes, even when functional CRE sequences were identified in the promoter regions of the target genes (21). These studies indicated either that CcpA of S. mutans does not function in CCR or that there are redundancies in CCR systems in this organism (49). More recently, we presented data showing that diauxic growth of S. mutans on inulin and glucose was not evident when cells lacking the EIIAB component of a mannose-PTS system (ManL) were used (4), supporting the idea that there are redundancies in the systems for CCR in this organism. Importantly, a role for CcpA in regulation of established virulence determinants was revealed when our laboratory showed that the gtfB and ftf genes, which encode glucan and fructan synthetic enzymes, respectively, required CcpA for optimal expression (9). In this study, the knowledge gained by studying the fruA gene allowed us to develop a system that revealed a role for CcpA in CCR in S. mutans, and the use of microarrays revealed that the scope of the CcpA regulon in S. mutans is substantial and dependent upon the carbohydrate source. Subsequently, using biochemical and physiological assays, we showed that CcpA has a profound influence on the expression of traits that are critical for the establishment, persistence, and virulence of S. mutans.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. mutans UA159 is a cariogenic human isolate whose whole genome sequence is available (6). Strain TW1 was derived from UA159 and contains a kanamycin resistance cassette replacing nucleotides 56 to 687 of the ccpA gene (49). We previously described the engineering of S. mutans UA159 so that it carried a reporter gene fusion of the fruA promoter to a chloramphenicol acetyltransferase (CAT) gene (cat) (PfruA-cat) to create strain MMZ12 (49, 50). The gene fusion was cloned adjacent to a polar kanamycin resistance marker (ΩKm), and a single copy was integrated into the chromosome by replacing the mtlA-mtlD genes of the mannitol operon. A second ccpA deletion-insertion mutant was made compatible with the reporter gene integration vector by replacing the ccpA coding sequence (nucleotides 56 to 687) with a spectinomycin resistance marker (spc) in strain MMZ12 to create strain MMZ13. S. mutans strains were cultured in brain heart infusion (BHI) media at 37°C in a 5% CO2 atmosphere. When required, 1 mg ml−1 kanamycin was added to the growth medium. To grow cells for isolation of RNA and enzymatic assays and to assess the ability of cells to grow with different sugar sources, tryptone-vitamin base (TV) medium (14) supplemented with the desired carbohydrate was used.
PTS and reporter gene assays.
Permeabilized cells of S. mutans strains that were grown to an optical density at 600 nm (OD600) of 0.6 in TV medium supplemented with 0.5% (wt/vol) glucose, fructose, or mannose were assayed for sugar-specific PTS activity, as described elsewhere (28). S. mutans strains containing a PfruA-cat fusion were inoculated into TV medium supplemented with 0.5% galactose or glucose and grown to early exponential phase (OD600, 0.2). Then fructose was added to the medium at various concentrations (0, 0.05, 3, 5, and 10 mM) for 3.5 h to induce fruA expression (50). Cells were then harvested, and CAT activity was assayed by the method of Shaw (37), with modifications described elsewhere (17).
EMSA.
An electrophoretic mobility shift assay (EMSA) was carried out by using a previously published protocol (43). Briefly, a DNA fragment containing the promoter of fruA or the same fragment in which the catabolite response element was mutated (49) was amplified by PCR and radiolabeled with [γ-32P]ATP using T4 polynucleotide kinase. Approximately 1.2 fmol of radiolabeled probe was used in combination with different concentrations of purified recombinant His6-tagged S. mutans CcpA protein in a 10-μl reaction mixture containing 10 mM HEPES (pH 7.9), 50 mM KCl, 5 mM MgCl2, 1 mM EDTA, 5 mM dithiothreitol, 2 μg poly(dI-dC), and 10% glycerol. After incubation at room temperature for 20 min, the DNA-protein samples were resolved in a nondenaturing, low-ionic-strength polyacrylamide gel. Signals were developed from X-ray film after 5 h of exposure to the gel. The N-terminally tagged CcpA protein was obtained by amplifying the entire CcpA structural gene from S. mutans UA159 and cloning it in frame in pQE30 (Qiagen). The protein was overproduced in Escherichia coli by induction with isopropyl-β-d-thiogalactopyranoside (IPTG) and purified as a soluble protein on a Ni2+ affinity column using the protocol recommended by the supplier.
Acid tolerance assays.
The glycolytic profiles of UA159 and TW1 were monitored by performing pH drop experiments (8). Briefly, cells were grown in 50 ml of BHI broth to an OD600 of 0.5, washed twice with ice-cold water, and resuspended in 4.75 ml of 50 mM KCl, 5 mM MgCl2, and the pH was titrated to 7.2 by addition of 0.1 M KOH until it was stable. Then 0.25 ml of 1 M glucose was added, and the pH was recorded for 30 min. To assess the capacity of each strain to lower the pH in the absence of exogenous carbohydrates, cells were grown as described above, washed twice with ice-cold water, and resuspended in 5 ml of 50 mM KCl, 5 mM MgCl2, and the pH was recorded for 30 min. The ability of strains to grow at low pH was assessed by diluting (1:20) an overnight culture grown in BHI medium into fresh BHI medium or BHI medium that had been acidified to pH 5.5 by addition of HCl. Growth was monitored with a Bioscreen C (Oy Growth Curves) at 37°C, as described elsewhere (4). The ability of S. mutans to survive exposure to pH 2.8 was assessed as described elsewhere (3). Briefly, cells were grown in 50 ml of BHI broth at pH 7.0 or pH 5.0 (acid-adapted cells) to an OD600 of 0.5, washed twice with 0.1 M glycine buffer (pH 7.0), and resuspended in 8 ml of 0.1 M glycine buffer (pH 2.8). Viability was monitored by plating serial dilutions onto BHI agar after 30 and 60 min.
Enzymatic assays.
Cells of S. mutans UA159 and TW1 that were grown in TV broth containing 0.5% glucose or galactose were collected in the mid-exponential phase of growth (OD600, 0.5) and permeabilized with toluene. For F-ATPase assays, the cells were incubated for 30 min with 5 mM ATP in ATPase buffer, as previously described (8). The release of inorganic phosphate from ATP was monitored using a QuantiChrom phosphate assay kit (Bioassay System, Hayward, CA). Pyruvate dehydrogenase (PDH) assays were performed by using the protocol described by Fine and Costello (20).
RNA isolation.
RNA was isolated from S. mutans (1) by using 50-ml cultures that were grown under the desired conditions and harvested by centrifugation at 4°C. Pelleted cells were resuspended in 400 μl of diethyl pyrocarbonate-treated water, 800 μl of RNAprotect reagent (Qiagen, Inc., Chatsworth, CA) was added, and the samples were incubated at room temperature for 5 min with vortexing for 10 s at 1-min intervals. Cells were then pelleted, resuspended in 250 μl Tris-EDTA (50:10) buffer, and transferred to 1.5-ml screw-cap tubes containing sterile glass beads (average diameter, 0.1 mm; Biospec, Bartlesville, OK), 10 μl of 1% sodium dodecyl sulfate, and 300 μl of acid phenol-chloroform (5:1). After centrifugation for 15 min at maximum speed at 4°C, RNA was further purified using an RNeasy mini kit (Qiagen, Inc., Chatsworth, CA), including on-column DNase digestion with RNase-free DNase (Qiagen), as recommended by the supplier.
