Abstract
CD4C/human immunodeficiency virus (HIV) transgenic mice develop an AIDS-like disease. We used this model to study the effects of HIV-1 on dendritic cells (DC). We found a progressive decrease in total DC numbers in the lymph nodes, with a significant accumulation of CD11bHi DC. In the thymus, the recovery of transgenic CD8α+ DC had a tendency to be lower. Spleen DC were augmented in the marginal zone. Transgenic DC showed a decreased capacity to present antigen in vitro, consistent with their reduced major histocompatibility complex class II expression and impaired maturation profile. The accumulation of immature DC may contribute to disease and may reflect an adaptive advantage for the virus by favoring its replication and preventing the generation of fully functional antiviral responses.
Dendritic cells (DC) are involved in the generation of immune responses as well as in the selection and maintenance of the T-cell repertoire (3, 46, 54, 75, 78, 81). During human immunodeficiency virus type 1 (HIV-1) infection, Langherans cells and DC at the mucosal surfaces are the earliest cell types to be exposed to HIV-1 in vivo and are thought to facilitate transmission of the virus to CD4+ T cells following their migration to the lymph nodes (6, 64; reviewed in references 43, 71, and 86). HIV-1 is known to replicate preferentially in immature DC (32), and HIV-1 footprints have been detected in blood-, adenoid/tonsil-, and skin-derived DC from HIV-positive individuals (12, 28, 45, 49, 68), but not in all studies (5, 40). This controversy about the extent of infection of human DC in vivo is also apparent regarding the effects of HIV-1 on DC differentiation, phenotype, and function, where a consensus has been difficult to reach, given the various sources of DC being studied, their stages of differentiation, their mode of isolation and culture, and the various stimuli used (7, 43, 86). It is therefore not clear whether and how HIV-1 affects DC populations and their function. Neither is it known whether “myeloid” and “plasmacytoid” DC subpopulations, the latter being most probably of lymphoid origin (79), are affected differently by HIV-1.
To gain insight into the pathogenesis of HIV-1, we previously generated CD4C/HIV transgenic mice, which express HIV-1 gene products in CD4+ T cells and in cells of the macrophage/dendritic lineages and develop a severe AIDS-like disease. This disease is characterized by atrophy of the lymphoid organs and by gradual and preferential loss of CD4+ T cells and of their functions (34, 35). These mice also exhibit an impaired capacity to generate germinal center reactions and harbor hyperresponsive B cells and serum autoantibody (65). Here we report our study on DC in these transgenic mice.
MATERIALS AND METHODS
Mice.
The CD4C/HIVMutA transgenic mice have been described previously (35). Sex-matched littermates were used between 6 and 20 weeks of age. In order to generate in vitro mixed leukocyte reaction, CD4+ lymphocytes from sex-matched BALB/c mice were used between 6 and 12 weeks of age. For in vitro antigen presentation studies, CD4+ T cells from sex-matched pigeon cytochrome c T-cell receptor-specific AD10 transgenic mice (41) (obtained from P. Hugo, formerly from our institute) were used between 6 and 12 weeks of age. The AD10 mice were checked for transgene expression by PCR and by fluorescence-activated cell sorting (FACS) with the rat anti-mouse Vβ3 monoclonal antibody (KJ-25), which was a gift from P. Hugo. Pigeon cytochrome c (20 kDa) was purchased from Sigma, and the pigeon cytochrome c peptide KAERADLIAYLKQATAK was a gift from P. Hugo.
Antibodies and reagents.
The antibodies RA36B2 (rat anti-mouse B220), GK1.5 (rat anti-mouse CD4), 53.6.78 (rat anti-mouse CD8), and M5-114 (rat anti-mouse major histocompatibility complex [MHC] class II) were obtained from the American Type Culture Collection (Rockville, Md.). The P7/7.1-fluorescein isothiocyanate monoclonal antibody (rat anti-mouse MHC II) was a gift from T. Owens (McGill University, Canada). Hamster anti-mouse CD11c was purchased from Pharmingen. Rat anti-mouse Mac-1 (CD11b), IAk, B7-1, and B7-2 were from Cedarlane. Goat immunoglobulin M (IgM)-Texas Red, indodicarbocyanine-streptavidin, and fluorescein isothiocyanate-streptavidin were from Southern Biotechnology Associates (Birmingham, Ala.). Mouse anti-rat IgG F(ab′)2-fluorescein isothiocyanate was obtained from Jackson Immunoresearch Laboratories (West Grove, Pa.). FGK-45 (rat anti-mouse CD40) was from A. Rolink (Basel Institute for Immunology, Switzerland). Irrelevant rat IgG1, rat IgG2a, and rat IgG2b monoclonal antibodies were used for isotype controls. The generation of the soluble CD8α-CD40 ligand has been reported previously (44). Mouse recombinant interleukin-2 (IL-2), IL-4, and granulocyte-macrophage colony-stimulating factor (GM-CSF) were from the X63-mIL-2, X63-mIL-4, and X63-mGM-CSF-transfected plasmacytomas (39), respectively.
Isolation of resting CD4+ T cells.
For isolation of resting CD4+T cells, lymph nodes from nonimmunized mice were mechanically disrupted. The high-density cells were isolated on Percoll gradients followed by a 1-h incubation at 37°C to remove any adherent cells. Further purification involved the depletion of contaminating cells with sheep anti-rat immunoglobulin-coated magnetic beads (Dynabeads; Dynal, Oslo, Norway) following incubation with a cocktail of hybridoma supernatants (anti-MHC II, anti-B220, and anti-CD8). This resulted in >95% pure CD4+ T-cell receptor αβ-positive cells.
Purification of spleen, lymph nodes, and thymic DC.
DC were isolated from spleen, lymph nodes, and thymus of nonimmunized mice. Briefly, following enzymatic digestion with collagenase 4188 (Worthington Biomedical Corp., Freehold, N.J.) plus DNase I (Sigma Chemical Corp., St. Louis, Mo.), the low-density (1.060 to 1.065 g/ml) cells were isolated on Percoll (Pharmacia, Uppsala, Sweden) gradients and allowed to adhere for 1 h at 37°C. Nonadherent cells were then removed, and the adhered DC and macrophages were further cultured overnight, after which time DC were no longer adherent, in contrast to macrophages. DC were collected and contaminating cells were removed by magnetic depletion. For thymic DC, washing following each step with a buffer containing phosphate-buffered saline, 5 mM EDTA, and 2% fetal bovine serum allowed efficient removal of attached thymocytes, as described before (88). The DC do not contain the B220+ plasmacytoid pre-DC, since anti-B220 monoclonal antibody was used to deplete contaminating B cells from our preparations. Also, these plasmacytoid pre-DC mature into CD8α+ CD205 (DEC-205)− DC only following microbial stimuli (56), and the enriched CD8α+ DC recovered from CD4C/HIV transgenic mice were DEC-205+.
Generation of bone marrow-derived DC.
DC were derived from bone marrow cultures with a slight modification of the method described previously (37). Briefly, the bone marrow was obtained by flushing the femurs of mice. The suspension was then depleted of mature lymphocytes and MHC class II-positive cells with sheep anti-rat immunoglobulin-coated magnetic beads (Dynal) following incubation with monoclonal antibody, and the remaining cells were cultured at 107 cells per 15-cm petri dish in Iscove's modified Dulbecco's medium (Gibco-BRL, Life Technologies) supplemented with 10% fetal bovine serum (CanSera, Rexdale, Ontario, Canada), l-glutamine (Gibco-BRL), 2-mercaptoethanol, and antibiotics. Supernatant from X63-mGM-CSF was added at a final dilution of 1:20. Nonadherent cells were removed at days 2 and 3 of culture by gently swirling the plates, removing 65% of the medium, and adding back fresh medium supplemented with GM-CSF. At day 6, nonadherent cells were recultured in fresh medium and GM-CSF with or without lipopolysaccharide (10 μg/ml) and/or anti-CD40 monoclonal antibody (FGK-45, 25% supernatant). Following overnight culture, the nonadherent fraction was obtained and determined to be DC by morphology and FACS (i.e., >95% CD11b+ CD11c+).
Assessment of CD4+ T-cell proliferation.
Cells were cultured in round-bottomed 96-well plates in Iscove's modified Dulbecco's medium supplemented with 5% fetal bovine serum, l-glutamine, 2-mercaptoethanol, and antibiotics. CD4+ T cells were cultured at a density of 2 × 105 cells/well, and DC were added at different ratios. IL-2 was added at 100 U/ml final concentration. Proliferation was assessed by the incorporation of [3H]thymidine (Amersham, Buckinghamshire, United Kingdom) for the final 18 h of a 42-h culture period.
Immunofluorescence.
Serial cryosections were prepared, fixed in acetone for 10 min, and stored at −20°C. For immunolabeling, sections were rehydrated in phosphate-buffered saline and then sequentially incubated with primary and secondary antibodies in a humidified chamber. Slides were mounted with Mowiol aqueous solution (Hoechst Aktiengesellschaft, Frankfurt, Germany), and analysis was performed by confocal microscopy (Zeiss LSM 510).
