Abstract
Recombinant adeno-associated viral (rAAV) vectors are frequently used for gene delivery to the central nervous system and are capable of transducing neurons and glia in vitro. In this study, seven serotypes of a rAAV vector expressing green fluorescent protein (GFP) were characterized for tropism and toxicity in primary cortical cells derived from embryonic rat brain. At 2 days after transduction, serotypes 1 and 5 through 8 expressed GFP predominately in glia, but by 6 days post-transduction expression was neuronal except for AAV5. AAV2 and 9 produced minimal GFP expression. Using cell viability assays, toxicity was observed at higher multiplicities of infection (MOI) for all serotypes except AAV2 and 9. The toxicity of AAV1 and 5-8 affected mostly glia as indicated by a loss of glial-marker immunoreactivity. A frameshift mutation in the GFP gene reduced overall toxicity for serotypes 1, 5 and 6, but not 7 and 8 suggesting that the toxicity was not solely due to the overexpression of GFP. Collectively, a differential tropism and toxicity was observed among the AAV serotypes on primary cortical cultures with an overall preferential glial transduction and toxicity.
Keywords: AAV, adeno-associated virus, gene therapy, serotype, tropism, toxicity, neuron, glia
Introduction
Adeno-associated viral (AAV) vectors are a versatile platform for gene delivery to the central nervous system (CNS) (Gao, Vandenberghe, and Wilson, 2005; McCown, 2005; Tenenbaum et al., 2004; Xiao et al., 1997). An AAV viral genome is approximately 4.7 kilobases (kb) and contains two sets of viral genes, the rep and cap genes important for replicating/packaging the viral DNA and producing the capsid proteins, respectively. The viral genes are flanked by two 145 bp inverted terminal repeats (ITRs) which are required for replication and packaging. Recombinant AAV vectors are most commonly generated by replacing all viral DNA between the ITRs with a transcriptional cassette of less than approximately 5 kb. The resulting construct is cotransfected into HEK293 cells with a plasmid containing helper virus genes and rep/cap contaning plasmid (Xiao, Li, and Samulski, 1998). The resulting rAAV lacks any viral genes and the serotype of the rAAV can be altered by using different rep/cap constructs during packaging.
Currently, over 100 non-redundant genotypes of AAV have been identified (Gao, Vandenberghe, and Wilson, 2005) and offer varied tropism In the rodent brain, Taymans et al (2007) compared serotypes 1, 2, 5, 7 and 8 for tropism in various brain regions and found that these serotypes were primarily neuronal (Taymans et al., 2007). Earlier studies also found neuronal expression by serotypes 1, 2, 5, 7 and 8l (Burger et al., 2004; Cearley and Wolfe, 2006; Davidson et al., 2000; Harding et al., 2006; Paterna, Feldon, and Bueler, 2004). Additional expression was observed in ependymal cells by serotypes 4 an 5 (Davidson et al., 2000), astrocytes by serotypes 5-8 (Davidson et al., 2000; Harding et al., 2006), microglia by serotypes 2 and 5 (Cucchiarini et al., 2003) and oligodendrocytes by serotype 2 (Chen et al., 1999). In vitro studies using rAAV vectors have identified transduced neurons (Cao et al., 2004; Garrity-Moses et al., 2005; Keir et al., 1999; Kugler et al., 2003; Michel et al., 2005; Nomoto et al., 2003; Shevtsova et al., 2005) and glia (Kugler et al., 2003; Nomoto et al., 2003; Shevtsova et al., 2005), but these studies were conducted under different conditions with limited serotypes (2 and 5). No reports to date have compared tropism of multiple serotypes on neurons and glia in vitro under the same controlled conditions.
In vitro studies using primary cells or tissue provide an opportunity to conduct complex analysis of molecular mechanisms and cellular process that would be impractical to test in vivo initially. Neurons and glia grown in vitro are extensively used to study the functions of genes and their proteins for understanding basic mechanisms, modeling neurodegenerative diseases and evaluating therapeutic potential of genes and compounds. One limitation to gene transfer studies in primary neurons is the inefficiency and toxicity associated with transfection methods (Jaworski et al., 2000; Martinez and Hollenbeck, 2003; Watanabe et al., 1999). AAV vectors can efficiently transduce primary neurons in vitro (see above) but the toxicity of AAV vectors on primary neurons and glia in vitro has not been well characterized.
One of the features that makes rAAV vectors a desired vector for gene therapy in the brain is the low toxicity and immunogenicity. Delivery of rAAV2 vector to rodent brain produces minimal cellular immune response (Feng et al., 2004; Lo et al., 1999; Mastakov et al., 2002). Lo et al (1999) reported that circulating antibodies to capsid, but not the transgene, were detectable at 2-4 months after single injection of AAV, but there was no correlation with antibody titers and transgene expression. In a recent phase I clinical trial, a rAAV2 vector delivered intraparenchymally showed no evidence of increased neutralizing antibodies to AAV2 capsid in 7 of 10 patients and all subjects showed minimal systemic signs of inflammation or immune stimulation (McPhee et al., 2006). Although AAV vectors have been used to transduce neurons and glia in vitro (Cao et al., 2004; Garrity-Moses et al., 2005; Keir et al., 1999; Kells et al., 2007; Kugler et al., 2003; Michel et al., 2005; Nomoto et al., 2003; Shevtsova et al., 2005), there are no reports examining the toxicity of different serotypes nor comparing tropism under the same culture conditions. In addition to the toxicity associated with the vector, the transgene can be responsible for toxic effects associated with viral vector transduction. Previous studies have shown that overexpression of GFP can be toxic to cells (Detrait et al., 2002; Klein et al., 2006; Liu et al., 1999), therefore, it is important to differentiate the toxicity of the transgene product from the vector/cell interactions.
The purpose of this study is to evaluate the tropism and toxicity of seven serotypes (1, 2, and 5 through 9) of a double-stranded rAAV vector expressing GFP in primary cultures containing both glia and neurons. The vectors are packaged, purified, titered and applied to primary cultures under the same conditions. The toxicity related to the GFP transgene is also examined by comparison to an AAV vector containing a frameshift mutation in the GFP gene (AAVΔGFP). Our results indicate a differential expression of the GFP transgene (tropism) and toxicity from AAV serotypes 1, 2 and 5 through 9 on primary neurons and glia in vitro. The pattern of toxicity and tropism is consistent with the relatedness of the capsid proteins.
