Abstract
During studies to determine a role for tumor necrosis factor (TNF) in herpes simplex virus type 1 (HSV-1) infection using TNF receptor null mutant mice, we discovered a genetic locus, closely linked to the TNF p55 receptor (Tnfrsf1a) gene on mouse chromosome 6 (c6), that determines resistance or susceptibility to HSV-1. We named this locus the herpes resistance locus, Hrl, and showed that it also mediates resistance to HSV-2. Hrl has at least two alleles, Hrlr, expressed by resistant strains like C57BL/6 (B6), and Hrls, expressed by susceptible strains like 129S6 (129) and BALB/c. Although Hrl is inherited as an autosomal dominant gene, resistance to HSV-1 is strongly sex biased such that female mice are significantly more resistant than male mice. Analysis of backcrosses between resistant B6 and susceptible 129 mice revealed that a second locus, tentatively named the sex modifier locus, Sml, functions to augment resistance of female mice. Besides determining resistance, Hrl is one of several genes involved in the control of HSV-1 replication in the eye and ganglion. Remarkably, Hrl also affects reactivation of HSV-1, possibly by interaction with some unknown gene(s). We showed that Hrl is distinct from Cmv1, the gene that determines resistance to murine cytomegalovirus, which is encoded in the major NK cell complex just distal of p55 on c6. Hrl has been mapped to a roughly 5-centimorgan interval on c6, and current efforts are focused on obtaining a high-resolution map for Hrl.
Most studies examining immunological control of herpes simplex virus type 1 (HSV-1) infection in humans and experimental animal models have focused on adaptive immune responses. Data accrued from these studies have shown that T-cell- meditated responses are crucial for resistance to HSV-1 infection (42, 58). For example, immunosuppressed individuals and those with defects in T-cell immunity, such as AIDS patients, are especially vulnerable to herpesvirus infections (51). However, recent studies suggest an equally important role for early innate responses, which are crucial for limiting virus spread to critical target organs and for orchestrating subsequent optimal adaptive immune responses (9, 50). Natural immunity is phylogenetically much older than adaptive immunity, and it differs from the latter in that the receptors responsible for pathogen recognition are germ line encoded and recognize invariant structural patterns on the surface of microorganisms (31).
Pioneering studies by Lopez and others (32, 36, 38) demonstrated that natural resistance of inbred mouse strains to HSV-1 formed a continuum, such that the strains could be classified as highly susceptible (A/J and PL), moderately susceptible (BALB/c, AKR/J, and DBA/2J), and resistant (C57BL/6 [B6], C57BL/10, DBA/1, and CBA) following intraperitoneal (i.p.) challenge. Analyses of F1 and F2 crosses and N2 backcrosses between resistant C57BL/6 and susceptible (BALB/c or A/J) mice revealed that resistance to i.p. challenge with HSV-1 was a dominant, autosomal trait determined by two independently segregating genes (38). Furthermore, resistance was not H-2 linked, as congenic strains expressing major histocompatibility complex alleles characteristic of the susceptible strains in a B6 background were as resistant as the parental B6 and B10 strains to HSV-1-induced mortality (30, 39). While HSV-1 strain differences did not influence the pattern of resistance seen in the inbred strains, the route of inoculation did, as A/J mice were significantly more resistant to intravenous challenge than to i.p. challenge with HSV-1 (36, 37).
To examine the genetics of natural resistance to HSV-1 neural infection, Simmons and colleagues (65) used the flank inoculation model as a better approximation of natural HSV-1 infection. Their results corroborated Lopez's prior results with one important exception: namely, that only one locus, rather than two loci, was found to determine resistance to mortality. A plausible explanation for this discrepancy is that the different routes of inoculation, i.p. versus flank (i.e., cutaneous), elicit different protective immune responses. i.p. inoculation may well involve spread to critical visceral organs such as the liver, in addition to infection of the central nervous system (CNS) via neural conduits. In contrast, after flank or cutaneous inoculation, HSV-1 infection is largely confined to the corresponding sensory nerve tracts and CNS, with minimal if any involvement of other critical organs in an immunocompetent host. Hence, protection from HSV-1 inoculated via cutaneous and i.p. routes might involve one and two genes, respectively. In contrast to mortality, resistance to ganglionic infection after flank inoculation of HSV-1 was shown to be genetically complex, involving multiple, independently segregating autosomal dominant loci (30, 66).
Genetic resistance to HSV-1 can be circumvented by direct intracerebral inoculation of HSV-1, resulting in uniform mortality in genetically resistant and susceptible mouse strains, which implies that resistance is immunologically mediated and functions to restrict the spread of virus to the CNS (38). Restriction of HSV-1 replication in peripheral nervous system ganglia of resistant mice (C57BL/6) is observed at early stages of infection, days 2 to 5 after inoculation, consistent with the involvement of innate immune responses (37, 66). Several studies have suggested a prominent role for NK cells, an important component of the innate immune system, in the control of herpesvirus infections (12). However, a complicating factor in these studies involving in vivo antibody-mediated depletion of NK cells is that the targeted lineage marker, NK1.1, lacks the required specificity for NK cells (5, 29, 67). Moreover, there is evidence that HSV, like cytomegalovirus (CMV), has evolved strategies to escape NK cell killing (3, 19). Early reports on innate resistance to HSV implicated a role for macrophages (37). Macrophages activated by HSV-1 infection produce tumor necrosis factor (TNF), NO, and alpha/beta interferon (IFN-α/β), all of which can potently inhibit HSV-1 in vitro and in vivo. Thus, it is not surprising that in vivo depletion of macrophages results in enhanced susceptibility to HSV-1 infection (33) (E. Cantin, unpublished observations). Interestingly, macrophage infiltration and early IFN-γ production in the peripheral nervous system were blocked by depletion of γδ T cells, suggesting that cooperation between macrophages and γδ T cells is an important component of the innate response to HSV-1 (33). A comprehensive understanding of natural resistance to HSV-1 infection will not be forthcoming until the critical gene(s) that determine innate resistance is identified and functionally characterized.
Previously, inflammatory responses characterized by expression of proinflammatory cytokines, including IFN-γ and TNF, were observed to persist in the nervous system of HSV-1-infected mice well into latency (16). Our prior studies of HSV-1 infection in IFN-γ−/− and IFN-γR−/− mice revealed that the IFN-γ receptor, rather than IFN-γ itself, was required for protection from mortality (14), whereas both IFN-γ and the IFN-γ receptor were important for in vivo control of reactivated HSV-1 (13).
TNF is the prototypical member of a large family of immunoregulatory proteins, and its diverse functions include regulation of T-cell trafficking and apoptosis, activation of dendritic cells and macrophages, and antiviral activity (17, 28, 35, 40, 52, 70). We report here that, in the course of studies to ascertain a role for TNF in HSV infection, we serendipitously discovered a locus on mouse chromosome 6 (c6) that determines resistance to HSV-1 mortality. We named this locus the herpes resistance locus (Hrl). We demonstrated that Hrl is closely linked to the p55 TNF receptor (TNFR) gene (Tnfrsf1a) on c6 and that alone it mediates resistance to HSV mortality in male mice, whereas a second locus, the sex modifier locus (Sml) is responsible for enhanced resistance of female mice. Remarkably, Hrl also influences in vitro reactivation of HSV-1 and affects aspects of both innate and adaptive immunity to HSV. The identification of Hrl and Sml and functional characterization of their gene products will provide novel insights into innate immunity, immune factors that modulate reactivation and sex-based differences in HSV-1 infection that were previously reported (26).
MATERIALS AND METHODS
Mouse strains.
TNFR p55 (Tnfrsf1a) null mutant mice backcrossed 5 (p55−/− N5) or 13 (p55−/− N13) times to C57BL/6 (B6) were obtained from Amgen Inc. (Thousand Oaks, Calif.). TNFR p75−/− N5 (Tnfrsf1b) and double receptor knockout (p55p75−/− N4) mice, originally derived by Peschon et al. (46), were obtained from Lyle Moldawer (University of Florida, Gainesville). B6 mice and C.B8-Klra8Cmv1-r/Uwa congenic mice were obtained from Jackson Laboratory (Bar Harbor, Maine) and 129S6 (previously 129SvEvTac), (B6 × 129)F1, and BALB/c mice were from Taconic (Germantown, N.Y.).
Virus stocks and inoculation of mice.
Master stocks of HSV-1 strain 17+ or HSV-2 strain MS comprised only of cell-released virus were prepared in and were titered on mycoplasma-free CV-1 cell monolayers; single-use aliquots of virus in Hanks balanced salt solution (HBSS) were stored at −80°C.
The City of Hope animal care committee approved of all animal procedures. Mice were inoculated with HSV-1 or HSV-2 by corneal scarification. The right cornea of mice deeply anesthetized by i.p. injection of ketamine and xylazine was gently scarified by using a 27-gauge needle: 10 vertical strokes followed by application of HSV in a volume of 4 μl of HBSS followed by another 10 horizontal strokes and gentle massaging of the eye with the eyelid to promote virus uptake (14). To minimize experimental variation, the same individual inoculated all of the mice used in studies reported here and mice were always inoculated before noon. The same virus master stock was used for all experiments reported here. For determination of 50% lethal dose (LD50), groups of five to eight mice were challenged with 10-fold dilutions of virus ranging from 5 to 105 PFU per mouse and LD50 values were calculated as described by Reed and Muench previously (15, 53).
Murine cytomegalovirus (MCMV) Smith strain (K181+) stocks, prepared from salivary glands of MCMV-infected BALB/c mice harvested on day 14 postinfection as previously described (22), were obtained from Chris Morello (University of California at San Diego, La Jolla). MCMV titers were determined by plaque assay on NIH 3T3 cells as previously described (41).
HSV shedding and necropsy titers.
To monitor HSV shedding in the tear film, the inoculated cornea was swabbed with an alginate swab moistened in HBSS. The swab was vigorously agitated in a vial containing 0.2 ml of Dulbecco's modified Eagle's medium (DMEM) supplemented with 2% fetal bovine serum (FBS), and HSV was detected by plaque assay on rabbit skin (RS) cell monolayers.
