Abstract
Infection with Helicobacter pylori is associated with different human gastric diseases. Biochemical studies, in vitro adherence assays, and in vivo animal models revealed that epithelial attachment of H. pylori can be mediated by the blood-group antigen-binding adhesin (BabA) targeting human Lewisb surface epitopes. Studies with transgenic mice expressing the Lewisb epitope have shown that such attachment can alter disease outcome. In the current study, the presence of the babA2 gene encoding the adhesin was investigated in clinical isolates from a German population by using PCR and reverse transcription–PCR. A positive genotype was correlated to allelic variations in the genes encoding VacA and CagA and also to the prevalence of duodenal ulcer, distal gastric adenocarcinoma, mucosa-associated lymphoid tissue lymphoma, and antral gastritis. The presence of babA2 was significantly associated with duodenal ulcer (P = 0.0002) and adenocarcinoma (P = 0.033). In contrast, type 1 strains (vacAs1- and cagA-positive) were associated with only duodenal ulcer (P = 0.004) but not adenocarcinoma (P = 0.235). Genotype presence of babA2, vacAs1, and cagA (“triple-positive” strains) showed a highly significant correlation to the prevalence of ulcer (P = 0.000002) and adenocarcinoma (P = 0.014) and discriminated significantly better between disease outcome than did the current type 1 classification. These results indicate that the babA2 gene is of high clinical relevance and would be a useful marker to identify patients who are at higher risk for specific H. pylori-related diseases.
Keywords: VacA, CagA, ulcer, adenocarcinoma
Helicobacter pylori is a Gram-negative bacterium that colonizes the human stomach, mainly persisting within the gastric mucus layer (1). After acquisition in childhood, the organism persists for decades, and mixed infections are uncommon (2–5). Few bacteria can be detected in membrane-to-membrane contact with epithelial cells; contact enables them to interact directly with the human gastric epithelium (6). H. pylori infection regularly leads to active gastritis, which may progress to chronic gastritis with atrophy (7), duodenal ulcer (8), gastric adenocarcinoma (9, 10), or mucosa-associated lymphoid tissue (MALT) lymphoma (11). Environmental circumstances, host factors, as well as bacterial virulence factors may be important for the differential development of these diseases. The relevance of bacterial virulence factors was suggested by epidemiological studies in which a strong association between the virulence factors vacuolating cytotoxin (VacA) and cytotoxin-associated antigen (CagA) and the presence of duodenal ulcer was detected (12, 13).
VacA is a protein of 87 kDa that has been shown to vacuolate epithelial cells (14). CagA is a 120-kDa immunodominant antigen that elicits a strong immunological response to H. pylori strains (15, 16) and is used as a marker for the insertion of a large pathogenicity island encoding many proteins, several of which have been implicated in pathogenesis (17). Strains harboring the VacA phenotype or vacAs1 genotype and the CagA protein or cagA gene have been detected at a higher frequency in patients with duodenal ulcer (18), atrophic gastritis, and gastric carcinoma (19–21). Because of the clinical importance of these virulence factors, H. pylori strains were classified as type 1 (VacA- and CagA-positive) and type 2 (both negative; ref. 22), but this classification alone did not allow for a proper clinical distinction between pathogenic and other strains because of the high prevalence of type 1 strains in western populations.
Recent studies provide evidence that bacterial adherence factors may contribute further to the specific tropism and pathogenicity of H. pylori in the human gastric epithelium. The blood-group antigen-binding adhesin, BabA, has been shown to mediate adherence of H. pylori to human Lewisb (α-1,3/4-difucosylated) blood-group antigens on gastric epithelial cells (23, 24). In vitro adherence assays revealed that H. pylori bound in a lineage-specific manner to gastric surface mucous cells mediated by fucosylated blood-group antigens (25). Moreover, studies with transgenic mice expressing the human Lewisb epitope in gastric epithelial cells indicated that Lewisb functions as a receptor for a H. pylori adhesin and mediates its attachment to gastric pit and surface mucous cells (26). Attachment of H. pylori to gastric epithelial cells in such transgenic mice resulted in the development of chronic gastritis and gastric atrophy (27). Recently, the gene encoding BabA has been cloned (and termed babA2; ref. 28), which thus allows identification of H. pylori strains harboring the babA2 genotype by PCR.
The clinical relevance of the H. pylori adherence factor BabA has not yet been determined in a larger series of clinical isolates obtained through routine endoscopy. Therefore, we investigated the presence and transcription of babA2, cagA, and vacA in clinical H. pylori isolates and correlated these data to the presence of gastritis, duodenal ulcer, gastric adenocarcinoma, or MALT lymphoma. Our data indicate that adherence of H. pylori to gastric epithelial cells may be crucial for the efficient delivery of bacterial toxins, resulting in specific H. pylori-related diseases.
Materials and Methods
Patient Population.
H. pylori isolates were obtained from 114 patients with histologically confirmed distal gastric adenocarcinoma (total of n = 27; n = 20 intestinal type; n = 7 diffuse type), duodenal ulcer (n = 23), MALT lymphoma of the stomach (n = 29), or antral gastritis (n = 35). Cancers of the gastric cardia were excluded. Patients (55 male, 59 female) were age matched with median ages of 65 and 60.5 as well as 59 and 59 years, respectively. H. pylori isolates from patients with MALT lymphoma (23, 29) and distal gastric cancer (30) were recruited from previous studies, whereas isolates from patients with duodenal ulcer or gastritis were taken from routine diagnostics. All patients presented for routine endoscopy because of abdominal pain or dyspeptic complaints. Patients receiving antisecretory therapy (proton pump inhibitors or H2-receptor antagonists) or nonsteroidal antiinflammatory drugs were excluded. All biopsies were collected at the Technical University of Munich and one related teaching hospital.
