Abstract
Aggregation chimeras were formed between C57BL/6 mice heterozygous for the Apcmin (Min) mutation and wild-type SWR mice, that differ in their Pla2g2a status, a modifier of Apcmin, and also in their resistance to intestinal polyp formation. Variation in the dolichos biflorus agglutinin-staining patterns of the intestines of these mouse strains was used to determine the chimeric composition of the intestine in individual mice and to examine the clonal composition of adenomas. Macroscopic adenoma numbers in chimeric mice were compared with the expected adenoma numbers based on the percentage of C57BL/6J-Apcmin/+ epithelium in individual mice. These results unexpectedly show that there was no apparent inhibitory effect of the SWR-derived (Pla2g2a wild-type) tissue on adenoma formation in the C57BL/6J-Apcmin/+ epithelium. This suggests that the main genetic modifiers of the Min phenotype act at a cellular or crypt-restricted level with no discernable systemic effect. All adenomas were seen to contain C57BL/6J-Apcmin/+-derived epithelium, confirming that the germ-line mutation of the mApc gene is necessary to initiate tumorigenesis in this model system, and that the mApc gene acts in a cell autonomous fashion.
Familial adenomatous polyposis (FAP) is an autosomal dominant disease in which affected individuals develop hundreds to thousands of adenomatous polyps throughout their gastrointestinal tract. This disease has been shown to result from mutations in the adenomatous polyposis coli (APC) gene. Closer inspection of some polyposis kindreds has shown a marked variation in the FAP phenotype between individual members of the same family carrying identical APC mutations. This finding has led to the suggestion that there may be genetic modifiers of FAP that affect the FAP phenotype within individual patients.
The discovery of the multiple intestinal neoplasia (Min) mouse (1), a model of FAP identified during a germ-line mutagenesis experiment, has enabled the search for genetic modifiers of the FAP phenotype. Back crossing C57BL/6J-Apcmin/+ to other inbred strains has shown significant variations in adenoma multiplicity on different genetic backgrounds. A single genetic modifier called Mom1 appears responsible for around 50% of this variation in adenoma number (2). This modifier has been mapped to distal mouse chromosome 4, where a strong candidate for this modifying effect is the secretory phospholipase A2 (Pla2g2a) gene (3). Further experimental evidence supporting a modifying effect of this secretory phospholipase has been provided by using transgenic mice overexpressing Pla2g2a. Overexpression of this cosmid transgene caused some reduction in tumor multiplicity and size, comparable to the effects conferred by a single copy of the resistance allele Mom1 (4).
Recent research has shown that human FAP adenomas may be polyclonal (5) as well as chimeric Min intestinal adenomas (6). Several possible explanations may account for this observed polyclonality: “field” effects may cause adenomas to cluster (nonrandom collision); multiple adenomatous clones may be required for (or strongly favor) early adenoma growth; or perhaps, early adenomas may induce adenomatous growth in surrounding crypts. To address these issues and study the potential effects of genetic modifiers of Apcmin on intestinal polyp numbers, Min mouse aggregation chimeras have been formed by using a combination of C57BL/6-Apcmin/+ mice with wild-type SWR mice, two mouse strains differing in their Pla2g2a status, and so in their resistance to Apcmin polyp formation.
Materials and Methods
Mouse Husbandry and the Establishment of the Imperial Cancer Research Fund (ICRF) Min C57BL/6J Colony.
Min (C57BL/6J-Apcmin/+) heterozygote mice were originally obtained in 1992 (gift from A. Moser, McArdle Laboratories, University of Wisconsin, Madison, WI). Male mice were initially back-crossed to female C57BL/6J mice (ICRF Biological Resources, Clare Hall, South Mimms, Hertfordshire, United Kingdom), and the resultant embryos were transferred by aseptic hysterectomy to foster mothers in specified pathogen-free isolators. All breeding was subsequently performed in specified pathogen-free units by brother (C57BL/6J-Apcmin/+) mated with sister (C57BL/6J-Apc+/+). All mice were then reared on open shelves (nonspecified pathogen-free) and allowed food and water ad libitum.
Chimera Formation.