Trigeminal ganglia (Tg), brain stem, and eyes harvested from mice that died from HSV infection or were euthanatized because of pronounced symptoms of HSV encephalitis were homogenized in media containing 2% FBS (DMEM-2), and HSV necropsy titers were determined in the supernatants. Necropsy tissues were processed immediately or were stored at 4°C. Control experiments demonstrated that there was no significant loss of titer for tissues stored at 4°C or at room temperature for up to 24 h compared to tissues that were processed immediately after death.
In vitro reactivation of HSV.
Latently infected mice were sacrificed on days 28 to 30 postinfection, and Tg were cultured intact as explants in DMEM supplemented with 10% FBS (DMEM-10) for 5 days. The Tg were homogenized in DMEM-2, and reactivated HSV-1 present in the homogenate and culture supernatants was detected by plaque assay on RS cells.
Quantitative PCR for HSV DNA in latently infected ganglia.
Total ganglionic DNA was extracted from the Tg homogenate pellets or from Tg that were not subject to reactivation in vitro by digestion of the tissue with 250 μg of proteinase K in 250 μl of proteinase K buffer followed by phenol extraction and ethanol precipitation. DNA concentration and purity were determined spectrophotometrically from the ratio of absorbance at 260 nm to that at 280 nm.
Semiquantitative PCR for HSV gD sequences in Tg was done by comparing amplified gD sequences to that of the single-copy-number cellular adipsin gene as previously described (26). A quantitative fluorescence-based PCR assay derived from the commercially available TaqMan PCR was used to determine the quantity of HSV DNA in latently infected nonreactivated Tg and Tg pellets obtained after in vitro reactivation of explanted Tg cultures. The TaqMan PCR assay, originally optimized for amplification of HSV-1 gG sequences in human Tg samples (47), had to be reoptimized for efficient amplification of gG sequences in mouse Tg DNA. The optimized TaqMan PCR assay conditions for mouse Tg included the use of 4 mM Mg2+, 500 nM primers, 5% dimethyl sulfoxide, 2 U of AmpliTaq Gold with 100 ng of total ganglionic DNA per reaction, and TaqMan probe at 100 nM final concentration, all other conditions being as originally specified. The gG-specific primer sequences have been reported elsewhere (47). A TaqMan PCR Core kit (Applied Biosystems) was used, and the samples were run on a Bio-Rad iCycler. A standard curve, generated by amplification of HSV DNA ranging from 5 to 5 × 107 copies per 100 ng of normal mouse DNA, was linear throughout the range. All standards were in triplicate, all samples were in duplicate, and PCR standard interassay correlation coefficient variability was less than 0.7%.
Determination of NO levels in macrophage cultures.
Resident peritoneal exudate (PE) macrophages were obtained by lavage with RPMI medium supplemented with 5% FBS. The cells were washed and plated in 100-cm-diameter tissue culture dish in RPMI medium-10% FBS. On the next day the culture was washed and the adherent cells were removed by scraping in cell dissociation buffer and were replated at a density of 2.5 × 105 cells per well of a 96-well plate. Following treatments to activate the macrophages, NO levels in macrophage culture supernatants were determined as nitrite concentration ([NO]) by using the Greiss reagent and were quantitated by comparison to a standard curve generated by using sodium nitrate (69). Briefly, a 100-μl aliquot of medium from the macrophage cultures was mixed with an equal volume of Greiss reagent (1% sulfanilamide, 0.1% N-[1-napthyl] etheylenediamine dihydrochlororide, and 2.1% phosphoric acid), and after 5 min at room temperature, the absorbance was read at 540 nm. The data presented are averages plus or minus standard errors of the means of duplicate cultures assayed in duplicate and are representative of three to six experiments.
RESULTS
Susceptibility of p55−/− mice to HSV-1 is influenced by variation in genetic background.
In a series of experiments intended to elucidate the role of TNF in HSV infection, we observed high mortality in the null mutant TNFR1 (p55−/−) mice but not in TNFR2 (p75−/−) and, surprisingly, double receptor knockout (p55−/− p75−/−) mice. The LD50 for HSV-1 strain 17+ was determined to be 320 PFU for the p55−/− strain compared to >105 PFU for the control B6 strain and the p75−/− and p55−/− p75−/− mice. Mortality in the p55−/− strain ranged from 20% at 0.1 times the LD50 to 80% at 10 times the LD50, whereas both the p55−/− p75−/− and p75−/− strains challenged in parallel were as resistant as the control B6 strain, with mortality ranging from 0 to 14% at the LD50 of p55−/− mice (Table 1).
TABLE 1.
Anomalous resistance to HSV-1 in TNFR knockout strains
| HSV-1(17+) doseb | Mortality rate (%)a for:
|
|||
|---|---|---|---|---|
| C57BL/6 | p55−/− N5 | p75−/− N5 | p55−/− p75−/− N4 | |
| 0.1× | 0 | 20 | 0 | 0 |
| 1× | 0 | 50 | 0 | 0 |
| 10× | 6 | 80 | 13 | 14 |
Number of mice for each group varied from 5 (0.1× and 1× experiments) to 13 (10× experiments). The percentage of mortality is based on combined results from five experiments.
Viral dose indicated (×) is based on LD50 determined previously for p55−/− N5 mice to be 320 PFU. LD50 for C57BL/6 is > 105 PFU.
Results of experiments with soluble TNFR1 preparations (not shown) did not support our initial hypothesis that TNF signaling via p75 accounted for the discrepancy in mortality comparing p55−/− and p55−/− p75−/− mice. This was because the untreated p55−/− mice used for the soluble-TNFR experiments were unexpectedly as resistant as the B6 control mice. And, rather than ameliorating mortality as anticipated, soluble TNFR1 caused a dose-dependent increase in mortality in both B6 and the resistant p55 mice (unpublished data). We thus became suspicious that differences in genetic background of these null mutant mice might explain the anomalous mortality and account for the loss of phenotype in the p55−/− mice. The various TNFR null mutant lines had been generated conventionally by using 129 ES cells carrying a targeted mutation in either Tnfrsf1a (p55) or Tnfrsf1b (p75). Subsequently the different mutant lines were backcrossed four or five times to the B6 strain, resulting in >95% of their background genes being B6 derived; therefore, B6 mice were used as controls. The p55−/− line that displayed high susceptibility to HSV-induced mortality had been backcrossed five times to the B6 strain (B6p55−/− N5 or p55−/− N5 mice) (48), as had the resistant p75−/− strain (B6p75−/− N5) (46). However, the p55−/− p75−/− double knockout was derived by crossing the p75−/− N5 line to a p55−/− line that was produced entirely in the B6 background by using a B6 ES cell clone harboring a targeted mutation in the p55 gene; the p55−/− p75−/− line was backcrossed four times to give B6p55−/− p75−/− N4 (or p55−/− p75−/−) (46).
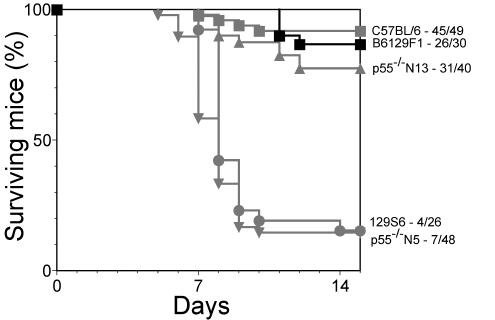
Significantly, we established that the resistant p55−/− mice had been backcrossed a further eight times to the B6 strain (B6p55−/− N13 mice (Tak Mak, personal communication). When p55−/− N13 mice were inoculated with HSV, 31 of 40 (78%) mice survived, compared to only 7 of 48 (15%) of p55−/− N5 mice (P < 0.001). There was a statistically significant difference in mortality when B6 and p55−/− N5 mice but not B6 and p55−/− N13 mice were compared. Based on this result and knowledge of the genetic background of the various TNFR lines, we hypothesized that different alleles of a locus closely linked to tnfrsf1a (p55, TNFR1) on mouse c6 confer resistance or susceptibility to HSV-1-induced mortality depending on whether the allele was derived from the resistant B6 or the susceptible 129 strain background, respectively. We have named the locus on c6 Hrl. This hypothesis predicts that HSV-1-induced mortality in the parental B6 and 129S6 strains should mirror that of the p55−/− N13 and p55−/− N5 strains, respectively. Identical mortality of 85% was observed for the susceptible p55−/− N5 and 129 strains, whereas the resistant p55−/− N13 and B6 strains had mortality rates of 22 and 8%, respectively, that were statistically indistinguishable (P > 0.05, log rank test), consistent with our hypothesis (Fig. 1).
FIG. 1.
HSV-1 induced mortality in the p55−/− congenic lines, the parental B6 and 129 strains and (B6 × 129)F1. Mortality in male (B6 × 129)F1 mice aged 8 to 10 weeks challenged with 10 times the LD50 of HSV-1 is shown in black squares, and mortality data for the parental B6 (grey squares) and 129 strain (grey circles) and the p55−/− Hrl congenic strains N5 (grey upward-pointed triangles) and N13 (grey downward-pointed triangles) are included for comparison purposes. Mice given 10 times the LD50 were monitored for mortality. The log rank test was used to determine P.
Evidence for linkage of Hrl to p55.