Results
Tropism of AAV serotypes on primary cortical cultures
To broadly characterize the tropism of the various serotypes of dsAAVGFP, primary cortical cultures were transduced on the 6th day in vitro (DIV) at muliplicities of infection (MOIs) of 2×102, 2×103, 2×104 and 2×105 viral genomes (vg)/cell. At DIV8 or DIV12 cultures were fixed and visualized after immunofluorescent amplification of GFP signal and immunolabeling neurons (NeuN) or astrocytes (GFAP). Serotypes 2 and 9 exhibited minimal GFP signal at the two highest MOIs tested on both DIV8 (Fig 1) and DIV12 (Fig 2) and showed 5-10% colocalization with either GFAP-immunoreactive (IR) or NeuN-IR cells. Serotype 5 resulted in GFAP-IR and non-NeuN-IR cell expression of GFP at DIV8 (Fig 1). At DIV12, serotype 5 continued to show colocalization to predominantly GFAP-IR and non-NeuN-IR cells and the expression of GFAP appeared reduced (Fig 2). On DIV8 at each MOI, serotypes 1 and 6 exhibited the highest intensity of GFP signal compared to all other serotypes which was primarily colocalized to GFAP-IR cells and non-NeuN-IR cells, but NeuN-IR cells expressing low GFP signal were present at the two highest MOIs (Fig 1 a,d). Serotypes 7 and 8 produced similar pattern of GFP expression as serotypes 1 and 6 but less efficiently i.e. serotypes 1 and 6 at 2×104 was similar to 7 and 8 at 2×105(Figs 1 and 2). On DIV8 at higher MOIs, serotypes 1 and 6 produced GFP+ cells that were highly vacuolized suggesting a cytopathic effect (Fig 1p). At DIV12, the GFP expression in cells transduced with serotypes 1, 6, 7 and 8 showed a high degree of colocalization with NeuN-IR and a loss of colocalization with GFAP-IR (Fig 2a, b).
Figure 1. Serotype-specific expression of GFP in primary cortical cultures.
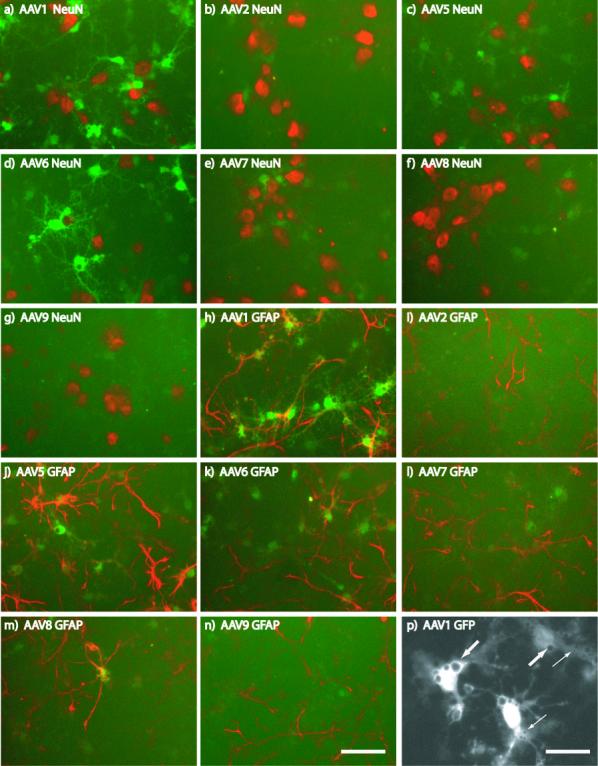
Primary cortical cultures (DIV6) were transduced with different serotypes of dsAAVGFP. Two days after transduction, cells were fixed and immunostained for NeuN (red; a-g) or GFAP (red; h-n). Results from MOI = 2×104. P) At 2×105 MOI, AAV1-transduced cells contained vacuoles of varying size (small and large arrows) devoid of GFP fluorescence (white) were observed. Scale bars: a-n) = 50 um, p) = 25 um
Figure 2. Loss of cell-specific marker immunoreactivity in response to AAV serotypes.
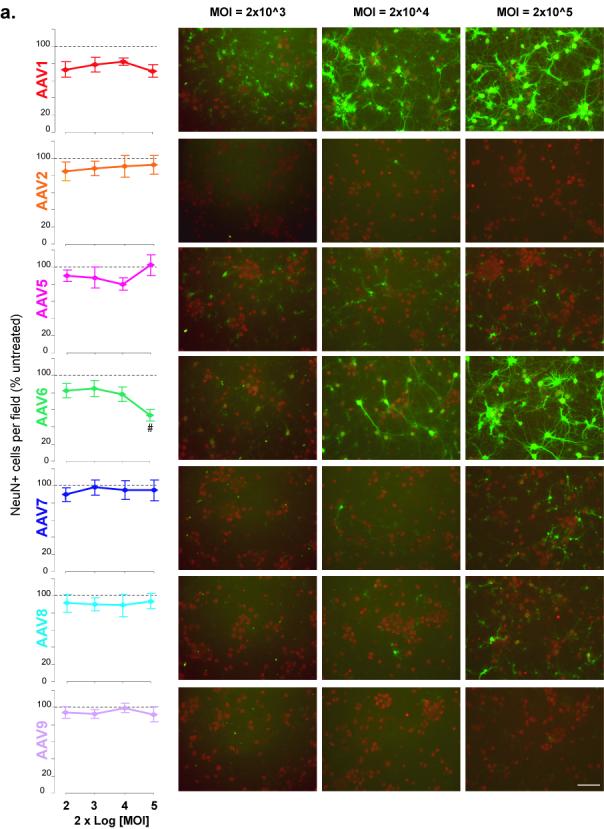
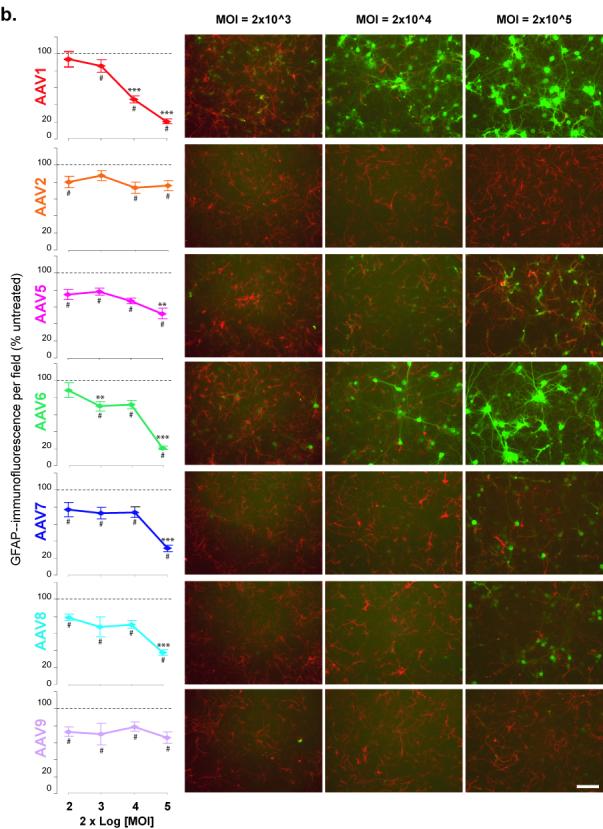
(a) NeuN or (b) GFAP immunoreactivity of primary cortical cultures transduced with AAV serotypes on DIV6 and immunostained 6 days after transduction. Graphs (left) show the average number of (A) NeuN+ cells per field or (b) GFAP immunoreactivity per field in the indicated AAV-treated group as percentage of the untreated group (dashed line) for different MOIs. Panels (right) are representative fields of (a) NeuN+ (red) or (b)GFAP-IR (red) and (a, b) GFP+(green) cells for MOI of 2×103, 2×104 and 2×105. # p<0.05 versus control by One-way ANOVA, Dunnett’s post-hoc test;. Scale bar = 100 um
Effects of AAV serotypes on immunoreactivity of cell specific markers