Chimeras were made by aggregation (7). C57BL/6-Apcmin/+ mice were mated with C57BL/6-Apc+/+ mice and the resulting morulae aggregated with wild-type SWR morulae.
Genotyping of Mice.
Tail snips were obtained from mice at 3 weeks of age, and genomic DNA was prepared by proteinase K digestion using standard techniques. All mice were typed for their Min status by using an allele-specific PCR analysis as described (2). A PCR methodology was designed for assessment of the Mom1 resistance allele status. From current evidence, the secretory phospholipase A2 gene (Pla2g2a) gene maps to the Mom1 region and appears to confer a similar or equivalent resistance to Mom1. The Pla2g2a allele is null homozygous in the C57BL/6J genetic background and contains an A/T insertion in exon three of Pla2g2a leading to premature termination and extremely low levels of intestinal secretory phospholipase A2 mRNA. This absence or low level of expression is presumed to confer sensitivity to polyp formation with Apcmin/+. An intragenic allele-specific PCR, spanning exons three and four of the Pla2g2a gene detecting the presence or absence of the null allele of the Pla2g2a gene, was designed to produce an internal control Pla2g2a DNA product (≈355 bp) and second DNA product (≈260 bp) specific for the Pla2g2a null allele. Three oligonucleotide primers: 5′-GTCCAAGGGAACATTGCG-3′, 5′-GCCTGGGTGGCAAAGGATT-3′, and 5′-CAGTCATGAGTAACACAGCACC-3′ (called 98E3.F, 192MUT.F, and 252E4.R, respectively) were run under standard PCR conditions with an annealing temperature of 60°C. The internal control band of 355 bp also spans a BamHI digestion site. Homozygosity for the wild-type Pla2g2a could therefore be further confirmed by digesting 10 μl of the 355-bp PCR product with BamHI, whereas PCR products from mice homozygous for mutant Pla2g2a were resistant to digestion. By this method, the SWR strain, known to be a polyp-resistant strain (8), was shown to be Pla2g2a+/+. Any C57BL/6 ↔ SWR chimeras would, therefore, be expected to show two PCR bands for Pla2g2a at 355 bp and 260 bp, with BamHI digestion of the 355-bp band.
Postmortem Examination of Mice.
Mice were killed by CO2 inhalation. The relative contributions of each mouse strain to the coat color of individual chimeric mice were assessed to the nearest 5% by an experienced animal technician. A postmortem examination of all major organs was then performed on each mouse. The small intestine was divided into three portions of equal length (SB1, proximal; SB2, middle; and SB3, distal). Intestinal tumors were then assessed in each small bowel segment (SB1, SB2, and SB3) and in the large bowel (LB) by using a magnification lens (×10) to determine intestinal tumor number, site, and size (9). The entire small and large intestines and samples from other major organs were fixed overnight in methacarn, transferred to 70% ethanol, and then routinely processed to paraffin for histological analysis.
Staining with Dolichos Biflorus Agglutinin (DBA).
Tissue sections (4 μm) from the intestine were stained with horseradish peroxidase-conjugated DBA (1:100) (previously United States Biochemical, now Amersham International) and developed with 3′,3′-diaminobenzidine by using a one-step immunohistochemical technique. Nuclei were counterstained with hematoxylin. Intestinal sections were then examined and the staining pattern of individual crypts was assessed. The numbers of DBA-positive and -negative crypts were then assessed for each bowel segment. Serial sections were also stained with hematoxylin and eosin for detailed histopathological investigation.
Control DBA staining was performed on tissue sections derived from eight C57BL/6 mice (three Apcmin/+ and two Apc+/+ control mice obtained from the ICRF breeding unit, and three Apcmin/+ mice derived from the chimera experiment) and four control SWR mice.
Results
Formation of Chimeras.
Aggregations (238) were performed resulting in 206 apparently chimeric blastocysts. These were transferred into 15 pseudopregnant F1 (CBA crossed with C57BL/6) foster mothers, resulting in 88 live-born mice. Coat color examination of the 79 mice surviving to adulthood showed that there were 9 C57BL/6 (black) mice, 17 SWR (white) mice, and 53 C57BL/6 ↔ SWR chimeras. The percentage of coat color derived from each mouse strain was highly variable in different chimeric mice, with further variation in the patch sizes and patterns of patches.
