Abstract
During recent studies of ribonucleolytic “degradosome” complexes of Escherichia coli, we found that degradosomes contain certain RNAs as well as RNase E and other protein components. One of these RNAs is ssrA (for small stable RNA) RNA (also known as tm RNA or 10Sa RNA), which functions as both a tRNA and mRNA to tag the C-terminal ends of truncated proteins with a short peptide and target them for degradation. Here, we show that mature 363-nt ssrA RNA is generated by RNase E cleavage at the CCA-3′ terminus of a 457-nt ssrA RNA precursor and that interference with this cleavage in vivo leads to accumulation of the precursor and blockage of SsrA-mediated proteolysis. These results demonstrate that RNase E is required to produce mature ssrA RNA and for normal ssrA RNA peptide-tagging activity. Our findings indicate that RNase E, an enzyme already known to have a central role in RNA processing and decay in E. coli, also has the previously unsuspected ability to affect protein degradation through its role in maturation of the 3′ end of ssrA RNA.
Ribonuclease E (RNase E), which is essential for cell growth and was initially characterized as the enzyme that processes 9S RNA to yield a 5S product, p5S RNA (1), has been shown to control the rate-limiting step in the degradation of a variety of Escherichia coli mRNAs (for reviews, see refs. 2–5), and a small regulatory RNA, RNAI—an antisense repressor of the replication primer of ColE1-type plasmids (6–8). Temperature-sensitive mutants of RNase E have been isolated (9–12) and mapped to its enzymatic active domain (13), which is located at the N-terminal half of the polypeptide (14, 15). Inactivation of RNase E activity in these mutants by culture at nonpermissive temperature prolongs the decay of bulk mRNA (11, 12).
Recently, an RNase E-containing multicomponent ribonucleolytic complex termed the “RNA degradosome” has been isolated and characterized by two distinct approaches (16, 17), both of which show that degradosomes contain the protein components RNase E, PNPase, RhlB RNA helicase, and enolase. In addition to these proteins, the degradosome was found to contain the chaperonins DnaK (16, 18) and GroEL (16), polyphosphate kinase (18), mRNA, and certain structural RNA species (16, 19), including RNAI, fragmented rRNAs, 9S RNA, and 10Sa/ssrA RNA [a small stable RNA encoded by the ssrA gene (20–22)]. Most of these RNAs are known to be substrates for RNase E (19, 23–26).
Nascent polypeptides translated in E. coli from truncated mRNAs lacking stop codons commonly receive a short carboxyl-terminal peptide tag (27, 28), synthesized in trans (28, 29), that targets the resulting fusion polypeptide for rapid degradation (28). Except for the first alanine, the amino acid sequence of this tag (AANDENYALAA) is encoded by 10Sa/ssrA RNA (20–22), also known as tmRNA because of its dual tRNA-like and mRNA-like activities (22, 28–30). Mature 363-nt ssrA RNA, which has its 3′ and 5′ ends paired in a tRNA-like structure, is charged with an alanine residue (22, 30), which then is linked covalently to the nascent polypeptide chain, facilitating co-translational switching of the ribosome to the ssrA-encoded mRNA segment specifying the last 10 amino acids of the chain (28).
Generation of mature ssrA RNA molecules involves processing at the 5′ end of a 457-nt pretRNA-like precursor by RNase P (22) and, according to a recent report, trimming at the 3′end by the activities of ribonucleases T and PH (31). Although ssrA RNA precursors were observed to accumulate in cells after inactivation of RNase E (32, 33), a direct role for RNase E in ssrA RNA processing was excluded (33).
A recent discovery of ssrA RNA along with RNase E and known RNase E substrates in degradosomes (19) prompted us to reinvestigate of the role of RNase E in ssrA RNA maturation. In the present study, we find that RNase E cuts the ssrA RNA precursor site specifically at three locations near its CCA-3′ end, generating both mature ssrA RNA and slightly longer intermediates. Moreover, we show that processing of ssrA RNA by RNase E is required for ssrA RNA-mediated tagging and proteolysis of truncated peptide chains in E. coli cells.
Experimental Procedures
Bacterial Strains and Plasmids.