Quantitation of fluorescent CD11c+ cells by image analysis.
Serial cryosections of lymph nodes and spleen from nontransgenic (n = 5) and transgenic (n = 7) mice 5 months of age were prepared and labeled as stated above with biotinylated anti-CD11c monoclonal antibody (Pharmingen) followed by streptavidin-Alexafluor 488-conjugate (Molecular Probes) to visualize DC. Slides were mounted with Vectashield solution. Sections were scanned and color images were captured with a Zeiss Axiovert S100TV microscope supported with Northern Eclipse 6.0 software. For study of lymph nodes, images of a mean of three lymph nodes per animal were taken in the cortical and paracortical zones (mean of eight images by 20× objective per animal). For studies of the spleen, images were taken in the white pulp (mean of nine images by 20× objective per animal). The nonspecific background labeling was subtracted prior to data acquisition. Quantitation of the area taken up by green fluorescent cells (square micrometers) was calculated and compared between nontransgenic and transgenic mice.
Flow cytometry.
Immunolabeling was performed on ice in FACS buffer (1× PBS, 1% bovine serum albumin, and 0.01% sodium azide), and fluorescence intensity was analyzed with a FACScan apparatus (Becton Dickinson, San Jose, Calif.).
Statistics.
A two-way analysis of variance was performed to study the effect of age and group (nontransgenic and transgenic) on the variables (Table 1). A Student's t test with Satterthwaite-Welch approach was performed to study the effect of group (Table 4). The Student's t test for single samples was used to test if the ratios were equal to or different than 1 (Tables 2, 3, and 5). For the data presented in Fig. 4, statistical analyses were performed according to Student's t test. P values of <0.05 were considered significant.
TABLE 1.
DC recovery from peripheral lymph nodes of CD4C/HIVMutA transgenic mice
| Expt no. | Mouse age (mo) | No. of micea | Nontransgenic
|
Transgenic
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total no. (105) | % of DC
|
Total no. (105) | % of DC
|
|||||||
| CD11bneg-int | CD11bhi | CD8α+ | CD11bneg-int | CD11bhi | CD8α+ | |||||
| 1 | 1.5 | 2 | 1.3 | 81 | 15 | 35 | 3.0 | 75 | 21 | 20 |
| 2 | 2 | 2 | 0.7 | 69 | 29 | 25 | 0.7 | 65 | 35 | 16 |
| 3 | 2 | 2 | 0.8 | 65 | 29 | 25 | 0.8 | 60 | 39 | 24 |
| 4 | 2 | 4 | 1.4 | 66 | 34 | ND | 0.8 | 59 | 41 | ND |
| 5 | 2.5 | 2 | 0.5 | 49 | 48 | ND | 0.5 | 38 | 60 | ND |
| 6 | 2.5 | 2 | 1.5 | 58 | 16 | 22 | 0.7 | 50 | 16 | 9 |
| 1 to 6 | 1.5-2.5 | 14 | 1.0 ± 0.2 | 65 ± 4 | 28 ± 5 | 27 ± 3 | 1.0 ± 0.4 | 58 ± 5* | 35 ± 6** | 17 ± 3 |
| 7 | 3 | 3 | 0.6 | 68 | 32 | ND | 0.3 | 45 | 55 | ND |
| 8 | 3 | 2 | 1.4 | 69 | 29 | ND | 0.5 | 24 | 75 | ND |
| 9 | 3 | 4 | 2.2 | 37 | 64 | ND | 1.0 | 22 | 79 | ND |
| 10 | 3 | 2 | 3.0 | 72 | 27 | 23 | 2.4 | 68 | 31 | 17 |
| 11 | 3.5 | 3 | 1.3 | 83 | 17 | ND | 1.5 | 72 | 28 | ND |
| 12 | 4 | 2 | 1.2 | 60 | 34 | ND | 1.0 | 46 | 50 | ND |
| 13 | 4 | 3 | 0.6 | 71 | 29 | 10 | 0.5 | 46 | 54 | 6 |
| 14 | 5.5 | 2 | 1.7 | 74 | 23 | ND | 1.0 | 53 | 46 | ND |
| 7 to 14 | 3-5.5 | 21 | 1.5 ± 0.3 | 67 ± 5 | 32 ± 5 | 17 ± 7 | 1.0 ± 0.2 | 47 ± 6* | 52 ± 6** | 12 ± 6 |
The number indicated applies to both nontransgenic and transgenic mice (i.e., 2 indicates two of each). Total, data represent absolute numbers of DC recovered per mouse. CD11b levels of expression appear as high (CD11bHi) or negative to intermediate (CD11bneg-int). The data are presented as mean ± standard error of the mean. ND, not done. Each experiment was done with sex-matched littermate controls. *, P = 0.023, **, P = 0.03 (two-way analysis of variance).
TABLE 4.
Recovery of CD8α+ DC from thymus of CD4C/HIVMutA transgenic micea
| Expt no. | Age (mo) | No. of miceb | No. of cells recoveredc (104)
|
|
|---|---|---|---|---|
| Nontransgenic | Transgenic | |||
| 15 | 1.5 | 2 | 6.5 | 3.2 |
| 16 | 2 | 2 | 5.0 | 3.0 |
| 6 | 2.5 | 2 | 9.0 | 1.6 |
| 7 | 3 | 3 | 2.0 | 1.3 |
| 10 | 3 | 2 | 2.2 | 1.4 |
| 12 | 4 | 2 | 11.0 | 3.0 |
| Total (mean ± SEM) | 1.5 - 4 | 13 | 6.0 ± 1.5 | 2.0 ± 0.4cd |
Experiment numbers are the same as for Table 1. Each experiment was done with sex-matched littermate controls.
See Table 1, footnote a.
Total recovery of CD11c+ MHC II+ CD8α+ DC in thymus. Data represent absolute numbers of DC recovered per mouse.
A t test with the Satterthwaite-Welch approach was performed to study the effect of group; P = 0.054.
TABLE 2.
Phenotypic characterization of lymph node DC of CD4C/HIVMutA transgenic micea
| Marker | No. of expt | Age (mo) | Mean fluorescence intensityb ± SEM | Pc |
|---|---|---|---|---|
| MHC class II | 16 | 2-4 | 1.5 ± 0.1 | <0.001 |
| CD40 | 8 | 2-4 | 1.4 ± 0.1 | 0.042 |
| B7-2 | 13 | 2-4 | 1.2 ± 0.1 | 0.041 |
| MHC class I | 10 | 2-4 | 1.2 ± 0.06 | 0.006 |
| CD11b | 13 | 2-4 | 0.6 ± 0.25 | 0.012 |
FACS analysis. Each experiment was done with pooled sex-matched transgenic (n = 3) and control nontransgenic (n = 3) littermates.
The data are expressed as the ratio of nontransgenic to transgenic DC.
Student's t test for single samples.
TABLE 3.
Phenotypic characterization of bone marrow-derived DC of CD4C/HIVMutA transgenic micea
| Marker | No. of expt | Age (mo) | Mean fluorescence intensity ± SEM | P |
|---|---|---|---|---|
| MHC class II | 8 | 2-4 | 1.6 ± 0.1 | 0.001 |
| CD40 | 4 | 2-4 | 1.6 ± 0.2 | 0.103 |
| B7-2 | 7 | 2-4 | 1.2 ± 0.1 | 0.02 |
| MHC class I | 8 | 2-4 | 1.3 ± 0.06 | 0.001 |
See Table 2, footnotes a, b, and c.
TABLE 5.
Phenotypic characterization of DC from thymus of CD4C/HIVMutA transgenic micea
| Marker | No. of expt | Age range (mo) | Mean fluorescence intensity ± SEM | P |
|---|---|---|---|---|
| MHC class II | 9 | 1.5-4 | 1.3 ± 0.1 | 0.052 |
| CD8α | 8 | 1.5-4 | 1.4 ± 0.2 | 0.037 |
| CD11b | 5 | 1.5-4 | 1.0 ± 0.1 | 0.797 |
FACS analysis of thymic DC. See Table 2, footnotes a, b, and c, for other details.
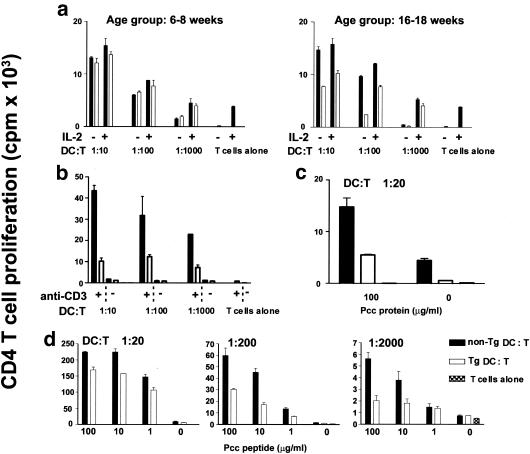
FIG. 4.