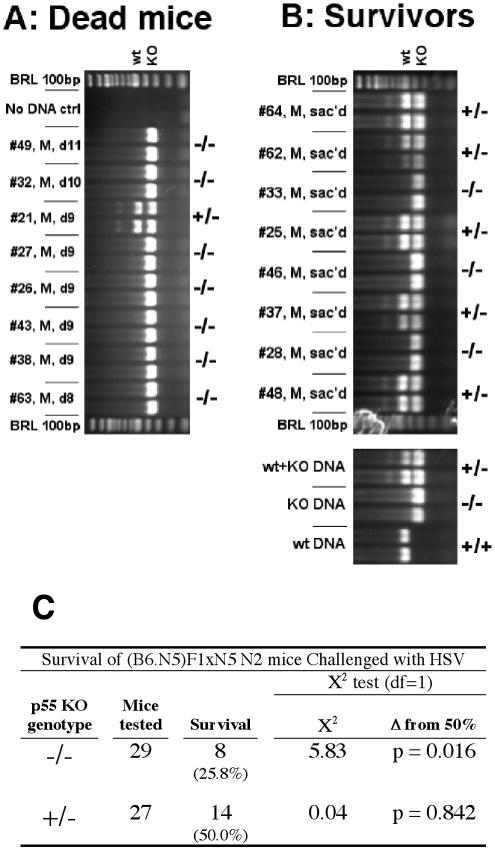
The profound difference in resistance between p55−/− N5 and p55−/− N13 and the observation that p55−/− N5 and the parental 129S6 strain are equally susceptible to HSV-1 mortality (≥87.5% mortality in susceptible animals) provide compelling evidence for linkage of Hrl to the p55 gene on c6. However, since the possibility that Hrl is on some other chromosome could not be excluded, it was important to confirm linkage before proceeding with mapping studies focused on c6. The null hypothesis to test for linkage predicts that, if Hrl were not linked to p55, susceptibility to HSV-1 would segregate independently of genotype at the p55 locus and would occur with equal frequency (50%) in heterozygous and homozygous N2 progeny of a (B6 × p55−/− N5)F1 × p55−/− N5 backcross (64). Conversely, linkage of Hrl to Tnfrsf1a predicts that susceptibility to HSV-1 challenge in the N2 backcross mice should segregate with homozygosity for the p55 null mutation. In experiment 1, 88% (seven of eight) of male N2 mice that died of HSV infection were homozygous p55−/− (Fig. 2A) compared to only 38% (three of eight) of littermates that survived a challenge by two times the LD50 (Fig. 2B). Because the difference in mortality between p55 homozygous and heterozygous null mutant mice was not statistically significant, this result supports but does not prove linkage. In experiment 2, in which 29 homozygous and 27 heterozygous p55 null mutant male N2 backcross mice were challenged at 10 times the LD50, susceptibility (Hrls) segregated with the p55−/− genotype at a frequency that was significantly different from 50% (P = 0.016), which is consistent with linkage of Hrl to p55 (Fig. 2C). Data from male N2 mice were used to assess linkage of Hrl to p55, because the combined effects of enhanced resistance of female mice (see below), together with the potential for sex-biased TNF signaling effects (49, 56), were considered too complex for interpretation of the data from female mice.
FIG. 2.
Linkage of Hrl to the p55 locus. Progeny from a (B6 × p55−/− N5)F1 × p55−/− N5 N2 backcross were challenged with 10 times the LD50 of HSV-1. Susceptibility (A) and resistance (B) correlated with homozygosity and heterozygosity, respectively, for the p55 null mutation (−/− and +/−) as determined by PCR on tail DNA samples, and this correlation was statistically significant (C).
The mode of inheritance of Hrl and sex-biased resistance to HSV.
To determine the pattern of inheritance of Hrl, B6 and 129 mice were crossed and mortality of the (B6 × 129)F1 males was determined after HSV-1 challenge by corneal inoculation. As shown in Fig. 1, 87% (26 of 30) of the (B6 × 129)F1 mice survived, which is indistinguishable from survival of B6 or p55−/− N13 Hrlr mice but is significantly different (P < 0.001) from survival of 129 or p55−/− N5 Hrls mice, observed to be 15% (4 of 26 or 7 of 48, respectively). Identical mortality was seen for female (B6 × 129)F1 mice (not shown); thus, these results indicate autosomal dominant inheritance of Hrl.
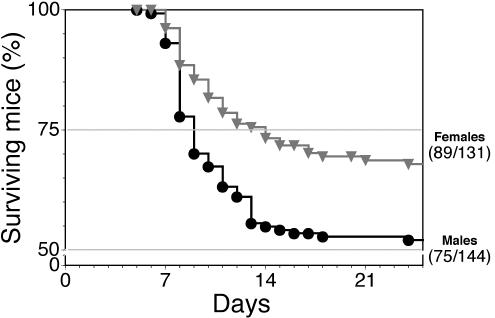
For genetic mapping of Hrl, 275 129 × (B6 × 129)F1 N2 backcross mice were phenotyped for resistance to HSV-1 challenge. A profound sex-based difference in resistance was evident in that 52% (75 of 144) of male mice, compared to 68% (89 of 131) of female mice, were resistant to HSV-1-induced mortality (Fig. 3). Mortality in male mice was not significantly different from 50% (P = 0.80), but it was significantly different from 75% (P < 0.0001), which is consistent with a single locus determining resistance. In contrast, mortality in female mice was significantly different from 50% (P < 0.0001) but not from 75% (P = 0.43), which would be expected if at least two loci were responsible for resistance in female mice. The second locus was named the sex modifier locus (Sml), and sex-based differences in innate resistance to HSV-1 were ascribed to Sml functioning to augment resistance in susceptible female mice that are genotypically Hrls.
FIG. 3.
Resistance to HSV-1 is sex biased. A total of 275 male and female 129 × (B6 × 129)F1 N2 backcross mice were challenged with 10 times the LD50 of HSV-1 and were monitored for mortality and symptoms of encephalitis. Female (grey triangles) mice were approximately 50% more resistant (P = 0.018) to HSV-1 than were male mice (black circles).
Hrl also confers resistance to HSV-2.
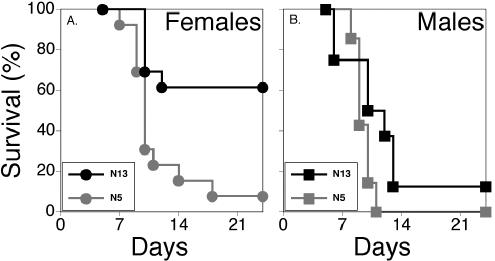
To determine whether Hrl mediates resistance to the closely related HSV-2, p55−/− N5 and p55−/− N13 mice were challenged with the highly virulent MS strain. A significantly higher mortality rate was observed in p55−/− N5 mice than in p55−/− N13 female mice (P = 0.0022) (Fig. 4A), but for the more susceptible male mice, the differential susceptibility between N5 and N13 mice was obscured by excessive mortality in male mice that were more susceptible to HSV (P = 0.081) (Fig. 4B). It is interesting that a sex difference in resistance was observed after challenge of the p55−/− Hrl congenic lines with HSV-2, most clearly evidenced by the significantly different mortality rates of male and female N13 mice (P = 0.0293) (Fig. 4). Although the same trend was evident for N5 mice challenged with HSV-2, the greater overall susceptibility of these mice tended to obscure sex differences (P = 0.154). Similarly for HSV-1, mortality was either too high, as in the case of p55−/− N5, or too low, as in the case of p55−/− N13, to reveal significant sex differences in mortality, although the tendency for greater resistance in females was evident (not shown). It remains to be determined if, as suggested by these results, sex differences in the outcome of infection are more pronounced for HSV-2 than for HSV-1. Thus, Hrl confers resistance to HSV-2 as expected, and resistance is sex biased in favor of females as shown for HSV-1 challenge of 129 × (B6 × 129)F1 N2 mice in Fig. 3 and as previously reported (26).
FIG. 4.
Hrl mediates resistance to HSV-2. p55−/− Hrl congenic N5 and N13 mice were challenged with 3,200 PFU of the virulent MS strain of HSV-2 and were monitored for symptoms of encephalitis and mortality. Mortality for females is shown in panel A and for males in panel B.
Hrl is distinct from Cmv1, the gene that determines resistance to MCMV.
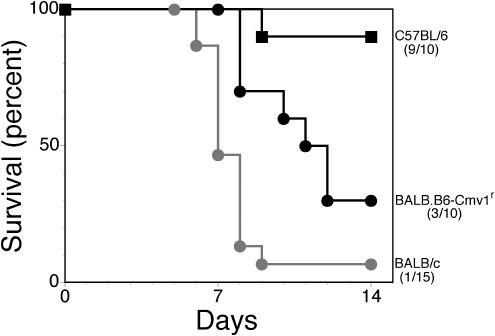
The major NK cell complex (NKC), where Cmv1 (the gene responsible for resistance to MCMV) is encoded, lies immediately distal of p55 (62). Several groups reported recently that Cmv1 encodes Klra8 (formerly Ly49H), an NK cell-activating receptor expressed by the resistant B6 strain but not the susceptible BALB/c or 129 strain (10, 34). Several earlier studies demonstrated that NK cells mediate resistance of B6 mice to MCMV infection, and considering that NK cells have also been implicated in resistance to human CMV and HSV, it was important to test whether Hrl and Cmv1 were distinguishable. Challenge of BALB/c and B6 mice and the C.B8-Klra8Cmv1-r/Uwa congenic strain, which expresses the B6-derived resistance allele of Cmv1 with a lethal dose of HSV-1, resulted in mortality only in the BALB/c and Cmv1r strains, as shown in Fig. 5. BALB/c (grey circles) and C.B8-Klra8Cmv1-r/Uwa (black circles) mice were both significantly more susceptible to HSV-1 than were B6 (squares) mice (P < 0.001 and P = 0.0092, respectively). Interestingly, the C.B8-Klra8Cmv1-r/Uwa congenic strain was more resistant than the parental BALB/c strain (P = 0.0037). These results show that Cmv1 does not confer resistance to HSV-1 induced mortality. Additionally, challenge of the p55−/− Hrl congenic lines, N5 and N13, and the parental 129S6 and B6 strains with different doses of MCMV resulted in mortality only in the susceptible 129 strain (Table 2). The LD50 for 129 mice (1.1 × 105 PFU) was at least 12-fold lower than for the p55−/− Hrl congenic lines and the resistant B6 strain (>1.3 × 106 PFU), which demonstrates that Hrl does not confer resistance to MCMV infection. These results establish unequivocally that Hrl and Cmv1 are distinct loci on c6 that mediate resistance to HSV-1 and MCMV, respectively.
FIG. 5.
Cmv1 does not mediate resistance to HSV-1. Mortality in the C.B8-Klra8Cmv1-r/Uwa congenic strain was compared to that of the resistant B6 and susceptible BALB/c parental strains after challenge with 10 times the LD50 of HSV-1. BALB/c (grey circles) and C.B8-Klra8Cmv1-r/Uwa (black circles) animals are both susceptible to HSV-1, compared to C57BL/6 (black squares) mice.