PCR Analysis of Mice.
Triplicate PCR analysis showed that 19 mice carried the Apcmin mutation and the remaining 60 mice were totally Apc+/+. Of the 19 mice carrying the Apcmin mutation, there were 16 C57BL/6 ↔ SWR chimeras and 3 C57BL/6 mice. PCR analysis of Pla2g2a status confirmed that the C57BL/6 mouse strain was homozygous null for Pla2g2a. The SWR mouse strain was Pla2g2a+/+, whereas the C57BL/6 ↔ SWR chimeras had both null and wild-type alleles for Pla2g2a.
Postmortem Examination of Mice.
A single C57BL/6-Apcmin/+ mouse and an SWR mouse resulting from the chimera experiment were killed at 3 months of age to act as controls for DBA staining. A C57BL/6-Apc+/+ and a C57BL/6-Apcmin/+↔SWR were found dead without obvious pathology at postmortem. Two C57BL/6 Min mice developed rapidly enlarging mammary tumors and were killed, but none of the 16 C57BL/6-Apcmin/+ ↔ SWR chimeric mice developed breast tumors. All surviving Apcmin/+ mice were killed at approximately 6 months of age (mean age 175 ± 2.6 days). Because of the limited number of C57BL/6-Apcmin/+ mice resulting from the experiment for the formation of chimeras, three more C57BL/6-Apcmin/+ mice bred in the ICRF facility under similar rearing conditions were used as controls for DBA staining. These mice were killed at 5 months of age. Macroscopic polyp counts were obtained from a parallel group of approximately 100 control C57BL/6-Apcmin/+ mice [small intestine: SB1 = 4.5 (±00.76 SEM), SB2 = 8.1 (±1.15), SB3 = 13.9 (±1.58); large intestine = 1.8 (±0.2)] (9). The mean age at death of these control mice was 231 ± 9.5 days.
Control DBA Staining of SWR and C57BL/6 Tissues.
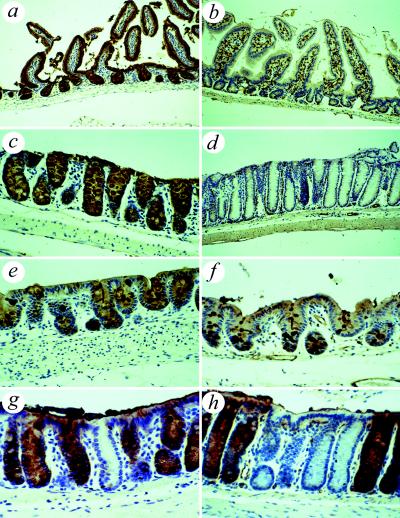
Sections showed that, as described in C57BL/6 mice, the intestinal epithelium stained strongly positive, whereas the vascular endothelium stained negative. Conversely in the SWR mouse, the intestinal epithelium stained negative, whereas the vascular endothelium stained strongly positive (10) (Fig. 1). On close examination, however, there appeared to be an area of anomalous staining in the cecum of both strains with weakly DBA-positive enterocytes and scattered strongly DBA-positive goblet cells (Fig. 1 e and f). This area was of varying size, located primarily in the cecum, but extended into the ascending colon in some animals. Examination of the rectum and distal colon of C57BL/6 mice also showed occasional single crypts or small collections of crypts that were entirely DBA-negative (Fig. 1g). Similar close examination of the staining pattern of the small intestine of both C57BL/6 and SWR mice showed no evidence of variation with all C57BL/6 small intestinal epithelium staining strongly positive (Fig. 1a) and all SWR small intestinal epithelium staining negative for DBA (Fig. 1b). The DBA staining pattern of all other tissues examined was identical between these two mouse strains (data not shown).
Figure 1.
