Abstract
Steroidogenic factor 1 (SF-1) plays an essential role in adrenal development, although the exact molecular mechanisms are unclear. Our previous work established that Ff1b is the zebra fish homologue of SF-1 and that its disruption by antisense morpholinos leads to a complete ablation of the interrenal organ. In this study, results of biochemical analyses suggest that Ff1b and other Ff1 members interact with Prox1, a homeodomain protein. Fine mapping using site-directed mutants showed that this interaction requires an intact Ff1b heptad 9 and AF2, as well as Prox1 NR Box I. In vivo, this physical interaction led to the inhibition of Ff1-mediated transactivation of pLuc3XFRE, indicating that Prox1 acts to repress the transcriptional activity of Ff1b. In situ hybridization demonstrates that prox1 colocalizes with ff1a and ff1b in the liver and interrenal primordia, respectively. Embryos microinjected with prox1 morpholino displayed a consistent partial reduction of 3β-Hsd activity in the interrenal organ, while ff1b morpholino led to a disappearance of prox1. Based on these results, we propose that during the course of interrenal organogenesis, Prox1 functions as a tissue-specific coregulator of Ff1b and that the subsequent inhibition of Ff1b activity, after its initial roles in the specification of interrenal primordium, is critical for the maturation of the interrenal organ.
Molecular and genetic evidence from experiments with mammals suggests that steroidogenic factor 1 (SF-1), dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X-chromosome gene 1 (DAX-1), and Wilms' tumor 1 (WT-1) are absolutely required for the development of the adrenal cortex (32, 52, 53). They are expressed in the fetal adrenal at the earliest stages and appear to act in a concerted manner, probably by activating common development pathways to direct normal adrenal development (32, 53). SF-1, an orphan nuclear receptor (NR) with no known ligand, is expressed in the earliest precursors of the adrenal gland, the adrenogonadal progenitor cells (25, 32), and SF-1−/− knockout mice lack the adrenal gland (42, 59, 66). As a key regulator of adrenal development, it regulates the transcription of a number of genes involved in gonadal and adrenal development, including DAX-1 and MIS, and many of the enzymes involved in the steroidogenic pathway (25, 30, 47, 53, 64).
WT-1 encodes a Kruppel-type zinc finger transcription factor (7, 16), and mutations of this gene in humans lead to congenital abnormalities of urogenital development (21). Experiments with knockout mice confirmed the importance of WT-1, which is expressed only in the adrenogonadal precursors but not in the fetal adrenal, since mice homozygous for a null allele lacked kidneys, gonads, and adrenals (35, 45). DAX-1 encodes a highly divergent orphan nuclear receptor (82). Although knockout mice lacking DAX-1 do not exhibit adrenocortical deficiency and show apparently normal development of the adrenal cortex, the expression of Cyp11A1 in the zona fasciculata is reduced (81). In contrast, the loss of DAX-1 functions in humans has much more severe effects on adrenal development, causing primary adrenocortical insufficiency and the absence of adult zones in the adrenal cortex (6, 46, 82). Both WT-1 and DAX-1 can be recruited by SF-1 to modulate its transcriptional activity (12, 47). Indeed, the antagonistic effects of DAX-1 and WT-1 on SF-1 are necessary to determine the sex-specific expression of Mullerian-inhibiting substance (49). Despite their developmental significance, we do not know if physical interactions with DAX-1 and WT-1 are necessary for the roles of SF-1 in adrenal development. The importance of DAX-1, which is tightly coexpressed with SF-1 in the fetal and adult adrenal, in the regulation of steroidogenic enzymes is well documented (3, 31, 32, 53, 70).
Although the vast majority of NRs and their coregulators are expressed ubiquitously (2, 18, 57), it has been postulated that coregulators might be able to determine the tissue- and promoter-specific responses exerted by NRs (43). Only a few NR coregulators display a tissue- or cell-specific expression pattern. In Drosophila, the formation of segmentation requires the widely expressed fushi tarazu factor 1β (Ftz-F1β) to act as a coactivator for Ftz, whose expression is restricted to the even stripes (22, 63, 69, 80). Hairless is expressed in hair follicles and skin, and mutation of this gene leads to a hairless phenotype in mice and humans (1, 54). Recently, Hairless has been characterized as a corepressor for thyroid hormone receptor (TR), which is widely expressed (56). These indicate that ubiquitously expressed NRs could mediate tissue-specific functions through interacting partners that have restricted expression patterns. For SF-1, modulation by coregulators such as DAX-1, WT-1, GATA4, and Ptx1 appears to be sufficient to achieve promoter specificity in the endocrine tissues (53, 72, 73).
In mammals, although SF-1 is essential for adrenal cortex development, the mechanisms by which it plays its essential roles are not known, although its modulation by DAX-1 is important for the transcriptional regulation of steroidogenic enzymes (3, 32, 53). Our previous work has established that Ff1b (officially designated as NR5a4 by the Nuclear Receptors Nomenclature Committee) is the earliest known molecular marker for the zebra fish interrenal organ (8). Ff1b plays an essential role in the initiation of the interrenal primordium and its subsequent development and acquisition of steroidogenic capacity (9). Besides ff1b, two other ff1 genes, ff1a (41) and ff1c (X. Jun and W. K. Chan, unpublished data), have been identified in zebra fish. Both the DNA binding domain (DBD) and ligand binding domain (LBD) are highly conserved for all three zebra fish ff1 genes, and they transactivate the promoter containing a consensus SF-1 responsive element (FRE). While ff1a is the homologue of mammalian liver receptor homologue 1 (LRH-1), ff1c is basal to both the SF-1 and LRH clades. Only ff1a (40, 41) and ff1b (8, 9) are expressed during early embryonic development, with ff1a showing a broad tissue expression pattern (40, 41) while ff1b has a more restricted expression profile and is expressed only in the hypothalamus, interrenal gland, and gonads. ff1c is expressed only after the completion of embryonic development, predominantly in the intestine, liver, ovary, muscle, and heart.
In this study, we investigated whether Prox1, a prospero-related homeodomain protein, which is colocalized with Ff1b in the interrenal primordium, could interact with and modulate the transcriptional activity of Ff1b. We report here that zebrafish Prox1 could repress the transcriptional activity of Ff1b, which is unlike the coactivator role that we have described previously for Prox1 with respect to TRs (15). This led us to carry out further analyses using antisense morpholinos. The antisense prox1 morpholino reduces the size of the interrenal organ as revealed by Δ5-3β-hydroxysteroid hydrogenase (3β-Hsd) enzymatic activity and ff1b expression, while the ff1b morpholino restricts the expression domain of prox1. Taken together, it appears that Prox1 functions as a tissue-specific coregulator of Ff1b and that the formation of an SF-1/Prox1 complex is crucial for interrenal development.
MATERIALS AND METHODS
Yeast two-hybrid assay.
pLexA-Ff1a, pLexA-Ff1b, and pLexA-Ff1c were constructed by subcloning PCR fragments, containing the entire open reading frames (ORFs), into pLexA (Clontech) with EcoRI and XhoI. PCR fragments were obtained using Pfu Turbo polymerase (Stratagene). Mutations of the heptad 9 and AF2 domains of zebra fish Ff1s were carried out using the QuikChange XL site-directed mutagenesis kit as specified by the manufacturer (Stratagene). The zebrafish pB42AD-Prox1 NR box mutants have been previously reported (15). The EGY48 yeast strain was transformed with various pLexA-Ff1 and pB42AD-Prox1 constructs sequentially, plated onto selective medium, and incubated for 3 to 5 days at 30°C. Five independent yeast clones were selected for each set of transformation and grown overnight in selective medium. The yeast cells were transferred to inductive medium the next morning and cultured for 6 h at 30°C, until the optical density at 600 nm reached 0.5 to 0.8. Cell extracts were prepared using three freeze-thaw cycles. The β-galactosidase activity that resulted from the interaction of the pLexA-Ff1 and pB42AD-Prox1 was measured by using the quantitative liquid assay, with o-nitrophenyl-β-d-galactopyranoside (ONPG) as the substrate and expressed in Miller units (42). Each experiment was repeated at least twice, and the results obtained were reproducible. Only data from a single experiment were presented as the mean (± standard error of the mean [SEM]) of five replicates.
Zebra fish maintenance and mutant lines.
Zebra fish (Danio rerio) were raised by standard methods (75). Embryos were obtained by natural spawning and cultured in embryo medium at 28.5°C. The embryos were staged as previously described (33). Embryos to be used for histological analyses were treated with 0.03% phenylthiourea (Sigma) from 16 h postfertilization (hpf) to inhibit melanin pigment formation. Homozygous mutant embryos were obtained from mating using fish heterozygous for the no tail (ntlb160) (23), floating head (flhtk241) (49), and one-eyed pinhead (oepm134) (60) alleles, respectively.
Microinjection of mRNAs and coimmunoprecipitation.
The ORF of zebra fish prox1 was cloned into pCMV-Tag2, while those of ff1a, ff1b, and ff1c were cloned into pCMV-Tag3 (Stratagene). The cloning strategy added a FLAG and c-myc tag to Prox1 and the Ff1s, respectively. These constructs were then used as templates for mRNA synthesis. FLAG-tagged prox1 and c-myc-tagged ff1 capped mRNAs were prepared from the linearized pCMV-Tag expression plasmids by using the mCAP mRNA capping kit (Stratagene). Capped mRNA of FLAG-tagged prox1 (1.2 ng) alone, or with an equal molar ratio of the various c-myc-tagged ff1 genes were injected into embryos at the one- to two-cell stage. Injected embryos (n = 100) were deyolked at 16 h p.f. and homogenized in 200 μl of ice-cold IP buffer (1% Triton X-100 in phosphate-buffered saline (PBS) supplemented with complete protease inhibitor mixture [Roche]). Extracts were incubated at 4°C for 1 h and centrifuged at 12,000 × g for 10 min. The soluble protein supernatants were recovered, biotinylated with biotin-7-NHS (Roche), and subsequently incubated with anti-FLAG M2-agarose affinity gel (Sigma) at 4°C for 9 h. One-tenth of each protein supernatant after immunoprecipitation was saved to verify that the labeled proteins were present in equal amounts. The FLAG beads were washed three times with ice-cold IP buffer. Immunoprecipitates, equivalent to 33 injected zebra fish embryos, were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting.
Plasmid microinjection and transactivation assay.