TABLE 2.
p55−/− Hrl congenic strains are resistant to MCMV infection
| Virus dose (PFU)b | Mortalitya rate for:
|
|||
|---|---|---|---|---|
| 129S6 | p55−/− N5 | p55−/− N13 | C57BL/6 | |
| 1.3 × 106 | NDc | ND | ND | 0/5 |
| 6.4 × 105 | 4/5 | 0/6 | 0/5 | 0/5 |
| 3.2 × 105 | 4/5 | 0/5 | 0/5 | 0/5 |
| 1.6 × 105 | 4/5 | 0/5 | 0/4 | 0/5 |
| 0.8 × 105 | 0/5 | 0/5 | 0/3 | ND |
LD50 for 129S6 male mice = 1.1 × 105 PFU and > 1.3 × 106 PFU for the other strains tested.
Indicated dose administered i.p.
ND, not done.
A genetic map for Hrl.
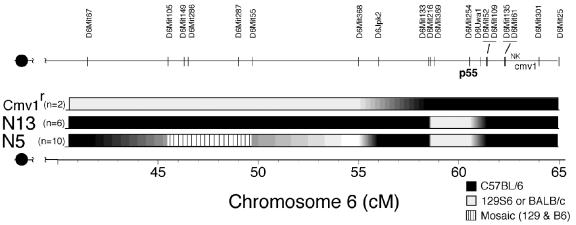
We used PCR amplification of simple-sequence-length polymorphic DNA markers capable of discriminating B6 from 129 sequences to construct a genetic map of Hrl on mouse c6 by using classic linkage analysis. The approach involves correlating DNA markers with resistance in a 129 × (B6 × 129)F1 N2 backcross population, as well as in the p55−/− and BALB × B6Cmv1r congenic strains (54). Preliminary MapManager QTX linkage analysis of phenotypic and genotypic data generated from a subset of the 275 N2 mice gave the highest logarithm of odds (LOD) score for Hrl being located centromeric to p55, with a lower score suggesting a telomeric location (not shown). Such “end effects” may be the consequence of using a limited data set that failed to produce significant correlations. Nonetheless, the predicted proximal location (with a higher LOD score) for Hrl fits fairly well with the low-resolution genetic map presented in Fig. 6, which was derived from analysis of the p55−/− and C.B8-Klra8Cmv1-r/Uwa congenic strains. The stippled region extending from roughly 40 to 45 centimorgans defines a mosaic region, where individual p55−/− N5 mice expressed either B6 or 129 or both marker alleles. The high penetration of the resistance and susceptibility phenotypes clearly excludes Hrl being encoded in the mosaic region. Genotyping of C.B8-Klra8Cmv1-r/Uwa mice in the interval spanning the major NKC revealed that B6 sequences extend approximately 2 centimorgans beyond D6Mit254 (p55), the previously mapped limit (61, 63). Since Hrl cannot lie within any region that is homozygous for B6 or 129 DNA sequences, this leaves the interval extending from approximately 50 to 55 cM as the favored candidate region for encoding Hrl (Fig. 6).
FIG. 6.
A genetic map for Hrl. Confirmed polymorphic markers spanning 40 to 65 centimorgans of mouse chromosome 6 that were used to delineate the parental origin of genetic material in the p55−/− Hrl congenic N5 and N13 strains and the C.B8-Klra8Cmv1-r/Uwa congenic strain. Chromosome segments are shaded black for B6 derived, light grey for 129 or BALB/c derived, and striped for mosaic regions present in the three strains. The locations of p55, the major NK complex (NK), and the murine cytomegalovirus resistance (Cmv1) loci are indicated. DNA marker locations were retrieved from http://www.informatics.jax.org/searches/marker_form.shtml.
Phenotypic characteristics of Hrl.
Selected virological and immunological parameters were monitored in control and TNFR knockout mice during the acute and latent stages of HSV infection. The goal was to discern phenotypes that were differentially expressed in the p55−/− N5 and p55−/− N13-infected, Hrl congenic strains, as identification of phenotypes regulated by Hrl might provide insight as to the function of Hrl and the cell types in which it might be expressed.
More pronounced weight loss in susceptible than in resistant mouse strains.
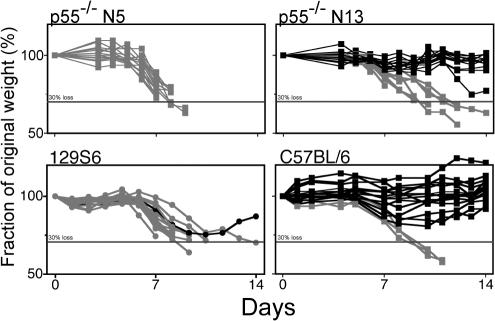
During acute-stage infection with HSV-1, weight loss began earlier and was more rapid for susceptible p55−/− N5 and 129S6 mice than for resistant p55−/− N13 and B6 mice when it occurred, which correlates with the susceptible strains dying more rapidly than the resistant strains (Fig. 7). In fact, for the majority of p55−/− N13 and B6 mice, there was a tendency for weight gain during the acute phase of infection, which attests to their resistance to HSV-1. An important observation was that weight loss in excess of 30% of initial body weight (day 0) was an excellent prognostic indicator of death; only 2% (3 of 150) of mice that lost more than 30% of their body weight subsequently recovered and survived. By utilizing the criterion of weight loss in excess of 25% of initial body weight in conjunction with clinical signs of encephalitis, it was possible to identify and euthanatize HSV-1-infected mice that would otherwise die from encephalitis, thus avoiding unnecessary suffering for terminally ill mice.
FIG. 7.
HSV-induced weight loss in susceptible and resistant mouse strains. HSV-infected animals were monitored daily for weight loss. Individual mice are shown and data are given as percentage of original (preinoculation) weight for each of four strains used. Individual mice that died from infection or were euthanatized due to symptoms of encephalitis are shown as grey symbols and lines. The horizontal line in each panel indicates 30% body weight loss. Data shown are for 14 to 19 mice per group and are representative of results from three experiments.
Necropsy HSV titers in individual mice do not correlate with mortality.
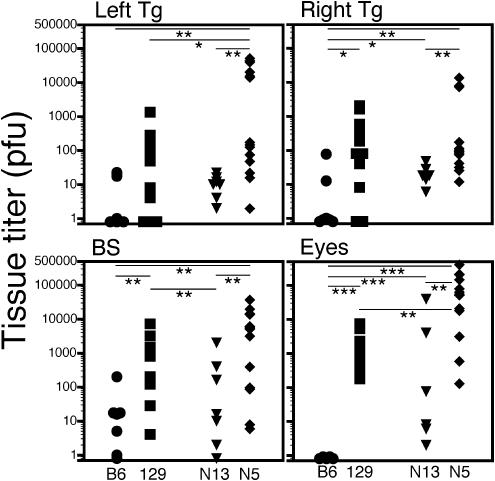
Mice that either died or were euthanatized because of impending death due to encephalitis were necropsied and HSV-1 titers were determined in the eyes, ganglia, and brain stem. Most striking was the complete absence of virus from the eyes of B6 mice compared to the eyes of p55−/− N13 mice, even though these mice were equally resistant to HSV-1 (Fig. 8). The relative ratio of HSV titers in the eyes and Tg comparing B6 to p55−/− N13 reflects control of HSV replication by TNF signaling via p55, whereas the ratio of titers comparing p55−/− N5 and p55−/− N13 reflects control mediated by the different alleles of Hrl. The observation of significant differences in brain stem titers seen in comparison of p55−/− N5 and p55−/− N13 mice but not B6 and p55−/− N13 mice suggests that Hrl is involved in control of HSV in the brain stem while signaling via p55 is not. It has been reported that multiple loci are involved in control of HSV infection in the nervous system (30), and our data suggest that Hrl and p55 are two loci involved in control of HSV-1 in the eye and Tg, whereas only Hrl is involved in the brain stem. Although, on a population basis, titers in the tested tissues tended to be higher in susceptible than in resistant mouse strains, titers in the ganglion or brain stem of individual mice did not correlate with mortality, even though death resulted from encephalitis involving the CNS (55). An important corollary of these results is that HSV titers in the ganglion or brain stem cannot be used as a surrogate marker to phenotype mice as susceptible or resistant in strategy to genetically map Hrl, since classical linkage analysis depends on correlations between the phenotypes and genotypes of individual mice.
FIG. 8.
HSV-1 titers in necropsy tissue samples. Necropsy tissues were collected from mice that died after ocular inoculation or were euthanatized because of severe encephalitis. HSV-1 load in eye, Tg, and brain stem tissues was determined by plaque assay on CV-1 cells. Parental strains (B6 circles, and 129, squares) are shown in black, and the p55−/− congenic strains are shown in grey (p55−/− N13, triangles; and p55−/− N5, diamonds). Eye titers are the sum of left and right eyes. Time of death ranged from days 5 to 14, with the majority of susceptible (129 and p55−/− N5) mice usually dying between days 7 and 10. No correlation was seen between time of death and HSV titer. Horizontal bars with asterisks above represent P for comparison of the indicated mouse groups; *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Greater HSV shedding in the tear film of p55−/− N5 mice than in that of N13 mice.
Determination of the presence of HSV-1 in corneal tear film swabs taken daily from day 1 through day 9 postinfection showed that the frequency of shedding and the amount of virus shed were greater for p55−/− N5 than for p55−/− N13 mice, with a tendency toward prolonged shedding for the p55−/− N5 mice (Table 3). Only 1 of 12 p55−/− N13 mice compared to 10 of 12 p55−/− N5 mice shed virus on 2 or more consecutive days. In a comparison of p55−/− N13 and p55−/− N5 mice, total shedding was demonstrated in 13 of 57 and 44 of 57 possible shedding opportunities, respectively, which demonstrates more stringent control of HSV-1 in the resistant p55−/− N13 mice. Given that virus shed at later times postinfection is thought to emanate from virus replicated in the ganglion that is subsequently seeded to the cornea, the data in Table 3 suggest that HSV-1 is cleared from ganglia of the two strains with equivalent efficacy, as the duration of shedding was not different.
TABLE 3.