DBA staining of mouse intestines. (a) C57BL/6 small intestine showing uniform positive staining of epithelial cells. (b) SWR small intestine showing uniform negative staining of epithelial cells. (c) C57BL/6 colon showing uniform positive staining of epithelial cells but no staining of vascular endothelium. (d) SWR colon showing uniform negative staining of epithelial cells but strong positivity of vascular endothelium. (e) C57BL/6 showing aberrant staining in the cecum with strongly positive goblet cells and weak staining of enterocytes. (f) SWR showing aberrant staining in cecal epithelium similar in appearance to that seen in C57BL/6 mice. (g) C57BL/6 rectum showing loss of staining in occasional colonic crypts. (h) Staining of colon of chimera showing a patch of DBA-negative SWR-derived crypts surrounded by C57BL/6-derived DBA-positive crypts.
DBA Staining of Intestinal Crypts in C57BL/6-Apcmin ↔ SWR Chimeras.
Examination of the DBA staining pattern of the intestine in chimeras containing C57BL/6-Apcmin/+ epithelium showed that crypts were entirely DBA-positive or entirely DBA-negative. No mixed crypts were seen. DBA-positive and -negative crypts were arranged in patches of varying size, ranging from single crypts to patches more than 100 crypts wide (Fig. 1h). An intermediate DBA staining pattern was detected in the cecum and ascending colon as noted in both C57BL/6 and SWR control mice.
For each of the 15 adult chimeras containing C57BL/6-Apcmin/+ epithelium, the numbers of DBA-positive and -negative crypts were counted from each of the intestinal sections (SB1, SB2, SB3, and LB). Areas of intermediate DBA staining in the cecum and ascending colon were not assessed. A total of 88,399 intestinal crypts were examined, with a mean of 5,893 crypts counted per chimera. The mean percentage of DBA-positive crypts in individual chimeras was calculated and compared with the percentage of black coat color. Results showed that there was a positive correlation between chimera coat color and intestinal crypt-staining pattern (n = 15, r2 = 0.681, correlation coefficient r = 0.825, P < 0.001; Fig. 2).
Figure 2.
Scattergraph showing percentage of coat color black (Min positive) vs. percentage of intestinal crypts staining with DBA.
By using figures for the macroscopic tumor numbers from a large parallel series of control C57BL/6-Apcmin/+ mice reared under identical conditions and assessed in an identical fashion, the expected number of tumors, based on the percentage of DBA-positive crypts, was calculated for each bowel section for individual chimeras. Expected polyp no. = control C57BL/6-Apcmin/+polyp no. × (DBA-positive crypts/total crypts).
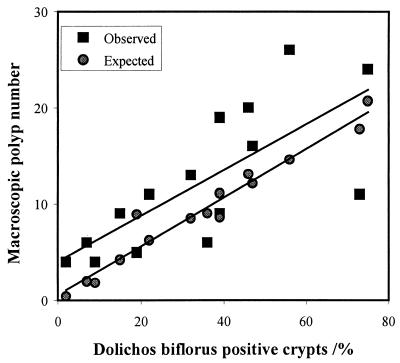
The total number of small intestinal tumors expected for each animal was then calculated and compared with the actual number of tumors detected. Results from these calculations show that overall, there were more small intestinal polyps occurring in the Min chimeras than would have been expected if polyp number depended simply on the number of DBA-positive, and so C57BL/6-Apcmin/+ crypts, present (Wilcox matched pairs sign test, P = 0.035; Fig. 3).
Figure 3.
A graph of observed and expected macroscopic polyp number against percentage of DBA-positive crypts. Wilcox matched pairs sign test, P = 0.035.
Furthermore, the ratio of the observed-to-expected polyp number is significantly negatively correlated with the percentage of DBA-positive crypts (correlation coefficient −0.545, P = 0.035; Fig. 4). This suggests that either the presence of normal SWR tissues or the relative absence of C57BL/6 tissues increases the probability of polyps arising on C57BL/6-Apcmin/+ epithelium.
Figure 4.
Ratio of observed-to-expected polyp number against percentage of DBA-positive crypts. Correlation coefficient = −0.545 and P < 0.03.
Very few 4-μm macroscopic colonic polyps were detected (7 macroscopic polyps arose in 16 mice, which is within the expected historical range). With such small colonic polyp numbers, no meaningful statistical analysis was possible.
Control Staining of Intestinal Adenomas.