Microarray experiments.
S. mutans UA159 microarrays were provided by The Institute for Genomic Research (TIGR). The microarrays consisted of 1,948 70-mer oligonucleotides representing 1,960 open reading frames printed four times on the surface of each microarray slide (http://pfgrc.tigr.org/mutansinfo.shtml). A reference RNA that had been isolated from 2 liters of UA159 cells grown in BHI broth to an OD600 of 0.5 was used in every experiment (1). The advantages of using a reference RNA in microarray studies are described elsewhere (30, 38). The experimental conditions consisted of S. mutans strains UA159 and TW1 grown in TV broth containing 0.5% glucose or galactose and collected at the mid-exponential phase of growth (OD600, 0.5). All RNAs were purified as described above and used to generate cDNA by the protocol provided by TIGR (http://pfgrc.tigr.org/protocols.shtml), with the following minor modifications. The amount of RNA in each reaction mixture was increased to 10 μg, and the molar ratio of dTTP to aminoacyl-dUTP was increased to 1:2. SuperScript III reverse transcriptase (Invitrogen, Gaithersburg, MD) was used to increase cDNA yields. Purified cDNAs from experimental groups were coupled with indocarbocyanine (Cy3)-dUTP, while reference cDNA was coupled with indodicarbocyanine (Cy5)-dUTP (Amersham Biosciences, Piscataway, NJ). Four individual Cy3-labeled cDNA samples originating from four different cultures of UA159 or TW1 grown in each experimental condition were hybridized to the arrays along with Cy5-labeled reference cDNA, generating a total of 16 slides. Hybridization was carried out with a Maui four-chamber hybridization system (BioMicro Systems, Salt Lake City, UT) for 16 h at 42°C. The slides were then washed by using TIGR protocols and scanned using a GenePix scanner (Axon Instruments Inc., Union City, CA).
S. mutans microarray data analysis.
After the slides were scanned, single-channel images were loaded into TIGR Spotfinder software (http://www.tigr.org/software/) and overlaid. A spot grid was created according to TIGR specifications and manually adjusted to fit all spots within the grid, and then the intensity values of each spot were determined. Data were normalized using Microarray Data Analysis Software (MIDAS) (http://www.tigr.org/software/) by using LOWESS and iterative log mean centering with default settings, followed by in-slide replicate analysis. Statistical analysis was carried out using BRB Array Tools (http://linus.nci.nih.gov/BRB-ArrayTools.html) with a cutoff P value of 0.001 for class prediction and class comparison. Microarray data have been deposited in the NCBI (accession no. GSE8850).
Real-time quantitative reverse transcription-PCR.
One microgram of RNA from UA159 and TW1 cells grown under each experimental condition was used to generate cDNA, and real-time quantitative reverse transcription-PCR was used to validate microarray experiments with controls and internal standards as described elsewhere (1).
RESULTS AND DISCUSSION
CcpA affects CCR of fruA.
In previous studies on the regulation of fructan hydrolase (fruA) gene expression (49), deletion of CRE sequences in the fruA promoter region led to nearly complete alleviation of CCR. In contrast, when the ccpA mutant strain was grown in the presence of glucose and the inducing substrate inulin, which is a predominantly β-2,1-linked homopolymer of fructose, no alleviation of CCR was evident (49). Similar results were obtained with other CCR-sensitive genes when strains of S. mutans lacking CcpA (RegM) were examined (21, 39). It was proposed previously that either CcpA does not play a major role in CCR or S. mutans possesses redundant systems for CCR (21, 39), likely by mechanisms involving the PTS (18, 49). Recently, we described a novel four-component signal transduction pathway that senses fructose and governs the activation of fruA (50). With the disclosure of the mechanisms of induction of fruA, it was possible to design experiments to more definitively assess the contribution of CcpA to CCR of the fruA operon.
Promoter fusions were used to measure the expression levels of fruA in S. mutans strains that were grown to early exponential phase (OD600, 0.2) in TV medium supplemented with 0.5% glucose or galactose (galactose is far less effective at eliciting CCR than glucose [50]) and then exposed to various concentrations of fructose for 3.5 h to induce expression of fruA (50). It should be noted that it is not possible to grow S. mutans in the absence of carbohydrates, because this organism lacks a complete respiratory chain and cannot carry out oxidative phosphorylation. As shown in Table 1, when a relatively high concentration of glucose (0.5%) was used to grow S. mutans MMZ12, which contains a single copy of a cat fusion to the fruA promoter integrated in the wild-type genetic background, expression was repressed regardless of the concentration of fructose used to induce fruA. In contrast, when MMZ12 was grown on the poorly repressing substrate galactose, low levels of fructose efficiently induced expression of fruA. Addition of higher concentrations of fructose resulted in onset of CCR, as shown by the decrease in CAT activity at a fructose concentration of 5 mM or higher. In contrast, the overall expression of the reporter gene activity in S. mutans MMZ13 (ccpA PfruA-cat) was substantially greater than that in the wild-type background in either TV-glucose or TV-galactose medium. Specifically, the CAT activity in cells grown in TV-glucose medium was about 60-fold higher in the strain carrying the deletion of ccpA than in the strain with the wild-type background. Also, expression of fruA in the ccpA mutant background was less sensitive to CCR triggered by fructose concentrations higher than 5 mM. Clearly, deletion of ccpA resulted in substantial relief of the CCR of fruA expression exerted by both glucose and fructose.
TABLE 1.
Expression of PfruA-cat fusion in fructose pulse experimenta
| Preculture | Background | PfruA::cat CAT sp act (nmol min−1 mg protein−1) after pulsing for 3.5 h with fructose at a concn of:
|
||||
|---|---|---|---|---|---|---|
| 0 mM | 0.05 mM | 3 mM | 5 mM | 10 mM | ||
| TV-0.5% glucose | UA159 | 0 (0) | 0 (0) | 0.13 (0.09) | 0.05 (0.05) | 0.11 (0.12) |
| ccpA | 0 (0) | 0 (0) | 6.53 (0.91) | 6.50 (0.87) | 6.61 (0.35) | |
| TV-0.5% galactose | UA159 | 0 (0) | 2.49 (0.03) | 2.48 (0.40) | 1.15 (0.15) | 1.08 (0.03) |
| ccpA | 0 (0) | 3.96 (0.54) | 9.95 (0.44) | 14.60 (1.67) | 13.23 (2.28) | |
| TV-0.5% fructose | UA159 | 0.44 (0.06) | ND | ND | ND | ND |
| ccpA | 7.16 (0.90) | ND | ND | ND | ND | |
Bacteria were cultured to early exponential phase (OD600, 0.2) in TV medium supplemented with 0.5% glucose or galactose, and fructose was added at the indicated concentrations. After incubation for an additional 3.5 h at 37°C, cells were harvested and subjected to CAT assays. For precultures with fructose, no fructose was added before the 3.5-h incubation. The values are averages and standard deviations (in parentheses) of at least three independent experiments. ND, not determined.