To determine whether the observed in changes in the GFP expression patterns from 2 to 6 days post-transduction where due to loss of GFAP-IR, the cultures were examined at 2 and 6 days after transduction for the presence of neurons (NeuN immunoreactive cells) or astrocytes (GFAP immunoreactivity). At 2 days post-transduction, there was no significant difference in the number of NeuN-IR cells between non-transduced cells and all serotypes tested at all MOIs (data not shown). Similarly, at 6 days post-transduction there was no change in NeuN+ cell counts for all serotypes except AAV6 which exhibited a significant decrease (p<0.05,Oneway ANOVA-Dunnett’s) compared to non-transduced cells (Fig 2a). At 6 days post-transduction, cultures treated with serotypes 1, 6, 7 and 8 exhibit GFP+ cells that mainly colocalize with NeuN-IR cells at the higher MOIs, however AAV5 shows minimal colocalization with NeuN (Fig 2a panels).
Two days after transduction, the GFAP-IR showed no significant difference among the serotypes and controls (data not shown) At 6 days post-transduction, all serotypes showed slight loss of GFAP-IR (15-20%) compared to controls at nearly all MOIs (Fig 2b) A MOI-dependent decrease in GFAP-IR was observed at 6 days post-transduction for AAV1 and AAV6 (Fig 2b). At the highest MOI (2×105), serotypes 5, 7 and 8 exhibited a further decrease in GFAP-IR compared to the next lowest MOI, 2×104 (Fig 2b). As observed at 2 days post-transduction, AAV5 colocalized to GFAP-IR cells and minimal expression was detected in NeuN-IR cells.(Fig 2b).
Acute toxicity of AAV serotypes on primary cortical cultures
The acute toxicity associated with transduction by the various serotypes was next examined as a function of MOI at 2 days post-transduction. Primary cortical cultures were transduced on DIV6 with various serotypes of dsAAVGFP at MOIs of 2×102, 2×103, 2×104 and 2×105 vg/cell. At 2 days post-transduction, overall cell viability was assessed by lactate dehydrogenase (LDH) release assay on the media and the bioreduction of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) on cells. All serotypes tested except 2 and 9 exhibited a MOI-dependent decline in cell viability (Figs 3a,b). At an MOI of 2×104, MTS levels were significantly less in cultures treated with serotypes 1, 5, 6 and 7 compared to uninfected controls (p<0.05, Oneway-ANOVA-Dunnett’s; Fig 4a) Similarly, at an MOI of 2×104, LDH release was significantly increased by serotype 1 (p<0.001,ANOVA-SNK), 5 and 6 (p<0.05,ANOVA-SNK; Fig 4c). Unlike the MTS assay results at MOI of 2×104 (Fig 4a) serotype 7 did not show significant increase in toxicity over control by LDH assay (Fig 4c). Overall, serotype 1 exhibited the highest toxicity at all MOIs. Serotypes 5 through 8 had less, but significant toxicity compared to AAV1 and serotype 2 and 9 had minimal toxic effects.
Figure 3. Toxicity of AAV serotypes on primary cortical cultures.
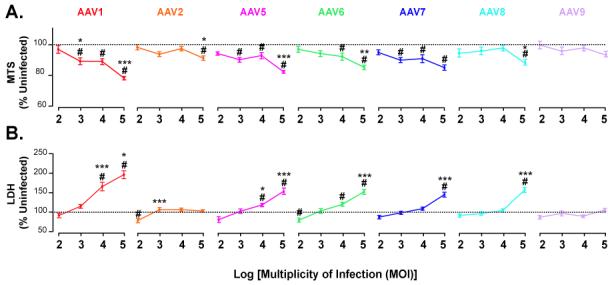
Primary cortical cultures (DIV6) were transduced with different serotypes of dsAAVGFP and assayed 2 days later for viability. MTS assay (a) and LDH assay (b) of primary cortical cells and media, respectively. Serotype comparison of MTS (c) and LDH (d) at MOI of 2×104. # p<0.05 versus control by One-way ANOVA, Dunnett’s post-hoc test; * p<0.05, **p<0.01 and ***p<0.001 versus adjacent lower MOI by One-way ANOVA, SNK post-hoc test.
Figure 4. Effects of mutant GFP transgene on cellular toxicity.
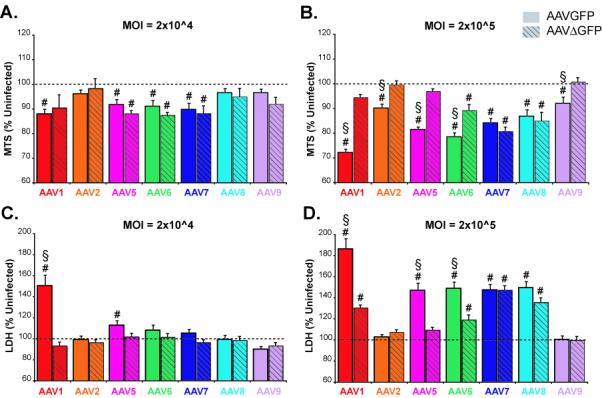
Primary cortical cultures (DIV6) were transduced with different serotypes of dsAAVGFP (solid bars) or dsAAVΔGFP (hatched bars) at a MOI of 2×104 (a,c) and 2×105 (b,d). Two days after transduction, cell viability was measured by MTS assay (a,b) and LDH assay (c,d). # p<0.05 versus control by One-way ANOVA, Dunnett’s post-hoc test § p<0.05 Student t-test versus dsAAVΔGFP of same serotype.