WCL01 (rne-3071, ssrA1∷cat) was constructed by P1 transduction of ssrA− from E. coli strain X90 ssrA1∷cat (28) into N3431, which carries the rne-3071 temperature-sensitive mutation (9). Plasmid pGP1-2 contains the T7 RNA polymerase gene under the control of a temperature-sensitive bacteriophage λ repressor (34). Plasmids pRE196 and pRE205 contain the rne wild-type and rne-3071 mutant gene, respectively, which were fused to the FLAG-epitope sequence that allows the fusion protein to be purified by M2 affinity column (19). The ssrA gene was cloned via PCR amplification of a 457-bp DNA fragment that contains the ssrA gene (coordinates from nucleotide −7 to nucleotide 450) from E. coli chromosomal DNA, using primers 10Sa-4 (5′CTT AAG CTT ACA CAT TGG GGC) and 10Sa-5 (5′GGA GAA TTC GAG GGC ACA AAA). The PCR-amplified DNA fragment then was cloned into the TA-vector pCR2.1 (Invitrogen). The resulting plasmid was named pCR2.1-10Sa. Construction of pT7/T3-10Sa for in vitro synthesis of ssrA RNA precursor involved cutting pCR2.1-10Sa with HindIII and EcoRI. The DNA fragment containing the ssrA gene then was ligated into a HindIII-EcoRI linearized pT7/T3α-19 plasmid (Life Technologies, Rockville, MD), in which the ssrA expression is under the control of the T7 promoter. pPW510F, a derivative of pPW500 (28), was constructed by inserting a recombinant Ptrc-ssrA DNA fragment with ssrA-expression under the control of the Ptrc promoter into the SmaI site of pPW500, and it was used for the expression of ssrA RNA and the N-terminal domain of the λ-repressor lacking terminal codons. The recombinant Ptrc-ssrA DNA fragment was derived from a pPW500 derivative, pPW501, in which the λ repressor-gene between the NcoI and SmaI of pPW500 was replaced with the EcoRI DNA fragment carrying the ssrA gene of pCR2.1-10Sa.
Isolation of RNase E Complexes and RNAs, Primer Extension, and Northern Blot Analyses.
To detect mature ssrA RNA and its precursor in both E. coli cells and the RNase E-degradosome complexes, the wild-type (pRE196) and mutant (pRE205) FLAG-RNase E were over-expressed in E. coli N3431 (rne-3071) carrying pGP1-2 (34), and the RNase E complexes were purified as described (16). Culture cells were grown in Luria–Bertani broth with kanamycin (50 μg/ml) and ampicillin (50 μg/ml) at 30°C to an OD600 of 0.5, and then were shifted to 44°C for 30 min to express FLAG-RNase E. To inactivate RNase E activity, the culture of N3431 carrying pGP1-2 and pRE205 was used because temperature up-shift to 44°C also inactivated the host’s endogeneous RNase E activity. RNase E-degradosome complexes were purified as described (16) except that 5 mM EDTA was added to the TBS buffer during purification. RNase E-degradosome complexes used for in vitro cleavage assay were purified from E. coli BL21(DE3) containing pRE196 as described (16, 19).
Isolation of degradosome RNA and total RNA from E. coli cell lysates was as described (16, 35). Northern blot and primer extension analyses were performed as described (35). The internally labeled riboprobe used for the detection of ssrA RNA and its precursors contained the nucleotide sequences complementary to nucleotides 114–318 of ssrA RNA. Primers used for primer extension analysis were 5′-end labeled with [γ-32P]ATP by T4 polynucleotide kinase (New England Biolabs) as described (35).
In Vitro RNase E Cleavage Assay.
EcoRI-linearized pT7/T3-10Sa was used as the template for T7 RNA polymerase in vitro synthesis of [α-32P]UTP internally labeled ssrA RNA precursors. The synthesized ssrA RNA precursor contained extra nucleotides at both ends (5′GGG AAG CTT, 3′CAA TT) due to PCR cloning. [γ-32P]ATP labeled 5′-end ssrA RNA precursors were prepared from the phosphorylation of cold ssrA RNA precursors whose 5′ends were pretreated with calf intestinal phosphatase (Promega). Radioactively labeled ssrA RNA precursors were purified from an 8% polyacrylamide sequencing gel, and the position of full length RNA was determined by autoradiography. For purification, RNA was eluted from gel slabs into 400 μl of TE buffer in DEPC water at 37°C overnight. After extraction with phenol and phenol/chloroform, RNA in the aqueous phase was mixed with 20 μg of yeast tRNA (Boehringer Mannheim), 1/10 volume of 3 M NaOAc (pH 7.8), and an equal volume of isopropanol, and then was aliquoted into four tubes and was stored at −20°C for 1 hour for precipitation before use. Substrate (20,000 cpm) was incubated with 4 μl of the RNase E-complexes in RNase E buffer (36) in a total volume of 10 μl. The mixture was incubated at 30°C for the indicated time period, was extracted with 40 μl of phenol, and was mixed with 10 μl of sequencing loading buffer. The samples were heated at 85°C for 5 min, were chilled on ice, and were loaded onto an 8% polyacrylamide sequencing gel.
Pulse–Chase Experiments.