Immunohistochemistry for Lewisb.
Immunostaining was performed according to standard protocols (31). Briefly, paraffin-embedded biopsies were deparaffinized and rehydrated. After blocking of endogenous peroxidase with 1% hydrogen peroxide, sections were incubated in normal serum for 30 min and then incubated with optimal diluted Lewisb antibody (Signet Laboratories, Dedham, MA) for 2 h at room temperature. After washing, secondary antibodies were applied for 30 min and detected by peroxidase reaction.
H. pylori Culture.
Gastric biopsies were obtained from patients undergoing gastric endoscopy after informed consent. Each biopsy was homogenized in 0.5 ml of brucella broth with 10% (vol/vol) FCS without antibiotics. The suspension was inoculated subsequently onto three different media: Wilkins Chalgren agar (Oxoid, Basingstoke, U.K.) containing 10% (vol/vol) horse blood, Dent supplement (Oxoid), and 0.4 g/liter KNO3 (Merck); Pylori agar (BioMérieux, Charbonnier les Bains, France); and nonselective Columbia chocolate agar (GCII, Becton Dickinson) without antibiotics. The media were incubated for up to 10 days at 36°C in an microaerobic atmosphere generated by gas insufflation: 0.6 bar of air (1 bar = 100 kPa) was evacuated from a jar and replaced by a mixture of 15% CO2, 5% O2, and 80% N2 resulting in an atmosphere of 9% CO2, 11% O2, and 80% N2. H. pylori was identified by colony morphology, Christensen urease (Bacto urea base, Difco), oxidase (Oxidase Dry Slide, Difco), catalase [3% (vol/vol) H2O2, Merck], and phase-contrast microscopy. Isolates were stored at −70°C in brucella broth plus 30% (vol/vol) FCS and 20% (vol/vol) glycerol. All frozen isolates were controlled for contamination.
In Vitro Adherence Assay.
Isolates were harvested from agar plates at stationary phase (during which BabA is mainly expressed in culture), resuspended in 0.2 M carbonate buffer, and digoxigenin labeled as described (32). Stocks were diluted to a density of 1 OD600 and frozen at −20°C in 100-μl aliquots. Universal Covalent 96-well microtiter-plates (Corning Costar, Cambridge, MA) were coated with 50 ng per well Lewisb conjugated to human serum albumin (IsoSep AB, Stockholm) diluted in 0.2 M carbonate buffer (pH 9.6) to 1 ng/μl. A 50-μl aliquot of the solution (or 50 μl of buffer for controls) was added to each well, incubated for 1 h in the dark, and then removed with a pipette. Plates were exposed to UV light for 30 s in a Stratalinker (Stratagene) to immobilize the glycoproteins, and 100 μl of blocking buffer (0.5% nonfat dry milk/0.2% Tween 20) was added. After incubation for 1 h at room temperature, plates were decanted without washing, and 50 μl of bacterial suspension [diluted 1:2, including 10% (vol/vol) FCS] was added and incubated for 1 h at room temperature with gentle agitation (100 rpm) to reduce nonspecific binding. Each well was then washed three times with 100 μl of PBS, and detection was performed by using anti-DIG-HRP-antibody and ABTS-solution (both from Roche Diagnostics) according to the manufacturer’s instructions. Extinction was quantified in a microplate reader (Bio-Rad) at 405 nm and normalized to controls (uncoated wells). In each single experiment, all strains were tested in two coated wells and two controls, and at least two independent assays were performed on each strain.
Preparation of DNA and RNA from H. pylori Cultures.
For RNA preparation, agar plates were rinsed with 1 ml of lysis buffer [7 M urea/1.2% (vol/vol) SDS/35 mM NaCl/15 mM EDTA/10 mM Tris⋅HCl], and 800 μl of the initial volume was collected for phenol/chloroform extraction. Alternatively, bacteria grown in culture broth were pelleted by centrifugation for 5 min at 10,000 × g. The bacterial pellets were washed twice with PBS and then resuspended in 800 μl of lysis buffer (pH 5.2). RNA was extracted by phenol/chloroform and stored until use at −70°C. DNA was precipitated from the organic phase after removal of the aqueous phase by addition of 300 μl of 100% (vol/vol) ethanol, shaking, and centrifugation at 11,000 × g for 30 min. The pellet was washed twice with 70% (vol/vol) ethanol.
Reverse Transcription and PCR.
PCR amplification of H. pylori gene loci was performed for ureB (as a positive control), cagA, vacA mosaics vacAs1/2 and vacAm1/2, and babA2. Because allelic variations of the cagA gene locus have been described (33, 34), we used two different primer sets to amplify the middle region and 3′ end of the cagA gene (not shown). We obtained identical results in our German population and therefore used primers located in the middle region of the cagA gene to determine the cagA genotype status.