HSV shedding in tear film of p55−/− micea
| Mouse | Shedding status at different times after infectionb or total of shedding mice after given day
|
No. of total shedding mice/no. of shedding opportunities | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | ||
| N13-1 | +c | ++ | NDd | + | + | + | + | − | − | 7/8 |
| N13-2 | − | + | ND | − | − | + | − | − | − | 2/8 |
| N13-3 | − | − | ND | − | − | − | − | − | − | 0/8 |
| N13-4 | − | − | ND | − | − | − | − | Euthe | 0/6 | |
| N13-5 | + | − | ND | − | − | − | + | Euth | 2/6 | |
| N13-6 | − | − | ND | − | − | − | Euth | 0/6 | ||
| N13-7 | − | − | ND | − | Euth | 0/3 | ||||
| N13-8 | − | − | ND | − | Euth | 0/3 | ||||
| N13-9 | + | − | ND | − | Euth | 1/3 | ||||
| N13-10 | − | − | ND | Euth | 0/2 | |||||
| N13-11 | + | − | ND | Euth | 1/2 | |||||
| N13-12 | − | − | ND | Euth | 0/2 | |||||
| No. of positive N13 mice/total of N13 mice | 4/12 | 2/12 | N/Af | 1/9 | 1/6 | 2/6 | 2/6 | 0/3 | 0/3 | 13/57 |
| N5-1 | − | − | ND | ++ | + | − | − | − | − | 3/8 |
| N5-2 | + | + | ND | + | + | + | + | − | − | 6/8 |
| N5-3 | + | + | ND | + | − | ++ | − | + | − | 6/8 |
| N5-4 | + | + | ND | + | − | − | + | Euth | 4/6 | |
| N5-5 | + | − | ND | ++ | + | + | − | Euth | 5/6 | |
| N5-6 | + | +++ | ND | + | − | − | + | Euth | 6/6 | |
| N5-7 | − | +++ | ND | − | Euth | 3/3 | ||||
| N5-8 | + | ++ | ND | + | Euth | 4/3 | ||||
| N5-9 | − | ++ | ND | + | Euth | 3/3 | ||||
| N5-10 | − | − | ND | Euth | 0/2 | |||||
| N5-11 | + | ++ | ND | Euth | 3/2 | |||||
| N5-12 | − | − | ND | Euth | 0/2 | |||||
| No. of positive N5 mice/total of N5 mice | 7/12 | 8/12 | N/A | 8/9 | 3/6 | 3/6 | 3/6 | 1/3 | 0/3 | 44/57 |
Right eyes were swabbed with moistened alginate swabs (HBSS + 2% calf serum) that were immediately plated on RS cell monolayers seeded 24 h earlier.
Mice were infected with 320 PFU of HSV-1(17+) by corneal scarification in the right eye.
Scores are indicated as + for 1 to 49 plaques, ++ for 50 to 200 plaques, and +++ for >200 plaques. −, negative.
Not done.
Mouse euthanatized for use in a separate experiment.
Not applicable.
p55−/− N5 and p55−/− N13 macrophages differ in NO production in response to LPS or IFN-γ.
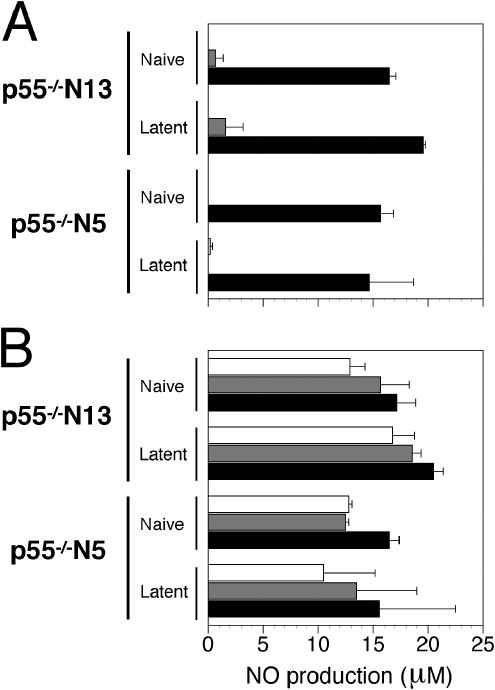
Depletion of macrophages in resistant B6 and p55−/− N13 mice resulted in increased mortality after HSV-1 infection (unpublished results), attesting to the importance of macrophages in resistance to HSV-1 infection (37). It was of interest, therefore, to determine possible differences in macrophage function between p55−/− N5 and p55−/− N13 mice. This was accomplished by assessing NO production by PE macrophages from uninfected or latently infected mice stimulated with lipopolysaccharide (LPS) or IFN-γ. There was no significant difference in NO production in overall comparisons (n = 6) of p55−/− N5 uninfected macrophages and infected macrophages (P = 0.37) or in comparisons of p55−/− N5 and p55−/− N13 uninfected macrophages (P = 0.11) (Fig. 9). However, differences in NO production were significant in comparison of macrophages from naive and latent p55−/− N13 mice (P = 0.013) and also when comparing macrophages from latent p55−/− N5 and p55−/− N13 mice (P = 0.014) (Fig. 9). These differences were attributable to the greater responsiveness of macrophages from latently infected p55−/− N13 mice when HSV-1 was present in the in vitro culture, as P was > 0.25 without HSV-1 for both comparisons above and P < 0.01 when HSV-1 was present (n = 3). These differences in the response of N5 and N13 macrophages were determined by using the Student paired t test (two-tailed) to compare overall NO production from macrophages within strains (with or without HSV-1) and across strains per treatment set (IFN-γ or LPS). The enhanced responsiveness of PE macrophages from latently infected p55−/− N13 mice to activating stimuli (IFN-γ, LPS, or HSV) was not due to contamination with virus-specific memory T cells, as the adherent macrophage cultures were extensively washed prior to stimulation with IFN-γ/LPS and infection with HSV-1. In Fig. 9 it can be seen that HSV-1 synergizes with IFN-γ/LPS to augment NO production by PE macrophages from uninfected or latently infected mice, as previously reported by Paludan and colleagues for uninfected macrophages infected with HSV-2 (44). However, in vivo, infection of PE macrophages is not expected for corneally inoculated mice; thus, how prior HSV-1 infection of mice can lead to enhanced macrophage NO production in response to IFN-γ, LPS or HSV-1 infection in vitro is not understood at present. The observed differences in NO production by PE macrophages from latently infected p55−/− N5 and N13 mice suggest involvement of Hrl.
FIG. 9.
Differences in NO production comparing p55−/− N5 and p55−/− N13 macrophages. Peritoneal macrophages from naïve and latently infected mice (four mice per group) seeded at a density of 2.5 × 105 cells per well in a 96-well dish were cultured overnight with IFN-γ (10 U/ml) to activate the cells. The next morning, the cultures were mock infected (A) or were infected with HSV-1 (multiplicity of infection = 2) (B). After removal of virus inoculum, the cultures were treated with medium alone, IFN-γ (20 U/ml), or LPS (100 ng/ml), and 20 h later culture supernatants were assayed for NO production by using the Greiss reaction. The data presented are averages plus or minus standard errors of the means of two or three experiments for the different mouse groups.
Impaired in vitro reactivation of latent HSV-1 in ganglia of p55−/− N5 mice.
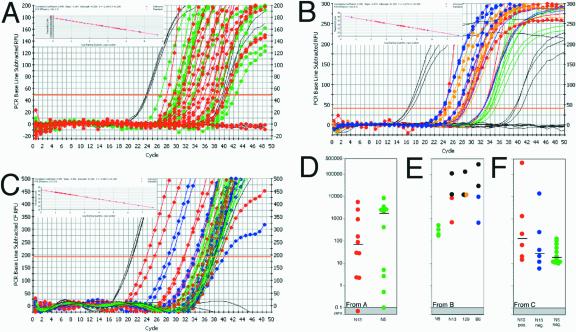
To assess in vitro reactivation of latent HSV-1, ganglia taken from mice sacrificed on days 28 to 30 postinfection were cultured in vitro for 5 days before assays of ganglion homogenate supernatants for infectious virus by plaque assay on RS cells. HSV-1 was efficiently reactivated in ganglia from all of the mouse strains except for p55−/− N5 (Table 4). Impaired reactivation in ganglia of p55−/− N5 mice could have resulted from a failure to establish latent infections in surviving mice or, alternatively, to the load of latent viral genomes in latently infected ganglia being below some critical threshold required for efficient reactivation (60). To discriminate among these possibilities, DNA extracted from ganglion homogenate pellets was assayed for HSV-1 gD sequences by semiquantitative DNA PCR (26). When p55−/− N13 and p75−/− ganglia that reactivated were compared to p55−/− N5 ganglia that did not, the expectation was for a significantly greater HSV DNA load in the former as a result of genome amplification by replication during the 5 days of in vitro explant culture. However, PCR analysis showed that HSV DNA was present at similar levels in p55−/− N13 and p75−/− ganglia that reactivated and p55−/− N5 ganglia that did not reactivate (data not shown). As this result implies that reactivation was either blocked after viral DNA synthesis or, alternatively, that latency was established at high copy numbers in N5 ganglia, it was important to accurately measure HSV-1 DNA levels in individual nonreactivated (baseline) and in vitro reactivated ganglia; this was done by quantitative TaqMan PCR assays for HSV-1 gG sequences (47). No significant differences in baseline HSV-1 DNA levels were found in comparisons of p55−/− N5 and N13 ganglia from mice inoculated at 0.2 times the LD50 (Fig. 10A); the average genome copy number for N5 ganglia was 2,596 ± 706, compared to 2,070 ± 1,271 for N13 ganglia. Therefore, impaired reactivation in p55−/− N5 mice is apparently not due to latency being established with subcritical levels of HSV-1 DNA compared to p55−/− N13 ganglia. Additionally, compared to nonreactivated ganglia (Fig. 10A), the HSV-1 genome copy number was not drastically increased in p55−/− N13 and 129 ganglia from mice inoculated at the LD50 or 0.2 times the LD50 (Fig. 10B and C, respectively), in which HSV-1 was reactivated by in vitro explant culture. In general, the HSV genome copy number tended to be lower in p55−/− N5 ganglia than in p55−/− N13 or 129 ganglia subjected to in vitro reactivation (Fig. 10B and C). However, the significance of this observation is at present unclear, as there were p55−/− N13 and 129 ganglia that failed to reactivate despite having genome copy numbers higher than for N5 and, conversely, ganglia with lower genome copy numbers than for N5 that did reactivate. Thus, it appears that the quantity of total latent HSV-1 DNA per ganglion is not strictly correlated with the propensity for reactivation. Studies in progress are aimed at discerning the basis for impaired reactivation of HSV-1 in p55−/− N5 ganglia compared to that in p55−/− N13 ganglia.
