Abstract
Our research team and laboratories have concentrated on two inherited endocrine disorders, congenital adrenal hyperplasia (CAH) and apparent mineralocorticoid excess, in thier investigations of the pathophysiology of adrenal steroid hormone disorders in children. CAH refers to a family of inherited disorders in which defects occur in one of the enzymatic steps required to synthesize cortisol from cholesterol in the adrenal gland. Because of the impaired cortisol secretion, adrenocorticotropic hormone levels rise due to impairment of a negative feedback system, which results in hyperplasia of the adrenal cortex. The majority of cases is due to 21-hydroxylase deficiency (21-OHD). Owing to the blocked enzymatic step, cortisol precursors accumulate in excess and are converted to potent androgens, which are secreted and cause in utero virilization of the affected female fetus genitalia in the classical form of CAH. A mild form of the 21-OHD, termed nonclassical 21-OHD, is the most common autosomal recessive disorder in humans, and occurs in 1/27 Ashkenazic Jews. Mutations in the CYP21 gene have been identified that cause both classical and nonclassical CAH. Apparent mineralocorticoid excess is a potentially fatal genetic disorder causing severe juvenile hypertension, pre- and postnatal growth failure, and low to undetectable levels of potassium, renin, and aldosterone. It is caused by autosomal recessive mutations in the HSD11B2 gene, which result in a deficiency of 11β-hydroxysteroid dehydrogenase type 2. In 1998, we reported a mild form of this disease, which may represent an important cause of low-renin hypertension.
Our laboratory has dedicated itself to two severe disorders of childhood—congenital adrenal hyperplasia (CAH) and apparent mineralocorticoid excess (AME)—which can be seen as paradigms of the process of identification, characterization, and treatment of an autosomal recessive disease. For both of these steroid disorders, we have used a combination of clinical observation and investigation, hormonal assays, epidemiological and population studies, and molecular genetics analyses.
CAH Caused by 21-Hydroxylase Deficiency (21-OHD).
CAH refers to a family of inherited disorders in which defects occur in one of the enzymatic steps required to synthesize cortisol from cholesterol in the adrenal gland. Because of the impaired cortisol secretion, adrenocorticotropic hormone (ACTH) levels rise via a negative feedback system, which results in hyperplasia of the adrenal cortex. In 21-OHD, responsible for 90–95% of CAH cases, there is an accumulation of the precursors immediately proximal to the 21-hydroxylation step in the pathway of cortisol synthesis. These excess precursors are converted to potent androgens, which cause in utero virilization of the external genitalia of the female fetus in the classical form of CAH. Newborn males have normal genitalia although, as with females, they may develop other signs of androgen excess in childhood.
The earliest documented description of CAH was in 1865 by a Neapolitan anatomist named Luigi De Crecchio (1), in which he described a cadaver as having a penis with urethral openings on its underside and undescended testes. To the surprise of De Crecchio, the post mortem dissection also revealed a vagina, a uterus, fallopian tubes, and ovaries, as well as markedly enlarged adrenal glands. The patient had had a sex reassignment, having been declared a female at birth and a male 4 years later. As an adult, he conducted himself as a male socially and sexually. The patient died in his 40s after the last of several episodes of vomiting, diarrhea, and prostration. This was certainly a case of a genetic female with masculinization of the external genitalia, caused by excess adrenal androgens, who had symptoms consistent with adrenal insufficiency (2). This remarkable description, written almost 135 years ago, could apply to present-day cases.
Biochemical Features.
Adrenal steroidogenesis and fetal development.
The adrenal cortex produces the glucocorticoid, cortisol, and the mineralocorticoid, aldosterone, under the control of regulatory systems that largely function independently. The cortex is divided into three distinct zones—the outer zona glomerulosa, the middle zona fasciculata, and the inner zona reticularis—defined by different cellular arrangements. These zones are functionally distinct: i.e., mineralocorticoids are synthesized in the zona glomerulosa, glucocorticoids are produced by the zona fasciculata/reticularis, and androgens are synthesized in the zona reticularis.
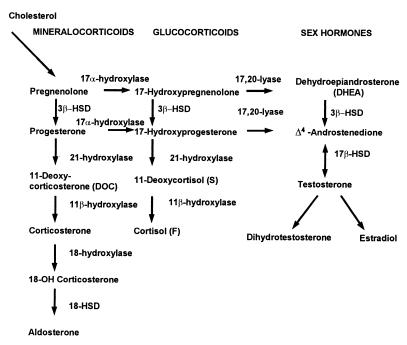
The steroidogenic acute regulatory protein shuttles cholesterol to the inner mitochondrial membrane (3). The production of cortisol in the zona fasciculata occurs in five steps: cleavage of the cholesterol side chain by the cholesterol desmolase enzyme, cytochrome CYP11A1 (P450scc), to yield pregnenolone; conversion of pregnenolone to progesterone by 3β-hydroxysteroid dehydrogenase with accompanying Δ5,4-isomerization; and successive hydroxylations at the 17α, 21, and 11β positions, each mediated by a distinct cytochrome P450, resulting in cortisol (Fig. 1) (4). Cortisol is synthesized under the trophic control of ACTH and in turn regulates ACTH synthesis via a negative feedback loop.
Figure 1.
Pathways of steroid biosynthesis.
Male genital differentiation in embryonic and fetal life depends on two functions of the testes (5): (i) the secretion of testosterone by the Leydig cells directs the formation of the internal male urogenital tract (i.e., the epididymides, vasa deferentia, seminal vesicles, and ejaculatory ducts) from the Wolffian (mesonephric) ducts, and testosterone, which is reduced to dihydrotestosterone by 5α-reductase, virilizes the external genitalia; and (ii) the secretion of the anti-Mullerian hormone (AMH) from the Sertoli cells suppresses development of the Mullerian ducts, thus preventing formation of the female internal structures (i.e., the fallopian tubes, uterus, cervix, and upper vagina). AMH is not secreted by the fetal ovary. Thus, even females with extreme external virilization from androgen excess will have normal development of the uterus and fallopian tubes, making later childbearing possible with proper treatment.
In females with CAH, the degree of virilization of external genitalia toward the male type in genetic females has been classified in five stages by Prader (6), where on a scale of 1 to 5 (I–V) the genitalia can be scored from slightly virilized (e.g., mildly enlarged clitoris) to indistinguishable from a male. Most classical cases of 21-OHD are born with Prader IV genitalia.
Abnormalities in steroid production.
The hormonal imbalances in CAH result from the combination of impaired enzymatic activity and subsequent impaired cortisol synthesis. Clinical syndromes reflect the resultant increased levels of steroids proximal to the nonfunctioning enzymatic step and hyperstimulation of the adrenal gland by ACTH. In 21-OHD, the conversion of 17α-hydroxyprogesterone (17-OHP), the main substrate of the 21-hydroxylase enzyme, to 11-deoxycortisol in the pathway of cortisol synthesis is impaired (Fig. 1). The enzyme defect also impairs the conversion of progesterone to 11-deoxycorticosterone in the pathway of aldosterone synthesis. When cortisol production is decreased, pituitary secretion of ACTH increases via the negative feedback system. Thus, the unblocked precursors 17-OHP, pregnenolone, 17-hydroxypregnenolone, and progesterone accumulate. These steroid precursors can serve as substrates for androgen biosynthesis and are diverted in the adrenals to androgen pathways, resulting in excess secretion of the androgens dehydroepiandrosterone, Δ4-androstenedione, and testosterone. In classic 21-OHD, the production of these androgens early in gestation virilizes the external genitalia in the genetic female fetus.
Clinical Features.
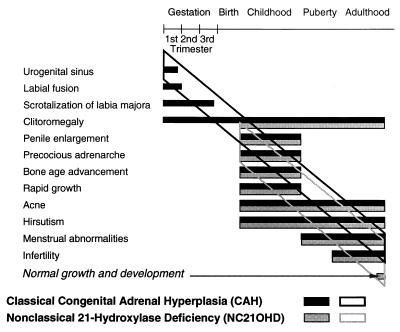
There are three forms of 21-OHD: (i) classic simple virilizing; (ii) classic salt wasting; and (iii) nonclassical, a mild form of the disease (Fig. 2).