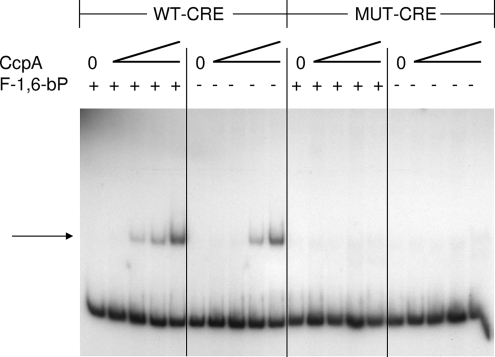
To demonstrate that CcpA could directly interact with the fruA promoter region, EMSAs were performed with a purified, histidine-tagged S. mutans CcpA protein. The protein was obtained by PCR amplifying the S. mutans UA159 ccpA structural gene, cloning it in frame in pQE30, and overexpressing and affinity purifying the soluble protein from E. coli. The EMSA was performed as described in Materials and Methods. As shown in Fig. 1, CcpA was able to induce a shift in mobility of the fruA promoter region when the CRE sequence was intact. However, when a fragment carrying a mutated CRE sequence (49) was utilized, there was a dramatic reduction in the ability of CcpA to induce a shift. F-1,6-bP has been shown to be an allosteric effector of CcpA binding (43), and we observed that F-1,6-bP enhanced the binding of CcpA to the fruA promoter region carrying the intact CRE. A purified His6-ArcR protein from Streptococcus gordonii, which specifically binds in vitro to the arginine deiminase promoter region (51), was unable to induce a shift in the migration of the fruA promoter fragment (data not shown). Collectively, these results support the hypothesis that CCR of fruA is mediated directly by the binding of CcpA to the fruA CRE.
FIG. 1.
EMSAs. DNA fragments of the fruA promoter carrying wild-type (WT-CRE) or mutated CRE (MUT-CRE) sequences (42) were end labeled, and the abilities of different amounts of purified His6-CcpA (the lanes contained 0, 27, 54, 109, and 218 pmol of protein) to induce a mobility shift in the presence (+) or absence (−) of 2 mM F-1,6-bP (Sigma) were examined. The arrow indicates the migration position of the shifted product.
The inability to induce fruA expression in the presence of high concentrations of glucose and the down-regulation of fruA in the presence of elevated levels of fructose confirmed the sensitivity of the operon to CCR. Consistent with the idea that glycolytic intermediates, usually F-1,6-bP, trigger CCR through activation of HPr kinase rather than through the presence of specific sugars (19, 32, 35, 43), the observation that fructose was able to trigger CCR through CcpA was expected, and this likely functions as a feedback mechanism to down-regulate the amount of FruA produced when sufficient quantities of the enzyme accumulate to liberate steady-state levels of fructose greater than 3 mM. Notably, as shown by a comparison of CAT activities in galactose- and glucose-grown cells, some sensitivity to CCR was retained in the absence of CcpA, which indicated that there are additional pathways for down-regulation of fruA expression when elevated levels of readily metabolizable carbohydrates are present. In fact, the following four additional gene products have been found to participate in negative regulation of fruA transcription initiation, albeit not nearly to extent shown here for CcpA: EIIABman (ManL) of the PTS (4), the LevDEFG mannose/fructose permease (50), and two fructose PTS permeases, FruI and FruCD (48). We propose that these PTS enzymes exert their influence on fruA expression similar to other PTS-regulated pathways (18, 42, 43), such that the activity of either LevR, LevQST, or some as-yet-unidentified regulatory protein is governed by phosphorylation or allosteric interactions with the PTS permeases.
Finally, it is clear that growth in the presence of the relatively high concentration of galactose (0.5%) used in this study resulted in some CcpA-dependent repression of gene expression (Table 1). We examined multiple nonpreferred carbohydrates in an attempt to obtain conditions that alleviate CCR, but galactose gave the best combination of reasonably good growth and enhanced expression of CCR-sensitive operons. The fact that galactose can apparently trigger CcpA-dependent repression is not surprising, since we have shown that when galactose is provided to cells instead of lactose, catabolism occurs through the Leloir pathway (2), which converts the galactose to glucose and funnels the carbohydrate through the Embden-Meyerhoff-Parnas pathway, thus creating allosteric activators of HPr kinase.
Microarray analysis of the role of CcpA in gene expression during growth on glucose.
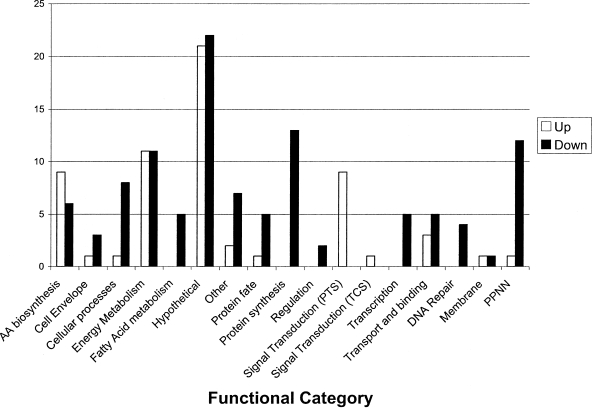
To begin to define the scope of the CcpA regulon, the transcriptomes of S. mutans UA159 and TW1 grown under catabolite-repressing conditions (glucose) and catabolite-derepressing conditions (galactose) were compared. When glucose was the sole carbohydrate for growth, 170 genes were differentially expressed at least twofold as a result of loss of CcpA (Fig. 2; see Table S1 in the supplemental material). Of the gene products, 22 were predicted to participate in energy metabolism, 9 were PTS components, and 43 were hypothetical proteins (Fig. 2). Among the most profoundly up-regulated genes in the ccpA mutant strain were the genes that encoded the components of the PDH enzyme complex (Smu.1421 to Smu.1424), pyruvate-formate lyase, enzymes in the tricarboxylic acid (TCA) cycle, selected sugar-specific PTS permeases, and components of the glycogen biosynthetic machinery (see Table S1 in the supplemental material). Consistent with the profile data, TW1 cells grown in glucose had 78% higher PDH activity than the wild-type strain (data not shown). Given the impact on expression of PDH, TCA enzymes, and selected glycolytic enzymes, CcpA clearly legislates gene expression to discriminate between carbohydrate-replete and carbohydrate-limiting conditions. In particular, when there is excess carbohydrate, S. mutans moves carbohydrates primarily through lactate dehydrogenase, sacrificing an ATP and generating NAD+. When carbohydrate is limiting, S. mutans produces mainly formic and acetic acids, shunts carbohydrate through pyruvate formate lyase, and uses its partial TCA cycle to regenerate reducing equivalents to maintain an appropriate NAD/NADH balance. This regulatory control was clearly short-circuited in the strain growing on glucose and lacking CcpA.