TABLE 4.
Impaired HSV-1 reactivation in p55−/− N5 micea
| Inoculum or total | Tissueb | No. of positive results/no. of samples for:
|
|||||
|---|---|---|---|---|---|---|---|
| C57BL/6 | p55−/− p75−/− | p75−/− | p55−/− N13 | p55−/− N5 | 129S6 | ||
| 0.1 × LD50b (32 PFU) | Right Tg | 2/10 | 2/3 | 2/4 | 6/12 | 0/16 | ND |
| LD50 (320 PFU) | Right Tg | 11/14 | 3/3 | 5/5 | 2/4 | 0/7 | 4/6 |
| 10 × LD50 (3,200 PFU) | Right Tg | ND | ND | ND | 4/4 | ND | ND |
| Totalc | Right Tg | 13/24 | 5/6 | 7/9 | 12/20 | 0/23 | 4/6 |
| Overall % | Right Tg | 54% | 83% | 78% | 60% | 0% | 67% |
Tg were cultured for 5 days, homogenized, and freeze-thawed three times, and cell debris was pelleted. Samples were scored positive for reactivation if the homogenate supernatant resulted in plaques on RS cells. ND, not determined.
LD50 for p55−/− N5 as reference.
Total gives number of positive ganglia/number of ganglia.
FIG. 10.
Quantitative PCR for HSV DNA load in latently infected Tg. Each Tg DNA sample (100 ng) was analyzed in duplicate by TaqMan real-time PCR as described in Materials and Methods. Standards ranged from 5 to 5 × 107 copies of HSV virion DNA in 100 ng of normal mouse DNA. Raw data shown in panels A, B and C are summarized in panels D, E and F, respectively, as HSV-1 genome copies present in individual Tg. For graphs A, B and C, flanking standards and blanks are shown as black lines without symbols; standard curves are shown as inset with standards in blue and samples in red. (A) Baseline (nonreactivated) PCR on 0.2 times the LD50 Tg: N5 in green and N13 in red. (B) Reactivated PCR on the LD50 Tg: N5, none reactivated (green lines); N13, two did not (red lines), two reactivated (25, 28; with symbols); and 129, two did not (orange lines), two reactivated (24, 28; with symbols), two did not (blue lines), two reactivated (24, 26.5; with symbols). (C) Reactivated PCR on 0.2 times the LD50 Tg: N5, none reactivated (shown in green lines without symbols); N13, 6 of 12 reactivated, shown as red circles; and N13, 6 of 12 did not reactivate, shown as blue circles. (D) Baseline (nonreactivated) N13 in red and N5 in green. (E) After 5 days of Tg explant culture, reactivation-positive Tg for each strain are shown in black while those that did not reactivate are shown in color (N5 is green, N13 is red, 129 is yellow, and B6 is blue). (F) After 5 days of Tg explant culture, reactivation-positive N13 Tg are shown in red, and reactivation-negative N13 and N5 Tg are shown in blue and green, respectively.
DISCUSSION
The results presented here establish that Hrl, a locus closely linked to Tnfrsf1a (p55) on mouse c6, functions to confer resistance or susceptibility to HSV-1 infection, depending on whether the allele derives from the resistant B6 or susceptible 129 strain background, respectively. Hrl behaves as a dominant autosomal gene that alone determines resistance in male mice, while an additional modifier locus, Sml, functions to augment resistance in susceptible female mice. Sml might encode a sex hormone or a sex hormone receptor that effectively enhances resistance of female mice expressing Hrls. Alternatively, Sml might encode a gene whose activity is sex biased or Sml might directly affect replication of HSV-1 in female but not male mice. Considering that roughly 50% of Hrls mice survive, it may be that, of two Sml alleles, only one is capable of mediating enhanced resistance, or if Sml is hormonally related, it could be that its effects may be restricted to females in a particular phase of the estrous cycle.
Earlier studies of genetic resistance to HSV infection demonstrated involvement of either one or two major dominant autosomal loci in resistance of inbred mouse strains to mortality induced by cutaneous or i.p. inoculation of HSV, respectively (37, 38, 66). Our results differ in one important aspect, which is that analysis of the various backcrosses in these prior studies failed to reveal sex-based differences in resistance. This discrepancy could arise because too few backcross mice were analyzed and/or because the effect of Sml varies with different mouse strain backgrounds; earlier crosses were B6 × A/J, B6 × BALB/c, B10 × A/J, and B10 × BALB/c (37, 66), whereas we used B6 × 129 crosses. Sex-based differences in innate resistance to HSV-1 reported here provide a genetic basis for rationalizing the observed greater susceptibility, in terms of mortality and in vivo reactivation of HSV-1, of male than of female mice, that was previously reported (26). Sex-based differences in mortality were accentuated for HSV-2 compared to HSV-1 infection of p55−/− N5 and N13 Hrl congenic strains for unknown reasons, but differential sensitivity of HSV-1 and HSV-2 replication to sex hormone effects is one possible explanation. The potential benefit of identifying and characterizing Sml functionally is evident from results of recent clinical trails of a gD-2 subunit vaccine, which demonstrated protection against both HSV-2 infection and disease in women but not in men who were seronegative for both HSV-1 and HSV-2 (68).
A comparison of HSV-1 infection in the p55−/− N5 and N13 lines revealed several phenotypes that were differentially expressed, suggesting regulation by Hrl; these included differences in necropsy HSV titers in the eyes, in vitro NO production by PE macrophages, and in vitro reactivation of latent HSV-1. While the p55−/− N5 mice were impaired relative to N13 mice for all the aforementioned traits, it is instructive to focus on reactivation, as the data are particularly informative. HSV was efficiently reactivated in vitro in ganglia from male B6, 129, p75−/−, p55p75−/−, and p55−/− N13 but not p55−/− N5 latently infected mice sacrificed on days 28 to 35 postinfection; insufficient data are available for female mice at present. Based on the levels of latent HSV-1 DNA in ganglia determined by quantitative TaqMan PCR for HSV gG sequences, latency was established with equal efficiency in p55−/− N5 and p55−/− N13 ganglia. However, the quantitative PCR results do not exclude establishment of latency, with an increased frequency of neurons harboring amounts of HSV-1 DNA below a critical threshold required for efficient reactivation in p55−/− N5 ganglia compared to p55−/− N13 ganglia as the reason for impaired reactivation in p55−/− N5 ganglia (60). Experiments to investigate this possibility are in progress. Counterintuitively, the HSV genome copy number in ganglia reactivated in vitro was not dramatically higher than in nonreactivated ganglia. Considering the potential for extensive HSV-1 replication during 5 days of in vitro ganglion explant culture, this result suggests that only a subset of latently infected neurons reactivated in vitro or alternatively that only a fraction of the latent genomes can reactivate.
Although the p55−/− congenic mice express different alleles of Hrl, this cannot account for impaired reactivation in p55−/− N5 mice, since reactivation is normal in parental 129 mice that are also genotypically Hrls like p55−/− N5 mice. Therefore, we speculate that for efficient reactivation Hrl needs to interact with some unknown gene(s) or that Hrls may be dependent on p55 signaling for efficient reactivation. Such interactions may be blocked in p55−/− N5 mice, as in only this strain is there discordance between the expressed Hrl allele and the overall genetic background. Thus, the 129-derived Hrls allele is expressed in an overall B6 background in p55−/− N5 mice, while the B6-derived Hrlr allele is expressed in the B6 background in p55−/− N13 mice. Studies are in progress to determine the validity of this mismatch hypothesis in explaining impaired reactivation in p55−/− N5. Nonetheless, to our knowledge, this is the first report of a genetic locus that influences HSV-1 reactivation.
Mapping studies, involving correlation of genotypic and phenotypic data obtained from analysis of HSV-1 and MCMV infection of p55−/− N5 and N13 congenic strains, the C.B8-Klra8Cmv1-r/Uwa congenic strain, and F1 and N2 crosses between the B6 and 129 strains, established (i) that linkage of Hrl to Tnfrsf1a occurs on c6; (ii) that Hrl and Cmv1 are distinct loci; (iii) that Hrl is not encoded in the major NKC locus; (iv) that HSV-1 titers in the ganglion or brain stem of individual mice do not correlate with mortality and thus cannot be used as a surrogate marker for mortality to phenotype individual mice as resistant or susceptible; and (v) that Hrl is located in the interval spanning D6Mit55 and D6Jpk2, representing ∼6 centimorgans of DNA.
There are several genes involved in host immunity within the mapped interval that are candidates for Hrl, including Tnfr superfamily members of unknown function. The Tnfr-related genes are of particular interest, because in studies to be reported elsewhere we showed that TNF, but neither p55 nor p75, is required for protection against HSV-1 (unpublished data). In this context, it is notable that herpesviruses have evolved mechanisms specifically targeting TNF superfamily members as an immune evasion strategy (7, 8). Thus, expression of HveA (HveM), a recently described Tnfr superfamily member, on activated T cells facilitates HSV-1 infection, resulting possibly in cell death or impaired functionality that could compromise the host adaptive response. Furthermore, macrophages have frequently been implicated in resistance to HSV infection (18, 27, 33, 37, 57), which is consistent with them being the major producer of TNF and high-output NO, effector molecules with documented beneficial and/or detrimental effects in different HSV-1 infection models (1, 23). Resistance of B6 mice to MCMV infection was recently shown to depend on expression of Klra8, an activating NK cell receptor encoded by Cmv1, on NK cells. Remarkably, enhanced NK cell lysis of infected cells is triggered by an MCMV-encoded protein ligand for Klra8 (4, 10, 20). A protective role for NK cells in resistance to HSV-1 infection was inferred from observations that in vivo elimination of NK cells by treatment of mice with anti-NK1.1 antibodies resulted in a significant increase in mortality (2, 25, 45). However, results from these in vivo neutralization experiments require reevaluation in light of recent reports that NK1.1 expression is upregulated on virus specific CD4+ and CD8+ T cells (5, 29, 67). Hence, the importance of NK cells in resistance to HSV-1 remains unresolved, and in this context it is important to note that our data exclude the NKC as a major contributor to resistance.