Figure 2.
Clinical spectrum of steroid 21-OHD. There is a wide spectrum of clinical presentations ranging from prenatal virilization with labial fusion to precocious adrenarche to pubertal or postpubertal virilization. [Reproduced with permission from ref. 113 (copyright 1983, McGraw–Hill)].
Classic simple virilizing.
The prominent features of classic simple virilizing 21-OHD are progressive virilism with accelerated growth and advanced bone ages but no evidence of mineralocorticoid deficiency. Females with this type of CAH have ambiguity of the external genitalia (including clitoromegaly and fusion and scrotalization of the labial folds with a urogenital sinus).
Diagnosis at the birth of a female with virilizing CAH is usually made immediately because of the apparent genital ambiguity. For newborn males, however, differentiation of the external genitalia is not affected. Diagnosis in the newborn male depends on screening. Postnatally, genitalia may continue to virilize because of an excess of adrenal androgens, and pseudoprecocious puberty can occur. Signs of hyperandrogenism include facial, axillary, and pubic hair; adult body odor; temporal balding; and severe acne. Poor control of the disease in boys with classic CAH has been associated with small testes and infertility with reduced sperm counts. This occurs because the excess androgens are aromatized peripherally to estrogens, which suppress pituitary gonadotropins and impair the growth and function of the testes.
The high levels of androgens can also accelerate growth in early childhood, producing an unusually tall and muscular child. However, this early growth spurt is followed by premature epiphyseal maturation and closure, resulting in a final height that is below that expected from parental heights (7). Thus, the patients are tall children but short adults.
Classic salt wasting.
Blocks in the activity of 21-hydroxylase result in genital ambiguity in the simple virilizing form and additionally in salt wasting in three-fourths of cases (4). With the use of double isotope-dilution techniques, our team demonstrated that aldosterone synthesis is impaired in some patients with 21-OHD CAH, which is associated with electrolyte abnormalities, but is normal in those with no electrolyte abnormalities (8, 9), thus biochemically distinguishing a salt-wasting form from the simple virilizing form.
Because of a deficiency of aldosterone, a salt-retaining hormone, renal salt wasting causes low serum concentrations of sodium (hyponatremia), high serum potassium levels (hyperkalemia), high plasma renin levels, and fluid volume depletion. This hormonal milieu may be manifested by a life-threatening shock-like hypotension and hyperkalemia. We and others have shown that salt losing in infancy from an aldosterone biosynthetic defect may improve with age in some cases (10–13).
Nonclassical.
An attenuated late-onset form of adrenal hyperplasia, which we have called “nonclassical CAH,” was first suspected during the 1950s by gynecologists in clinical practice who treated women with glucocorticoids for physical signs of hyperandrogenism, including infertility. The first biochemical documentation of a late-appearing 21-OHD was by Decourt and colleagues in 1957 (14). It was determined by the studies of our group and others that this form was in fact an allelic variant at the genetic locus of the 21-hydroxylase enzyme (15–21).
In nonclassical 21-OHD, partial deficiencies of 21-hydroxylation cause postnatal androgen excess and milder symptoms. Females do not have genital ambiguity at birth, though both males and females may manifest signs of androgen excess at any phase of postnatal development, such as precocious pubic hair. In pubertal-age girls, menarche may be delayed, and in adolescent and young adult women, secondary amenorrhea is common. In women, hirsutism, male-pattern baldness, oligomenorrhea or amenorrhea, and/or polycystic ovary disease may occur. In males, oligospermia has been found in some cases. For men and women, short adult stature, insulin resistance, severe cystic acne, and reduced fertility can also be seen in untreated groups.
Epidemiology.
Newborn screening worldwide of almost 6.5 million babies has demonstrated an overall incidence of 1:15,000 live births for the classic form of 21-OHD (22–24). The incidence of classic CAH in either homogeneous or heterogeneous general populations is as high as 1 in 7,500 live births (Brazil) (25).
In 1985, we demonstrated that the overall frequency of nonclassic 21-OHD is surprisingly high in the population at large and even higher in certain ethnic groups (25). It is in fact the most common human autosomal recessive disorder in humans. The disease frequency in the general heterogeneous population of New York City was 1/100. The highest ethnic-specific disease frequency was found among Ashkenazic Jews at 1/27. Other ethnic groups with high disease frequency included Hispanics (1/40), Slavs (1/50), and Italians (1/300) (25). These results have been confirmed by others (26, 27).
Diagnosis.
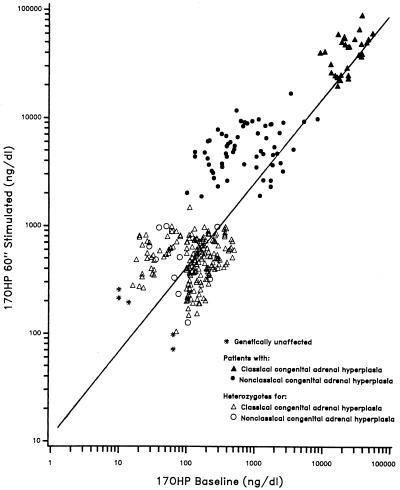
In classical 21-OHD, progesterone, 17-OHP, androstenedione, and testosterone are secreted in excess. Diagnosis can be made by measuring the urinary excretion of the metabolites of C19 steroids, which are increased (28, 29). However, hormonal diagnosis of all types of 21-OHD is best achieved by an ACTH stimulation test, in which the serum 17-OHP concentration is measured before and 60 min after intravenous administration of synthetic ACTH (Cortrosyn). In 1983, we set the hormonal standards for a diagnostic ACTH-stimulation test: plots on a logarithmic scale of baseline vs. ACTH-stimulated 17-OHP concentrations result in a regression line with three distinguishable groups (30, 31) (Fig. 3). These nomograms clearly distinguish the patient with (i) classical 21-OH deficiency from (ii) those with the milder symptomatic and asymptomatic nonclassical forms of 21-OH deficiency, as well as (iii) heterozygotes for all of the forms and those subjects predicted by genotyping to be unaffected. The nomograms can also identify individuals heterozygous for 21-OH deficiency in the general population who have a characteristic heterozygote response. These nomograms provide a powerful tool by which to assign the 21-OH deficiency genotype.
Figure 3.
17-OHP nomogram for the diagnosis of steroid 21-OHD (60-min cortrosyn stimulation test). The data for this nomogram was collected between 1982 and 1991 at the Department of Pediatrics. The New York Hospital–Cornell Medical Center, New York, NY 10021.
Genetics.
CYP21 mutations.
The gene encoding 21-hydroxylase (a microsomal cytochrome P450 termed CYP21 [previously P450c21]) is located on the short arm of chromosome 6 in the human lymphocyte antigen complex (32), and the gene for the 21-OH enzyme is termed CYP21 (33). Our group advanced the understanding of the CYP21 gene, adding to its characterization in terms of location, duplication in tandem with complement C4 isotypes, structure, and the sequencing of the gene and its pseudogene and their arrangement on the chromosome (34–38). CYP21 and its homologue, the pseudogene CYP21P, alternate with two genes called C4B and C4A (37, 39) that encode the two isoforms of the fourth component (C4) of serum complement (40). CYP21 and CYP21P, which each contain 10 exons, share 98% sequence homology in exons and approximately 96% sequence homology in introns (38, 41).
Approximately 40 mutations in the CYP21 gene causing 21-OHD have been identified thus far (42). Of those, we demonstrated deletional mutations of the 21-OHD genes and characterized specific point mutations in many patients at our center (38, 43–48). The most common mutations appear to be the result of either of two types of meiotic recombination between CYP21 and CYP21P: (i) misalignment and unequal crossing over, resulting in large-scale DNA deletions, and (ii) apparent gene conversion events that result in the transfer to CYP21 of smaller-scale deleterious mutations present in the CYP21P pseudogene.
Correlation/noncorrelation of genotype to phenotype.