The [35S]methionine pulse–chase experiments were performed as described (28), with modifications. WCL01 containing either pPW500 or pPW510F and N3433 containing pPW510F were grown in M9/glucose medium (37) containing thiamine (20 μM), l-arginine⋅HCl (40 μg/ml), and appropriate antibiotics [chloramphenicol (34 μg/ml), ampicillin (50 μg/ml), or both]. At an OD460 of 0.4, isopropyl β-d-thiogalactoside (1 mM) was added to the cell culture. The culture was divided into two flasks and was grown at 30°C and 44°C, respectively, for 20 min. For pulse labeling, [35S]methionine (75 μCi/ml) was added for 1 min, and then was chased with l-methionine (2.8 mg/ml). Immediately before the pulse labeling, 2 units of OD460 growing culture was removed for the RNA preparation analyzed in Fig. 4. At various times, 1 ml of each culture was removed for cell lysate preparation. The cell pellets were immediately resuspended in 1 × SDS sample buffer and was lysed by boiling for 5 min. The proteins were separated on a 12% SDS/PAGE gel, and 35S-labeled proteins were detected by autoradiography.
Figure 4.
Northern blot analysis of ssrA RNA and ssrA RNA precursors before (U lanes) or after (I lanes) induction. The lower panel shows the internal control of ethidium bromide stained 5S rRNA. The positions of ssrA RNA (SsrA), ssrA RNA precursor (pSsrA), and a read-through fusion-transcript of the λ repressor variant mRNA-ssrA RNA precursor (λ-pSsrA) also are shown. The λ-pSsrA fusion transcript was confirmed by Northern blot detection using a 32P-labeled riboprobe complementary to λ repressor variant mRNA (data not shown). This experiment was carried out in parallel to the experiment of Fig. 3: isopropyl β-d-thiogalactoside (1 mM) was added at an OD460 of 0.4 and was divided into two cultures incubated at 30 and 44°C, respectively, for 20 min (see Experimental Procedures, “Pulse–chase experiments”). Immediately before the pulse–chase, 2 OD460 units of each culture were removed for total RNA preparation for Northern blot analysis (35). It is expected to detect small amount of stable, matured ssrA RNA, which were made on isopropyl β-d-thiogalactoside induction and prior complete inactivation of RNase E.
Results and Discussion
Precursor ssrA RNA in the RNA Degradosome.
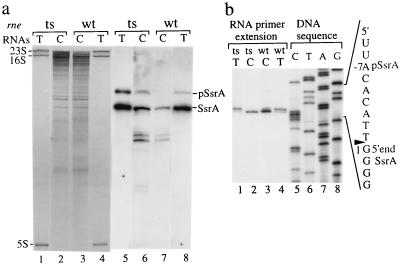
Earlier work has detected ssrA RNA sequences in degradosomes obtained from cells expressing functional RNase E (19). As seen in Fig. 1, Northern blot analysis shows the presence of two RNA species complementary to an ssrA RNA probe in degradosomes isolated from E. coli cells containing active (N3431/pGP1-2/pRE196) or inactive (N3431/pGP1-2/pRE205) forms of RNase E. However, the larger species was present in relatively greater amounts in degradosomes obtained from cells lacking RNase E activity (Fig. 1a, lane 6 vs. 7), suggesting that it is a precursor RNA whose cleavage by RNase E generates the shorter species. Primer extension revealed that the 5′ ends of both the truncated and precursor species of ssrA RNA present in these degradosomes consist of the (5′)-GGGG sequence (Fig. 1b, lanes 2 and 3), which is known to be generated by RNase P processing of ssrA RNA precursor molecules (22). Thus, both RNA species had undergone 5′ end maturation by RNase P, and, thus, the slower migration of the ssrA RNA species that accumulates in degradosomes of RNase E-defective cells is the result of incomplete maturation at the 3′ rather than 5′ end.
Figure 1.
RNase E inactivation causes the accumulation of ssrA RNA precursors with an unmatured 3′ end. rne wild-type (wt) or temperature-sensitive (rne-3071 ts) FLAG-RNase E, pRE196, or pRE205 (16), respectively, was overexpressed in the N3431 (rne-3071) carrying pGP1−2 (34) at 44°C for 30 min. Total RNAs (T) or RNAs associated with RNase E complexes (C) were isolated from cell lysates or affinity-purified RNase E complexes as described in Experimental Procedures. (a) The purified RNAs were loaded onto a 5% polyacrylamide sequencing gel and were stained with ethidium bromide (lanes 1–4), and the RNAs were transferred onto a Zeta-Probe blotting membrane (Bio-Rad) and were hybridized with riboprobe complementary to ssrA RNA (lanes 5–8). The positions of 23S, 16S, and 5S rRNAs, mature ssrA RNA (SsrA), and ssrA RNA precursors (pSsrA) are shown. (b) Matured 5′ end termini of ssrA RNAs were detected by primer extension analysis (lanes 1–4) using a synthetic DNA primer 10Sa-1 complementary to the sequences of nucleotides 27–51 of ssrA RNA as indicated in Fig. 2d [10Sa-1, 5′TCG GCA TGC ACC TTG GGT TTC GCA A (22)]. DNA sequencing ladders generated by using the identical primer, i.e., 10Sa-1, are shown (lanes 5–8). The 5′-end sequences of the ssrA RNA precursor (labeled as pSsrA at the −7 nucleotide position) or matured ssrA RNA (shown as 1) and RNase P processing site (labeled with an arrow; also see Fig. 2d) also are shown.