Mutating GFP partially attenuates toxicity of AAV serotypes on primary cultures
To examine whether the observed toxicity was related to toxic effects of GFP, a mutation was successfully introduced into the GFP gene creating a frameshift after the 10th amino acid of GFP that resulted in a protein (ΔGFP) that was 5 amino acids longer than GFP but only homologous at the first 10 amino acid. Primary cultures were transduced with the different serotypes of dsAAVΔGFP at four MOIs. For all MOIs tested, there was no detectable fluorescence from the dsAAVΔGFP. Two days after transduction, the overall viability was assessed by LDH release assay on the media and MTS assay on cells. At 2×104 MOI, dsAAVΔGFP serotypes 1,5,6,7 exhibited significant decrease (approximately 10%, p<0.05, Oneway ANOVA, Dunnett’s post-hoc), in viability by MTS compared to untreated cells (Fig 4a). However, there was no significant difference compared to the serotype equivalent of dsAAVGFP at the same MOI (Fig 4a). Compared to untreated cells, no significant change in LDH release was observed at 2×104 MOI for all serotypes of dsAAVΔGFP, however, dsAAVGFP showed significant increase in LDH levels for serotypes 1, 5 and 6 (Fig 4c). At the highest MOI (2×105), dsAAVΔGFP exhibited significantly less toxicity (p<0.05, t-test) than AAVGFP for serotypes 1, 5, and 6 by both LDH and MTS assays. For serotypes 7 and 8, both dsAAVGFP and dsAAVΔGFP caused a significant decrease in cell viability compared to untreated cells by both LDH and MTS assays (p<0.05, Oneway ANOVA, Dunnett’s post-hoc), but there was no difference between dsAAVGFP and dsAAVΔGFP by Student t-test (Figs 4b,d). Serotypes 2 and 9 of either dsAAVGFP or dsAAVΔGFP produced no significant change in cell viability at 2×104 MOI by MTS and LDH assay (Figs 4a, c) or 2×105 by LDH assay (Fig 4d). There was a significant decrease in viability compared to untreated cells by MTS assay at 2×105 MOI for serotypes 2 and 9 of dsAAVGFP (p<0.05, Oneway ANOVA, Dunnett’s post-hoc), but dsAAVΔGFP was significantly different from dsAAVGFP of the same serotype (p<0.05, Student t-test; Fig 4b).
Discussion
In this study the tropism and toxicity of AAV serotypes 1, 2, 5, 6, 7, 8 and 9 on primary cortical cultures derived from rat embryos were examined. A double-stranded rAAV vector was used to reduce the delay from viral exposure to transgene expression by removing the requirement of the second-strand DNA synthesis (Wang et al., 2003). In primary cortical cultures transduced with dsAAVGFP, GFP expression could be detected as early as 8 hours after transduction using immunofluorescence to amplify GFP fluorescent signal and at 2 days without amplification (data not shown). Using GFP fluorescence as an index of tropism, at 2 days post-transduction there was a MOI-dependent increase in GFP expression for all serotypes except 2 and 9. The use of GFP transgene expression for comparing the tropism(s) of different serotypes of rAAVs has been described (Burger et al., 2004; Cearley and Wolfe, 2006; Paterna, Feldon, and Bueler, 2004; Taymans et al., 2007). When examined 2 days post-transduction, the tropism (GFP expression) for all serotypes was predominantly glia, however, by 6 days post-transduction, the GFP expression was largely in NeuN-IR cells at the higher MOIs. Previous studies have shown that AAV2 or AAV5 containing the CMV promoter produced strong expression in glia compared to neurons, but exchanging the CMV promoter for the neuronal synapsin promoter restricted the GFP expression to neurons.(Kugler et al., 2003; Shevtsova et al., 2005). In the current study, we examined the relative differences among serotypes of GFP expression by the CMV promoter and observed primarily glial expression initially which changes to neuronal over time with the exception of serotype 5.
The toxicity of the serotypes was characterized at 2 days post-transduction using MTS and LDH assays which showed a MOI-related toxicity for all serotypes except 2 and 9. Previous studies have indicated that GFP overexpression has been linked to neuronal toxicity (Detrait et al., 2002; Klein et al., 2006; Liu et al., 1999). We found that, at the highest MOIs used for serotypes 1 and 5 through 8, GFP expression was correlated with LDH (R=.0.936; p=0.034). There is an inverse correlation between GFP expression and MTS (R=0.8324; p=0.069). To address whether the overexpression of GFP was contributing to the overall toxicity, a frameshift mutation in the GFP gene of the dsAAV vector was made to create a mutant protein of similar size but only the first 10 amino acids of GFP. At the highest MOI of 2×105, dsAAVΔGFP significantly reduced overall toxicity for serotypes 1, 5 and 6, but did not affect the toxicity of 7 or 8. At the next lowest MOI of 2×104, dsAAVΔGFP exhibited no toxicity by LDH assay but similar toxicity to dsAAVGFP. Together, the overexpression of GFP at the highest MOI is a large contributor to the observed toxicity, but the differential effects the mutant GFP among serotypes suggests that other mechanisms of toxicity remain possibly mediated via viral vector protein(s)-cell interactions. Although cells treated with AAV1 or AAV2 empty capsids with equivalent capsid proteins to those seen with MOIs used above did not alter MTS or GFAP-IR compared to untreated cells (data not shown). These data suggest that serotype 1 toxicity is primarily due to the toxic effects of GFP, but additional studies are needed to identify the toxic effects observed with all serotypes tested.
The toxicity at the higher MOIs, particularly with serotypes 1 and 6, correspond to an eventual loss of GFAP-IR. The GFP expression in cells transduced with highest MOI of AAV1 or AAV6 for two days reveals the presence of large intracellular vacuoles in GFAP-IR cells. The vacuole may result from excessive endocytosis and accumulation of virion in multiple endosomal pathways (Ding et al., 2005). Although the vacuoles are likely clear predictors of cell death based on their early presence in GFAP-IR cells and subsequent loss of GFAP-IR cells, the exact mechanism by which they are formed and whether they contribute to the cell death is not known.