The ORFs of mouse SF-1 and zebra fish prox1, prox1-NR1m2m3m, ff1a, ff1b, and ff1c were cloned into pcDNA3.1(+) (Invitrogen). One- or two-cell embryos were injected with 4.6 nl of solution containing 0.5 μg of pcDNA3.1-Prox1 expression plasmid per μl, 0.1 μg of the respective pcDNA3.1-Ff1 expression plasmids per μl, and 50 ng of luciferase reporter plasmid, pLuc3XFRE, per μl in 1× Danieau buffer containing 0.25% phenol red. The Renilla luciferase plasmid, pRL-CMV (5 ng/μl), was also included to allow the normalization of firefly luciferase activity. After 14 h, healthy embryos were harvested for luciferase assays. For each sample, five embryos were homogenized by repeated pipetting in 20 μl of 1× passive lysis buffer provided with the Dual Luciferase reporter assay system (Promega). Luciferase activity in 20 μl of homogenate was assayed using a Lumat LB9507 luminometer (Berthold).
Electrophoretic mobility shift assay.
A nonradioactive electrophoretic mobility shift assay was carried out using the digoxigenin (DIG) gel shift kit (Roche). Oligonucleotides containing the monomeric SF-1-responsive element (5′-CACAGGGCACTGTCCCCCAAGGTCAATGATATCGCGGGAA-3′) are generated by annealing complementary oligonucleotides and end labeled with digoxigenin.
In vitro-translated proteins of mouse SF-1 and zebra fish Prox1, Ff1a, Ff1b, and Ff1c were prepared from the respective pcDNA3.1(+) constructs using the TNT coupled reticulocyte lysate system (Promega). The translated proteins were biotinylated by adding Transcend tRNA (Promega) to the coupled transcription-translation reaction mixtures, and the proteins were verified by chemiluminescence before the electrophoretic mobility shift assay was performed.
For the binding reaction, 8 μl of the translated mixture was mixed with 4 μl of 5× binding buffer [100 mM HEPES (pH 7.6), 5 mM EDTA, 50 mM (NH4)2SO4, 5 mM dithiothreitol DTT, Tween 20, 1% (wt/vol), 150 mM KCl], 1 μl of 1-mg/ml poly(dI-dC), 1 μl of 1-mg/ml poly-l-lysine, and 1 μl of labeled probe in the presence or absence of unlabeled competitor, and double-distilled water was added to a total volume of 20 μl. The mixture was incubated for 30 min at 20°C. The reaction products were electrophoresed on 8% polyacrylamide gel in 0.5× Tris-bovate-EDTA (TBE), transferred onto a nylon membrane by electroblotting, and UV cross-linked. Chemiluminescence was performed to detect the DIG-labeled bands.
Whole-mount in situ hybridization and cryostat sectioning.
Whole-mount in situ hybridization was performed as described previously with minor modifications (8). DIG-labeled riboprobes were synthesized from SacII-linearized prox1 plasmid using SP6 RNA polymerase. Fluorescein-labeled riboprobes were synthesized from plasmids containing ff1b, ff1a, and proinsulin cDNAs. Plasmids for ff1b and ff1a were linearized with SalI and transcribed with T7 RNA polymerase. The proinsulin plasmid was linearized with SphI and transcribed with SP6 RNA polymerase. DIG-labeled riboprobes were detected with alkaline phosphatase-conjugated anti-DIG antibody (Roche), while fluorescein-labeled probes were detected with alkaline phosphatase-conjugated antifluorescein antibody (Roche). Visualization was performed either with nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP) (Promega) or with Fast Red (Roche). For two-color in situ hybridization, the first antibody was inactivated by heating the stained embryos at 65°C for 30 min. Stained embryos were postfixed in 4% paraformaldehyde (PFA) in PBS and washed in PBS supplemented with 1% Triton X-100 (PBST). This was followed by tissue clarification in 50% glycerol in PBS.
Cryostat sectioning was performed essentially as described previously (75). Sections 10 μm thick were collected on a CM1900 cryostat (Leica). Specimens prepared by either whole-mount in situ hybridization or cryostat sectioning were mounted on glass slides and photographed under Normaski optics on an Olympus AX710 microscope system.
Chromogenic histochemical staining for 3β-Hsd.
Histochemical staining for 3β-Hsd was performed on whole embryos by using a protocol adapted from Levy's method as previously described (19). Embryos were fixed overnight at 4°C in 4% PFA-PBS and washed twice with PBST. Chromogenic reaction was carried out at 37°C for 3 h in staining solution containing 0.1 mg of etiocholan-3β-o1-17-one per ml as the substrate, 0.2 mg of NAD per ml, 0.1 mg of nicotinamide per ml, and 0.1 mg of of nitroblue tetrazolium (Promega) per ml, in PBST. Staining reactions were terminated by washing in PBST followed by fixing in 4% PFA-PBS. Chemicals were from Sigma unless otherwise stated.
Microinjection of MO.
Morpholino oligonucleotides (MO) were synthesized at Genetools LLC. A 2 mM stock solution was prepared by dissolving lyophilized MO powder in 1x Danieau solution before further dilution into the required concentrations for microinjection. The nucleotide sequences of the prox1MO, ff1bMO, iaff1bMO, and STDMO are 5′-ATGTGCTGTCATGGTCAGGCATCAC-3′ (58), 5′-AATCCTCATCTGCTCTGAAGTCCAT-3′, 5′-TACCTGAAGTCTCGTCTACTCCTAA-3′, and 5′-CCTCTTACCTCAGTTACAATTTATA-3′, respectively. MO-containing solutions (∼4.6 nl) were injected into one-cell stage embryos by using a Nanoject injector (Drummond).
Generation of Prox1 antiserum and Western blotting.
Western analysis for immunoprecipitates or for total proteins extracted from embryos was performed as described in The Zebrafish Book (75). After fractionation by SDS-PAGE and electrotransfer onto a nitrocellulose membrane, biotinylated proteins were detected with horseradish peroxidase-conjugated antibiotin antibody (Pierce), while FLAG-tagged and c-myc-tagged proteins were detected with anti-FLAG M2 (Sigma) and anti-c-myc (Roche) monoclonal antibodies, respectively. Polyclonal antibodies were raised in rabbit, against the entire homeodomain (amino acids 583 to 638) of zebra fish Prox1 (15) (BioSource/QCB). Antibodies were partially purified from the immune sera by using protein A affinity columns.
RESULTS
Prox1 physically interacts with Ff1 isoforms.
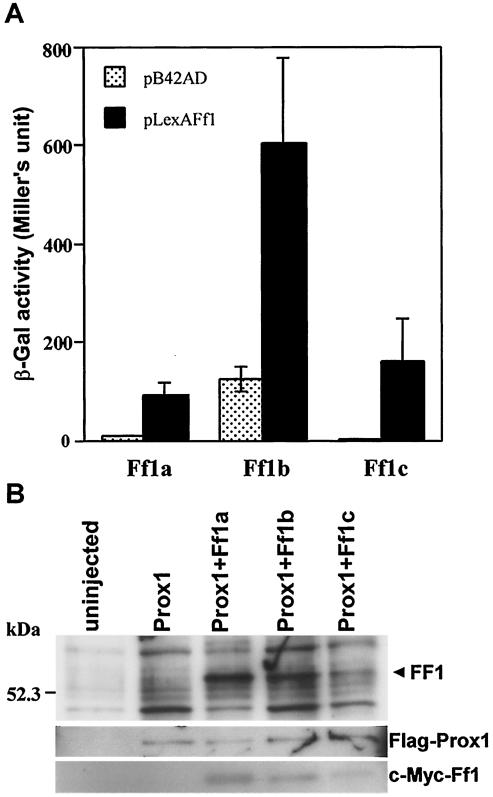
We have previously identified Prox1, a divergent homeobox protein, as a novel coactivator of TRs and retinoic acid receptors (RARs) (15). To assess if Prox1 also interacts with zebra fish Ff1s, the ORF of ff1a, ff1b, and ff1c were inserted into pLexA and tested for their ability to interact with Prox1 in yeast (Fig. 1A). Of the three Ff1s, Ff1b displayed the strongest physical interaction with Prox1, although there was a considerable level of background β-galactosidase activity due to an autonomous activation domain within Ff1b. The interaction strength of Prox1 with Ff1a and Ff1c was about 15 and 28%, respectively, compared to that with Ff1b.
FIG. 1.
Prox1 interacts with various Ff1 isoforms in yeast and zebra fish embryos. (A) Interaction of Ff1 proteins with Prox1 in a yeast two-hybrid assay. β-Galactosidase (β-Gal) activities were calculated based on duplicate measurements of five independent yeast transformants. Note that Ff1b has an autonomous activation domain. (B) Ff1 proteins coimmunoprecipitate with Prox1. Capped mRNAs of FLAG-tagged Prox1 alone, or in combination with c-myc-tagged Ff1a, Ff1b, or Ff1c, were injected into zebra fish embryos at the one- or two-cell stage. The injected embryos were harvested at 16 hpf, and extracted proteins were biotinylated. The lysates were subjected to immunoprecipitation with anti-FLAG agarose beads. The immunoprecipitates were analyzed by Western blotting, and the specific protein bands were detected with streptavidin, FLAG-specific antibody, or c-myc-specific antibody.
To confirm that Prox1 interacts with Ff1 proteins in vivo, we used a coimmunoprecipitation assay. Capped mRNAs of FLAG-tagged prox1 were microinjected into zebra fish embryos alone or with c-myc-tagged ff1a, ff1b, or ff1c mRNAs. At 16 h p.f., protein extracts were prepared from the microinjected embryos, biotinylated, and used for immunoprecipitation with anti-FLAG agarose matrix (Fig. 1B). When Prox1 was immunoprecipitated from embryos that were coinjected with ff1 mRNAs, an additional band of biotinylated protein corresponding to the Ff1 proteins was detected. Anti-FLAG and anti-c-myc antibodies were used to verify the FLAG-tagged-Prox1 and c-myc-tagged Ff1 isoforms in the immunoprecipitation complex. We confirmed that Ff1 proteins could be detected by anti-c-myc antibody only in embryos coinjected with FLAG-tagged prox1 and c-myc-tagged ff1 mRNAs but not in those that were uninjected or injected with FLAG-tagged prox1 alone. Thus, in the presence of coexpressed Ff1 and Prox1, they coimmunoprecipitated, indicating that they associated in vivo.
LXXLL motifs of Prox1 are required for interaction with Ff1 isoforms.