Primer sequences were ureB sense (5′-TTCACCCCAACAAATCCCTACAG-3′), ureB antisense (5′-ACGGCCCATCGCTTGAGAGT-3′), cagA sense (5′-GTATGGGGGCAATGGTGGTC-3′), and cagA antisense (5′-GATTCTTGGAGGCGTTGGTGTAT-3′; vacA primers were synthesized as described (13). The babA2 primers were designed on the basis of the recently published signal sequence of the babA2 gene (28) and were babA2 sense (5′-AAT CCA AAA AGG AGA AAA AGT ATG AAA-3′) and babA2 antisense (5′-TGT TAG TGA TTT CGG TGT AGG ACA-3′); identical primers for DNA and RNA amplification of cagA and babA2 were used. For reverse transcription, 1 μg of total RNA (in a volume of 11 μl) and 1 μl of random hexamer primers (Amersham Pharmacia) were incubated at 70°C for 10 min and placed on ice for 5 min. cDNA synthesis was performed in a total volume of 20 μl with Superscript II (GIBCO/BRL) according to the manufacturer’s instructions. cDNA (2 μl) or genomic DNA (20 ng) was used for amplification. Amplification was carried out in a total volume of 50 μl containing PCR buffer [50 mM KCl/10 mM Tris⋅HCl, pH 8.7/200 μM of each deoxynucleotide/2 units of AmpliTaq polymerase (Perkin–Elmer/Cetus)/1 μl of each primer (20 μM)]. MgCl2 concentrations were adjusted for each primer pair. Reaction mixtures were overlaid with 25 μl of mineral oil (Serva) and amplified as follows: an initial denaturation for 5 min at 94°C; 30 cycles of 94° for 1 min, 55–62°C for 1 min, 72°C for 1 min; and a final extension at 72°C for 10 min. PCR products were analyzed on 1–2% agarose gels stained with ethidium bromide. Sequences were confirmed by restriction analysis and direct sequencing.
Statistical Analysis.
The χ2 test and the Fisher’s exact test (because the numbers of isolates in the vacAs1, cagA, or babA2 negative ulcer groups were determined to be zero) were used to compare the differences among the groups. P values are indicated in the text and in the figure legends. P values <0.05 were considered to be significant.
Results
PCR Analysis of the Different H. pylori Genotypes.
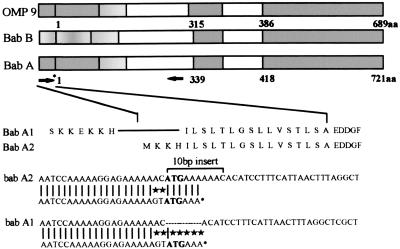
H. pylori was successfully isolated and cultured from 114 patients. Integrity of DNA was confirmed by urease PCR, yielding clear signals from all isolates (not shown). Initially, sequence variations of the babA gene were investigated. The babA2 genotype was detected by mismatch PCR. The upstream primer was located from −20 to +3 in the initial signal sequence, containing three mismatches to the babA1 gene and two additional mismatches at −2/−3 (shown in Fig. 1). All PCR products were analyzed by sequencing, which confirmed the presence of babA2 genotype. The results of DNA amplification for the babA2 were confirmed by reverse transcription–PCR after RNA isolation, indicating that this gene was efficiently transcribed. The babA2 genotype was detected in 82 of 114 isolates (71.9%).
Figure 1.
BabA gene organization, nucleotide sequence (adapted from ref. 28), and primer positions for mismatch PCR. The binding of the sense primer to babA1 and babA2 genes is shown in relation to repeat motif. Matches are indicated by vertical bars; mismatches are indicated by asterisks. Regions of high homology to other genes (OMP9) are indicated by gray shading of open bars.
Correlation of babA2 Genotyping and in Vitro Adhesion Properties.
A subset of 54 H. pylori strains was investigated for adhesion to Lewisb in a microtiter assay. Of these, 23 strains were babA2 negative, and 31 strains were babA2 positive. Strains were considered positive if the ratio of extinctionlewis/extinctioncontrol was >1.5. BabA2-positive strains showed values between 1.8 and 2.0; negative strains showed values around 1.0. No binding was observed to Lewisa. None of the babA2-negative strains showed any binding in the adherence assay. Of 31 babA2-positive strains, 28 showed a significant increase in extinction, indicating efficient binding, whereas 3 babA2-positive strains did not bind Lewisb (Table 1).
Table 1.
Correlation between the babA2 genotype and adherence to Lewisb epitopes in an in vitro adherence assay in a subset of 54 H. pylori strains
| Adhesion to Lewisb epitopes | babA2 genotype-negative (n = 23 strains) | babA2 genotype-positive (n = 31 strains) |
|---|---|---|
| Adhesion to Lewisb | 0 | 28 |
| No adhesion to Lewisb | 23 | 3 |
| Correlation | 100% | 91% |
Simultaneous Expression of babA2, cagA, and vacA Genotype.