Because of the great stability of ssrA RNA (20–22), we suspected that the mature ssrA RNA present in degradosomes isolated from rnets cells after 30 min of growth at 44°C represents molecules processed before the temperature up-shift. To eliminate possible effects of preexisting ssrA RNA in our evaluation of effects of RNaseE inactivation on ssrA activity, we generated a N3431 derivative, WCL01, that lacks the chromosomal ssrA gene for use in subsequent in vivo experiments (see below).
Site-Specific Cleavage of ssrA RNA Near the CCA-3′ End of the Precursor RNA in Vitro.
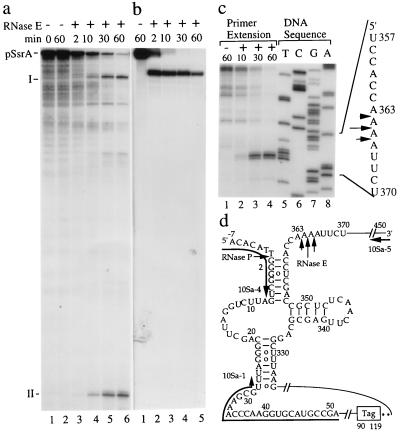
In vitro cleavage of [α-32P]UTP internally labeled 460-nt ssrA RNA precursors synthesized from a cloned ssrA gene under the control of bacteriophage T7 promoter (pT7/T3-10Sa; see Experimental Procedures for its construction) was examined by using purified RNase E degradosome complexes (see Experimental Procedures) (16, 19). The RNA precursor was found to be cut by RNase E site-specifically, yielding two predominant 32P-labeled RNA species: one ≈370 nt and the other 90 nt in length (Fig. 2a, labeled as I and II, respectively, lanes 3–6). Additionally, RNase E cleavage of a [γ-32P] 5′-labeled 460-nt ssrA RNA precursor resulted in a single 5′-labeled product 370 nt in length (Fig. 2b, labeled as I, lanes 2–5). Collectively, the results showed that RNase E cleaves the ssrA RNA precursor at a site ≈90 nt from its 3′-end, which is at or near the location of the CCA-3′-tail of mature 363 nt ssrA RNA (22).
Figure 2.
RNase E cuts site-specifically near the CCA-(3′)-end of the ssrA RNA. In vitro cleavage assays were performed for 0, 2, 10, 30, and 60 min durations with (+) or without (−) RNase E complexes. ssrA RNA precursors (pSsrA) used in these assays were continuously labeled with [α-32P]UTP (a), were 5′ labeled with [γ-32P]ATP (b), or were unlabeled (c). (a and b) The cleavage reactions were separated by gel electrophoresis using an 8% polyacrylamide sequencing gel and were subjected to autoradiography. The positions of ssrA RNA precursors (pSsrA) and RNase E cleavage products (I and II) are shown. (c) 5′-end termini of RNase E cleavage products were detected by primer extension analysis as described (23) using a 5′ labeled 10Sa-5 primer whose sequences are complementary to the sequences of nucleotides 439–450 of ssrA RNA precursor (see Experimental Procedures and d). The DNA sequencing ladders (c, lanes 5–8) of the ssrA gene corresponding to the 5′-end termini detected for the primer extension are shown. The RNase E processing sites of the ssrA RNA precursor near the CCA-(3′)-end of matured ssrA RNA are indicated with arrows (c and d), in which the arrow with a longer bar indicates a dominant cutting site. The ssrA RNA precursor, some of the coordinators of the ssrA RNA precursor, the RNase P, and RNase E processing sites and the DNA primers used for the experiments are shown (d). The region containing the ORF of the carboxyl-terminal tag (Tag) and its stop codons (asterisks) are marked.
Precise cleavage sites in precursor ssrA RNA analyzed by primer extension showed that the RNase E cleavage of the ssrA RNA precursor occurs at three separate sites in an AU-rich region distal to the CCA-3′-tail of mature ssrA RNA (Fig. 2 c, lanes 2–4, and d). Cleavage at the most proximal of these sites directly generates a CCA-3′ terminus in which charging by the amino acid alanine can occur, thus allowing ssrA RNA to act as a tRNA that promotes the trans-translation of the carboxyl-terminal peptide tag fusion protein. Thus, RNaseE activity is sufficient to produce mature ssrA RNA. Cleavage at the more distal two sites requires further 3′ end trimming, as also was found for monomeric tRNA precursors cleaved at exactly corresponding sites by RNase E (e.g., tRNA1Ser and tRNAAsn) (38).
Inactivation of RNase E Interferes with ssrA RNA-Mediated Peptide-Tagging Synthesis and Proteolysis of Truncated Peptides.