When compared to the phylogenetic analysis of the capsid viral protein 1 (VP1) for each serotype (Fig 5), the intensity of expression, cellular tropism and toxicity observations are consistent with the relative capsid protein homologies. For example, the pairs 1/6, 7/8 and 2/9 had similar characteristics in tropism, expression and toxicity and each shows closest homology to each other. In fact, the homology of AAV1 and AAV6 capsid proteins are nearly identical (Gao et al., 2002) and show similar tropism and toxicity on the cortical cultures in the current study. The toxicity of both serotypes 7 and 8 where unaffected by mutating GFP and these two serotypes are closely related. The parallels of serotype tropism, toxicity and capsid homology implies the observed differences in the serotypes is a reflection of the capsid-cell interactions and is consistent with observations that single amino acid changes can alter tropism of AAV vectors (Wu et al., 2006). Cellular receptors have been identified for serotypes 1 and 6 (sailic acid(Chen et al., 2005; Seiler et al., 2006)), serotype 2 (heparan sulfate proteoglycans(Summerford and Samulski, 1998)), fibroblast growth factor 1(Qing et al., 1999), integrin (Summerford, Bartlett, and Samulski, 1999), hepatocyte growth factor receptor(Kashiwakura et al., 2005)and laminin receptor (Akache et al., 2006)), serotype 5 (platelet derived growth factor receptor(Di Pasquale et al., 2003)), serotypes 8 and 9 (laminin (Akache et al., 2006)). Many of these receptors are capable of signaling upon activation by a ligand. AAV2 has been shown to promote pro-death pathways in the absence of transgene expression (Duverger et al., 2002). Future studies examining the capsid-receptor interactions for the various serotypes may provide a better understanding of how cell signaling and viability is altered by the process of vector attachment and entry into the cell.
Figure 5. Phylogram of capsid proteins for AAV serotypes 1, 2 and 5 through 9.
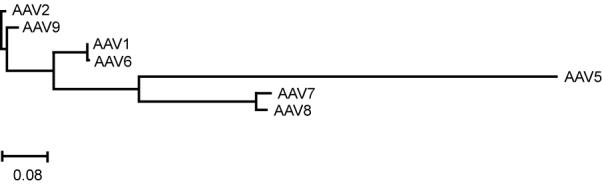
Tree rooted to AAV2. Units represent frequency of changes per 100 residues.
Based on the observations of this study, AAV vectors can be toxic to cultured neurons and glia; therefore, a MOI at or below 2×104 vg/cell is recommended to create minimal toxicity to glia and observable expression in both glia and neurons. This recommended MOI is comparable to the MOIs (103 to 105) in other in vitro studies using AAV-mediated gene transfer to neurons (Cao et al., 2004; Doroudchi et al., 2005; Gong et al., 2004; Kells et al., 2007; Kugler et al., 2003; Shevtsova et al., 2005). To achieve further specificity and physiologically relevant expression, the use of cell specific promoters will be needed. AAV vectors have been generated to provide cell-restricted gene expression by virtue of a cellular promoter (Kugler et al., 2003; Shevtsova et al., 2005). By incorporating cellular promoters into select pseudotyped AAV vectors and transducing cells at MOIs of minimal toxicity, the physiological or therapeutic levels of gene product may be achieved in vitro.
Materials and Methods
Primary Cortical Cultures
Cells were isolated from E15 embryos collected from timed-pregnant Sprague-Dawley rats as described previously with modifications(Cox et al., 2004) and in accordance with approved procedures by the NIH Animal Care and Usage Committee. Specifically, brain cortices from E15 embryos were pooled and digested 20 minutes in prewarmed (37°C) 1 ml/embryo of 0.05% trypsin-ETDA (0.2% (Invitrogen). Cortices were triturated and diluted in plating media [Neurobasal media (Invitrogen), 2% heat-inactivated fetal bovine serum (Sigma-Aldrich, Milwaukee, WI), 2% B27 supplement (Invitrogen), 200 mM L-glutamine and 25 mM L-glutamate] added at approximately 2 ml per embryo. Viability was assessed by trypan blue staining (Invitrogen) and cells were plated at 3×104 viable cells/well in 0.2 ml plating media into 96 well plates coated with 0.15-0.2% polyethyleneimine in 150mM sodium borate, pH 8.5 (Sigma-Aldrich). Plated cells were placed in 37°C humidified incubator with 5.5% CO2. Cells were fed by 50% media exchange on 4th day in vitro (DIV4) with feed media (plating media without serum or glutamate).
Plasmids
The construction of pdsAAVGFP has been described previously (Wang et al., 2003). To generate pdsAAVΔGFP, pdsAAVGFP was digested with BseRI and T4 polymerase to remove the 3′ overhang of 2 bases. This created a frameshift mutation at amino acid 11 of GFP and an overall protein of 243 amino acids (compared to 238 aa of GFP). For the serotypes 1, 2, 5, 6, 7, 8 and 9 the respective plasmids were used in transfections described below: pXR1 (aka pXX12; (Rabinowitz et al., 2002)), pXX2 (Xiao, Li, and Samulski, 1998), pXR5 (Rabinowitz et al., 2002), pAAV2/6 (Rutledge, Halbert, and Russell, 1998), pAAV7 (Gao et al., 2002), pAAV8 (Gao et al., 2002), and pAAV9 (Gao et al., 2004). Plasmids used for packaging AAV were generously provided by Dr. Xiao Xiao (UNC, Chapel Hill, NC).
AAV Packaging
All vectors were prepared by triple transfection method as described (Xiao, Li, and Samulski, 1998). Specifically, HEK293 cells are grown in 293 media [DMEM-HG (Invitrogen) containing 5% bovine growth serum, (BGS; HyClone, Logan, UT) and 1% penicillin-streptomycin in 20 × 150 mm dishes until 80-90% confluent. For one 150 mm dish, 25 ug pHelper (Stratagene, La Jolla, CA, USA), 18 ug of pdsAAVGFP or pdsAAVΔGFP, and 7 ug of rep/cap plasmid which varies with serotype (see above) were added to 2 ml of 0.25M CaCl2. The solution was gently vortexed while a 2X buffer (280 mM NaCl, 50 mM HEPES and 1.5mM Na2HPO4) was slowly dripped into the solution. After 15-17 hours, transfection media was replaced with 293 media. Forty eight hours post-transfection, the cells and media were harvested and centrifuged for 5 minutes at 800xg, 4°C. The pellet was resuspended in 1 ml of 50 mM Tris HCl pH 8.0, 150 mM NaCl, 2 mM MgCl2 (resuspension buffer) per 150mm dish. The resuspended cells were frozen and stored at -80°C until purification.