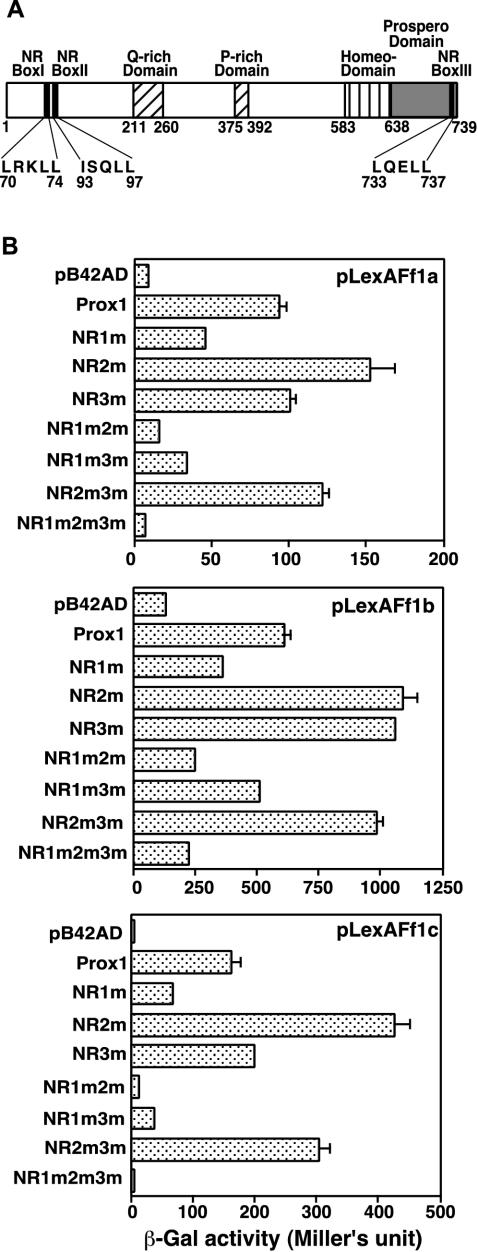
The mutation of the LXXLL motifs to LXXAA has been reported to abolish NR interaction function (28). The presence of functional LXXLL motifs in Prox1 (Fig. 2A) has been investigated previously in the context of its interaction ability with TRs (15). Seven zebra fish Prox1 NR box site-directed mutants, in which the three NR boxes were mutated individually or in various combinations, were examined for their ability to interact with the Ff1 proteins in yeast (Fig. 2B). As expected, when all three NR boxes were mutated (Prox1-NR1m2m3m), the ability of Prox1 to interact with Ff1 proteins was completely abolished. Single Prox1 NR box II and III mutants did not lose their ability to interact with Ff1 isoforms. Indeed, the Prox1 NR box II mutant consistently enhanced the interaction of Prox1 with Ff1 proteins. Double Prox1 mutants that carried a mutated NR box III also behaved identically in their interaction with all three Ff1 proteins compared to the respective single mutants (NR2m versus NR2m3m; NR1m versus NR1m3m). The results confirm the noninvolvement of the Prox1 NR box III, as previously established for TRα1 and TRβ1 (15). However, when NR box I was mutated, Prox1 interaction ability with Ff1s was reduced, and if NR box II was also mutated (NR1m2m), the ability of Prox1 to interact was further reduced, especially for Ff1a and Ff1c.
FIG. 2.
Ff1 proteins interact with Prox1 through LXXLL motifs. (A) Schematic representation of Prox1 functional domains, including the three putative NR box motif sequences. The LXXLL and IXXLL motifs were mutagenized to (I/L)XXAA to generate seven full-length Prox1 NR box site-directed mutants. (B) The NR boxes of zebra fish Prox1 show differential interaction abilities with Ff1 proteins. Seven Prox1 NR box site-directed mutants were tested for their ability to interact with Ff1a, Ff1b, and Ff1c in yeast. The results are presented as mean and SEM (n = 5). NR1m, NR2m, and NR3m are single NR box mutants at the first, second, and third NR box, respectively. NR1m2m, NR1m3m, NR2m3m, are double NR box mutants, while NR1m2m3m is the triple NR box mutant.
In conclusion, we have shown that the NR boxes are involved in the interaction between Prox1 and Ff1s. The results suggest that NR box I is the primary LXXLL motif employed by Prox1 in its interaction with Ff1s. However, NR box II (ISQLL) is also able to function as a secondary interaction site when NR box I is inactivated by mutation. It is also quite probable that amino acid residues surrounding NR box I and II are also important. Since the LRKLL motif is located in the second α helix, which is just 19 amino acids upstream of the ISQLL motif, what we have observed might be a reflection of this requirement.
Ff1 heptad 9 and AF2 core domains are essential for interaction with Prox1.
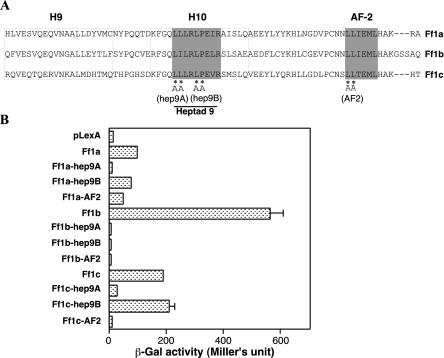
Within the LBD of zebra fish Ff1s, there are two highly conserved domains, the heptad 9 and AF2 domains (Fig. 3A). AF2 determines the major activation function of the Ff1 proteins, while heptad 9 is required for AF2 activity in zebra fish Ff1a (38, 39, 41, 63). Also, AF2 of mouse SF-1 and heptad 9 of zebra fish Ff1a are required for interaction with SRC-1, and AF2 of Drosophila Ftz-F1 is essential for interaction with Ftz (10, 41, 63). To understand if leucine residues within heptad 9 and AF2 of Ff1 proteins are essential for interactions with Prox1, site-directed mutagenesis was carried out for Ff1a, Ff1b, and Ff1c. Two heptad 9 mutants and one AF2 mutant were constructed (Fig. 3A). The heptad 9A mutants carried LL-to-AA mutations for Ff1a and Ff1c and LI-to-AA mutations for Ff1b, while the heptad 9B mutants carried LP-to-AA mutations. In the AF2 mutants, the first two leucine residues were changed to alanine residues.
FIG. 3.
The heptad 9 and AF2 domains of Ff1 proteins are required for interaction with Prox1. (A) Mutations of Ff1 proteins at hepted 9 and AF2 were generated by site-directed mutagenesis. hep9A and hep9B are heptad 9 site-directed mutants with either LL or LP replaced by AA. (B) Interaction of Prox1 with the hep9A, hep9B, and AF2 mutants of the three Ff1 proteins in yeast. The results are from one representative experiment (n = 3) and presented as mean and SEM (n = 5).
These site-directed Ff1 mutants were tested for interactions with Prox1 in yeast (Fig. 3B). All Ff1b heptad 9 and AF2 mutants had completely lost their ability to interact with Prox1. In contrast, some heptad 9A and AF2 mutants of Ff1a and Ff1c were still able to interact with Prox1. Ff1a-heptad 9A was unable to interact with Prox1, while Ff1c-heptad 9A showed a >80% reduction in interaction strength. For AF2 mutants, the reduction of Prox1 interaction activity was smaller for Ff1a (50%) than for Ff1c (96%). For the heptad 9B mutants, while the hydrophobic LP residues were absolutely essential for Ff1b, their mutation to AA for Ff1a or Ff1c did not seem to affect their interaction with Prox1. Our results suggest that only Ff1b has an absolute requirement for both heptad 9 and AF2 domains in its interaction with Prox1 and that their cooperative effects may account for the strong interaction between Prox1 and Ff1b. The differential effects of the heptad 9A and AF2 mutants on the interaction of the Ff1 proteins with Prox1 are very intriguing, although we cannot provide a good biological explanation.
Prox1 is a corepressor of Ff1s in zebra fish embryos.
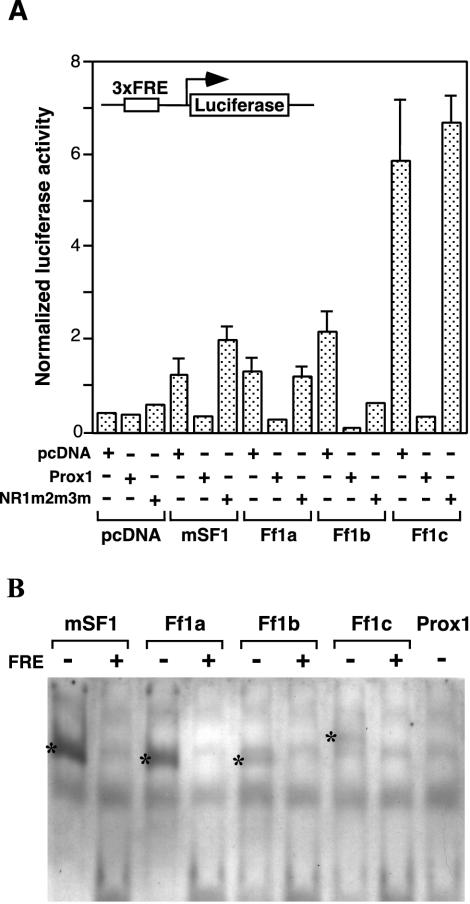
To understand if the physical interaction between Prox1 and Ff1 would modify the transcriptional activity of Ff1 proteins, we tested the ability of Prox1 to modulate Ff1-mediated transcriptional activation. Since ff1 genes and prox1 are not expressed maternally and the zygotic expression of ff1 genes initiates only after the 10-somite stage, zebra fish embryos provide a good in vivo context for evaluating the effects of Prox1 on Ff1 transcriptional activities. Zebra fish ff1 and mouse Sf-1 expression plasmids were microinjected into zebra fish embryos together with prox1 expression plasmid and pLuc3xFRE reporter (Fig. 4A). Although mouse Sf-1 and zebra fish Ff1 proteins activated the pLuc3xFRE reporter differentially, the repressing ability of Prox1 was consistent, affecting Ff1b transcriptional activity most severely (94.5% inhibition), followed by that of Ff1c (93.5%) and finally those of Ff1a (72.7%) and mouse Sf-1 (67.6%).
FIG. 4.
Prox1 represses Ff1-mediated transactivation in zebra fish embryos. (A) Embryos at the one- to two-cell stage were injected with the pLuc3xFRE reporter and different combinations of Ff1 and Prox1 expression vectors. pRL-CMV was included as a normalization control for firefly luciferase activity. Embryos (n = 5) were harvested at 14 hpf, and dual-luciferase assays were performed. The normalized luciferase activity was calculated from three experiments. (B) Electrophoretic mobility shift assay for zebra fish Ff1 proteins and Prox1, using a consensus FRE. In vitro-translated zebra fish Ff1a, Ff1b, Ff1c, and Prox1 and mouse SF-1 were allowed to bind DIG-labeled oligonucleotides carrying a consensus FRE. All zebra fish Ff1 proteins and mouse SF-1 bound FRE, but no binding was observed for zebra fish Prox1. Unlabeled FRE oligonucleotides (25-fold excess) were used as competitors to examine the specificity of the protein-FRE binding. The FRE-Ff1 complexes are denoted by an asterisk.