FIG. 2.
Numbers of genes in functional categories differentially expressed in strain TW1 compared to UA159 when cells are cultivated in TV medium containing 0.5% glucose. AA, amino acid; TCS, two-component systems; PPNN, purines, pyrimidines, nucleosides, nucleotides.
Among the more interesting genes that were down-regulated, significant decreases in expression were noted for the genes encoding components of the proton-translocating F-ATPase, phosphoglycerate kinase (Pgk), and fructosyltransferase (ftf), which catalyzes the conversion of sucrose to high-molecular-weight polymers of fructose, as well as gtfD, which encodes the glucosyltransferase D enzyme that produces predominantly α-1,6-linked polymers of glucose from sucrose. Since acid tolerance (ATPase), acid production from carbohydrate (Pgk), and glucan and fructan production (Gtf and Ftf), as well as the ability to transport carbohydrates and produce intracellular storage compounds (glycogen), as noted above (PTS and Glg), are central to the pathogenic potential of S. mutans, CcpA represents a major control point for expression of critical virulence attributes in cells presented with glucose.
Growth in derepressing conditions induces major changes in the UA159 transcriptome.
To contrast the effects of growth under conditions that weakly and strongly trigger CCR, the transcriptomes of UA159 grown in TV-galactose and TV-glucose media, respectively, were examined. When the transcriptome of UA159 grown in TV-galactose medium was compared with that of the same strain grown in TV-glucose medium, 90 genes belonging to 12 functional categories (see Fig. S1 in the supplemental material) were differentially expressed (P < 0.001). It is noteworthy that only three genes in the energy metabolism category were down-regulated, whereas 23 genes in this category were markedly up-regulated. In addition, the expression of 28 genes encoding hypothetical proteins was affected, and 19 of them were up-regulated. The genes encoding proteins belonging to the pathways for lactose and galactose utilization (2) were the most strongly induced genes in cells growing on galactose (see Table S2 in the supplemental material). The galKTE genes, encoding the enzymes for galactose utilization via the Leloir pathway (2), were up-regulated between 10- and 36-fold, but the genes of the lactose (lac) operon, encoding the lactose PTS permease and the tagatose-6-phosphate pathway, were up-regulated 70- to 300-fold. Since the products of the galKTE operon contribute to the generation of UDP-galactose, which is required for cell wall biogenesis, the lower level of induction of galKTE than of the lac operon may reflect a need for higher constitutive levels of expression of the Leloir pathway in the absence of galactose. Components of the multiple-sugar metabolism operon (msm) were also induced in cells growing on galactose. The Msm pathway is induced by raffinose and melibiose, both of which are α-galactosides (5).
The enhanced expression of gene products involved in energy metabolism, including pyruvate formate lyase and components of the TCA cycle, in cells growing on galactose is consistent with the idea that the cells perceive carbohydrate limitation. S. mutans UA159 grows more slowly on 0.5% galactose (generation time, 193.2 ± 13.5 min) than on 0.5% glucose (generation time, 126 ± 10.5 min), and TW1 grows only slightly more slowly on galactose (generation time, 222 ± 19.3 min) than the wild-type strain. As noted above, when S. mutans is grown with sufficient amounts of a rapidly metabolized carbohydrate, the levels of glycolytic intermediates are high and carbohydrate flows principally to lactate. In limiting carbohydrate conditions, acetate and formate are dominant and the cells engage the partial TCA cycle to regenerate NADH for biosynthetic reactions. Based on comparison of the transcriptomes of the wild-type strain growing on glucose and on galactose, CcpA appears to be a central control point for genetic regulation of the switch between feast metabolism and famine metabolism (15). The switch is likely triggered by the level of glycolytic intermediates, which would be predicted to be lower in galactose-grown cells because of the slower transport and metabolism of this carbohydrate (2). Consistent with this idea is the fact that many of the genes induced by growth in galactose in the parental strain that are not directly involved in galactose catabolism are also induced in cells growing on glucose in a CcpA-deficient strain (see Table S1 in the supplemental material). Catabolite modification of the transcriptome of Lactobacillus acidophilus has also been reported, and a role for CcpA was inferred from the gene expression data (7). It should also be noted that we found no evidence of induction of a stringent response in cells growing on galactose when the transcriptomes were compared with those of S. mutans treated with mupirocin, which induces a vigorous stringent response by S. mutans (data not shown), so the slower-growth phenotype on galactose probably contributes little to the changes in gene expression, a conclusion which is reinforced by data described below.
Other noteworthy results include the finding that the ftf gene encoding the fructosyltransferase enzyme involved in production of extracellular storage polymers of fructose was up-regulated nearly threefold and the fruR gene, which controls expression of a fructose PTS permease and possibly other genes, was up-regulated around twofold. On the other hand, the expression of luxS, which we and other workers have shown to have global effects on gene expression in S. mutans, was down-regulated about twofold. Finally, a putative ammonia transporter, which was up-regulated in the TW1 strain grown in glucose, was down-regulated roughly 10-fold in cells grown on galactose, indicating possible coordination of C and N assimilation. A link between CcpA and regulation of nitrogen assimilation has been established in other organisms (31, 52).
CcpA is also critical for regulation of gene expression under derepressing conditions.
A somewhat surprising finding was obtained when the transcriptomes of the CcpA-deficient strain growing in galactose and in glucose were compared. Under these conditions, 515 genes in 22 distinct functional categories were differentially expressed at least twofold (P ≤ 0.001). Of these 515 genes, 59 were involved in energy metabolism, but 162 encoded hypothetical proteins and 50 were involved in transport and binding (see Fig. S2 in the supplemental material). As noted above for the wild-type strain growing on galactose, the most substantially induced genes were those of the lac and gal operons (see Table S3 in the supplemental material). It is also noteworthy that the msm operon, whose products catabolize certain α-galactosides and a variety of surface-localized proteins (WapA, glyceraldehyde-3-phosphate dehydrogenase, and GbpC), as well as a variety of glycolytic enzymes and a variety of carbohydrate transporters and catabolic pathways, were also substantially induced in cells growing on galactose. Also, genes encoding subunits of the F-ATPase, a number of key stress genes (dnaK and clpX), and DNA repair enzymes were also expressed at higher levels in the presence of galactose in the strain lacking CcpA. Of the many down-regulated genes, a number of genes encoding PTS permeases, including levDEFG and scrB, which encode fructose/mannose and sucrose transporters, respectively, and a number of genes encoding ion transporters were significantly affected, as were selected components of the competence pathway (see Table S3 in the supplemental material). It is also interesting that the expression of glucose and fructose transporters was down-regulated in galactose-grown cells independent of the presence of CcpA. This finding implies that these systems require induction by glucose, which would be consistent with previous findings for glucose, mannose, and fructose porters (1, 48).