Pereira et al. (45) reported recently that a NKC-linked locus, Rhs1, was responsible for rapid control of acute HSV-1 ganglionic infection and for an increased frequency of latently infected neurons. After flank inoculation, HSV-1 replication was restricted in dorsal root ganglia in resistant B6 mice expressing Rhs1r compared to susceptible BALB/c mice that express Rhs1s, as assessed by the extent of zosteriform skin lesions at day 5 postinfection (45). Importantly, analysis of B6 × BALB backcrosses indicated that at least three other genes besides Rhs1 were involved in control of HSV-1 zosteriform spread, which is consistent with results from prior studies showing that multiple genes control HSV-1 ganglionic infection and the establishment of latency (30, 66). Two results suggested that Rhs1 is located on c6 linked to the NKC: (i), compared to BALB/c mice, zosteriform spread was retarded in the C.B8-Klra8Cmv1-r/UWA congenic strain carrying the B6-derived NKC, and (ii), C.B8-Klra8Cmv1-r/Uwa mice were resistant to i.p. challenge with HSV-1 (LD50 > 107 PFU compared to LD50 < 104 PFU for susceptible BALB/c mice). In contrast, we showed that C.B8-KlraiCmv1-r/Uwa mice were susceptible to HSV-1, although slightly more resistant than the BALB/c parental strain (Fig. 5). Introduction of the B6-NKC could explain the slightly increased resistance of C.B8-Klra8Cmv1-r/Uwa mice, as the NKC is highly polymorphic between inbred mouse strains (11). HSV-1 strain differences cannot account for the discrepant mortality results, since the LD50 values for 129 mice inoculated on the cornea with HSV-1(17+) (used in our studies) or HSV-1 SC16 (used in studies by Pereira et al.) were similar, i.e., 330 or 795 PFU, respectively. Differences in inoculation route in the two studies, corneal versus i.p., are a possible explanation, even though earlier studies found that this parameter did not significantly alter the ranking of inbred mouse strains in terms of susceptibility to HSV-1 (38). Although initially identified as one of four genes controlling zosteriform spread, Rhs1 was mapped by determining the pattern of resistance to HSV i.p. inoculation in a panel of B6 and BALB/c background NKC congenic strains. Based on results from HSV-1 challenge of C.B8-Klra8Cmv1-r/Uwa congenic mice and reports that one and two genes, respectively, determine resistance to HSV inoculated peripherally versus i.p. (39), we conclude that Rhs1 is distinct from Hrl. However, the data do not exclude Rhs1 being a linked modifier locus for Hrl.
In summary, Hrl is genetic locus linked to Tnfrsf1a on mouse c6, which has two alleles: Hrlr, which dominantly determines resistance of mice with the B6 background, and Hrls, which confers susceptibility to 129S6 mice and possibly other susceptible strains. There is a strong sex bias to HSV resistance due to effects of Sml, a modifier locus that acts to augment resistance of susceptible female mice. Hrl also affects reactivation of latent HSV as well as aspects of the host immune response, possibly by interaction with some unknown gene(s). Detection of regions of extensive synteny between the mapped Hrl candidate region and several different human chromosomal segments, primarily 3p and 12p, suggests strongly the existence of a human ortholog for Hrl. The eventual identification and characterization of Hrl and Sml will advance understanding of innate resistance to HSV and possibly lead to identification of immune factors that are important for efficient reactivation and hence represent potential therapeutic targets. Eventually, unraveling the interactions between Hrl and Sml will provide a unique opportunity for understanding sex-based differences in HSV-1 infection (26), and this is clearly important for vaccine design (68).
Finally, the results of this study highlight the importance of carefully considering the potentially confounding effects of genetic background on interpretation of results from studies of mice with targeted null mutations, particularly in genes with pleiotropic effects (24, 43, 59). The problem arises because 129-derived ES cells are commonly used for gene targeting and because the targeted mutation is usually transferred to the B6 strain background by repeated backcrossing, which results in the rapid replacement of 129-derived genes (21). However, even after 10 backcrosses, as much as 40 Mb of 129 genetic material can remain linked to the knockout locus (≤0.5% of the genome at N12) and can influence the phenotype of the targeted mutation. An ideal solution to the problem is to produce the targeted mutation in the desired strain background, thus avoiding backcrossing, but presently suitable ES cell lines are available only for the B6 and 129 strains (6).
Acknowledgments
We thank D. Spector and C. Morello (University of California at San Diego) for help with preparation of salivary gland-derived stocks of MCMV.
This work was supported by Public Health Service grant EY-013814 from the National Eye Institute and by a grant from Amgen Inc.
REFERENCES
- 1.Adler, H., J. L. Beland, N. C. Del-Pan, L. Kobzik, J. P. Brewer, T. R. Martin, and I. J. Rimm. 1997. Suppression of herpes simplex virus type 1 (HSV-1)-induced pneumonia in mice by inhibition of inducible nitric oxide synthase (iNOS, NOS2). J. Exp. Med. 185:1533-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler, H., J. L. Beland, N. C. Del-Pan, L. Kobzik, R. A. Sobel, and I. J. Rimm. 1999. In the absence of T cells, natural killer cells protect from mortality due to HSV-1 encephalitis. J. Neuroimmunol. 93:208-213. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad, A., E. Sharif-Askari, L. Fawaz, and J. Menezes. 2000. Innate immune response of the human host to exposure with herpes simplex virus type 1: in vitro control of the virus infection by enhanced natural killer activity via interleukin-15 induction. J. Virol. 74:7196-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arase, H., E. S. Mocarski, A. E. Campbell, A. B. Hill, and L. L. Lanier. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296:1323-1326. [DOI] [PubMed] [Google Scholar]
- 5.Assarsson, E., T. Kambayashi, J. K. Sandberg, S. Hong, M. Taniguchi, L. Van Kaer, H. G. Ljunggren, and B. J. Chambers. 2000. CD8+ T cells rapidly acquire NK1.1 and NK cell-associated molecules upon stimulation in vitro and in vivo. J. Immunol. 165:3673-3679. [DOI] [PubMed] [Google Scholar]
- 6.Banbury Conference on Genetic Background in Mice. 1997. Mutant mice and neuroscience: recommendations concerning genetic background. Neuron 19:755-759. [DOI] [PubMed] [Google Scholar]
- 7.Benedict, C., T. Banks, and C. Ware. 2003. Death and survival: viral regulation of TNF signaling pathways. Curr. Opin. Immunol. 15:59-65. [DOI] [PubMed] [Google Scholar]
- 8.Benedict, C. A., and C. F. Ware. 2001. Virus targeting of the tumor necrosis factor superfamily. Virology 289:1-5. [DOI] [PubMed] [Google Scholar]
- 9.Biron, C. A. 1999. Initial and innate responses to viral infections—pattern setting in immunity or disease. Curr. Opin. Microbiol. 2:374-381. [DOI] [PubMed] [Google Scholar]
- 10.Brown, M. G., A. O. Dokun, J. W. Heusel, H. R. C. Smith, D. L. Beckman, E. A. Blattenberger, C. E. Dubbelde, L. R. Stone, A. A. Scalzo, and W. M. Yokoyama. 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 292:934-937. [DOI] [PubMed] [Google Scholar]
- 11.Brown, M. G., A. A. Scalzo, L. R. Stone, P. Y. Clark, Y. Du, B. Palanca, and W. M. Yokoyama. 2001. Natural killer gene complex (Nkc) allelic variability in inbred mice: evidence for Nkc haplotypes. Immunogenetics 53:584-591. [DOI] [PubMed] [Google Scholar]
- 12.Brutkiewicz, R. R., and R. M. Welsh. 1995. Major histocompatibility complex class I antigens and the control of viral infections by natural killer cells. J. Virol. 69:3967-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantin, E., B. Tanamachi, and H. Openshaw. 1999. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J. Virol. 73:3418-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantin, E., B. Tanamachi, H. Openshaw, J. Mann, and K. Clarke. 1999. Gamma interferon (IFN-γ) receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFN-γ ligand null-mutant mice. J. Virol. 73:5196-5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantin, E. M., R. Eberle, J. L. Baldick, B. Moss, D. E. Willey, A. L. Notkins, and H. Openshaw. 1987. Expression of herpes simplex virus 1 glycoprotein B by a recombinant vaccinia virus and protection of mice against lethal herpes simplex virus 1 infection. Proc. Natl. Acad. Sci. USA 84:5908-5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantin, E. M., D. R. Hinton, J. Chen, and H. Openshaw. 1995. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J. Virol. 69:4898-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, G., and D. V. Goeddel. 2002. TNF-R1 signaling: a beautiful pathway. Science 296:1634-1635. [DOI] [PubMed] [Google Scholar]
- 18.Cheng, H., T. M. Tumpey, H. F. Staats, N. van Rooijen, J. E. Oakes, and R. N. Lausch. 2000. Role of macrophages in restricting herpes simplex virus type 1 growth after ocular infection. Investig. Ophthalmol. Vis. Sci. 41:1402-1409. [PubMed] [Google Scholar]
- 19.Confer, D. L., G. M. Vercellotti, D. Kotasek, J. L. Goodman, A. Ochoa, and H. S. Jacob. 1990. Herpes simplex virus-infected cells disarm killer lymphocytes. Proc. Natl. Acad. Sci. USA 87:3609-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniels, K. A., G. Devora, W. C. Lai, C. L. O'Donnell, M. Bennett, and R. M. Welsh. 2001. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 194:29-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doetschman, T. 1999. Interpretation of phenotype in genetically engineered mice. Lab. Anim. Sci. 49:137-143. [PubMed] [Google Scholar]
- 22.Elliott, R., C. Clark, D. Jaquish, and D. H. Spector. 1991. Transcription analysis and sequence of the putative murine cytomegalovirus DNA polymerase gene. Virology 185:169-186. [DOI] [PubMed] [Google Scholar]
- 23.Fujii, S., T. Akaike, and H. Maeda. 1999. Role of nitric oxide in pathogenesis of herpes simplex virus encephalitis in rats. Virology 256:203-212. [DOI] [PubMed] [Google Scholar]
- 24.Gerlai, R. 1996. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 19:177-181. [DOI] [PubMed] [Google Scholar]
- 25.Ghiasi, H., S. Cai, G. C. Perng, A. B. Nesburn, and S. L. Wechsler. 2000. The role of natural killer cells in protection of mice against death and corneal scarring following ocular HSV-1 infection. Antivir. Res. 45:33-45. [DOI] [PubMed] [Google Scholar]
- 26.Han, X., P. Lundberg, B. Tanamachi, H. Openshaw, J. Longmate, and E. Cantin. 2001. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J. Virol. 75:3048-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heise, M. T., and H. W. Virgin IV. 1995. The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J. Virol. 69:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbein, G., and W. A. O'Brien. 2000. Tumor necrosis factor (TNF)-alpha and TNF receptors in viral pathogenesis. Proc. Soc. Exp. Biol. Med. 223:241-257. [DOI] [PubMed] [Google Scholar]
- 29.Kambayashi, T., E. Assarsson, J. Michaelsson, P. Berglund, A. D. Diehl, B. J. Chambers, and H. G. Ljunggren. 2000. Emergence of CD8(+) T cells expressing NK cell receptors in influenza A virus-infected mice. J. Immunol. 165:4964-4969. [DOI] [PubMed] [Google Scholar]
- 30.Kastrukoff, L. F., A. S. Lau, and M. L. Puterman. 1986. Genetics of natural resistance to herpes simplex virus type 1 latent infection of the peripheral nervous system in mice. J. Gen. Virol. 67:613-621. [DOI] [PubMed] [Google Scholar]
- 31.Kimbrell, D. A., and B. Beutler. 2001. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2:256-267. [DOI] [PubMed] [Google Scholar]
- 32.Kirchner, H., M. Kochen, K. Munk, H. M. Hirt, S. E. Mergenhagen, and D. L. Rosenstreich. 1978. Differences in susceptibility to herpes simplex virus infection of inbred strains of mice. IARC Sci. Publ. 24:783-788. [PubMed] [Google Scholar]
- 33.Kodukula, P., T. Liu, N. V. Rooijen, M. J. Jager, and R. L. Hendricks. 1999. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J. Immunol. 162:2895-2905. [PubMed] [Google Scholar]
- 34.Lee, S. H., S. Girard, D. Macina, M. Busa, A. Zafer, A. Belouchi, P. Gros, and S. M. Vidal. 2001. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat. Genet. 28:42-45. [DOI] [PubMed] [Google Scholar]
- 35.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 36.Lopez, C. 1975. Genetics of natural resistance to herpesvirus infections in mice. Nature 258:152-153. [DOI] [PubMed] [Google Scholar]
- 37.Lopez, C. 1985. Natural resistance mechanisms in herpes simplex virus infections, p. 37-68. In B. Roizman (ed.), The herpesviruses, vol. 4. Plenum, New York, N.Y.