Coinjections of zebra fish ff1 and mouse Sf-1 expression plasmids with the mutant prox1-NR1m2m3m construct were also performed to examine whether the physical interaction mediated by the NR boxes was essential for the repression. In contrast to wild-type Prox1, the coexpression of Prox1-NR1m2m3m did not lead to similar repression of Ff1 transactivation activities, implicating that physical interaction is required for Prox1 to repress Ff1 activities. It is noteworthy that although the Prox1-NR1m2m3m mutant is presumably unable to interact with Ff1b, it is still capable of repressing Ff1b transcriptional activity.
An electromobility shift assay was performed to rule out the possibility that the repression of Ff1 transcriptional activity by Prox1 was due to a competition for FRE binding (Fig. 4B). SF-1 is known to bind FRE as a monomer to activate transcription (74, 79). The ability of zebra fish Ff1a, Ff1b, and Ff1c to recognize and bind a consensus FRE has been previously shown (C. Chai and W. K. Chan, unpublished data). The zebra fish ff1a, ff1b, ff1c, and prox1 and the mouse Sf-1 mRNAs were transcribed in vitro and translated. Equal amounts of in vitro translation reaction mixture were incubated with DIG-labeled FRE oligonucleotides, and the formation of protein-DNA complexes was analyzed on a 8% polyacrylamide gel. All the Ff1 and Sf1 proteins formed gel-retarded complexes with FRE, which could be competed efficiently (80%) by unlabeled FREs at a molar excess of 25-fold, indicating that the Ff1 proteins binding to FRE were specific. Prox1, however, failed to form stable complexes with FRE, ruling out the possibility that Prox1 could compete with Ff1 proteins for binding to FRE. We conclude that the repression of Ff1 transcriptional activity by Prox1 does not result from the competition for FRE but is mediated through physical interactions and is also likely to be independent of the DNA binding ability of Prox1.
Prox1 colocalizes with ff1b in the interrenal primordium during embryogenesis.
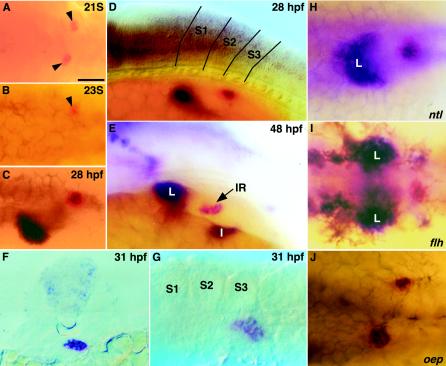
During embryogenesis, prox1 colocalizes with ff1a and ff1b expression domains in the trunk region. ff1b is the earliest known marker of the interrenal primordium, which gives rise to the adrenocortical steroidogenic cells (9). The expression of ff1b in the presumptive interrenal primordium first appeared as two bilateral patches close to the midline at the 21-somite stage (Fig. 5A). By the 23-somite stage, they converged to form a single cluster located immediately to the right of the midline of the third somite (Fig. 5B). By the 28-somite stage, prox1 could be detected in the hepatic and interrenal primordia (Fig. 5C and D), and prox1 expression at the cluster of cells forming the intestinal primordium, which is posterior and ventral to the interrenal organ, was initiated as late as 48 hpf (Fig. 5E). However, we were unable to locate prox1 expression in the pancreatic primordium. A cross-section of the interrenal primordium (Fig. 5F) revealed that ff1b and prox1 transcripts appeared to be coexpressed within the cluster. The ff1b-expressing interrenal primordium could be easily identified by the enzymatic activity of 3β-Hsd. 3β-Hsd was detectable as early as 27.5 hpf, which is after the convergence of the interrenal tissue primordia and temporally coincided with the initiation of prox1 expression. At 31 hpf, prox1 expression demarcated the steroidogenic cells, as revealed by 3β-Hsd histochemical staining (Fig. 5G).
FIG. 5.
Colocalization of prox1 and ff1b at interrenal tissue in wild-type embryo and midline mutants. (A to E) Dorsal (A to C) and lateral, (D and E) view of wild-type embryos after two-color in situ hybridization with prox1 (blue) and ff1b (red) at the 21-somite stage (B), the 23-somite stage (B), 28 hpf (C), and 48 hpf (D and E). The ff1b-expressing interrenal primordium at the 21- and 23-somite stages is denoted by an arrowhead. Boundaries defining the first three somites are indicated in panel D. (F) A cross-section of a 31-hpf embryo at the midlevel of the interrenal primordium after double staining of prox1 (blue) and ff1b (red). (G) Lateral view of a 31-hpf embryo showing 3β-Hsd enzymatic activity (histochemistry) and prox1 expression (in situ hybridization). The yolk was manually removed. (H to J) Mutants with defects in midline specification including no tail (ntl) (H), floating head (flh) (I), and one-eyed pinhead (oep) (J) after double in situ hybridization of prox1 (blue) and ff1b (red). All panels show the trunk of the embryo and, except for panel F, are oriented with the anterior to the left. L, liver; S1 to S3, somites 1 to 3. Bar, 50 μm.
The initiation of prox1 expression at interrenal tissue occurred after the convergence of the left and right bilateral interrenal primordia. We decided to investigate if proper midline specification is a prerequisite for the developing interrenal tissue to express prox1. Zebra fish mutants with various degrees of midline defects, including no tail (ntl), floating head (flh), and one-eyed-pinhead (oep), were examined for the expression of both prox1 and ff1b by double in situ hybridization (Fig. 5H to J). Mutations at the ntl/Brachyury locus lead to defects in notochord (axial mesoderm) differentiation (23, 62), whereas the notochord in flh/Xnot mutant embryos is replaced by muscles (paraxial mesoderm) (24, 71). The merger of the bilateral interrenal primordia and the colocalization of prox1 and ff1b were unperturbed in these two zebra fish mutants. In contrast, the prox1-expressing hepatic cells located anterior to the interrenal tissue showed a loss of asymmetry during the process of liver morphogenesis. Consistent with the notion that notochord signaling is important for liver asymmetry (34), flh displayed a stronger phenotype, with the two symmetric liver primordia failing to merge, while a partial merger of bilateral liver primordia was found in ntl. The expression patterns of prox1 in ntl and flh mutants revealed that notochord signaling is essential for the convergence of the hepatic but not interrenal primordia, despite the expression of prox1 in both.
oep/EGF-CFC, the homolog of the mouse cripto and criptic genes (83), affects the nodal signaling required for proper specification of endoderm and midline tissues (20, 61). We found that the bilateral interrenal tissue primordia failed to fuse in oep mutant embryos and that two clusters of ff1b-expressing cells remained on either side of the midline at 24 hpf. Interestingly, the colocalization of ff1b and prox1 expression was not affected because the convergence of interrenal primordia did not take place in oep mutants. The colocalization of prox1 and ff1b in oep mutants, despite the perturbed interrenal development due to defective midline signaling, further supports the idea that a tight regulatory or biochemical relationship exists between Prox1 and Ff1b.
prox1 colocalizes with ff1a at the liver and intestine with a later onset of expression.
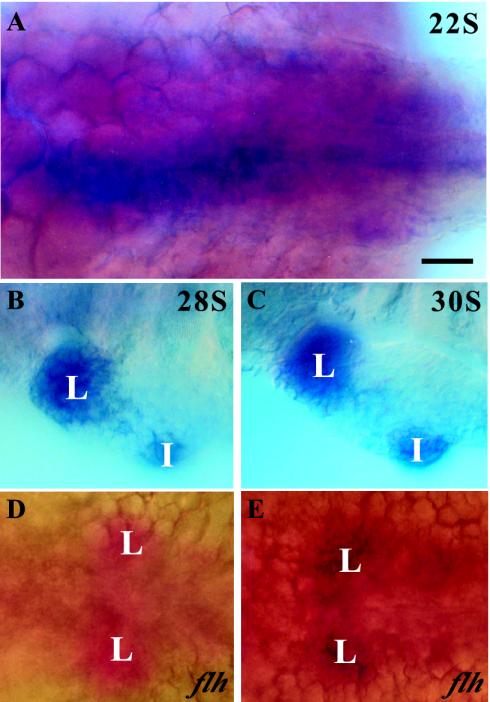
By the 22-somite stage, ff1a was detected at the posterior endoderm, to the left of the midline. The ff1a-expressing endodermal cells first appeared as a thin, continuous layer on the ventral side of the embryo, in close contact with the yolk sac (Fig. 6A). They became restricted to hepatic and intestinal primordia by the 28-somite stage. The colocalization of prox1 in the liver primordium was first evident at this stage (Fig. 6B and C). A sequential two-color staining performed on an flh mutant, where the hepatic primordia appeared bilaterally symmetrical to the midline, showed that prox1 and ff1a were colocalized (Fig. 6D and E). Although the ff1a expression at the intestinal primordium started as early as its aggregation by the 28-somite stage, prox1 could be detected only around 48 hpf.
FIG. 6.
Colocalization of ff1a and prox1 in the liver primordium. Wild-type and flh mutant embryos were double labeled with ff1a (blue) and prox1 (red). (A) At the 22-somite stage, ff1a but not prox1 was detected at various regions of the posterior endoderm. (B and C) From the 28-somite stage (B) to the 30-somite stage (C), ff1a was expressed at both hepatic and intestinal primordia while prox1 colocalized with ff1a at the hepatic but not the intestinal primordium. The fast red staining of prox1 was difficult to distinguish from the strong intensity of ff1a staining. (D and E) A sequential double in situ hybridization was performed on the flh mutant, initially with prox1 (red) (D) and then with ff1a (blue) (E), with both stains delineating the symmetric hepatic primordia across the midline. L, liver; I, intestine. Bar, 50 μm.
prox1 morphants led to a milder degree of disruption at the interrenal primordium than ff1b morphants.