Considering the scope and magnitude of the changes in gene expression induced by the combination of loss of CcpA and growth in galactose, it is reasonable to postulate that the roles of the CcpA regulon may be most significant in cellular growth and homeostasis during low carbohydrate availability, which may be mimicked by the presence of nonpreferred carbohydrates. One possible explanation for the widespread changes in gene expression induced by the combination of galactose and lack of CcpA is that the consequences of inappropriate derepression of genes are magnified when cells are required to grow on a poorly metabolizable carbohydrate like galactose compared to growth on glucose. Consistent with this idea is the finding that only 90 genes are different in wild-type cells growing on galactose compared with cells growing on glucose (see Table S1 in the supplemental material). Thus, the loss of CcpA in galactose-grown cells may cause derepression or lack of activation by CcpA of genes controlling core homeostatic functions, which in turn may trigger expression of global stress response pathways. Overlap of CcpA and stress regulons of B. subtilis was noted in a previous global transcriptional analysis (31). It seems unlikely, however, that the differences in gene expression are related to growth rate in any significant way. First, as noted above, there is not much difference in the growth rates of UA159 and TW1 in TV medium with 0.5% galactose. More importantly, we have shown that there are essentially no differences in gene expression patterns between a relA mutant of S. mutans, which grows more slowly than the parental strain, and the wild-type strain when cells are growing exponentially (33a).
Effect of CcpA on gene regulation is more pronounced under conditions that favor CCR.
We next compared the transcriptomes of UA159 and TW1 grown in TV-galactose medium. When a cutoff P value of 0.001 was used, only 96 genes showed altered expression, compared with the 170 genes whose expression was different in these two strains when glucose was the growth carbohydrate. As observed for the transcriptome comparisons described above, the majority of gene products affected by the mutation fell in the hypothetical category (see Fig. S3 and Table S4 in the supplemental material). However, the genes that showed the greatest degree of up-regulation during the comparison of cells grown in galactose included genes encoding a putative Na+-dependent transporter (Smu.602), a predicted Na+-alanine symporter, and a predicted ammonium transporter. Components of PDH and lactate dehydrogenase were also up-regulated, but selective TCA cycle enzymes, including citrate lyase were down-regulated, as were some glycogen metabolism genes. It is also noteworthy that the expression of ftf and the expression of an intracellular amylase gene were decreased. The latter gene is immediately downstream of ccpA (39) but is also apparently transcribed from its own promoter. The fact that the expression of this amylase gene was affected in the mutant only in the presence of galactose provides strong evidence that polar effects on amylase expression are not relevant to the outcomes determined here.
The fact that there were fewer differences in gene expression between the wild-type and mutant strains when cells were grown on galactose could be interpreted as suggesting that there is some specificity in the response of the CcpA pathway to glucose. However, a number of observations argue against this idea. The first is that, as shown here, growth on fructose is able to induce CCR through the CcpA pathway (Table 1), so CcpA-dependent CCR is not specific to glucose. Second, we have demonstrated that galactose alone is metabolized by S. mutans exclusively through the GalKTE pathway. In contrast, the galactose liberated from internalized lactose-6-phosphate is utilized through the tagatose pathway and potentially through the GalKTE pathway at a much lower level. Thus, when S. mutans is grown on galactose, the sugar does not bypass glycolysis, as it does in the tagatose pathway, and F-1,6-bP should be produced, which is the usual trigger for activation of HPr kinase. The reason that we believe galactose is a poor substrate for triggering CCR, however, is that it is metabolized more slowly than glucose and fructose (2), so the steady-state levels of glycolytic intermediates remain below the threshold needed for efficient activation of HPr kinase. Taking into consideration all of the data accumulated for CcpA of S. mutans presented here and elsewhere and since S. mutans has an HPr kinase and inactivation of the hprK gene results in very poor growth of the organisms (R. A. Burne, unpublished data), it seems that the primary trigger for CcpA is sensing of the levels of glycolytic intermediates, as has been well documented in other organisms (19, 43), and that there is not a particular effect of glucose that is exerted through the CcpA pathway.
Growth and carbohydrate utilization by the CcpA-deficient strain.
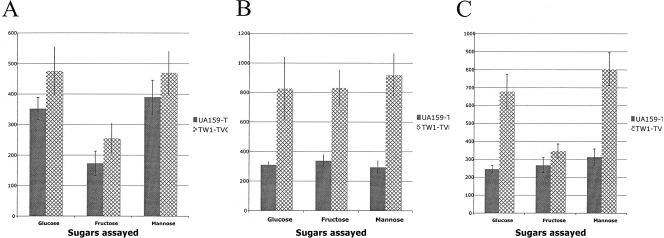
In light of our functional studies showing that CcpA governs genes involved in carbohydrate utilization (fruA, gtfBC, and ftf) (9, 49) and in light of the profound effects of CcpA on genes involved in carbohydrate transport and metabolism, as revealed here by microarrays, we explored in more detail some of the relevant physiological properties of the CcpA-deficient strain. First, the doubling times and sugar-specific PTS activities of UA159 and TW1 grown in TV medium supplemented with 0.2% glucose, fructose, or mannose as the sole carbohydrate source were determined. Compared to the wild-type strain, TW1 grew significantly more slowly (P ≤ 0.05) on all sugars (Table 2). However, TW1 had significantly higher PTS activity (P ≤ 0.05) for all the sugars assayed when it was grown in TV broth supplemented with 0.5% glucose, fructose, or mannose (Fig. 3), and the largest differences were observed in cells grown in the presence of fructose or mannose. The slow-growth phenotype of the ccpA mutant strain is likely attributable to the derepression of a variety of catabolic pathways that are normally repressed during growth in the presence of the readily metabolizable carbohydrates tested. Glucose, mannose, and fructose are all likely to induce CcpA-dependent changes in gene regulation to similar extents if the main pathway for stimulation of binding of CcpA is the HPr kinase pathway, so the slow-growth phenotype on all of these sugars appears to be consistent with our data. Another potential explanation for the slower-growth phenotype is that the cells shunt larger amounts of carbohydrate into storage compounds, as suggested by changes in expression for the genes in the glycogen biosynthetic pathway (Table 2). Alternatively, the inactivation of ccpA in cells growing on glucose should have the net effect of shunting carbohydrate away from lactate production and into formate and acetate production and to biosynthetic pathways. Such a rerouting of carbohydrate could slow cell growth by driving unnecessary anabolic reactions or, more likely, by perturbing the NAD/NADH balance in the cells in an unfavorable way. The increases in PTS transport that we observed may in part be explained by the increases in the expression of the levDEFG genes, which encode a fructose/mannose PTS system that likely can also transport glucose (50). However, effects on the overall PTS activity could also be due to allosteric regulation or posttranscriptional control of the synthesis or activity of porters.