The clinical isolates were analyzed further with regard to the simultaneous presence of the cagA, vacA, and babA genotype. CagA gene expression was also verified by amplification of RNA with reverse transcription–PCR in identical isolates. These PCR experiments yielded identical products, indicating efficient transcription of this gene in cagA-positive strains. vacA mosaics were determined with regard to s1/s2 and m1/m2 sequences according to previously published gene variations (13). The vacAs1 genotype has been correlated to the presence of the VacA toxin in vivo (13). Thus, the vacAs1 genotype served as an indicator for toxin-positive strains and was detected in a total of 99 isolates (Table 2), whereas 15 had the vacAs2 genotype. A statistically significant correlation was observed between vacAs1 and cagA genotypes (P < 0.001; χ2 = 34.5; not shown), between the babA2 and cagA genotypes (P < 0.01; χ2 = 15.3), and between the babA2 and vacAs1 genotypes (P < 0.01; χ2 = 17.5), as shown in Table 2. No correlation, however, was observed between the presence of the vacAm1 or vacAm2 genotype and cagA status (not shown). When the simultaneous presence of vacAs1, cagA, and babA2 genes was investigated, we found that 78 of 99 isolates with vacAs1 genotype were babA2 positive, as were 70 of 86 isolates with cagA genotype. Of 82 isolates with babA2 genotype, 68 were triple positive and thus contained all three genes (Table 2). Thus, the presence of the babA2 genotype was significantly associated with the simultaneous presence of type 1 strain genotypes (P < 0.01; χ2 = 12.87).
Table 2.
Frequency and correlation of the vacAs1, cagA, and babA2 genotypes in a total of 114 H. pylori isolates
| Genotype (n = 114) | babA2-positive (n = 82) | babA2-negative (n = 32) | χ2 value | P value |
|---|---|---|---|---|
| vacs1-positive (n = 99) | 78 | 21 | 17.5 | <0.01 |
| cagA-positive (n = 86) | 70 | 16 | 15.3 | <0.01 |
| Type 1 (n = 84) | 68 | 16 | 12.8 | <0.01 |
Genotype Distribution in Different Gastric Diseases.
Table 3 shows the distribution of various genotypes in different gastric diseases. The overall babA2 genotype prevalence ranged at 71.9% in the current study. The babA2 genotype was detected in all patients with duodenal ulcer (23 of 23 ulcer isolates, 100%) and in 77.8% of patients with distal gastric adenocarcinomas (21 of 27 isolates) but only in 69% of isolates from patients with MALT lymphoma (20 of 29 isolates). BabA2 was detected at a significantly lower frequency in isolates from patients with chronic gastritis (18 of 35, 51.4%). A highly statistically significant correlation was obtained between the babA2 genotype and the presence of duodenal ulcer (Fisher’s exact two-sided P = 0.00024). In addition, babA2 genotype showed a significant correlation with the presence of gastric adenocarcinoma (χ2 = 4.535; P = 0.033).
Table 3.
Correlation between virulence factor genotypes and disease prevalence
| Gene | Total, n = 114 (%) | Gastric adenocarcinoma, n = 27 (%) | Duodenal ulcer, n = 23 (%) | MALT, n = 29 (%) | Gastritis, n = 35 (%) | P value gastric adenocarcinoma vs. antral gastritis | P value duodenal ulcer vs. antral gastritis |
|---|---|---|---|---|---|---|---|
| vacAs1 | 99 (86.8) | 26 (96.3) | 23 (100) | 21 (72.4) | 29 (82.9) | 0.126 | 0.036 |
| vacAs2 | 15 (13.2) | 1 (3.7) | 0 (0) | 8 (27.6) | 6 (17.1) | n.s. | n.s. |
| vacAm1 | 54 (47.4) | 20 (74.1) | 8 (34.7) | 10 (34.5) | 16 (45.7) | 0.025 | n.s. |
| vacAm2 | 60 (52.6) | 7 (25.9) | 15 (65.2) | 19 (65.5) | 19 (54.3) | n.s. | n.s. |
| cagA | 89 (78.9) | 25 (92.6) | 23 (100) | 14 (48.3) | 27 (77.1) | 0.094 | 0.008 |
| babA2 | 82 (71.9) | 21 (77.8) | 23 (100) | 20 (69.0) | 18 (51.4) | 0.033 | 0.0002 |
| Type 1 | 84 (73.7) | 23 (85.1) | 23 (100) | 12 (41.4) | 25 (71.4) | 0.235 | 0.0041 |
| Triple-positive | 68 (59.6) | 20 (74.1) | 23 (100) | 20 (34.5) | 15 (42.9) | 0.014 | 2 × 10−6 |
The P value of gastric adencarcinoma vs. antral gastritis was calculated with the χ2 test. The P value of duodenal ulcer vs. antral gastritis was calculated with Fisher’s exact two-sided P test. n.s., not significant.
The vacAs1 genotype prevalence ranged at 86.8% in the current study (Table 3) and was detected at high frequency in isolates from patients with gastritis (82.9%). The presence of the VacAs1 genotype correlated with the presence of duodenal ulcer when a two-sided χ2 test was applied (χ2 = 4.3; P = 0.036), whereas Fisher’s exact test did not reveal statistical significance (two-sided P value of 0.072). No correlation was found between vacAs1 and adenocarcinoma (χ2 = 2.7; P = 0.126). Interestingly, the vacAm1 genotype was detected at a higher frequency in isolates from patients with gastric adenocarcinoma (74.1% vs. 34–44% in other gastric diseases) and thereby showed a significant association to only this disease (χ2 = 4.65; P = 0.025).