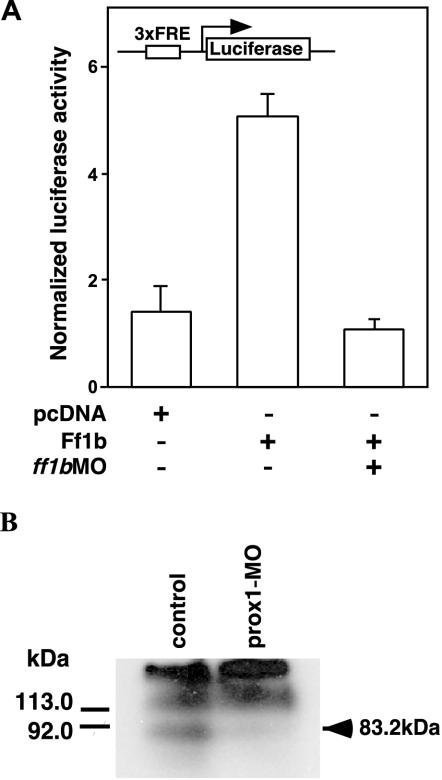
Based on the colocalization of prox1 with ff1b, the phenotypes of prox1 and ff1b morphants (prox1MO and ff1bMO) were compared to determine if prox1 and ff1b are functionally required in the development of the interrenal organ. The effectiveness of ff1bMO and prox1MO in knocking down Ff1b and Prox1 protein function was first verified. Consistent with our earlier results (9), the Ff1b-mediated transcriptional activity was markedly reduced in embryos coinjected with ff1bMO and the ff1b expression and luciferase reporter plasmids (Fig. 7A). prox1MO was also effective in reducing Prox1 levels, as revealed by Western blot analysis (Fig. 7B). Antibodies raised against zebra fish Prox1 detected three bands in the lysate. The lowest band (∼83.2 kDa) correlated with the estimated size of zebra fish Prox1 and was specifically knocked down by prox1MO. In contrast, two other protein bands (>113 kDa) which reacted with the Prox1 antibodies were quantitatively unchanged.
FIG. 7.
ff1bMO and prox1MO inhibited protein translation in zebra fish embryos. (A) The pLuc3xFRE luciferase reporter and the expression plasmid, pcDNA3.1-ff1b were coinjected into one-cell-stage embryos, with or without ff1bMO (1 pmol per embryo). pRL-CMV was included as a normalization control. pcDNA3.1 was also coinjected with the luciferase reporter to determine the background value. Embryos (n = 5) were harvested at 14 hpf, and dual luciferase assays were performed. The normalized luciferase activity was calculated from three experiments. (B) prox1MO (1 pmol per embryo) was injected into one-cell-stage embryos. Both uninjected and injected embryos were harvested at 31 hpf for protein extraction. An aliquot (equivalent to 30 embryos) was separated by SDS-PAGE and analyzed by Western blotting with anti-prox1 antibody. An 83.2-kDa band, which is equivalent to the putative molecular mass of Prox1, was knocked down by prox1MO, while two nonspecific high-molecular-mass bands remained unaffected.
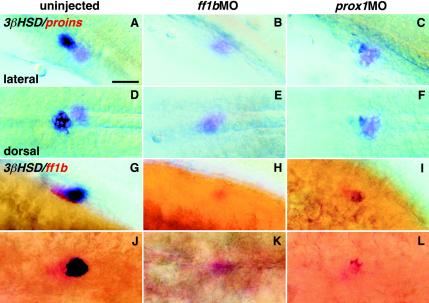
We have shown previously that ff1bMO led to the loss of 3β-Hsd enzymatic activity in the interrenal tissues of embryos (9). We analyzed both prox1 and ff1b morphants at 31 hpf, a stage immediately after the initiation of prox1 and ff1b colocalization (Fig. 8; Table 1). Injection of ff1bMO (1.2 to 2.5 pmol per embryo) did not cause gross morphological aberration but resulted in the complete ablation of ff1b-expressing cells in some embryos (Table 2). The ff1b morphants had a complete reduction of 3β-Hsd enzymatic activity (Fig. 8B and E; Table 1). As a comparison, prox1MO led to only a partial reduction of 3β-Hsd activity in a large number of embryos (Fig. 8C and F; Table 1). However, coinjection of ff1bMO and prox1MO resulted in the complete loss of 3β-Hsd activities (Table 1). The number of ff1b-expressing cells were partially reduced in ff1b morphants injected at 1 pmol per embryo (Fig. 8H and K; Table 2). No doses of ff1bMO, however, perturbed endocrine islet cells, as revealed by proinsulin expression (Fig. 8B and E). The reduction in the number of ff1b-expressing cells by ff1bMO is caused by the ablation of interrenal tissue or could be due to an interruption of some unknown mechanism of ff1b autoregulation (9).
FIG. 8.
Effects of ff1bMO and prox1MO on the enzymatic activity of 3β-Hsd. Either ff1bMO or prox1MO was injected into one-cell-stage embryos (1 pmol per embryo). Uninjected and injected embryos were fixed at 31 hpf and subjected to chromogenic staining of 3β-Hsd activity (blue) and in situ hybridization for either proinsulin (red) (A to F) or ff1b (red) (G to L). The cluster of proinsulin-expressing cells is situated immediately caudal to the interrenal primordium, which expresses both ff1b and 3β-Hsd. Bar, 50 μm.
TABLE 1.
Number of zebra fish larvae retaining 3β-Hsd activity in the interrenal tissues after injection with various antisense MOs
| MO injected | Dosage (pmol) per embryo | No (%) with 3β-Hsd activity in interrenal tissue:
|
n | ||
|---|---|---|---|---|---|
| Complete loss | Partial loss | Wild type | |||
| ff1b | 1.0 | 212 (88.0) | 10 (4.1) | 19 (7.9) | 241 |
| 1.2 | 170 (98.2) | 1 (0.6) | 2 (1.2) | 173 | |
| prox1 | 1.0 | 2 (1.3) | 116 (73.4) | 40 (25.3) | 158 |
| 1.2 | 3 (3.2) | 88 (93.6) | 3 (3.2) | 94 | |
| ff1b + prox1 | 1.0 each | 104 (100) | 0 (0) | 0 (0) | 104 |
| STDa | 1.2 | 0 (0) | 1 (0.6) | 160 (99.4) | 161 |
| Uninjected | 1 (0.5) | 7 (3.7) | 181 (95.8) | 189 | |
STD, standard control MO.
TABLE 2.
Number of ff1b-MO-injected zebra fish larvae retaining prox1 expression in the interrenal tissue
| MO injected | Dosage (pmol) per embryo | No. (%) with prox1 expressionc:
|
n | |||
|---|---|---|---|---|---|---|
| Complete loss
|
Wild type
|
|||||
| ff1b(+) | ff1b(−) | ff1b(+) | ff1b(−) | |||
| ff1b | 0.8 | 44 (67.7) | 0 (0) | 21 (32.3) | 0 (0) | 65 |
| 1.2 | 86 (48.9) | 65 (36.9) | 25 (14.2) | 0 (0) | 176 | |
| 2.5 | 69 (38.1) | 92 (50.8) | 20 (11.1) | 0 (0) | 181 | |
| iaff1aa | 1.2 | 0 (0) | 0 (0) | 53 (100) | 0 (0) | 53 |
| STDb | 1.2 | 0 (0) | 0 (0) | 82 (100) | 0 (0) | 82 |
| Uninjected | 0 (0) | 0 (0) | 189 (100) | 0 (0) | 189 | |
iaff1a, the complement of the antisense sequence of ff1b-MO.
STD, standard control MO.
ff1b(+) denotes no or minimal reduction of ff1b transcripts at interrenal tissue; ff1b(−) denotes significant or total reduction of ff1b transcripts at interrenal tissue.
Injection of prox1MO (1.0 and 1.2 pmol) did not result in growth or morphological defects. Neither a complete ablation of interrenal cells nor the absence of ff1b expression was observed in prox1 morphants (Fig. 8I and L; Table 1). The size of the interrenal organ was, however, reduced, as judged by ff1b and 3β-Hsd activity (Fig. 8I and L), albeit to a lesser extent than in ff1b morphants. While the enzymatic activity of 3β-Hsd showed a marked decrease, the proinsulin expression appeared to be normal in the prox1 morphants (Fig. 8C and F).
Ff1b morpholino specifically downregulates prox1 at interrenal tissue.
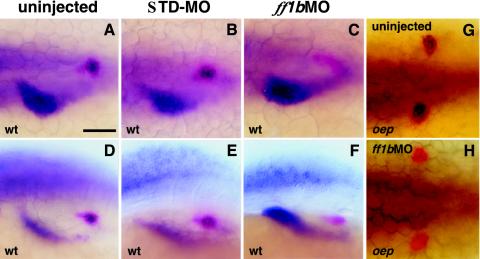
Lower doses of ff1bMO (<1 pmol per embryo) caused partial deletion of the interrenal primordium and left the ff1b largely unperturbed, but prox1 expression was knocked down completely in >67.7% of injected embryos (Fig. 9C and F; Table 2). Furthermore, prox1 expression was never observed in ff1b morphants that were defective in ff1b (Table 2). The down-regulation of prox1 expression by ff1bMO was highly specific, since prox1 expression in the liver and other tissues including the anterior neural plate and retina remained unchanged. In oep mutants, where the bilateral interrenal primordia failed to merge, ff1bMO (0.8 pmol) also led to a complete absence of prox1 expression without affecting the size of interrenal primordia significantly (Fig. 9G and H). Based on the prox1 down-regulation by ff1bMO in the wild type and oep mutants, we speculate that a close regulatory relationship between ff1b and prox1 exists in interrenal development.
FIG. 9.
fflbMO perturbs prox1 expression at the interrenal organ but not the liver. Either STDMO (B and E) or ff1bMO (C and F) was injected into wild-type (A to F) and oep (G and H) embryos at the one-cell stage (0.8 pmol per embryo). Uninjected and injected embryos were fixed at 31 hpf, and in situ hybridization was carried out using prox1 (blue) and ff1b (red) as probes. The apparently strong liver prox1 staining in the ff1bMO-injected embryo (C and F) resulted from our effort to highlight the thin layer of ff1b-expressing cells. Bar, 50 μm.
DISCUSSION
The polarized expression of Prox1 is pivotal for the specification of the lymphatic fate and subsequent development of the lymphatic vasculature in mice (77, 78). More importantly, human PROX1 has been identified as a master regulator that could promote a lymphatic endothelial phenotype by overriding the default blood vasculature phenotype (29, 55). Mice lacking Prox1 also display defects in lens fiber elongation and hepatocyte migration in the embryonic liver (67, 76). The molecular mechanisms responsible for the developmental functions of Prox1 have not been elucidated. It is probable that many of these developmental effects are dependent on the ability of Prox1 to transactivate downstream target genes such as γ-crystallin (37). Recently, we discovered that zebra fish Prox1 could act as a novel NR coactivator partner of TRs and RARs (15); this added a new dimension to the regulatory repertoire of Prox1. We further extend the repertoire of Prox1 here to include an ability to disrupt interrenal development by acting as a novel coregulator of Ff1b.