Our finding that RNase E is required to produce the CCA-3′-tail of mature ssrA RNA suggests that inactivation of RNase E should interfere with the ssrA RNA-mediated tail-tag proteolysis pathway. To test this prediction, it was necessary to carry out RNase E inactivation in an rnets-strain lacking preexisting mature ssrA RNA, which persists stably for extended periods in rnets-E. coli cells after shifting to an elevated nonpermissive temperature (see Fig. 1a, lane 5). We therefore introduced a ssrA null mutation allele [X90 ssrA1∷cat (28), a gift from R. T. Sauer (Massachusetts Institute of Technology, Cambridge, MA)] by P1 transduction into the rnets strain, strain WCL01. Using WCL01, we investigated the effect of RNase E on SsrA-targeted carboxyl-terminal tag-mediated proteolysis by synthesizing the ssrA RNA from an inducible promoter (plasmid pPW510F; see Experimental Procedures for its construction). In addition to containing an inducible ssrA gene, pPW510F also encodes an inducible N-terminal domain of a bacteriophage λ repressor protein that lacks translational stop codons and thus allows assay of trans-translation protein synthesis mediated by mature ssrA RNAs (28).
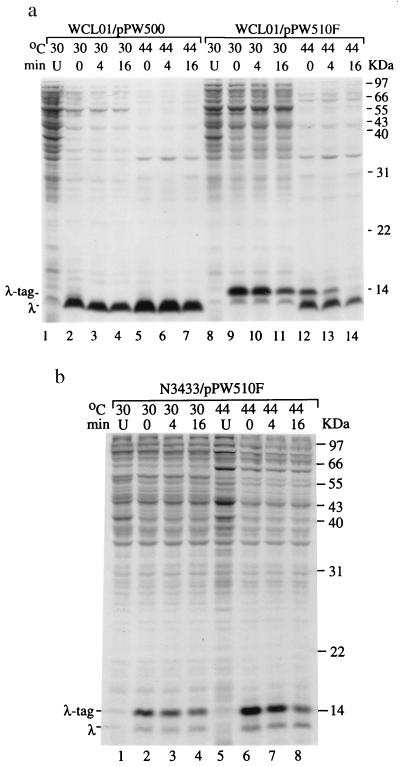
In the presence of active RNase E in E. coli WCL01/pPW510F cells cultured at 30°C, the synthesis of SsrA peptide-tagged λ repressor variant was evident (indicated as λ-tag; Fig. 3a, lanes 9–11). In contrast, along with peptide-tagged λ repressor variants, inactivation of RNase E by shifting the temperature to 44°C resulted in the synthesis of truncated, untagged-λ repressor variants (indicated as λ; Fig. 3a, lanes 12–14). In a parallel experiment, the synthesis of peptide-tagged λ repressor variants were revealed at both 30°C and 44°C in an RNase E wild-type strain (N3433) containing the pPW510F (Fig. 3b). These results demonstrate that untagged λ repressor variants detectable in an rne-temperature mutant WCL01/pPW510F after temperature shift-up to 44°C resulted from the inactivation of RNase E and not from the temperature shift itself. Thus, RNase E is required for normal ssrA RNA activity in vivo.
Figure 3.
RNase E activity is required for the ssrA RNA mediating carboxyl-terminal tagged proteolysis. (a) Degradation of λ repressor (residues 1–93)-M2-H6-trpAt protein in WCL01 (ssrA− rne-3071) containing pPW500 [for overexpression of λ repressor variant (28)] or pPW510F (for overexpression of λ repressor variant and ssrA RNA precursor) cultured at 30°C (active RNase E) or 44°C (inactive RNase E) assayed by pulse–chase experiments. The λ repressor variant mRNA and ssrA RNA precursors were expressed by isopropyl β-d-thiogalactoside induction as described in Experimental Procedures. The positions of [35S]Met-pulse-labeled newly synthesized polypeptides, the λ repressor variant protein (λ), and the ssrA RNA-mediated peptide tag (λ-tag) are indicated at 0 min (lanes 2, 5, 9, and 12). Lane U, uninduced control. Lanes indicated as 4 and 16 min: time after chase. (b) Degradation of the pulse-labeled λ repressor occurs in the rne wild type containing pPW510F cultured at both 30°C (lanes 2–4) or 44°C (lanes 5–8).
Blockage of Proteolysis of Truncated Peptides Associated with Impeded Processing of ssrA Precursor RNA After RNase E Inactivation in WCL01/pPW510F Cells.