- 38.Lopez, C. 1981. Resistance to herpes simplex virus— type 1 (HSV-1). Curr. Top. Microbiol. Immunol. 92:15-24. [DOI] [PubMed] [Google Scholar]
- 39.Lopez, C. 1980. Resistance to HSV-1 in the mouse is governed by two major, independently segregating, non-H-2 loci. Immunogenetics 11:87-92. [DOI] [PubMed] [Google Scholar]
- 40.Ma, X. 2001. TNF-alpha and IL-12: a balancing act in macrophage functioning. Microbes Infect. 3:121-129. [DOI] [PubMed] [Google Scholar]
- 41.Morello, C. S., L. D. Cranmer, and D. H. Spector. 2000. Suppression of murine cytomegalovirus (MCMV) replication with a DNA vaccine encoding MCMV M84 (a homolog of human cytomegalovirus pp65). J. Virol. 74:3696-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nash, A. A., and P. Cambouropoulos. 1993. The immune response to herpes simplex virus. Semin. Virol. 4:181-186. [Google Scholar]
- 43.Olson, E. N., H. H. Arnold, P. W. Rigby, and B. J. Wold. 1996. Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell 85:1-4. [DOI] [PubMed] [Google Scholar]
- 44.Paludan, S. R., L. Malmgaard, S. Ellermann-Eriksen, L. Bosca, and S. C. Mogensen. 2001. Interferon (IFN)-gamma and herpes simplex virus/tumor necrosis factor-alpha synergistically induce nitric oxide synthase 2 in macrophages through cooperative action of nuclear factor-kappa B and IFN regulatory factor-1. Eur. Cytokine Netw. 12:297-308. [PubMed] [Google Scholar]
- 45.Pereira, R. A., A. Scalzo, and A. Simmons. 2001. Cutting edge: a NK complex-linked locus governs acute versus latent herpes simplex virus infection of neurons. J. Immunol. 166:5869-5873. [DOI] [PubMed] [Google Scholar]
- 46.Peschon, J. J., D. S. Torrance, K. L. Stocking, M. B. Glaccum, C. Otten, C. R. Willis, K. Charrier, P. J. Morrissey, C. B. Ware, and K. M. Mohler. 1998. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 160:943-952. [PubMed] [Google Scholar]
- 47.Pevenstein, S. R., R. K. Williams, D. McChesney, E. K. Mont, J. E. Smialek, and S. E. Straus. 1999. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J. Virol. 73:10514-10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfeffer, K., T. Matsuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73:457-467. [DOI] [PubMed] [Google Scholar]
- 49.Pfeiffer, A., J. Janott, M. Mohlig, M. Ristow, H. Rochlitz, K. Busch, H. Schatz, and E. Schifferdecker. 1997. Circulating tumor necrosis factor alpha is elevated in male but not in female patients with type II diabetes mellitus. Horm. Metab. Res. 29:111-114. [DOI] [PubMed] [Google Scholar]
- 50.Pien, G. C., K. B. Nguyen, L. Malmgaard, A. R. Satoskar, and C. A. Biron. 2002. A unique mechanism for innate cytokine promotion of T cell responses to viral infections. J. Immunol. 169:5827-5837. [DOI] [PubMed] [Google Scholar]
- 51.Posavad, C. M., D. M. Koelle, M. F. Shaughnessy, and L. Corey. 1997. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc. Natl. Acad. Sci. USA 94:10289-10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rath, P. C., and B. B. Aggarwal. 1999. TNF-induced signaling in apoptosis. J. Clin. Immunol. 19:350-364. [DOI] [PubMed] [Google Scholar]
- 53.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 54.Reeves, R. H., and P. D'Eustachio. 1999. Genetic and comparative mapping in mice, p. 71-133. In E. D. G. B. Birren, P. Hieter, S. Klapholz, R. M. Myers, H. Riethman, and J. Roskams (ed.), Genome analysis: a laboratory manual, vol. 4. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 55.Richards, J. T., E. R. Kern, J. C. Overall, Jr., and L. A. Glasgow. 1981. Differences in neurovirulence among isolates of Herpes simplex virus types 1 and 2 in mice using four routes of infection. J. Infect. Dis. 144:464-471. [DOI] [PubMed] [Google Scholar]
- 56.Roberts, C. W., S. M. Cruickshank, and J. Alexander. 1995. Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infect. Immun. 63:2549-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossol-Voth, R., S. Rossol, K. H. Schutt, S. Corridori, W. de Cian, and D. Falke. 1991. In vivo protective effect of tumour necrosis factor alpha against experimental infection with herpes simplex virus type 1. J. Gen. Virol. 72:143-147. [DOI] [PubMed] [Google Scholar]
- 58.Rouse, B. T., and L. A. Babiuk. 1978. Mechanisms of recovery from Herpesvirus infections—a review. Can. J. Comp. Med. 42:414-427. [PMC free article] [PubMed] [Google Scholar]
- 59.Ruuls, S. R., and J. D. Sedgwick. 1999. Unlinking tumor necrosis factor biology from the major histocompatibility complex: lessons from human genetics and animal models. Am. J. Hum. Genet. 65:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawtell, N. M., D. K. Poon, C. S. Tansky, and R. L. Thompson. 1998. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J. Virol. 72:5343-5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scalzo, A., M. Brown, D. Chu, J. Heusel, W. Yokoyama, and C. Forbes. 1999. Development of intra-natural killer complex (NKC) recombinant and congenic mouse strains for mapping and functional analysis of NK cell regulatory loci. Immunogenetics 49:238-241. [DOI] [PubMed] [Google Scholar]
- 62.Scalzo, A. A., N. A. Fitzgerald, A. Simmons, A. B. La Vista, and G. R. Shellam. 1990. Cmv-1, a genetic locus that controls murine cytomegalovirus replication in the spleen. J. Exp. Med. 171:1469-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scalzo, A. A., P. A. Lyons, N. A. Fitzgerald, C. A. Forbes, and G. R. Shellam. 1995. The BALB. B6-Cmv1r mouse: a strain congenic for Cmv1 and the NK gene complex. Immunogenetics 41:148-151. [DOI] [PubMed] [Google Scholar]
- 64.Silver, A. M. 1995. Mouse genetics: concepts and applications. Oxford University Press, New York, N.Y.
- 65.Simmons, A. 1989. H-2-linked genes influence the severity of herpes simplex virus infection of the peripheral nervous system. J. Exp. Med. 169:1503-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simmons, A., and A. B. La Vista. 1989. Neural infection in mice after cutaneous inoculation with HSV-1 is under complex host genetic control. Virus Res. 13:263-270. [DOI] [PubMed] [Google Scholar]
- 67.Slifka, M. K., R. R. Pagarigan, and J. L. Whitton. 2000. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J. Immunol. 164:2009-2015. (Erratum, 164: 3445.) [DOI] [PubMed] [Google Scholar]
- 68.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, G. Dubin, and the GlaxoSmithKline Herpes Vaccine Efficacy Study Group. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652-1661. [DOI] [PubMed] [Google Scholar]
- 69.Stuehr, D. J., S. S. Gross, I. Sakuma, R. Levi, and C. F. Nathan. 1989. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J. Exp. Med. 169:1011-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wallach, D. 1997. Cell death induction by TNF: a matter of self control. Trends Biochem. Sci. 22:107-109. [DOI] [PubMed] [Google Scholar]