DC functions are impaired in CD4C/HIVMutA transgenic mice. DC were purified from peripheral lymph nodes of nontransgenic (non-Tg) and transgenic (Tg) mice at 6 to 8 and 16 to 18 weeks of age. DC were irradiated with 3,000 rads prior to culture. (a) For the allogeneic mixed leukocyte reaction, DC were cocultured at ratios of 1:10, 1:100, or 1:1,000 with purified resting CD4+ T cells from BALB/c mice in the presence or absence of IL-2 (100 U/ml). Proliferation was measured by [3H]thymidine incorporation for the last 18 h of a 48-h culture period. Experiments are shown separately for younger mice (6 to 8 weeks old) (left panel) and older (16 to 18 weeks old) mice (right panel). (b) To assess costimulatory capacity, DC isolated from nontransgenic (non-Tg) and transgenic (Tg) 16- to 18-week-old mice were cocultured on anti-CD3 (0.1 μg/ml)-coated plates with syngeneic resting CD4+ T cells purified from nontransgenic mice at ratios of 1:10, 1:100, and 1:1,000. T-cell proliferation was measured as in a. (c and d) The capacity to present pigeon cytochrome c (Pcc) or pigeon cytochrome c peptide to purified resting syngeneic pigeon cytochrome c-specific T-cell receptor CD4+ T cells from AD10 mice was assessed. Prior to culture, DC were incubated overnight with either medium alone, pigeon cytochrome c, or pigeon cytochrome c peptide at the indicated concentrations. DC were then cocultured with CD4+ T cells at the indicated DC-to-T-cell ratio. T-cell proliferative responses were measured as in a. Experiments are shown for older mice (16 to 18 weeks old). (a to d) Bars on graphs represent the mean ± standard error of the mean. For each experiment shown, the data are representative of at least four experiments, each involving DC from pooled lymph nodes of sex- and age-matched transgenic (n = 3) and control nontransgenic (n = 3) mice. Statistical analyses were performed according to Student's t test on data from all experiments. Differences between nontransgenic and transgenic DC that reached significance are shown in panels a (right panel), c, and d at P < 0.05 and in panel b at P < 0.001.
RESULTS
DC from CD4C/HIV transgenic mice express the HIV-1 transgene.
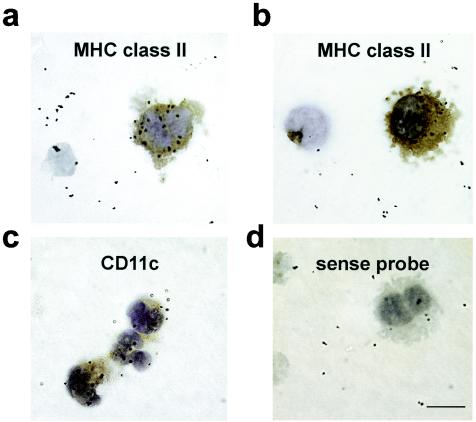
Human DC express CD4 (60, 87). Since the CD4C promoter used to express HIV-1 in CD4C/HIV transgenic mice was of human origin and allowed expression of human CD4 on the surface of lymph node, spleen, and thymic DC in CD4C/CD4 transgenic mice (36), we anticipated that it might allow expression of HIV-1 in DC of CD4C/HIV transgenic mice. Indeed, strong expression of the HIV-1 transgene as assessed by in situ hybridization was found on peripheral lymph node DC from CD4C/HIVMutA transgenic mice (Fig. 1), which express rev, env, and nef of HIV-1 (35).
FIG. 1.
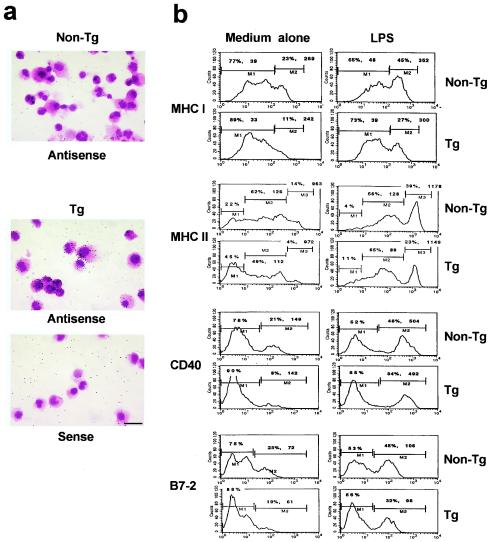
Expression of the HIV-1 transgene in murine lymph node DC. Transgene expression in lymph node DC of CD4C/HIVMutA transgenic mice was detected by in situ hybridization with an HIV-1-specific antisense riboprobe (a to c) and a sense probe as a control (d). The expression of MHC II (a and b) and CD11c (c) on the DC was determined by immunohistochemistry and appears in brown. Magnification, ×100; bar, 25 μm. Data are representative of at least nine experiments, each involving cells from pooled lymph nodes of sex- and age-matched transgenic (n = 3) and control nontransgenic (n = 3) mice.
Lymph nodes of CD4C/HIVMutA transgenic mice progressively lose their DC but accumulate a subpopulation of CD11bHi DC.
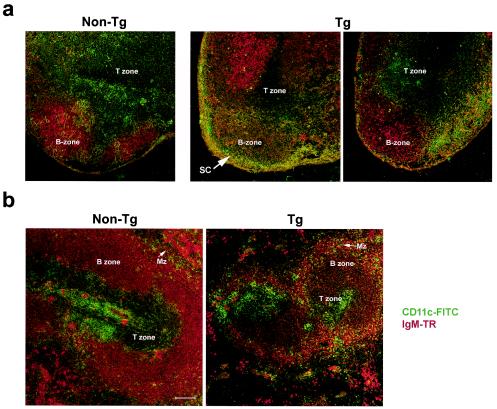
The absolute numbers of peripheral lymph node DC recovered from young (1.5 to 2.5 months) transgenic mice was not altered (Table 1). However, in older transgenic animals (3 to 5.5 months) and in mice with severe disease, the number of recovered DC had a tendency to be lower (1.5-fold) than in nontransgenic controls, suggesting loss of DC as disease progresses. The number of DC was further evaluated by an alternative technique, with a quantitative analysis of CD11c-positive cells on lymph node cryosections, as described in Materials and Methods. A significant 1.4-fold reduction (P = 0.025) of the area taken up by green fluorescent cells (CD11c+) was found in older transgenic animals (n = 10). The assessment of DC on transgenic lymph node cryosections also showed that they were greatly decreased in the T-cell zone and accumulated in the subcapsular sinuses compared to nontransgenic controls (Fig. 2a). This distribution of transgenic DC follows the pattern of transgene expression (65).
FIG. 2.
Detection of DC in cryosections of lymph nodes and spleen of CD4C/HIVMutA transgenic mice. The presence of DC was assessed on cryosections of peripheral lymph nodes (a) and spleen (b) of nontransgenic (non-Tg) and transgenic (Tg) mice at 3 months of age. IgM expression is in red (Texas Red [TR]) and CD11c expression is in green (fluorescein isothiocyanate [FITC]). Magnification, ×10; bar, 100 μm. Sections were analyzed by confocal microscopy. MZ, marginal zone; SC, subcapsular sinuses. Data are representative of at least three experiments, each involving the analysis of lymph node and spleen sections from sex- and age-matched transgenic (n = 2) and control nontransgenic (n = 2) mice.
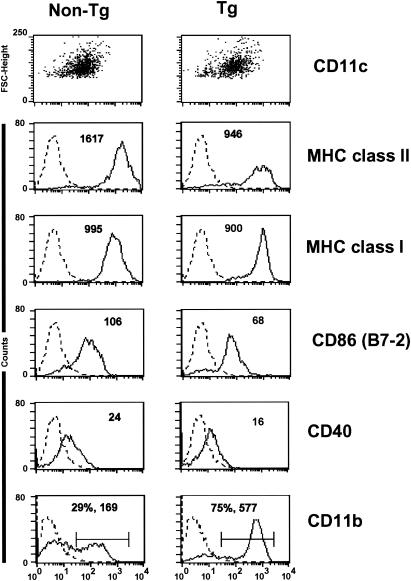
Phenotypic analysis of transgenic lymph node CD11c+ DC showed that they expressed MHC class I, CD40, and B7-2 with lower mean fluorescence intensity than nontransgenic DC (Fig. 3 and Table 2). Interestingly, a significant reduction in MHC class II mean fluorescence intensity was consistently observed, even early in the course of the disease (Fig. 3 and Table 2). Additional studies on DC from lymph nodes of CD4C/HIVMutG transgenic mice (which only express Nef of HIV-1) (35) also showed lower levels of MHC class II (mean fluorescence intensity ratio, nontransgenic/transgenic, 1.4 ± 0.1). A high proportion of the transgenic DC population showed a significant increase in mean fluorescence intensity for CD11b expression (CD11bHi) compared to nontransgenic DC (Fig. 3 and Tables 1 and 2). Therefore, the lymph nodes of transgenic mice accumulate CD11bHi MHC class IILo HIV+ DC, reflective of an immature phenotype (81).