TABLE 2.
Doubling times of S. mutans strains growing exponentially in TV medium supplemented with glucose, fructose, or mannose as the sole carbohydrate
| S. mutans strain | Doubling time (min) with:
|
||
|---|---|---|---|
| Glucose | Fructose | Mannose | |
| UA159 | 102 ± 11 | 114 ± 6 | 209 ± 17 |
| TW1 | 148 ± 17 | 189 ± 22 | 310 ± 12 |
FIG. 3.
Sugar transport by the PTS in S. mutans UA159 and the CcpA-deficient derivative TW1. Cells were cultivated in TV medium with glucose (A), fructose (B), or mannose (C) and harvested in the mid-exponential phase of growth, and PTS-dependent sugar transport was assayed as described in Materials and Methods. Data are expressed as means of at least three separate cultures that were assayed in triplicate. The error bars indicate standard deviations. Statistical significance (see text) was determined by the Student t test.
Previously, we demonstrated that loss of CcpA did not affect diauxic growth of S. mutans on combinations of glucose and inulin (49). To determine whether loss of ccpA could impact diauxic growth on sugars that have catabolic pathways under the control of CCR, UA159 and TW1 were grown in TV broth supplemented with 0.05% glucose plus 0.5% inulin or cellobiose. Loss of CcpA did not eliminate diauxic growth of S. mutans on these carbohydrates (data not shown). This finding supports the hypothesis that redundant systems for CCR do occur in S. mutans and that these systems are not restricted to CCR of fruA.
CcpA deficiency enhances glycolysis and intracellular stores of carbohydrate.
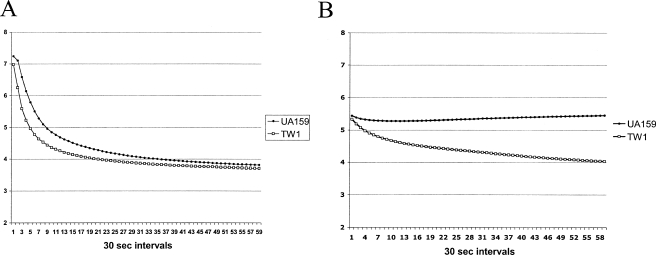
To determine whether CcpA could influence the glycolytic rates of S. mutans, pH drop experiments were performed as described in Materials and Methods. TW1 was capable of lowering the pH faster and of achieving a slightly lower final pH than UA159 (Fig. 4A). Before the pH drop is initiated by addition of glucose, the protocol calls for titration of the cells with KOH to achieve a relatively stable pH around pH 7.2 (8). Interestingly, TW1 consistently required a larger volume of KOH to achieve a stable, neutral pH. When S. mutans is grown in the presence of excess carbohydrate, the cells can produce significant quantities of a glycogenlike intracellular polysaccharide (IPS) (11, 22), and some of the genes for glycogen production were up-regulated in TW1 (Table 2). We therefore reasoned that the need for additional KOH to stabilize the pH of the mutant was due to overproduction of IPS by TW1. To evaluate this idea, we performed pH drop experiments without titration with KOH or addition of glucose to determine how well each strain could produce acid from endogenous stores of carbohydrate. TW1 was able to lower the pH faster and to reach a terminal pH that was more than 1 pH unit lower than that of the wild-type strain (Fig. 4B). Since S. mutans makes only exopolysaccharides from sucrose and does not produce a true capsule, this finding provides evidence that TW1 accumulated substantially greater internal reserves of carbohydrate than the parental strain accumulated, consistent with the gene expression profile. Of particular interest was the finding that accumulation of IPS enhanced survival not only during nutrient starvation but also at low pH, environmental conditions that directly disturb the functionality of the major system for internalizing sugar into the streptococcal cell, the PTS (14, 40, 41, 44, 45). Given the importance of IPS to the persistence and virulence of S. mutans (40), CcpA was once again found to be a central regulatory protein for control of virulence traits. As noted above, the slow-growth phenotype of the ccpA mutant strain could in fact be partially attributable to the fact that the organism shunts larger amounts of glucose into storage compounds and away from ATP generation for growth. Finally, regulation of glycogen metabolism by both CcpA and ManL, as shown previously (1), potentially allows a hierarchy of control that allows the organisms to synthesize or degrade glycogen in response to glycolytic flux via CcpA and external pools of carbohydrate via ManL, respectively.
FIG. 4.
Glycolytic acidification by S. mutans UA159 and TW1 in the presence of added glucose (A) or using endogenous stores (B). Experiments were performed as described in Materials and Methods, and the data are representative of no fewer than three separate replicates that all produced the same results.
Loss of CcpA enhances acid tolerance.
Because TW1 had enhanced glycolytic profiles and decreased the pH more than the parental strain, traits which are closely associated with enhanced acid tolerance, we investigated whether loss of CcpA affects the low-pH tolerance of S. mutans by comparing the growth rates of the strains in acidified medium and by comparing their sensitivities to acid killing. Although there were no statistically significant differences in the death of acid-adapted cells at pH 2.8 (data not shown), the doubling times of UA159 and TW1 differed in BHI broth acidified with HCl to pH 5.5 were 284.3 ± 24 and 231.25 ± 17.5 min, respectively, indicating that the CcpA-deficient strain is substantially more acid resistant. Enhanced acid resistance of oral streptococci is typically characterized by elevated expression of the proton-translocating ATPase and by an ability to decrease the pH through glycolysis more rapidly and to a greater extent (29). Since no significant increases in ATPase gene expression were noted and the ATPase activity of TW1 was shown to be lower than that of the parental strain (the TW1 activity was 62% that of the wild-type strain) (data not shown), the enhanced acid resistance phenotype of TW1 likely is a result of increases in the expression of PTS permeases. In fact, Nascimento et al. (33) have shown that acid adaptation of Streptococcus sobrinus is not accompanied by increased ATPase activity or atp gene expression but is characterized by increases in PTS-dependent glucose transport and up-regulation of the glucose PTS permease encoded by ptsG.
Conclusion.
Carbohydrate availability is probably the single most important factor affecting the composition and pathogenic potential of supragingival oral biofilms. The ability of oral bacteria, particularly cariogenic species, such as S. mutans, to respond rapidly and effectively to changes in carbohydrate source and availability is believed to be central to the competitive fitness of the organisms and their ability to cause disease (11). Previously, the role of CcpA in S. mutans was underappreciated, but this study demonstrates the profoundly important role played by CcpA in gene regulation of key virulence attributes of this organism. Dissecting the relationships of CcpA with other regulatory factors, including the PTS, the stress regulon, and quorum-sensing systems, should help provide a comprehensive view of the strategies used by S. mutans to become established, persist, and cause disease.