In general, the genotype of CYP21 correlates with the phenotype of 21-OHD. In 1995, we compared the genotypes and phenotypes in approximately 200 patients and divided them into mutation-identical groups (49). This study demonstrated that the 10 most common mutations observed in CYP21 cause variable phenotype effects and are not always concordant with genotype. We have subsequently genotyped over 600 patients and identified 85 mutational groups, 23 groups of which had more than one phenotype. DNA sequencing analysis could enable us to rule out rare undetected mutations on the same allele.
The noncorrelation of genotype to phenotype may present a difficulty for the clinician in directing prenatal treatment. However, in general, prenatal treatment for CAH is safe and is warranted even when the doubt exists.
Prenatal Diagnosis and Treatment.
Prenatal diagnosis is appropriate in families where a previous family member has been affected. Prenatal treatment with dexamethasone suppresses the formation of androgens by the fetal adrenal gland and can prevent or minimize the ambiguity of the female genitalia, thus precluding an incorrect sex assignment, and the ensuing psychiatric problem of gender confusion in affected females. Prenatal diagnosis has now been utilized for over a decade in the prenatal treatment of 21-OHD (approximately 780 at-risk pregnancies were referred to our hospital). Dexamethasone was chosen because it crosses the placenta, crossing from the maternal to the fetal circulation. It enters the fetus to suppress ACTH because it is not bound to high-affinity transport proteins in the blood and because it cannot be metabolized by the placental 11β-hydroxysteroid dehydrogenase.
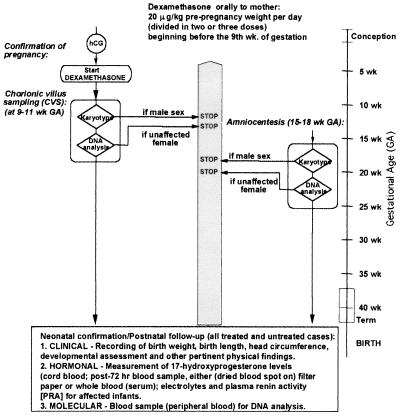
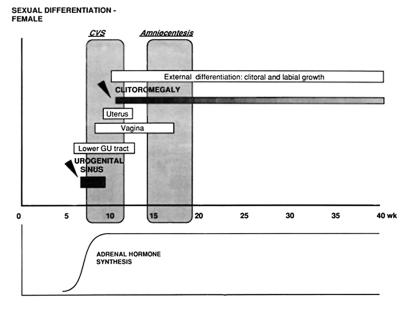
Our group developed an algorithm for the prenatal diagnosis of 21-OHD using direct molecular analysis of the CYP21 gene and for treatment of the disorder with dexamethasone (Fig. 4) (50). Dexamethasone (20 μg/kg/day in three divided doses) is administered to the pregnant mother beginning before 10 weeks gestation, blind to whether the fetus is female or is affected, to suppress excess adrenal androgen secretion and to prevent virilization should the fetus be an affected female (Fig. 4). From the timetable of sexual differentiation, it is evident that the urogenital sinus has already begun to be formed by the ninth week of gestation, and thus treatment must begin before this to prevent virilization of the genitalia (Fig. 5). Diagnosis by DNA analysis can be made after chorionic villus sampling in the eighth to tenth week gestation or by sampling amniotic fluid cells (amniocentesis) in the second trimester. The fetal DNA is analyzed by PCR (51) and Southern blotting (52). Treatment is discontinued if the fetus is shown to be an unaffected female on DNA analysis or a male on karyotype analysis.
Figure 4.
Algorithm depicting prenatal management of pregnancy in families at risk for a fetus with 21-OHD. hCG, human chorionic gonadotropin [Reproduced with permission from ref. 115 (Copyright 1995, the Endocrine Society)].
Figure 5.
Timetable of prenatal sexual differentiation.
Since 1986, prenatal diagnosis and treatment for CAH caused by 21-OHD has been carried out in over 400 pregnancies at The New York Hospital–Cornell Medical Center. Of those pregnancies evaluated, approximately 85 fetuses were found to be affected with classical 21-OHD; of those, approximately 50 were female, and of those 35 were treated prenatally with dexamethasone until term. Dexamethasone administered at or before the ninth week of gestation was effective in reducing virilization in the genetic female so that genitoplasty was not needed (Fig. 6). As reported in previous studies (53–55), prenatally treated newborns did not differ significantly in birth weight from untreated newborns in our study. Mean birth weight was 3.4 kg for dexamethasone-treated fetuses and 3.5 kg (P = 0.26) for controls. Fetal wastage was slightly less for dexamethasone-treated than untreated pregnancies. Followup studies are in progress to evaluate cognition, gender, temperament, and handedness (an indicator of prenatal androgen effect) in children and adults to evaluate the long-term consequences of prenatal dexamethasone treatment.
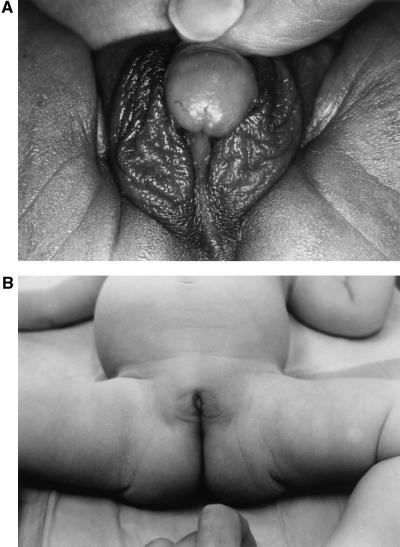
Figure 6.
Genitalia of prenatally untreated (A) and treated (B) sisters with salt-wasting 21-OHD. The untreated newborn girl exhibits ambiguous genitalia with an enlarged clitoris and scrotalization of the labia majora (Prader IV). The sib treated prenatally with dexamethasone was born with normal genitalia, and surgical recession will not be necessary.
In initial studies, we have not identified significant or enduring differences in side effects in the mothers who were treated with dexamethasone from the mothers who were not. However, by report, mothers who were not treated with dexamethasone gained an average of 28.6 lb, whereas treated mothers gained an average of 36.8 lbs. (P < 0.005). There were no significant differences in regard to the presence of striae (P = 0.14), edema (P = 0.56), hypertension (P = 0.60), or gestational diabetes (P = 0.42) by report, either. Further, when we surveyed 100 treated mothers at random, 14 of them had had CAH-affected girls, and all 14 were satisfied with the outcome of prenatal dexamethasone treatment.
Based on our experience and other large human studies (53–55), proper prenatal diagnosis and treatment of 21-OHD is safe and is effective in significantly reducing or eliminating virilization in the affected female.
Now that a mouse model for steroid 21-OHD has been found, we have begun a project to develop gene therapy for CAH.
Apparent Mineralocorticoid Excess (AME).
AME is a genetic disorder that typically causes severe hypertension in children, pre- and postnatal growth failure, low to undetectable levels of potassium, renin, and aldosterone levels, and is caused by a deficiency of 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2). This potentially fatal disease is caused by autosomal recessive mutations in the HSD11B2 gene. The exploration and elucidation of this disease at our center and others has opened up a new area in receptor biology as a result of the demonstration that the specificity of the mineralocorticoid receptor (MR) function depends on a metabolic enzyme rather than the receptor itself.
Historical Background.
Although Werder et al. described a patient with similar clinical features in 1974, the first biochemical description of this disease was made by us in a 3-year-old Native American child from the Zuni tribe (56, 57). Detailed clinical and endocrine evaluations of this child established the presence of features that could not be explained by any known syndrome.
Aldosterone regulates electrolyte excretion and intravascular volume by stimulating increased resorption of sodium from the urine. It is the most potent mineralocorticoid synthesized, yet, despite strong evidence of mineralocorticoid excess and hyperaldosteronism, aldosterone was undetectable in the prismatic case and in similar cases that were subsequently identified. Thus it was initially thought that the condition was caused by an unknown mineralocorticoid (57, 58). However, our attempts to identify one were unsuccessful. Using tritiated cortisol, we then observed that the metabolism of cortisol to biologically inactive cortisone was decreased and serum cortisol half-life was prolonged, whereas the conversion of cortisone to cortisol was normal (59). This implied that 11β-hydroxysteroid dehydrogenase, the enzyme that converts cortisol to cortisone, may be deficient.