FIG. 3.
Phenotypic characterization of lymph node DC in CD4C/HIVMutA transgenic mice. DC were purified from peripheral lymph nodes of nontransgenic (non-Tg) and transgenic (Tg) mice. The data show FACS analysis on CD11c+ gated lymph node DC from nontransgenic and transgenic mice. Results are shown as mean fluorescence intensity. Data are shown for DC from animals 3 months of age and are representative of at least 12 experiments with mice ranging between 2 and 4 months of age, each experiment involving cells from pooled lymph nodes of sex- and age-matched transgenic (n = 3) and control nontransgenic (n = 3) mice.
Lymph node DC of CD4C/HIVMutA transgenic mice are impaired in their antigen-presenting capacity.
The low MHC class II expression levels of transgenic lymph node DC could reflect altered functions. We therefore measured their capacity to stimulate a mixed leukocyte reaction following coculture with allogeneic nontransgenic CD4+ T cells. Transgenic lymph node DC from young mice stimulated the mixed leukocyte reaction comparably to nontransgenic DC (Fig. 4a). However, DC from older transgenic mice were less potent than DC from age- and sex-matched controls at any DC/T-cell ratio tested (Fig. 4a). A similar reduced mixed leukocyte reaction was also observed for lymph node DC from CD4C/HIVMutG transgenic mice (data not shown). Addition of IL-2 only partially restored these responses (Fig. 4a).
Transgenic DC from older animals also showed a reduced capacity to deliver costimulatory signals to syngeneic CD4+ T cells in the presence of low concentrations of anti-CD3 (0.01 to 0.1 μg/ml), consistent with the lower levels of costimulatory molecules found on these transgenic DC (Fig. 4b and Table 2). DC from older transgenic mice were also impaired in their capacity to present both pigeon cytochrome c protein (Fig. 4c) and peptide (Fig. 4d) to pigeon cytochrome c-specific CD4+ T cells compared to nontransgenic DC, at concentrations of pigeon cytochrome c protein or peptide ranging from 1 to 100 μg/ml and at any DC/T-cell ratio tested. Interestingly, DC from younger transgenic mice were capable of presenting both pigeon cytochrome c protein and peptide in a fashion similar to that of nontransgenic DC (data not shown). This differential response to pigeon cytochrome c presented by DC from young versus older transgenic mice was similar to that found for the mixed leukocyte reaction.
Increased DC numbers in the spleens of CD4C/HIVMutA transgenic mice.
We then determined whether the number of DC in transgenic spleens was affected. The absolute number of splenic DC (CD11c+ MHC class II+) recovered in young as well as in older transgenic animals or in mice with severe disease was increased (2.2-fold ± 0.9-fold) over that obtained from nontransgenic spleens. This contrasts with the tendency to lose DC in lymph nodes as disease progresses. The assessment of DC on splenic cryosections showed that they were increased in the transgenic compared to the nontransgenic marginal zone and that the T-cell zone was not deprived of DC (Fig. 2b). Interestingly, the marginal zone and T-cell zone correspond to the areas where high levels of HIV-1 transgene expression was found (65). With the alternative approach of fluorescence quantitation in situ, we did not find a significant increase in total DC (CD11c+) numbers in the spleens of transgenic animals (n = 12). These apparently contradictory results raise the possibility that transgenic spleen DC could be more resistant to the ex vivo isolation procedure.
FACS analysis of transgenic splenic DC showed that they expressed CD11c, MHC class I, MHC class II, CD40, and B7-2 as did DC from nontransgenic controls (data not shown). Again, a ≈1.5-fold reduction in MHC class II mean fluorescence intensity (compared to nontransgenic mice) was consistently observed, even early in the course of the disease (data not shown). Also, a large population of transgenic splenic DC showed an increase in mean fluorescence intensity for CD11b expression (data not shown). Therefore, as in transgenic lymph nodes, DC of an immature CD11bHi phenotype are augmented in transgenic spleens.
Impaired maturation of bone marrow-derived DC from CD4C/HIVMutA transgenic mice.
Since the majority of DC purified from transgenic lymph nodes and spleens showed a phenotype which resembled that described for immature “myeloid” DC (81), we studied the in vitro maturation of bone marrow-derived DC from transgenic mice of different ages. After 7 days in culture, the yield of recovered transgenic CD11c+ DC was similar to that of nontransgenic DC, suggesting that their precursor pool may be intact. The transgenic bone marrow-derived DC expressed high levels of HIV-1 RNA, as detected by in situ hybridization (Fig. 5a), and of Nef protein, as assessed by Western blotting (data not shown). FACS analysis showed similar levels of expression of CD11b and CD11c on transgenic as on nontransgenic DC (data not shown) and revealed that the mean fluorescence intensities for MHC class II, B7-2, and MHC class I were significantly lower on bone marrow-derived DC from transgenic mice compared to nontransgenic controls (Fig. 5b and Table 3). The mean fluorescence intensity for CD40 also showed a tendency to be lower on bone marrow-derived transgenic DC (Fig. 5b and Table 3). Similar studies on CD4C/HIVMutG transgenic mice also showed a reduction (1.8-fold ± 0.4-fold) in MHC class II expression on bone marrow-derived transgenic DC compared to nontransgenic DC.
FIG. 5.
Bone marrow-derived DC from CD4C/HIVMutA transgenic mice express the HIV transgene and are impaired in their maturation process. Bone marrow progenitors derived from the femur of nontransgenic (non-Tg) and transgenic (Tg) mice were allowed to mature for 6 days in the presence of 10% fetal bovine serum and GM-CSF. Cells were then recultured for an additional 24 h with (b) (right) or without (a and b) (left) lipopolysaccharide (10 μg/ml). The recovered cells showed dendritic morphology and expressed CD11c, as assessed by FACS. (a) These bone marrow-derived DC were analyzed for transgene expression by in situ hybridization with an HIV-1-specific antisense probe and a sense probe as a control. Counterstaining was done with hematoxylin and eosin. Magnification, ×40; bar, 50 μm. (b) Bone marrow-derived DC were analyzed by FACS for surface expression of MHC I, MHC II, CD40, and B7-2 (CD86) molecules. The data are representative of at least four different experiments, each involving cells from the bone marrow of sex- and age-matched transgenic (n = 3) and control nontransgenic (n = 3) mice.
To determine whether maturation could be enhanced in vitro, DC were stimulated with either lipopolysaccharide or via CD40, known to favor DC differentiation (81). Lipopolysaccharide induced an increased expression of MHC class I, MHC class II, CD40, and B7-2 relative to untreated cells in both transgenic and nontransgenic DC (Fig. 5b). Stimulation with either anti-CD40 monoclonal antibody or soluble CD8α-CD40 ligand gave comparable results, although to a weaker extent (data not shown). However, even after these treatments, lower percentages of DC expressing higher levels of these molecules were obtained in transgenic than in nontransgenic populations (Fig. 5b). The mean fluorescence intensity for these molecules was also lower on transgenic DC (Fig. 5b). Bone marrow-derived DC from transgenic and nontransgenic mice were as efficient at endocytosing fluorescein isothiocyanate-labeled latex beads, and lymph node and spleen transgenic DC did not endocytose more extensively than nontransgenic DC, despite their “immature” phenotype (data not shown).
CD8α+ DC in CD4C/HIVMutA transgenic mice.
In view of our findings that CD11bHi DC accumulate in transgenic mice, we also studied the CD8α+ DC (75), referred to here as lymphoid DC, despite the fact that CD8α can no longer definitively distinguish murine lymphoid DC (46), although it appears to be faithful for the characterization of murine thymic DC (13). The DC we studied do not include the B220+ plasmacytoid pre-DC described recently (56) (see Materials and Methods) and shown to be of lymphoid origin (13). The CD8α+ DC recovered from CD4C/HIVMutA transgenic mice were found to express high levels of the HIV-1 transgene, as quantitated by counting grains over purified thymic DC after in situ hybridization with an HIV-1-specific probe [nontransgenic DC: 0.5 ± 0.1/cell (n = 111); transgenic DC: 12 ± 0.7/cell (n = 102); data presented as mean ± standard error of the mean, P < 0.001]. In the lymph nodes of transgenic mice, the percentage of CD8α+ DC had a tendency to be lower than in their nontransgenic controls (Table 1).
In the thymus, all DC recovered were CD8α+ and the numbers of DC recovered also had a tendency to be lower in transgenic than nontransgenic animals (Table 4). The CD11bHi CD8α− population was not augmented (data not shown), in contrast to our observation in transgenic lymph nodes (Fig. 3 and Tables 1 and 2). Although the total DC loss was more apparent in older animals (Table 1), the low number of CD8α+ DC in transgenic lymph nodes and thymus was observed even in young animals (Tables 1 and 4).