Supplementary Material
Acknowledgments
This study was supported by NIDCR grant DE12236 to R.A.B.
We thank Henry Baker for assistance with establishing the microarray technology and protocols for data analysis and Vanessa Lin for technical assistance.
Footnotes
Published ahead of print on 25 January 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Abranches, J., M. M. Candella, Z. T. Wen, H. V. Baker, and R. A. Burne. 2006. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J. Bacteriol. 1883748-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abranches, J., Y. Y. Chen, and R. A. Burne. 2004. Galactose metabolism by Streptococcus mutans. Appl. Environ. Microbiol. 706047-6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abranches, J., J. A. Lemos, and R. A. Burne. 2006. Osmotic stress responses of Streptococcus mutans UA159. FEMS Microbiol. Lett. 255240-246. [DOI] [PubMed] [Google Scholar]
- 4.Abranches, J. A., Y. M. Chen, and R. A. Burne. 2003. Characterization of Streptococcus mutans strains deficient in EIIABMan of the sugar phosphotransferase system. Appl. Environ. Microbiol. 694760-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aduse-Opoku, J., L. Tao, J. J. Ferretti, and R. R. Russell. 1991. Biochemical and genetic analysis of Streptococcus mutans alpha-galactosidase. J. Gen. Microbiol. 137757-764. [DOI] [PubMed] [Google Scholar]
- 6.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 9914434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrangou, R., M. A. Azcarate-Peril, T. Duong, S. B. Conners, R. M. Kelly, and T. R. Klaenhammer. 2006. Global analysis of carbohydrate utilization by Lactobacillus acidophilus using cDNA microarrays. Proc. Natl. Acad. Sci. USA 1033816-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belli, W. A., and R. E. Marquis. 1991. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl. Environ. Microbiol. 571134-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browngardt, C. M., Z. T. Wen, and R. A. Burne. 2004. RegM is required for optimal fructosyltransferase and glucosyltransferase gene expression in Streptococcus mutans. FEMS Microbiol. Lett. 24075-79. [DOI] [PubMed] [Google Scholar]
- 10.Bruckner, R., and F. Titgemeyer. 2002. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol. Lett. 209141-148. [DOI] [PubMed] [Google Scholar]
- 11.Burne, R. A. 1998. Oral streptococci: products of their environment. J. Dent. Res. 77445-452. [DOI] [PubMed] [Google Scholar]
- 12.Burne, R. A., Y. M. Chen, D. W. Wexler, H. Kuramitsu, and W. H. Bowen. 1996. Cariogenicity of Streptococcus mutans strains with defects in fructan metabolism assessed in a program-fed specific pathogen free rat model. J. Dent. Res. 751572-1577. [DOI] [PubMed] [Google Scholar]
- 13.Burne, R. A., K. Schilling, W. H. Bowen, and R. E. R. E. Yasbin. 1987. Expression, purification, and characterization of an exo-β-d-fructosidase of Streptococcus mutans. J. Bacteriol. 1694507-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burne, R. A., Z. T. Wen, Y. Y. Chen, and J. E. Penders. 1999. Regulation of expression of the fructan hydrolase gene of Streptococcus mutans GS-5 by induction and carbon catabolite repression. J. Bacteriol. 1812863-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlsson, J. 1984. Regulation of sugar metabolism in relation to the “feast-and famine” existence of plaque, p. 205-211. In B. Guggenheim (ed.), Cariology. Karger, Basel, Switzerland.
- 16.Chauvaux, S., I. T. Paulsen, and M. H. Saier. 1998. CcpB, a novel transcription factor implicated in catabolite repression In Bacillus subtilis. J. Bacteriol. 180491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, Y. Y., M. J. Betzenhauser, and R. A. Burne. 2002. cis-Acting elements that regulate the low-pH-inducible urease operon of Streptococcus salivarius. Microbiology 1483599-3608. [DOI] [PubMed] [Google Scholar]
- 18.Dahl, M. K. 2002. CcpA-independent carbon catabolite repression in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 4315-321. [PubMed] [Google Scholar]
- 19.Deutscher, J., E. Kuster, U. Bergstedt, V. Charrier, and W. Hillen. 1995. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria. Mol. Microbiol. 151049-1053. [DOI] [PubMed] [Google Scholar]
- 20.Fine, I. H., and L. A. Costello. 1963. The use of starch electrophoresis in dehydrogenase studies. Methods Enzymol. 7958-912. [Google Scholar]
- 21.Griswold, A. R., M. Jameson-Lee, and R. A. Burne. 2006. Regulation and physiologic significance of the agmatine deiminase system of Streptococcus mutans UA159. J. Bacteriol. 188834-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamada, S., and H. D. Slade. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44331-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henkin, T. M. 1996. The role of the CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis. FEMS Microbiol. Lett. 1359-15. [DOI] [PubMed] [Google Scholar]
- 24.Iyer, R., N. S. Baliga, and A. Camilli. 2005. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J. Bacteriol. 1878340-8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, S. I., C. Jourlin-Castelli, S. R. Wellington, and A. L. Sonenshein. 2003. Mechanism of repression by Bacillus subtilis CcpC, a LysR family regulator. J. Mol. Biol. 334609-624. [DOI] [PubMed] [Google Scholar]
- 26.Kotrba, P., M. Inui, and H. Yukawa. 2001. Bacterial phosphotransferase system (PTS) in carbohydrate uptake and control of carbon metabolism. J. Biosci. Bioeng. 92502-517. [DOI] [PubMed] [Google Scholar]
- 27.Kuramitsu, H. K. 1987. Recent advances in defining the cariogenicity of mutans streptococci: molecular genetic approaches. Eur. J. Epidemiol. 3257-260. [DOI] [PubMed] [Google Scholar]
- 28.LeBlanc, D. J., V. L. Crow, L. N. Lee, and C. F. Garon. 1979. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J. Bacteriol. 137878-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemos, J. A., J. Abranches, and R. A. Burne. 2005. Responses of cariogenic streptococci to environmental stresses. Curr. Issues Mol. Biol. 795-107. [PubMed] [Google Scholar]
- 30.Liu, Z. L., and P. J. Slininger. 2007. Universal external RNA controls for microbial gene expression analysis using microarray and qRT-PCR. J. Microbiol. Methods 68486-496. [DOI] [PubMed] [Google Scholar]