We and others postulated that the mineralocorticoid specificity of the MR was lost in these patients, allowing cortisol to bind to the MR and act as a mineralocorticoid (59–61). It was demonstrated that the MR has equal affinity for aldosterone and cortisol in vitro (62–65). However, circulating levels of cortisol are 100- to 1,000-fold higher than those of aldosterone, hence it appeared that endogenous exposure of the MR to cortisol could preempt the ability of aldosterone to be its ligand. In 1982, we proposed that cortisol was the mineralocorticoid inducing hypertension. (66).
The role 11β-HSD plays in mineralocorticoid action was confirmed from studies of the effects of the drug carbenoxolone and of extracts from the root of the licorice plant, Glycyrrhiza glabra. Licorice ingestion in large amounts can cause sodium retention, elevated blood pressure, and potassium wasting, resulting in a hypertensive state resembling AME (67). Glycyrrhetinic acid, the hydrolytic derivative of the active steroid in licorice extracts, is a competitive inhibitor of 11β-HSD (68, 69). Carbenoxolone is a semisynthetic analog of glycyrrhetinic acid and can also induce AME-like side effects, including sodium retention, hypokalemic alkalosis, low levels of plasma renin, and hypertension. However, unlike glycyrrhetinic acid, which inhibits only the dehydrogenase function of 11β-HSD, carbenoxolone inhibits both the dehydrogenase and the oxoreductase (i.e., converting cortisone to cortisol) function of the enzyme in the liver (68, 70).
Although defective 11β-HSD appeared to be a likely candidate for the cause of AME, the only form of the enzyme, the gene for which had been cloned at that time, was shown to be normal (71). The mRNA was found to be expressed predominantly in the liver (71). Because aldosterone acts primarily through its effects on the renal distal convoluted tubules and cortical collecting ducts, it was predicted that the MRs should exist in these locations. However, this 11β-HSD, which was NADP dependent, was shown to be expressed only in the proximal tubules (72–74), suggesting the presence of another isoform of 11β-HSD in the distal tubules. Further, no mutations were detected in the gene for “HSD11 ” (later described as type 1) in patients with AME (75).
A separate NAD-dependent isoform (type 2) that colocalized with the MR in the distal nephron was subsequently discovered by Naray-Fejes-Toth (76–79).† It possessed all of the properties necessary for protecting the MR: a very high affinity for endogenous glucocorticoids, a high abundance in target cells, and irreversible dehydrogenase activity. These data suggested that a defect in a type 2 isoform was responsible for AME (77, 79–81).
In 1995, cDNA encoding rabbit-collecting duct 11β-HSD type 2 was isolated, sequenced, and characterized by Naray-Fejes-Toth (82). The human cDNA for the type 2 isoform (11β-HSD2) was cloned, sequenced (83), and localized to chromosome 16q22 by Krozowski et al. (84) and by Agarwal et al. (85) by 1995 as well. The 11β-HSD2 enzyme is NAD-dependent and appears to have dehydrogenase activity only (80, 83). The Km of 11β-HSD2 for cortisol is 1–100 nM (78, 80, 83, 86), indicating that 11β-HSD2 has a 20- to 2,000-fold higher affinity for cortisol than does 11β-HSD1. In humans, 11β-HSD2 mRNA was expressed in kidney cortex and medulla, sigmoid and rectal colon, and salivary gland (87), and in the pancreas, prostate, ovary, small intestine, placenta, spleen, and testes (83).
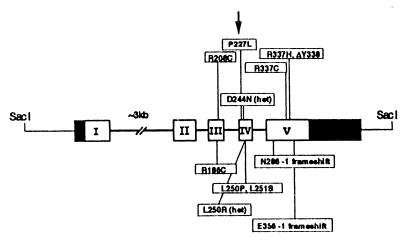
In 1995, we discovered the first mutation in the HSD11B2 gene in a consanguineous Iranian family with three sibs suffering from AME (a C to T transition resulting in a R337C mutation) (88). We and others have subsequently identified 17 mutations in the HSD11B2 gene in patients affected with AME (Fig. 7) (refs. 88–94; P. M. Stewart, personal communication). We have also shown, by in vitro expression studies, that the mutations impair the conversion of cortisol to cortisone (95, 96).
Figure 7.
Mutations in the gene for 11β-hydroxysteroid dehydrogenase type 2 in patients with AME who were investigated by our group. The HSD11B2 gene has five exons, is 6.2 kb long, and has been mapped to chromosome 16q22. All mutations found in affected patients are homozygous except for one patient, who is a compound heterozygote (D244N/L250R).
Epidemiology.
AME is rare, having been identified in only approximately 40 patients worldwide in the past 20 years. Owing to our primary role in the discovery and characterization of AME, we have been referred a large number of these patients and have been able to analyze the phenotype and genotype of these cases (90).
To date, most patients with AME who have had molecular genetic analysis have been homozygotes for one of the different mutations. Rare autosomal recessive mutations are classically explained by consanguinity, endogamy (a high coefficient of inbreeding), or by a founder effect. In our study in 1995 of eight families with members affected by AME, seven of the families appeared to fit one of these three explanations (89). Four families came from ancestry where consanguinity and tribal inbreeding were the custom, and three came from a Zoroastrian population that originated in Iran and was driven out by Muslims in the seventh century. In the remaining family, African-Americans from North Carolina, consanguinity was not proven. Few compound heterozygotes have been identified.
Clinical and Biochemical Features.
Classic AME.
We are one of the few centers in which a comprehensive AME study population was examined clinically, biochemically, and genetically.
AME usually presents early in life. Clinical features include severe hypertension, failure to thrive, and persistent polydipsia and polyuria. Biochemical profiles demonstrate metabolic alkalosis and severe hypokalemia. Plasma renin activity is low, suggesting a volume-expansion hypertension, which responds to dietary sodium restriction. All steroid levels, including aldosterone, are very low.
Biochemical diagnosis of AME can be made by measuring the ratio of cortisol to cortisone by the ratios of their urinary metabolites. In our report in 1998 of 14 AME patients studied at our center, urinary metabolites of cortisol demonstrated an abnormal ratio of tetrahydrocortisone/tetrahydrocortisone (THF/THE) with a predominance of THF (90). The ratio of THF+5αTHF to THE was 6.7 to 33, whereas the normal ratio is 1.0. The optimal diagnostic test is to measure the generation of tritiated water in plasma samples when 11-tritiated cortisol is injected, as described by Hellman et al. (97). Infusion of [11-3H]cortisol in our patients revealed the conversion of cortisol to cortisone to be 0–6% in typical AME patients, whereas the normal conversion is 90–95% (Table 1).
Table 1.