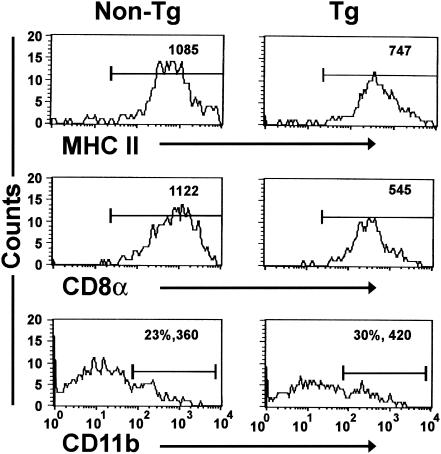
Marked reductions in MHC class II and CD8α mean fluorescence intensities were observed on DC isolated from transgenic thymus (Fig. 6 and Table 5) and lymph nodes (data not shown). However, the mean fluorescence intensity of CD11b was increased only on a subpopulation of thymic transgenic DC, present in both transgenic and nontransgenic mice (Fig. 6 and Table 5).
FIG. 6.
Thymic DC from CD4C/HIVMutA transgenic mice have reduced levels of MHC II and CD8α on their surface. DC were purified from the thymus of nontransgenic (non-Tg) and transgenic (Tg) mice 8 weeks of age and analyzed by FACS for surface expression of MHC II, CD8α, and CD11b. Data are representative of at least seven experiments with animals 6 to 16 weeks of age and are shown as a percentage and mean fluorescence intensity. Each experiment involved cells from pooled thymuses of sex- and age-matched transgenic (n = 3) and control nontransgenic (n = 3) mice.
DISCUSSION
Our study of DC populations in CD4C/HIV transgenic mice reveals that most of them express HIV-1 and show an impaired distribution, immature phenotype (MHC class IILo, CD8αLo, CD11bHi) (75, 81), and altered functions. Total DC in lymph nodes and thymus have a tendency to be less numerous as disease progresses. Also, oral mucosa MHC class II+ cells, possibly DC, have been found to be low in these transgenic mice (17). However, transgenic DC of a myeloid phenotype accumulate in the spleen and in the lymph node subcapsular sinuses.
Comparison of these results with those reported for human DC in HIV-infected individuals is not obvious. First, it is still not clear whether DC populations described in mice have their exact equivalents in humans (46, 54, 75, 78). Second, it seems that the number of HIV-1-infected DC is not high in infected individuals; it was even initially controversial whether they were infected at all (43, 86). Third, the DC populations studied here in lymph nodes, thymus, bone marrow, and spleen have not been extensively studied in HIV-1-infected humans. Finally, most reports with human DC do not distinguish infected from noninfected cells in their DC preparations. Despite these caveats, our findings in transgenic mice are in agreement with some reports for HIV-positive individuals. Indeed, in some HIV-positive individuals, DC were not found to be decreased in the spleen (51), while their numbers were reported to be low in blood (10, 20, 21, 25, 33, 38, 49, 57, 59), skin (55), lymphoid tissue (47), and oral mucosa (80).
Also, DC with an immature phenotype have been reported in some HIV-positive individuals (24, 51, 62). However, infection of human DC with HIV-1 in vitro has not been found to induce an immature phenotype (9, 53, 84), although Nef was reported to induce a distinct activation program (53). Despite the fact that the effects of HIV-1 expression on human DC in vivo have not all been studied, our results would suggest that in HIV-positive individuals, DC populations may also be affected in their distribution, be less numerous, and/or show an immature phenotype, thus contributing significantly to AIDS progression.
Impaired DC functions in CD4C/HIV transgenic mice.
Consistent with their general incapacity to undergo full maturation, DC from transgenic mice exhibit a lowered capacity to stimulate a mixed leukocyte reaction and present antigen. These results are reminiscent of some but not all (9, 11, 14, 84) studies on the reduced mixed leukocyte reaction or antigen presentation obtained with blood-derived DC from HIV-positive individuals (1, 20, 24, 49), with in vitro-infected blood-derived human DC (50), with monocytes (48), and even with MHC class II/Nef-transduced HeLa cells (83; reviewed in references 42 and 43). Our data are also similar to the decreased antigen presentation capacities reported for macrophages infected with HIV-1 (63). In addition, the finding that transgenic DC are impaired in their delivery of costimulatory signals, which is consistent with their low levels of CD40 and CD86 surface expression, is similar to the findings of Loré et al. (47) that DC in lymph nodes and tonsils of HIV-positive subjects and AIDS patients express lower levels of CD80, CD86, and CD40 on their surface.
These reduced functions are unlikely to be caused simply by the low expression levels of costimulatory or MHC class II molecules on transgenic DC, since the low CD40, CD86, and MHC class II phenotype is observed throughout disease in these transgenic mice, while the impairment of DC functions is progressive. The reduced antigen presentation by transgenic DC does not seem to reside at the level of antigen processing, given that presentation of both pigeon cytochrome c protein and peptide is reduced.
Interestingly, the functional defects of the transgenic DC population occur concomitantly with an apparent decrease in CD8α+ DC and increase in myeloid DC, suggesting that DC subpopulations are differentially affected by HIV-1. Similarly, human DC populations also seem to be affected differently by HIV-1. Indeed, plasmacytoid DC, which may be of lymphoid origin (79), have been found to be reduced in the blood of HIV-positive patients, whereas myeloid DC numbers and function remained normal in the same individuals (10). In addition, HIV-1 replication in human immature plasmacytoid DC has been shown to be triggered upon activation with CD40 ligand, and this DC subpopulation appears to be differently affected following in vitro infection by HIV-1 compared to human myeloid DC, which can replicate HIV-1 without CD40 ligand-induced maturation (26).
Thus, it seems that transgenic DC fail to undergo full maturation and to progressively lose their capacity to fully activate T cells. Whether this is due to direct or indirect effects of HIV gene products, transgenic DC probably have intrinsic functional defects which impair cellular interactions and signaling and/or expression or secretion of factors affecting other cells.
What could cause DC impairment in CD4C/HIV transgenic mice?
The variation in cell number and immaturity of DC in transgenic mice could be consequent to a direct effect of HIV-1 gene products, most probably Nef. Nef is mainly responsible for the development of the AIDS-like disease in the CD4C/HIVMutA and CD4C/HIVMutG transgenic mice (35). Indeed, CD4C/HIVMutG transgenic mice, which express only Nef, develop the same disease, and DC from the lymph nodes and bone marrow of these mice showed reduced levels of MHC class II, as was found for DC from CD4C/HIVMutA transgenic mice. Interestingly, Nef expression by immature human and macaque DC triggered cytokine and chemokine release as well as activation of STAT3 and NF-κB without upregulating molecules characteristic of mature DC such as HLA-DR and CD86 (52, 53). This disabled phenotypic maturation is similar to what we found in DC of CD4C/HIVMutA and CD4C/HIVMutG transgenic mice. However, in contrast to our findings, the latter Nef-expressing human DC were also very efficient at activating autologous CD4+ T cells (53). These contrasting results may simply reflect the DC origin and their in vitro culture conditions. Also, the fact that DC populations studied in CD4C/HIV transgenic mice have been altered by expression of HIV-1 gene products at earlier stages of differentiation may explain these differences.
It has been shown that the downregulation of endocytic activity during the process of DC maturation is controlled by Rho family GTPases, especially Cdc42 (30), which, once activated, binds to p21-activated kinase (16). p21-activated kinase is known to associate with HIV-1 Nef (72). We recently found that p21-activated kinase is highly activated in transgenic thymocytes and macrophages and is also activated in transgenic DC expressing Nef (Vincent, P., J. Poudrier, Z. Hanna, and P. Jolicoeur, unpublished data). Moreover, our studies with bone marrow-derived DC also support a direct effect of HIV gene products on inhibiting DC maturation.
However, our experiments cannot rule out indirect effects on DC and contributions from the impaired environment in these transgenic mice. Indeed, the low CD4 expression levels on transgenic T cells as well as their incapacity to express CD40 ligand (65) and their progressive depletion in the lymphoid organs (34, 35, 65), could affect DC localization to the T-cell zones and reduce availability of survival and maturation signals (3, 43, 54, 81). This may explain the tendency to recover lower numbers of CD8α+ DC in transgenic organs, since they preferentially localize in T-cell zones of lymphoid organs (81). Consistent with these views are the findings that in Rag2−/− and SCID mice, deficient in mature T cells, DC are impaired in their distribution, MHC class II expression levels, and function (76), a phenotype resembling that of DC from CD4C/HIV transgenic mice. Furthermore, accumulation of DC in the splenic marginal zone and in the lymph node subcapsular sinuses of transgenic mice suggests a homing defect, which may also be related to their disorganized and dysfunctional microenvironment (65).
Effect of DC alterations on the pathogenesis of CD4C/HIV transgenic mice.