DBA-stained sections of small and large intestine were examined from six C57BL/6-Apcmin/+ control mice. Intestinal adenomas were examined for the presence of foci of loss of epithelial DBA positivity. There was no loss of DBA positivity seen in any adenomas under 1 mm in diameter. Of 54 small intestinal adenomas 1 mm or greater in diameter, 52 (96%) showed no loss, and 2 tumors (4%; 2 and 4 mm in diameter) showed focal loss of DBA positivity. For these two small intestinal tumors showing loss of DBA staining, both occurred in a single mouse and showed almost complete focal absence of DBA staining in deep dysplastic glands (Fig. 5 a and b).
Figure 5.
Staining patterns of intestinal adenomas. (a) Hematoxylin and eosin staining of a control C57BL/6-Apcmin/+ small intestinal tumor. (b) DBA staining of a control C57BL/6-Apcmin/+ small intestinal tumor showing focal loss of DBA positivity (see arrow). (c) Hematoxylin and eosin staining of a C57BL/6-Apcmin/+ ↔ SWR small intestinal tumor. (d) DBA staining of a C57BL/6-Apcmin/+ ↔ SWR small intestinal tumor showing numerous DBA-negative glands. (e) Hematoxylin and eosin staining of a control C57BL/6-Apcmin/+ colonic tumor. (f) DBA staining of a control C57BL/6-Apcmin/+ colonic tumor showing extensive loss of DBA positivity.
Similar examination of colonic adenomas showed that three out of six adenomas of 1 mm or greater in diameter showed focal loss of DBA positivity, with widespread loss in some tumors (Fig. 5 e and f).
Examination of DBA Staining of Intestinal Adenomas in C57BL/ 6 Min/+ ↔ SWR Chimeras.
Each of the DBA-stained sections from 15 chimeras with C57BL/6-Apcmin/+ epithelium was examined for the presence, and DBA staining pattern, of adenomas and intravillus cystic structures (11). The epithelium within adenomas was also assessed for dysplasia. Mildly dysplastic changes commonly seen were glandular irregularity, increased cellular nuclear to cytoplasmic ratio, and normal mitoses at a high crypt position. Moderately dysplastic features noted included severe glandular irregularity with glandular “back-to-backing,” and nuclear crowding and enlargement.
The dysplastic epithelium of all tumors and intravillus cysts examined stained at least partially positive with DBA, with no entirely DBA-negative tumors identified. In most tumors, the dysplastic epithelium stained predominantly DBA-positive, with only occasional DBA-negative crypts. The epithelium of 53% (76/144) of small intestinal tumors of 1 mm or greater diameter stained entirely DBA-positive, whereas 47% (68/144) of such tumors contained DBA-negative crypts. The great majority of these DBA-negative glands showed hyperplastic or mildly dysplastic changes. Foci of DBA-negative moderate dysplasia were seen in 12% (18/144) of the tumors (Fig. 5 c and d). Comparison of these results with those obtained in control C57BL/6-Apcmin/+ mice shows no statistically significant difference in the DBA staining pattern of adenomas in control and chimeric Apcmin/+ mice (χ2, P = 0.067; Fishers Exact Test, P = 0.1).
Discussion
The purpose of this study is to use the unique biological properties of chimeric mice to allow investigation of both the action of genetic modifiers of the Min phenotype and the clonality of early intestinal tumors. Our results suggest that the major suppressors of the Min phenotype function at a crypt or cell autonomous level with no discernable distant or systemic affect. Examination of the clonality of adenomas was complicated by the spontaneous loss of DBA staining seen in adenomas in control Apcmin/+ mice; however, all adenomas stained at least partially positive for DBA, suggesting that mutation of the Apc gene in epithelial cells is necessary for adenoma formation in this model.