Prox1 expression in the interrenal primordium.
Prox1 expression in mammals is consistent with a bipotential precursor cell fate for the pancreas and liver (5, 14). The expression of prox1 in the liver is conserved throughout the vertebrates, including mice (5, 50), humans (84), and zebra fish. However, although prox1 is detected in mouse pancreatic precursors, as visualized by LacZ staining (5), we are unable to establish its pancreatic expression in zebra fish by using in situ hybridization. Thus, prox1 expression in early zebra fish endoderm might be restricted only to the liver precursors. Also, we should not discount the possibility that prox1 expression is too weak to be detected by in situ hybridization and that a lineage-tracing approach is required. More importantly, we report here for the first time that prox1 is coexpressed with Ff1b in the zebra fish interrenal organ several hours after its initial specification. The expression of prox1 in the mammalian fetal adrenal remains to be established.
The initiation of prox1 colocalization with ff1b in the interrenal cells coincides with the rapid accumulation of 3β-Hsd enzymatic activity, which suggests that they might have differentiated or are committed to a steroidogenic fate. The relatively late expression of prox1 in the interrenal organ, which also lags behind ff1a expression in the liver, appears to rule out a functional role in the initiation or specification of the interrenal primordium. Similarly to its role in the maturation and terminal differentiation of slow skeletal fibers (58), Prox1 probably functions at a rather late stage of initiation or maturation in the interrenal. Since the “knockdown” Prox1 activity does not lead to a complete loss of 3β-Hsd activity, Prox1 is unlikely to mediate or act immediately downstream of Ff1b. In the ubo phenotype, interrenal development appears not to be affected or show any osmoregulatory defects. It appears that a signaling pathway distinct from the Shh pathway identified in the ubo phenotype (58) is responsible for activating the expression of prox1 in the interrenal primordium, which, incidentally, shares a mesodermal origin with skeletal muscles.
Prox1 interaction with Ff1b.
While the vast majority of NR coregulators are structurally diverse, they nevertheless have a recurring structural feature, an α-helical LXXLL motif that is used to interact with NRs (28). Prox1 interaction with Ff1b is also dependent on such an LXXLL motif, LRKLL, which forms part of NR box I. However, NR box II must also be mutated for Prox1 to lose the ability to interact with Ff1b. What is unusual is that an intact second IXXLL motif (ISQLL) is absolutely required for the ligand-dependent recruitment by RARs and TRs (15), while NR box III retains absolutely no activity. Such divergent properties of NR boxes within a single coregulator have never been previously reported. The differential requirement for the heptad 9 and AF2 domains among the three zebra fish Ff1s is also intriguing. Interaction of Ff1b and Ff1c with Prox1 requires intact AF2 and heptad 9 domains, which is clearly different from the requirement of Ff1a: an intact heptad 9 domain more than an intact AF2 domain. Thus, unlike what has been demonstrated for Ff1a with human SRC-1 (41), only in Ff1b is there a strict requirement for the heptad 9 domain for interaction with Prox1.
Prox1 is a corepressor of Ff1b.
Although Ff1b is crucial for the specification of the interrenal primordium and initiation of steroidogenesis (9), overexpression of Ff1b during early zebra fish embryogenesis does not lead to the appearance of ectopic steroidogenic cells, as judged by 3β-Hsd activity (data not shown). In murine embryonic stem cells, however, SF-1 overexpression confers a steroidogenic fate (11). It appears that the recruitment of specific coregulators by zebra fish Ff1b might be required for the induction and specification of the interrenal organ. Besides the well-characterized interactions with DAX-1 and WT-1 (12, 47), many other transcriptional activators like Egr-1, Ptx1, GATA4, USF, TReP-132, DEAD box protein DP103, and SOX9 could functionally modulate SF-1 (13, 17, 26, 36, 51, 64, 65, 72, 73). In gonadotrophs, for instance, Egr-1 synergizes with SF-1 to regulate the β subunit of lutenizing hormone while Ptx1 acts in concert with SF-1 and Pit1 to regulate the Lim-homeodomain gene, Lim3/Lhx3 (36, 72).
Consistent with the view that an intricate relationship exists between Prox1 and Ff1b, prox1 morphants display defective differentiation of the interrenal cells as judged by the loss of 3β-Hsd activity and ff1b expression, while in ff1b morphants, prox1 is not expressed and the entire interrenal is ablated (9). What is intriguing is that the knock down of Prox1 did not lead to an increase or expansion of the interrenal ff1b expression in prox1 morphants. This would have been consistent with the ability of Prox1 to repress the transcriptional activity of Ff1b, as described in this paper. The failure to find such a direct correlation indicates that a complex interaction exists between Prox1 and Ff1b in the interrenal primordium. We favor the existence of multiple levels of regulatory interaction between Prox1 and Ff1b, which are likely to extend beyond the corepressor role that we have described for Prox1 on Ff1b. The down-regulation of prox1 expression in ff1b morphants reinforces this notion and suggests that Ff1b could act either directly to regulate the transcription of prox1 in the interrenal primordium or indirectly due to the destruction of interrenal precursor cells brought about by the loss of Ff1b functions.
The physical interaction between zebra fish Prox1 and Ff1b and the subsequent formation of a complex that could repress Ff1b-mediated transcriptional activity suggest that the down-regulation of specific Ff1b-responsive genes may be an integral part of the differentiation of the zebra fish interrenal organ. These downstream target genes might be involved in the induction, proliferation, or even apoptosis of early interrenal cells (32, 53). A putative FRE has been identified in the promoter of the zebra fish ff1b gene (Chai and Chan, unpublished), suggesting that Ff1b expression could be autoregulated, which has also been demonstrated for the mammalian SF-1 (48). Thus, the down-regulation of ff1b expression by the Prox1-Ff1b repressor complex might also conceivably serve as a means of interfering with the autoregulation of ff1b and allowing the final maturation and acquisition of steroidogenic capacity by the interrenal cells.
Besides DAX-1 and Prox1, RIP 140 is also a corepressor for SF-1 in the adrenal (68). The relationships among all three corepressors are, however, unclear. Since DAX-1 serves as an adaptor in recruiting the N-CoR corepressor complex to SF1 (12), it remains to be seen if Prox1 could similarly recruit corepressor complexes containing N-CoR or histone deacetylase complexes to Ff1b and cause the subsequent displacement of coactivator complexes. Another aspect that requires careful consideration is that with the knock down of Prox1 functions in prox1 morphants, Dax-1 or RIP 140 could replace Prox1 as the predominant interacting partner and exert an even greater inhibitory effect on the Ff1b-mediated transcriptional activity, leading to a decrease of ff1b and 3β-hsd expression. The disruption of Dax-1 and RIP 140 functions in the zebra fish interrenal organ would therefore be important for the further elucidation of the functional roles of Prox1.
Role of Prox1 as a transcription factor.
As a sequence-specific transcription factor, Prox1 could also activate downstream target genes that might play a role in interrenal development. The homeodomain is very highly conserved in the vertebrate Prox1 and Drosophila prospero. However, zebra fish Prox1 did not bind the Drosophila prospero binding site or the Prox1-responsive element identified in the murine γ-crystallin promoter (27, 37) in our hands (data not shown). The determination of the putative binding motif of Prox1 will not only serve to further our understanding of the gene expression regulatory network coordinated by Prox1 but will also facilitate the search for putative transcription units which might harbor adjacent DNA binding sites for Ff1 and Prox1.
While the impact of the Prox1-Ff1b corepressor complex has not been fully determined and specific target genes that are repressed in the interrenal organ have not been identified, it is tempting to speculate that the formation of this repressor complex is essential to bring about the terminal differentiation of the adrenocortical cells in the zebra fish interrenal organ. The involvement of transcription repression in order for NRs to exert developmental functions is not without precedent. For instance, Hairless, which is a corepressor for TRs, is required for hair follicle development (56), while the Drosophila TIF1 homologue, bonus, which forms a corepressor complex with Ftz-F1β, is required for numerous events in metamorphosis including leg elongation, bristle development, and pigmentation (4). The relatively uncomplicated morphological organization of the zebra fish interrenal organ will allow us to formulate a more precise definition of the developmental blueprint that governs the specification and fate of the steroidogenic cells.
Acknowledgments
This work was supported by the Institute of Molecular Agrobiology and a BMRC grant (to C.W.K.). G.W. is supported by a NUS scholarship and NUS research grant (R-154-000-124-112).
We thank B. C. Chung (Institute of Molecular Biology, Academia Sinica, Taiwan) for her generosity in sharing her unpublished data with us, and K. L. Parker (University of Texas Southwestern Medical Center) for providing us with the mouse Sf-1.