Transgenic DC are also likely to affect their microenvironment. The segregation of myeloid DC in B-cell-rich areas may allow them to provide each other with signals for their persistence. DC have been shown to signal to B cells (4, 15, 23, 89) and plasmablasts (29). The expanded splenic marginal zone with numerous DC and IgMHi IgDLo B cells, as well as the increase in large IgMHi B cells surrounded by DC in the red pulp of transgenic mice (65) support this view. The altered transgenic DC phenotype and function, as well as factors expressed and/or released by transgenic DC, could contribute to the expanded B-cell compartment and autoantibody production found in these transgenic mice (65) and often reported in AIDS patients (19, 58, 70, 73). In support of these views, the immaturity of splenic DC has been reported in autoimmune disease-prone biobreeding rats (18). Also, expansion of the splenic marginal zone has recently been associated with overexpression of BlyS, which is expressed by DC (15). This led to B-cell hyperactivity, decreased T-cell numbers, and development of a systemic lupus erythematosus-like autoimmune disease, a phenotype resembling that of the CD4C/HIV transgenic mice.
Additionally, the altered distribution and function of transgenic DC in the periphery and in the thymus most probably contribute to the altered phenotype and function as well as the loss of CD4+ T cells in transgenic mice and may affect the outcome of the T-cell repertoire, leading to T-cell anergy and suppression (22, 31, 82, 85). Also, the fact that a large number of cells are lost by apoptosis in the transgenic lymphoid organs (35) and that capture of apoptotic cells by DC can trigger autoimmune reactions (66) or T-cell anergy (67; reviewed in reference 81) would be consistent with the production of autoantibodies (65) and the defective CD4+ T-cells response (65) observed in these transgenic mice.
Finally, it has recently been shown that immature DC favor the development of CD4+ immunosuppressive “regulatory” T cells (69, 74). It is therefore possible that the significant population of transgenic CD4+ T cells which express activation markers produce high amounts of gamma interferon (65) and are hyporesponsive to in vitro stimulation (Weng X., E. Paicepuiu, J. Poudrier, P. Chrobak, D. G. Kay, Z. Hanna, T. W. Mak, and P. Jolicoeur, unpublished data), similar to what has been reported for regulatory T cells (69, 74), may be selected and/or induced by transgenic DC.
Transgenic DC phenotype and virus replication.
Most of the work on the interaction of HIV-1 with DC has been done to test whether DC are infected or not in HIV-positive individuals or whether DC can be infected with HIV-1 (43, 86). Since the preintegration events of the HIV-1 viral cycle are bypassed in CD4C/HIV transgenic mice, our results are not relevant to these issues but are especially relevant to postintegration events. In this regard, the finding that Nef is required for efficient replication of HIV-1 in human DC-T-cell clusters, through upregulation of DC-SIGN (53, 61, 77), highlights the relevance of Nef expression in DC for optimal viral replication.
The striking immature phenotype of transgenic DC may be quite relevant to human AIDS by providing a selective replicative advantage to the virus. It has indeed been reported that HIV-1 preferentially replicates in immature DC (2, 8, 32) and that virions released from immature DC are far more infectious than those released from mature DC (27). Furthermore, the immature phenotype and loss of DC in transgenic mice may provide an additional adaptive advantage for HIV-1, preventing the generation of fully functional antiviral responses.
Conclusion.
CD4C/HIV transgenic mice develop numerous phenotypes which are very similar to those of human AIDS. This suggests that the underlying cellular and molecular causes of these phenotypes, including the DC alterations, may be the same in humans and in these mice. In this case, these transgenic mice should represent an adequate model to probe these pathways.
Acknowledgments
The first two authors contributed equally to this work.
We thank Isabelle Corbin, Karina Lamarre, and Eve-Lyne Thivierge for excellent animal care assistance and Patrick Couture, Stéphane Gagnon, Chunyan Hu, Pascale Jover, Benoit Laganière, and Ginette Massé for technical assistance. We are grateful to Christian Charbonneau and Hélène Lienard for excellent confocal microscopy and image-processing expertise. We thank Nathalie Tessier for flow cytometry services. We thank Miguel Chagnon (Dept. of Mathematics and Statistics, U. of Montreal, Canada) for statistical analyses. We also thank Rita Gingras for help in preparing the manuscript. We thank Patrice Hugo (presently at Metriogene, Canada) and Trevor Owens (McGill University, Canada) for providing some reagents and Marie H. Kosco-Vilbois (presently at Novimmune, Geneva) and Pavel Chrobak for reviewing the manuscript and for helpful comments.
This work was supported by grants to P.J. from the MRC of Canada and from the NHLBI (NIH) (HL59846) and to P.J. and J.P. from the FRSQ-SIDA.
REFERENCES
- 1.Andrieu, M., D. Chassin, J. F. Desoutter, I. Bouchaert, M. Baillet, D. Hanau, J. G. Guillet, and A. Hosmalin. 2001. Downregulation of major histocompatibility class I on human dendritic cells by HIV Nef impairs antigen presentation to HIV-specific CD8+ T lymphocytes. AIDS Res. Hum. Retrovir. 17:1365-1370. [DOI] [PubMed] [Google Scholar]
- 2.Bakri, Y., C. Schiffer, V. Zennou, P. Charneau, E. Kahn, A. Benjouad, J. C. Gluckman, and B. Canque. 2001. The maturation of dendritic cells results in postintegration inhibition of HIV-1 replication. J. Immunol. 166:3780-3788. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Bjorck, P., L. Flores-Romo, and Y. J. Liu. 1997. Human interdigitating dendritic cells directly stimulate CD40-activated naive B cells. Eur. J. Immunol. 27:1266-1274. [DOI] [PubMed] [Google Scholar]
- 5.Cameron, P. U., U. Forsum, H. Teppler, A. Granelli-Piperno, and R. M. Steinman. 1992. During HIV-1 infection most blood dendritic cells are not productively infected and can induce allogeneic CD4+ T cells clonal expansion. Clin. Exp. Immunol. 88:226-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 7.Cameron, P. U., M. G. Lowe, S. M. Crowe, U. O'Doherty, M. Pope, S. Gezelter, and R. M. Steinman. 1994. Susceptibility of dendritic cells to HIV-1 infection in vitro. J. Leukoc. Biol. 56:257-265. [DOI] [PubMed] [Google Scholar]
- 8.Canque, B., Y. Bakri, S. Camus, M. Yagello, A. Benjouad, and J. C. Gluckman. 1999. The susceptibility to X4 and R5 human immunodeficiency virus-1 strains of dendritic cells derived in vitro from CD34(+) hematopoietic progenitor cells is primarily determined by their maturation stage. Blood 93:3866-3875. [PubMed] [Google Scholar]
- 9.Canque, B., M. Rosenzwajg, S. Camus, M. Yagello, M. L. Bonnet, M. Guigon, and J. C. Gluckman. 1996. The effect of in vitro human immunodeficiency virus infection on dendritic-cell differentiation and function. Blood 88:4215-4228. [PubMed] [Google Scholar]
- 10.Chehimi, J., D. E. Campbell, L. Azzoni, D. Bacheller, E. Papasavvas, G. Jerandi, K. Mounzer, J. Kostman, G. Trinchieri, and L. J. Montaner. 2002. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J. Immunol. 168:4796-4801. [DOI] [PubMed] [Google Scholar]
- 11.Chougnet, C., S. S. Cohen, T. Kawamura, A. L. Landay, H. A. Kessler, E. Thomas, A. Blauvelt, and G. M. Shearer. 1999. Normal immune function of monocyte-derived dendritic cells from HIV-infected individuals: implications for immunotherapy. J. Immunol. 163:1666-1673. [PubMed] [Google Scholar]
- 12.Cimarelli, A., G. Zambruno, A. Marconi, G. Girolomoni, U. Bertazzoni, and A. Giannetti. 1994. Quantitation by competitive PCR of HIV-1 proviral DNA in epidermal Langerhans cells of HIV-infected patients. J. Acquir. Immune Defic. Syndr. 7:230-235. [PubMed] [Google Scholar]
- 13.Corcoran, L., I. Ferrero, D. Vremec, K. Lucas, J. Waithman, M. O'Keeffe, L. Wu, A. Wilson, and K. Shortman. 2003. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J. Immunol. 170:4926-4932. [DOI] [PubMed] [Google Scholar]
- 14.Cramer, L. A., and J. A. Frelinger. 2001. Dendritic cells transduced with HIV Nef express normal levels of HLA-A and HLA-B class I molecules. J. Acquir. Immune Defic. Syndr. 27:417-425. [DOI] [PubMed] [Google Scholar]