In parallel to Fig. 3a, Northern blot analysis of ssrA RNAs purified from rne-temperature mutant WCL01/pPW510F grown at 44°C revealed the concurrent accumulation of unprocessed ssrA RNA precursors on inactivation of RNase E (from ≈25 to 90%; indicated as pSsrA and λ-pSsrA in Fig. 4, lane 6). Consistently, these incompletely processed ssrA RNA precursors failed to mediate the tagging of incomplete peptides (Fig. 3a, lanes 13 and 14). WCL01 cells contain plasmid pPW500, which lacks the ssrA gene and therefore does not produce ssrA RNA (Fig. 3b, lanes 2 and 3). Consequently, SsrA-tagged λ repressor variants are absent in WCL01/pPW500 cells (Fig. 3a, lanes 2–7). Collectively, our results show that the RNase E enzyme cleaves at the 3′end of the precursor RNA to generate mature ssrA RNA and that cells lacking RNase E activity fail to accomplish the maturation of ssrA RNA required for its tRNA-like function and thus fail to carry out SsrA-mediated peptide-tagging proteolysis.
Not withstanding an earlier conclusion that RNase E is not involved in the maturation of ssrA RNA (11), our findings show that RNase E-processing at the CCA-3′ end generates the ssrA RNA terminus required for the synthesis of carboxyl-terminal tagging peptides. Recently, it was reported that exonucleolytic trimming by multiple RNases is required for the 3′ maturation of ssrA RNA (31), although their ability to affect peptide-tagging was not demonstrated. In that report, strains mutated in genes for multiple RNases, including RNase T, PH, D, and BN, but containing RNase E, accumulated an immature ssrA RNA species that contained extra nucleotides at the 3′end. However, an even greater amount of mature ssrA RNA was also evident in the mutated bacteria, in seeming contradiction to the conclusion (31) that trimming by some or all of these exonriboucleases is a prerequisite for maturation of the CCA-3′-tail of ssrA RNA. Our results show that the RNase E-cleavage occurring at the most proximal of three cleavage sites we have identified in the ssrA RNA precursor directly generates the CCA-3′ terminus necessary of SsrA-mediated proteolysis. However, RNase E cleavages at the two distal sites may be followed by secondary exonucleolytic trimming to produce this terminus.
In addition to the monomeric tRNA1Ser and tRNAAsn (38), and ssrA RNA (present study), RNase E is responsible for the 3′ processing and biosynthesis of another small stable RNA species, M1 RNA/ssrB RNA, which is the RNA component and catalytic subunit of E. coli RNase P (39, 40). As RNase P is known to mature the 5′-end of ssrA RNA (22), inactivation of RNase E may interfere indirectly with the processing of the 5′end of ssrA RNA. However, inactivation of RNase E during our experiments showed no effects on 5′end processing (Fig. 1b), possibly because preexisting mature M1 RNA molecules may have allowed continued RNase P processing of ssrA RNA at the 5′end.
Endonucleolytic cleavage of mRNAs by RNases in actively dividing bacterial cells can lead to the formation of broken mRNA fragments lacking translation stop codons (e.g., refs. 41 and 42). Translation of these resulting mRNA segments may produce N-terminal peptide fragments trapped with 70S ribosomes (28, 30, 43), possibly impeding further mRNA decay of the partially degraded mRNA. Tagging of incomplete peptides by ssrA RNA results in their release from the 70S ribosomes and subsequent peptide degradation, which we suggest may in turn release mRNA fragments that encode these peptides. The release of partially degraded mRNA molecules from ribosomes may expose the fragmented mRNA’s 3′ end, rendering it susceptible to polyadenylation and consequent enhancement of 3′ to 5′ exonucleolytic decay (8, 44–47).
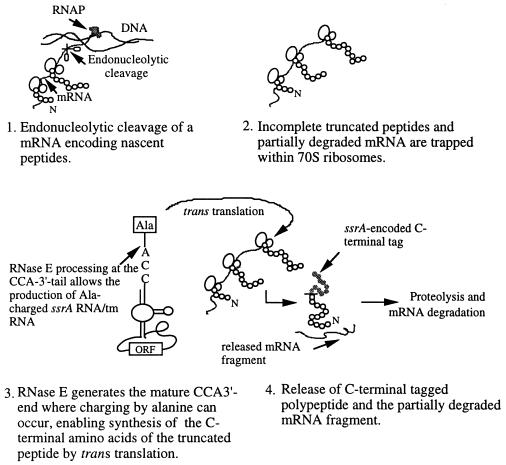
By carrying out a key step in the production of mature CCA-3′-end of ssrA RNA, RNase E now has been shown to be required for normal ssrA RNA-mediated degradation of incomplete peptides (Fig. 5). Homologs of both rne and ssrA have been detected in a variety of Gram-negative and Gram-positive bacterial species (48–50). The role of RNase E in ssrA RNA processing also may help ensure that the endonucleolytic cleavages generated in mRNA molecules by RNase E or other RNases do not adversely affect later steps in mRNA decay (Fig. 5).
Figure 5.