REFERENCES
- 1.Ahmad, W., M. Faiyaz ul Haque, V. Brancolini, H. C. Tsou, S. ul Haque, H. Lam, V. M. Aita, J. Owen, M. deBlaquiere, J. Frank, P. B. Cserhalmi-Friedman, A. Leask, A. J. McGrath, M. Peacocke, M. Ahmad, J. Ott, and A. M. Christiano. 1998. Alopecia universalis associated with a mutation in the human hairless gene. Science 279:720-724. [DOI] [PubMed] [Google Scholar]
- 2.Aranda, A., and A. Pascual. 2001. Nuclear hormone receptors and gene expression. Physiol. Rev. 81:1270-1304. [DOI] [PubMed] [Google Scholar]
- 3.Babu, P. S., D. L. Bavers, F. Beuschlein, S. Shah, B. Jeffs, J. L. Jameson, and G. D. Hammer. 2002. Interaction between Dax-1 and steroidogenic factor-1 in vivo: increased adrenal responsiveness to ACTH in the absence of Dax-1. Endocrinology 143:665-673. [DOI] [PubMed] [Google Scholar]
- 4.Beckstead, R., J. A. Ortiz, C. Sanche, S. N. Prokopenko, P. Chambon, R. Losson, and H. J. Bellen. 2001. Bonus, a Drosophila homolog of TIF1 proteins, interacts with nuclear receptors and can inhibit betaFTZ-F1-dependent transcription. Mol. Cell 7:753-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke, Z., and G. Oliver. 2002. Prox1 is an early specific maker for the developing liver and pancreas in the mammalian foregut endoderm. Mech. Dev. 118:147-155. [DOI] [PubMed] [Google Scholar]
- 6.Burris, T. B., W. Guo, and E. R. McCabe. 1996. The gene responsible for adrenal hypoplasia congenita, DAX-1, encodes a nuclear hormone receptor that defines a new class within the superfamily. Recent Prog. Horm. Res. 51:241-259. [PubMed] [Google Scholar]
- 7.Call, K. M., T. Glaser, C. Y. Ito, A. J. Buckler, J. Pelletier, D. A. Haber, E. A. Rose, A. Kral, H. Yeger, W. H. Lewis, C. Jones, and D. E. Housman. 1990. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell 60:509-520. [DOI] [PubMed] [Google Scholar]
- 8.Chai, C., and W. K. Chan. 2000. Developmental expression of a novel Ftz-F1 homologue, ff1b (5A4), in the zebra fish Danio rerio. Mech. Dev. 91:421-426. [DOI] [PubMed] [Google Scholar]
- 9.Chai, C., Y. W. Liu, and W. K. Chan. 2003. ff1b is required for the development of steroidogenic component of the zebra fish interrenal organ. Dev. Biol. 260:226-244. [DOI] [PubMed] [Google Scholar]
- 10.Crawford, P. A, J. A. Polish, G. Ganpule, and Y. Sadovsky. 1997. The activation function-2 hexamer of steroidogenic factor-1 is required, but not sufficient, for potentiation by SRC-1. Mol. Endocrinol. 11:1626-1635. [DOI] [PubMed] [Google Scholar]
- 11.Crawford, P. A., Y. Sadovsky, and J. Milbrandt. 1997. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells towards the steroidogenic lineage. Mol. Cell. Biol. 17:3997-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford, P. A., C. Dorn, Y. Sadovsky, and J. Milbrandt. 1998. Nuclear receptor DAX-1 recruits nuclear receptor corepressor N-CoR to steroidogenic factor 1. Mol. Cell. Biol: 18:2949-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Santa Barbara, P., N. Bonneaud, B. Boizet, M. Desclozeaux, B. Moniot, P. Sudbeck, G. Scherer, F. Poulat, and P. Berta. 1998. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Mullerian hormone gene. Mol. Cell. Biol. 18:6653-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deutsch, G., J. Jung, M. Zheng, J. Lora, and K. S. Zaret. 2001. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 128:871-881. [DOI] [PubMed] [Google Scholar]
- 15.Gao, W. 2002. M.Sc. thesis. National University of Singapore, Singapore.
- 16.Gessler, M., A. Poustka, W. Cavenee, R. L. Neve, S. H. Orkin, and G. A. Bruns. 1990. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature 343:774-778. [DOI] [PubMed] [Google Scholar]
- 17.Gizard, F., B. Lavallee, F. DeWitte, E. Teissier, B. Staels, and D. W. Hum. 2002. The transcriptional regulating protein of 132 kDa (TReP-132) enhances P450scc gene transcription through interaction with steroidogenic factor-1 in human adrenal cells. J. Biol. Chem. 277:39144-39155. [DOI] [PubMed] [Google Scholar]
- 18.Glass, C. K., and M. G. Rosenfeld. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14:121-141. [PubMed] [Google Scholar]
- 19.Grassi Milano, E., F. Basari, and C. Chimenti. 1997. Adrenocortical and adrenomedullary homologs in eight species of adult and developing teleosts: morphology, histology, and immunohistochemistry. Gen. Comp. Endocrinol. 108:483-496. [DOI] [PubMed] [Google Scholar]
- 20.Gritsman, K., J. Zhang, S. Cheng, E. Heckscher, W. S. Talbot, and A. F. Schier. 1999. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell 97:121-132. [DOI] [PubMed] [Google Scholar]
- 21.Gubler, M. C., Y. Yang, C. Jeanpierre, S. Barbaux, and P. Niaudet. 1999. WT-1, renal development, and glomerulopahies. Adv. Nephrol. Necker Hosp. 29:299-315. [PubMed] [Google Scholar]
- 22.Guichet, A., J. W. Copeland, M. Erdelyi, D. Hlousek, P. Zavorszky, J. Ho, S. Brown, A. Percival-Smith, H. M. Krause, and A. Ephrussi. 1997. The nuclear receptor homologue Ftz-F1 and the homeodomain protein Ftz are mutually dependent cofactors. Nature 385:548-352. [DOI] [PubMed] [Google Scholar]
- 23.Halpern, M. E., R. K. Ho, C. Walker, and C. B. Kimmel. 1993. Induction of muscle pioneers and floor plate is distinguished by the zebra fish no tail mutation. Cell 75:99-111. [PubMed] [Google Scholar]
- 24.Halpern, M. E., C. Thisse, R. K. Ho, B. Thisse, B. Riggleman, B. Trevarrow, E. S. Weinberg, J. H. Postlethwait, and C. B. Kimmel. 1995. Cell-autonomous shift from axial to paraxial mesodermal development in zebra fish floating head mutants. Development 121:4257-4264. [DOI] [PubMed] [Google Scholar]
- 25.Hanley, N. A., W. E. Rainey, D. I. Wilson, S. G. Ball, and K. L. Parker. 2001. Expression profiles of SF-1, DAX1, and CYP17 in the human fetal adrenal gland: potential interactions in gene regulation. Mol. Endocrinol. 15:57-68. [DOI] [PubMed] [Google Scholar]
- 26.Harris, A. N., and P. L. Mellon. 1998. The basic helix-loop-helix, leucine zipper transcription factor, USF (upstream stimulatory factor), is a key regulator of SF-1 (steroidogenic factor-1) gene expression in pituitary gonadotrope and steroidogenic cells. Mol. Endocrinol. 12:714-726. [DOI] [PubMed] [Google Scholar]
- 27.Hassan, B., L. Li, K. A. Bremer, W. Chang, J. Pinsonneault, and H. Vaessin. 1997. Prospero is a panneural transcription factor that modulates homeodomain protein activity. Proc. Natl. Acad. Sci. USA 94:10991-10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heery, D. M., E. Kalkhoven, S. Hoare, and M. G. Parker. 1997. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733-736. [DOI] [PubMed] [Google Scholar]
- 29.Hong, Y. K., N. Harvey, Y. H. Noh, V. Schacht, S. Hirakawam, M. Detmar, and G. Oliver. 2002. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 225:351-357. [DOI] [PubMed] [Google Scholar]
- 30.Hoyle, C., V. Narvaez, G. Alldus, R. Lovell-Badge, and A. Swain. 2002. Dax1 expression is dependent on steroidogenic factor 1 in the developing gonad. Mol. Endocrinol. 16:747-756. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda, Y., A. Swain, T. J. Weber, K. E. Hentges, E. Zanaria, E. Lalli, K. T. Tamai, P. Sassone-Corsi, R. Lovell-Badge, G. Camerino, and K. L. Parker. 1996. Steroidogenic factor 1 and Dax-1 colocalize in multiple cell lineages: potential links in endocrine development. Mol. Endocrinol. 10:1261-1272. [DOI] [PubMed] [Google Scholar]
- 32.Keegan, C. E., and G. D. Hammer. 2002. Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol. Metab. 13:200-208. [DOI] [PubMed] [Google Scholar]
- 33.Kimmel, C. B., W. W. Ballard, S. R. Kimmel, B. Ullmann, and T. F. Schilling. 1995. Stages of embryonic development of the zebra fish. Dev. Dyn. 203:253-310. [DOI] [PubMed] [Google Scholar]
- 34.Korzh, S., A. Emelyanov, and V. Korozh. 2001. Developmental analysis of ceruloplasmin gene and liver formation in zebra fish. Mech. Dev. 103:137-139. [DOI] [PubMed] [Google Scholar]
- 35.Kreidberg, J. A., H. Sariola, J. M. Loring, M. Maeda, J. Pelletier, D. Housman, and R. Jaenisch. 1993. WT-1 is required for early kidney development. Cell 744:679-691. [DOI] [PubMed] [Google Scholar]
- 36.Lee S. L., Y. Sadovsky A. H. Swirnoff, J. A. Polish, P. Goda, G. Gavrilina, and J. Milbrandt. 1996. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science 273:1219-1221. [DOI] [PubMed] [Google Scholar]
- 37.Lengler, J., E. Krausz, S. Tomarev, A. Prescott, R. A. Quinlan, and J. Graw. 2001. Antagonistic action of Six3 and Prox1 at the gamma-crystallin promoter. Nucleic Acids Res. 29:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, L. A., D. Lala, and B. C. Chung. 1998. Function of steroidogenic factor 1 (SF1) ligand-binding domain in gene activation and interaction with AP1. Biochem. Biophys. Res. Commun. 250:318-320. [DOI] [PubMed] [Google Scholar]
- 39.Li, L. A., E. F. Chiang, J. C. Chen, N. C. Hsu, Y. J. Chen, and B. C. Chung. 1999. Function of steroidogenic factor 1 domains in nuclear localization, transactivation, and interaction with transcription factor TFIIB and c-Jun. Mol. Endocrinol. 13:1588-1598. [DOI] [PubMed] [Google Scholar]
- 40.Lin, W. W., H. W. Wang, C. Sum, D. Liu, C. L. Hew, and B. C. Chung. 2000. Zebrafish ftz-f1 gene has two promoters, is alternatively spliced, and is expressed in digestive organs. Biochem. J. 348:439-446. [PMC free article] [PubMed] [Google Scholar]
- 41.Liu, D., M. Chandy, S. K. Lee, Y. Le Drean, H. Ando, F. Xiong, L. E. Woon, and C. L. Hew. 2000. A zebra fish Ftz-F1 (Fushi tarazu factor 1) homologue requires multiple subdomains in the D and E regions for its transcriptional activity. J. Biol. Chem. 275:16758-16766. [DOI] [PubMed] [Google Scholar]
- 42.Luo, X., Y. Ikeda, and K. L. Parker. 1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481-490. [DOI] [PubMed] [Google Scholar]