- 15.Cyster, J. G. 2000. B cells on the front line. Nat. Immunol. 1:9-10. [DOI] [PubMed] [Google Scholar]
- 16.Daniels, R. H., and G. M. Bokoch. 1999. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem. Sci. 24:350-355. [DOI] [PubMed] [Google Scholar]
- 17.de Repentigny, L., F. Aumont, J.-S. Ripeau, M. Fiorillo, D. G. Kay, Z. Hanna, and P. Jolicoeur. 2002. Mucosal candidiasis in transgenic mice expressing human immunodeficiency virus type 1. J. Infect. Dis. 185:1103-1114. [DOI] [PubMed] [Google Scholar]
- 18.Delemarre, F. G., P. J. Simons, H. J. de Heer, and H. A. Drexhage. 1999. Signs of immaturity of splenic dendritic cells from the autoimmune prone biobreeding rat: consequences for the in vitro expansion of regulator and effector T cells. J. Immunol. 162:1795-1801. [PubMed] [Google Scholar]
- 19.Ditzel, H. J., S. M. Barbas, C. F. Barbas 3rd, and D. R. Burton. 1994. The nature of the autoimmune antibody repertoire in human immunodeficiency virus type 1 infection. Proc. Natl. Acad. Sci. USA 91:3710-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donaghy, H., B. Gazzard, F. M. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 101:4505-4511. [DOI] [PubMed] [Google Scholar]
- 21.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c(+) myeloid and CD11c(-) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 98:2574-2576. [DOI] [PubMed] [Google Scholar]
- 22.Drakesmith, H., B. Chain, and P. Beverley. 2000. How can dendritic cells cause autoimmune disease? Immunol. Today 21:214-217. [DOI] [PubMed] [Google Scholar]
- 23.Dubois, B., B. Vanbervliet, J. Fayette, C. Massacrier, C. Van Kooten, F. Briere, J. Banchereau, and C. Caux. 1997. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J. Exp. Med. 185:941-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eales, L. J., J. Farrant, M. Helbert, and A. J. Pinching. 1988. Peripheral blood dendritic cells in persons with AIDS and AIDS related complex: loss of high intensity class II antigen expression and function. Clin. Exp. Immunol. 71:423-427. [PMC free article] [PubMed] [Google Scholar]
- 25.Fan, Z., X. L. Huang, L. Zheng, C. Wilson, L. Borowski, J. Liebmann, P. Gupta, J. Margolick, and C. Rinaldo. 1997. Cultured blood dendritic cells retain HIV-1 antigen-presenting capacity for memory CTL during progressive HIV-1 infection. J. Immunol. 159:4973-4982. [PubMed] [Google Scholar]
- 26.Fong, L., M. Mengozzi, N. W. Abbey, B. G. Herndier, and E. G. Engleman. 2002. Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J. Virol. 76:11033-11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank, I., L. Kacani, H. Stoiber, H. Stossel, M. Spruth, F. Steindl, N. Romani, and M. P. Dierich. 1999. Human immunodeficiency virus type 1 derived from cocultures of immature dendritic cells with autologous T cells carries T-cell-specific molecules on its surface and is highly infectious. J. Virol. 73:3449-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frankel, S. S., B. M. Wenig, A. P. Burke, P. Mannan, L. D. Thompson, S. L. Abbondanzo, A. M. Nelson, M. Pope, and R. M. Steinman. 1996. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science 272:115-117. [DOI] [PubMed] [Google Scholar]
- 29.Garcia, D., V, A. Gulbranson-Judge, M. Khan, P. O'Leary, M. Cascalho, M. Wabl, G. G. Klaus, M. J. Owen, and I. C. MacLennan. 1999. Dendritic cells associated with plasmablast survival. Eur. J. Immunol. 29:3712-3721. [DOI] [PubMed] [Google Scholar]
- 30.Garrett, W. S., L. M. Chen, R. Kroschewski, M. Ebersold, S. Turley, S. Trombetta, J. E. Galan, and I. Mellman. 2000. Developmental control of endocytosis in dendritic cells by Cdc42. Cell 102:325-334. [DOI] [PubMed] [Google Scholar]
- 31.Goldrath, A. W., and M. J. Bevan. 1999. Selecting and maintaining a diverse T-cell repertoire. Nature 402:255-262. [DOI] [PubMed] [Google Scholar]
- 32.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grassi, F., A. Hosmalin, D. McIlroy, V. Calvez, P. Debre, and B. Autran. 1999. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS 13:759-766. [DOI] [PubMed] [Google Scholar]
- 34.Hanna, Z., D. G. Kay, M. Cool, S. Jothy, N. Rebai, and P. Jolicoeur. 1998. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J. Virol. 72:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanna, Z., D. G. Kay, N. Rebai, A. Guimond, S. Jothy, and P. Jolicoeur. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163-175. [DOI] [PubMed] [Google Scholar]
- 36.Hanna, Z., N. Rebai, J. Poudrier, and P. Jolicoeur. 2001. Distinct regulatory elements are required for faithful expression of human CD4 in T cells, macrophages and dendritic cells of transgenic mice. Blood 98:2275-2278. [DOI] [PubMed] [Google Scholar]
- 37.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones, G. J., C. Watera, S. Patterson, A. Rutebemberwa, P. Kaleebu, J. A. Whitworth, F. M. Gotch, and J. W. Gilmour. 2001. Comparative loss and maturation of peripheral blood dendritic cell subpopulations in African and non-African HIV-1-infected patients. AIDS 15:1657-1663. [DOI] [PubMed] [Google Scholar]
- 39.Karasuyama, H., and F. Melchers. 1988. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, with modified cDNA expression vectors. Eur. J. Immunol. 18:97-104. [DOI] [PubMed] [Google Scholar]
- 40.Karhumaki, E., M. E. Viljanen, M. Cottler-Fox, A. Ranki, C. H. Fox, and K. J. Krohn. 1993. An improved enrichment method for functionally competent, highly purified peripheral blood dendritic cells and its application to HIV-infected blood samples. Clin. Exp. Immunol. 91:482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaye, J., N. J. Vasquez, and S. M. Hedrick. 1992. Involvement of the same region of the T-cell antigen receptor in thymic selection and foreign peptide recognition. J. Immunol. 148:3342-3353. [PubMed] [Google Scholar]
- 42.Knight, S. C. 1994. AIDS. A problem of antigen presentation? Curr. Biol. 4:1131-1134. [DOI] [PubMed] [Google Scholar]
- 43.Knight, S. C., and S. Patterson. 1997. Bone marrow-derived dendritic cells, infection with human immunodeficiency virus, and immunopathology. Annu. Rev. Immunol. 15:593-615. [DOI] [PubMed] [Google Scholar]
- 44.Lane, P., T. Brocker, S. Hubele, E. Padovan, A. Lanzavecchia, and F. McConnell. 1993. Soluble CD40 ligand can replace the normal T-cell-derived CD40 ligand signal to B cells in T-cell-dependent activation. J. Exp. Med. 177:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langhoff, E., E. F. Terwilliger, H. J. Bos, K. H. Kalland, M. C. Poznansky, O. M. Bacon, and W. A. Haseltine. 1991. Replication of human immunodeficiency virus type 1 in primary dendritic cell cultures. Proc. Natl. Acad. Sci. USA 88:7998-8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu, Y. J., H. Kanzler, V. Soumelis, and M. Gilliet. 2001. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2:585-589. [DOI] [PubMed] [Google Scholar]
- 47.Lore, K., A. Sonnerborg, C. Brostrom, L. E. Goh, L. Perrin, H. McDade, H. J. Stellbrink, B. Gazzard, R. Weber, L. A. Napolitano, Y. van Kooyk, and J. Andersson. 2002. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS 16:683-692. [DOI] [PubMed] [Google Scholar]
- 48.Louie, A. T., L. M. Wahl, I. K. Hewlett, J. S. Epstein, and S. Dhawan. 1996. Impaired antigen presentation to CD4+ T cells by HIV-infected monocytes is related to down-modulation of CD4 expression on helper T cells: possible involvement of HIV-induced cellular factors. FEBS Lett. 398:1-6. [DOI] [PubMed] [Google Scholar]
- 49.Macatonia, S. E., R. Lau, S. Patterson, A. J. Pinching, and S. C. Knight. 1990. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology 71:38-45. [PMC free article] [PubMed] [Google Scholar]
- 50.Macatonia, S. E., S. Patterson, and S. C. Knight. 1989. Suppression of immune responses by dendritic cells infected with HIV. Immunology 67:285-289. [PMC free article] [PubMed] [Google Scholar]
- 51.McIlroy, D., B. Autran, J. P. Clauvel, E. Oksenhendler, P. Debre, and A. Hosmalin. 1998. Low CD83, but normal MHC class II and costimulatory molecule expression, on spleen dendritic cells from HIV-positive patients. AIDS Res. Hum. Retrovir. 14:505-513. [DOI] [PubMed] [Google Scholar]