AAV Purification and Titering
All serotypes of AAV vectors were purified by CsCl ultracentrifugation based on established methods (Xiao, Li, and Samulski, 1998) with modifications. Cell pellets were freeze-thawed 3 times, vortexing after each thaw. Benzonase was then added (50units / mL of cell solution; Sigma-Aldrich) for 1 hour at 37°C with occasional mixing. Cell debris was centrifuged for 20 minutes at 2450 x g, 4°C. Supernatant was adjusted to 30 ml using resuspension buffer followed by 10 ml 2.5 M NaCl and 10 ml 40% PEG 8000 and incubated at 4°C for 18-20 hours. The solution was centrifuged for 20 minutes at 2450 x g, 4°C and the pellet was resuspended in 20 ml of 50 mM HEPES, 150 mM NaCl, 20 mM EDTA, pH 8.0 and 1% Sarcosyl. The solution was transferred to 36 ml ultracentrifuge tube and under laid consecutively with 1.3 g/ml and 1.5 g/ml CsCl in PBS. The CsCl interfaces were marked and mineral oil was used for balancing. The tubes were centrifuged (Discovery 100SE; Sorvall, Newton, CT) in a Surespin 630/36 rotor (Sorvall) at 166,900xg 10°C for 20-24 hours. Approximately 8 mls was removed using an 18 g needle inserted 2mm below the 1.5-1.3g/ml CsCl interface. The solution was transferred to a 17 ml ultracentrifuge tube, under laid with 2ml of 1.5 g/ml CsCl, balanced and centrifuged for 24-48 hours at 110,000xg, 10°C. Fractions were collected via an 18g needle inserted below the 1.5g/mL interface. Using the refractive index, fractions with a Brix % between 21.0 and 28.0% were pooled and dialyzed using a 10,000 MWCO dialysis cassette (Pierce, Rockford, IL, USA) PBS containing 0.5mM MgCl2 with three exchanges over 25-30 hours. The equilibrated virus was aliquoted, frozen, and stored at -80°C. Viral titers were determined using Real-time quantitative PCR with “TaqMan™” chemistry and analyzed by Opticon2 thermalcycler (Biorad, Hercules, CA, USA). Titers were calculated as viral genomes based on a standard curve of pdsAAVGFP plasmid linearized with MluI. The concentration of the linearized plasmid was determined using a ND-1000 spectrophotometer (Nanodrop, Wilminton, DE, USA) and visually checked on agarose gel containing a Low Mass DNA ladder (Invitrogen). The linearized plasmid was diluted in PBS to obtain concentrations ranging from 1×10-1 to 10-5vg/ml equivalents. A viral aliquot was thawed, sonicated for 10 seconds and serially diluted from 1:1,000 to 1:10,000 in PBS. Standards and 10-3 and 10-4 dilutions (triplicates) were assayed using Taqman Master mix (Applied Biosystems, Foster City, CA, USA) with eGFP primers and probe (Biosearch Technologies, Inc. Novato, CA, USA) under the following reaction conditions: 95°C, 5 min; 94°C, 20 sec and 60°C, 1 min, 41 times. Primer and probe sequences: 5′ AGCAAAGACCCCAACG AGAA 3′(fwd), 5′ FAM-CGCGATCACAT GGTCCTGCTGG-BH1 3′ (probe), and 5′GGCGGCGGTCACGAACT’3′(rev).
AAV transduction of Primary Cortical Cultures
Cultures were transduced on DIV6 using each rAAV serotype normalized to 2×1012 vg/ml and serially diluted in the PBS containing 0.5mM MgCl to obtain 2×1011, 2×1010, and 2×109 viral genomes (vg)/ml. Five ul of media was added to each well of a 96 well plate containing approximately 50,000 cells in 100 ul. Plates were gently mixed and returned to 37°C incubator for two hours followed by a replacement of 100 ul of fresh feed media. For cultures harvested at DIV12, cells were fed by 50% media exchange on DIV8 and DIV11 using feed media.
LDH and MTS assays
For LDH assay, media was collected from DIV8 cultures and assayed using the Cytotoxicity Detection Kit (LDH; Roche Applied Sciences, Indianapolis, IN, USA) according to manufacturers recommendations (Koh and Choi, 1987). For MTS assay, 100uL of media was removed from each well on DIV8 for LDH then 20 ul of MTS reagent from the CellTiter 96 AQueous One Solution cell proliferation assay (Promega, Madison, WI) was added to each well. Plates were returned to the 37°C incubator for 1 hour then the A490 nm was read using a Biotek ELx 808iu spectrophotometer (BioTek Instruments).
Immunofluorescent labeling and image analysis
At indicated time(DIV8 or DIV12), cells were fixed in 200uL of 4% paraformaldehyde in PBS for 1 hour at room temperature, washed and stored in PBS at 4°C. For immunofluorescent labeling cells were permeabilized 15 minutes in room temperature in PBS containing 0.1% Triton X-100 (Sigma) and 0.2% bovine serum albumin (BSA; Sigma). Cells were incubated in PBS containing 0.1% Triton X-100, 2% BSA, and 5% goat serum (Sigma) for 1 hour. Primary antibodies to GFAP (mouse anti-GFAP; #MAB360; Millipore, Billerica, MA) or NeuN (mouse anti-NeuN; #MAB377; Millipore) were diluted 1:500, and/or GFP (chicken anti-GFP; AB16901; Millipore) was diluted 1:5000 in PBS containing 0.1%Triton X-100 and 5% goat serum. Cells were incubated in primary antibodies overnight at 4°C with gentle shaking. After 3×5 minute washes in PBS, secondary antibodies (Alexa fluor 568 goat anti-mouse and/or Alexa fluor 488 goat anti-chicken; Invitrogen) were diluted 1:500 in PBS containing 0.1% Triton X-100 and 5% goat serum and added to cells for 1 hr at room temperature. After 2×5 minute washes in PBS, 0.001% DAPI (Invitrogen) in PBS was added 10 minutes at room temperature. Cells were washed 2×5min and stored away from light at 4°C until imaging
Immunolabelled cells were imaged with a Nikon Eclipse TE2000-E inverted microscope (Nikon, Melville, NY) using a Spot RT slider camera (Diagnostic Instruments, Sterling Heights, MI) and MetaMorph v6.2 software (Molecular Devices, Sunnyvale, CA). Briefly, the microscope stage was programmed to move to the center of each well for manual focusing. Once focused, the stage moved to 4 locations per well and acquire images using UV filter (DAPI), FITC filter (GFP and Alexa 488) and rhodamine (Alexa 568). Exposure times were kept constant for each filter. Immunoreactive pixel density (GFAP) or cell count (NeuN or DAPI) were made using the integrated morphometry feature of Metamorph.