Review of AME patients studied at the New York Hospital–Cornell Medical Center: Signs and biochemical features at presentation and subsequent biochemical and genetic evaluation
| Patient | Kindred | Ethnicity | Age, yr | Sex | Birth weight, kg | BP, mmHg | BP (90th centile for age) | Serum K+, mmol/l | THF+ 5αTHF/THE | F secretion rate, mg/d | % Conversion F → E | HSD11B2 mutation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 1 | American Indian | 1.0 | F | 1.8 | 180/140 | 105/69 | 2.2 | 32.1 | 0.08 | 0 | E356 − 1 frameshift |
| 2 | 2 | Zoroastrian | 9.0 | M | 2.0 | 250/180 | 110/71 | 3.5 | 9.1† | 0.47 | 0 | R337H, ΔY338 |
| 3 | 3 | Italian-Moroccan | 4.0 | M | 2.3 | 160/110 | 104/67 | 3.1 | 33.0 | 0.72 | ND | L250R/D244N |
| 4 | 4 | African-American | 9.3 | F | 2.1 | 130/90 | 109/71 | 2.7 | 8.9 | 0.12 | 2 | R186C |
| 5 | 4 | African-American | 4.3 | F | 2.6 | 142/98 | 98/70 | 2.8 | 14.9 | 0.15 | 4.2 | R186C |
| 6 | 5 | East Indian | 0.8 | M | 2.0 | 150/100 | 105/67 | 2.4 | 20.1 | 0.05 | 6 | R337H, ΔY338 |
| 7 | 6 | East Indian | 2.1 | M | 2.3 | 150/100 | 91/68 | 1.79 | 12.5 | 0.83 | 2 | R337H, ΔY338 |
| 8 | 7 | Middle Eastern | 10.9 | M | 2.1 | 170/110 | 115/73 | 1.7 | 27.9 | 0.36 | ND | R208C |
| 9 | 7 | Middle Eastern | 9.3 | M | 2.4 | 160/118 | 110/71 | 2.9 | 27.3 | 0.24 | ND | R208C |
| 10 | 8 | American Indian | 3.3 | M | 2.0 | 205/130 | 99/60 | 0.9 | 26.8 | 0.51 | 1.5 | L250P, L251S |
| 11 | 9 | Persian | 14.0 | F | 2.2 | 220/160 | 116/79 | 2.8 | 8.91 | ND | ND | R337C |
| 12 | 9 | Persian | 11.6 | M | 2.1 | 170/110 | 110/72 | 2 | 6.85 | ND | ND | R337C |
| 13 | 9 | Persian | 4.0 | F | 2.4 | 160/100 | 98/70 | 3.1 | 6.7 | ND | ND | R337C |
| 14 | 10 | Turkish | 0.1 | M | 2.5 | 155/115 | 99/60 | 3.0 | 13.8 | ND | 4.5 | N286 − 1 frameshift |
| 15 | 11 | Mennonite | 12.6 | F | 3.6 | 160/90 | 110/72 | 5.0 | 3.0 | 0.55 | 58.4 | P227L |
| Normal values | >2.5 | 3.2–5.2 | 1.0 | 11.5 | 90–95% | |||||||
ND, not done; BP, blood pressure; F, cortisol; B, cortisone.
*, Patient 1 died;
, THF/THE.
In our report (90), all 14 patients studied had characteristic signs of a severe 11β-HSD2 defect, which were consistent with patients reported worldwide (56, 57, 59, 75, 88–91, 98–111): birth weights were lower than in their unaffected sibs, and the patients were short, underweight, and hypertensive for age. Damage of one or more organs (kidneys, retina, heart, and central nervous system) because of hypertension was found in all of the patients except one. In addition, most of these patients also had nephrocalcinosis and left ventricular hypertrophy. The followup studies of end-organ damage in six patients who had had 2 to 13 years of treatment revealed significant improvement in all patients, demonstrating the importance of early diagnosis and treatment. In our review of AME and suspected AME patients worldwide, 5 of 42 died of AME-related illnesses, and 2 diagnosed genetically were stillborn (112).
Mild form of AME.
All of the AME patients reported until 1998 had the characteristic signs of a severe 11β-HSD2 defect. We have recently reported the first patient with a mild form of AME.
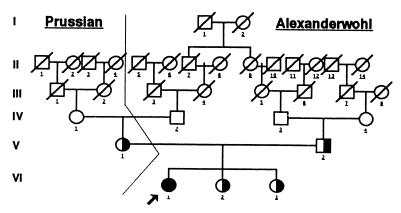
Asymptomatic hypertension was diagnosed in an American girl at age 12.5 years during a sports physical in 1995. She had normal serum electrolytes, undetectable plasma renin, serum aldosterone of <1.2 ng/dl, normal urinalysis, normal urine culture, normal intravenous pyelography, normal arteriogram of the kidney, normal renal scan, and normal heart size on chest x-ray. Birth weight was 7 lb 1 oz. The parents are consanguineous Mennonites of Prussian descent (Alexanderwohl Church) (Fig. 8). The only family member with hypertension is the maternal grandmother. Although the patient lacked hypokalemia and low birth weight and had only mild hypertension, we established a diagnosis of AME genetically. She exhibited only a moderately abnormal ratio of cortisol to cortisone metabolites [THF+5αTHF/THE was 3.0] and metabolism of cortisol to cortisone as determined by the measurement of tritiated water release after [11-3H]cortisol infusion (58%) (typical patients are <6%) (Table 1). The patient clearly had AME based on biochemical evidence and later was proven to be homozygous for the P227L mutation in the HSD11B2 gene.
Figure 8.
Pedigree of Mennonite family showing consanguinity (114).
Kinetic analysis, performed by our collaborator Z. Krozowski, showed an intermediary Km in this patient as compared with a severe AME patient (112). In whole cells, the P227L construct gave a Km for cortisol of 300 nM as compared with a Km 62 nM for the wild-type construct; in cell homogenates, the P227L construct gave a Km for cortisol of 350 nM and a Km of 54 nM for the wild-type construct. Another study of a severe AME patient demonstrated a Km of 1,010 nM as compared with the normal control of 110 nM (95, 96). The Km of 300 nM in this patient suggests a less severe defect than in other patients, resulting in her mild phenotype.
We believe that this mild form of AME may be prevalent in the inbred Mennonite population to which our patient belongs. We are in the process of studying this population of 2,000 members (i) to determine whether there are other cases of this mild form of AME and (ii) to establish the heterozygote frequency of the mutation found in the HSD11B2 gene of our patient in the Mennonites to ascertain the frequency of this disease in the European counterpart. So far, approximately 500 blood samples have been sent to us for DNA analysis from the Mennonite population in Goessel, KS, from which our patient comes. In our preliminary findings of 369 samples tested thus far, we identified nine carriers of the P227L mutation. This results in a heterozygote frequency of 2.4%.§ Considering there are only approximately 40 patients known worldwide with AME, this heterozygote frequency is very high.
Essential hypertension (the second highest cause of death) has been estimated to occur in 15 million residents in the United States, and approximately 40% are associated with low renin. If patients unrelated to the Mennonite community have similar mild mutations in the HSD11B2 gene, we may find that this disorder is an unrecognized cause of low-renin hypertension.
Acknowledgments
Significant sections of the work on which the data are reported herein were supported by U.S. Public Health Service Grant HD00072 and General Clinical Research Center Grant RR 06020. We express our appreciation to Laurie Vandermolen for her editorial assistance in the preparation of this manuscript.
Abbreviations
- CAH
congenital adrenal hyperplasia
- AME
apparent mineralocorticoid excess
- ACTH
adrenocorticotropic hormone
- 11β-HSD2
11β-hydroxysteroid dehydrogenase type 2
- 21-OHD
21-hydroxylase deficiency
- 17-OHP
17α-hydroxyprogesterone
- MR
mineralocorticoid receptor
- THF
tetrahydrocortisol
- THE
tetrahydrocortisone
Footnotes
Ugrasbul-Eksinar, F., New, M. I. & Wilson, R. C., Endocrine Society 81st Annual Meeting, June 12–15, 1999, San Diego, CA.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 30, 1996.
Provencher, P. H., Mercer, W. R., Funder, J. W. & Krozowski, Z. S., Endocrine Society 74th Annual Meeting, June 24–27, 1992, San Antonio, TX, Vol. 305, p. 128 (abstr).