The lectin DBA binds to N-acetyl galactosamine residues on endothelial and intestinal epithelial cells (12). DBA binds to intestinal epithelial cells in C57BL/6 mice but not in SWR mice, thus DBA can be used as a marker to determine the derivation of intestinal epithelium in C57BL/6↔SWR chimeras (13). The finding of an aberrant DBA staining pattern in the cecum and ascending colon of both the C57BL/6 and SWR control mice was unexpected and has not been described in mouse clonality studies. This DBA-staining variation in the proximal large bowel pattern may have been missed in previous studies because they have mainly concentrated on examination of the small intestine (13). Other studies have shown such variation in the DBA-staining pattern in the colon of both the rat, with loss of staining in the distal colon (14), and in humans, with variation of the DBA-staining pattern in the ascending colon (15). Another unexpected finding was loss of DBA positivity in occasional single crypts within the distal colon. It is possible that these changes may be related to somatic mutation of the Dlb-1 locus, which is responsible for the pattern of DBA staining. Similar single-crypt changes have been described in the human colon with aging, after spontaneous somatic mutation at the OAT locus (16); however, the C57BL/6 mouse strain is reported to be homozygous for the Dlb-1b allele, which confers DBA-positive staining to intestinal epithelium. Because this allele acts in a dominant fashion (10), to lose DBA staining would require loss or mutation of both Dlb-1b alleles, which seems an unlikely explanation for these findings. A more likely possibility is that changes in another factor indirectly involved in the DBA staining of the intestinal epithelium might possibly also lead to loss of DBA positivity (e.g., pattern of mucin expression).
The assessment of the mosaic composition of the normal intestine was not, however, seriously affected by this variation in staining pattern. The aberrant staining pattern in the cecum was readily recognized, and these areas were excluded from counts of normal crypt staining pattern. Potential errors introduced by the occurrence of occasional “false negative” crypts in the distal colon are minimal as a total of over 5,000 crypts was examined in each chimera.
Crossing the Apcmin mutation onto the different genetic backgrounds of other inbred mouse strains has suggested that there are multiple genetic modifiers of the Min phenotype (17). Variations in adenoma burden between FAP patients with identical APC mutations has led to suggestions that there may be similar genetic loci that modify the FAP phenotype in humans (18–20). The strongest genetic modifier identified in mice, Mom1, is absent in the C57BL/6 strain but present in many other strains of laboratory mice. The genetic change underlying polyp sensitivity has been thought to be attributable to a mutation in the gene secretary phospholipase A2, which maps to the Mom1 region on mouse chromosome 4 (syntenic to human 1p35–36). A single base thymidine insertion in exon 3 causes a frameshift mutation that results in a premature stop codon and truncated Pla2g2a protein. C57BL/6J mice have been shown to be homozygous for this mutant allele (3). SWR mice carry Mom1 resistance (i.e., homozygous wild-type Pla2g2a), so the Min mutation on an SWR background would be expected to result in a marked decrease in intestinal adenomas. In agreement with this, first generation Apcmin/+ offspring from crosses of C57BL/6-Apcmin/+ with SWR are resistant to the development of intestinal tumors, with a major part of this resistance mapping to the Mom1 locus (personal communication from A. Moser, University of Wisconsin, Madison, WI).
It has been suggested that as Pla2g2a encodes a secretary phospholipase and is most active in the intestinal lumen, its action is probably noncell autonomous (3). Increasing the percentage of SWR-derived crypts that produce and secrete Pla2g2a in a C57BL/6 Min↔SWR mouse might therefore be expected to reduce the number of intestinal adenomas proportionately as the SWR component increases. More recently, however, it has been shown that the action of the Mom1 modifier is more likely to be restricted to individual crypts (21). Examination of polyp counts in our chimeric mice agrees with these results, with no evidence that increasing the percentage of SWR intestinal crypts decreases the number of polyps arising per unit area of C57BL/6 epithelium. This strongly suggests that the net effect of Mom1 and other major modifiers of Apcmin carried by SWR act at least at a crypt-restricted, if not cell-autonomous, level. Our macroscopic polyp counts show that more polyps are seen in the chimeras than would be expected from the percentage of C57BL/6 crypts present, with a significant increase in the ratio of actual-to-expected polyps with increasing percentage of SWR crypts. The explanation of this result is not straightforward. Chimeric mice were reared under the same conditions as C57BL/6-Apcmin/+ control mice, and were killed at an earlier age when similar, if not fewer macroscopic polyps would be expected. Postmortem examination and assessment of polyp number was performed blind by the same operator; thus, environmental conditions, age at death, and variations in experimental assessment are unlikely to account for the increased polyp numbers seen in these chimeras. Possible explanations to account for these findings include the following: (i) either the presence of SWR-derived tissues increases polyp number through an effect independent of Mom1 or an excess of C57BL/6-Apcmin/+-derived tissues reduces polyp number (this could be because of the effects of relatively weak genetic modifiers acting at a systemic level or to varying specific tissue sensitivities to growth factors in the different mouse strains); and (ii) there is clustering of polyps on C57BL/6-Apcmin/+ epithelium, so that apparently single macroscopic polyps are formed by the coalescence of multiple microscopic adenomas. A reduction in C57BL/6-Apcmin/+ epithelium might then be expected to reduce the number of microscopic adenomas at a greater rate than the number of macroscopic polyps. Presently, there is no obvious experimental evidence to particularly favor either of these possibilities.