- 52.Messmer, D., J. Bromberg, G. Devgan, J. M. Jacque, A. Granelli-Piperno, and M. Pope. 2002. Human immunodeficiency virus type 1 Nef mediates activation of STAT3 in immature dendritic cells. AIDS Res. Hum. Retrovir. 18:1043-1050. [DOI] [PubMed] [Google Scholar]
- 53.Messmer, D., J. M. Jacque, C. Santisteban, C. Bristow, S. Y. Han, L. Villamide-Herrera, E. Mehlhop, P. A. Marx, R. M. Steinman, A. Gettie, and M. Pope. 2002. Endogenously expressed nef uncouples cytokine and chemokine production from membrane phenotypic maturation in dendritic cells. J. Immunol. 169:4172-4182. [DOI] [PubMed] [Google Scholar]
- 54.Moser, M., and K. M. Murphy. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1:199-205. [DOI] [PubMed] [Google Scholar]
- 55.Muller, H., S. Weier, G. Kojouharoff, M. Grez, S. Berger, R. Kappus, P. M. Shah, H. J. Stutte, and H. L. Schmidts. 1993. Distribution and infection of Langerhans cells in the skin of HIV-infected healthy subjects and AIDS patients. Res. Virol. 144:59-67. [DOI] [PubMed] [Google Scholar]
- 56.O'Keeffe, M., H. Hochrein, D. Vremec, I. Caminschi, J. L. Miller, E. M. Anders, L. Wu, M. H. Lahoud, S. Henri, B. Scott, P. Hertzog, L. Tatarczuch, and K. Shortman. 2002. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function that differentiate into CD8(+) dendritic cells only after microbial stimulus. J. Exp. Med. 196:1307-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pacanowski, J., S. Kahi, M. Baillet, P. Lebon, C. Deveau, C. Goujard, L. Meyer, E. Oksenhendler, M. Sinet, and A. Hosmalin. 2001. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood 98:3016-3021. [DOI] [PubMed] [Google Scholar]
- 58.Pantaleo, G., and A. S. Fauci. 1995. New concepts in the immunopathogenesis of HIV infection. Annu. Rev. Immunol. 13:487-512. [DOI] [PubMed] [Google Scholar]
- 59.Patterson, S., N. R. English, H. Longhurst, P. Balfe, M. Helbert, A. J. Pinching, and S. C. Knight. 1998. Analysis of human immunodeficiency virus type 1 (HIV-1) variants and levels of infection in dendritic and T cells from symptomatic HIV-1-infected patients. J. Gen. Virol. 79:247-257. [DOI] [PubMed] [Google Scholar]
- 60.Patterson, S., J. Gross, N. English, A. Stackpoole, P. Bedford, and S. C. Knight. 1995. CD4 expression on dendritic cells and their infection by human immunodeficiency virus. J. Gen. Virol. 76:1155-1163. [DOI] [PubMed] [Google Scholar]
- 61.Petit, C., F. Buseyne, C. Boccaccio, J. P. Abastado, J. M. Heard, and O. Schwartz. 2001. Nef is required for efficient HIV-1 replication in cocultures of dendritic cells and lymphocytes. Virology 286:225-236. [DOI] [PubMed] [Google Scholar]
- 62.Pimpinelli, N., L. Borgognoni, R. Riccardi, G. Ficarra, M. Mori, D. Gaglioti, and P. Romagnoli. 1991. CD36(OKM5)+ dendritic cells in the oral mucosa of HIV- and HIV-positive subjects. J. Investig. Dermatol. 97:537-542. [DOI] [PubMed] [Google Scholar]
- 63.Polyak, S., H. Chen, D. Hirsch, I. George, R. Hershberg, and K. Sperber. 1997. Impaired class II expression and antigen uptake in monocytic cells after HIV-1 infection. J. Immunol. 159:2177-2188. [PubMed] [Google Scholar]
- 64.Pope, M., M. G. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. G. S. Hoffman, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78:389-398. [DOI] [PubMed] [Google Scholar]
- 65.Poudrier, J., X. Weng, D. G. Kay, G. Paré, E. L. Calvo, Z. Hanna, M. H. Kosco-Vilbois, and P. Jolicoeur. 2001. The AIDS disease of CD4C/HIV transgenic mice shows impaired germinal centers and autoantibodies and develops in the absence of IFN-γ and IL-6. Immunity 15:173-185. [DOI] [PubMed] [Google Scholar]
- 66.Propato, A., G. Cutrona, V. Francavilla, M. Ulivi, E. Schiaffella, O. Landt, R. Dunbar, V. Cerundolo, M. Ferrarini, and V. Barnaba. 2001. Apoptotic cells overexpress vinculin and induce vinculin-specific cytotoxic T-cell cross-priming. Nat. Med. 7:807-813. [DOI] [PubMed] [Google Scholar]
- 67.Quaratino, S., L. P. Duddy, and M. Londei. 2000. Fully competent dendritic cells as inducers of T-cell anergy in autoimmunity. Proc. Natl. Acad. Sci. 97:10911-10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rinfret, A., H. Latendresse, R. Lefebvre, G. St-Louis, P. Jolicoeur, and L. Lamarre. 1991. HIV-infected multinucleated histiocytes in oropharyngeal lymphoid tissues from two asymptomatic patients. Am. J. Clin. Pathol. 138:421-426. [PMC free article] [PubMed] [Google Scholar]
- 69.Roncarolo, M. G., M. K. Levings, and C. Traversari. 2001. Differentiation of T regulatory cells by immature dendritic cells. J. Exp. Med. 193:F5-F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Root-Bernstein, R. S. 1990. Multiple-antigen-mediated autoimmunity (MAMA) in AIDS: A possible model for postinfectious autoimmune complications. Res. Immunol. 141:321-339. [DOI] [PubMed] [Google Scholar]
- 71.Rowland-Jones, S. L. 1999. HIV: The deadly passenger in dendritic cells. Curr. Biol. 9:R248-R250. [DOI] [PubMed] [Google Scholar]
- 72.Sawai, E. T., C. Cheng-Mayer, and P. A. Luciw. 1997. Nef and the Nef-associated kinase. Res. Virol. 148:47-52. [DOI] [PubMed] [Google Scholar]
- 73.Shearer, G. M. 1998. HIV-induced immunopathogenesis. Immunity 9:587-593. [DOI] [PubMed] [Google Scholar]
- 74.Shevach, E. M. 2001. Certified professionals: CD4(+)CD25(+) suppressor T cells. J. Exp. Med. 193:F41-F46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shortman, K., D. Vremec, L. M. Corcoran, K. Georgopoulos, K. Lucas, and L. Wu. 1998. The linkage between T-cell and dendritic cell development in the mouse thymus. Immunol. Rev. 165:39-46. [DOI] [PubMed] [Google Scholar]
- 76.Shreedhar, V., A. M. Moodycliffe, S. E. Ullrich, C. Bucana, M. L. Kripke, and L. Flores-Romo. 1999. Dendritic cells require T cells for functional maturation in vivo. Immunity 11:625-636. [DOI] [PubMed] [Google Scholar]
- 77.Sol-Foulon, N., A. Moris, C. Nobile, C. Boccaccio, A. Engering, J. P. Abastado, J. M. Heard, Y. van Kooyk, and O. Schwartz. 2002. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity 16:145-155. [DOI] [PubMed] [Google Scholar]
- 78.Sousa, C. 2001. Dendritic cells as sensors of infection. Immunity 14:495-498. [DOI] [PubMed] [Google Scholar]
- 79.Spits, H., F. Couwenberg, A. Q. Bakker, K. Weijer, and C. H. Uittenbogaart. 2000. Id2 and Id3 inhibit development of CD34(+) stem cells into predendritic cell (pre-DC)2 but not into pre-DC1. Evidence for a lymphoid origin of pre-DC2. J. Exp. Med. 192:1775-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sporri, B., J. von Overbeck, C. U. Brand, J. Schmidli, M. L. Sanchez, and R. B. LR. Grunow. 1994. Reduced number of Langerhans cells in oral mucosal washings from HIV-1 seropositives. J. Oral Pathol. Med. 23:399-402. [DOI] [PubMed] [Google Scholar]
- 81.Steinman, R. M., M. Pack, and K. Inaba. 1997. Dendritic cells in the T-cell areas of lymphoid organs. Immunol. Rev. 156:25-37. [DOI] [PubMed] [Google Scholar]
- 82.Steinman, R. M., S. Turley, I. Mellman, and K. Inaba. 2000. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 191:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 98:12144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verhasselt, B., E. Naessens, C. Verhofstede, M. De Smedt, S. Schollen, T. Kerre, D. Vanhecke, and J. Plum. 1999. Hum. immunodeficiency virus nef gene expression affects generation and function of human T cells, but not dendritic cells. Blood 94:2809-2818. [PubMed] [Google Scholar]
- 85.Viret, C., F. S. Wong, and C. A. Janeway, Jr. 1999. Designing and maintaining the mature T-cell receptor repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity 10:559-568. [DOI] [PubMed] [Google Scholar]
- 86.Weissman, D., and A. S. Fauci. 1997. Role of dendritic cells in immunopathogenesis of human immunodeficiency virus infection. Clin. Microbiol. Rev. 10:358-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wood, G. S., N. L. Warner, and R. A. Warnke. 1983. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J. Immunol. 131:212-216. [PubMed] [Google Scholar]
- 88.Wu, L., D. Vremec, C. Ardavin, K. Winkel, G. Suss, H. Georgiou, E. Maraskovsky, W. Cook, and K. Shortman. 1995. Mouse thymus dendritic cells: kinetics of development and changes in surface markers during maturation. Eur. J. Immunol. 25:418-425. [DOI] [PubMed] [Google Scholar]
- 89.Wykes, M., A. Pombo, C. Jenkins, and G. G. MacPherson. 1998. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 161:1313-1319. [PubMed] [Google Scholar]