References
- 1.De Crecchio L. Morgagni. 1865;7:154–188. [Google Scholar]
- 2.New M I, Dupont B, Grumbach K, Levine L S. In: The Metabolic Basis of Inherited Disease. Stanbury J B, Wyngaarden J B, Fredrickson D S, Goldstein J L, Brown M D, editors. New York: McGraw–Hill; 1982. pp. 973–1000. [Google Scholar]
- 3.Lin D, Sugawara T, Strauss J F, 3rd, Clark B J, Stocco D M, Saenger P, Rogol A, Miller W L. Science. 1995;267:1821–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- 4.New M I, White P C. In: Genetic and Molecular Biological Aspects of Endocrine Disease. Thakker R V, editor. London: Bailliere Tindall; 1995. pp. 525–554. [Google Scholar]
- 5.Jost A. In: Hermaphroditism, Genital Anomalies and Related Endocrine Disorders. Jones H W, Scott W W, editors. Baltimore: Williams & Wilkins; 1971. p. 16. [Google Scholar]
- 6.Prader A. Helv Paediatr Acta. 1958;13:2313. [PubMed] [Google Scholar]
- 7.New M I, Gertner J M, Speiser P W, Del Balzo P. J Endocrinol Invest. 1989;12:91–95. [PubMed] [Google Scholar]
- 8.New M I, Miller B, Peterson R E. J Clin Invest. 1966;45:412–428. doi: 10.1172/JCI105356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.New M I, Seaman M P. J Clin Endocrinol Metab. 1970;30:361–371. doi: 10.1210/jcem-30-3-361. [DOI] [PubMed] [Google Scholar]
- 10.Luetscher J A. Recent Prog Horm Res. 1956;12:175. [PubMed] [Google Scholar]
- 11.Rosler A, Levine L S, Schneider B, Novogroder M, New M I. J Clin Endocrinol Metab. 1977;45:500–512. doi: 10.1210/jcem-45-3-500. [DOI] [PubMed] [Google Scholar]
- 12.Stoner E, Dimartino-Nardi J, Kuhnle U, Levine L S, Oberfield S E, New M I. Clin Endocrinol (Oxford) 1986;24:9–20. doi: 10.1111/j.1365-2265.1986.tb03249.x. [DOI] [PubMed] [Google Scholar]
- 13.Speiser P W, Agdere L, Ueshiba H, White P C, New M I. N Engl J Med. 1991;324:145–149. doi: 10.1056/NEJM199101173240302. [DOI] [PubMed] [Google Scholar]
- 14.Decourt M J, Jayle M F, Baulieu E. Ann Endocrinol (Paris) 1957;18:416. [PubMed] [Google Scholar]
- 15.New M I, Lorenzen F, Pang S, Gunczler P, Dupont B, Levine L S. J Clin Endocrinol Metab. 1979;48:356–359. doi: 10.1210/jcem-48-2-356. [DOI] [PubMed] [Google Scholar]
- 16.Rosenwaks Z, Lee P A, Jones G S, Migeon C J, Wentz A C. J Clin Endocrinol Metab. 1979;49:335. doi: 10.1210/jcem-49-3-335. [DOI] [PubMed] [Google Scholar]
- 17.Levine L S, Dupont B, Lorenzen F, Pang S, Pollack M, Oberfield S, Kohn B, Lerner A, Cacciari E, Mantero F, et al. J Clin Endocrinol Metab. 1980;51:1316–1324. doi: 10.1210/jcem-51-6-1316. [DOI] [PubMed] [Google Scholar]
- 18.Laron Z, Pollack M S, Zamir R, Roitman A, Dickerman Z, Levine L S, Lorenzen F, O’Neill G J, Pang S, New M I, et al. Hum Immunol. 1980;1:55–66. doi: 10.1016/0198-8859(80)90009-9. [DOI] [PubMed] [Google Scholar]
- 19.Pollack M S, Levine L S, O’Neill G J, Pang S, Lorenzen F, Kohn B, Rondanini G F, Chiumello G, New M I, Dupont B. Am J Hum Genet. 1981;33:540–550. [PMC free article] [PubMed] [Google Scholar]
- 20.Levine L S, Dupont B, Lorenzen F, Pang S, Pollack M, Oberfield S E, Kohn B, Lerner A, Cacciari E, Mantero F, et al. J Clin Endocrinol Metab. 1981;53:1193–1198. doi: 10.1210/jcem-53-6-1193. [DOI] [PubMed] [Google Scholar]
- 21.Kohn B, Levine L S, Pollack M S, Pang S, Lorenzen F, Levy D, Lerner A J, Rondanini G F, Dupont B, New M I. J Clin Endocrinol Metab. 1982;55:817–827. doi: 10.1210/jcem-55-5-817. [DOI] [PubMed] [Google Scholar]
- 22.Pang S Y, Wallace M A, Hofman L, Thuline H C, Dorche C, Lyon I C, Dobbins R H, Kling S, Fujieda K, Suwa S. Pediatrics. 1988;81:866–874. [PubMed] [Google Scholar]
- 23.Pang S, Clark A. Trends Endocrinol Metab. 1990;1:300–307. doi: 10.1016/1043-2760(90)90068-e. [DOI] [PubMed] [Google Scholar]
- 24.Pang S, Clark A. Screening. 1993;2:105–139. [Google Scholar]
- 25.Speiser P W, Dupont B, Rubinstein P, Piazza A, Kastelan A, New I M. Am J Hum Genet. 1985;37:650–667. [PMC free article] [PubMed] [Google Scholar]
- 26.Sherman S L, Aston C E, Morton N E, Speiser P W, New M I. Am J Hum Genet. 1988;42:830–838. [PMC free article] [PubMed] [Google Scholar]
- 27.Zerah M, Ueshiba H, Wood E, Speiser P W, Crawford C, McDonald T, Pareira J, Gruen D, New M I. J Clin Endocrinol Metab. 1990;70:1662–1667. doi: 10.1210/jcem-70-6-1662. [DOI] [PubMed] [Google Scholar]
- 28.Bongiovanni A M, Eberlein W R, Cara J. J Clin Endocrinol Metab. 1954;14:409. doi: 10.1210/jcem-14-4-409. [DOI] [PubMed] [Google Scholar]
- 29.Butler G C, Marrian G F. J Biol Chem. 1937;119:565. [Google Scholar]
- 30.New M I, Lorenzen F, Lerner A J, Kohn B, Oberfield S E, Pollack M S, Dupont B, Stoner E, Levy D J, Pang S, et al. J Clin Endocrinol Metab. 1983;57:320–326. doi: 10.1210/jcem-57-2-320. [DOI] [PubMed] [Google Scholar]
- 31.White P C, New M I, Dupont B. N Engl J Med. 1987;316:1519–1524. doi: 10.1056/NEJM198706113162406. [DOI] [PubMed] [Google Scholar]
- 32.Dupont B, Oberfield S E, Smithwick E M, Lee T D, Levine L S. Lancet. 1977;2:1309–1312. doi: 10.1016/s0140-6736(77)90362-2. [DOI] [PubMed] [Google Scholar]
- 33.Nebert D W, Nelson D R, Coon M J. DNA Cell Biol. 1991;10:1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- 34.White P C, New M I, Dupont B. Proc Natl Acad Sci USA. 1984;81:1986–1990. doi: 10.1073/pnas.81.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White P C, New M I, Dupont B. Proc Natl Acad Sci USA. 1984;81:7505–7509. doi: 10.1073/pnas.81.23.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White P C, Chaplin D D, Weis J H, Dupont B, New M I, Seidman J G. Nature (London) 1984;312:465–467. doi: 10.1038/312465a0. [DOI] [PubMed] [Google Scholar]
- 37.White P C, Grossberger D, Onufer B J, Chaplin D D, New M I, Dupont B, Strominger J L. Proc Natl Acad Sci USA. 1985;82:1089–1093. doi: 10.1073/pnas.82.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White P C, New M I, Dupont B. Proc Natl Acad Sci USA. 1986;83:5111–5115. doi: 10.1073/pnas.83.14.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carroll M C, Campbell R D, Porter R R. Proc Natl Acad Sci USA. 1985;82:521–525. doi: 10.1073/pnas.82.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belt K T, Carroll M C, Porter R R. Cell. 1984;36:907–914. doi: 10.1016/0092-8674(84)90040-0. [DOI] [PubMed] [Google Scholar]