Phylogenetic analysis of AAV capsid-encoding genes
A phylogenetic tree was generated using Discovery Studio Gene v1.5 (Accelyrs, San Diego, CA) by first aligning with ClustalW analysis of the translated capsid region of AAV1 (Accession#AF063497, nts2223-4433), AAV2 (AF043303, nts2203-4410), AAV5 (AF085716, nts2207-4381), AAV6 (AF028704, nts 2208-4418), AAV7 (AF513851, nts 2122-4435), AAV8 (AF513852, nts 2121-4437) and AAV9 (AY530629). Phylogenetic reconstruction of the AAV capsids was generated using neighbor-joining method in best tree mode with distances calculated as uncorrected “p” with gaps distributed proportionally. The resulting phylogenetic tree was rooted to AAV2.
Statistics
All experiments were conducted independently 3-6 times using n= 6-18 wells (MTS and LDH) or 2 times using n = 3-9 wells (immunolabeling). Statistical analyses were performed with one-way ANOVA and either post-hoc Student-Newman-Keuls test or Dunnett’s. Significance was inferred at p<0.05. Data are presented as mean +/- S.E.M.
Acknowledgements
This research was supported by the IRP of NIDA, NIH, DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80(19):9831–6. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10(2):302–17. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Cao G, Xiao M, Sun F, Xiao X, Pei W, Li J, Graham SH, Simon RP, Chen J. Cloning of a novel Apaf-1-interacting protein: a potent suppressor of apoptosis and ischemic neuronal cell death. J Neurosci. 2004;24(27):6189–201. doi: 10.1523/JNEUROSCI.1426-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13(3):528–37. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Chen H, McCarty DM, Bruce AT, Suzuki K. Oligodendrocyte-specific gene expression in mouse brain: use of a myelin-forming cell type-specific promoter in an adeno-associated virus. J Neurosci Res. 1999;55(4):504–13. doi: 10.1002/(SICI)1097-4547(19990215)55:4<504::AID-JNR10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Chen S, Kapturczak M, Loiler SA, Zolotukhin S, Glushakova OY, Madsen KM, Samulski RJ, Hauswirth WW, Campbell-Thompson M, Berns KI, Flotte TR, Atkinson MA, Tisher CC, Agarwal A. Efficient transduction of vascular endothelial cells with recombinant adeno-associated virus serotype 1 and 5 vectors. Hum Gene Ther. 2005;16(2):235–47. doi: 10.1089/hum.2005.16.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S, Harvey BK, Sanchez JF, Wang JY, Wang Y. Mediation of BMP7 neuroprotection by MAPK and PKC IN rat primary cortical cultures. Brain Res. 2004;1010(12):55–61. doi: 10.1016/j.brainres.2004.02.068. [DOI] [PubMed] [Google Scholar]
- Cucchiarini M, Ren XL, Perides G, Terwilliger EF. Selective gene expression in brain microglia mediated via adeno-associated virus type 2 and type 5 vectors. Gene Ther. 2003;10(8):657–67. doi: 10.1038/sj.gt.3301925. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, Zabner J, Ghodsi A, Chiorini JA. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci U S A. 2000;97(7):3428–32. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrait ER, Bowers WJ, Halterman MW, Giuliano RE, Bennice L, Federoff HJ, Richfield EK. Reporter gene transfer induces apoptosis in primary cortical neurons. Mol Ther. 2002;5(6):723–30. doi: 10.1006/mthe.2002.0609. [DOI] [PubMed] [Google Scholar]
- Di Pasquale G, Davidson BL, Stein CS, Martins I, Scudiero D, Monks A, Chiorini JA. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med. 2003;9(10):1306–12. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- Ding W, Zhang L, Yan Z, Engelhardt JF. Intracellular trafficking of adeno-associated viral vectors. Gene Ther. 2005;12(11):873–80. doi: 10.1038/sj.gt.3302527. [DOI] [PubMed] [Google Scholar]
- Doroudchi MM, Liauw J, Heaton K, Zhen Z, Forsayeth JR. Adeno-associated virus-mediated gene transfer of human aromatic L-amino acid decarboxylase protects mixed striatal primary cultures from L-DOPA toxicity. J Neurochem. 2005;93(3):634–40. doi: 10.1111/j.1471-4159.2005.03048.x. [DOI] [PubMed] [Google Scholar]
- Duverger V, Sartorius U, Klein-Bauernschmitt P, Krammer PH, Schlehofer JR. Enhancement of cisplatin-induced apoptosis by infection with adeno-associated virus type 2. Int J Cancer. 2002;97(5):706–12. doi: 10.1002/ijc.10077. [DOI] [PubMed] [Google Scholar]
- Feng X, Eide FF, Jiang H, Reder AT. Adeno-associated viral vector-mediated ApoE expression in Alzheimer’s disease mice: low CNS immune response, long-term expression, and astrocyte specificity. Front Biosci. 2004;9:1540–6. doi: 10.2741/1323. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78(12):6381–8. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5(3):285–97. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(18):11854–9. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity-Moses ME, Teng Q, Liu J, Tanase D, Boulis NM. Neuroprotective adeno-associated virus Bcl-xL gene transfer in models of motor neuron disease. Muscle Nerve. 2005;32(6):734–44. doi: 10.1002/mus.20418. [DOI] [PubMed] [Google Scholar]
- Gong Y, Chen S, Sonntag CF, Sumners C, Klein RL, King MA, Hughes JA, Meyer EM. Recombinant adeno-associated virus serotype 2 effectively transduces primary rat brain astrocytes and microglia. Brain Res Brain Res Protoc. 2004;14(1):18–24. doi: 10.1016/j.brainresprot.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Harding TC, Dickinson PJ, Roberts BN, Yendluri S, Gonzalez-Edick M, Lecouteur RA, Jooss KU. Enhanced gene transfer efficiency in the murine striatum and an orthotopic glioblastoma tumor model, using AAV-7- and AAV-8-pseudotyped vectors. Hum Gene Ther. 2006;17(8):807–20. doi: 10.1089/hum.2006.17.807. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Figiel I, Proszynski T, Kaczmarek L. Efficient expression of tetracycline-responsive gene after transfection of dentate gyrus neurons in vitro. J Neurosci Res. 2000;60(6):754–60. doi: 10.1002/1097-4547(20000615)60:6<754::AID-JNR7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Kashiwakura Y, Tamayose K, Iwabuchi K, Hirai Y, Shimada T, Matsumoto K, Nakamura T, Watanabe M, Oshimi K, Daida H. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J Virol. 2005;79(1):609–14. doi: 10.1128/JVI.79.1.609-614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir SD, House SB, Li J, Xiao X, Gainer H. Gene transfer into hypothalamic organotypic cultures using an adeno-associated virus vector. Exp Neurol. 1999;160(2):313–6. doi: 10.1006/exnr.1999.7236. [DOI] [PubMed] [Google Scholar]