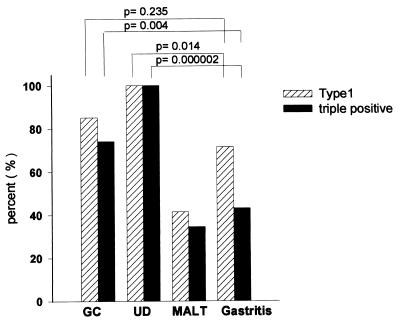
The cagA genotype (Table 3) was significantly associated with duodenal ulcer (100%; Fisher’s exact two-sided P = 0.008) but not with gastric adenocarcinoma (total of 92.6%; χ2 = 3.5; P = 0.094). Type 1 strains (vacAs1- and cagA-positive) were found in 23 of 23 cases with ulcer and in 23 of 27 cases with adenocarcinoma. Type 1 strains were significantly associated with duodenal ulcer (Fisher’s exact two-sided P = 0.0041) but not with gastric adenocarcinoma (χ2 = 1.65; P = 0.235). A two-group χ2 test with a one-sided significance level indicated a 91% power to detect the difference between the ulcer and gastritis group in type 1 strains, whereas the identical test yielded only a 28% power to discriminate gastric adenocarcinoma and gastritis, which was not statistically significant.
Distribution of Triple-Positive Strains.
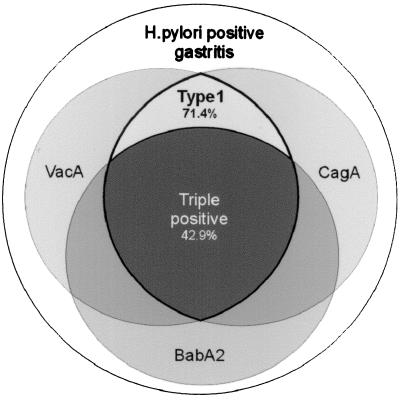
H. pylori strains that harbored the vacAs1 gene (toxin-positive strains) and the cagA genotype and that were also positive in regard to babA2 expression showed a highly significant correlation to the presence of duodenal ulcer (total of 100%; Fisher’s exact two-sided P = 0.000002) and a significant correlation to gastric adenocarcinoma (total of 74.1%; χ2 = 6.04; P = 0.014). Triple-positive strains were detected only in 34.5% of isolates from patients with MALT or 42.9% of isolates from patients with gastritis. The distribution of vacAs1-, cagA-, and babA2-positive strains in the gastritis group is illustrated in Fig. 2. A two-group χ2 test with a one-sided significance level indicated a 99% power to detect the difference between the ulcer and gastritis group and a 77% power to detect the difference between adenocarcinomas and gastritis. The statistical significance levels and the discriminating power exceeded the results from type 1 strains by far. Hence, a different strain-typing definition is suggested (Fig. 3), based on the close correlation of the triple-positive strains to ulcer and gastric adenocarcinoma.
Figure 2.
Schematic illustration of the distribution of vacAs1, cagA, and babA2 strains in a total of 35 H. pylori isolates from patients with gastritis.
Figure 3.
babA2, type 1, and triple-positive genotypes in H. pylori isolates from patients with different diseases. GC, gastric adenocarcinoma; UD, duodenal ulcer.
Lewisb Epitope Expression in Patients with Gastritis.
Lewisb epitope expression was determined in all 35 patients with gastritis. Immunohistochemistry was performed as described in Materials and Methods. By using a monoclonal antibody against Lewisb, 33 patients showed positive staining for Lewisb in the gastric mucosa; only 2 were Lewisb negative. All 15 patients harboring triple-positive strains showed expression of Lewisb antigens in the gastric mucosa. Of the 17 triple-negative patients, 2 were Lewisb negative.
Follow-Up of the Gastritis Group.
Finally, a follow-up study was performed with the patients included in the gastritis group (n = 35) for a median of 5 years (4.0–5.5 years). Three patients were excluded, because no data were available. Of the remaining 32 isolates from the gastritis group, 15 were initially triple positive, harboring babA2, vacAs1, and cagA genes. From these patients, one patient developed gastric adenocarcinoma; one had a bleeding ulcer 2 years after initial endoscopy; and two developed intestinal metaplasia as determined in routine gastric biopsies. Furthermore, two patients were treated by eradication triple therapy because of a positive family history for gastric cancer; two others were subjected to eradication triple therapy, because they complained about chronic abdominal pain and felt improvement after therapy (total of eight patients with complaints). In contrast, 1 of the triple-negative patients (n = 17) developed intestinal metaplasia, but none had any history of ulcer or gastric malignancy. One patient in this group was treated by eradication triple therapy because of dyspeptic complaints.
Discussion
Key factors in the specific tropism and pathogenicity of H. pylori in the stomach include mechanisms of adherence and secretion of bacterial toxins. Several lines of evidence suggest a key role of Lewisb antigens as receptors for adherence of H. pylori to gastric surface mucosal cells. Biochemical studies have identified a 78-kDa protein from H. pylori strains (termed BabA) that allows binding to the blood-group antigen Lewisb present on the surface of gastric epithelial cells (24). Two corresponding genes encoding BabA have been cloned: babA1 and babA2 (28). Only the babA2 gene is functionally active. These two genes have almost complete sequence homology, with the exception of a 10-bp insert, found only in babA2, which creates a translational initiation codon in the signal peptide sequence (28). We used this sequence difference to amplify the babA2 gene selectively by mismatch PCR. In all H. pylori strains investigated, results obtained from DNA and RNA amplification of the babA2 gene were concordant, indicating that when the babA2 gene is present, it is efficiently transcribed. Sequencing confirmed the specificity of the assay in all cases.