Assessment of the clonality of adenomas in this study was complicated by the spontaneous loss of DBA staining in tumors. In the human colon, studies have demonstrated a decrease in DBA staining in both FAP tumors (22) and with increasing dysplasia in sporadic colorectal tumors (23); however, other studies have failed to document such loss of DBA staining in both human colorectal tumors (24) and carcinogen-induced rat colorectal tumors (25). Variation in the DBA-staining pattern of the normal colon in both the C57BL/6 and SWR mice suggested that interpretation of clonality in colonic adenomas would be problematic. The small total number of colonic adenomas available for inspection, together with the finding that 50% (3/6) of adenomas from control C57BL/6-Apcmin/+ mice showed loss of DBA staining, confirmed these suspicions. The clonality of large intestinal adenomas was not considered further.
The rate of DBA false-negative small intestinal adenomas from control C57BL/6- Apcmin/+ mice was approximately 4% (2/54 adenomas). Examination of the staining pattern of adenomas in chimeras showed that 12% (18/144) of small intestinal adenomas showed loss of DBA staining in moderately dysplastic glands. Statistical analysis of these results shows no statistically significant difference in the DBA-staining pattern of adenomas in the C57BL/6-Apcmin/+ and chimeric-Apcmin/+ mice; thus, despite the fact that in chimeric mice, multiple adenomas were seen to consist of both DBA-positive and -negative dysplastic crypts, the possibility that these changes were because of loss of DBA staining in C57BL/6-derived dysplastic epithelium rather than to polyclonality cannot be excluded.
Whereas no firm conclusions can presently be made concerning the clonal composition of adenomas in the chimeric mice, examination of the DBA-staining pattern of small and large intestinal tumors has shown that the dysplastic epithelium of most tumors stained strongly positive for DBA. Only occasional DBA-negative dysplastic glands were seen and no tumors with entirely DBA-negative dysplastic epithelium identified. These observations strongly suggest that all tumors are at least initiated in C57BL/6-Apcmin/+ epithelium, and that the Apc mutation in epithelial cells is a requirement for adenoma formation in this model. These results are in agreement with widely held models of tumorigenesis and counter suggestions that tumors may arise because of field effects, or abnormal stromal/epithelial interactions (26) rather than as a direct consequence of DNA mutation. Our results also show that in chimeric mice, the presence of SWR-derived tissues (expressing wild-type Pla2g2a) does not have any inhibitory effect on the formation of adenomas in C57BL/6-Apcmin/+ epithelium. These results confirm that the main genetic modifiers of the Min phenotype act at a crypt-restricted, if not at a cell-autonomous, level with no discernible systemic effects.