- Kells AP, Henry RA, Hughes SM, Connor B. Verification of functional AAV-mediated neurotrophic and anti-apoptotic factor expression. J Neurosci Methods. 2007;161(2):291–300. doi: 10.1016/j.jneumeth.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol Ther. 2006;13(3):517–27. doi: 10.1016/j.ymthe.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987;20(1):83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- Kugler S, Lingor P, Scholl U, Zolotukhin S, Bahr M. Differential transgene expression in brain cells in vivo and in vitro from AAV-2 vectors with small transcriptional control units. Virology. 2003;311(1):89–95. doi: 10.1016/s0042-6822(03)00162-4. [DOI] [PubMed] [Google Scholar]
- Liu HS, Jan MS, Chou CK, Chen PH, Ke NJ. Is green fluorescent protein toxic to the living cells? Biochem Biophys Res Commun. 1999;260(3):712–7. doi: 10.1006/bbrc.1999.0954. [DOI] [PubMed] [Google Scholar]
- Lo WD, Qu G, Sferra TJ, Clark R, Chen R, Johnson PR. Adeno-associated virus-mediated gene transfer to the brain: duration and modulation of expression. Hum Gene Ther. 1999;10(2):201–13. doi: 10.1089/10430349950018995. [DOI] [PubMed] [Google Scholar]
- Martinez CY, Hollenbeck PJ. Transfection of primary central and peripheral nervous system neurons by electroporation. Methods Cell Biol. 2003;71:339–51. doi: 10.1016/s0091-679x(03)01016-1. [DOI] [PubMed] [Google Scholar]
- Mastakov MY, Baer K, Symes CW, Leichtlein CB, Kotin RM, During MJ. Immunological aspects of recombinant adeno-associated virus delivery to the mammalian brain. J Virol. 2002;76(16):8446–54. doi: 10.1128/JVI.76.16.8446-8454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ. Adeno-associated virus (AAV) vectors in the CNS. Curr Gene Ther. 2005;5(3):333–8. doi: 10.2174/1566523054064995. [DOI] [PubMed] [Google Scholar]
- McPhee SW, Janson CG, Li C, Samulski RJ, Camp AS, Francis J, Shera D, Lioutermann L, Feely M, Freese A, Leone P. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med. 2006;8(5):577–88. doi: 10.1002/jgm.885. [DOI] [PubMed] [Google Scholar]
- Michel U, Malik I, Ebert S, Bahr M, Kugler S. Long-term in vivo and in vitro AAV-2-mediated RNA interference in rat retinal ganglion cells and cultured primary neurons. Biochem Biophys Res Commun. 2005;326(2):307–12. doi: 10.1016/j.bbrc.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Nomoto T, Okada T, Shimazaki K, Mizukami H, Matsushita T, Hanazono Y, Kume A, Katsura K, Katayama Y, Ozawa K. Distinct patterns of gene transfer to gerbil hippocampus with recombinant adeno-associated virus type 2 and 5. Neurosci Lett. 2003;340(2):153–7. doi: 10.1016/s0304-3940(03)00095-8. [DOI] [PubMed] [Google Scholar]
- Paterna JC, Feldon J, Bueler H. Transduction profiles of recombinant adeno-associated virus vectors derived from serotypes 2 and 5 in the nigrostriatal system of rats. J Virol. 2004;78(13):6808–17. doi: 10.1128/JVI.78.13.6808-6817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5(1):71–7. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, Samulski RJ. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76(2):791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72(1):309–19. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MP, Miller AD, Zabner J, Halbert CL. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum Gene Ther. 2006;17(1):10–9. doi: 10.1089/hum.2006.17.10. [DOI] [PubMed] [Google Scholar]
- Shevtsova Z, Malik JM, Michel U, Bahr M, Kugler S. Promoters and serotypes: targeting of adeno-associated virus vectors for gene transfer in the rat central nervous system in vitro and in vivo. Exp Physiol. 2005;90(1):53–9. doi: 10.1113/expphysiol.2004.028159. [DOI] [PubMed] [Google Scholar]
- Summerford C, Bartlett JS, Samulski RJ. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5(1):78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72(2):1438–45. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, Wilson JM, Debyser Z, Baekelandt V. Comparative Analysis of Adeno-Associated Viral Vector Serotypes 1, 2, 5, 7, And 8 in Mouse Brain. Hum Gene Ther. 2007;18(3):195–206. doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- Tenenbaum L, Chtarto A, Lehtonen E, Velu T, Brotchi J, Levivier M. Recombinant AAV-mediated gene delivery to the central nervous system. J Gene Med. 2004;6(Suppl 1):S212–22. doi: 10.1002/jgm.506. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10(26):2105–11. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- Watanabe SY, Albsoul-Younes AM, Kawano T, Itoh H, Kaziro Y, Nakajima S, Nakajima Y. Calcium phosphate-mediated transfection of primary cultured brain neurons using GFP expression as a marker: application for single neuron electrophysiology. Neurosci Res. 1999;33(1):71–8. doi: 10.1016/s0168-0102(98)00113-8. [DOI] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Grieger JC, Govindasamy L, Agbandje-McKenna M, Samulski RJ. Single Amino Acid Changes Can Influence Titer, Heparin Binding, and Tissue Tropism in Different Adeno-Associated Virus (AAV) Serotypes. J Virol. 2006 doi: 10.1128/JVI.01288-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Li J, McCown TJ, Samulski RJ. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol. 1997;144(1):113–24. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72(3):2224–32. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