Our current results further substantiate the observation that bacteria harboring the babA2 gene express the adhesin and can thereby bind to Lewisb antigens on gastric epithelial cells. The correlation between babA2 genotype and in vitro binding to Lewisbantigens was determined in a subset of strains (n = 54) by using an adherence assay performed in Lewisb-coated microtiter plates. In these experiments, we determined that babA2-negative strains did not bind Lewisb epitopes, indicating the specificity of our PCR assay. Also, no binding to Lewisa was observed. Moreover, the correlation between babA2 gene presence and adhesin expression was greater than 90%, indicating that, in most babA2-positive strains, the adhesin is expressed and functionally active. Some strains may lack the protein because of differential gene regulation, as suggested previously (35). These strains may be able to adapt their outer membrane protein expression to conditions in the environment by switching gene expression on and off. The presence of highly homologous genes could allow allelic replacement between genomic areas of different transcriptional activity. Thus, the in vitro binding activity may not necessarily reflect the in vivo situation. Furthermore, the expression of the protein may depend further on bacterial growth and may differ at various time points of culture or in vivo conditions.
The presence of the babA2 genotype can therefore be regarded as a good indicator for the ability of strains to express the Lewisb-binding adhesin. The presence of the corresponding Lewis antigens was verified in the current study in a subset of patients with gastritis. As shown by immunohistochemistry, the majority of patients (>90%) expressed Lewisb in gastric biopsies. This result is in concordance with histopathological studies that found Lewisb expression in up to 95% of gastric tissue (36). Absence of Lewisb expression in the gastric mucosa seems to be rare, and the predictive value therefore may not be increased substantially by determination of Lewis status.
babA2 genotype distribution was determined in different gastric diseases and was a good marker for the presence of duodenal ulcer (100% vs. 51.4% in gastritis) and adenocarcinoma (77.8%). The close correlation between babA2 and the presence of duodenal ulcer was not unexpected, because it had been anticipated in previous biochemical studies (23) and in vitro assays (25). Thus, our current clinical and experimental data confirm previous experimental studies indicating a central role of H. pylori’s adhesin and Lewis antigens in the pathogenesis of ulcer disease.
Our finding regarding the correlation between babA2 gene presence and gastric cancer suggests that the presence of H. pylori’s adhesin and simultaneous Lewisb epitope expression in the gastric mucosa may play an important role in the pathogenesis of gastric cancer as well. Previous studies have shown that H. pylori can attach directly to areas of intestinal metaplasia in the stomach (37). This finding may be explained the fact that Lewisb expression is also detected at a high percentage in areas of intestinal metaplasia, potential precursor lesions of gastric cancer (36). Therefore, adherence of babA2-positive strains to areas of intestinal metaplasia and the direct interaction of bacteria with these cells may be a critical factor for the development of distal gastric cancer.
Another study could not find any effect of Lewis antigen expression on the adherence of H. pylori to human gastric epithelial cells (38). This study, however, investigated only a small number of individuals (n = 19) and used isolated gastric cells, which might not reflect in vivo conditions in different gastric diseases. Additional adherence factors independent of Lewisb have also been shown to mediate adherence of H. pylori, but the clinical relevance of these has not been determined yet. Thus, BabA is not the only adherence factor of H. pylori. Our current data, however, give evidence for the clinical relevance of BabA and further suggest that this factor may have special importance for the induction of pathogenicity when additional factors, such as vacA and cagA, are present.
Therefore, we investigated the simultaneous presence of the bacterial virulence factor genes cagA, vacA, and babA2 among clinical isolates of different patient groups. We initially found that the babA2 status was significantly associated with cagA-genotype-positive strains. This finding is in accordance with observations made by Ilver et al. (28), who found expression of both cagA and babA in 70% of their isolates investigated. The vacAs1 genotype was also significantly associated with the presence of the babA2 genotype (Fig. 2), indicating that, in some (triple-positive) strains, the presence of all three factors might be important for mediating pathogenicity. On the other hand, this observation might reflect only a coincidence because of the high prevalence of cagA-, vacAs1-, and babA2-positive strains (>70%). Our current data, however, clearly indicate that the simultaneous presence of these three genes represents a specific intersection within several groups of strains with defined virulence, as illustrated in Fig. 2.
Classification of H. pylori strains by the simultaneous expression of vacAs1, cagA, and babA2 allowed identification of a subgroup of type 1 strains that are associated with specific gastric diseases in human adults. The statistical correlation of these triple-positive strains showed a remarkable difference in regard to disease distribution and significance levels compared with type 1 strains alone.
The association of type 1 strains with duodenal ulcer or gastric carcinoma has been reported in several studies before (7, 12, 18, 21, 39, 40). We also confirmed the association between type 1 strains and duodenal ulcer in our study, although the absolute difference between these groups was relatively small. In contrast, we found that type 1 strains were not associated with adenocarcinoma, a finding that might be explained by the high prevalence of type 1 strains in our population. However, because only 10–20% of H. pylori-infected patients with chronic gastritis will develop duodenal ulcer and less then 1% may develop gastric adenocarcinoma, our current data confirm that genotyping of H. pylori strains by the presence vacA and cagA only in patients with gastritis (>70% type 1) cannot identify a small subgroup of patients with chronic gastritis who might benefit from H. pylori eradication strategies.