- 41.Higashi Y, Yoshioka H, Yamane M, Gotoh O, Fujii-Kuriyama Y. Proc Natl Acad Sci USA. 1986;83:2841–2845. doi: 10.1073/pnas.83.9.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krawczak M, Cooper D N. Trends Genet. 1997;13:121–122. doi: 10.1016/s0168-9525(97)01068-8. [DOI] [PubMed] [Google Scholar]
- 43.Werkmeister J W, New M I, Dupont B, White P C. Am J Hum Genet. 1986;39:461–469. [PMC free article] [PubMed] [Google Scholar]
- 44.Amor M, Parker K L, Globerman H, New M I, White P C. Proc Natl Acad Sci USA. 1988;85:1600–1604. doi: 10.1073/pnas.85.5.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Globerman H, Amor M, Parker K L, New M I, White P C. J Clin Invest. 1988;82:139–144. doi: 10.1172/JCI113562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White P C, Vitek A, Dupont B, New M I. Proc Natl Acad Sci USA. 1988;85:4436–4440. doi: 10.1073/pnas.85.12.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Speiser P W, New M I, White P C. N Engl J Med. 1988;319:19–23. doi: 10.1056/NEJM198807073190104. [DOI] [PubMed] [Google Scholar]
- 48.Tusie-Luna M, Speiser P W, Dumic M, New M I, White P C. Mol Endocrinol. 1991;5:685–692. doi: 10.1210/mend-5-5-685. [DOI] [PubMed] [Google Scholar]
- 49.Wilson R C, Mercado A B, Cheng K C, New M I. J Clin Endocrinol Metab. 1995;80:2322–2329. doi: 10.1210/jcem.80.8.7629224. [DOI] [PubMed] [Google Scholar]
- 50.Speiser P W, Laforgia N, Kato K, Pareira J, Khan R, Yang S Y, Whorwood C, White P C, Elias S, Schriock E, et al. J Clin Endocrinol Metab. 1990;70:838–848. doi: 10.1210/jcem-70-4-838. [DOI] [PubMed] [Google Scholar]
- 51.Wilson R C, Wei J Q, Cheng K C, Mercado A B, New M I. J Clin Endocrinol Metab. 1995;80:1635–1640. doi: 10.1210/jcem.80.5.7745011. [DOI] [PubMed] [Google Scholar]
- 52.White P C, Vitek A, Dupont B, New I. Proc Natl Acad Sci USA. 1988;85:4436–4440. doi: 10.1073/pnas.85.12.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forest M G, Betuel H, David M. Endocr Res. 1989;15:277–301. doi: 10.1080/07435808909039101. [DOI] [PubMed] [Google Scholar]
- 54.Forest M G, David M, Morel Y. J Steroid Biochem Mol Biol. 1993;45:75–82. doi: 10.1016/0960-0760(93)90125-g. [DOI] [PubMed] [Google Scholar]
- 55.Mercado A B, Wilson R C, Cheng K C, Wei J Q, New M I. J Clin Endocrinol Metab. 1995;80:2014–2020. doi: 10.1210/jcem.80.7.7608248. [DOI] [PubMed] [Google Scholar]
- 56.Werder E A, Zachmann M, Vollmin J A, Veyrat R, Prader A. Res Steroids. 1974;6:385–389. [Google Scholar]
- 57.New M I, Levine L S, Biglieri E G, Pareira J, Ulick S. J Clin Endocrinol Metab. 1977;44:924–933. doi: 10.1210/jcem-44-5-924. [DOI] [PubMed] [Google Scholar]
- 58.Ulick S, Ramirez L C, New M I. J Clin Endocrinol Metab. 1977;44:799–802. doi: 10.1210/jcem-44-4-799. [DOI] [PubMed] [Google Scholar]
- 59.Ulick S, Levine L S, Gunczler P, Zanconato G, Ramirez L C, Rauh W, Rosler A, Bradlow H L, New M I. J Clin Endocrinol Metab. 1979;49:757–764. doi: 10.1210/jcem-49-5-757. [DOI] [PubMed] [Google Scholar]
- 60.New M I, Oberfield S E, Carey R M, Greig F, Ulick S, Levine L S. In: Endocrinology of Hypertension, Serono Symposia No. 50. Mantero F, Biglieri E G, Edwards E R W, editors. New York: Academic; 1982. pp. 85–101. [Google Scholar]
- 61.Oberfield S E, Levine L S, Carey R M, Greig F, Ulick S, New M I. J Clin Endocrinol Metab. 1983;56:332–339. doi: 10.1210/jcem-56-2-332. [DOI] [PubMed] [Google Scholar]
- 62.Funder J W, Pearce P T, Smith R, Smith A I. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- 63.Arriza J L, Weinberger C, Cerelli G, Glaser T M, Handelin B L, Housman D E, Evans R M. Science. 1987;237:268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- 64.Krozowski Z S, Funder J W. Proc Natl Acad Sci USA. 1983;80:6056–6060. doi: 10.1073/pnas.80.19.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marver D, Stewart J, Funder J W, Feldman D, Edelman I S. Proc Natl Acad Sci USA. 1974;71:1431–1435. doi: 10.1073/pnas.71.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.New M I, Oberfield S E, Carey R M, Greig F, Ulick S, Levine L S. Endocrinology of Hypertension, Serono Symposia No. 50. New York: Academic; 1982. pp. 85–101. [Google Scholar]
- 67.Stewart P M, Wallace A M, Valentino R, Burt D, Shackleton C H, Edwards C R. Lancet. 1987;ii:821–824. doi: 10.1016/s0140-6736(87)91014-2. [DOI] [PubMed] [Google Scholar]
- 68.Stewart P M, Wallace A M, Atherden S M, Shearing C H, Edwards C R. Clin Sci. 1990;78:49–54. doi: 10.1042/cs0780049. [DOI] [PubMed] [Google Scholar]
- 69.Farese R V, Jr, Biglieri E G, Shackleton C H, Irony I, Gomez-Fontes R. N Engl J Med. 1991;325:1223–1227. doi: 10.1056/NEJM199110243251706. [DOI] [PubMed] [Google Scholar]
- 70.Funder J W. Endocr Res. 1989;15:227–238. doi: 10.1080/07435808909039098. [DOI] [PubMed] [Google Scholar]
- 71.Tannin G M, Agarwal A K, Monder C, New M I, White P C. J Biol Chem. 1991;266:16653–16658. [PubMed] [Google Scholar]
- 72.Edwards C R, Stewart P M, Burt D, Brett L, McIntyre M A, Sutanto W S, de Kloet E R, Monder C. Lancet. 1988;2:986–989. doi: 10.1016/s0140-6736(88)90742-8. [DOI] [PubMed] [Google Scholar]
- 73.Castello R, Schwarting R, Muller C, Hierholzer K. Renal Physiol Biochem. 1989;12:320–327. doi: 10.1159/000173209. [DOI] [PubMed] [Google Scholar]
- 74.Rundle S E, Funder J W, Lakshmi V, Monder C. Endocrinology. 1989;125:1700–1704. doi: 10.1210/endo-125-3-1700. [DOI] [PubMed] [Google Scholar]
- 75.Nikkila H, Tannin G M, New M I, Taylor N F, Kalaitzoglou G, Monder C, White P C. J Clin Endocrinol Metab. 1993;77:687–691. doi: 10.1210/jcem.77.3.8370690. [DOI] [PubMed] [Google Scholar]
- 76.Naray-Fejes-Toth A, Watlington C O, Fejes-Toth G. Endocrinology. 1991;129:17–21. doi: 10.1210/endo-129-1-17. [DOI] [PubMed] [Google Scholar]
- 77.Mercer W R, Krozowski Z S. Endocrinology. 1992;130:540–543. doi: 10.1210/endo.130.1.1727721. [DOI] [PubMed] [Google Scholar]
- 78.Brown R W, Chapman K E, Edwards C R, Seckl J R. Endocrinology. 1993;132:2614–2621. doi: 10.1210/endo.132.6.8504762. [DOI] [PubMed] [Google Scholar]
- 79.Naray-Fejes-Toth A, Fejes-Toth G. Steroids. 1994;59:105–110. doi: 10.1016/0039-128x(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 80.Rusvai E, Naray-Fejes-Toth A. J Biol Chem. 1993;268:10717–10720. [PubMed] [Google Scholar]