Abbreviations
- FAP
familial adenomatous polyposis
- APC
adenomatous polyposis coli
- DBA
dolichos biflorus agglutinin
- ICRF
Imperial Cancer Research Fund
References
- 1.Moser A R, Pitot H C, Dove W F. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 2.Dietrich W F, Lander E S, Smith J S, Moser A R, Gould K A, Luongo C, Borenstein N, Dove W. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- 3.MacPhee M, Chepenik K P, Liddell R A, Nelson K K, Siracusa L D, Buchberg A M. Cell. 1995;81:957–966. doi: 10.1016/0092-8674(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 4.Cormier R T, Hong K H, Halberg R B, Hawkins T L, Richardson P, Mulherkar R, Dove W F, Lander E S. Nat Genet. 1997;17:88–91. doi: 10.1038/ng0997-88. [DOI] [PubMed] [Google Scholar]
- 5.Novelli M R, Williamson J A, Tomlinson I P M, Elia G, Hodgson S V, Talbot I C, Bodmer W F, Wright N A. Science. 1996;272:1187–1190. doi: 10.1126/science.272.5265.1187. [DOI] [PubMed] [Google Scholar]
- 6.Merritt A J, Gould K A, Dove W F. Proc Natl Acad Sci USA. 1997;94:13927–13931. doi: 10.1073/pnas.94.25.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarkowski A K. Nature(London) 1961;4779:857–860. [Google Scholar]
- 8.Shoemaker A R, Gould K A, Luongo C, Moser A R, Dove W F. Biochim Biophys Acta. 1997;1332:F25–F48. doi: 10.1016/s0304-419x(96)00041-8. [DOI] [PubMed] [Google Scholar]
- 9.Wasan H S, Novelli M, Bee J, Bodmer W F. Proc Natl Acad Sci USA. 1997;94:3308–3313. doi: 10.1073/pnas.94.7.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winton D J, Blount M A, Ponder B A. Nature (London) 1988;333:463–466. doi: 10.1038/333463a0. [DOI] [PubMed] [Google Scholar]
- 11.Shoemaker A R, Moser A R, Dove W F. Cancer Res. 1995;55:4479–4485. [PubMed] [Google Scholar]
- 12.Ponder B A, Wilkinson M M. Dev Biol. 1983;96:535–541. doi: 10.1016/0012-1606(83)90191-4. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt G H, Winton D J, Ponder B A. Development (Cambridge, UK) 1988;103:785–790. doi: 10.1242/dev.103.4.785. [DOI] [PubMed] [Google Scholar]
- 14.McGarrity T J, Peiffer L P, Colony P C. Exp Pathol (Jena) 1991;41:175–183. doi: 10.1016/s0232-1513(11)80085-x. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y S. Pathology. 1987;19:397–401. doi: 10.3109/00313028709103890. [DOI] [PubMed] [Google Scholar]
- 16.Campbell F, Appleton M A, Fuller C E, Greeff M P, Hallgrimsson J, Katoh R, Ng O L, Satir A, Williams G T, Williams E D. J Pathol. 1994;174:169–174. doi: 10.1002/path.1711740305. [DOI] [PubMed] [Google Scholar]
- 17.Moser A R, Dove W F, Roth K A, Gordon J I. J Cell Biol. 1992;116:1517–1526. doi: 10.1083/jcb.116.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paul P, Letteboer T, Gelbert L, Groden J, White R, Coppes M J. Hum Mol Genet. 1993;2:925–931. doi: 10.1093/hmg/2.7.925. [DOI] [PubMed] [Google Scholar]
- 19.Tomlinson I P M, Neale K, Talbot I C, Spigelman A D, Williams C B, Phillips R K S, Bodmer W F. J Med Genet. 1996;33:268–273. doi: 10.1136/jmg.33.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomlinson I P M, Beck N E, Neale K, Bodmer W F. Ann Hum Genet. 1996;60:369–376. doi: 10.1111/j.1469-1809.1996.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 21.Gould K A, Dove W F. Proc Natl Acad Sci USA. 1997;94:5848–5853. doi: 10.1073/pnas.94.11.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sams J S, Lynch H T, Burt R W, Lanspa S J, Boland C R. Cancer (Philadelphia) 1990;66:502–508. doi: 10.1002/1097-0142(19900801)66:3<502::aid-cncr2820660317>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Campo E, Condom E, Palacin A, Quesada E, Cardesa A. Dis Colon Rectum. 1988;31:892–899. doi: 10.1007/BF02554856. [DOI] [PubMed] [Google Scholar]
- 24.Yoshioka M, Yamaguchi K, Urano Y, Mori S. Jpn J Cancer Clin. 1985;31:45–51. [PubMed] [Google Scholar]
- 25.Caldero J, Campo E, Vinas J, Cardesa A. Lab Invest. 1989;61:670–676. [PubMed] [Google Scholar]
- 26.Rubin H. Cancer Res. 1985;45:2935–2942. [PubMed] [Google Scholar]