Genotyping of H. pylori strains by the additional presence of the adherence factor gene babA2 in type 1 strains, however, yielded a highly significant association with ulcer (P = 2 × 10−6) and distal gastric adenocarcinoma (P = 0.014). The association between triple-positive strains and duodenal ulcer is in accordance with the single correlation of type 1 and babA2 strains with this disease, whereas the significance level and the discriminating power exceed the previously reported values for type 1 strains by far. Our observation that triple-positive (in contrast to type 1) strains are associated with gastric adenocarcinoma indicates even more clearly that babA2 is able to discriminate between type 1 subgroups with different prognoses. In this context, definition of a triple-positive subgroup yielded a 77% power in a two-group χ2 test, whereas type 1 strains showed only a 28% power to detect carcinoma and thus were not significantly associated. Because the absolute percentage of triple-positive strains in patients with chronic gastritis was smaller than that of type 1 strains, H. pylori eradication of patients in this group seems to be more reasonable. Indeed, a follow-up analysis of the patients harboring triple-positive strains in the gastritis group showed that these patients developed gastric abnormalities more frequently. This observation may further support the view that triple-positive strains in patients with chronic gastritis should be eradicated, because the combined presence of these genes may lead to duodenal ulcer or, after years of infection, to the generation of adenocarcinoma.
Previously, the presence of triple-positive strains has been determined in a small population of children (41). In this study, no correlation between ulcer development or gastric inflammation and the presence of cag-pathogenicity island, cytotoxin production, and binding to Lewisb oligosaccharide was found. However, this lack of correlation may be a specific finding in children and may be explained by the fact that Lewisb expression seems to be less frequent in children than in adults (41).
In contrast to the correlation of babA2, cagA, and vacAs1 genotype with ulcer or adenocarcinoma, we found an inverse relation of triple-positive strains to the presence of MALT lymphoma. These three genotypes were significantly lower in patients with MALT lymphoma. In this group, vacAs2 strains were detected more frequently. This finding stands in contrast to previously reported associations (42); however, it may be explained by the fact that the pathomechanism for the development of MALT lymphoma is initiated by H. pylori infection but differs from the mechanism by which H. pylori induces ulcers or adenocarcinoma, such as clonal expansion of B cells induced by T cell stimulation (43, 44).
Our current data support the assumption that the virulence factors VacA and CagA may have special importance when bacteria are able to adhere tightly to gastric surface mucous cells via their adhesin, and subsequently, infection with these strains leads to epithelial damage, caused either by the direct delivery of bacterial toxins or by the indirect induction of an immune response. We further suggest that H. pylori can be classified as a triple-positive strain with simultaneous presence of vacAs1, cagA, and babA2 and that the combined expression of these genes is related to ulcer or adenocarcinoma development.
Acknowledgments
We thank Dr. Stephan Wagenpfeil for statistical advice. This work was supported by grants from the Kuratorium für klinische Forschung of the Technical University of Munich (Grant KKF 8733151), Gastroenterology Foundation (Munich, Germany), Astra Hässle (Wedel, Germany), and Deutsche Forschungsgemeinschaft (Grant DFG Pr 411/2-2 to C.P.).
Abbreviations
- MALT
mucosa-associated lymphoid tissue
- VacA
vacuolating cytotoxin
- CagA
cytotoxin-associated antigen
- BabA
blood-group antigen-binding adhesin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Blaser M J. J Clin Invest. 1997;100:759–762. doi: 10.1172/JCI119588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Covacci A, Telford J L, Del G G, Parsonnet J, Rappuoli R. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 3.Miehlke S, Thomas R, Guiterrez O, Graham D Y, Go M F. J Clin Microbiol. 1999;37:245–247. doi: 10.1128/jcm.37.1.245-247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suerbaum S, Smith J M, Bapumia K, Morelli G, Smith N H, Kunstmann E, Dyrek I, Achtman M. Proc Natl Acad Sci USA. 1998;95:12619–12624. doi: 10.1073/pnas.95.21.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kersulyte D, Chalkauskas H, Berg D E. Mol Microbiol. 1999;31:31–43. doi: 10.1046/j.1365-2958.1999.01140.x. [DOI] [PubMed] [Google Scholar]
- 6.Thomsen L L, Gavin J B, Tasman J C. Gut. 1990;31:1230–1236. doi: 10.1136/gut.31.11.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuipers E J, Uyterlinde A M, Pena A S, Roosendaal R, Pals G, Nelis G F, Festen H P, Meuwissen S G. Lancet. 1995;345:1525–1528. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. J Am Med Assoc. 1994;272:65–69. (abstr.). [Google Scholar]
- 9.Forman D. Scand J Gastroenterol Suppl. 1996;214:31–33. [PubMed] [Google Scholar]