Schematic model showing events in which a partially degraded mRNA is released from 70S ribosomes by ssrA RNA charging of the CCA-3′ terminus generated by RNase E with an alanine that promotes trans translation of an ssrA-encoded ORF specifying peptide tag used in proteolysis. 70S ribosomes (indicated as two joining circles), elongating nascent polypeptides (streams of small circles), broken mRNA lacking a stop codon (curved line), carboxyl terminal ssrA-encoded peptide-tag (filled circles), RNA polymerase complex (RNAP), and DNA template are shown. N indicates the NH2-terminal of peptides. Ala-charged ssrA RNA/tm RNA is shown.
Acknowledgments
We thank Dr. S. N. Cohen and Mr. G. M. Cohen for critical comments on the manuscript. The study was supported by a Frontier Research Award from the National Science Council and by an intramural fund from Academia Sinica of the Republic of China. C.-L.W. received a graduate fellowship from the National Science Council, the Republic of China.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ghora B K, Apirion D. Cell. 1978;15:1055–1066. doi: 10.1016/0092-8674(78)90289-1. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S N, McDowall K J. Mol Microbiol. 1997;23:1099–1106. doi: 10.1111/j.1365-2958.1997.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 3.Melefors O, Lundberg U, von Gabain A. In: Control of Messenger RNA Stability. Belasco J, Brawerman G, editors. San Diego: Academic; 1993. pp. 53–70. [Google Scholar]
- 4.Nierlich D P, Murakawa G J. Prog Nucleic Acid Res Mol Biol. 1996;52:153–216. doi: 10.1016/s0079-6603(08)60967-8. [DOI] [PubMed] [Google Scholar]
- 5.Coburn G A, Mackie G A. Prog Nucleic Acid Res Mol Biol. 1999;62:55–108. doi: 10.1016/s0079-6603(08)60505-x. [DOI] [PubMed] [Google Scholar]
- 6.Tomcsanyi T, Apiron D. J Mol Biol. 1985;185:713–720. doi: 10.1016/0022-2836(85)90056-7. [DOI] [PubMed] [Google Scholar]
- 7.Lin-Chao S, Cohen S N. Cell. 1991;65:1233–1242. doi: 10.1016/0092-8674(91)90018-t. [DOI] [PubMed] [Google Scholar]
- 8.Xu F, Lin-Chao S, Cohen S N. Proc Natl Acad Sci USA. 1993;90:6756–6760. doi: 10.1073/pnas.90.14.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apirion D, Lassar A B. J Biol Chem. 1978;253:1738–1742. [PubMed] [Google Scholar]
- 10.Apirion D. Genetics. 1978;90:659–671. doi: 10.1093/genetics/90.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwano M, Ono M, Endo H, Hori K, Nakamura K, Hirota Y, Ohnishi Y. Mol Gen Genet. 1977;154:279–285. doi: 10.1007/BF00571283. [DOI] [PubMed] [Google Scholar]
- 12.Ono M, Kuwano M. J Mol Biol. 1979;129:343–357. doi: 10.1016/0022-2836(79)90500-x. [DOI] [PubMed] [Google Scholar]
- 13.McDowall K J, Hernandez R G, Lin-Chao S, Cohen S N. J Bacteriol. 1993;175:4245–4249. doi: 10.1128/jb.175.13.4245-4249.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taraseviciene L, Bjork G R, Uhlin B E. J Biol Chem. 1995;270:26391–26398. doi: 10.1074/jbc.270.44.26391. [DOI] [PubMed] [Google Scholar]
- 15.McDowall K J, Cohen S N. J Mol Biol. 1996;255:349–355. doi: 10.1006/jmbi.1996.0027. [DOI] [PubMed] [Google Scholar]
- 16.Miczak A, Kaberdin V R, Wei C-L, Lin-Chao S. Proc Natl Acad Sci USA. 1996;93:3865–3869. doi: 10.1073/pnas.93.9.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Py B, Higgins C F, Krisch H M, Carpousis A J. Nature (London) 1996;381:169–172. doi: 10.1038/381169a0. [DOI] [PubMed] [Google Scholar]
- 18.Blum E, Py B, Carpousis A J, Higgins C H. Mol Microbiol. 1997;26:387–398. doi: 10.1046/j.1365-2958.1997.5901947.x. [DOI] [PubMed] [Google Scholar]
- 19.Bessarab D A, Kaberdin V R, Wei C-L, Liou G-G, Lin-Chao S. Proc Natl Acad Sci USA. 1998;95:3157–3161. doi: 10.1073/pnas.95.6.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chauhan A K, Apirion D. Mol Microbiol. 1989;3:1481–1485. doi: 10.1111/j.1365-2958.1989.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 21.Komine Y, Inokuchi H. J Bacteriol. 1991;173:5252. doi: 10.1128/jb.173.17.5252.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komine Y, Kitabatake M, Yokogawa T, Nishikawa K, Inokuchi H A. Proc Natl Acad Sci USA. 1994;91:9223–9227. doi: 10.1073/pnas.91.20.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin-Chao S, Wong T-T, McDowall K J, Cohen S N. J Biol Chem. 1994;269:10797–10803. [PubMed] [Google Scholar]