- 43.McKenna, N. J., and B. W. O'Malley. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465-474. [DOI] [PubMed] [Google Scholar]
- 44.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Moore, A. W., L. McInnes, J. Kreidberg, N. D. Hastie, and A. Schedl. 1999. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 126:1845-1857. [DOI] [PubMed] [Google Scholar]
- 46.Muscatelli, F., T. M. Strom, A. P. Walker, E. Zanaria, D. Recan, A. Meindl, B. Bardoni, S. Guioli, G. Zehetner, W. Rabl, H. P. Schwartz, J.-C. Kaplan, G. Camerino, T. Meitinger, and A. P. Monaco. 1994. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 372:672-676. [DOI] [PubMed] [Google Scholar]
- 47.Nachtigal, M. W., Y. Hirokawa, D. L. Enyeart-VanHouten, J. N. Flanagan, G. D. Hammer, and H. A. Ingraham. 1998. Wilms' tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 93:445-454. [DOI] [PubMed] [Google Scholar]
- 48.Nomura, M., H. Nawata, and K. Morohash. 1996. Autoregulatory loop in the regulation of the mammalian ftz-f1 gene. J. Biol. Chem. 271:8243-8249. [DOI] [PubMed] [Google Scholar]
- 49.Odenthal, J., P. Haffter, E. Vogelsang, M. Brand, F. J. van Eeden, M. Furutani-Seiki, M. Granato, M. Hammerschmidt, C. P. Heisenberg, Y. J. Jiang, D. A. Kane, R. N. Kelsh, M. C. Mullins, R. M. Warga, M. L. Allende, E. S. Weinberg, and C. Nusslein-Volhard. 1996. Mutations affecting the formation of the notochord in the zebra fish, Danio rerio. Development 123:103-115. [DOI] [PubMed] [Google Scholar]
- 50.Oliver, G., B. Sosa-Pineda, S. Geisendorf, E. P. Spana, C. Q. Doe, and P. Gruss. 1993. Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech. Dev. 44:13-16. [DOI] [PubMed] [Google Scholar]
- 51.Ou, Q., J. F. Mouillet, X. Yan, C. Dorn, P. A. Crawford, and Y. Sadovsky. 2001. The DEAD box protein DP103 is a regulator of steroidogenic factor-1. Mol. Endocrinol. 15:1569-1579. [DOI] [PubMed] [Google Scholar]
- 52.Parker, K. L. 1998. The roles of steroidogenic factor 1 in endocrine development and function. Mol. Cell. Endocrinol. 145:15-20. [DOI] [PubMed] [Google Scholar]
- 53.Parker, K. L., and B. P. Schimmer. 2001. Genetics of the development and function of the adrenal cortex. Rev. Endocr. Metab. Disord. 2:245-252. [DOI] [PubMed] [Google Scholar]
- 54.Panteleyev A. A., R. Paus, W. Ahmad, J. P. Sundberg, and A. M. Christiano. 1998. Molecular and functional aspects of the hairless (hr) gene in laboratory rodents and humans. Exp. Dermatol. 7:249-267. [DOI] [PubMed] [Google Scholar]
- 55.Petrova, T. V., T. Makinen, T. P. Makela, J. Saarela, I. Virtanen, R. E. Ferrell, D. N. Finegold, D. Kerjaschki, S. Yla-Herttuala, and K. Alitalo. 2002. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 21:4593-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Potter, G. B., G. M. Beaudoin III, C. L. DeRenzo, J. M. Zarach, S. H. Chen, and C. C. Thompson. 2001. The hairless gene mutated in congenital hair loss disorders encodes a novel nuclear receptor corepressor. Genes Dev. 15:2687-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robyr, D., A. P. Wolffe, and W. Wahli. 2000. Nuclear hormone receptor coregulators in action: diversity for shared tasks. Mol. Endocrinol. 14:329-347. [DOI] [PubMed] [Google Scholar]
- 58.Roy, S., C. Wolff, and P. W. Ingham. 2001. The u-boot mutation identifies a Hedgehog-regulated myogenic switch for fiber-type diversification in the zebra fish embryo. Genes Dev. 15:1563-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadovsky, Y., P. A. Crawford, K. G. Woodson, J. A. Polish, M. A. Clements, L. M. Tourtellotte, K. Simburger, and J. Milbrandt. 1995. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc. Natl. Acad. Sci. USA 92:10939-10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schier, A. F., S. C. Neuhauss, M. Harvey, J. Malicki, L. Solnica-Krezel, D. Y. Stainier, F. Zwartkruis, S. Abdelilah, D. L. Stemple, Z. Rangini, H. Yang, and W. Driever. 1996. Mutations affecting the development of the embryonic zebra fish brain. Development 123:165-178. [DOI] [PubMed] [Google Scholar]
- 61.Schier, A. F., S. C. Neuhauss, K. A. Helde, W. S. Talbot, and W. Driever. 1997. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebra fish and interacts with no tail. Development 124:327-342. [DOI] [PubMed] [Google Scholar]
- 62.Schulte-Merker, S., F. J. van Eeden, M. E. Halpern, C. B. Kimmel, and C. Nusslein-Volhard. 1994. no tail (ntl) is the zebra fish homologue of the mouse T (Brachyury) gene. Development 120:1009-1015. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz, C. J., H. M. Sampson, D. Hlousek, A. Percival-Smith, J. W. Copeland, A. J. Simmonds, and H. M. Krause. 2001. FTZ-Factor 1 and Fushi tarazu interact via conserved nuclear receptor and coactivator motifs. EMBO J. 20:510-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen, W. H., C. C. Moore, Y. Ikeda, K. L. Parker, and H. A. Ingraham. 1994. Nuclear receptor steroidogenic factor 1 regulates the mullerian inhibiting substance gene: a link to the sex determination cascade. Cell 77:651-661. [DOI] [PubMed] [Google Scholar]
- 65.Shen, H. C., and H. A. Ingraham. 2002. Regulation of the orphan nuclear receptor steroidogenic factor 1 by Sox proteins. Mol. Endcrinol. 16:529-540. [DOI] [PubMed] [Google Scholar]
- 66.Shinoda, K., H. Lei, H. Yoshii, M. Nomura, M. Nagano, H. Shiba, H. Sasaki, Y. Osawa, Y. Ninomiya, O. Niwa, K.-I. Morohashi, and E. Li. 1995. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev. Dyn. 204:22-29. [DOI] [PubMed] [Google Scholar]
- 67.Sosa-Pineda, B., J. T. Wigle, and G. Loiver. 2000. Hepatocyte migration during liver development required Prox1. Nat. Genet. 25:254-255. [DOI] [PubMed] [Google Scholar]
- 68.Sugawara, T., S. Abe, N. Sakuragi, Y. Fujimoto, E. Nomura, K. Fujieda, M. Saito, and S. Fujimoto. 2001. RIP 140 modulates transcription of the steroidogenic acute regulatory protein gene through interactions with both SF-1 and DAX-1. Endocrinology 142:3570-3577. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki, T., H. Kawasaki, R. T. Yu, H. Ueda, and K. Umesono. 2001. Segmentation gene product Fushi tarazu is an LXXLL motif-dependent coactivator for orphan receptor FTZ-F1. Proc. Natl. Acad. Sci. USA 98:12403-12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swain, A., E. Zanaria, A. Hacker, R. Lovell-Badge, and G. Camerino. 1996. Mouse Dax1 expression is consistent with a role in sex determination as well as in adrenal and hypothalamus function. Nat. Genet. 12:404-409. [DOI] [PubMed] [Google Scholar]
- 71.Talbot, W. S., B. Trevarrow, M. E., Halpern, A. E. Melby, G. Farr, J. H. Postlethwait, T. Jowett, C. B. Kimmel, and D. Kimelman. 1995. A homeobox gene essential for zebra fish notochord development. Nature 378:150-157. [DOI] [PubMed] [Google Scholar]
- 72.Tremblay, J. J., C. Lanctot, and J. Drouin. 1998. The pan-pituitary activator of transcription, Ptx1 (pituitary homeobox 1), acts in synergy with SF-1 and Pit1 and is an upstream regulator of the Lim-homeodomain gene Lim3/Lhx3. Mol. Endocrinol. 12:428-441. [DOI] [PubMed] [Google Scholar]
- 73.Tremblay, J. J., and R. S. Viger. 1999. Transcription factor GATA-4 enhances Mullerian inhibiting substance gene transcription through a direct interaction with the nuclear receptor SF-1. Mol. Endocrinol. 13:1388-1401. [DOI] [PubMed] [Google Scholar]
- 74.Ueda, H., G. C. Sun, T. Murata, and S. Hirose. 1992. A novel DNA-binding motif abuts the zinc finger domain of insect nuclear hormone receptor FTZ-F1 and mouse embryonal long terminal repeat-binding protein. Mol. Cell. Biol. 12:5667-5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Westerfield, M. 2000. The zebra fish book, 4th ed. University of Oregon Press, Eugene.
- 76.Wigle, J. T., K. Chowdhury, P. Gruss, and G. Oliver. 1999. Prox1 function is crucial for mouse lens-fibre elongation. Nat. Genet. 21:318-322. [DOI] [PubMed] [Google Scholar]
- 77.Wigle, J. T., and G. Oliver. 1999. Prox1 function is required for the development of the murine lymphatic system. Cell 98:769-778. [DOI] [PubMed] [Google Scholar]
- 78.Wigle, J. T., N. Harvey, M. Detmar, I. Lagutina, G. Grosveld, M. D. Gunn, D. G. Jackson, and G. Oliver. 2002. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 21:1505-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson, T. E., T. J. Fahrner, and J. Milbrandt. 1993. The orphan receptors NGFI-B and steroidogenic factor 1 establish monomer binding as a third paradigm of nuclear receptor-DNA interaction. Mol. Cell. Biol. 13:5794-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu, Y., W. Li, K. Su, M. Yussa, W. Han, N. Perrimon, and L. Pick. 1997. The nuclear hormone receptor Ftz-F1 is a cofactor for the Drosophila homeodomain protein Ftz. Nature 385:552-555. [DOI] [PubMed] [Google Scholar]
- 81.Yu, R. N., M. Ito, T. L. Saunders, S. A. Camper, and J. L. Jameson. 1998. Role of Ahch in gonadal development and gametogenesis. Nat. Genet. 20:353-357. [DOI] [PubMed] [Google Scholar]
- 82.Zanaria, E., F. Muscatelli, B. Bardoni, T. M. Strom, S. Guioli, W. Guo, E. Lalli, C. Moser, A. P. Walker, E. R. McCabe, T. Meitinger, A. P. Monaco, P. Sassone-Corsi, and G. Camerino. 1994. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 372:635-641. [DOI] [PubMed] [Google Scholar]
- 83.Zhang, J., W. S. Talbot, and A. F. Schier. 1998. Positional cloning identifies zebra fish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell 92:241-251. [DOI] [PubMed] [Google Scholar]
- 84.Zinovieva, R. D., M. K. Duncan, T. R. Johnson, R. Torres, M. H. Polymeropoulos, and S. I. Tomarev. 1996. Structure and chromosomal localization of the human homeobox gene Prox1. Genomics 35:517-522. [DOI] [PubMed] [Google Scholar]