- 10.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 11.Wotherspoon A C, Ortiz H C, Falzon M R, Isaacson P G. Lancet. 1991;338:1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 12.Blaser M J. Aliment Pharmacol Ther. 1996;10, Suppl. 1:73–77. doi: 10.1046/j.1365-2036.1996.22164008.x. [DOI] [PubMed] [Google Scholar]
- 13.Atherton J C, Cao P, Peek R M, Jr, Tummuru M K, Blaser M J, Cover T L. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 14.Papini E, de B M, Milia E, Bugnoli M, Zerial M, Rappuoli R, Montecucco C. Proc Natl Acad Sci USA. 1994;91:9720–9724. doi: 10.1073/pnas.91.21.9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peek R M, Jr, Miller G G, Tham K T, Perez P G, Cover T L, Atherton J C, Dunn G D, Blaser M J. J Clin Microbiol. 1995;33:28–32. doi: 10.1128/jcm.33.1.28-32.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabtree J E, Taylor J D, Wyatt J I, Heatley R V, Shallcross T M, Tompkins D S, Rathbone B J. Lancet. 1991;338:332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 17.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atherton J C, Peek R M, Jr, Tham K T, Cover T L, Blaser M J. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 19.Crabtree J E, Farmery S M, Lindley I J, Figura N, Peichl P, Tompkins D S. J Clin Pathol. 1994;47:945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuipers E J, Perez P G, Meuwissen S G, Blaser M J. J Natl Cancer Inst. 1995;87:1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 21.Blaser M J, Perez P G, Kleanthous H, Cover T L, Peek R M, Chyou P H, Stemmermann G N, Nomura A. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 22.Xiang Z, Censini S, Bayeli P F, Telford J L, Figura N, Rappuoli R, Covacci A. Infect Immun. 1995;63:94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boren T, Falk P, Roth K A, Larson G, Normark S. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 24.Boren T, Normark S, Falk P. Trends Microbiol. 1994;2:221–228. doi: 10.1016/0966-842x(94)90626-2. [DOI] [PubMed] [Google Scholar]
- 25.Falk P, Roth K A, Boren T, Westblom T U, Gordon J I, Normark S. Proc Natl Acad Sci USA. 1993;90:2035–2039. doi: 10.1073/pnas.90.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falk P G, Bry L, Holgersson J, Gordon J I. Proc Natl Acad Sci USA. 1995;92:1515–1519. doi: 10.1073/pnas.92.5.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guruge J L, Falk P G, Lorenz R G, Dans M, Wirth H P, Blaser M J, Berg D E, Gordon J I. Proc Natl Acad Sci USA. 1998;95:3925–3930. doi: 10.1073/pnas.95.7.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilver D, Arnquist A, Ögren J, Frick I, Kersulyte D, Incecik E T, Berg D, Govacci A, Engstrand L, Boren T. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 29.Neubauer A, Thiede C, Morgner A, Alpen B, Ritter M, Neubauer B, Wundisch T, Ehninger G, Stolte M, Bayerdorffer E. J Natl Cancer Inst. 1997;89:1350–1355. doi: 10.1093/jnci/89.18.1350. [DOI] [PubMed] [Google Scholar]
- 30.Miehlke S, Hackelsberger A, Meining A, Hatz R, Lehn N, Malfertheiner P, Stolte M, Bayerdorffer E. Br J Cancer. 1998;78:263–266. doi: 10.1038/bjc.1998.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason D Y, Sammons R. J Clin Pathol. 1978;31:454–460. doi: 10.1136/jcp.31.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falk P, Boren T, Haslam D, Caparon M. Methods Cell Biol. 1994;45:166–188. doi: 10.1016/s0091-679x(08)61851-8. [DOI] [PubMed] [Google Scholar]
- 33.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, et al. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miehlke S, Kibler K, Kim J G, Figura N, Small S M, Graham D Y, Go M F. Am J Gastroenterol. 1996;91:1322–1325. [PubMed] [Google Scholar]
- 35.Alm R A, Ling L S, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, et al. Nature (London) 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi K, Sakamoto J, Kito T, Yamamura Y, Koshikawa T, Fujita M, Watanabe T, Nakazato H. Am J Gastroenterol. 1993;88:919–924. [PubMed] [Google Scholar]
- 37.Genta R M, Gurer I E, Graham D Y, Krishnan B, Segura A M, Gutierrez O, Kim J G, Burchette J L., Jr Gastroenterology. 1996;111:1206–1211. doi: 10.1053/gast.1996.v111.pm8898634. [DOI] [PubMed] [Google Scholar]
- 38.Clyne M, Drumm B. Gastroenterology. 1997;113:72–80. doi: 10.1016/s0016-5085(97)70082-9. [DOI] [PubMed] [Google Scholar]
- 39.Blaser M J, Crabtree J E. Am J Clin Pathol. 1996;106:565–567. doi: 10.1093/ajcp/106.5.565. [DOI] [PubMed] [Google Scholar]
- 40.Blaser M J. Scand J Gastroenterol Suppl. 1994;205:1–5. [PubMed] [Google Scholar]
- 41.Celik J, Su B, Tiren U, Finkel Y, Thoresson A C, Engstrand L, Sandstedt B, Bernander S, Normark S. J Infect Dis. 1998;177:247–252. doi: 10.1086/517365. [DOI] [PubMed] [Google Scholar]
- 42.Eck M, Schmausser B, Haas R, Greiner A, Czub S, Muller H H. Gastroenterology. 1997;112:1482–1486. doi: 10.1016/s0016-5085(97)70028-3. [DOI] [PubMed] [Google Scholar]
- 43.Zucca E, Bertoni F, Roggero E, Bosshard G, Cazzaniga G, Pedrinis E, Biondi A, Cavalli F. N Engl J Med. 1998;338:804–810. doi: 10.1056/NEJM199803193381205. [DOI] [PubMed] [Google Scholar]
- 44.Zucca E, Bertoni F, Roggero E, Cazzaniga G, Bosshard G, Biondi A, Cavalli F. Leukemia. 1998;12:247–249. doi: 10.1038/sj.leu.2400908. [DOI] [PubMed] [Google Scholar]