- 31.Lorca, G. L., Y. J. Chung, R. D. Barabote, W. Weyler, C. H. Schilling, and M. H. Saier, Jr. 2005. Catabolite repression and activation in Bacillus subtilis: dependency on CcpA, HPr, and HprK. J. Bacteriol. 1877826-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-Verstraete, I., J. Deutscher, and A. Galinier. 1999. Phosphorylation of HPr and Crh by HprK, early steps in the catabolite repression signaling pathway for the Bacillus subtilis levanase operon. J. Bacteriol. 1812966-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nascimento, M. M., J. A. Lemos, J. Abranches, R. B. Goncalves, and R. A. Burne. 2004. Adaptive acid tolerance response of Streptococcus sobrinus. J. Bacteriol. 1866383-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Nascimento, M. M., J. A. Lemos, J. Abranches, V. K. Lin, and R. A. Burne. 2008. Role of RelA of Streptococcus mutans in global control of gene expression. J. Bacteriol. 19028-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Postma, P. W., J. W. Lengeler, and G. R. Jacobsen. 1993. Phosphenolpyruvate:carbohydrate phosphotransferase of bacteria. Microbiol. Rev. 57543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saier, M. H., Jr. 1996. Cyclic AMP-independent catabolite repression in bacteria. FEMS Microbiol. Lett. 13897-103. [DOI] [PubMed] [Google Scholar]
- 36.Seidl, K., M. Stucki, M. Ruegg, C. Goerke, C. Wolz, L. Harris, B. Berger-Bachi, and M. Bischoff. 2006. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 501183-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw, W. V. 1975. Chloramphenicol acetyltransferase activity from chloramphenicol-resistant bacteria. Methods Enzymol. 43737-755. [DOI] [PubMed] [Google Scholar]
- 38.Shi, L., L. H. Reid, W. D. Jones, R. Shippy, J. A. Warrington, S. C. Baker, P. J. Collins, F. de Longueville, E. S. Kawasaki, K. Y. Lee, Y. Luo, Y. A. Sun, J. C. Willey, R. A. Setterquist, G. M. Fischer, W. Tong, Y. P. Dragan, D. J. Dix, F. W. Frueh, F. M. Goodsaid, D. Herman, R. V. Jensen, C. D. Johnson, E. K. Lobenhofer, R. K. Puri, U. Schrf, J. Thierry-Mieg, C. Wang, M. Wilson, P. K. Wolber, L. Zhang, S. Amur, W. Bao, C. C. Barbacioru, A. B. Lucas, V. Bertholet, C. Boysen, B. Bromley, D. Brown, A. Brunner, R. Canales, X. M. Cao, T. A. Cebula, J. J. Chen, J. Cheng, T. M. Chu, E. Chudin, J. Corson, J. C. Corton, L. J. Croner, C. Davies, T. S. Davison, G. Delenstarr, X. Deng, D. Dorris, A. C. Eklund, X. H. Fan, H. Fang, S. Fulmer-Smentek, J. C. Fuscoe, K. Gallagher, W. Ge, L. Guo, X. Guo, J. Hager, P. K. Haje, J. Han, T. Han, H. C. Harbottle, S. C. Harris, E. Hatchwell, C. A. Hauser, S. Hester, H. Hong, P. Hurban, S. A. Jackson, H. Ji, C. R. Knight, W. P. Kuo, J. E. LeClerc, S. Levy, Q. Z. Li, C. Liu, Y. Liu, M. J. Lombardi, Y. Ma, S. R. Magnuson, B. Maqsodi, T. McDaniel, N. Mei, O. Myklebost, B. Ning, N. Novoradovskaya, M. S. Orr, T. W. Osborn, A. Papallo, T. A. Patterson, R. G. Perkins, E. H. Peters, R. Peterson, et al. 2006. The microarray quality control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat. Biotechnol. 241151-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson, C. L., and R. R. B. Russell. 1998. Identification of a homolog of CcpA catabolite repressor protein in Streptococcus mutans. Infect. Immun. 662085-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spatafora, G., K. Rohrer, D. Barnard, and S. Michalek. 1995. A Streptococcus mutans mutant that synthesizes elevated levels of intracellular polysaccharide is hypercariogenic in vivo. Infect. Immun. 632556-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spatafora, G. A., M. Sheets, R. June, D. Luyimbazi, K. Howard, R. Hulbert, D. Barnard, M. el Janne, and M. C. Hudson. 1999. Regulated expression of the Streptococcus mutans dlt genes correlates with intracellular polysaccharide accumulation. J. Bacteriol. 1812363-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stülke, J., M. Arnaud, G. Rapoport, and I. Martin-Verstraete. 1998. PRD—a protein domain involved in PTS-dependent induction and carbon catabolite repression of catabolic operons in bacteria. Mol. Microbiol. 28865-874. [DOI] [PubMed] [Google Scholar]
- 43.Titgemeyer, F., and W. Hillen. 2002. Global control of sugar metabolism: a gram-positive solution. Antonie van Leeuwenhoek 8259-71. [PubMed] [Google Scholar]
- 44.Vadeboncoeur, C., D. Brochu, and J. Reizer. 1991. Quantitative determination of the intracellular concentration of the various forms of HPr, a phosphocarrier protein of the phosphoenolpyruvate:sugar phosphotransferase system in growing cells of oral streptococci. Anal. Biochem. 19624-30. [DOI] [PubMed] [Google Scholar]
- 45.Vadeboncoeur, C., and L. Gauthier. 1987. The phosphoenolpyruvate:sugar phosphotransferase system of Streptococcus salivarius. Identification of a IIIman protein. Can. J. Microbiol. 33118-122. [DOI] [PubMed] [Google Scholar]
- 46.Varga, J., V. L. Stirewalt, and S. B. Melville. 2004. The CcpA protein is necessary for efficient sporulation and enterotoxin gene (cpe) regulation in Clostridium perfringens. J. Bacteriol. 1865221-5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weickert, M. J., and G. H. Chambliss. 1990. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc. Natl. Acad. Sci., USA 876238-6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wen, Z. T., C. Browngardt, and R. A. Burne. 2001. Characterization of two operons that encode components of fructose-specific enzyme II of the sugar:phosphotransferase system of Streptococcus mutans. FEMS Microbiol. Lett. 205337-342. [DOI] [PubMed] [Google Scholar]
- 49.Wen, Z. T., and R. A. Burne. 2002. Analysis of cis- and trans-acting factors involved in regulation of the Streptococcus mutans fructanase gene (fruA). J. Bacteriol. 184126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng, L., Z. T. Wen, and R. A. Burne. 2006. A novel signal transduction system and feedback loop regulate fructan hydrolase gene expression in Streptococcus mutans. Mol. Microbiol. 62187-200. [DOI] [PubMed] [Google Scholar]
- 51.Zeng, L., Y. Dong, and R. A. Burne. 2006. Characterization of cis-acting sites controlling arginine deiminase gene expression in Streptococcus gordonii. J. Bacteriol. 188941-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zomer, A. L., G. Buist, R. Larsen, J. Kok, and O. P. Kuipers. 2007. Time-resolved determination of the CcpA regulon of Lactococcus lactis subsp. cremoris MG1363. J. Bacteriol. 1891366-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.