- 24.McDowall K J, Kaberdin V R, Wu S-W, Cohen S N, Lin-Chao S. Nature (London) 1995;374:287–290. doi: 10.1038/374287a0. [DOI] [PubMed] [Google Scholar]
- 25.McDowall K J, Lin-Chao S, Cohen S N. J Biol Chem. 1994;269:10790–10796. [PubMed] [Google Scholar]
- 26.Cormack R S, Mackie G A. J Mol Biol. 1992;228:1078–1090. doi: 10.1016/0022-2836(92)90316-c. [DOI] [PubMed] [Google Scholar]
- 27.Tu G-F, Reid G E, Zhang J-G, Moritz R L, Simpson R J. J Biol Chem. 1995;270:9322–9326. doi: 10.1074/jbc.270.16.9322. [DOI] [PubMed] [Google Scholar]
- 28.Keiler K C, Waller P R H, Sauer R T. Science. 1996;271:990–993. doi: 10.1126/science.271.5251.990. [DOI] [PubMed] [Google Scholar]
- 29.Himeno H, Sato M, Tadaki T, Fukushima M, Ushida C, Muto A. J Mol Biol. 1997;268:803–808. doi: 10.1006/jmbi.1997.1011. [DOI] [PubMed] [Google Scholar]
- 30.Ushida C, Himeno H, Watanabe T, Muto A. Nucleic Acids Res. 1994;22:3392–3396. doi: 10.1093/nar/22.16.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Pandit S, Deutscher M P. Proc Natl Acad Sci USA. 1998;95:2856–2861. doi: 10.1073/pnas.95.6.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subbarao M N, Apirion D. Mol Gen Genet. 1989;217:499–504. doi: 10.1007/BF02464923. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava R K, Miczak A, Apirion D. Biochimie. 1990;72:791–802. doi: 10.1016/0300-9084(90)90188-m. [DOI] [PubMed] [Google Scholar]
- 34.Tabor S, Richardson C C. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin-Chao S, Chen W-T, Wong T-T. Mol Microbiol. 1992;6:3385–3393. doi: 10.1111/j.1365-2958.1992.tb02206.x. [DOI] [PubMed] [Google Scholar]
- 36.Kaberdin V R, Chao Y-H, Lin-Chao S. J Biol Chem. 1996;271:13103–13109. doi: 10.1074/jbc.271.22.13103. [DOI] [PubMed] [Google Scholar]
- 37.Miller J H. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. p. 431. [Google Scholar]
- 38.Ray B K, Apirion D. J Mol Biol. 1981;149:599–617. doi: 10.1016/0022-2836(81)90349-1. [DOI] [PubMed] [Google Scholar]
- 39.Lundberg U, Altman S. RNA. 1995;1:327–334. [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S, Kim H, Park I, Lee Y. J Biol Chem. 1996;271:19330–19337. doi: 10.1074/jbc.271.32.19330. [DOI] [PubMed] [Google Scholar]
- 41.Mackie G A. J Bacteriol. 1991;173:2488–2497. doi: 10.1128/jb.173.8.2488-2497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo W-M, Lin-Chao S. J Biol Chem. 1997;272:15516–15520. doi: 10.1074/jbc.272.24.15516. [DOI] [PubMed] [Google Scholar]
- 43.Tadaki T, Fukushima M, Ushida C, Himeno H, Muto A. FEBS Lett. 1996;399:223–226. doi: 10.1016/s0014-5793(96)01330-0. [DOI] [PubMed] [Google Scholar]
- 44.Cohen S N. Cell. 1995;80:829–832. doi: 10.1016/0092-8674(95)90284-8. [DOI] [PubMed] [Google Scholar]
- 45.Xu F, Cohen S N. Nature (London) 1995;374:180–183. doi: 10.1038/374180a0. [DOI] [PubMed] [Google Scholar]
- 46.Hajnsdorf E, Braun F, Haugel-Nielsen J, Regnier P. Proc Natl Acad Sci USA. 1995;92:3973–3977. doi: 10.1073/pnas.92.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Hara E B, Chekanova J A, Ingle C A, Kushner Z R, Peters E, Kushner S R. Proc Natl Acad Sci USA. 1995;92:1807–1811. doi: 10.1073/pnas.92.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen S N, McDowall K J. Mol Microbiol. 1997;23:1099–1106. doi: 10.1111/j.1365-2958.1997.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 49.Kaberdin V R, Miczak A, Jakobsen J S, Lin-Chao S, McDowall K J, von Gabain A. Proc Natl Acad Sci USA. 1998;95:11637–11642. doi: 10.1073/pnas.95.20.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muto A, Sato M, Tadaki T, Fukushima M, Ushida C, Himeno H. Biochimie. 1996;78:985–991. doi: 10.1016/s0300-9084(97)86721-1. [DOI] [PubMed] [Google Scholar]