- 81.Krozowski Z, Ma Guire J A, Stein-Oakley A N, Dowling J, Smith R E, Andrews R K. J Clin Endocrinol Metab. 1995;80:2203–2209. doi: 10.1210/jcem.80.7.7608280. [DOI] [PubMed] [Google Scholar]
- 82.Naray-Fejes-Toth A, Fejes-Toth G. Endocrinology. 1995;136:2579–2586. doi: 10.1210/endo.136.6.7750480. [DOI] [PubMed] [Google Scholar]
- 83.Albiston A L, Obeyesekere V R, Smith R E, Krozowski Z S. Mol Cell Endocrinol. 1994;105:R11–R17. doi: 10.1016/0303-7207(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 84.Krozowski Z S, Baker E, Obeyesekere V, Callen D F. Cytogenet Cell Genet. 1995;71:124–125. doi: 10.1159/000134089. [DOI] [PubMed] [Google Scholar]
- 85.Agarwal A K, Rogerson F M, Mune T, White P C. Genomics. 1995;29:195–199. doi: 10.1006/geno.1995.1231. [DOI] [PubMed] [Google Scholar]
- 86.Agarwal A K, Mune T, Monder C, White P C. J Biol Chem. 1994;269:25959–25962. [PubMed] [Google Scholar]
- 87.Whorwood C B, Mason J I, Rickets M L, Howie A J, Stewart P M. Mol Cell Endocrinol. 1995;110:R7–R11. doi: 10.1016/0303-7207(95)03546-j. [DOI] [PubMed] [Google Scholar]
- 88.Wilson R C, Krozowski Z S, Li K, Obeyesekere V R, Razzaghy-Azar M, Harbison M D, Wei J Q, Shackleton C H L, Funder J W, New M I. J Clin Endocrinol Metab. 1995;80:2263–2266. doi: 10.1210/jcem.80.7.7608290. [DOI] [PubMed] [Google Scholar]
- 89.Wilson R C, Harbison M D, Krozowski Z S, Funder J W, Shackleton C H L, Hanauske-Able H M, Wei J Q, Hertecant J, Moran A, Neiberger R E, et al. J Clin Endocrinol Metab. 1995;80:3145–3150. doi: 10.1210/jcem.80.11.7593417. [DOI] [PubMed] [Google Scholar]
- 90.Dave-Sharma S, Wilson R C, Harbison M D, Newfield R, Razzaghy-Azar M, Krozowski Z, Funder J W, Shackleton C H L, Bradlow H L, Wei J, et al. J Clin Endo Metab. 1998;83:2244–2254. doi: 10.1210/jcem.83.7.4986. [DOI] [PubMed] [Google Scholar]
- 91.Mune T, Rogerson F M, Nikkila H, Agarwal A K, White P C. Nat Genet. 1995;10:394–399. doi: 10.1038/ng0895-394. [DOI] [PubMed] [Google Scholar]
- 92.Stewart P M, Krozowski Z S, Gupta A, Milford D V, Howie J A, Sheppard M C. Lancet. 1996;347:88–91. doi: 10.1016/s0140-6736(96)90211-1. [DOI] [PubMed] [Google Scholar]
- 93.Kitanaka S, Katsumata N, Tanae A, Hibi I, Takeyama K, Fuse H, Kato S, Tanaka T. J Clin Endocrinol Metab. 1997;82:4054–4058. doi: 10.1210/jcem.82.12.4455. [DOI] [PubMed] [Google Scholar]
- 94.Li A, Li K X Z, Marui S, Krozowski Z S, Batista M C, Whorwood C B, Arnhold I J P, Shackleton C H L, Mendonca B B, Stewart P M. J Hypertens. 1997;15:1397–1402. doi: 10.1097/00004872-199715120-00005. [DOI] [PubMed] [Google Scholar]
- 95.Obeyesekere V R, Ferrari P, Andrews R K, Wilson R C, New M I, Funder J W, Krozowski Z S. J Clin Endocrinol Metab. 1995;80:3381–3383. doi: 10.1210/jcem.80.11.7593456. [DOI] [PubMed] [Google Scholar]
- 96.Ferrari P, Obeyesekere V R, Li K, Wilson R C, New M I, Funder J W, Krozowski Z S. Mol Cell Endocrinol. 1996;119:21–24. doi: 10.1016/0303-7207(96)03787-2. [DOI] [PubMed] [Google Scholar]
- 97.Hellman L, Nakada F, Zumoff B, Fukushima D, Bradlow H L, Gallagher T F. J Clin Endocrinol Metab. 1971;33:52–62. doi: 10.1210/jcem-33-1-52. [DOI] [PubMed] [Google Scholar]
- 98.Shackleton C H, Rodriguez J, Arteaga E, Lopez J M, Winter J S. Clin Endocrinol (Oxford) 1985;22:701–712. doi: 10.1111/j.1365-2265.1985.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 99.Stewart P M, Corrie J E, Shackleton C H, Edwards C R. J Clin Invest. 1988;82:340–349. doi: 10.1172/JCI113592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Batista M C, Mendonca B B, Kater C E, Arnhold I J, Rocha A, Nicolau W, Bloise W. J Pediatr. 1986;109:989–993. doi: 10.1016/s0022-3476(86)80282-7. [DOI] [PubMed] [Google Scholar]
- 101.Monder C, Shackleton C H, Bradlow H L, New M I, Stoner E, Iohan F, Lakshmi V. J Clin Endocrinol Metab. 1986;63:550–557. doi: 10.1210/jcem-63-3-550. [DOI] [PubMed] [Google Scholar]
- 102.Kitanaka S, Tanae A, Hibi I. Clin Endocrinol (Oxford) 1996;44:353–359. doi: 10.1046/j.1365-2265.1996.677500.x. [DOI] [PubMed] [Google Scholar]
- 103.Sann L, Revol A, Zachmann M, Legrand J C, Bethenod M. J Clin Endocrinol Metab. 1976;43:265–271. doi: 10.1210/jcem-43-2-265. [DOI] [PubMed] [Google Scholar]
- 104.Shackleton C H, Honour J W, Dillon M J, Chantler C, Jones R W. J Clin Endocrinol Metab. 1980;50:786–702. doi: 10.1210/jcem-50-4-786. [DOI] [PubMed] [Google Scholar]
- 105.Honour J W, Dillon M J, Levin M, Shah V. Arch Dis Child. 1983;58:1018–1020. doi: 10.1136/adc.58.12.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Milford D V, Shackleton C H L, Stewart P M. Clin Endocrinol (Oxford) 1995;43:241–246. doi: 10.1111/j.1365-2265.1995.tb01923.x. [DOI] [PubMed] [Google Scholar]
- 107.Muller-Berghaus J, Homoki J, Michalk D V, Querfeld U. Acta Paediatr. 1996;85:111–113. doi: 10.1111/j.1651-2227.1996.tb13903.x. [DOI] [PubMed] [Google Scholar]
- 108.Fiselier T J, Otten B J, Monnens L A, Honour J W, van Munster P J. Horm Res. 1982;16:107–114. doi: 10.1159/000179490. [DOI] [PubMed] [Google Scholar]
- 109.Harinck H I, van Brummelen P, Van Seters A P, Moolenaar A J. Clin Endocrinol (Oxford) 1984;21:505–514. doi: 10.1111/j.1365-2265.1984.tb01388.x. [DOI] [PubMed] [Google Scholar]
- 110.Winter J S D, McKenzie J K. In: Juvenile Hypertension. New M I, Levine S, editors. New York: Raven; 1977. pp. 123–132. [Google Scholar]
- 111.Gourmelen M, Saint-Jacques I, Morineau G, Soliman H, Julien R, Fiet J. Eur J Endocrinol. 1996;135:238–244. doi: 10.1530/eje.0.1350238. [DOI] [PubMed] [Google Scholar]
- 112.Wilson R C, Dave-Sharma S, Wei J, Obeyesekere V R, Li K, Ferrari P, Krozowski Z S, Shackleton C H L, Bradlow L, Wiens T, et al. Proc Natl Acad Sci USA. 1998;95:10200–10205. doi: 10.1073/pnas.95.17.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.New M I, DuPont B, Grumbach K, Levine L S. In: The Metabolic Basis of Inherited Disease. Stanbury J B, Wyngaarden J B, Fredrickson D S, Goldstein J L, Brown M S, editors. New York: McGraw–Hill; 1983. pp. 973–1000. [Google Scholar]
- 114.Wedel D C. The Story of Alexanderwohl. Newton, KA: Mennonite Press; 1974. [Google Scholar]
- 115.Mercado A B, Wilson R C, Chung K C, Wei J-Q, New M I. J Clin Endocrinol Metab. 1995;80:2014–2020. doi: 10.1210/jcem.80.7.7608248. [DOI] [PubMed] [Google Scholar]